Abstract
Previous studies have shown that inhibition of receptor-interacting serine/threonine kinase (RICK) (also known as RIP2) results in amelioration of experimental colitis. This role has largely been attributed to nucleotide-binding oligomerization domain 2 (NOD2) signaling since the latter is considered a major inducer of RICK activation. In this study, we explored the molecular mechanisms accounting for RICK-mediated inhibition of inflammatory bowel disease (IBD). In an initial series of studies focused on trinitrobenzene sulfonic acid (TNBS)-colitis and dextran sodium sulfate (DSS)-colitis we showed that down-regulation of intestinal RICK expression in NOD2-intact mice by intra-rectal administration of a plasmid expressing RICK-specific siRNA was accompanied by down-regulation of pro-inflammatory cytokine responses in the colon and protection of the mice from experimental colitis. Somewhat surprisingly, intra-rectal administration of RICK-siRNA also inhibited TNBS-colitis and DSS-colitis in NOD2-deficient and in NOD1/NOD2-double deficient mice. In complementary studies of humans with IBD we found that expression of RICK, cellular inhibitor of apoptosis protein 2 (cIAP2) and downstream signaling partners were markedly increased in inflamed tissue of IBD compared to controls without marked elevations of NOD1 or NOD2 expression. In addition, the increase in RICK expression correlated with disease activity and pro-inflammatory cytokine responses. These studies thus suggest that NOD1- or NOD2-independenent activation of RICK plays a major role in both murine experimental colitis and human IBD.
Keywords: inflammatory bowel diseases, NOD2, RICK/RIP2
RICK/RIP2 can mediate NOD2-independent gut inflammation
Introduction
The association of loss-of-function nucleotide-binding oligomerization domain 2 (NOD2) gene polymorphisms with increased risk for Crohn’s disease (CD) development strongly implies that NOD2 function, by whatever mechanism, protects against the development of the gut inflammation characterizing this disease (1–6). This notion is supported by the possibility that NOD2 has a host defense function in the gut mucosa and thus limits the survival of gut organisms potentially capable of inducing inflammation in the lamina propria or that it down-regulates excessive innate responses also capable of causing inflammation in this milieu (1–10). On this basis one might predict that inhibition of receptor-interacting serine/threonine kinase (RICK, also called RIP2) function, the obligate downstream signaling partner of NOD2, would have the same effect as loss of NOD2 function. Paradoxically, this has proven not to be the case (11): both in early studies in which RICK was inhibited by a relatively non-specific inhibitor or in more recently studies in which RICK was inhibited by highly specific inhibitors, RICK inhibition resulted in reduced levels of mouse models of colitis such as trinitrobenzene sulfonic acid (TNBS)-colitis (12), dextran sodium sulfate (DSS)-colitis (13) and SAMP1/Yit-colitis (14) as well as in reduced cytokine production by explants of tissue obtained from inflammatory bowel disease (IBD) patients (15).
One way of explaining this apparent contradiction is to postulate that RICK activation plays a central role in experimental gut inflammation by a mechanism that does not rely solely on NOD1 or NOD2 signaling. This possibility was initially supported by studies of RICK-deficient mice conducted by Kobayashi et al., in which it was shown that RICK deficiency was marked by decreased T-cell receptor-mediated T-cell proliferation and decreased Toll-like receptor (TLR) responses (16); similarly, in studies of RICK-deficient mice conducted by Chin et al., decreased T helper type 1 responses and defective IL-12-induced signal transducer and activator of transcription 4 activation were observed in the absence of RICK (17). These studies, however, have been challenged by more recent studies of RICK function in which it was found that cells lacking RICK exhibited changes neither in TLR-induced responses in macrophages nor in T-cell responses but did manifest reduced responses to NOD1 and NOD2 ligands (18, 19). These studies thus implied that, in spite of the earlier findings, RICK activation is specifically dependent on NOD1 and NOD2 stimulation and that RICK plays a minor role in gut inflammation in the absence of such stimulation.
To resolve these conflicting data regarding RICK function, we initially conducted studies of TNBS-colitis and DSS-colitis in mice in which RICK levels were depleted by in vivo administration of a plasmid expressing siRNA targeting RICK (embedded in a hemagglutinating virus of Japan-envelope, HVJ-E) and showed that such depletion was accompanied by greatly diminished experimental colitis. TNBS-colitis and DSS-colitis induced in NOD2-deficient or NOD1/NOD2-double deficient mice were also ameliorated by administration of siRNA targeting RICK, indicating that the effect of RICK depletion on colitis can occur independently of either NOD1 or NOD2 signaling. In companion studies of humans with ulcerative colitis (UC) and CD we examined the expression of NOD1, NOD2 and RICK mRNA in gut tissues from patients with both active and quiescent disease. In addition, we assessed the relation of RICK expression to cytokine synthesis. We found that mean NOD1 mRNA levels were marginally increased and mean NOD2 mRNA levels were unchanged or marginally decreased in IBD patients compared to controls. In contrast, mean RICK mRNA levels were quite clearly increased in IBD patients, especially those with active disease, and RICK was expressed in cells producing cytokines. Overall, these studies show that activation of RICK is involved in the immunopathogenesis of both experimental intestinal inflammation and human IBD and that such activation can occur independently of NOD1/NOD2 signaling.
Methods
Patients
Patients with IBD (CD; n = 28, UC; n = 118) were diagnosed as previously described (20). Clinical characteristics of these patients are summarized in Supplementary Table 1. Disease activity of CD and UC was determined as previously described (20). Three and 25 patients with CD were defined as remission and active disease, respectively, based on the endoscopic examinations. Forty-nine and 69 patients with UC were defined as remission and active disease, respectively, as previously described (20). Biopsy samples were obtained from these patients at the time of endoscopy and subjected to mRNA preparation also as previously described (20). Colonic biopsy samples from non-tumorous portions were obtained from patients with colonic polyps or early colon cancer at the time of colonoscopy and were used as control specimens. Colonic surgical specimens obtained from patients with CD (n = 9) or UC (n = 8) who underwent surgical operations were used for immunofluorescence analysis. Surgical operations were performed in these patients because of the following reasons; CD: perforation (n = 2), severe stricture (n = 3), ileus (n = 3) and diagnostic laparotomy for colitis (n = 1); UC: uncontrollable disease (n = 4), toxic megacolon (n = 2) and massive hemorrhage (n = 2). Non-cancerous portions of early colorectal cancers (n = 4) were used as controls for immunofluorescence analysis. Ethical permission of this study was granted by the review boards of Kindai University Faculty of Medicine.
Induction of colitis
TNBS-colitis was induced in C57BL/10 mice obtained from Japan SLC (Hamamatsu, Japan) as described previously (6). On day −2, −1 and 0, mice received intra-rectal administration of a plasmid expressing RICK-specific siRNA (InvivoGen, San Diego, CA, USA, 100 μg) or a control [luciferase (LUC)-specific siRNA, InvivoGen, 100 μg] plasmid encapsulated in a HVJ-E (Ishihara Sangyo, Osaka, Japan) for a total of three times before intra-rectal administration of 3.75 mg of TNBS (Sigma, St Louis, MO, USA) in 100 μl of 45% ethanol (6). In some experiments, mice received intra-peritoneal administration of pan-IAP inhibitor (AT406, 100 μg, Funakoshi, Tokyo, Japan) (21) or DMSO for a total of three times before intra-rectal administration of 3.75 mg of TNBS in 100 μl of 45% ethanol. TNBS-colitis was induced in C57BL/6 mice (Japan SLC), NOD2-deficient mice (6) and NOD1/NOD2-double deficient mice through intra-rectal application of 3.75 mg of TNBS in 100 μl of 50% ethanol on day 0 and day 2. NOD1/NOD2-double deficient mice were created by crossing NOD1- (22) or NOD2-deficient mice (6). DSS-colitis was induced as described previously (6). Mice were treated with 4% DSS in the drinking water from day 0 to day 6. On day 0, 1 and 2, mice received intra-rectal administration of a plasmid expressing RICK-specific siRNA (75 μg) or expressing LUC-specific siRNA (75 μg) encapsulated in a HVJ-E for a total of three times. At the indicated time points, mice were sacrificed and colon tissue samples were stained with hematoxylin and eosin (H&E). The scoring of the inflammation was performed as described previously (5, 6). Protocols of animal experiments were approved by the review boards of Kindai University Faculty of Medicine.
RNA isolation and quantitative PCR
Trizol reagent (Invitrogen, Carlsbad, CA, USA) was used to extract total RNA and Superscript III (Invitrogen) was used to synthesize single-stranded cDNA. The mRNA level for each target gene was determined by SYBR Green-based quantitative PCR (qPCR) using a LightCycler 480 system (Roche, Tokyo, Japan) and Quantitect primer assays (Qiagen, Valencia, CA, USA) followed by the normalization using GAPDH as a reference gene. Each PCR primer for Quantitect primer assays was purchased from Qiagen.
