Abstract
Gynecologic malignancies account for 10%–15% of all malignancies in females. A variety of oncologic options are available depending on organ of origin, histologic diagnosis, and disease grade and stage. Gynecologic malignancies are usually treated with surgery, chemotherapy, or radiation therapy. Posttreatment imaging plays a crucial role in the assessment of treatment response and tumor recurrence. Imaging of the female pelvis following chemotherapy and radiation therapy is particularly challenging due to alteration of the normal anatomy and loss of tissue planes. Expected changes in appearance occur following chemotherapy–radiation therapy, as do complications such as fistulas, proctitis, enteritis, typhlitis, cystitis, and insufficiency fractures. Radiologists should be familiar with both the expected posttreatment imaging findings and the imaging features of common complications to help make the correct interpretation and avoid possible pitfalls.
© RSNA, 2010
LEARNING OBJECTIVES FOR TEST 3
After reading this article and taking the test, the reader will be able to:
-
•.
Discuss the expected CT and MR imaging appearances of the female pelvis following chemotherapy or radiation therapy.
-
•.
Describe the CT and MR imaging findings of the most common complications of chemotherapy or radiation therapy for gynecologic malignancies.
-
•.
List the potential pitfalls in the interpretation of posttreatment imaging findings.
Introduction
Gynecologic malignancies are common, accounting for 10%–15% of all malignancies in females (1). Pretreatment imaging focuses on staging and treatment planning. Imaging performed following treatment—whether surgery, chemotherapy, or radiation therapy—plays a crucial role in the assessment of treatment response and tumor recurrence. Computed tomography (CT) has been shown to be reliable in the assessment of disease recurrence in ovarian carcinoma (2). Magnetic resonance (MR) imaging—in particular, dynamic contrast material–enhanced MR imaging—has a high sensitivity (91%) in the detection of recurrent gynecologic malignancy (3).
There are a variety of oncologic options available to patients with gynecologic malignancies depending on organ of origin, histologic diagnosis, and disease grade and stage. Therefore, it is important that the radiologist be aware of the possible imaging appearances following chemotherapy, radiation therapy, or a combination thereof, to accurately interpret the study and to avoid the pitfalls of reporting normal posttreatment anatomy as disease recurrence. In this article, we review the use of chemotherapy and radiation therapy in the treatment of gynecologic malignancies, briefly describe radiation therapy planning, and discuss and illustrate the expected posttreatment imaging appearances of the abdomen and pelvis as well as the appearances of common posttreatment complications.
Chemotherapy and Radiation Therapy
Chemotherapy and radiation therapy are used as adjuncts to surgery in the treatment of gynecologic malignancies. Chemotherapy is used as a neoadjuvant treatment for advanced ovarian cancer and, at some centers, for advanced cervical cancer. Adjuvant chemotherapy is used following surgery for ovarian cancer and advanced endometrial cancer. Primary chemotherapy–radiation therapy is used for the management of advanced cervical cancer (stages 2–4) (4). Adjuvant radiation therapy is used for surgically treated node-positive cervical cancer or when the margins are close, and can consist of either brachytherapy or combined external beam therapy and brachytherapy. In endometrial carcinoma, radiation therapy is used following surgery in patients with disease extending into the outer myometrium or outside the uterus. In ovarian carcinoma, radiation therapy is recommended only as a palliative treatment for stage IV disease (4,5).
