Abstract
Fused positron emission tomography (PET)/computed tomography (CT) is a recently developed technology that couples the functional information of PET with the anatomic details of CT. Integrated PET/CT scanners produce both PET and contrast material–enhanced CT images of the entire body in one setting. Typically, the amount of fluorine 18 (18F) fluorodeoxyglucose (FDG) uptake in normal pancreatic parenchyma is insignificant compared with that of the liver. However, both malignant (eg, adenocarcinoma) and benign (eg, acute pancreatitis) pancreatic conditions may demonstrate intense FDG uptake. PET/CT provides an opportunity to depict pancreatic tumors and distant metastases, perform preoperative staging, and monitor response to treatment, and it has proved useful in distinguishing postoperative fibrosis from recurrence. In selected cases, PET/CT findings may be used to help diagnose autoimmune pancreatitis mimicking a mass by depicting systemic involvement. PET/CT may also be used to direct biopsy to sites more likely to yield representative tumor tissue. Novel radiolabeled molecules, such as sigma-receptor ligands and 18F-3′-fluoro-3′-deoxy-l-thymidine (FLT), may play an even greater role in distinguishing tumor recurrence from postoperative fibrosis or inflammation.
© RSNA, 2012
Invited Commentary See discussion on this article by Yeh and Yee.
LEARNING OBJECTIVES FOR TEST 6
After completing this journal-based CME activity, participants will be able to:
-
•.
Review the current clinical applications of PET/CT in evaluating various pancreatic conditions.
-
•.
Describe the role of PET/CT in pancreatic cancer staging.
-
•.
Discuss the strengths and weaknesses of PET/CT in assessing various pancreatic conditions.
Introduction
Abdominal multidetector computed tomography (CT) is the mainstay of diagnostic imaging in patients with suspected pancreatic cancer. Although it provides excellent anatomic detail, it may not depict small tumors, which are more likely to be successfully resected if detected (1,2). Positron emission tomography (PET) is a functional imaging modality that has shown promise in tumor depiction, but it is unable to provide detailed high-spatial-resolution images (3).
PET/CT combines the functional information of PET with the detailed anatomic information of multidetector CT. Although the role of PET/CT in the diagnostic evaluation of patients with various abdominal malignancies is established, its role in pancreatic imaging is still evolving. PET/CT may be useful in the initial diagnosis, staging, or restaging of pancreatic adenocarcinomas, as well as in evaluating the response to treatment. In a few recent studies, it has also been shown to be useful in depicting malignant or invasive changes in mucinous cystic neoplasms and intraductal papillary neoplasms (IPMNs), as well as various rare tumors (4–7). In this article, we discuss the current and emerging indications for PET/CT in evaluating the pancreas.
Multidetector CT
The goal of pancreatic cancer imaging is to depict lesions in patients with suspected pancreatic malignancy and determine whether tumors are resectable. Because of the widespread availability of multidetector CT and its ability to image the entire abdomen and pelvis in a single breathhold with high spatial resolution, abdominal multidetector CT is considered the mainstay of diagnostic imaging in patients with suspected pancreatic cancer (2,8). Moreover, with multiplanar reformations such as three-dimensional and angiographic reconstruction, multidetector CT facilitates accurate preoperative staging of pancreatic tumors, and its ability to depict the entire abdomen and pelvis has been demonstrated to be useful for preoperative imaging of pancreatic adenocarcinomas (8,9). The reported positive predictive values of multidetector CT for helping determine whether tumors are resectable or not are 73%–91% and 95%–100%, respectively, with sensitivity of 95%–100% and specificity of 72%–100% for helping determine whether tumors are resectable or not.(2,8–13).
Despite these advances, multidetector CT has some limitations. Alone, it offers little functional information and largely depends on the size and morphologic characteristics to differentiate a tumor from normal structures. Lesion characterization is another glaring limitation of CT, and differentiating mass-forming pancreatitis (MFP) from adenocarcinomas is often difficult (14,15). Likewise, it is often challenging to detect small (<2 cm) tumors at multidetector CT, which are the most likely to be resectable (1). Moreover, in cases in which enhancement of the normal pancreas and peripancreatic vasculature is particularly pronounced, hypovascular solid tumors may be mistaken for cystic tumors (15).
Positron Emission Tomography
PET is an established molecular imaging modality, and fluorine 18 (18F) fluorodeoxyglucose (FDG), a glucose analog, is the most widely used radiotracer (16). Malignant lesions generally demonstrate avid FDG uptake, whereas most benign lesions are characterized by normal or minimally increased FDG accumulation. Focal areas of abnormally increased FDG uptake are considered suspicious for malignant disease, and in many cases, metabolic alterations precede the morphologic changes associated with malignant tumors (16,17).
In distinguishing chronic MFP from pancreatic cancer, several studies have shown that FDG PET is better for characterizing tissue than contrast material–enhanced CT (18–20). Likewise, FDG PET is more sensitive than contrast-enhanced CT for monitoring response to radiochemotherapy and depicting tumor recurrence after pancreatic adenocarcinoma resection (21–24). However, false-positive and false-negative results also may occur with FDG PET, and its inherent low spatial resolution may interfere with precise anatomic localization of findings (25,26). The reported sensitivity and specificity of FDG PET for depiction of pancreatic adenocarcinoma are 46%–71% and 63%–100%, respectively (18). Serum glucose levels also affect FDG PET findings. It has been reported that, among patients with pancreatic malignancy, FDG PET has relatively better sensitivity (83%–86%) for tumor depiction in patients who are euglycemic than in those with elevated glucose levels (42%–69%) (16,25–27). Relatively higher levels of ionizing radiation are also a consideration in whole-body PET. Likewise, long scanning times may affect patient compliance and increase patient motion. Finally, quantification and reproducibility of standardized uptake value (SUV) may be inaccurate because of noise attenuation correction methods.
PET/CT: Technical Considerations
The functional imaging of FDG PET and the anatomic detail of multidetector CT are concurrently obtained with a hybrid imaging device, combining the benefits of both modalities (16,25,26). Fused PET/CT provides precise anatomic delineation of FDG-avid lesions, improving overall image interpretation, accuracy, and confidence. In addition, lesions that do not demonstrate FDG uptake may be depicted and characterized by the CT component (16,25,26,28).
Although there is no consensus on the appropriate protocol for PET/CT examinations, both the CT and PET components should be performed with an optimal approach, particularly in patients with pancreatic indications. For a comprehensive imaging work-up, the protocol for dedicated diagnostic scanning should be used for the contrast-enhanced CT component. At our institution, oral and intravenous contrast material are integral to PET/CT protocols. To distend bowel loops, a commercially available neutral oral contrast agent that contains sorbitol and 0.1% barium sulfate (Volumen; Bracco, Princeton, NJ) is routinely administered. Because of its low attenuation, this contrast agent does not interfere with the attenuation correction of FDG activity (29). Nonionic iodinated contrast material is intravenously administered in all patients, unless it is contraindicated. Intravenous contrast material is invaluable for generating diagnostic-quality CT images for vascular evaluation and lesion depiction, characterization, and local staging for surgical planning.
After standard PET preparation, 15 mCi (normal range, 13.5–16.5) of FDG are intravenously injected 1 hour before the imaging evaluation; at our institution, a 64-section PET/CT scanner is used. First, an unenhanced low-dose image is obtained with no breathholding and the following parameters: section thickness, 5 mm; section interval, 5 mm; table feed, 18; pitch, 1.5; voltage, 140 kVp; current, 60 mA; rotation, 0.5 sec. PET images are then acquired with bed positions that match those used to obtain the scout view. As many as six bed positions are used, including a 3-min transmission image and variable emission images obtained on the basis of the patient’s body weight. The time to record input is 4 min per bed position for patients who weigh up to 77 kg, 5 min per bed position for patients who weigh 77–95 kg, and 6 min per bed position for those who weigh more than 95 kg. A typical PET examination lasts 18–36 min. After low-osmolar nonionic contrast material (Isovue 370; Bracco Diagnostics, Princeton, NJ) is administered at a rate of 2.5–5.0 mL/sec and 20 mL saline flush is administered at a similar rate, multiphase multidetector CT is performed. Contrast material dosage is determined on the basis of the patient’s body weight; we typically administer 80–120 mL. A dedicated pancreatic phase image of the upper abdomen is obtained after a delay of 35–45 sec (35 sec for patients with neuroendocrine tumor and 45 sec for patients with adenocarcinoma) from initiation of contrast material administration at a rate of 4–5 mL/sec.
Hypervascular lesions (neuroendocrine tumors and renal cell cancer metastases) are more conspicuous in the early phase (35-sec delay), whereas pancreatic parenchyma demonstrates optimal enhancement in the pancreatic phase (45-sec delay), allowing better definition of relatively hypovascular adenocarcinoma and nearby opacified critical vascular structures for preoperative staging. After a 60–65-sec delay, venous phase images are acquired from the level of the external auditory meatus to the symphysis pubis.
Role of PET/CT in Pancreatic Imaging
Each year, more than 43,000 patients receive a diagnosis of pancreatic ductal adenocarcinoma in the United States, with approximately 36,800 deaths resulting from the disease. In the United States, pancreatic ductal adenocarcinoma is the fourth leading cause of cancer-related death among both men and women and is the second most common cause (after colorectal cancer) of digestive cancer–related death (30,31). The overall incidence of pancreatic cancer is approximately 12 cases per 100,000 persons per year, with a 5-year survival rate of less than 5% after the initial diagnosis. Among all patients, the collective median length of survival is 4–6 months (32). Pancreatic adenocarcinoma is relatively aggressive, demonstrating early infiltration of the retroperitoneum and surrounding anatomic structures, including nerves and vessels, even when smaller than 2 cm. Depending on the stage of the tumor at the time of diagnosis, pancreatic adenocarcinoma is localized (ie, confined to the primary site) in only 8% of patients, with a 5-year relative survival rate of 21.5%; locally advanced in 27% of patients, with a 5-year relative survival rate of 8.6%; and advanced with metastases (eg, in the liver and peritoneum) in 53% of patients, with a 5-year relative survival rate of 1.8%. The tumor stage is unknown in 13% of patients, with a 5-year relative survival rate of 4.2% (32).Thus, less than 10%–20% of pancreatic adenocarcinomas are deemed surgically resectable, and many manifest at an advanced pathologic stage (30,33).
Diagnosis and treatment of pancreatic adenocarcinoma continue to be challenging. Serum CA 19-9, a mucin-associated carbohydrate antigen, may be detected in about 75% of patients with pancreatic adenocarcinoma and in about 10% of patients with a benign condition, rendering it neither sensitive nor specific for early detection of pancreatic adenocarcinoma. Unfortunately, an effective screening test for detecting pancreatic adenocarcinoma remains elusive (30,33,34). Contrast-enhanced multidetector CT, endoscopic ultrasonography (US), endoscopic retrograde cholangiopancreatography (ERCP), FDG PET, PET/CT, magnetic resonance (MR) imaging, and MR cholangiopancreatography (MRCP) are the recognized imaging modalities available for diagnosis and staging of pancreatic adenocarcinoma.
Surgery remains the only curative treatment for locally resectable and nonmetastatic pancreatic cancer. Although perioperative mortality rates have markedly decreased during the past 2 decades, surgical complication rates remain high (33). It is mandatory to determine the resectability (ie, resectable, borderline, or unresectable) of a tumor. In patients with pancreatic adenocarcinoma, it is critical that the presence or absence of metastases (eg, peritoneal or hepatic) be determined and the degree of superior mesenteric vein involvement and the relationship of the tumor with the superior mesenteric artery, celiac axis, hepatic artery, and gastroduodenal artery be evaluated (35). In patients with locally advanced adenocarcinoma, a palliative treatment option is chemoradiation therapy consisting of induction chemotherapy followed by chemoradiation therapy (33,36). Patients with metastatic pancreatic adenocarcinoma, which tends to metastasize to the liver, peritoneum, and regional lymph nodes, are best treated with chemotherapy alone or other palliative therapies (33).
FDG PET and, recently, PET/CT have been shown to be valuable in the management of pancreatic adenocarcinoma. The potential indications for FDG PET or PET/CT include imaging-guided biopsy planning, equivocal CT or nondiagnostic fine-needle aspiration findings in patients with suspected pancreatic cancer, tumor staging, depicting tumor recurrence, and monitoring response to therapy.
Biopsy of pancreatic masses is usually performed with endoscopic US guidance. However, in patients who require percutaneous biopsy, CT guidance is often the preferred approach (37). The success of imaging-guided biopsy mainly depends on the size of the suspected pancreatic mass and the ability to distinguish tumor from normal surrounding pancreatic parenchyma or associated chronic inflammatory changes. Desmoplastic reaction, which is inherent to most pancreatic cancers and recurrent tumors, may also introduce a sampling error and lead to an incorrect diagnosis. Because benign and malignant lesions may coexist in the same patient, it is crucial to select an appropriate site for tissue sampling (37).
FDG-intense foci of malignant tumors that are localized to the CT portion of a concurrent PET/CT study or that are coregistered at independent CT may provide morphologic correlation to direct tissue sampling to the most metabolically active areas in the pancreas. Tatli et al (38) confirmed that the use of previously acquired PET/CT images that are registered with intraprocedure CT to guide biopsy in the abdomen is feasible and improves the diagnostic success of CT-guided biopsy.