Immunohistochemical and immunofluorescence analysis
Colon samples were fixed in 10% formalin. Deparaffinized sections were incubated with anti-CD11b antibody (Abcam, Cambridge, MA, USA), anti-CD3 antibody (Abcam) and anti-phospho-IκBα antibody (pIκBα, Cell Signaling Technology, CST, Cambridge, MA, USA). Protein expression was visualized by the Dako Envision system (Dako Japan, Tokyo, Japan) as previously described (23, 24). The numbers of cells positive for these proteins were counted in high-power fields in each slide as previously described (23, 24). For the immunofluorescence analysis, deparaffinized sections were incubated with rabbit anti-IL-6 antibody (Abcam), rabbit anti-CD11c antibody (Abcam), mouse anti-TNF-α antibody (Abcam), mouse or rabbit anti-RICK antibody (Abcam), rabbit anti-cellular inhibitor of apoptosis protein 1 (cIAP1) antibody (Abcam), rabbit anti-cIAP2 antibody (Abcam) and rabbit anti-TGF-β-activated kinase 1 (TAK1) antibody (Abcam) followed by the incubation with Alexa 488- or Alexa 546-conjugated anti-mouse or rabbit IgG (Invitrogen). IgG from mouse or rabbit serum (Sigma) was used for isotype antibody staining. For visualization of interaction between cIAP2 and RICK or between TAK1 and RICK in human samples, deparaffinized sections were subjected to the protocols by using combinations of rabbit anti-cIAP2 antibody (Abcam), rabbit anti-TAK1 antibody (Abcam) and mouse anti-RICK antibody (BD Biosciences, San Jose, CA, USA) suggested by the Duolink In Situ kit (5). For visualization of interaction between cIAP2 and RICK or between cIAP1 and RICK in murine samples, rabbit anti-cIAP1 antibody (Abcam), rabbit anti-cIAP2 antibody (Abcam) and mouse anti-RICK antibody (BD Biosciences) were used. three to five representative immunohistochemical and immunofluorescence photographs were taken in each case by microscopy (Biozero BZ-8100, Keyence, Osaka, Japan) from each slide prepared and the numbers of positive cells were determined as previously described (23, 24).
Immunoblotting
Colonic lamina propria mononuclear cells (LPMCs) were isolated from mice at the indicated time points after the treatment with TNBS or DSS and protein lysates were prepared as previously described (5, 6). Anti-IκBα, anti-pIκBα, anti-phospho-p38 (pp38) anti-p38, anti-phospho-extracellular signal-regulated kinase (pERK), anti-ERK, anti-phospho-c-JUN N-terminal kinase (pJNK) and anti-RICK antibodies were obtained from CST. Anti-JNK1 antibody (Santa Cruz Biotechnology, Dallas, TX, USA), anti-actin antibody (Santa Cruz Biotechnology), anti-cIAP1 antibody (Santa Cruz Biotechnology) and anti-cIAP2 antibody (Abcam) were used.
Enzyme-linked immunosorbent assays
Colonic LPMCs and mesenteric lymph node (MLN) cells were isolated from mice 3 or 4 days after the treatment with TNBS or 8 days after the treatment with DSS as previously described (8) and stimulated with Pam3CSK4 (PAM, TLR2 ligand, 10 μg ml−1, InvivoGen), lipopolysaccharide (LPS, TLR4 ligand, 1 μg ml−1, Sigma), FK565 (NOD1 ligand, 10 μg ml−1, Astellas Pharma Inc., Tokyo, Japan), muramyl dipeptide (MDP, NOD2 ligand, 10 μg ml−1, InvivoGen) and anti-CD3 mAb (1 μg ml−1, BD Biosciences) for 48 h. Protein concentrations of cytokines were determined by eBioscience (San Diego, CA, USA) or R&D systems (Minneapolis, MN, USA) enzyme-linked immunosorbent assay (ELISA) kits for mouse IL-12p40, IL-6, TNF-α, IL-10, IL-17 and IFN-γ as previously described (5, 6).
Statistical analysis
Student’s t-test was used to evaluate the significance of the differences. Log-rank test was used to evaluate the significance of the differences in survival rates. Statistical analysis was performed with the Prism Program (Graphpad Software, La Jolla, CA, USA). A value of P < 0.05 was regarded as statistically significant. Correlation analysis was also performed with the Prism Program. The Bonferroni correction was used in the statistical analyses of data obtained from patients’ samples (Figs 3 and 4).
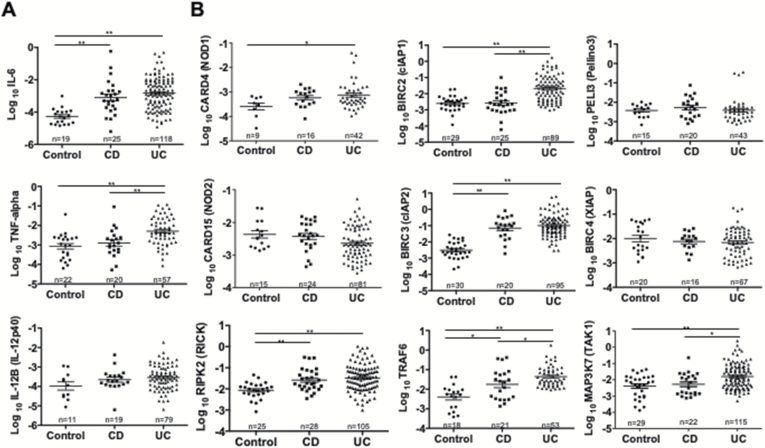
Fig. 3.
Colonic mucosa of patients with IBDs exhibits enhanced expression of RICK, cIAP1, cIAP2, TRAF6 and TAK1. Endoscopic biopsy samples were obtained from inflammatory portions of patients with CD and UC and subjected to qPCR analysis. Colonic biopsy samples obtained from non-tumorous portions of patients with colonic polyps or early colon cancer were used as controls. mRNA expression was normalized by using GAPDH as a reference gene. (A) Expression of pro-inflammatory cytokines (IL-6, TNF-α and IL-12p40) is shown. (B) Expression of NOD1, NOD2, RICK, cIAP1, cIAP2, TRAF6, Pellino 3, XIAP and TAK1 is shown. The number associated with each data set shows the number of patients studied. Results are expressed as mean ± SEM. Each dot represents a value of each patient sample. *P < 0.05, **P < 0.01.
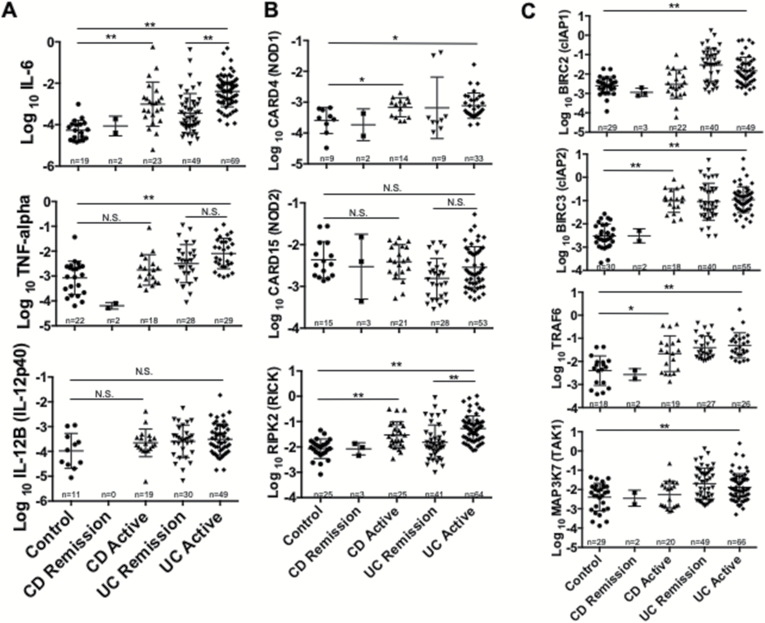
Fig. 4.
Colonic mucosa of patients with active UC and CD shows enhanced expression of RICK as compared with remission disease. Expression of pro-inflammatory cytokines (IL-6, TNF-α, IL-12p40) and RICK-associated molecules was measured by qPCR in patients with CD and UC with active or remitted disease as described in Fig. 3. (A) Expression of pro-inflammatory cytokines (IL-6, TNF-α, IL-12p40) is shown. (B, C) Expression of NOD1, NOD2, RICK, cIAP1, cIAP2, TRAF6 and TAK1 is shown. The number associated with each data set shows the number of patients studied. Results are expressed as mean ± SEM. *P < 0.05, **P < 0.01, N.S., not significant.
Results
Prevention of TNBS-colitis by down-regulation of RICK (RIP2)
In initial studies we sought to determine the role of RICK-mediated signaling in gut inflammation using a murine model previously shown to reproduce important aspects of human CD, TNBS-colitis (7, 25, 26). To this end, we administered plasmids expressing a RICK-specific siRNA as well as a control plasmid expressing LUC-specific siRNA, each encapsulated in a HVJ-E per rectum to mice prior to the induction of TNBS-colitis. As shown in our previous studies such encapsulated plasmids have excellent cellular penetration and as such can influence levels of intracellular components such as RICK (5, 6). Indeed, in the present study mice treated with encapsulated RICK-siRNA exhibited a marked reduction in RICK expression as compared to mice treated with control LUC-siRNA in colonic LPMCs (Supplementary Figure 1A).
We found that TNBS-colitis was greatly reduced in mice administered RICK-specific siRNA as judged by degree of body weight loss and tissue pathology score (Supplementary Figure 1B and C). The survival rate of the RICK-siRNA group was 100.0% (12/12), whereas that of the LUC-siRNA group was 75.0% (9/12, Supplementary Figure 1B). In addition, as revealed by immunoblotting (Supplementary Figure 1A), colonic LPMCs in these mice exhibited a marked reduction of RICK, cIAP1 and cIAP2 levels and, as detected by proximity ligation assay (PLA), marked reduction of intracellular interaction between cIAP1 or cIAP2 and RICK (Supplementary Figure 1D).
In accompanying studies, we found that knock-down of RICK expression was accompanied by decreased activation of nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) pathways as evidenced by a marked reduction of colonic LPMC expression of pIκBα, pp38, pERK and pJNK (Supplementary Figure 1A). Moreover, knock-down of RICK expression inhibited the accumulation of inflammatory cells such as CD11b+ myeloid cells and CD3+ T cells and reduced tissue expression of pIκBα (Supplementary Figure 2A) (note that isotype control antibody staining of tissues in these studies using mouse IgG or rabbit IgG was negative). Finally, mice subjected to RICK knock-down exhibited a marked reduction in colonic LPMC production of IL-12p40, TNF-α, IL-6 and IFN-γ following treatment of these cells with TLR and NOD2 ligands or anti-CD3 mAb (Supplementary Figure 2B). Taken together, these data strongly suggest that intra-rectal administration of RICK-siRNA leading to RICK knock-down inhibits TNBS-colitis via blockade of RICK activation and consequently its downstream activation of pro-inflammatory pathways.