Radiation Therapy Planning
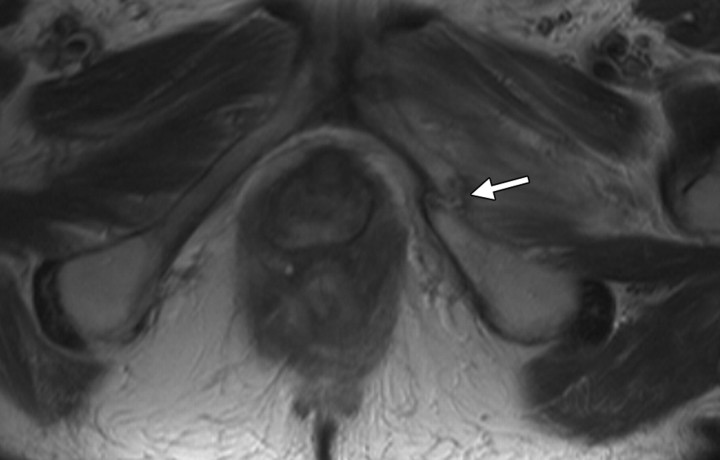
Pelvic irradiation affects other systems that are not intentionally targeted, possibly causing gastrointestinal or genitourinary complications in particular. Intrauterine and intracavitary brachytherapy is used following external beam radiation therapy to administer a focused high dose of radiation to a specific area of the uterus or cervix. It has been reported that uterine perforation occurs in 8% of brachytherapy procedures (Fig 1), usually at the time of tandem placement (6). The use of three-dimensional imaging to guide applicator placement helps avoid this complication and helps reduce radiation dose at brachytherapy performed for the treatment of cervical cancer by allowing more accurate placement with ultrasonography (US), MR imaging, positron emission tomography, or CT, depending on the institution (7). Recently, intensity-modulated radiation therapy has been implemented with the aim of achieving as high a target dose as possible without irradiating any nontargeted