Diagnosing Pancreatic Cancer When Other Imaging Findings Are Equivocal
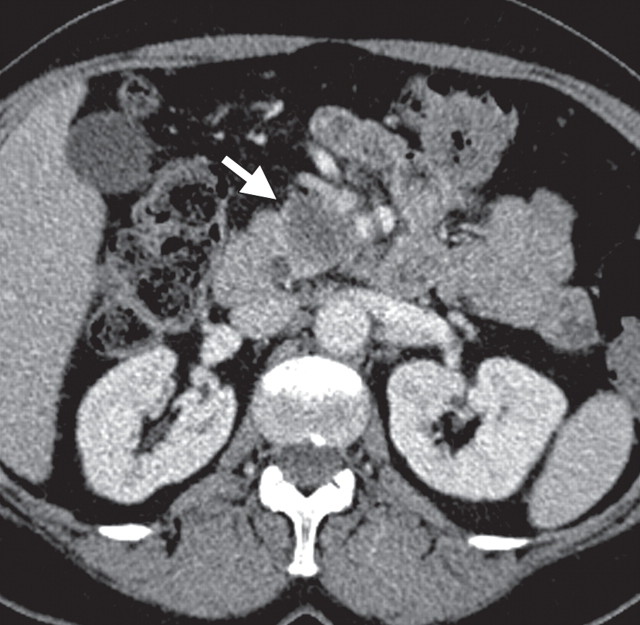
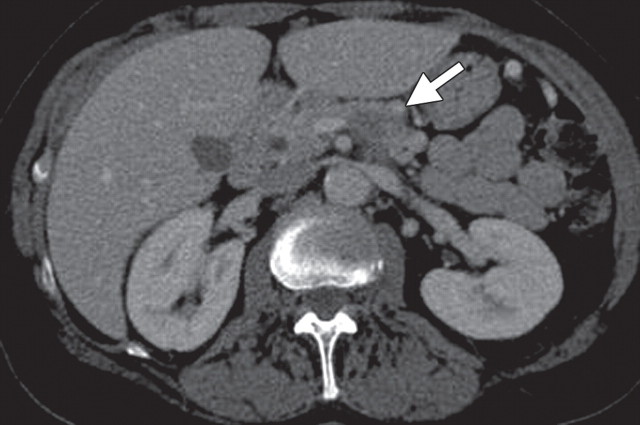
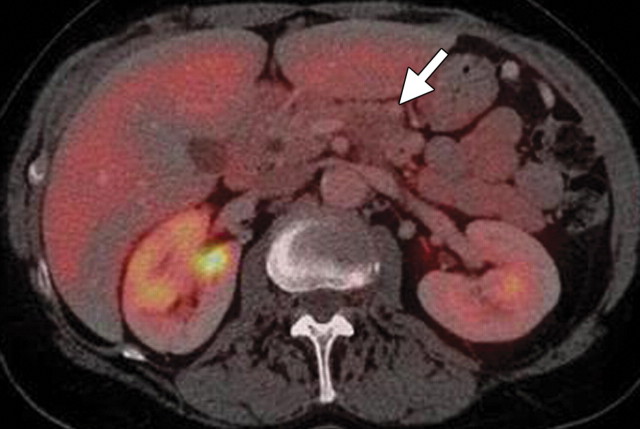
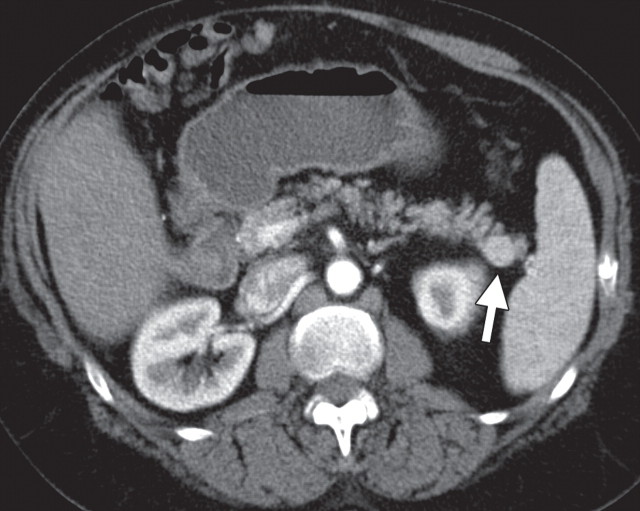
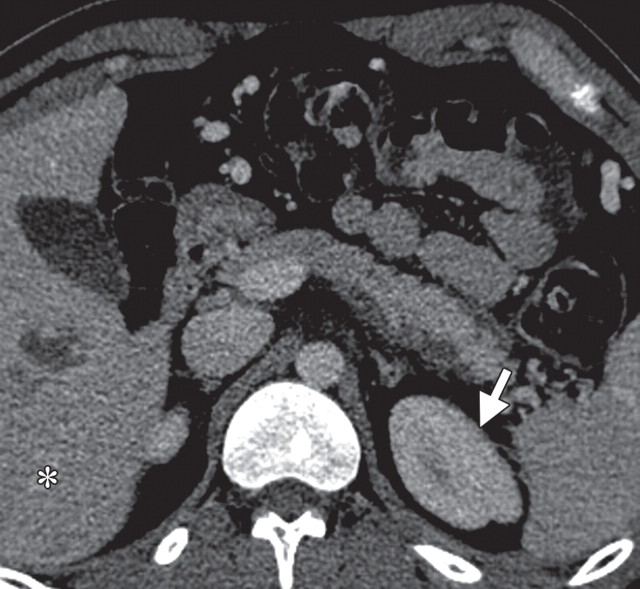
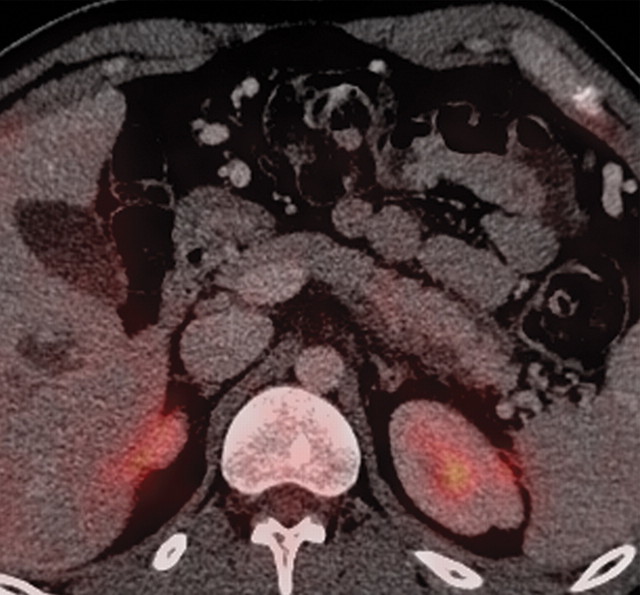
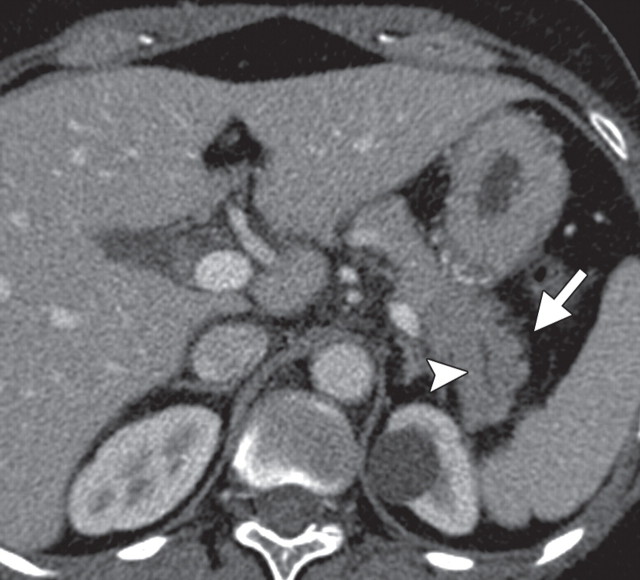
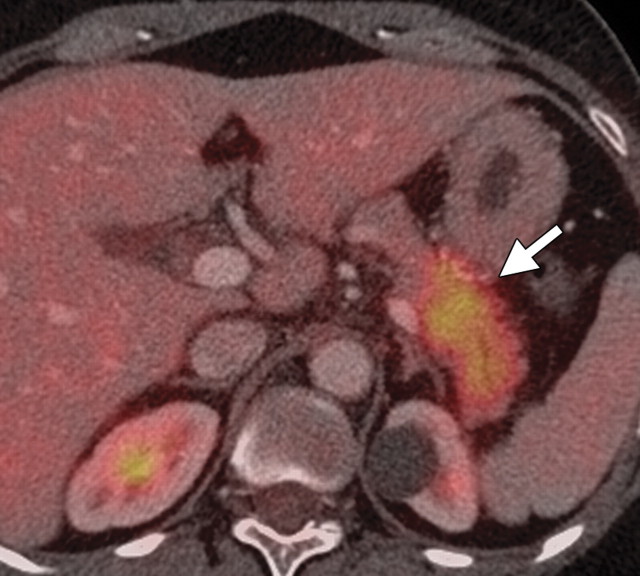
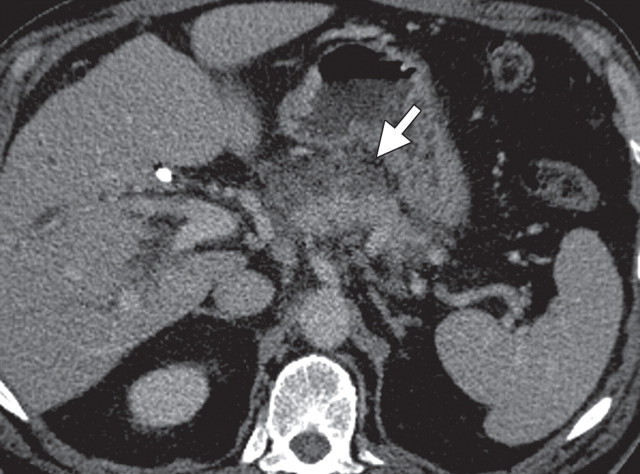
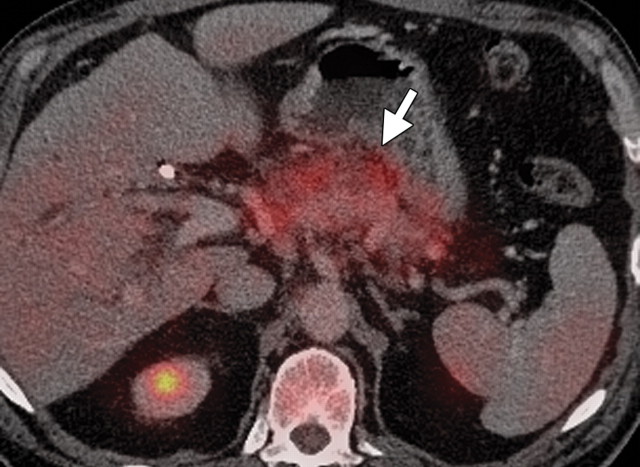
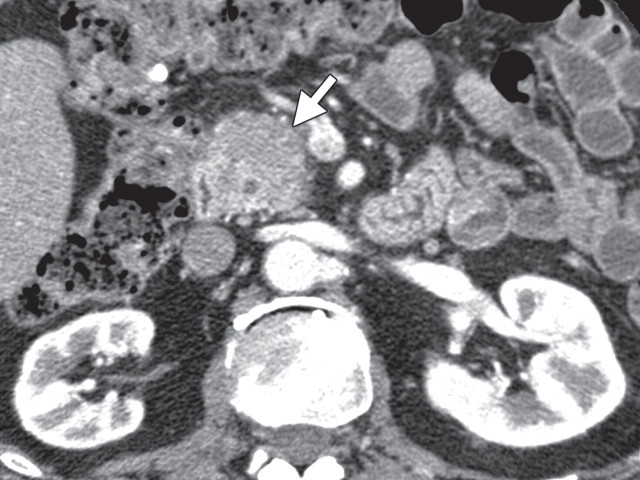
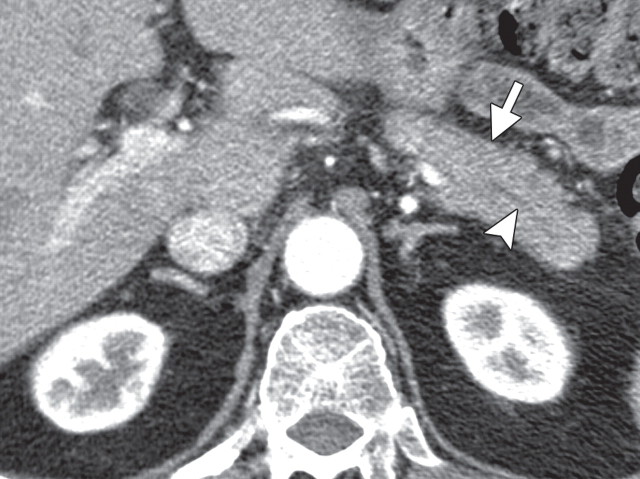
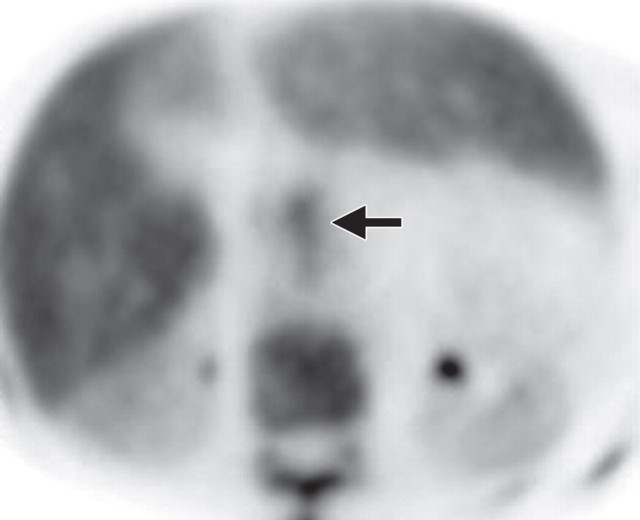
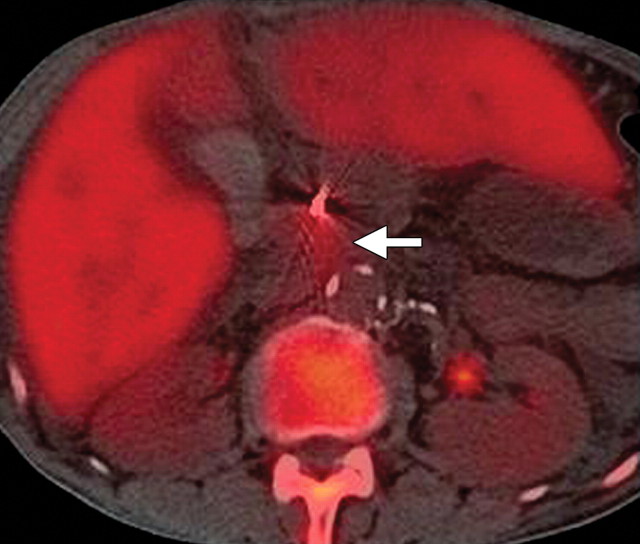
The limitations of CT for depicting pancreatic tumors are increasingly being recognized, and a correlation between tumor size and sensitivity (83% for depicting lesions <2 cm) exists (39). Moreover, about 10% of pancreatic adenocarcinomas and pancreatic metastases are isoattenuating at contrast-enhanced CT and, therefore, are not depicted, even when larger than 2 cm (Fig 1) (40).
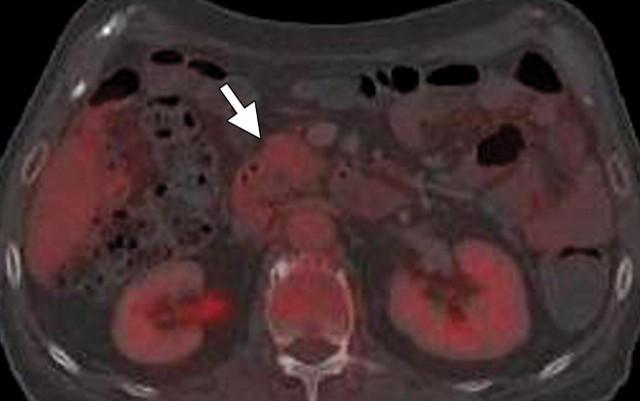
Isoattenuating pancreatic mass in a woman with breast cancer. (a, b) Fused PET/CT (a) and whole-body PET (b) images show a focal area of increased FDG uptake in the pancreatic body (arrow), a finding indicative of a malignant mass. (c) Corresponding coronal contrast-enhanced CT image shows no obvious lesion or main pancreatic duct dilatation. A diagnosis of metastatic deposit from a primary breast cancer was made on the basis of pathologic findings.
Figure 1a.
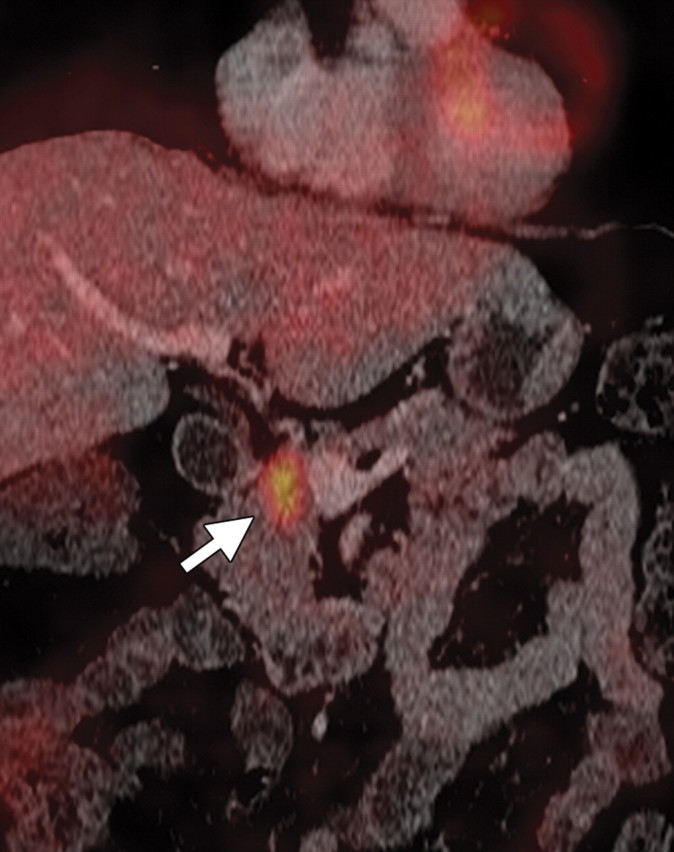
Figure 1b.
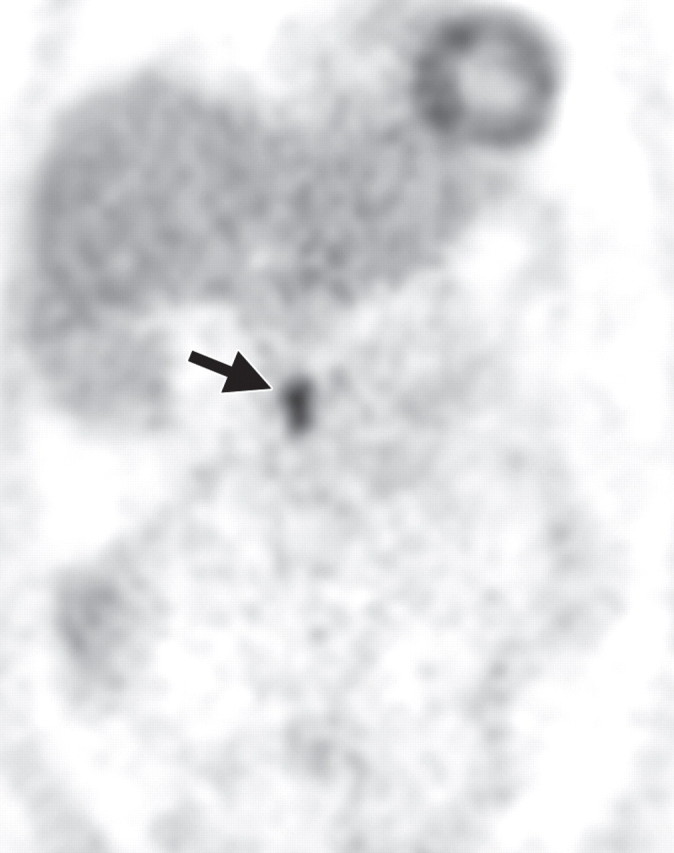
Figure 1c.
Through a combined qualitative and semiquantitative evaluation, FDG PET/CT may provide additional information over that provided by CT alone. Qualitative lesions are defined as demonstrating less, equal, or more FDG uptake than the liver (41). Pancreatic adenocarcinoma usually manifests as an area of increased uptake, appearing as a “hot spot” within the pancreas (Fig 2) (16,25,26,41). On the basis of tumor biology and the degree of desmoplastic response, pancreatic ductal adenocarcinoma may demonstrate a low level or no FDG uptake (Figs 3, 4).
Pancreatic adenocarcinoma. (a) Axial CT image shows a hypoattenuating mass (arrow) in the pancreatic body, a finding indicative of adenocarcinoma. (b) Coronal CT image shows associated vascular encasement (arrow). (c) Corresponding coronal PET/CT image shows increased FDG uptake (arrow) within the mass compared with that of the normal pancreatic parenchyma.
Figure 2a.
Figure 2b.
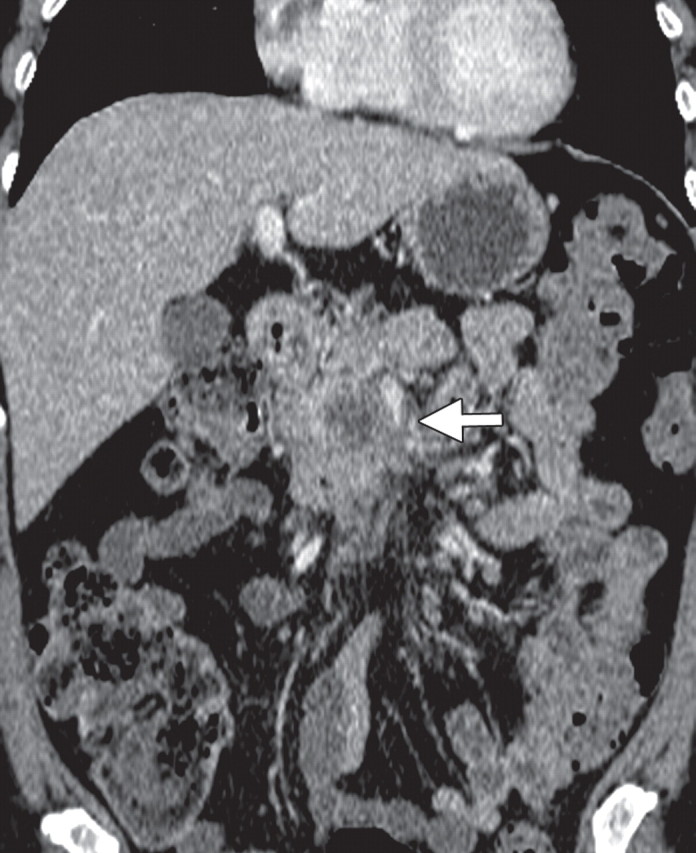
Figure 2c.