Prevention of TNBS-colitis by administration of a pan-IAP inhibitor (AT406)
RICK activation of NF-κB depends on its Lysine 63 (K63)-linked polyubiquitination by various kinds of E3-ligases such as cIAP1, cIAP2 and X-linked inhibitor of apoptosis protein (XIAP) (5, 27–31). In studies parallel to those described above we asked whether the systemic administration of AT406, an inhibitor of cIAP, cIAP2 and XIAP expression that impairs RICK ubiquitination and activation (21, 32, 33) attenuates TNBS-colitis. We found that, indeed, administration of AT406 inhibited the expression of both cIAP1 and cIAP2 in the lamina propria and, at the same time, inhibited the development of TNBS-colitis as judged by the degree of body weight loss and tissue pathology score (Supplementary Figure 3A–C). The survival rates of control DMSO and AT406 groups were comparable: DMSO group: 7/15 (46.7%); AT406 group: 7/15 (46.7%, Supplementary Figure 3A). Such attenuation of TNBS-colitis by AT406 was accompanied by a reduction of inflammatory immune cell infiltration into the colon as indicated by the number of LPMCs positive for CD3, CD11b or pIκBα (Supplementary Figure 3D) and MLN cells isolated from C57BL/10 mice treated with AT406 produced a much lower amount of IL-12p40 and IL-6 upon stimulation with TLR and NOD2 ligands as compared with those treated with DMSO (Supplementary Figure 3E). Taken together, these data provide additional evidence that inhibition of RICK (in this case by preventing its activation by IAPs) inhibits the development of TNBS-colitis.
Inhibition of TNBS-colitis through down-regulation of RICK (RIP2) expression in the absence of NOD2 or both NOD1 and NOD2
In studies parallel to those described above in which the effect of down-regulation of RICK expression was evaluated in NOD2-intact C57BL/10 mice with TNBS-colitis we evaluated the effect of RICK down-regulation on TNBS-colitis in C57BL/6 mice lacking NOD2 alone or both NOD1 and NOD2 (6). C57BL/10 mice are more susceptible to TNBS-colitis than C57BL/6 mice (5, 6); thus, two doses of TNBS were required to induce a comparable level of colitis in C57BL/6 mice (with NOD1/NOD2 deficiency) as induced by one dose in C57BL/10 mice.
In studies of mice lacking NOD2 alone we found that TNBS-colitis induced by a comparable regimen as that employed in NOD2-intact mice was as severe as in NOD2-intact mice as judged by weight loss following intra-rectal TNBS administration (Supplementary Figure 4). In addition, down-regulation of RICK expression in colonic LPMCs of NOD2-deficient mice by administration of encapsulated siRNA targeting RICK led to greatly decreased TNBS-colitis in such mice as evaluated by weight loss (Supplementary Figure 4A and B) and by a greatly decreased anti-CD3 mAb-induced IFN-γ response as well as by a decreased TLR-induced IL-12p40 response by colonic LPMCs (data not shown).
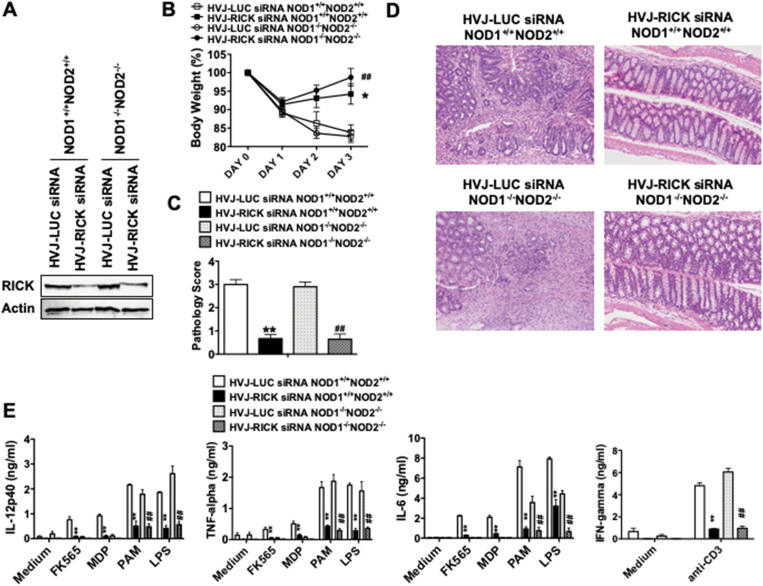
Similarly, in studies of NOD1/NOD2-double deficient mice we found that TNBS-colitis induced by a comparable regimen as that employed in NOD1/NOD2-intact mice [wild-type (WT) mice] was again as severe as that in NOD1/NOD2-intact mice and, as evaluated by weight loss and pathology scores, down-regulation of RICK expression in colonic LPMCs by administration of encapsulated siRNA targeting RICK in such mice again led to greatly decreased colitis (Fig. 1A–D). The survival rates of WT and NOD1/NOD2-double deficient (DKO) mice in TNBS-colitis were as follows: WT LUC-siRNA: 6/16 (37.5%); WT RICK-siRNA: 10/10 (100.0%); DKO LUC-siRNA: 10/12 (83.3%); DKO RICK-siRNA: 7/7 (100.0%, Supplementary Figure 5A). In addition, the decreased TNBS-colitis in both NOD1/NOD2-double deficient mice and WT mice treated with RICK-siRNA (as compared with those treated with LUC-siRNA) was accompanied by a greatly decreased anti-CD3 mAb-induced IFN-γ and IL-17 responses in LPMCs isolated from the colon as well as decreased TLR ligand-induced IL-12p40, TNF-α and IL-6 responses by these cells (Fig. 1E and Supplementary Figure 6A). As expected, decreased NOD1 ligand (FK565) or NOD2 ligand (MDP)-induced IL-12p40, TNF-α and IL-6 responses by colonic LPMCs were seen in WT mice treated with RICK-siRNA as compared with those treated with LUC-siRNA, whereas responses of colonic LPMCs from NOD1/NOD2-double deficient mice were low regardless of treatment. Finally, no significant changes in IL-10 production were observed in mice treated with siRNAs specific to RICK or LUC (Supplementary Figure 6A). Thus, TNBS-colitis induction was not dependent on the presence of NOD1 or NOD2 but was dependent on the presence of RICK.
Fig. 1.
Prevention of TNBS-colitis by administration of a RICK siRNA-expressing plasmid in NOD1 and NOD2-double deficient mice. NOD1 and NOD2-intact C57BL/6 mice (NOD1+/+NOD2+/+ mice) and NOD1 and NOD2-double deficient mice (NOD1−/−NOD2−/− mice) were administered HVJ-E-encapsulated RICK-siRNA expressing vector or control LUC-siRNA expressing vector via the intra-rectal route on days −2, −1 and 0 and then challenged with intra-rectal TNBS on day 0 and 2. (A) Expression of RICK in colonic LPMCs (cLPMCs) in TNBS-treated mice on day 3. Protein lysates were prepared from cLPMCs isolated from the colon of TNBS-treated mice on day 3 and then subjected to immunoblotting. (B) Changes of body weight in mice. Results shown are values from pooled data derived from two independent experiments (NOD1+/+NOD2+/+ LUC-siRNA; n = 16, NOD1+/+NOD2+/+ RICK-siRNA; n = 10, NOD1−/−NOD2−/− LUC-siRNA; n = 12, NOD1−/−NOD2−/− RICK-siRNA; n = 7). Results are expressed as mean ± SEM. *P < 0.05, as compared with NOD1+/+NOD2+/+ HVJ-LUC siRNA group. ##P < 0.01, as compared with NOD1−/−NOD2−/− HVJ-LUC siRNA group. (C, D) H&E staining of colon tissue obtained from mice on day 3; pathology scores calculated from examination of tissues obtained from mice on day 3; results are expressed as mean ± SEM. Results shown were obtained from pool tissues from two independent experiments. **P < 0.01, as compared with NOD1+/+NOD2+/+ HVJ-LUC siRNA group. ##P < 0.01, as compared with NOD1−/−NOD2−/− HVJ-LUC siRNA group. (E) Production of IL-12p40, TNF-α, IL-6 and IFN-γ by cLPMCs isolated from TNBS-challenged mice on day 3; cLPMCs (1 × 106 per ml) were stimulated with FK565, MDP, PAM, LPS or anti-CD3 mAb for 48 h after which culture fluids were assayed for cytokine levels by ELISA, as indicated. Results are expressed as mean ± SEM. **P < 0.01, as compared with NOD1+/+NOD2+/+ HVJ-LUC siRNA group. ##P < 0.01, as compared with NOD1−/−NOD2−/− HVJ-LUC siRNA group.