organs (8). This technique has been reported to decrease the radiation dose to the bladder and rectum by 23% and to the small bowel by 50% (9,10). Dose limitation during radiation therapy planning is also important in reducing the risk of future malignancy. The absolute risk of occurrence of sarcomas following radiation therapy for gynecologic malignancies has been estimated to be approximately 0.03%–0.8% (11).
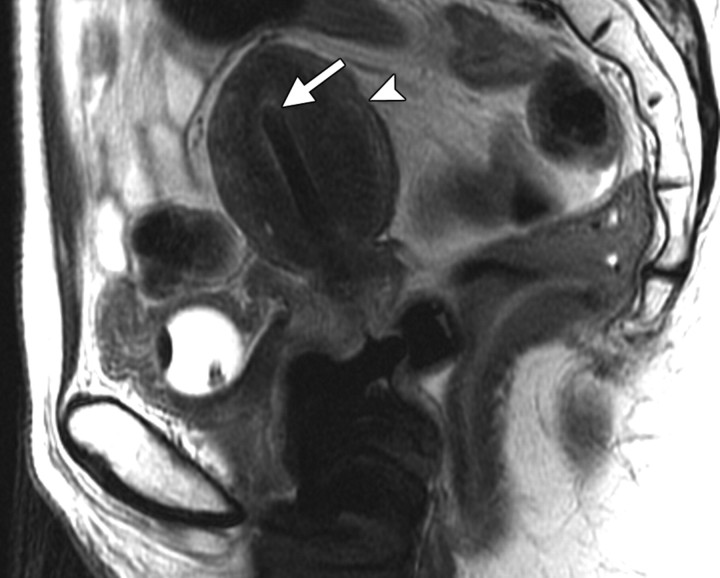
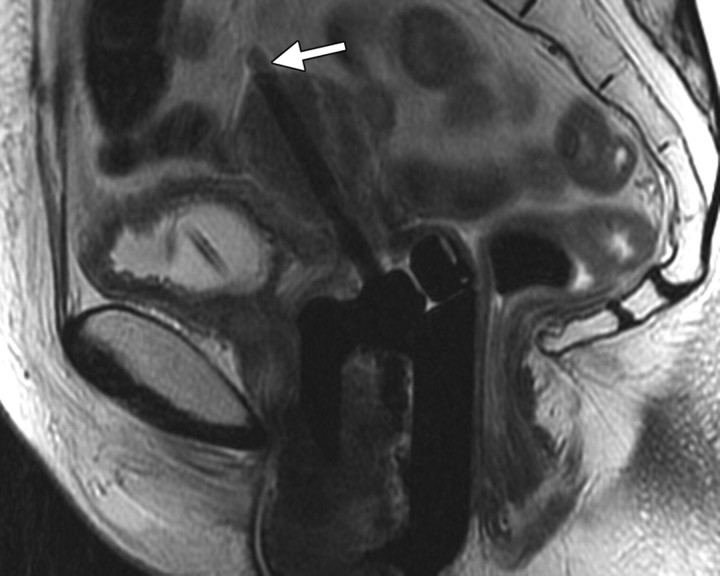
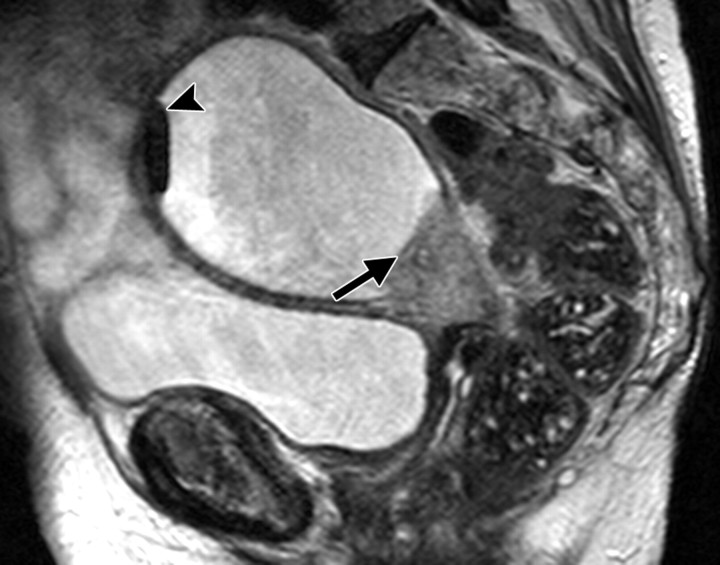
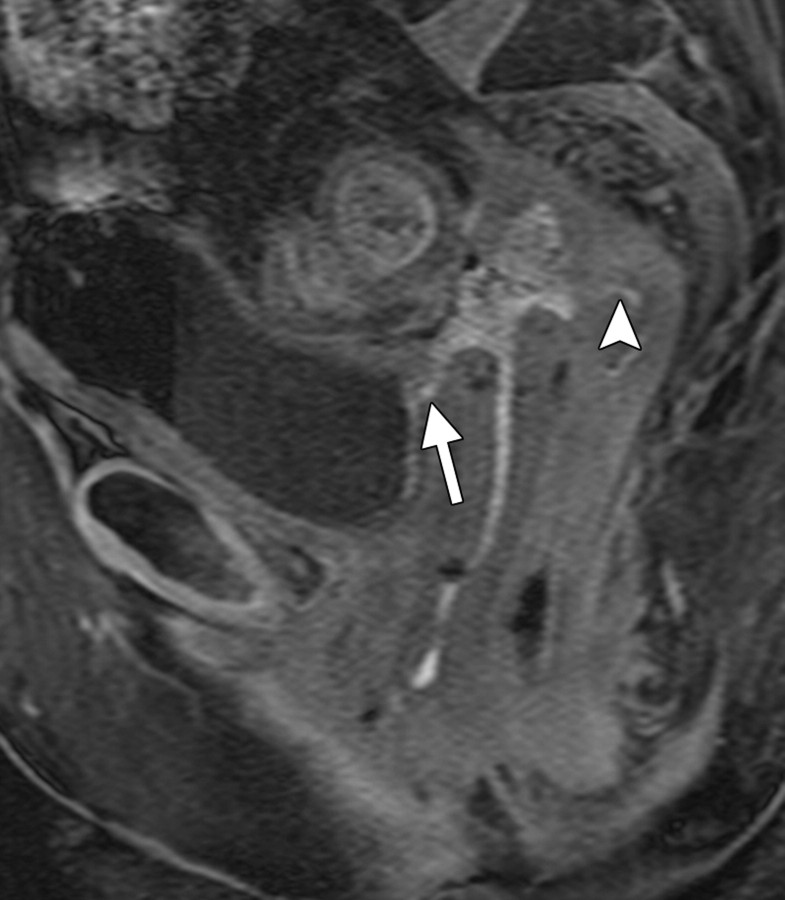
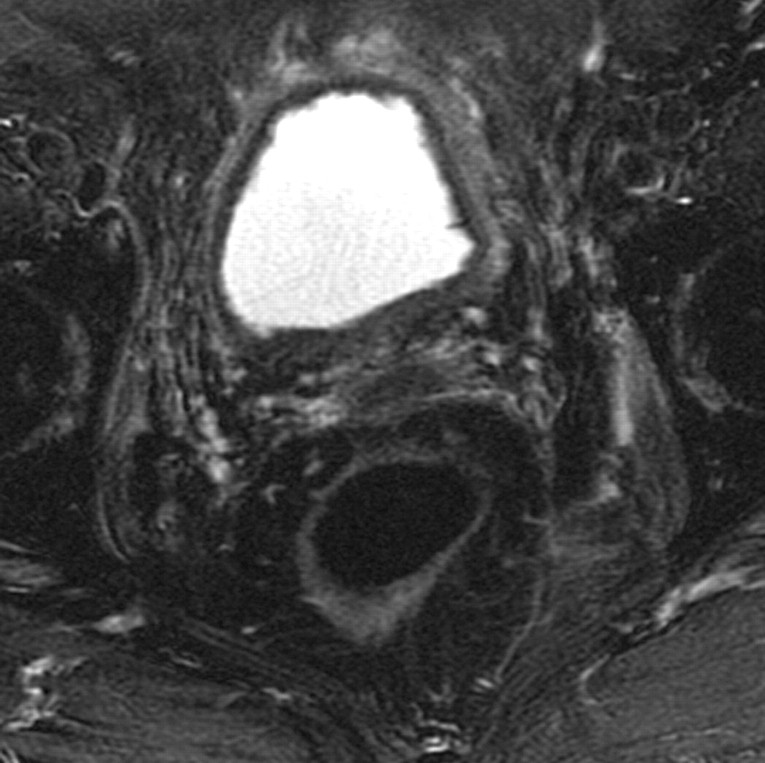
Positioning of the probe in brachytherapy. (a) Sagittal T2-weighted MR image obtained in a 38-year-old woman with stage IIIA cervical carcinoma shows a brachytherapy probe (arrow) correctly positioned within the endometrial cavity of the uterus (arrowhead). (b) Sagittal T2-weighted MR image obtained in a 42-year-old woman with stage IIB cervical carcinoma shows perforation of the uterine fundus (arrow) by a brachytherapy probe.