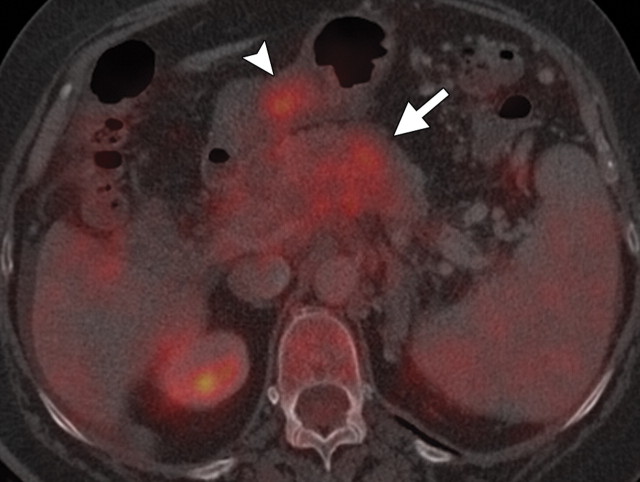
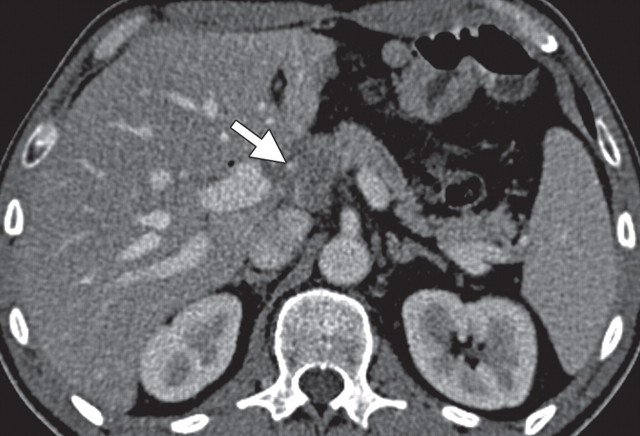
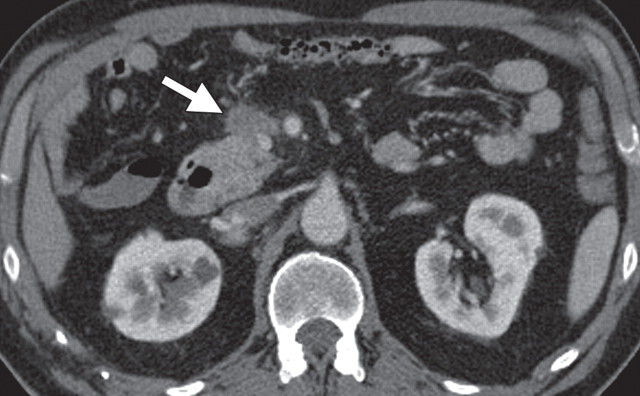
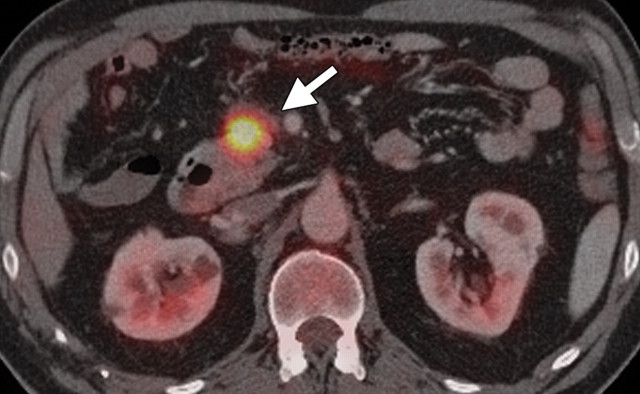
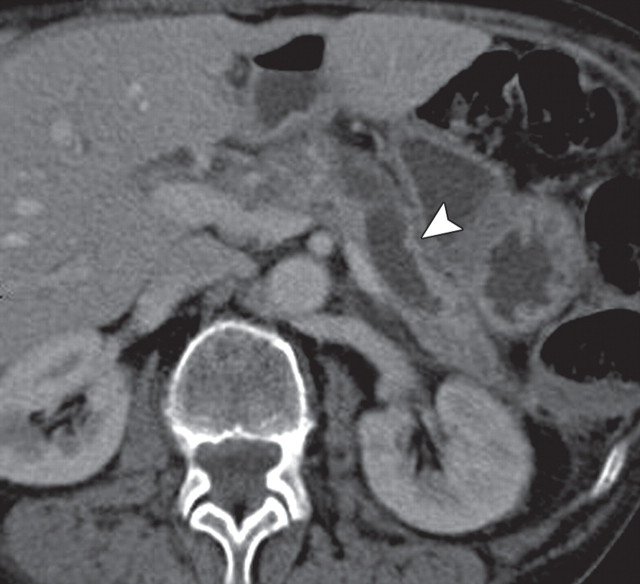
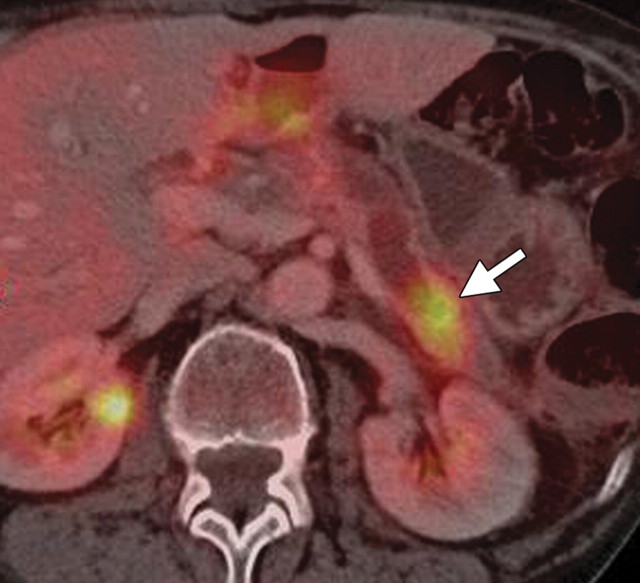
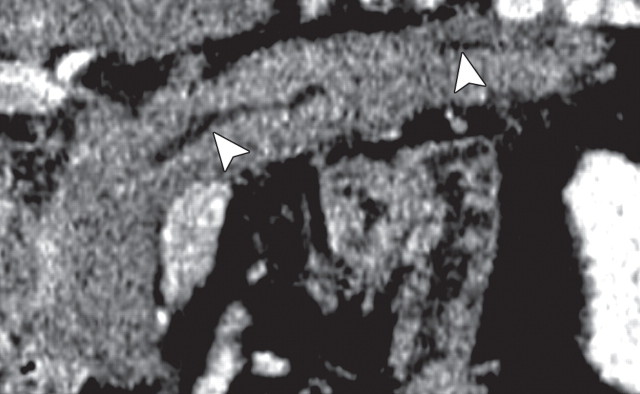
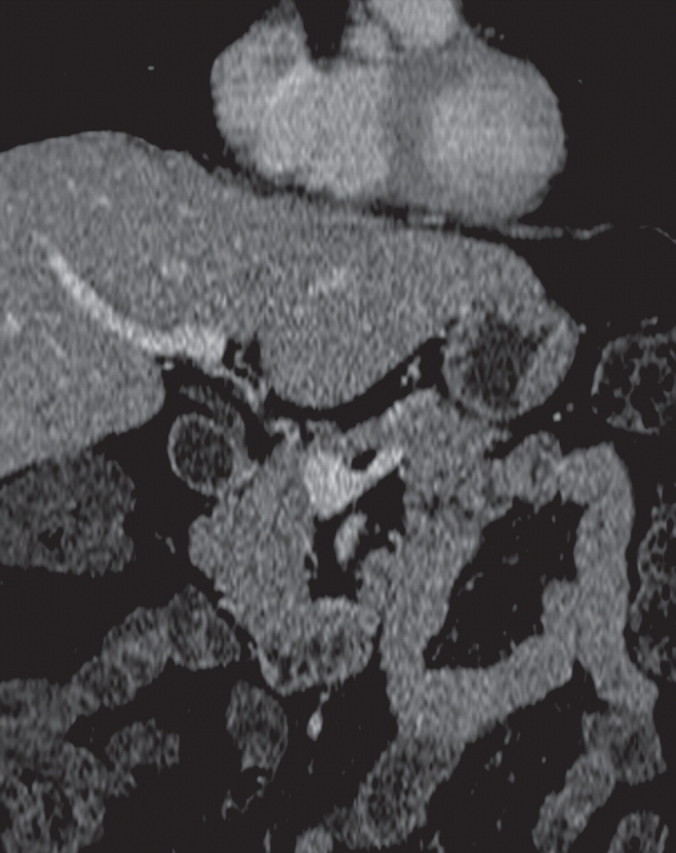
Pancreatic adenocarcinoma in a 46-year old woman with jaundice. (a, b) Axial (a) and coronal (b) contrast-enhanced multidetector CT images show indeterminate findings in the pancreatic head (arrow) and dilatation of the main pancreatic duct (arrowhead in b). A plastic stent is also seen in the common bile duct. (c, d) Axial (c) and coronal (d) fused PET/CT images show a focal area of slightly increased FDG activity in the pancreatic head (arrow). Initial endoscopic US–guided fine-needle aspiration findings were nondiagnostic; however, analysis of subsequent percutaneous biopsy specimens, obtained by directing sampling toward the location highlighted at PET/CT, confirmed the diagnosis of adenocarcinoma.
Figures 3a.
Figures 3b.
Figures 3c.
Figures 3d.
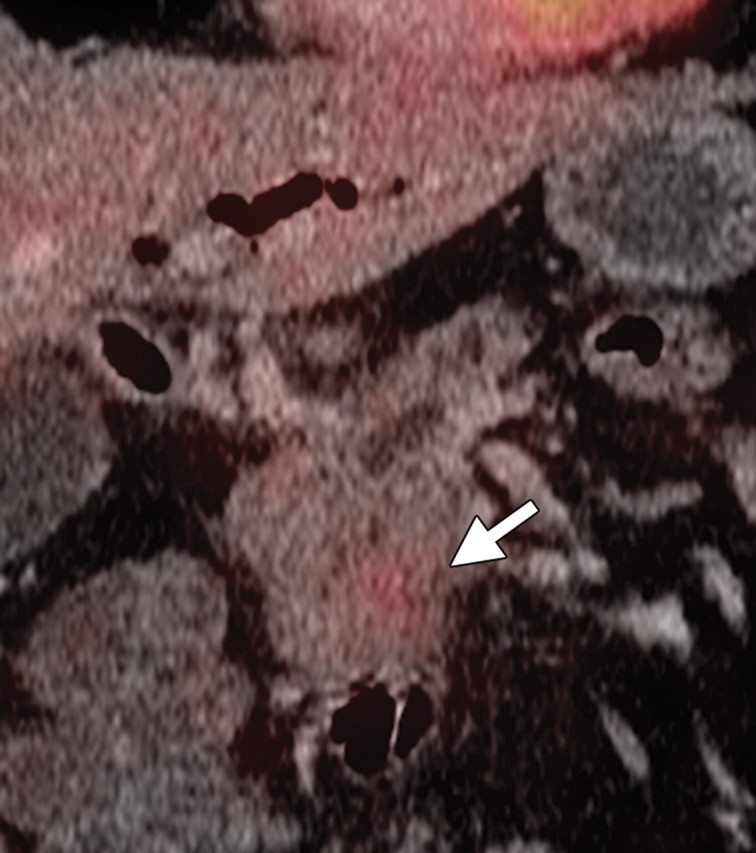
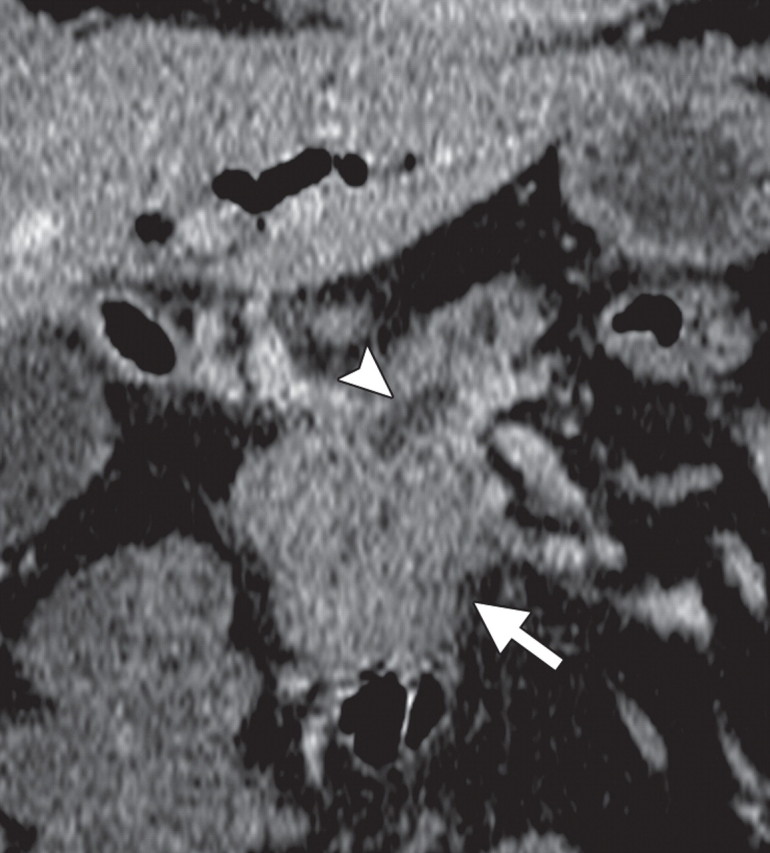
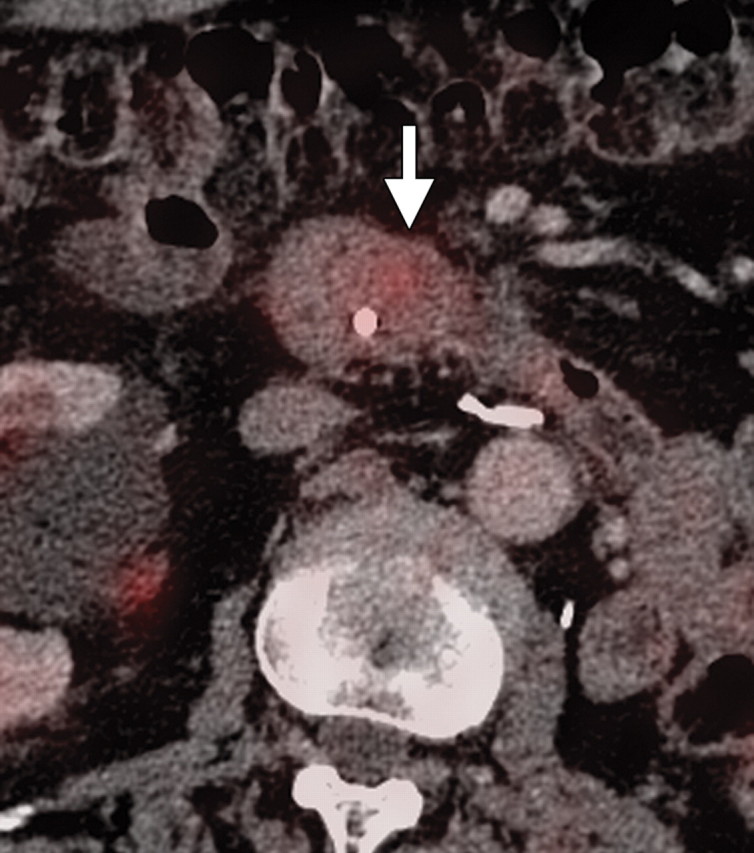
Non–FDG-avid pancreatic adenocarcinoma in a 49-year-old man experiencing weight loss and intense back pain. (a) Contrast-enhanced CT image shows a poorly defined hypoattenuating lesion (arrow). (b) Corresponding fused PET/CT image shows no appreciable FDG uptake in the lesion (arrow), which was confirmed to be adenocarcinoma.
Figures 4a.
Figures 4b.
Semiquantitative analysis relies on calculating the SUV of the lesion on the basis of activity in the region of interest (41). Typically, the maximum SUV (SUVmax) is higher in malignant lesions, irrespective of the size of the tumor (above a sub-centimeter minimum size threshold) (19,42). Thus, FDG PET may be useful in depicting small pancreatic lesions (<2 cm), which are difficult to detect at CT, or isoattenuating lesions, as well as for lesion characterization (16,20,25,26). A recent publication by Okano et al (42) is even more optimistic in its support of the use of FDG PET for depicting small pancreatic cancers, with reported sensitivities of 100% for FDG PET and 40% for contrast-enhanced CT for depicting lesions smaller than 2 cm. In another study by Lemke et al (43), it was reported that fused contrast-enhanced PET/CT is more sensitive for tumor depiction than PET and CT alone.
Reliably differentiating benign MFP from pancreatic adenocarcinoma may be difficult at CT. Both conditions are characterized by extensive fibrosis, with overlapping morphologic imaging findings. Moreover, pancreatic carcinoma may cause chronic obstructive inflammatory changes, and MFP is associated with an increased risk for adenocarcinoma (20% lifetime risk by the age of 60) (30,44). Performing pancreatic biopsy with endoscopic US or another form of imaging guidance may provide additional information about the nature of a pancreatic mass. However, endoscopic US may not depict discrete lesions, which are usually necessary to direct accurate tissue sampling, and sampling errors and uncommon complications such as pancreatitis are known limitations of endoscopic US–guided biopsy (37).
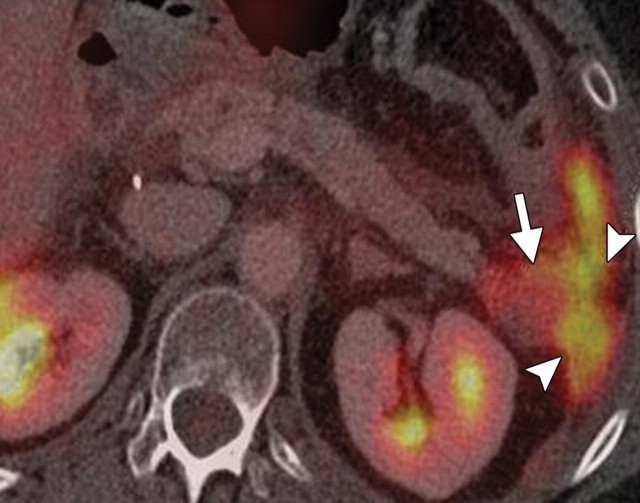
The utility of FDG PET for differentiating MFP from pancreatic adenocarcinoma has been explored by various investigators. Earlier studies reported that pancreatic adenocarcinoma tends to demonstrate higher FDG uptake than MFP. The SUV of pancreatic adenocarcinoma (3.5–5.1 ± 1.6–2.6) was found to be higher than that of benign lesions (1.9–0.8 ± 0.6–1.7) and the normal pancreas (45). When the SUV threshold for determining whether a lesion is malignant is decreased to 2.0 or more, the sensitivity for depicting adenocarcinoma increases from 86% to 100% at the expense of specificity, which decreases to 76%–77% because both inflammation and neoplasms demonstrate low FDG uptake (19,45). Thus, the SUV threshold for cancer diagnosis was raised to 4.0 to achieve a positive predictive value of 1.0 and a negative predictive value of 0.94. False-negative results may also be caused by low glucose tolerance and poorly controlled diabetes. Available data support the complementary roles of PET and CT. Focal FDG uptake is highly suspicious for cancer and requires further investigation, whereas in patients who are euglycemic, a lack of FDG uptake is more indicative of an MFP lesion (Fig 5) (20,46). In a recent study of patients with suspected pancreatic cancer, the SUVmax of malignant tumors was distinctly higher than that of benign lesions and chronic pancreatitis; PET/CT had sensitivity and specificity of 89% and 74%, respectively, for depicting such lesions (19). In another study of 38 patients, four had an MFP lesion that demonstrated no FDG uptake at PET/CT (18).