Inhibition of DSS-colitis through down-regulation of RICK (RIP2) expression in the absence of both NOD1 and NOD2
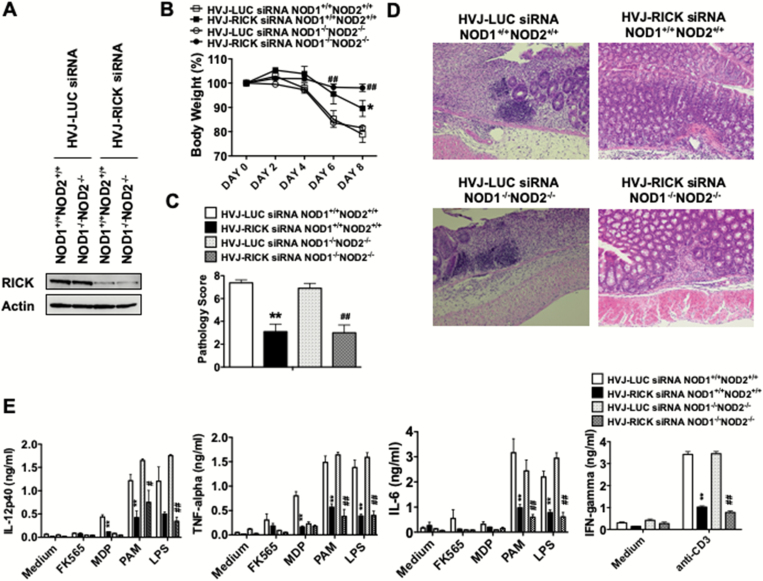
We next sought to determine the role of RICK-mediated signaling in a second murine model of gut inflammation shown to reproduce important aspects of human UC, DSS-colitis (7, 25, 26). To this end, we administered plasmids expressing a RICK-specific siRNA as well as control plasmids expressing LUC-specific siRNA, each encapsulated in a HVJ-E per rectum to mice during the initiation phase of DSS-colitis induction. Down-regulation of RICK expression was confirmed in colonic LPMCs by intra-rectal administration of RICK-siRNA in both WT and NOD1/NOD2-double deficient mice (Fig. 2A). As in the case of TNBS-colitis, down-regulation of RICK expression by siRNA protected both WT and NOD1/NOD2-double deficient mice from DSS-colitis as judged by body weight loss and pathology score (Fig. 2B–D). The survival rates of WT and DKO mice in DSS-colitis were as follows: WT LUC-siRNA: 8/10 (80.0%); WT RICK-siRNA: 10/10 (100.0%); DKO LUC-siRNA: 10/10 (100.0%); DKO RICK-siRNA: 8/8 (100.0%, Supplementary Figure 5B). Protection from DSS-colitis by down-regulation of RICK expression was associated with a marked reduction in pro-inflammatory cytokine responses (including IL-6, TNF-α, IL-12p40, IL-17 and IFN-γ) by colonic LPMCs isolated from WT and NOD1/NOD2-double deficient mice (Fig. 2E and Supplementary Figure 6B). A small increase was seen in IL-10 production by colonic LPMCs from NOD1/NOD2-double deficient mice treated with RICK-siRNA as compared with those with LUC-siRNA. These data show that the development of DSS-colitis (as in the case of TNBS-colitis) requires NOD1- and NOD2-independent RICK activation and that the decreased colitis observed in the presence of RICK inhibition was due to the loss of an inflammatory response rather than to the induction of an anti-inflammatory response.
Fig. 2.
Prevention of DSS-colitis by administration of a RICK siRNA-expressing plasmid in NOD1 and NOD2-double deficient mice. NOD1 and NOD2-intact C57BL/6 mice (NOD1+/+NOD2+/+ mice) and NOD1 and NOD2-double deficient mice (NOD1−/−NOD2−/− mice) were treated with DSS (4%) in the drinking water from day 0 to day 6. Mice were administered HVJ-E-encapsulated RICK-siRNA expressing vector or control LUC-siRNA expressing vector via the intra-rectal route on days 0, 1 and 2. (A) Expression of RICK in colonic LPMCs (cLPMCs) in DSS-treated mice on day 8. Protein lysates were prepared from cLPMCs isolated from the colon of DSS-treated mice on day 8 and then subjected to immunoblotting. (B) Changes of body weight in mice. Results shown are values from pooled data derived from two independent experiments (NOD1+/+NOD2+/+ LUC-siRNA; n = 10, NOD1+/+NOD2+/+ RICK-siRNA; n = 10, NOD1−/−NOD2−/− LUC-siRNA; n = 10, NOD1−/−NOD2−/− RICK-siRNA; n = 8). Results are expressed as mean ± SEM. *P < 0.05, as compared with NOD1+/+NOD2+/+ HVJ-LUC siRNA group. ##P < 0.01, as compared with NOD1−/−NOD2−/− HVJ-LUC siRNA group. (C, D) H&E staining of colon tissue obtained from mice on day 8; pathology scores calculated from examination of tissues obtained from mice on day 8; results are expressed as mean ± SEM. Results shown were obtained from pooled tissues derived from two independent experiments. **P < 0.01, as compared with NOD1+/+NOD2+/+ HVJ-LUC siRNA group. ##P < 0.01, as compared with NOD1−/−NOD2−/− HVJ-LUC siRNA group. (E) Production of IL-12p40, TNF-α, IL-6 and IFN-γ by cLPMCs isolated from DSS-challenged mice on day 8; cLPMCs (1 × 106 per ml) were stimulated with FK565, MDP, PAM, LPS or anti-CD3 mAb for 48 h after which culture fluids were assayed for cytokine levels by ELISA, as indicated. Results are expressed as mean ± SEM. **P < 0.01, as compared with NOD1+/+NOD2+/+ HVJ-LUC siRNA group. #P < 0.05, ##P < 0.01, as compared with NOD1−/−NOD2−/− HVJ-LUC siRNA group.
Expression levels of NOD1, NOD2, RICK and downstream signaling components in patients with IBD
To relate the results of the above studies of experimental colitis to intestinal inflammation in patients with IBD we next conducted extensive studies in which we employed qPCR analysis to estimate mRNA levels of NOD1, NOD2 and RICK as well as mRNA levels of related signaling components and cytokines in tissue specimens obtained by endoscopy from large groups of IBD patients and controls. Previous studies have shown that increased levels of NOD2 are observed following an inflammatory process induced by exposure to a pathologic organism (34–36); thus, the studies conducted here were based, in part, on the expectation that the participation of NOD2 in IBD inflammation would be accompanied by increased levels of NOD2 mRNA. This notion is supported by the fact that NF-κB generated by activated cells is a transcriptional activator of NOD2 and therefore could provide positive feedback for NOD2 synthesis during an inflammatory process (37, 38).
Of note, levels of expression varied considerably in both CD and UC patient groups as well as in controls. This could reflect variability in tissue sampling and in medications used in treatment of patients (5-aminosalicylic acid, prednisolone, TNF-α inhibitor, immunomodulator and calcineurin inhibitor; Supplementary Table 1). In addition, in the case of IBD patients, this could be due to variable levels of inflammation in the biopsy specimens. Because of this variability the validity of the values obtained in the studies depended on the large number of patient specimens evaluated and the strict reliance on statistical analysis of log-transformed expression data in which significant differences were established using mean values subjected to Bonferroni correction. On a related point, the number mRNA parameters assayed in each patient (and thus the sample size for the different parameters) varied somewhat due to the fact that total amount of mRNA extractable from endoscopic biopsy samples was at times limited by the amount of tissue obtained. However, for each parameter, a sufficient number of samples were obtained to ensure adequacy of sample size for the necessary statistical analyses. Finally, it is important to mention that IBD-associated NOD2 polymorphisms have not been found in Japanese or other Asian patient populations (39). Given that all of the biopsy samples analyzed in this study were obtained from Asian patients at Kindai University Hospital, this means that the data presented were not influenced by genetic effects on NOD2 levels (39). Based on the limited amount of data available, the same can be said for NOD1 (40).
In initial studies we evaluated both CD and UC patient tissue specimens obtained by endoscopy with respect to major pro-inflammatory cytokine expression (Fig. 3A). Both CD and UC patient tissues expressed increased mean levels of IL-6 compared to control tissues (obtained from uninvolved areas of patients with colonic polyps or early cancer). TNF-α expression levels in specimens from CD patients were lower than those from UC patients and not significantly different from control levels possibly reflecting the fact that >50% of CD patients (64.3%) were being treated with anti-TNF-α antibody (Supplementary Table 1). Overall, the mean cytokine values in the patient groups offer validation of the methodology used to evaluate NOD1/NOD2 and RICK gene expression since one would expect elevations in the pro-inflammatory cytokine expression in patient specimens compared to control specimens.
In further studies mRNA expression levels of NOD1 (CARD4), NOD2 (CARD15) and RICK (RIPK2) in the same tissue samples as those evaluated above for cytokine expression were assessed (Fig. 3B). Mean NOD1 expression levels were modestly increased in UC tissues (P < 0.05) compared to control tissues, whereas mean NOD2 expression levels were not increased in CD tissues compared to controls and were even modestly decreased in UC tissues compared to both CD and control tissues (Fig. 3B). In contrast, mean expression levels of RICK, the immediate downstream signaling partner of both NOD1 and NOD2 were distinctly increased in both CD and UC tissues compared to control tissues (P < 0.01, Fig. 3B). Thus, whereas NOD1 levels were only modestly elevated and NOD2 levels were unchanged or even decreased in patient tissues, RICK expression was clearly elevated in both patient groups; these data suggested that the latter elevation was independent of NOD2 activation and possibly NOD1 activation as well.
RICK activation of NF-κB depends on its K63-linked polyubiquitination by various E3-ligases such as cIAP1, cIAP2, XIAP, Pellino 3 and TNF receptor-associated factor 6 (TRAF6) (5, 27–31). We therefore measured the expression of the various ligases that have been shown to be involved in such ubiquitination. Both cIAP1 (BIRC2) and cIAP2 (BIRC3) were elevated in UC patients as compared with control patient tissues, whereas only cIAP2 was elevated in CD patients. In contrast, XIAP (BIRC4) was not increased in UC patient tissues corresponding to the fact that XIAP cofactors HOIP (RNF31) and Sharpin were also not increased; XIAP was also not increased in CD patient tissues but in this case HOIP was increased and Sharpin was marginally decreased (Fig. 3 and data not shown). Pellino 3 (PELI3), yet another ligase involved in RICK ubiquitination, was also not increased in either CD or UC patients as compared with control patient tissues; this finding thus does not support earlier work showing that patients with CD exhibit decreased expression of Pellino 3 at the protein level (27). Finally, IBD patients (particularly UC patients) displayed increased tissue expression of TRAF6, a component that functions both as an E3-ligase and as a downstream signaling molecule with respect to RICK. The latter finding is in accord with the fact that another signaling molecule, TAK1 (MAP3K7) was also increased in UC tissue (although not in CD tissue, Fig. 3B). Overall, the studies of NOD1/NOD2 mRNA expression as well as RICK and RICK-associated E3-ligase mRNA expression support the view that whereas NOD1 and NOD2 expression is not increased in IBD, RICK expression and at least some of its E3-ligases such as TRAF6 and cIAP2 are increased.