Figure 1a.
Figure 1b.
Expected Posttreatment Imaging Appearances of the Abdomen and Pelvis
Tumor
In cervical carcinoma, follow-up MR imaging after radiation therapy helps guide future treatment decisions. Response to radiation therapy is assessed on the basis of the interval decrease in tumor size (12), which can be seen as early as 2 months after treatment and is predictive of a good prognosis (13). Images obtained too soon after radiation therapy can be misleading. In a study of 44 patients with stage IB2/II cervical carcinoma who had undergone 45-Gy radiation therapy, chemotherapy, and brachytherapy, Vincens et al (12) found a risk of false-positive findings at MR imaging performed 3–8 weeks after treatment. Following radiation therapy, the primary tumor becomes more fibrotic and contracted, resulting in lower signal intensity, and the soft tissue and fat planes within the pelvis become more indistinct. The adjacent soft tissues may also undergo fibrotic changes and appear hypointense, thus becoming indistinguishable from the tumor, particularly in the first 6 months following radiation therapy. This post–radiation therapy imaging appearance may mimic tumor extension and parametrial invasion. In cases of advanced cervical carcinoma in which there was initial parametrial invasion, the intravenous administration of contrast material can help distinguish between radiation-induced parametrial fibrosis and residual or recurrent disease (14). Weber et al (15) found that the most helpful finding for differentiating recurrent cervical tumor from radiation fibrosis was the presence of a soft-tissue mass with higher signal intensity than that of adjacent pelvic sidewall muscle on T2-weighted MR images. The reconstitution of the normal hypointense cervical stroma is the most reliable indicator of a tumor-free postirradiation cervix (14).
Uterus
In postmenopausal patients, radiation therapy does not result in any significant changes in the appearance of the uterus at follow-up MR imaging. In premenopausal patients, the distinction between the junctional zone and outer myometrium is lost, and after 6 months the endometrium becomes thin and hypointense (16).