MFP in a 50-year-old man with a history of multiple episodes of pancreatitis. (a, b) Axial (a) and coronal (b) contrast-enhanced CT images show a hypo-attenuating mass in the pancreatic head (arrow in a), with no main pancreatic duct dilatation or vascular encasement. (c, d) Axial (c) and coronal (d) fused PET/CT images show low FDG uptake within the pancreatic head abnormality (arrow). Initial endoscopic US–guided biopsy yielded fibrous tissue and lymphocytes. The lesion was stable at follow-up imaging.
Figure 5a.
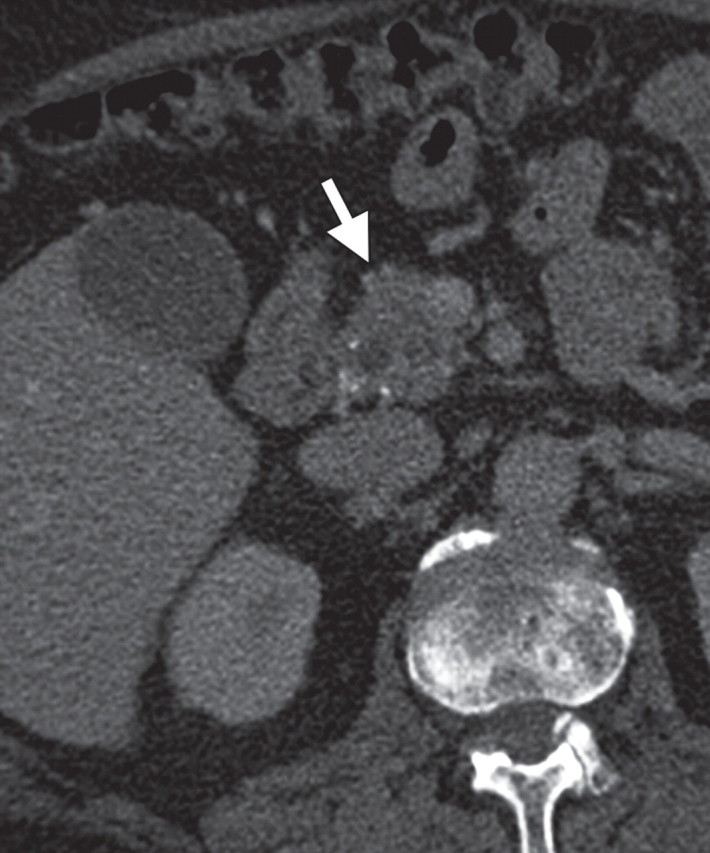
Figure 5b.
Figure 5c.
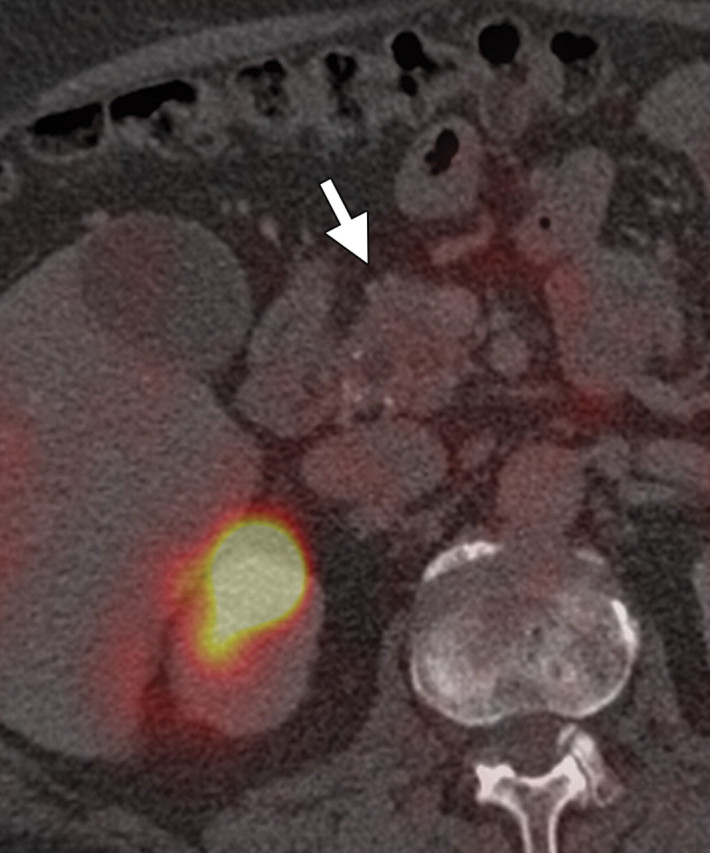
Figure 5d.
Preoperative Staging
Staging of pancreatic cancer is determined on the basis of local and distant spread of disease. Spread to regional or distant lymph nodes is usually indicative of a poor prognosis and may render the cancer inoperable (47). Multidetector CT is excellent for assessing local spread, with a reported positive predictive value of 73%–91% for resectability and 95%–100% for nonresectability (2,8–13,18,48). However, liver or peritoneal spread is discovered during surgery in about 20% of patients with tumors that are deemed resectable at CT. Likewise, lymph node spread is not optimally studied at CT.
Characterization of Lymph Nodes
Spread to lymph nodes is common and indicates a poor outcome (33,47). Current criteria for evaluating lymph node involvement at CT on the basis of size (>1 cm in the short axis) take limitations of differentiating between benign and malignant lymph nodes into account. Reactive lymph node enlargement around the pancreas commonly occurs in the setting of chronic liver disease, after biliary stent placement, or in the presence of stricture-induced cholangitis. Conversely, a substantial number of lymph nodes that harbor malignant cells do not meet the size criteria (>1 cm). Thus, lymph node staging remains difficult at CT, with a dismal 37% sensitivity and a more acceptable 79% specificity (49).
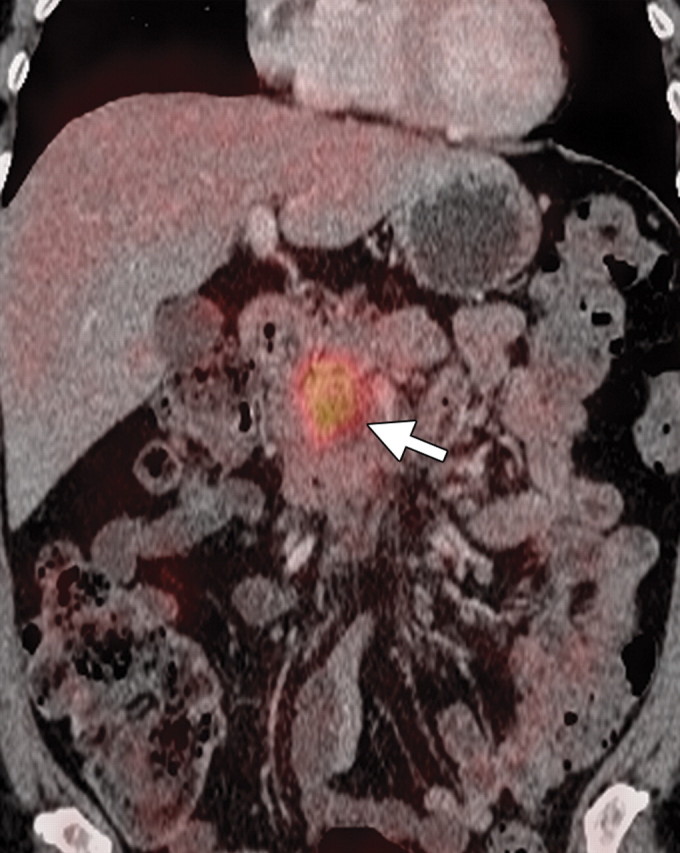
Few studies exist on the use of FDG PET for lymph node staging in patients with pancreatic cancer. Some studies reported moderate improvement in the performance of FDG PET compared with multidetector CT in patients with pancreatic masses, with sensitivity and specificity ranging from 30% to 49% and 63% to 93%, respectively, for evaluation of lymph nodes. The small tumor burden in affected lymph nodes and strong photon scatter from the primary tumor (a finding known as the penumbra effect) may partially explain the poor performance of FDG PET for lymph node staging (18,41,50,51). The performance of PET/CT for nodal staging in patients with pancreatic ductal adenocarcinoma has not been appropriately studied. However, it is conceivable that the functional information of FDG PET may complement that of CT for nodal staging, because even low-level activity at fused imaging may be indicative of nodal metastases. Fused PET/CT may improve the specificity of nodal staging compared with CT alone, helping identify metastatic deposits in lymph nodes that demonstrate nonspecific or borderline enlargement at CT (Fig 6) (52).
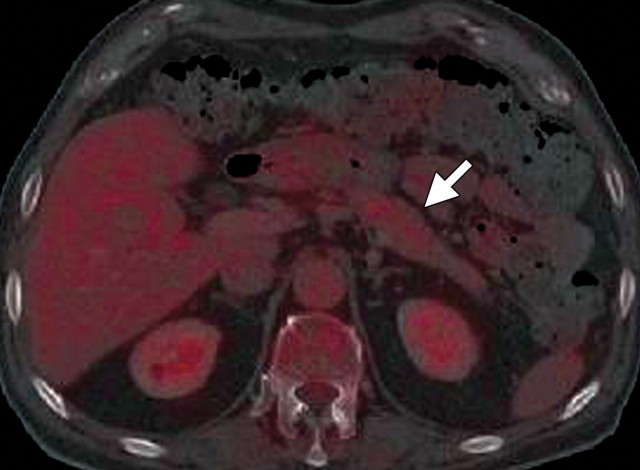
Metastatic lymphadenopathy in a 49-year-old man with pancreatic adenocarcinoma. (a) Axial contrast-enhanced multidetector CT image shows an enlarged lymph node (arrow) in the celiac region. (b) Corresponding axial fused PET/CT image shows increased FDG uptake (arrow) within the affected lymph node compared with that in the liver.
Figure 6a.
Figure 6b.
Depiction of Liver Metastases
Indeterminate lesions are frequently found in the liver at surveillance CT in patients with known malignancy. Depiction and characterization of hepatic lesions is challenging in patients with a fatty liver or contraindications for contrast material. Increased FDG activity in hepatic lesions is a strong indication of malignancy, and a lack of FDG uptake usually supports benignity; however, malignancy cannot be completely excluded in the absence of FDG uptake, especially in small lesions (53). Moreover, the performance of PET is influenced not only by the size of a lesion, but also by its biologic or histopathologic type and whether the patient has undergone therapy for the tumor (54). In a study comparing the performance of hepatobiliary (ie, mangafodipir trisodium) contrast-enhanced MR imaging and FDG PET, MR imaging was more accurate in depicting small liver metastases, with a reported accuracy of 97.1% compared with 85.3% for FDG PET (55). However, for depiction of distant metastases (ie, at other sites), FDG PET is superior to contrast-enhanced CT and MR imaging, with a reported sensitivity of 88% (18). Therefore, it is conceivable that PET/CT would provide accurate depiction of hepatic lesions and extrahepatic disease (Fig 7).
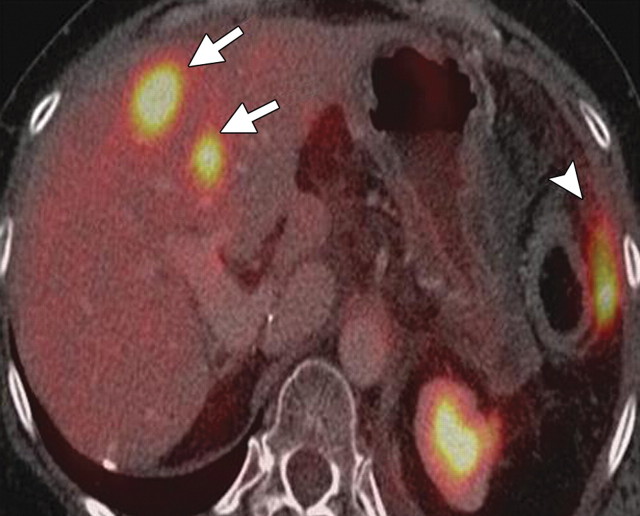
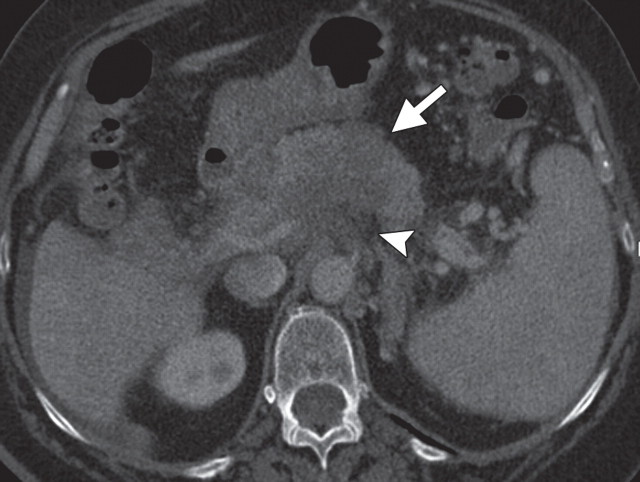
Complementary roles of CT and PET in depicting metastatic disease in a 63-year-old man with pancreatic adenocarcinoma who underwent chemotherapy. (a) Axial contrast-enhanced multidetector CT image shows obvious peritoneal disease around the stomach and abdominal wall (arrowheads). No appreciable liver lesions are seen against a background of reduced liver attenuation, a result of chemotherapy-induced steatosis. (b) Fused FDG PET/CT image shows two large FDG-avid metastatic areas in the liver (arrows). The obvious peritoneal implants are not FDG avid. A focal area of increased radiotracer uptake (arrowhead) is also seen, a result of misregistration from the splenic flexure.
Figure 7a.
Figure 7b.
Depiction of Peritoneal Metastases
Pancreatic adenocarcinoma tends to metastasize to the peritoneum, rendering patients ineligible for surgery or locoregional treatment, with the same treatment implications as other types of advanced systemic disease and a poor prognosis (mean survival, 6 months) (56). Depiction of peritoneal implants remains challenging at CT, with reported sensitivity of 65%–88% and specificity of 38%–63% (57). Even with modern multidetector CT technology, false-negative results are often encountered: Peritoneal implants were found at staging laparoscopy in 7% of patients with locally unresectable pancreatic cancer and no evidence of metastasis at multidetector CT (2,56,57). Clearly, the role of PET/CT in depicting metastases must be fully evaluated. Published data and our own observations find the functional information provided by PET promising, improving confidence in disease staging (58).
Depiction of Distant Metastases
After a diagnosis of pancreatic cancer is established and its local resectability confirmed, the main objective of pancreatic cancer staging is to identify distant metastases, which preclude surgery (33,50,59). In two small studies, the use of FDG PET for preoperative pancreatic cancer staging was reported to be cost beneficial because of its depiction of unexpected distant metastases in 43% of patients, thereby avoiding unnecessary surgical procedures (50,59). A few investigations have shown that the use of PET/CT may improve selection of patients for surgery by depicting primary pancreatic tumors not clearly evident at CT or MR imaging and prevent unnecessary pancreatic resection in as many as 25% of patients by depicting unsuspected metastases (Fig 8) (18,48).
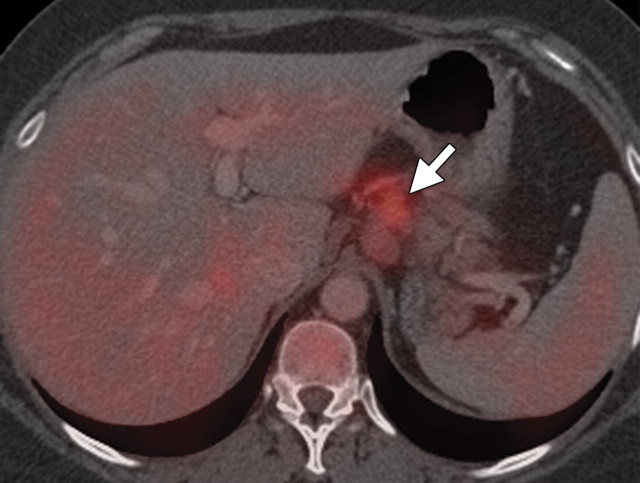
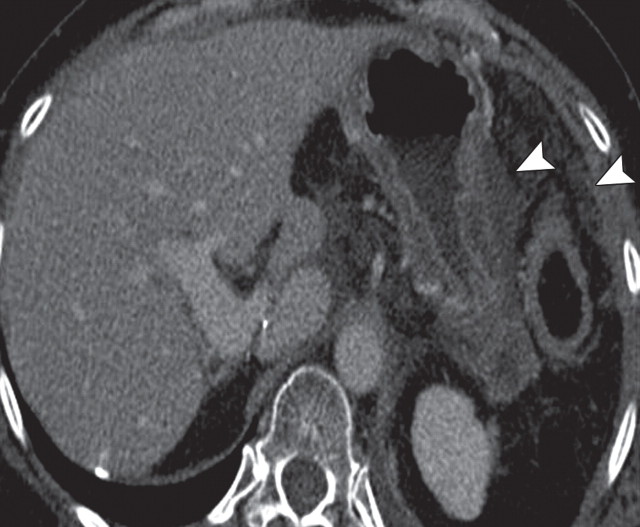
Pancreatic cancer with a peritoneal implant along the antrum of the stomach in a 52-year-old man. (a) Axial contrast-enhanced multidetector CT image shows a hypoattenuating invasive neoplastic lesion in the body of the pancreas (arrow) encasing the nearby mesenteric vessels (arrowhead). (b) Axial fused PET/CT image shows avid FDG uptake within the mass (arrow) and a focus of increased radiotracer uptake along the gastric wall (arrowhead) that was found to be a peritoneal implant. The implant was not seen at initial CT but increased in size at follow-up CT.
Figure 8a.
Figure 8b.