Relation of NOD1, NOD2, RICK and downstream signaling components to disease activity
The data displayed above were also analyzed with respect to disease activity (i.e. remission versus active disease). Only values obtained from UC patient tissues were statistically analyzed since only a small number of CD patients in remission were available. UC tissues obtained from patients with active disease expressed an increased amount of IL-6 mRNA compared to patients in remission, but not increased amounts of TNF-α or IL-12p40 mRNA (Fig. 4A). In keeping with the finding mentioned above that NOD1 and NOD2 expression levels were only marginally changed in patient tissues compared to control tissues, there were no differences between NOD1 levels in active and remission UC patient tissues. A small, but not significant increase in NOD2 levels was seen in active versus remission UC patient tissues (Fig. 4B). It should be noted, however, that this increase in NOD2 expression in active UC patients did not raise the mean value to a level greater than that exhibited by controls. Concomitant studies of RICK mRNA levels showed that these were increased in active versus remission UC patient tissues (Fig. 4B); in addition, while these increases were accompanied by a lack of change in levels of cIAP1, cIAP2, TRAF6 and TAK1, the levels of each of these factors remained higher than those in control patients (Fig. 4C). Thus, this analysis of active versus remission patient specimens suggests that whereas RICK expression levels are significantly increased in UC patients with active disease versus levels of patients in remission, levels of NOD1 or NOD2 are not increased.
A statistical analysis of mRNA levels in patients with remission and active CD, as mentioned, was not performed because of the very limited number of patients in remission available for study. However, a statistical comparison of patients with active CD and controls with respect to RICK levels was possible and showed that active CD patients had clearly higher RICK levels, consistent with the data obtained with the UC patient group (Fig. 4B). In addition, the expression value of NOD2 in the three CD patients in remission was in the same range as the 21 active CD patients.
Correlation of RICK signaling with pro-inflammatory cytokine expression in IBD tissues at the mRNA level
Given the observation above that tissue expression of RICK and its associated ubiquitinating ligases (TRAF6 and cIAP2) exceeds NOD1 and NOD2 expression in both UC and CD we focused on the relation of RICK to expression of pro-inflammatory cytokines in IBD tissues. In initial studies of this question, we constructed correlation curves reflecting individual patient RICK tissue expression with their tissue expression of pro-inflammatory cytokines.
This analysis with respect to the UC data showed clearly that RICK mRNA tissue levels exhibited significant correlation with the various pro-inflammatory cytokine mRNA levels (Supplementary Figure 7A) as well as with the various E3-ligase mRNA levels and TAK1 mRNA levels (Supplementary Figure 7B). A similar correlation analysis with respect to the CD data yielded inconsistent results; however, they did show that tissue RICK mRNA levels exhibited a significant correlation with the various pro-inflammatory cytokine (IL-12p40 and IL-6) mRNA levels as well as with cIAP2 mRNA levels (Supplementary Figure 8). These data suggest that RICK and its several E3-ligases (cIAP1, cIAP2 and TRAF6) are correlated with the pro-inflammatory cytokine response (IL-6, TNF-α and IL-12p40) in UC. The same is true in CD, but here the correlation was limited to levels of pro-inflammatory cytokines and with cIAP2.
Correlation of RICK signaling with pro-inflammatory cytokine expression in IBD tissues at the protein level
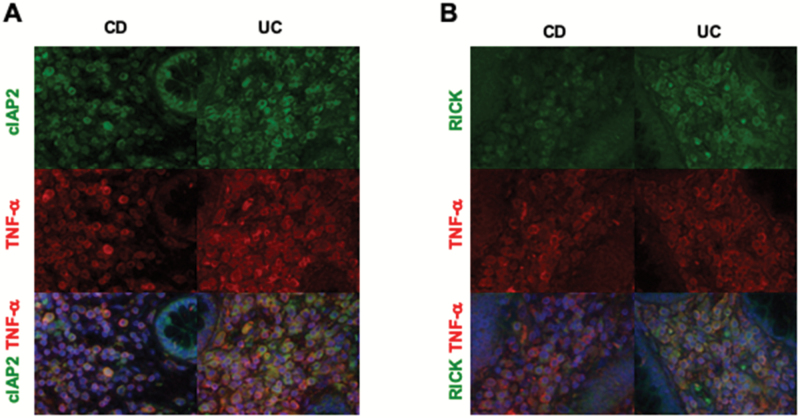
We next determined whether expression of pro-inflammatory cytokines (TNF-α and IL-6) is correlated with expression of RICK and/or one of its associated E3-ligases at the protein level in tissue samples obtained from UC or CD patients that had undergone surgical operation. In studies focused on TNF-α expression we performed dual immunofluorescence staining studies in which tissues were stained with either Alexa 488-labeled anti-RICK antibody or anti-cIAP2 antibody in combination with Alexa 546-labeled anti-TNF-α antibody. We found that in both CD and UC tissues, most cells staining positive for RICK or cIAP2 also stained positive for TNF-α and therefore exhibited a yellow fluorescence (Fig. 5). Thus, cells producing TNF-α were also expressing both RICK and cIAP2. Isotype control antibody staining using mouse IgG or rabbit IgG was negative (Supplementary Figure 9). Non-tumorous portions of early colorectal cancers were used as controls and two control samples were negative for RICK or cIAP2 staining, in parallel with the qPCR results (Supplementary Figure 10A).
Fig. 5.
Expression of TNF-α, cIAP2 and RICK in patients with IBDs. Expression of TNF-α, cIAP2 (A) and RICK (B) was examined by immunofluorescence analysis in surgical specimens obtained from CD or UC patients. Representative immunofluorescence photomicrograph of patient tissue stained with anti-TNF-α antibody (red color), anti-cIAP2 antibody (green color) or anti-RICK antibody (green color). Nuclei were stained with DAPI, magnification ×1200.
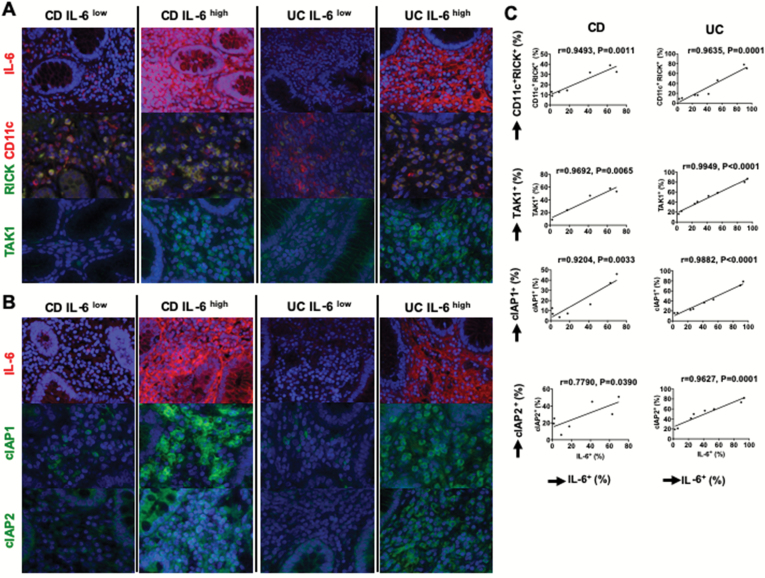
In parallel studies focusing on IL-6 we could not perform dual staining studies because an anti-human-IL-6 antibody (suitable for dual staining) that could be used in combination with suitable antibodies to RICK, cIAP2 or TAK1 was not available. Consequently, in this case we performed staining studies to determine if the numbers of mono-stained IL-6-expressing cells correlated with the numbers of mono-stained RICK, cIAP1, cIAP2 or TAK1 expressing cells. We found that tissue samples heavily infiltrated with IL-6-expressing cells detected by staining with Alexa 546-labeled anti-IL-6 antibody (right panels of both CD and UC tissues) contained greatly increased numbers of CD11c+ dendritic cells (DCs) visualized as yellow color upon staining with Alexa 546-labeled anti-CD11c antibody and Alexa 488-labeled RICK antibody in comparison with tissue containing few IL-6 expressing cells (left panels of both CD and UC tissues) (Fig. 6A). Similarly, green cIAP1- and cIAP2-expressing cells as well as green TAK1-expressing cells are greatly increased in tissue samples containing large numbers of red IL-6-expressing cells as compared to those containing few red IL-6-expressing cells (Fig. 6A and B). Consistent with the data obtained in qPCR studies, two control samples were negative for IL-6, cIAP1 or cIAP2 staining and contained few mononuclear cells expressing TAK1 (Supplementary Figure 10A). Semi-quantitative evaluation of these data obtained by counting of stained cells in representative tissues from patients with both UC and CD disclosed a positive correlation between the percentage of IL-6+ cells in the tissue and the percentage of CD11c+RICK+, cIAP1+, cIAP2+ and TAK1+ cells (Fig. 6C).
Fig. 6.