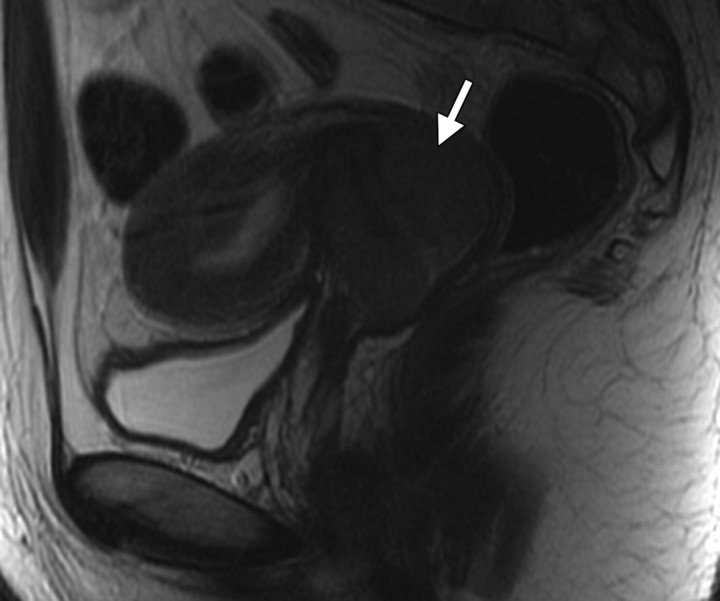
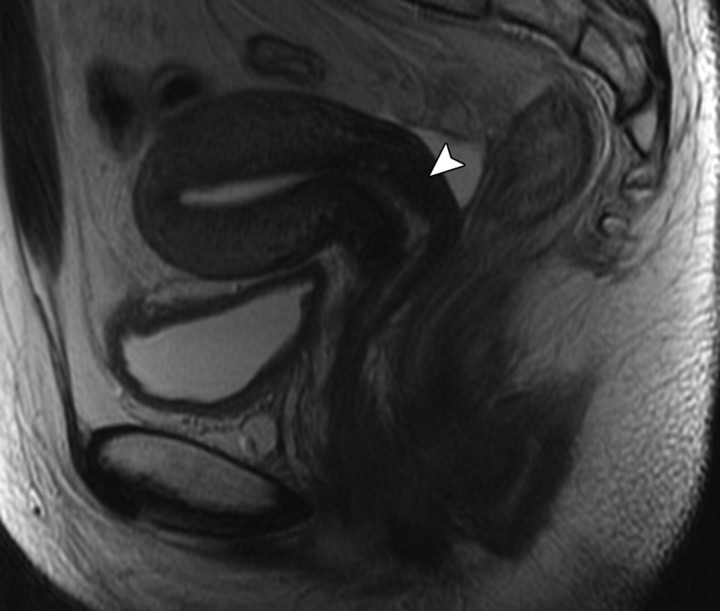
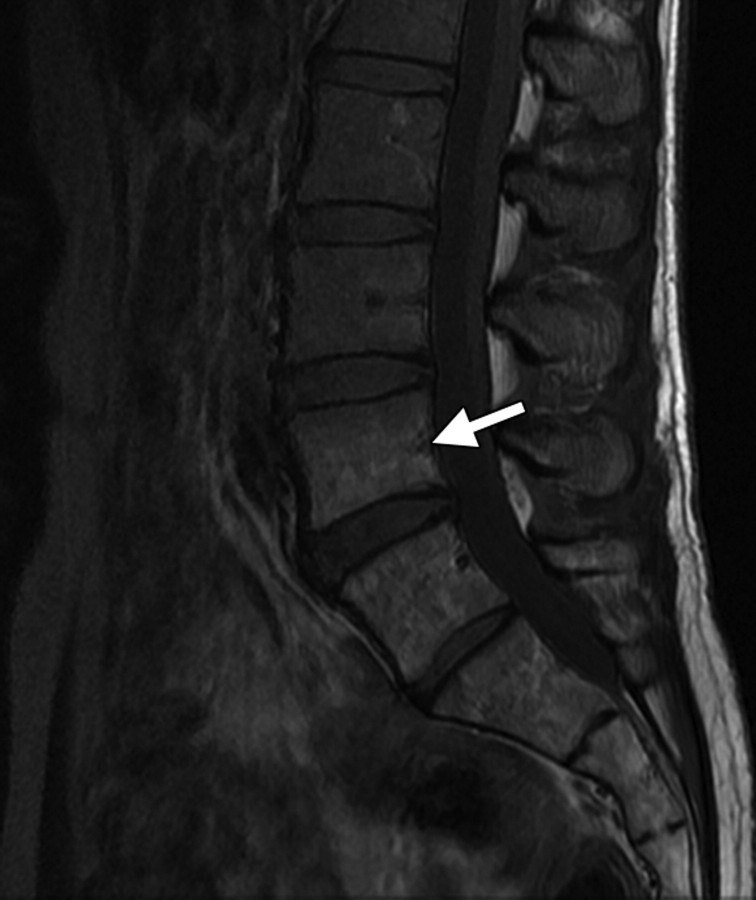
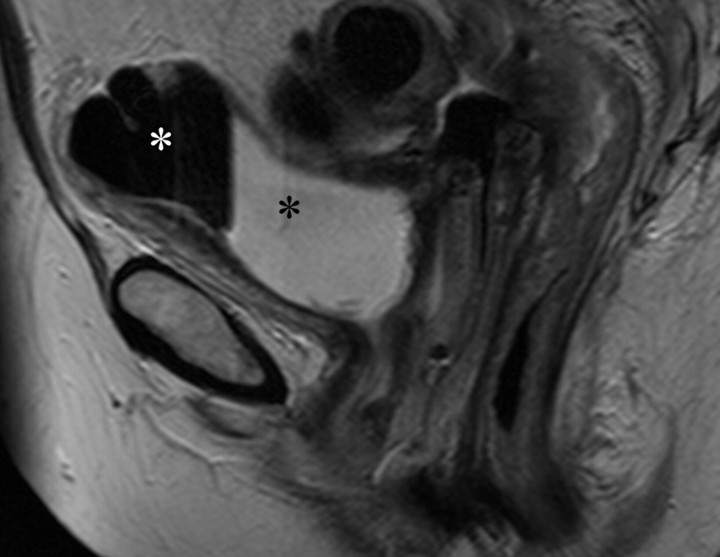
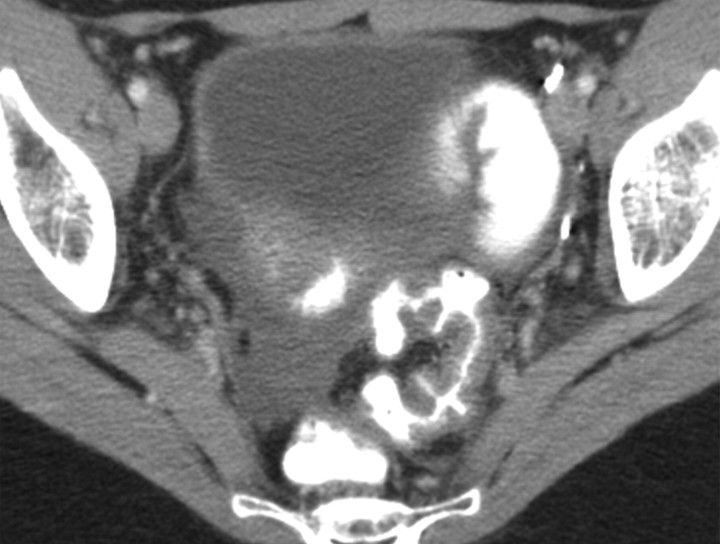
Changes in the cervix can be seen as early as 3 months after completion of therapy. Following treatment, the normal zonal anatomy may once again become well defined and the cervical stroma demonstrates homogeneous low signal intensity (Fig 2). A widened endocervical canal and hyperintense cervical stroma can occur at this stage, although in isolation neither of these findings is necessarily a sign of disease recurrence (13,17). At 3–6 months, cervical os stenosis is a recognized complication (Fig 3) (5). Uterine obstruction can lead to