Depiction of Tumor Recurrence and Monitoring Response to Therapy
After surgery, 72%–92% of pancreatic adenocarcinomas recur locally within 2 years (24,60). Locally recurrent tumors are usually not resectable; however, radiation therapy or ablation (eg, radiofrequency or cryoablation) may be a palliative option. Because the expected postoperative changes in the surgical bed and early tumor recurrence have similar morphologic characteristics, reliably differentiating between them is difficult at CT. Moreover, it is often difficult to obtain an adequate tissue sample because desmoplastic reaction is known to be associated with pancreatic cancers. The use of FDG PET to depict tumor recurrence is promising, particularly when CT findings are equivocal (Fig 9) (22, 24,61). Abnormal FDG uptake in the surgical bed 3 months after surgery is usually indicative of recurrence (Fig 10). The reported sensitivity of FDG PET for depicting tumor recurrence is 96% compared with 39% for CT and MR imaging (22). Moreover, after resection, tumor relapse is depicted at FDG PET earlier than it is at CT, with higher sensitivity (98%) and specificity (90%) (24).
Suspected recurrent pancreatic adenocarcinoma in a 52-year-old man with a history of Whipple resection. (a) Axial contrast-enhanced multidetector CT image shows a soft-tissue abnormality in the surgical bed (arrow) that was not considered to be a nondistended jejunal segment. Because a borderline increase in tumor marker CA 19-9 was also present, this finding is suspicious for tumor recurrence. (b) Fused PET/CT image shows no obvious increased FDG uptake in the surgical bed. At follow-up contrast-enhanced CT, the soft-tissue abnormality remained stable, ruling out tumor recurrence. This case highlights the role of PET/CT in the follow-up of patients with equivocal CT findings who underwent surgery for pancreatic adenocarcinoma.
Figure 9a.
Figure 9b.
Recurrent tumor in a patient with pancreatic adenocarcinoma who underwent distal pancreatectomy and splenectomy. (a) Axial contrast-enhanced multidetector CT image shows a soft-tissue abnormality in the surgical bed (arrow) and soft-tissue nodules (arrowheads) in the omental fat in the left upper quadrant. (b) Axial fused PET/CT image shows areas of clear FDG uptake that correspond with the soft-tissue abnormalities (arrow and arrowheads) seen at CT, a finding that confirms tumor recurrence.
Figure 10a.
Figure 10b.
Likewise, PET may play a role in monitoring response to chemo- and radiation therapy in patients with unresectable pancreatic cancer (21,23,62). As with other neoplasms, a significant reduction in FDG uptake may precede volumetric reduction at CT and may be proportional to the change in tumor size at subsequent follow-up examinations. Therefore, earlier depiction of tumor response to therapy at FDG PET could influence the continuation or withdrawal of treatment (21). Moreover, some recently published studies reported that FDG PET/CT may have prognostic value because tumors with a higher baseline SUVmax are more likely to recur in the early postoperative period. SUVmax is also an independent predictor for overall survival in patients with locally advanced pancreatic cancer (63,64). Postoperative inflammatory changes in the pancreas, radiation therapy, or stent placement may also cause some FDG uptake. To minimize these false-positive results, it is recommended that follow-up PET or PET/CT be performed at least 6 weeks after surgery (21,22,65).
Other Pancreatic Neoplasms
Pancreatic Endocrine Neoplasms
Pancreatic endocrine neoplasms are epithelial neoplasms with an organoid growth of cells that resemble pancreatic islet cells or other hormone-producing cells. They are usually well differentiated and are classified as functional or nonfunctional on the basis of the presence of an associated clinical endocrine paraneoplastic syndrome. Pancreatic endocrine neoplasms are named according to the predominant hormone they produce (eg, insulinoma, gastrinoma, vipoma, glucagonoma, and somatostatinoma).
Neuroendocrine tumors represent 1%–2% of all pancreatic neoplasms (30). More than a decade ago, functioning tumors represented 70% of neuroendocrine tumors; however, according to most recent studies, nonfunctioning tumors now account for 60%–80% of such tumors (66). Insulinoma and gastrinoma are the most common functioning islet cell tumors, accounting for about 32% and 9% of cases, respectively. Functioning tumors are detected earlier in their clinical course, when they are generally small in size.
Other pancreatic neuroendocrine tumors such as glucagonoma, somatostatinoma, vipoma, and nonfunctioning tumors are often large and malignant at the time of diagnosis (30). As many as 90% of nonfunctioning tumors are malignant at the time of diagnosis, with more indolent biologic behavior than pancreatic adenocarcinoma (67). Surgery is the best treatment option for patients with a solitary pancreatic neuroendocrine tumor; chemotherapy and other directed therapies are usually reserved for symptomatic patients with metastatic or locally advanced tumors (30).
Neuroendocrine tumors—with the exception of insulinomas and poorly differentiated lesions—are characterized by a dense layer of somatostatin receptors (SSRs) at the cell membrane, the reason functional imaging with somatostatin analogs is used to manage neuroendocrine tumors (66,68). Indium-111 octreotide scintigraphy may reliably depict SSR-expressing tumors with a sensitivity of approximately 80%–90% (66). Usually, well-differentiated, slow-growing neuroendocrine tumors demonstrate little or no FDG uptake, whereas poorly differentiated neuroendocrine tumors and metastases, which rarely express SSRs, are well depicted at FDG PET (Figs 11, 12). For depiction of pancreatic neuroendocrine tumors, contrast-enhanced MR imaging has sensitivity of 53%–79%, contrast-enhanced CT has sensitivity of 50%–73%, and FDG PET has sensitivity of 53%–57% (66,69–71).
Malignant, nonfunctioning, well-differentiated pancreatic neuroendocrine tumor in a 56-year-old woman who underwent resection of a carcinoid tumor in the ileum 15 years ago and recently received a diagnosis of breast cancer. (a) Axial contrast-enhanced multidetector CT image shows a well-defined, enhancing, solid lesion (arrow) in the pancreatic tail. (b) Axial PET/CT image shows a lack of focal FDG uptake within the lesion. At pathologic analysis, the lesion was determined to be a well-differentiated malignant pancreatic neuroendocrine tumor.
Figures 11a.
Figures 11b.
Figures 12.
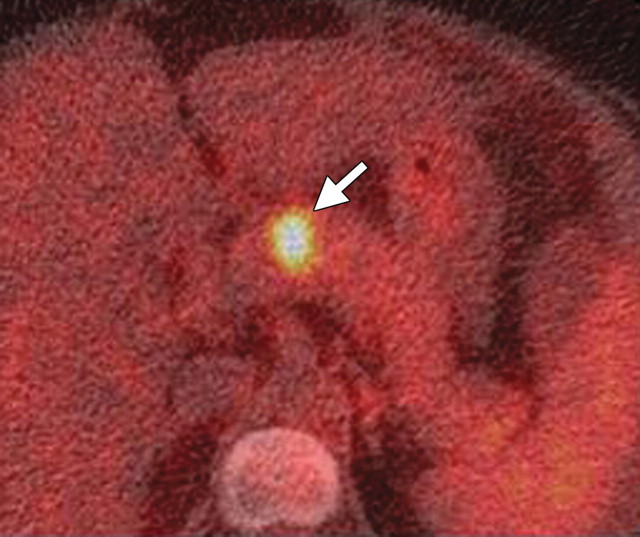
FDG-positive malignant neuroendocrine tumor. Fused axial PET/CT image shows an intense focal area of FDG uptake (arrow) in the mid pancreatic body. At pathologic analysis, the lesion was confirmed to be a poorly differentiated pancreatic neuroendocrine tumor.
Novel radiolabeled somatostatin analogs such as gallium-68 (68Ga) tetraazacyclododecanetetraacetic acid (DOTA)-DPhe-1-Tyr-3-ictreotate (TATE) (SSR-2 analogs), 68Ga DOTA-tyrosine-3-octreotide (TOC) (SSR-2 and SSR-5 analogs), and 68Ga DOTA-1-NaI-octreotide (NOC) (SSR-2, SSR-3, and SSR-5 analogs) are being made available for use in patients with neuroendocrine tumors. Kayani et al (68) reported that high-grade and poorly differentiated neuroendocrine tumors demonstrate more FDG uptake (sensitivity, 66%), whereas low-grade and well-differentiated tumors demonstrate more 68Ga DOTA-TATE uptake (sensitivity, 82%). Versari et al (72) reported that 68Ga DOTA-TOC PET has accuracy comparable to those of endoscopic US and multidetector CT for depicting primary neuroendocrine tumors in the duodenopancreatic area, with a sensitivity of 87% and specificity of 83%. New radiopharmaceutical agents such as carbon-11–labeled 5-hydroxytryptophan (5-HTP) and L-dihydroxyphenylalanine (L-DOPA) are promising in their ability help depict primary and metastatic neuroendocrine tumors, but their availability remains an impediment (69–71).
Pancreatic Lymphoma
Pancreatic lymphoma is a neoplasm characterized by lymphomatous involvement of the pancreas. It may arise primarily in the pancreas or secondarily involve the pancreas in the setting of systemic disease. Primary pancreatic lymphoma is rare (accounting for 0.5% of all pancreatic neoplasms) and is usually of the non-Hodgkin type, with both B- and T-cell lineages. The pancreatic head is the most common location, although the entire gland may be affected. Histologic analysis of this rare cancer is critical and requires adequate tissue samples for diagnosis (30). Patients with primary pancreatic lymphoma respond well to chemo- and radiation therapy. Surgery is not usually performed in patients with known lymphoma (73).
The usefulness of FDG PET in staging and differentiating primary pancreatic lymphoma from secondary lymphoma has already been established; PET/CT may be expected to confer the same benefits (74). At contrast-enhanced CT, the presence of intact fat planes between the lymph nodes and the pancreas and anterior displacement of the pancreas help differentiate peripancreatic lymphadenopathy from a primary pancreatic tumor (75). Lymphoma may manifest as a multifocal mass with various levels of increased FDG uptake or diffuse increased FDG uptake in the pancreas. Lymph nodes in the peripancreatic region also demonstrate FDG uptake.
Upon completion of chemo- or radiation therapy, response to therapy may be assessed at PET/CT (Fig 13). Persistent FDG uptake in the pancreas or lymph nodes is indicative of residual disease, whereas a lack of FDG uptake is indicative of a complete metabolic response. FDG PET findings may also be used to determine whether a favorable progression-free survival is likely. Two studies reported a 5-year progression-free survival rate of 88.8% in patients with negative PET findings and only 16.2% in patients with positive PET findings (76,77).
Pancreatic lymphoma in a 47-year-old woman. (a, b) Axial (a) and coronal (b) contrast-enhanced CT images show a hypoattenuating infiltrative mass (arrow) diffusely enlarging the pancreatic body and tail. A large hypoattenuating focal lesion (arrowhead) is also seen in the right hepatic lobe. (c, d) Representative axial (c) and coronal (d) fused PET/CT images show avid FDG uptake within the pancreatic mass (arrow) and the right lobe of the liver (arrowhead in d), findings indicative of disease spread. In d, misregistration is also seen in both lesions. (e, f) Posttreatment contrast-enhanced CT (e) and fused PET/CT (f) images obtained 6 months after chemotherapy show dramatic improvement in the tumor burden (* and arrow in e) and a favorable metabolic response.
Figure 13a.
Figure 13b.
Figure 13c.
Figure 13d.
Figure 13e.
Figure 13f.
Metastases
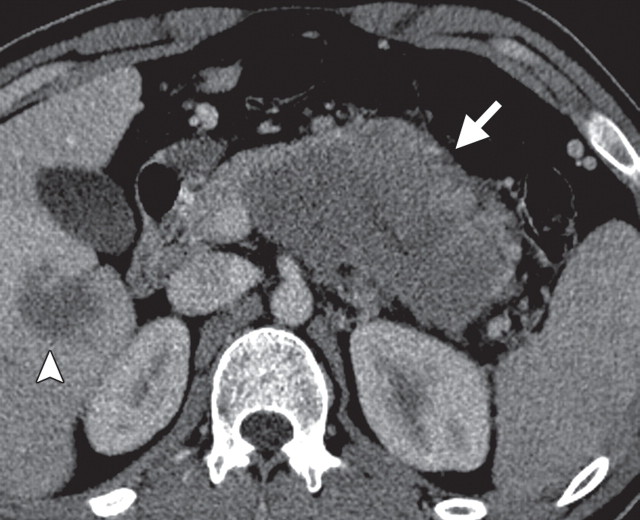
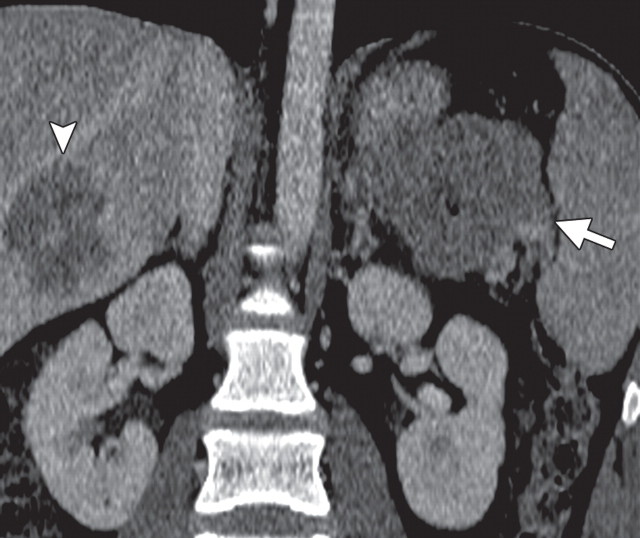
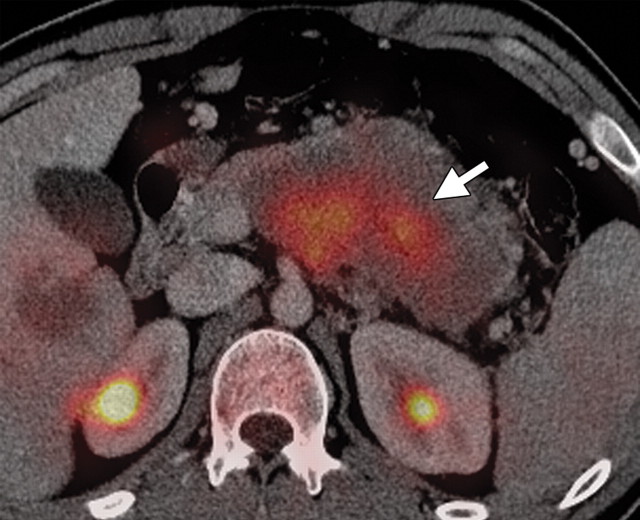
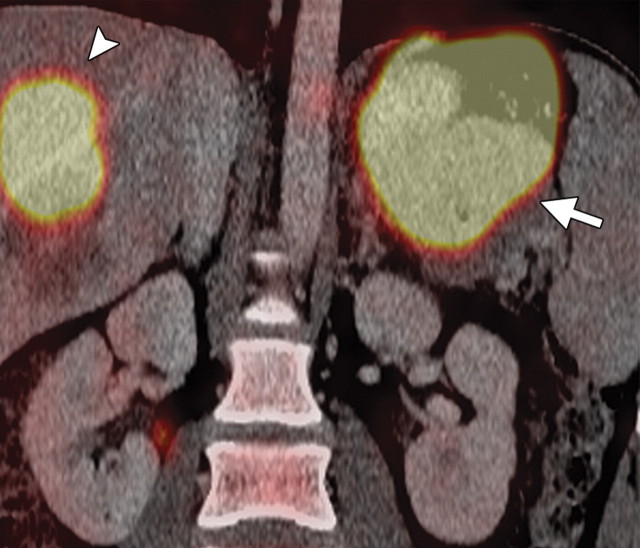
Pancreatic metastases are rare, accounting for 2% of all pancreatic neoplasms. They usually occur in the setting of advanced metastatic disease and, rarely, as isolated pancreatic metastases. They may arise synchronously or metachronously and may occur singly (in 25% of patients) or in multiples (in 75% of patients). In patients with single lesions, pancreatic metastases usually occur in the head (30,78). The primary malignancies that most commonly metastasize to the pancreas are lung, breast, melanoma, gastric, colorectal, renal, and ovarian cancers (30,79).
Solitary pancreatic metastases from renal cancer demonstrate high enhancement during the arterial or pancreatic phase at dynamic multidetector CT and MR imaging (78). At CT, most other pancreatic metastases are hypoattenuating with variable contrast enhancement, and at FDG PET they often demonstrate uptake similar to that in the primary tumor (Figs 14, 15) (80). In patients with many types of cancer, preoperative FDG PET may depict unknown pancreatic metastases. However, metastases from clear cell renal cancer often do not demonstrate FDG uptake; combined PET/CT may overcome the limitations of both modalities. Sato et al (79) reported that in patients with lung cancer, FDG PET/CT is advantageous both in the first stage and the follow-up stage in depicting unsuspected pancreatic metastases that have not yet manifested at CT.
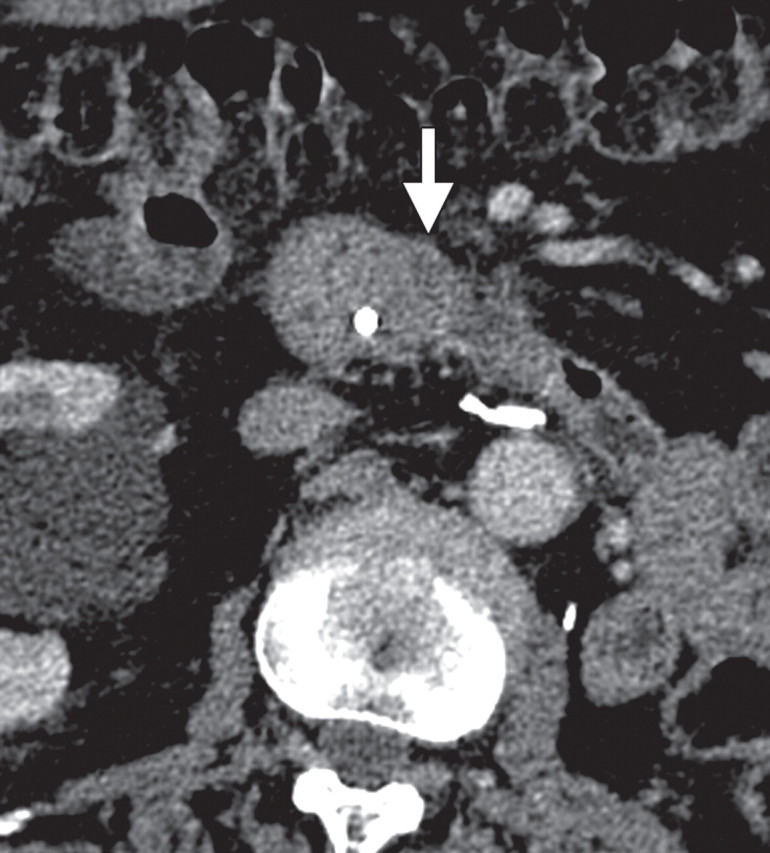
Pancreatic metastasis in a patient with colon carcinoma. (a, b) Axial (a) and coronal (b) CT images show a focal round hypoattenuating lesion (arrow) near the pancreatic head partially encasing the superior mesenteric artery. (c, d) Axial fused PET/CT (c) and whole-body PET (d) images show increased FDG uptake within the lesion (arrow). Biopsy was performed, and the lesion was confirmed to be a metastasis.