Expression of IL-6 is positively correlated to that of RICK, cIAP1, cIAP2 and TAK1 in patients with IBDs at a protein level. Expression of IL-6 and RICK-associated molecules was examined by immunofluorescence analysis using surgical specimens obtained from CD patients (n = 5 or 7) or UC patients (n = 8). (A) Representative immunofluorescence photomicrograph of patient tissue stained with anti-IL-6 antibody (red color), anti-RICK antibody (green color) and anti-CD11c antibody (red color), or anti-TAK1 antibody (green color); left panel of both CD and UC shows tissue expressing low numbers of IL-6+ (IL-6low) cells; right panel of both CD and UC shows tissue expressing high numbers of IL-6+ (IL-6high) cells. Nuclei were stained with DAPI, magnification ×1200 (RICK, CD11c, TAK1), ×800 (IL-6). (B) Representative immunofluorescence photomicrograph of patient tissue stained with anti-IL-6 antibody (red color), anti-cIAP1 antibody (green color) or anti-cIAP2 antibody (green color); left panel of both CD and UC shows tissue expressing low numbers of IL-6+ (IL-6low) cells; right panel of both CD and UC shows tissue expressing high numbers of IL-6+ (IL-6high) cells. Nuclei were stained with DAPI, magnification ×1200 (cIAP1, cIAP2), ×800 (IL-6). (C) The numbers of cells positive for IL-6, RICK, CD11c, cIAP1, cIAP2 and TAK1 was determined by counting in high-power fields and shown as the percentages of total DAPI-positive mononuclear cells. The percentages of cells positive for RICK, CD11c, cIAP1, cIAP2 and TAK1 were plotted against IL-6+ cells. Correlation analysis between cell populations shown was performed; each dot corresponds to a value of each patient sample. P value and Pearson r values are shown.
Molecular interactions between RICK and cIAP2 or RICK and TAK1 in cells expressing TNF-α or IL-6 in CD and UC tissues
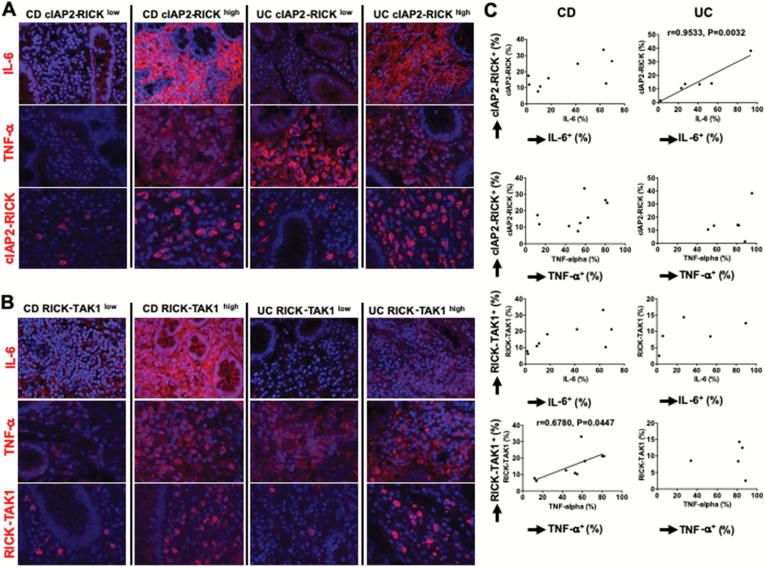
To explore further the involvement of RICK in pro-inflammatory cytokine production in CD and UC we performed PLA to clarify the relationship between cytokine expression and RICK-mediated signaling pathways in inflamed tissues from IBD patients (5). In this analysis molecular interactions between RICK and cIAP2 or RICK and TAK1 could be identified by the presence of cells containing a red fluorescence product generated by the proximity of RICK to cIAP2 or TAK1 and thus indicative of an interaction between RICK with cIAP2 or TAK1. We found that, in general, tissues containing a greater number of TNF-α+ or IL-6+ cells also contained a greater number of cells containing a red fluorescence product indicative of cells in which RICK is interacting with cIAP2 or TAK1 (Fig. 7A and B, right CD and UC panels). Two control samples were negative for the interaction between cIAP2 and RICK or between TAK1 and RICK (Supplementary Figure 10B).
Fig. 7.
Expression of IL-6 is positively correlated to the intensity of the molecular interaction between RICK and cIAP2 in patients with UC. Expression of IL-6 and TNF-α was examined by immunofluorescence analysis using surgical specimens obtained from CD (n = 9) or UC (n = 5 or 6) as described in Fig. 6. The molecular interaction between cIAP2 and RICK or between TAK1 and RICK was analyzed by PLAs. (A, B) Representative immunofluorescence photomicrograph of the patient tissue stained with anti-IL-6 antibody (red color, first and fourth row) or anti-TNF-α antibody (red color, second and fifth row). The molecular interaction between cIAP2 and RICK (third row) or between TAK1 and RICK (sixth row) was visualized as red color. Nuclei were stained with DAPI, magnification ×1200 (TNF-α, cIAP2–RICK interaction, RICK–TAK1 interaction), ×800 (IL-6). (C) The number of cells positive for IL-6 or TNF-α was determined by counting in high-power fields. The number of cells positive for the interaction between cIAP2 and RICK or between TAK1 and RICK was determined by counting in high-power fields and shown as the percentages of total DAPI-positive mononuclear cells. Correlation analysis between the percentage of cells positive for IL-6 or TNF-α and that of cells positive for the cIAP2–RICK interaction or RICK–TAK1 interaction was performed. Each dot corresponds to a value of each patient sample. P value and Pearson r values are shown.
Subsequent semi-quantitative evaluation of the PLA in relation to cells positive for TNF-α and IL-6 showed that the presence of increased numbers of cytokine positive cells was indeed generally associated with increased numbers of cells exhibiting red fluorescence indicative of interaction between RICK and cIAP2 or TAK1 (Fig. 7C). However, only in two instances was this correlation formally established by the presence of a linear correlation coefficient: the correlation between numbers of TNF-α+ cells and RICK-TAK1+ cells in CD tissue and the correlation between numbers of IL-6+ cells and cIAP2-RICK+ cells in UC. Overall, these findings suggested that the RICK signaling pathway is active in a substantial number of cells with active downstream synthesis of pro-inflammatory cytokines.
Discussion
In the initial series of studies of NOD signaling in relation to gut inflammation reported here we focused on RICK function in relation to two different established murine models of IBD, TNBS-colitis and DSS-colitis. In these studies we determined that intra-rectal delivery of a plasmid expressing siRNA specific for RICK encased in HVJ-E inhibits RICK expression in mucosal tissues and, in doing so, inhibits the induction of TNBS-colitis and DSS-colitis; in addition, the administration of a pan-inhibitor of IAPs, the latter factors essential for RICK activation, has a similar inhibitory effect. By showing that an agent with exquisite specificity for RICK inhibits two experimental models of colitis, TNBS-colitis and DSS-colitis, these studies augment previous evidence concerning the importance of RICK signaling in gut inflammation (12–14). In further studies utilizing the TNBS-colitis and DSS-colitis model we showed that inhibition of RICK function with RICK siRNA also inhibits both types of colitis in NOD2-deficient or NOD1/NOD2-double deficient mice in which these bacterial sensors cannot be playing a role in the intestinal inflammation. These studies thus offer the first evidence that the function of RICK in experimental colitis is not strictly dependent on prior activation of either NOD1 or NOD2 as implied in some previous studies relating to the activation of RICK (18, 19). In addition, they suggest that NOD1/NOD2-independent induction of gut inflammation (such as TLR-induced inflammation) is largely dependent on RICK activation along with other kinases conceivably activated by myeloid differentiation factor 88 (MyD88) and IL-1 receptor-associated kinase (IRAK) signaling.
To determine if the above conclusions concerning the importance of RICK activation and its independence from NOD1 and NOD2 derived from studies of murine experimental colitis applies to humans with IBD we assessed mRNA levels of various NOD, RICK and cytokine components in endoscopic biopsy specimens of a substantial number of IBD patients and controls by qPCR. The underlying assumption of this approach with respect to evaluation of activity of NOD1, NOD2 and RICK was that such activity would be reflected in (or marked by) increased levels of these components if they were participating in an inflammatory response. With respect to NOD2, this assumption is supported by evidence that NOD2 up-regulates NF-κB and the latter, in turn, up-regulates NOD2 transcription (37, 38). In addition, it has been shown that various kinds of gastrointestinal infections up-regulate NOD2 levels (34–36). Finally, since the gastrointestinal environment is replete with bacteria expressing NOD2 (and NOD1) ligands, the increased NOD2 levels would inevitably lead to increased NOD2 activity.
We found, as expected, that levels of NOD1 and NOD2 mRNA and other components varied within the patient groups studied and we therefore relied on log-transformed mean values and statistical analysis to assess changes in expression. The validity of this approach for assessment of mRNA levels of NOD1, NOD2 and RICK is supported by the fact that IL-6 and TNF-α mRNA levels in UC patients (not generally on anti-cytokine therapy) exhibited statistically significant and substantial elevations. Thus, the finding that NOD2 mRNA tissue levels from both active CD and UC patients are not elevated as compared with control tissues and NOD1 mRNA levels were at best marginally increased provides evidence that NOD2 or NOD1 has only a minor role in driving the pro-inflammatory process operative in these IBD patients. In contrast, the finding that RICK mRNA tissue levels from both active CD and UC are statistically greater than levels in control tissues, provides evidence that RICK is participating in the inflammatory response mediating IBD. In addition, evidence that RICK is activated in IBD patients is inherent in the finding that the increases in RICK mRNA tissue levels are associated with increases in mRNA levels of E3-ligases that are known to K63-polyubiquitinate RICK and thus facilitate its function as a signaling molecule capable of interacting with TAK1 (5, 29–31). Finally, both at the mRNA level and at the protein level in tissue from IBD patients, RICK levels correlated with pro-inflammatory cytokine levels and RICK expression was found in cells that synthesize such cytokines (see further discussion below). The overall conclusion that can be drawn from these studies is that there is little evidence that NOD2 (or indeed NOD1) is a necessary driving force of inflammation in patients with active IBD, whereas there is ample evidence that RICK activation is an essential participant in such inflammation; furthermore, these studies suggest, as do the studies of murine experimental colitis, that RICK activation is not necessarily dependent on NOD1 or NOD2 activity. These conclusions are again fully compatible with previous findings suggesting that NOD2 is not a necessary contributor to the pro-inflammatory cytokine responses causing IBD inflammation since loss-of-function polymorphisms are associated with increased rather than decreased susceptibility to CD (4, 26).