Figure 14a.
Figure 14b.
Figure 14c.
Figure 14d.
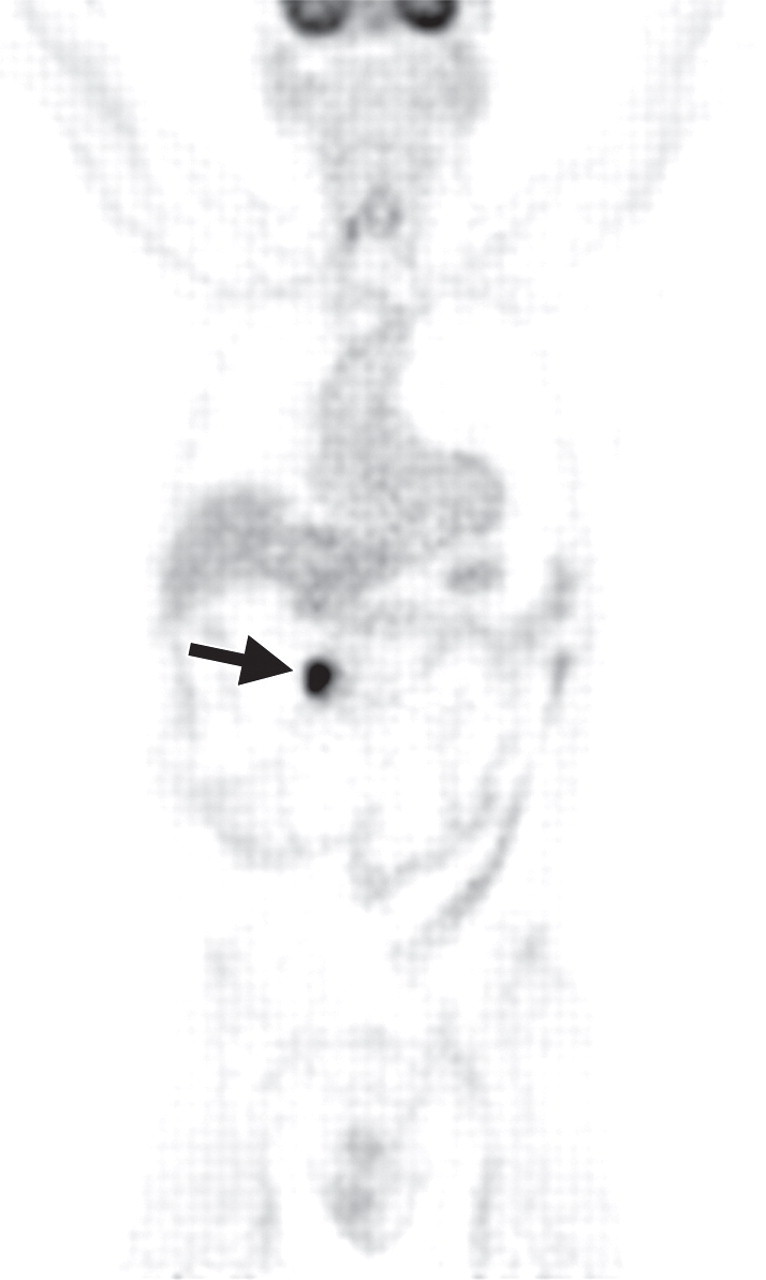
Pancreatic metastases in a patient with breast cancer and new symptoms of pancreatitis. (a) Axial contrast-enhanced multidetector CT image shows focal swelling in the pancreatic tail (arrow) and proximal mild pancreatic duct dilatation (arrowhead). (b) Axial PET/CT image shows swelling and increased FDG uptake in the entire distal pancreas. More avid focal uptake is seen at the site of the pancreatic duct transition (arrow). Biopsy was performed, and pathologic analysis confirmed a metastasis from breast cancer.
Figure 15a.
Figure 15b.
Cystic Neoplasms
Cystic neoplasms of the pancreas constitute less than 10% of all pancreatic neoplasms; however, they have become more commonly reported because of the increased use and improved accuracy of imaging techniques. They encompass a wide range of pathologic conditions ranging from benign lesions, such as serous cystoadenomas, to malignant, potentially malignant, and borderline tumors, such as neuroendocrine tumors with cystic features, mucinous cystic neoplasms, and IPMNs (81). Contrast-enhanced CT and MR imaging are the preferred modalities for the initial evaluation of cystic lesions, requiring the use of morphologic features to characterize cysts and determine the risk for malignancy. However, given the overlap in features of various cystic lesions, accurate classification and determination of benignity or malignancy is not always possible (82). The accuracy of multidetector CT for determining malignancy ranges from 53% to 86% on the basis of features such as marked main pancreatic duct dilatation of more than 10 mm, the presence of a large mural node larger than 1 cm, a cyst larger than 3 cm, irregular or septate features, and calcification (4,83).
Published studies have shown that FDG-positive cystic neoplasms are frankly malignant or invasive (Fig 16). Conversely, FDG-negative lesions may be benign, borderline malignant, or noninvasive malignant (5). By using a cut-off SUV of 2.5, differentiating between benign and malignant IPMNs was feasible, with malignant lesions (range, 6.7–2.7) demonstrating significantly higher SUVmax than benign lesions (range, 2.1–1.8) (4,6,7). The sensitivity (94%) and specificity (100%) of PET/CT for depicting malignant cystic pancreatic lesions have been shown to be superior to those of FDG PET (56% sensitivity and 83% specificity) and CT (81% sensitivity and 100% specificity) (84).
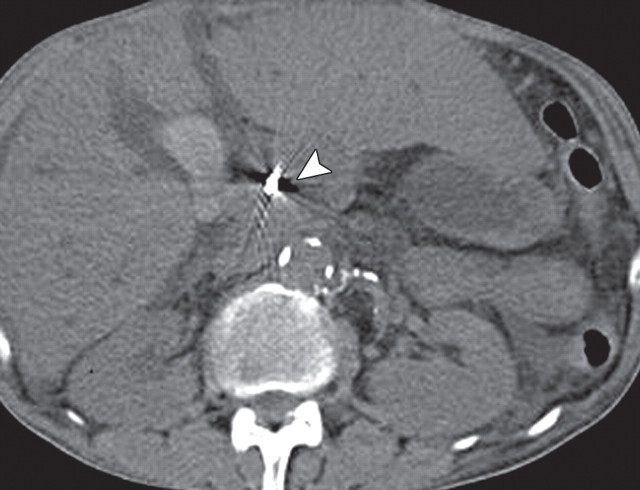
Malignant IPMN in a 67-year-old man with a recent onset of weight loss. (a) Axial contrast-enhanced multidetector CT image shows gross dilatation of the main pancreatic duct (arrowhead) and associated atrophy of pancreatic parenchyma, typical features of IPMN. (b) Fused PET/CT image shows an area of marked FDG uptake (arrow) within the pancreatic tail. A malignant IPMN with an invasive focus that corresponds with the area of FDG uptake was confirmed at surgery.
Figure 16a.
Figure 16b.
Despite the added benefits of FDG PET, the published data, as well as our own experience, suggest that the limitations of contrast-enhanced CT in discriminating borderline and noninvasive cancers cannot be overcome by PET, and the role of PET/CT is not yet established in managing cystic neoplasms. However, it has been suggested that the combination of the morphologic features of cystic lesions seen at multidetector CT and the concurrent functional information of FDG uptake provided by PET may improve diagnosis of malignant or invasive mucinous neoplasms of the pancreas (4,6,7).
Pancreatitis
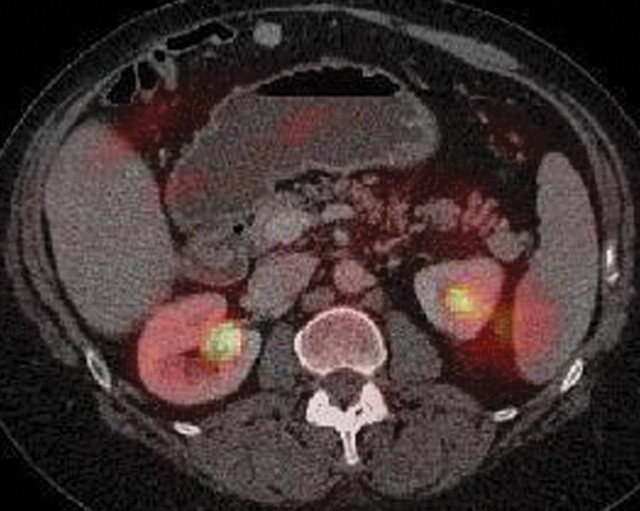
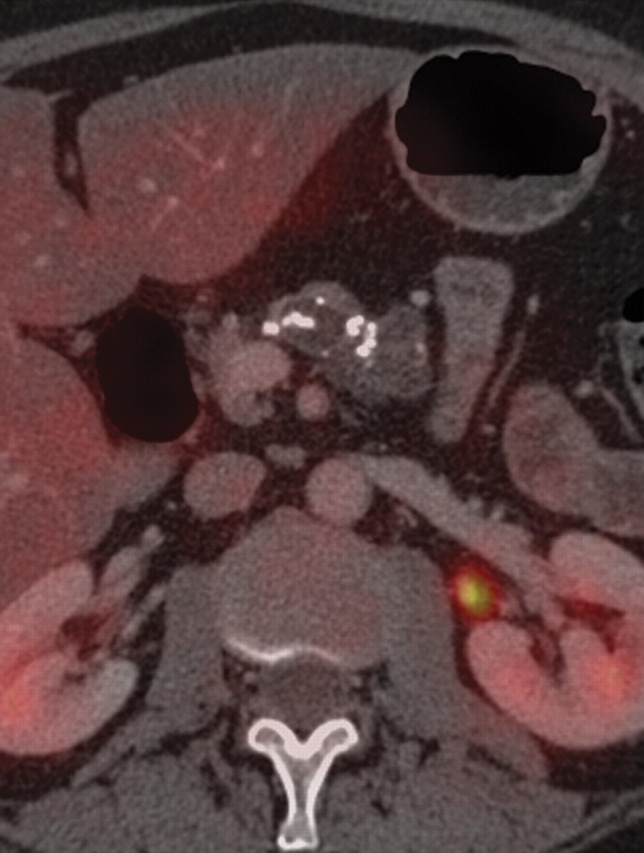
Pancreatitis is a well-known inflammatory process of the pancreas that is easily diagnosed on the basis of clinical, laboratory, and imaging findings. It may be incidentally found in patients who undergo PET/CT for oncologic follow up; thus, it is important to be aware of its imaging appearance to avoid misinterpreting it as metastatic or lymphomatous pancreatic involvement. According to the severity of the disease process and the presence of necrosis, acute pancreatitis may be classified as edematous or necrotic, with the latter conferring a much worse prognosis (85). Chronic pancreatitis is often related to alcohol consumption. Along with known morphologic features such as main pancreatic duct dilatation and parenchymal atrophy, calcification is a pathognomonic finding at CT in patients with longstanding inflammatory processes (Fig 17) (86).
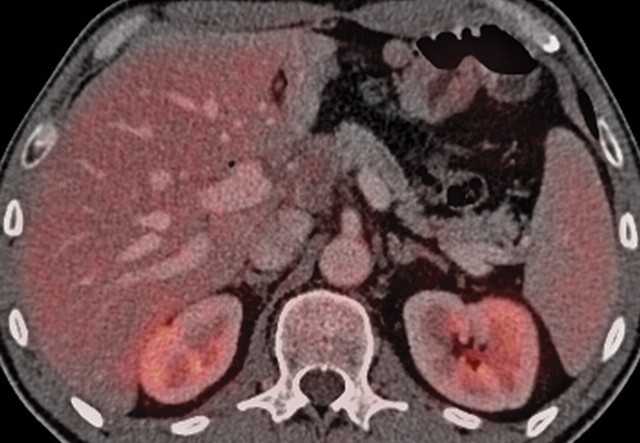
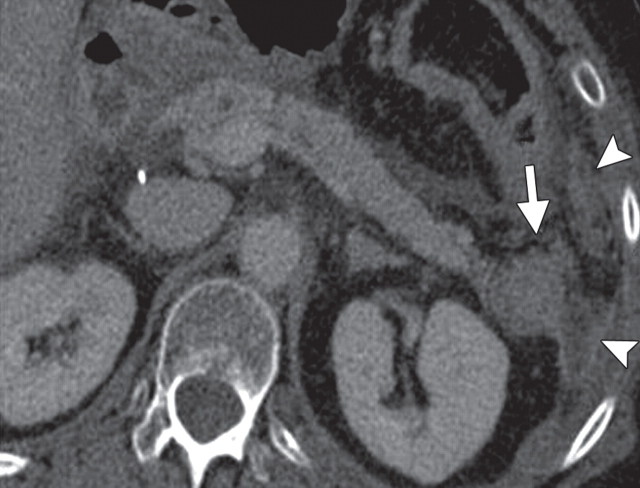
Chronic pancreatitis. (a) Axial multidetector CT image shows gross calcifications (arrow) within the pancreas, parenchymal atrophy, and dilatation of the main pancreatic duct, typical manifestations of chronic pancreatitis. (b, c) Coronal FDG PET (b) and axial PET/CT (c) images show no focal or diffuse FDG uptake in the pancreas.
Figure 17a.
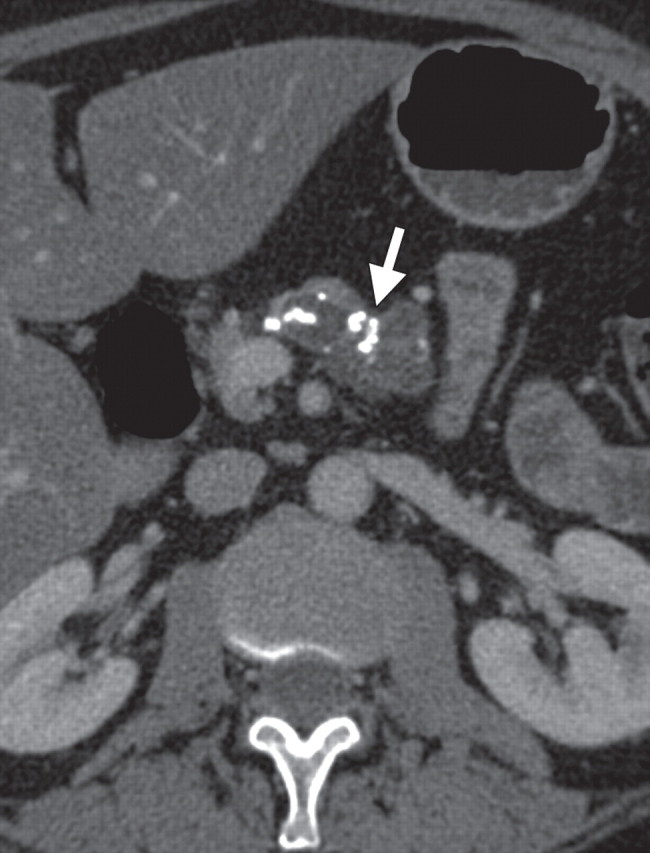
Figure 17b.
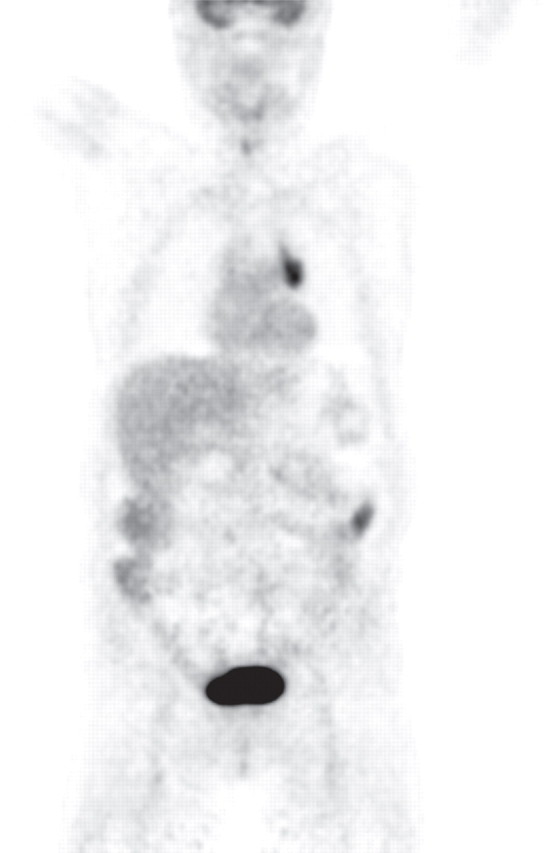
Figure 17c.
Autoimmune pancreatitis (AIP) is a distinct subtype of chronic pancreatitis and accounts for 2%–11% of all cases of chronic pancreatitis. Type 1 AIP is regarded as part of the immunoglobulin G4 (IgG4)–related disease spectrum (87). In as many as 30% of patients with pancreatic disease, other systems or organs, such as bile ducts, salivary and lachrymal glands, lymph nodes, and retroperitoneal tissue, are concurrently involved (87–89). Type I AIP is characterized by infiltration of tissues with IgG4-positive plasma cells, fibrosis with subsequent irregular narrowing of the main pancreatic duct, and obliterative phlebitis, as well as increased serum levels of one or more types of immunoglobulin, most commonly IgG4 (87,90,91). The typical CT and MR imaging features of AIP, such as diffuse or focal enlargement of the pancreas and the peripancreatic “halo sign,” are well recognized (87). The diagnostic criteria of AIP encompass typical histologic, imaging, and laboratory (ie, serum IgG4 levels) findings and response to corticosteroid therapy; however, typical imaging and laboratory findings are not present in 30% of patients (91,92). Moreover, AIP may manifest as a focal pancreatic mass, simulating a neoplasm (87,92).