The finding in the present study that NOD2 mRNA levels are not increased in colonic tissues of patients with active CD is at odds with previous studies that show with tissue-staining techniques that the number and types of cell expressing NOD2 is increased in CD and, in addition, NOD2 mRNA was increased in such patients (41–43). One important difference between these previous studies and the present study is that the previous studies were conducted in pediatric patients, whereas the studies conducted here were performed in adult patients. It may therefore be that in patients with more prolonged disease NOD2 levels fall and play a reduced role in the inflammation. A possible mechanistic basis for this possibility was reported by Rosenstiel et al., who showed that the NOD2 gene contains 5′ untranslated exons that are capable of translational repression and thus influence NOD2 protein expression (44). In addition, these investigators showed that expression of these ‘regulatory exons’ is determined by alternative splicing that is influenced by cellular exposure to inflammatory mediators (44). Whereas in the study reported by Rosenstiel et al., the expression of these down-regulatory exons was inhibited by rapamycin which thus up-regulated NOD2 expression, other factors not yet studied in this context may have the opposite effect (44).
The importance of RICK activation in the inflammation occurring in human IBD is supported by previous studies showing that levels of phosphorylated RICK (i.e. activated RICK) are increased in IBD tissue; in addition, these studies provide evidence that RICK inhibitors ameliorate inflammation in murine models of IBD and that inhibition of RICK down-regulates pro-inflammatory cytokine formation in mouse models of inflammation in vivo and in human IBD specimens in vitro (11–15). In the present study we provide additional data supporting the role of RICK in IBD inflammation with data showing that expression of RICK and associated E3-ligase (cIAP1, cIAP2 and TRAF6) mRNA in UC tissue correlates with expression of major pro-inflammatory cytokine (TNF-α, IL-6, IL-12p40) mRNAs. A similar correlation was also found in CD in that RICK and cIAP2 mRNA expression correlated with mRNA expression of IL-6, TNF-α and IL-12p40. These mRNA data were supported by protein data derived from both UC and CD patients in which tissue staining was used to assess RICK expression. These studies showed that expression of TNF-α occurred in cells expressing RICK and cIAP2 and that numbers of DCs expressing IL-6 were parallel to those of DCs expressing RICK, cIAP1 and cIAP2. This association of RICK with pro-inflammatory cytokines in inflamed IBD tissue was further highlighted with PLAs showing that RICK interacts with either cIAP2 or TAK1 in cells expressing cytokines in a pattern depending on the type of cytokine. Taken together, these data support the role of RICK in IBD by showing that activated RICK is expressed in cells producing pro-inflammatory cytokines and that such expression is accompanied by interactions between RICK and components of the inflammatory pathway. In line with this idea, we confirmed that pro-inflammatory cytokine responses (including IL-6, TNF-α and IL-12p40 responses) were markedly reduced in colonic LPMCs treated with RICK-specific siRNA as compared with those with control siRNA.
The lack of dependence of RICK-mediated pro-inflammatory function on NOD1 or NOD2 signaling pathways clearly shown in our studies of the role of RICK in TNBS-colitis and DSS-colitis is difficult to understand if one postulates that RICK activation is strictly tied to NOD1 and NOD2 signaling as suggested by studies of Park et al. and Hall et al. showing that neither receptor-induced T-cell proliferation and induction of T-cell differentiation nor TLR signaling is impaired in the absence of RICK, whereas NOD1 and NOD2 signaling is impaired in this circumstance (18, 19). However, these data do not take into account the initial studies by Kobayashi et al. and Chin et al. showing that cell stimulation that is clearly independent of NOD1 and NOD2 signaling such as stimulation by mitogens, cytokines and TLRs are impaired in cells from RICK-deficient mice (16, 17). That TLR stimulation can induce such NOD1- and NOD2-independent RICK signaling was subsequently demonstrated in mechanistic studies showing that LPS stimulation causes RICK binding to IRAK1 and TRAF6 as well as RICK phosphorylation accompanying pro-inflammatory cytokine production (45, 46). Additional evidence that RICK activation can be NOD1- and NOD2-independent has come from recent studies showing that RICK has a T-cell-intrinsic role in the induction and regulation of IL-17 and IFN-γ responses without affecting regulatory T-cell responses induced by Chlamydia pneumoniae (47). Consistent with this, down-regulation of IL-17 and IFN-γ responses accompanied by small increases in IL-10 responses were seen in the colon of RICK-siRNA-treated mice as compared with LUC-siRNA-treated mice. Finally, it is important to point out that the E3-ligases known to be involved in and necessary for RICK activation, such as cIAP1, cIAP2 and TRAF6 studied here, are also independent of NOD1 and NOD2 signaling as activation of such ligases is generally brought about by post-translational modifications such as phosphorylation or binding to ubiquitination targets; thus, their activity in the case of RICK may be governed by RICK activation itself (48).
The studies shown here imply that impaired colonic inflammation in the absence of RICK can in fact be due to dysfunction of signaling pathways independent of NOD1 and NOD2 activation and operative in DCs, macrophages or T cells. However, this should not be taken to mean that NOD1 or NOD2 activation does not contribute to RICK-mediated signaling; on the contrary, when NOD1/2 signaling is present and robust, it is quite likely to be a major contributor to such signaling. On the based of the data obtained in the studies of TNBS- and DSS-colitis (as well as the studies of IBD patients described here), we propose the following scheme as a shorthand for RICK signaling (Supplementary Figure 11): in the case of NOD1- and NOD2-dependent signaling of DCs or macrophages, NOD1/2 ligands activate NOD1 and NOD2 which then bind to RICK; this leads to auto-phosphorylation of RICK and subsequently its ubiquitination by various E3-ligases. Activated RICK then interacts with TAK1 and induces NF-κB and MAPK activation for the production of IL-6, IL-12/23p40 and TNF-α. In the case of NOD1 and NOD2-independent activation of RICK, RICK is activated in DCs, macrophages and T cells (Supplementary Figure 11); in DCs and macrophages, TLR ligands (such as LPS) activate TLRs and then their downstream MyD88-IRAK pathways for the production of IL-6, IL-12/23p40 and TNF-α (Supplementary Figure 11); RICK then binds to IRAK1 and is phosphorylated and ubiquitinated as in the NOD1/2 pathway (45, 46). Alternatively, RICK is activated in T cells via T-cell receptor signaling and then regulates cytokine synthesis such as that producing IFN-γ and IL-17 (Supplementary Figure 11); its initial binding partner(s) leading to its activation in this case is not yet known. Such cytokines may then induce E3-ligase activation.
In summary, the data reported here showing that experimental murine colitis is not diminished in the absence of NOD1 and NOD2 yet is still largely dependent on RICK signaling emphasizes that RICK occupies a central position in the induction of IBD and that TLRs conceivably driving such inflammation may utilize this kinase to gain access to downstream pro-inflammatory machinery. This, in turn, suggests that inhibition of RICK activation constitutes a viable approach to the treatment of IBD. It should be noted, however, that administration of RICK-siRNA utilized in this study is not a feasible alternative to the treatment of IBD at present since frequent intra-rectal administration of siRNAs is physically challenging and likely to be quite expensive. In addition, such RICK inhibition might impair host defense functions since RICK activation is involved in signaling pathways mediated not only by NODs but also by TLRs. Finally, it is important to point out that if indeed NOD2 dysfunction is associated with increased susceptibility to the occurrence of CD it is fair to say that inhibition of NOD2 function via inhibition of RICK function could ultimately lead to initiation or exacerbation of CD.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (19K08455) from the Japan Society for the Promotion of Science, the Naito Foundation, the SENSHIN Medical Research Foundation, the Yakult Bio-Science Foundation, the Smoking Research Foundation, the Kobayashi Foundation for Cancer Research, the Takeda Science Foundation, the Special Coordination Funds from the Ministry of Education, Culture, Sports, Science and Technology of Japan and Astellas Pharma Inc. in Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program, and by Japan Agency for Medical Research and Development (AMED) Grants for Research on Intractable Diseases.
Supplementary Material
Acknowledgements
We would like to thank Dr Yasutaka Chiba (Clinical Research Center, Kindai University Hospital) for his valuable advice on statistical analysis.
Conflicts of interest statement: the authors declared no conflicts of interest.
References
- 1. Chen G., Shaw M. H., Kim Y. G. and Nuñez G. 2009. NOD-like receptors: role in innate immunity and inflammatory disease. Annu. Rev. Pathol. 4:365. [DOI] [PubMed] [Google Scholar]
- 2. Cho J. H. 2008. The genetics and immunopathogenesis of inflammatory bowel disease. Nat. Rev. Immunol. 8:458. [DOI] [PubMed] [Google Scholar]
- 3. Philpott D. J., Sorbara M. T., Robertson S. J., Croitoru K. and Girardin S. E. 2014. NOD proteins: regulators of inflammation in health and disease. Nat. Rev. Immunol. 14:9. [DOI] [PubMed] [Google Scholar]
- 4. Strober W. and Watanabe T. 2011. NOD2, an intracellular innate immune sensor involved in host defense and Crohn’s disease. Mucosal Immunol. 4:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Watanabe T.,, Asano N.,, Meng G., et al. 2014. NOD2 downregulates colonic inflammation by IRF4-mediated inhibition of K63-linked polyubiquitination of RICK and TRAF6. Mucosal Immunol. 7:1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watanabe T.,, Asano N.,, Murray P. J., et al. 2008. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J. Clin. Invest. 118:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strober W., Asano N., Fuss I., Kitani A. and Watanabe T. 2014. Cellular and molecular mechanisms underlying NOD2 risk-associated polymorphisms in Crohn’s disease. Immunol. Rev. 260:249. [DOI] [PubMed] [Google Scholar]
- 8. Watanabe T., Kitani A., Murray P. J., Wakatsuki Y., Fuss I. J. and Strober W. 2006. Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity 25:473. [DOI] [PubMed] [Google Scholar]
- 9. Hedl M., Li J., Cho J. H. and Abraham C. 2007. Chronic stimulation of Nod2 mediates tolerance to bacterial products. Proc. Natl Acad. Sci. USA 104:19440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hedl M. and Abraham C. 2011. Secretory mediators regulate Nod2-induced tolerance in human macrophages. Gastroenterology 140:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jun J. C., Cominelli F. and Abbott D. W. 2013. RIP2 activity in inflammatory disease and implications for novel therapeutics. J. Leukoc. Biol. 94:927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hollenbach E., Vieth M., Roessner A., Neumann M., Malfertheiner P. and Naumann M. 2005. Inhibition of RICK/nuclear factor-kappaB and p38 signaling attenuates the inflammatory response in a murine model of Crohn disease. J. Biol. Chem. 280:14981. [DOI] [PubMed] [Google Scholar]
- 13. Hollenbach E., Neumann M., Vieth M., Roessner A., Malfertheiner P. and Naumann M. 2004. Inhibition of p38 MAP kinase- and RICK/NF-kappaB-signaling suppresses inflammatory bowel disease. FASEB J. 18:1550. [DOI] [PubMed] [Google Scholar]
- 14. Tigno-Aranjuez J. T.,, Benderitter P.,, Rombouts F., et al. 2014. In vivo inhibition of RIPK2 kinase alleviates inflammatory disease. J. Biol. Chem. 289:29651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haile P. A.,, Votta B. J.,, Marquis R. W., et al. 2016. The identification and pharmacological characterization of 6-(tert-butylsulfonyl)-N-(5-fluoro-1H-indazol-3-yl)quinolin-4-amine (GSK583), a highly potent and selective inhibitor of RIP2 kinase. J. Med. Chem. 59:4867. [DOI] [PubMed] [Google Scholar]
- 16. Kobayashi K.,, Inohara N.,, Hernandez L. D., et al. 2002. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416:194. [DOI] [PubMed] [Google Scholar]
- 17. Chin A. I., Dempsey P. W., Bruhn K., Miller J. F., Xu Y. and Cheng G. 2002. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature 416:190. [DOI] [PubMed] [Google Scholar]
- 18. Park J. H.,, Kim Y. G.,, McDonald C., et al. 2007. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J. Immunol. 178:2380. [DOI] [PubMed] [Google Scholar]
- 19. Hall H. T., Wilhelm M. T., Saibil S. D., Mak T. W., Flavell R. A. and Ohashi P. S. 2008. RIP2 contributes to Nod signaling but is not essential for T cell proliferation, T helper differentiation or TLR responses. Eur. J. Immunol. 38:64. [DOI] [PubMed] [Google Scholar]
- 20. Sakurai T.,, Kashida H.,, Watanabe T., et al. 2014. Stress response protein cirp links inflammation and tumorigenesis in colitis-associated cancer. Cancer Res. 74:6119. [DOI] [PubMed] [Google Scholar]
- 21. DiPersio J. F.,, Erba H. P.,, Larson R. A., et al. 2015. Oral Debio1143 (AT406), an antagonist of inhibitor of apoptosis proteins, combined with daunorubicin and cytarabine in patients with poor-risk acute myeloid leukemia–results of a phase I dose-escalation study. Clin. Lymphoma Myeloma Leuk. 15:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsuji Y., Watanabe T., Kudo M., Arai H., Strober W. and Chiba T. 2012. Sensing of commensal organisms by the intracellular sensor NOD1 mediates experimental pancreatitis. Immunity 37:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Watanabe T.,, Yamashita K.,, Arai Y., et al. 2017. Chronic fibro-inflammatory responses in autoimmune pancreatitis depend on IFN-α and IL-33 produced by plasmacytoid dendritic cells. J. Immunol. 198:3886. [DOI] [PubMed] [Google Scholar]
- 24. Watanabe T.,, Sadakane Y.,, Yagama N., et al. 2016. Nucleotide-binding oligomerization domain 1 acts in concert with the cholecystokinin receptor agonist, cerulein, to induce IL-33-dependent chronic pancreatitis. Mucosal Immunol. 9:1234. [DOI] [PubMed] [Google Scholar]
- 25. Strober W. and Fuss I. J. 2011. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 140:1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Strober W., Murray P. J., Kitani A. and Watanabe T. 2006. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol. 6:9. [DOI] [PubMed] [Google Scholar]
- 27. Yang S.,, Wang B.,, Humphries F., et al. 2013. Pellino3 ubiquitinates RIP2 and mediates Nod2-induced signaling and protective effects in colitis. Nat. Immunol. 14:927. [DOI] [PubMed] [Google Scholar]
- 28. Damgaard R. B.,, Fiil B. K.,, Speckmann C., et al. 2013. Disease-causing mutations in the XIAP BIR2 domain impair NOD2-dependent immune signalling. EMBO Mol. Med. 5:1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bertrand M. J., Doiron K., Labbé K., Korneluk R. G., Barker P. A. and Saleh M. 2009. Cellular inhibitors of apoptosis cIAP1 and cIAP2 are required for innate immunity signaling by the pattern recognition receptors NOD1 and NOD2. Immunity 30:789. [DOI] [PubMed] [Google Scholar]
- 30. Yang Y., Yin C., Pandey A., Abbott D., Sassetti C. and Kelliher M. A. 2007. NOD2 pathway activation by MDP or Mycobacterium tuberculosis infection involves the stable polyubiquitination of Rip2. J. Biol. Chem. 282:36223. [DOI] [PubMed] [Google Scholar]
- 31. Abbott D. W., Yang Y., Hutti J. E., Madhavarapu S., Kelliher M. A. and Cantley L. C. 2007. Coordinated regulation of Toll-like receptor and NOD2 signaling by K63-linked polyubiquitin chains. Mol. Cell. Biol. 27:6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang Y.,, Yang X.,, Xu T., et al. 2016. Overcoming resistance to TRAIL-induced apoptosis in solid tumor cells by simultaneously targeting death receptors, c-FLIP and IAPs. Int. J. Oncol. 49:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cai Q.,, Sun H.,, Peng Y., et al. 2011. A potent and orally active antagonist (SM-406/AT-406) of multiple inhibitor of apoptosis proteins (IAPs) in clinical development for cancer treatment. J. Med. Chem. 54:2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nagata E. and Oho T. 2017. Invasive Streptococcus mutans induces inflammatory cytokine production in human aortic endothelial cells via regulation of intracellular Toll-like receptor 2 and nucleotide-binding oligomerization domain 2. Mol. Oral Microbiol. 32:131. [DOI] [PubMed] [Google Scholar]
- 35. Liu X., Han Q. and Leng J. 2014. Analysis of nucleotide-binding oligomerization domain proteins in a murine model of pneumococcal meningitis. BMC Infect. Dis. 14:648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zheng B., Morgan M. E., van de Kant H. J., Garssen J., Folkerts G. and Kraneveld A. D. 2013. Transcriptional modulation of pattern recognition receptors in acute colitis in mice. Biochim. Biophys. Acta 1832:2162. [DOI] [PubMed] [Google Scholar]
- 37. Gutierrez O.,, Pipaon C.,, Inohara N., et al. 2002. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J. Biol. Chem. 277:41701. [DOI] [PubMed] [Google Scholar]
- 38. Hu C., Sun L., Hu Y., Lu D., Wang H. and Tang S. 2010. Functional characterization of the NF-kappaB binding site in the human NOD2 promoter. Cell. Mol. Immunol. 7:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Inoue N.,, Tamura K.,, Kinouchi Y., et al. 2002. Lack of common NOD2 variants in Japanese patients with Crohn’s disease. Gastroenterology 123:86. [DOI] [PubMed] [Google Scholar]
- 40. Chua K. H., Ng J. G., Ng C. C., Hilmi I., Goh K. L. and Kee B. P. 2016. Association of NOD1, CXCL16, STAT6 and TLR4 gene polymorphisms with Malaysian patients with Crohn’s disease. PeerJ 4:e1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Berrebi D.,, Maudinas R.,, Hugot J. P., et al. 2003. Card15 gene overexpression in mononuclear and epithelial cells of the inflamed Crohn’s disease colon. Gut 52:840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stronati L.,, Negroni A.,, Merola P., et al. 2008. Mucosal NOD2 expression and NF-kappaB activation in pediatric Crohn’s disease. Inflamm. Bowel Dis. 14:295. [DOI] [PubMed] [Google Scholar]
- 43. Negroni A.,, Stronati L.,, Pierdomenico M., et al. 2009. Activation of NOD2-mediated intestinal pathway in a pediatric population with Crohn’s disease. Inflamm. Bowel Dis. 15:1145. [DOI] [PubMed] [Google Scholar]
- 44. Rosenstiel P.,, Huse K.,, Franke A., et al. 2007. Functional characterization of two novel 5’ untranslated exons reveals a complex regulation of NOD2 protein expression. BMC Genomics 8:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lu C.,, Wang A.,, Dorsch M., et al. 2005. Participation of Rip2 in lipopolysaccharide signaling is independent of its kinase activity. J. Biol. Chem. 280:16278. [DOI] [PubMed] [Google Scholar]
- 46. Usluoglu N., Pavlovic J., Moelling K. and Radziwill G. 2007. RIP2 mediates LPS-induced p38 and IkappaBalpha signaling including IL-12 p40 expression in human monocyte-derived dendritic cells. Eur. J. Immunol. 37:2317. [DOI] [PubMed] [Google Scholar]
- 47. Shimada K.,, Porritt R. A.,, Markman J. L., et al. 2018. T-cell-intrinsic receptor interacting protein 2 regulates pathogenic T helper 17 cell differentiation. Immunity 49:873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vittal V., Stewart M. D., Brzovic P. S. and Klevit R. E. 2015. Regulating the regulators: recent revelations in the control of E3 ubiquitin ligases. J. Biol. Chem. 290:21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.