FDG avidity is usually influenced by the severity and cause of pancreatitis: Diffuse FDG uptake may be seen in patients with low-grade acute or subacute pancreatitis or AIP, whereas increased radiotracer uptake generally is not seen in patients with conventional chronic pancreatitis (Figs 18, 19) (18,20,92,93). Although both AIP and pancreatic ductal adenocarcinoma may be FDG avid, certain patterns of uptake, such as a longitudinal shape, heterogeneous or diffuse accumulation, and multifocal localization, are more typical of AIP. Moreover, concomitant extrapancreatic FDG uptake by other organs, such as the salivary glands and bile ducts, has recently been shown to be indicative of systemic disease (88,92). FDG PET or PET/CT may also help assess disease activity in the same way it is useful in patients with retroperitoneal fibrosis, that is, to support the decision of whether or not to administer corticosteroid therapy and monitor response to treatment (92,94). PET/CT may help in the diagnosis of AIP and assessment of response to corticosteroid therapy by depicting resolution of or decrease in diffuse FDG uptake in the pancreas (88,92,95).
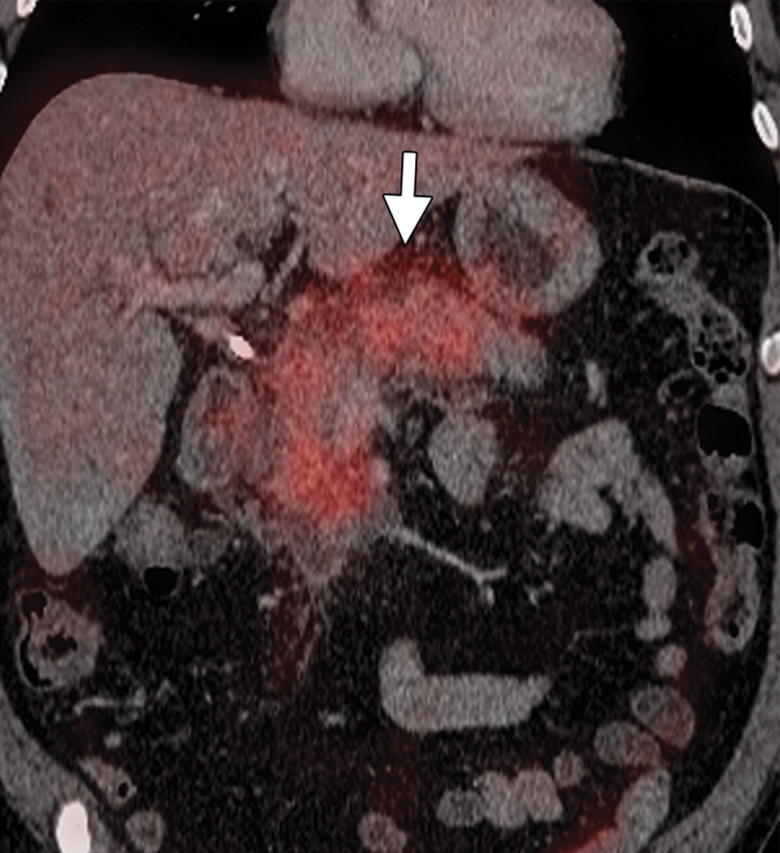
Subacute pancreatitis in a 62-year-old woman undergoing treatment for breast cancer. (a, b) Axial (a) and coronal (b) contrast-enhanced multidetector CT images show diffuse swelling and heterogeneity in the pancreatic parenchyma with peripancreatic edema (arrow). A calcified stone is also seen in the neck of the gallbladder (arrowhead in b). (c, d) Corresponding axial (c) and coronal (d) PET/CT images show a low level of diffuse heterogeneous FDG uptake in the pancreas and peripancreatic fat (arrow).
Figure 18a.
Figure 18b.
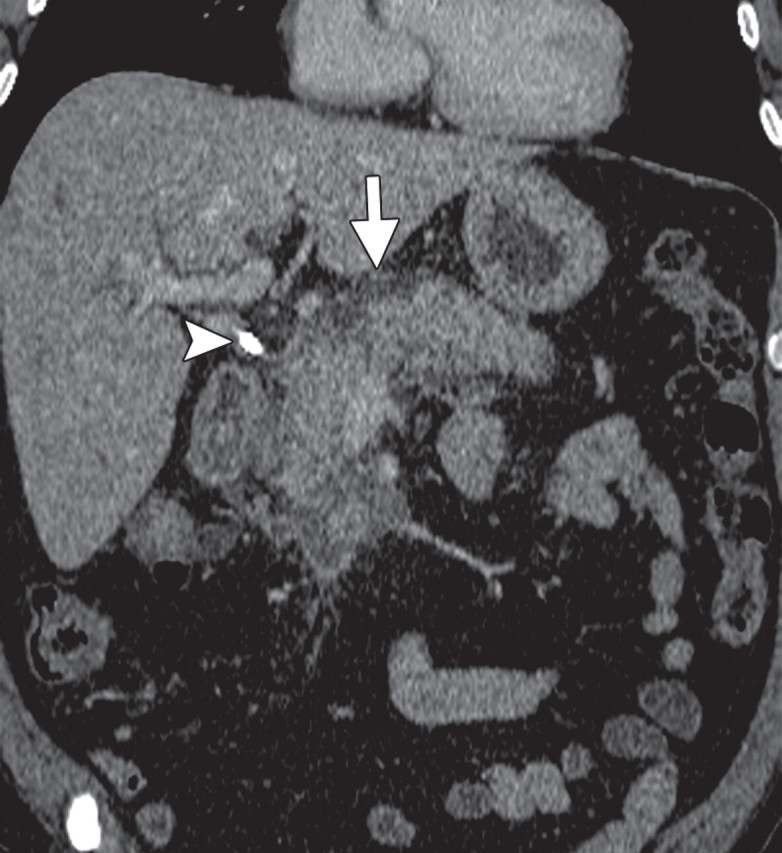
Figure 18c.
Figure 18d.
AIP in a 77-year-old man experiencing abdominal pain, weight loss, and jaundice. (a) Axial contrast-enhanced multidetector CT image shows a hypoattenuating mass in the pancreatic head (arrow). (b, c) Axial contrast-enhanced multidetector (b) and coronal reformatted (c) CT images show the pancreatic body and tail, which are slightly enlarged (arrow in b). Partial obliteration of the pancreatic outline and mild prominence of the main pancreatic duct (arrowheads) are also seen, findings indicative of pancreatic adenocarcinoma. (d, e) Axial PET/CT images show mild to moderate heterogeneous FDG uptake within the pancreatic head (arrow in d) and the rest of the body and tail (arrow in e), a finding indicative of a diffuse low-grade process, such as AIP. Serum CA 19-9 levels were not elevated, and endoscopic US–guided biopsy results were inconclusive. Corticosteroid therapy resulted in a favorable clinical and radiologic response.
Figure 19a.
Figure 19b.
Figure 19c.
Figure 19d.
Figure 19e.
Recent Developments in PET
Novel Radiotracers
Although FDG is a useful radiopharmaceutical, it is primarily a marker of glucose metabolism and may be taken up by nonneoplastic, highly metabolic tissues such as in acute inflammation. Furthermore, in the case of low metabolic tumors, its uptake may be less intense (16,25,26). New radiotracers that are more tumor-selective than 18F FDG have been developed, the most promising of which are sigma-receptor ligands and 18F 3′-fluoro-3′-deoxy-l-thymidine (FLT). Sigma receptors are overexpressed in rapidly proliferating cells, such as cancer cells. Radiolabeled sigma-receptor ligands are expected to be more specific in depicting tumor cells and may potentially be used for tumor depiction, staging, and evaluation of antitumor therapy.
FLT is an analog of thymidine. It is taken up by rapidly proliferating cells and, after phosphorylation, is trapped within cytosol (Fig 20). In a preclinical study by von Forstner et al (96) of radiotracers in severe combined immunodeficient (SCID) mice in 2008, it was reported that 18F FLT had the highest and most consistent uptake (evaluated with an MR imaging scanner) of various human pancreatic tumor cell lines compared with 18F FDG and 18F fluorethylcholine (FEC). In recent years, other preclinical studies have evaluated the ability of FLT to depict response to anticancer treatments, reporting early reductions in cellular FLT uptake after radio- and chemotherapy (97). Both σ-receptor ligands and FLT are more tumor-specific than FDG in animal models and performed better than FDG in distinguishing tumor from inflammatory tissue (98,99).
Use of FLT PET in a patient with a biliary stent and adenocarcinoma. (a) Axial multidetector CT image shows a beam hardening artifact (arrowhead), making it difficult to detect any suspicious focal lesion in the pancreas. (b) Axial FLT PET image shows avid uptake in the upper abdomen (arrow). (c) Fused PET/CT image accurately shows the area of increased uptake in the pancreatic head (arrow) due to an adenocarcinoma.
Figure 20a.
Figure 20b.
Figure 20c.
PET/MR Imaging
The combination of functional and morphologic imaging modalities, such as PET and CT, has shown its value in improving patient care, especially in oncology. However, CT exposes patients to a substantial amount of radiation and, unlike MR imaging, provides limited soft-tissue contrast for depiction and characterization of small lesions. For this reason, combining the soft-tissue contrast benefits of MR imaging with the functional information obtained with PET is attractive. Performing MR imaging and PET separately and then fusing the datasets is a viable but cumbersome option and introduces image misregistration. Although PET and MR images were fused with promising results in cases of chronic pancreatitis and pancreatic cancer, these claims need further confirmation with more clinical studies (100,101). The first combined whole-body PET/MR imaging scanner was recently approved by the Food and Drug Administration (FDA) for clinical use in humans. Potential indications for PET/MR imaging include conditions in which the high soft-tissue contrast of MR imaging and the whole-body imaging achieved with PET are required, such as musculoskeletal, intracranial, head and neck, breast, and liver tumors or metastases.
Summary
Because of the functional information it provides, PET is particularly useful in depicting unknown metastases and monitoring tumor recurrence, and FDG PET is an established problem-solving tool for the diagnosis and management of pancreatic adenocarcinoma. However, accurate localization of FDG uptake is sometimes difficult. CT provides superior morphologic information and allows assessment of vascular involvement. By itself, CT provides little functional information and largely depends on the size and morphologic characteristics of lesions to differentiate tumor from normal structures. Fused PET/CT serves as an all-in-one modality, bringing together the advantages of multidetector CT and FDG PET while overcoming some of their respective limitations. The role of PET/CT in diagnosing pancreatic cancer and other malignant tumors and premalignant lesions is emerging. New radioisotopes such as FLT and ′-receptor ligands may play a role in the assessment of various pancreatic tumors.
Recipient of a Certificate of Merit award for an education exhibit at the 2005 RSNA Annual Meeting.
For this journal-based CME activity, D.V.S and M.A.B. have disclosed various financial relationships (see “Disclosures of Potential Conflicts of Interest”); all other authors, the editor, and reviewers have no relevant relationships to disclose.
Disclosures of Potential Conflicts of Interest.- D.V.S.: Related financial activities: none. Other financial activities: consultant for Bracco Diagnostic. M.A.B.: Related financial activities: none. Other financial activities: royalties from Springer.
Abbreviations:
- AIP
- autoimmune pancreatitis
- FDG
- fluorine 18 fluorodeoxyglucose
- FLT
- 3′-fluoro-3′-deoxy-l-thymidine
- IPMN
- intraductal papillary neoplasms
- MFP
- mass-forming pancreatitis
- SSR
- somatostatin receptor
- SUV
- standardized uptake value
- SUVmax
- maximum SUV
References
- 1.Bronstein YL, Loyer EM, Kaur H, et al. Detection of small pancreatic tumors with multiphasic helical CT. AJR Am J Roentgenol 2004;182(3):619–623. [DOI] [PubMed] [Google Scholar]
- 2.Vargas R, Nino-Murcia M, Trueblood W, Jeffrey RB. MDCT in pancreatic adenocarcinoma: prediction of vascular invasion and resectability using a multiphasic technique with curved planar reformations. AJR Am J Roentgenol 2004;182(2):419–425. [DOI] [PubMed] [Google Scholar]
- 3.Chan SC, Ng SH, Chang JT, et al. Advantages and pitfalls of 18F-fluoro-2-deoxy-d-glucose positron emission tomography in detecting locally residual or recurrent nasopharyngeal carcinoma: comparison with magnetic resonance imaging. Eur J Nucl Med Mol Imaging 2006;33(9):1032–1040. [DOI] [PubMed] [Google Scholar]
- 4.Hong HS, Yun M, Cho A, et al. The utility of F-18 FDG PET/CT in the evaluation of pancreatic intraductal papillary mucinous neoplasm. Clin Nucl Med 2010;35(10):776–779. [DOI] [PubMed] [Google Scholar]
- 5.Sperti C, Bissoli S, Pasquali C, et al. 18-fluorodeoxyglucose positron emission tomography enhances computed tomography diagnosis of malignant intraductal papillary mucinous neoplasms of the pancreas. Ann Surg 2007;246(6):932–937; discussion 937–939. [DOI] [PubMed] [Google Scholar]
- 6.Takanami K, Hiraide T, Tsuda M, et al. Additional value of FDG PET/CT to contrast-enhanced CT in the differentiation between benign and malignant intraductal papillary mucinous neoplasms of the pancreas with mural nodules. Ann Nucl Med 2011; 25(7):501–510. [DOI] [PubMed] [Google Scholar]
- 7.Tomimaru Y, Takeda Y, Tatsumi M, et al. Utility of 2-[18F] fluoro-2-deoxy-d-glucose positron emission tomography in differential diagnosis of benign and malignant intraductal papillary-mucinous neoplasm of the pancreas. Oncol Rep 2010;24(3): 613–620. [DOI] [PubMed] [Google Scholar]
- 8.Zamboni GA, Kruskal JB, Vollmer CM, Baptista J, Callery MP, Raptopoulos VD. Pancreatic adenocarcinoma: value of multidetector CT angiography in preoperative evaluation. Radiology 2007;245(3): 770–778. [DOI] [PubMed] [Google Scholar]
- 9.Fukushima H, Itoh S, Takada A, et al. Diagnostic value of curved multiplanar reformatted images in multislice CT for the detection of resectable pancreatic ductal adenocarcinoma. Eur Radiol 2006;16(8): 1709–1718. [DOI] [PubMed] [Google Scholar]
- 10.Diehl SJ, Lehmann KJ, Sadick M, Lachmann R, Georgi M. Pancreatic cancer: value of dual-phase helical CT in assessing resectability. Radiology 1998;206(2):373–378. [DOI] [PubMed] [Google Scholar]
- 11.Lu DS, Reber HA, Krasny RM, Kadell BM, Sayre J. Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am J Roentgenol 1997;168(6):1439–1443. [DOI] [PubMed] [Google Scholar]
- 12.Manak E, Merkel S, Klein P, Papadopoulos T, Bautz WA, Baum U. Resectability of pancreatic adenocarcinoma: assessment using multidetector-row computed tomography with multiplanar reformations. Abdom Imaging 2009;34(1):75–80. [DOI] [PubMed] [Google Scholar]
- 13.Valls C, Andía E, Sanchez A, et al. Dual-phase helical CT of pancreatic adenocarcinoma: assessment of resectability before surgery. AJR Am J Roentgenol 2002;178(4):821–826. [DOI] [PubMed] [Google Scholar]
- 14.Kim T, Murakami T, Takamura M, et al. Pancreatic mass due to chronic pancreatitis: correlation of CT and MR imaging features with pathologic findings. AJR Am J Roentgenol 2001;177(2):367–371. [DOI] [PubMed] [Google Scholar]
- 15.Urban BA, McGhie PA, Fishman EK, Helical CT: diagnostic pitfalls of arterial phase imaging of the upper abdomen. AJR Am J Roentgenol 2000;174(2):455–461. [DOI] [PubMed] [Google Scholar]
- 16.Blodgett TM, Meltzer CC, Townsend DW. PET/CT: form and function. Radiology 2007;242(2): 360–385. [DOI] [PubMed] [Google Scholar]
- 17.Pandit-Taskar N, Schöder H, Gonen M, Larson SM, Yeung HW. Clinical significance of unexplained abnormal focal FDG uptake in the abdomen during whole-body PET. AJR Am J Roentgenol 2004;183(4):1143–1147. [DOI] [PubMed] [Google Scholar]
- 18.Kauhanen SP, Komar G, Seppänen MP, et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg 2009;250(6):957–963. [DOI] [PubMed] [Google Scholar]
- 19.Schick V, Franzius C, Beyna T, et al. Diagnostic impact of 18F-FDG PET-CT evaluating solid pancreatic lesions versus endosonography, endoscopic retrograde cholangio-pancreatography with intraductal ultrasonography and abdominal ultrasound. Eur J Nucl Med Mol Imaging 2008;35(10):1775–1785. [DOI] [PubMed] [Google Scholar]
- 20.van Kouwen MC, Jansen JB, van Goor H, de Castro S, Oyen WJ, Drenth JP. FDG-PET is able to detect pancreatic carcinoma in chronic pancreatitis. Eur J Nucl Med Mol Imaging 2005;32(4):399–404. [DOI] [PubMed] [Google Scholar]
- 21.Yoshioka M, Sato T, Furuya T, et al. Role of positron emission tomography with 2-deoxy-2-[18F]fluoro- d-glucose in evaluating the effects of arterial infusion chemotherapy and radiotherapy on pancreatic cancer. J Gastroenterol 2004;39(1):50–55. [DOI] [PubMed] [Google Scholar]
- 22.Ruf J, Lopez Hänninen E, Oettle H, et al. Detection of recurrent pancreatic cancer: comparison of FDG-PET with CT/MRI. Pancreatology 2005;5(2-3): 266–272. [DOI] [PubMed] [Google Scholar]
- 23.Kuwatani M, Kawakami H, Eto K, et al. Modalities for evaluating chemotherapeutic efficacy and survival time in patients with advanced pancreatic cancer: comparison between FDG-PET, CT, and serum tumor markers. Intern Med 2009;48(11): 867–875. [DOI] [PubMed] [Google Scholar]
- 24.Sperti C, Pasquali C, Bissoli S, Chierichetti F, Liessi G, Pedrazzoli S. Tumor relapse after pancreatic cancer resection is detected earlier by 18-FDG PET than by CT. J Gastrointest Surg 2010;14(1): 131–140. [DOI] [PubMed] [Google Scholar]
- 25.von Schulthess GK, Steinert HC, Hany TF. Integrated PET/CT: current applications and future directions. Radiology 2006;238(2):405–422. [DOI] [PubMed] [Google Scholar]
- 26.Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology 2004; 231(2):305–332. [DOI] [PubMed] [Google Scholar]
- 27.Diederichs CG, Staib L, Glatting G, Beger HG, Reske SN FDG PET: elevated plasma glucose reduces both uptake and detection rate of pancreatic malignancies. J Nucl Med 1998;39(6):1030–1033. [PubMed] [Google Scholar]
- 28.Cohade C, Osman M, Leal J, Wahl RL. Direct comparison of (18)F-FDG PET and PET/CT in patients with colorectal carcinoma. J Nucl Med 2003; 44(11):1797–1803. [PubMed] [Google Scholar]
- 29.Prabhakar HB, Sahani DV, Fischman AJ, Mueller PR, Blake MA. Bowel hot spots at PET-CT. RadioGraphics 2007;27(1):145–159. [DOI] [PubMed] [Google Scholar]
- 30.Hruban RH, Klimstra DS, Pitman MB. AFIP atlas of tumor pathology: tumors of the pancreas—series 4. Washington, DC: AFIP, 2007; 23–376. [Google Scholar]
- 31.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics: 2010. CA Cancer J Clin 2010;60(5):277–300. [DOI] [PubMed] [Google Scholar]
- 32.Howlader N, Noone AM, Krapcho M, et al. Anonymous Surveillance Epidemiology and End Results of the National Cancer Institute. SEER Cancer Statistics Review: 1975–2009. http://seer.cancer.gov/statfacts/html/pancreas.html. Published April 1 2012. Accessed April 30, 2012.
- 33.Duffy JP, Reber HA. Nonendocrine tumors of the pancreas. In: , Yamada T, ed. Textbook of Gastroenterology, 4th edition. Philadelphia, Pa: Lippincott Williams and Wilkins, 2003; 2091–2107. [Google Scholar]
- 34.Kang CM, Kim JY, Choi GH, et al. The use of adjusted preoperative CA 19-9 to predict the recurrence of resectable pancreatic cancer. J Surg Res 2007;140(1):31–35. [DOI] [PubMed] [Google Scholar]
- 35.Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009;16(7):1727–1733. [DOI] [PubMed] [Google Scholar]
- 36.Huguet F, Girard N, Guerche CS, Hennequin C, Mornex F, Azria D. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: a qualitative systematic review. J Clin Oncol 2009;27(13):2269–2277. [DOI] [PubMed] [Google Scholar]
- 37.Mallery JS, Centeno BA, Hahn PF, Chang Y, Warshaw AL, Brugge WR. Pancreatic tissue sampling guided by EUS, CT/US, and surgery: a comparison of sensitivity and specificity. Gastrointest Endosc 2002;56(2):218–224. [DOI] [PubMed] [Google Scholar]
- 38.Tatli S, Gerbaudo VH, Mamede M, Tuncali K, Shyn PB, Silverman SG. Abdominal masses sampled at PET/CT-guided percutaneous biopsy: initial experience with registration of prior PET/CT images. Radiology 2010;256(1):305–311. [DOI] [PubMed] [Google Scholar]
- 39.Tamm EP, Loyer EM, Faria SC, Evans DB, Wolff RA, Charnsangavej C. Retrospective analysis of dual-phase MDCT and follow-up EUS/EUS-FNA in the diagnosis of pancreatic cancer. Abdom Imaging 2007;32(5):660–667. [DOI] [PubMed] [Google Scholar]
- 40.Prokesch RW, Chow LC, Beaulieu CF, Bammer R, Jeffrey RB. Isoattenuating pancreatic adenocarcinoma at multi-detector row CT: secondary signs. Radiology 2002;224(3):764–768. [DOI] [PubMed] [Google Scholar]
- 41.Higashi T, Saga T, Nakamoto Y, et al. Diagnosis of pancreatic cancer using fluorine-18 fluorodeoxyglucose positron emission tomography (FDG PET): usefulness and limitations in “clinical reality”. Ann Nucl Med 2003;17(4):261–279. [DOI] [PubMed] [Google Scholar]
- 42.Okano K, Kakinoki K, Akamoto S, et al. 18F-fluorodeoxyglucose positron emission tomography in the diagnosis of small pancreatic cancer. World J Gastroenterol 2011;17(2):231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lemke AJ, Niehues SM, Hosten N, et al. Retrospective digital image fusion of multidetector CT and 18F-FDG PET: clinical value in pancreatic lesions—a prospective study with 104 patients. J Nucl Med 2004;45(8):1279–1286. [PubMed] [Google Scholar]
- 44.Boll DT, Merkle EM. Differentiating a chronic hyperplastic mass from pancreatic cancer: a challenge remaining in multidetector CT of the pancreas. Eur Radiol 2003;13(Suppl 5):M42–M49. [DOI] [PubMed] [Google Scholar]
- 45.Koyama K, Okamura T, Kawabe J, et al. Diagnostic usefulness of FDG PET for pancreatic mass lesions. Ann Nucl Med 2001;15(3):217–224. [DOI] [PubMed] [Google Scholar]
- 46.Imdahl A, Nitzsche E, Krautmann F, et al. Evaluation of positron emission tomography with 2-[18F]fluoro-2-deoxy- d-glucose for the differentiation of chronic pancreatitis and pancreatic cancer. Br J Surg 1999;86(2):194–199. [DOI] [PubMed] [Google Scholar]
- 47.Raut CP, Tseng JF, Sun CC, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg 2007;246(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saif MW, Cornfeld D, Modarresifar H, Ojha B. 18F-FDG positron emission tomography CT (FDG PET-CT) in the management of pancreatic cancer: initial experience in 12 patients. J Gastrointestin Liver Dis 2008;17(2):173–178. [PubMed] [Google Scholar]
- 49.Soriano A, Castells A, Ayuso C, et al. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol 2004;99(3):492–501. [DOI] [PubMed] [Google Scholar]
- 50.Heinrich S, Goerres GW, Schäfer M, et al. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg 2005; 242(2):235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diederichs CG, Staib L, Vogel J, et al. Values and limitations of 18F-fluorodeoxyglucose-positron-emission tomography with preoperative evaluation of patients with pancreatic masses. Pancreas 2000; 20(2):109–116. [DOI] [PubMed] [Google Scholar]
- 52.Gould MK, Kuschner WG, Rydzak CE, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med 2003;139(11):879–892. [DOI] [PubMed] [Google Scholar]
- 53.Nakamoto Y, Higashi T, Sakahara H, et al. Contribution of PET in the detection of liver metastases from pancreatic tumours. Clin Radiol 1999;54(4): 248–252. [DOI] [PubMed] [Google Scholar]
- 54.Akhurst T, Kates TJ, Mazumdar M, et al. Recent chemotherapy reduces the sensitivity of [18F]fluorodeoxyglucose positron emission tomography in the detection of colorectal metastases. J Clin Oncol 2005;23(34):8713–8716. [DOI] [PubMed] [Google Scholar]
- 55.Sahani DV, Kalva SP, Fischman AJ, et al. Detection of liver metastases from adenocarcinoma of the colon and pancreas: comparison of mangafodipir trisodium-enhanced liver MRI and whole-body FDG PET. AJR Am J Roentgenol 2005;185(1):239–246. [DOI] [PubMed] [Google Scholar]
- 56.Liu RC, Traverso LW. Diagnostic laparoscopy improves staging of pancreatic cancer deemed locally unresectable by computed tomography. Surg Endosc 2005;19(5):638–642. [DOI] [PubMed] [Google Scholar]
- 57.Tabuchi T, Itoh K, Ohshio G, et al. Tumor staging of pancreatic adenocarcinoma using early- and late-phase helical CT. AJR Am J Roentgenol 1999;173(2):375–380. [DOI] [PubMed] [Google Scholar]
- 58.Nishiyama Y, Yamamoto Y, Yokoe K, et al. Contribution of whole body FDG-PET to the detection of distant metastasis in pancreatic cancer. Ann Nucl Med 2005;19(6):491–497. [DOI] [PubMed] [Google Scholar]
- 59.Delbeke D, Rose DM, Chapman WC, et al. Optimal interpretation of FDG PET in the diagnosis, staging and management of pancreatic carcinoma. J Nucl Med 1999;40(11):1784–1791. [PubMed] [Google Scholar]
- 60.Kleeff J, Reiser C, Hinz U, et al. Surgery for recurrent pancreatic ductal adenocarcinoma. Ann Surg 2007;245(4):566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Casneuf V, Delrue L, Kelles A, et al. Is combined 18F-fluorodeoxyglucose-positron emission tomography/computed tomography superior to positron emission tomography or computed tomography alone for diagnosis, staging and restaging of pancreatic lesions? Acta Gastroenterol Belg 2007;70(4): 331–338. [PubMed] [Google Scholar]
- 62.Bang S, Chung HW, Park SW, et al. The clinical usefulness of 18-fluorodeoxyglucose positron emission tomography in the differential diagnosis, staging, and response evaluation after concurrent chemoradiotherapy for pancreatic cancer. J Clin Gastroenterol 2006;40(10):923–929. [DOI] [PubMed] [Google Scholar]
- 63.Schellenberg D, Quon A, Minn AY, et al. 18Fluorodeoxyglucose PET is prognostic of progression-free and overall survival in locally advanced pancreas cancer treated with stereotactic radiotherapy. Int J Radiat Oncol Biol Phys 2010;77(5):1420–1425. [DOI] [PubMed] [Google Scholar]
- 64.Okamoto K, Koyama I, Miyazawa M, et al. Preoperative 18[F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts early recurrence after pancreatic cancer resection. Int J Clin Oncol 2011;16(1):39–44. [DOI] [PubMed] [Google Scholar]
- 65.Blake MA, Singh A, Setty BN, et al. Pearls and pitfalls in interpretation of abdominal and pelvic PET-CT. RadioGraphics 2006;26(5):1335–1353. [DOI] [PubMed] [Google Scholar]
- 66.Tan EH, Tan CH. Imaging of gastroenteropancreatic neuroendocrine tumors. World J Clin Oncol 2011;2(1):28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rha SE, Jung SE, Lee KH, Ku YM, Byun JY, Lee JM. CT and MR imaging findings of endocrine tumor of the pancreas according to WHO classification. Eur J Radiol 2007;62(3):371–377. [DOI] [PubMed] [Google Scholar]
- 68.Kayani I, Bomanji JB, Groves A, et al. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1, Tyr3-octreotate) and 18F-FDG. Cancer 2008;112(11):2447–2455. [DOI] [PubMed] [Google Scholar]
- 69.Bombardieri E, Maccauro M, De Deckere E, Savelli G, Chiti A. Nuclear medicine imaging of neuroendocrine tumours. Ann Oncol 2001;12(Suppl 2): S51–S61. [DOI] [PubMed] [Google Scholar]
- 70.Ichikawa T, Peterson MS, Federle MP, et al. Islet cell tumor of the pancreas: biphasic CT versus MR imaging in tumor detection. Radiology 2000;216(1): 163–171. [DOI] [PubMed] [Google Scholar]
- 71.Nakamoto Y, Higashi T, Sakahara H, et al. Evaluation of pancreatic islet cell tumors by fluorine-18 fluorodeoxyglucose positron emission tomography: comparison with other modalities. Clin Nucl Med 2000;25(2):115–119. [DOI] [PubMed] [Google Scholar]
- 72.Versari A, Camellini L, Carlinfante G, et al. Ga-68 DOTATOC PET, endoscopic ultrasonography, and multidetector CT in the diagnosis of duodenopancreatic neuroendocrine tumors: a single-centre retrospective study. Clin Nucl Med 2010;35(5): 321–328. [DOI] [PubMed] [Google Scholar]
- 73.Nayer H, Weir EG, Sheth S, Ali SZ. Primary pancreatic lymphomas: a cytopathologic analysis of a rare malignancy. Cancer 2004;102(5):315–321. [DOI] [PubMed] [Google Scholar]
- 74.Karam M, Novak L, Cyriac J, Ali A, Nazeer T, Nugent F. Role of fluorine-18 fluoro-deoxyglucose positron emission tomography scan in the evaluation and follow-up of patients with low-grade lymphomas. Cancer 2006;107(1):175–183. [DOI] [PubMed] [Google Scholar]
- 75.Martin DR, Semelka RC. MR imaging of pancreatic masses. Magn Reson Imaging Clin N Am 2000;8(4):787–812. [PubMed] [Google Scholar]
- 76.Yoon SN, Lee MH, Yoon JK. F-18 FDG positron emission tomography findings in primary pancreatic lymphoma. Clin Nucl Med 2004;29(9):574–575. [DOI] [PubMed] [Google Scholar]
- 77.Mikhaeel NG, Hutchings M, Fields PA, O’Doherty MJ, Timothy AR. FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol 2005;16(9):1514–1523. [DOI] [PubMed] [Google Scholar]
- 78.Ghavamian R, Klein KA, Stephens DH, et al. Renal cell carcinoma metastatic to the pancreas: clinical and radiological features. Mayo Clin Proc 2000;75(6):581–585. [DOI] [PubMed] [Google Scholar]
- 79.Sato M, Okumura T, Kaito K, et al. Usefulness of FDG-PET/CT in the detection of pancreatic metastases from lung cancer. Ann Nucl Med 2009;23(1): 49–57. [DOI] [PubMed] [Google Scholar]
- 80.Merkle EM, Boaz T, Kolokythas O, Haaga JR, Lewin JS, Brambs HJ. Metastases to the pancreas. Br J Radiol 1998;71(851):1208–1214. [DOI] [PubMed] [Google Scholar]
- 81.Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med 2004;351(12):1218–1226. [DOI] [PubMed] [Google Scholar]
- 82.Takeshita K, Kutomi K, Takada K, et al. Unusual imaging appearances of pancreatic serous cystadenoma: correlation with surgery and pathologic analysis. Abdom Imaging 2005;30(5):610–615. [DOI] [PubMed] [Google Scholar]
- 83.Sahani DV, Sainani NI, Blake MA, Crippa S, Mino-Kenudson M, del-Castillo CF. Prospective evaluation of reader performance on MDCT in characterization of cystic pancreatic lesions and prediction of cyst biologic aggressiveness. AJR Am J Roentgenol 2011;197(1):W53–W61. [DOI] [PubMed] [Google Scholar]
- 84.Sainani N, Sahani DV, Blake M, Deshpande V, Fernandes-del Castillo C, Fischman A. Morphological and functional characterization of mucinous lesions of pancreas: is the combination PET-CT better than MDCT or PET alone? J Nucl Med Meeting Abstracts 2008;49:273P-a. [Google Scholar]
- 85.Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med 2006;354(20):2142–2150. [DOI] [PubMed] [Google Scholar]
- 86.Owyang C, Levitt M. Chronic Pancreatitis. In: , Yamada T, Alpers DH, Owyang C, Powell DH, Silverstein FE, eds. Textbook of gastroenterology Volume 2 New York, NY: JB Lippincott, 1991; 1874–1893. [Google Scholar]
- 87.Vlachou PA, Khalili K, Jang HJ, Fischer S, Hirschfield GM, Kim TK. IgG4-related sclerosing disease: autoimmune pancreatitis and extrapancreatic manifestations. RadioGraphics 2011;31(5): 1379–1402. [DOI] [PubMed] [Google Scholar]
- 88.Kamisawa T, Takum K, Anjiki H, et al. FDG-PET/CT findings of autoimmune pancreatitis. Hepatogastroenterology 2010;57(99-100):447–450. [PubMed] [Google Scholar]
- 89.Zen Y, Nakanuma Y. IgG4-related disease: a cross-sectional study of 114 cases. Am J Surg Pathol 2010;34(12):1812–1819. [DOI] [PubMed] [Google Scholar]
- 90.Finkelberg DL, Sahani D, Deshpande V, Brugge WR. Autoimmune pancreatitis. N Engl J Med 2006; 355(25):2670–2676. [DOI] [PubMed] [Google Scholar]
- 91.Chari ST, Smyrk TC, Levy MJ, et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol 2006;4(8):1010–1016; quiz 934. [DOI] [PubMed] [Google Scholar]
- 92.Lee TY, Kim MH, Park H, et al. Utility of 18F-FDG PET/CT for differentiation of autoimmune pancreatitis with atypical pancreatic imaging findings from pancreatic cancer. AJR Am J Roentgenol 2009;193(2):343–348. [DOI] [PubMed] [Google Scholar]
- 93.Jeong Yoon S, Lee B, Park CH. Imaging diagnosis of post-ERCP focal pancreatitis mimicking pancreatic carcinoma by follow-up F-18 FDG PET/CT. Clin Nucl Med 2011;36(1):70–72. [DOI] [PubMed] [Google Scholar]
- 94.Jansen I, Hendriksz TR, Han SH, Huiskes AW, van Bommel EF. (18)F-fluorodeoxyglucose position emission tomography (FDG-PET) for monitoring disease activity and treatment response in idiopathic retroperitoneal fibrosis. Eur J Intern Med 2010;21(3):216–221. [DOI] [PubMed] [Google Scholar]
- 95.Ozaki Y, Oguchi K, Hamano H, et al. Differentiation of autoimmune pancreatitis from suspected pancreatic cancer by fluorine-18 fluorodeoxyglucose positron emission tomography. J Gastroenterol 2008;43(2):144–151. [DOI] [PubMed] [Google Scholar]
- 96.von Forstner C, Egberts JH, Ammerpohl O, et al. Gene expression patterns and tumor uptake of 18F-FDG, 18F-FLT, and 18F-FEC in PET/MRI of an orthotopic mouse xenotransplantation model of pancreatic cancer. J Nucl Med 2008;49(8): 1362–1370. [DOI] [PubMed] [Google Scholar]
- 97.Buck AK, Kratochwil C, Glatting G, et al. Early assessment of therapy response in malignant lymphoma with the thymidine analogue [18F]FLT. Eur J Nucl Med Mol Imaging 2007;34(11):1775–1782. [DOI] [PubMed] [Google Scholar]
- 98.van Waarde A, Cobben DC, Suurmeijer AJ, et al. Selectivity of 18F-FLT and 18F-FDG for differentiating tumor from inflammation in a rodent model. J Nucl Med 2004;45(4):695–700. [PubMed] [Google Scholar]
- 99.van Waarde A, Jager PL, Ishiwata K, Dierckx RA, Elsinga PH. Comparison of sigma-ligands and metabolic PET tracers for differentiating tumor from inflammation. J Nucl Med 2006;47(1):150–154. [PubMed] [Google Scholar]
- 100.Malesci A, Balzarini L, Chiti A, Lucignani G. Pancreatic cancer or chronic pancreatitis? An answer from PET/MRI image fusion. Eur J Nucl Med Mol Imaging 2004;31(9):1352. [DOI] [PubMed] [Google Scholar]
- 101.Tatsumi M, Isohashi K, Onishi H, et al. 18F-FDG PET/MRI fusion in characterizing pancreatic tumors: comparison to PET/CT. Int J Clin Oncol 2011;16(4):408–415. [DOI] [PubMed] [Google Scholar]