the accumulation of fluid in the uterus, causing hematometrium or resulting in infection and pyometrium. In symptomatic patients, US can help identify these abnormalities (Fig 4a), although further evaluation with MR imaging to detect the cause of the obstruction is usually required (Fig 4b).
Stage IIB cervical carcinoma in a 27-year-old woman. (a) Sagittal T2-weighted MR image obtained prior to chemotherapy–radiation therapy shows a cervical tumor (arrow). (b) Sagittal T2-weighted MR image obtained 5 months after treatment shows resolution of the tumor and reconstitution of the normal low-signal-intensity cervical stroma (arrowhead). A widened endocervical canal is also seen.
Figure 2a.
Figure 2b.
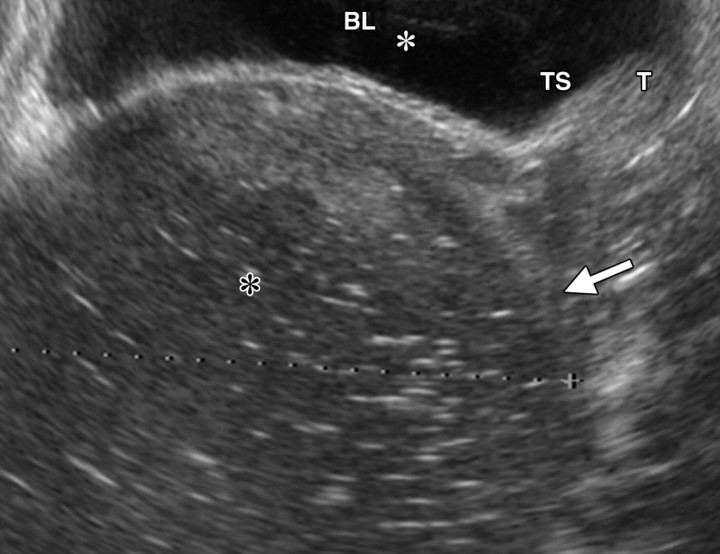
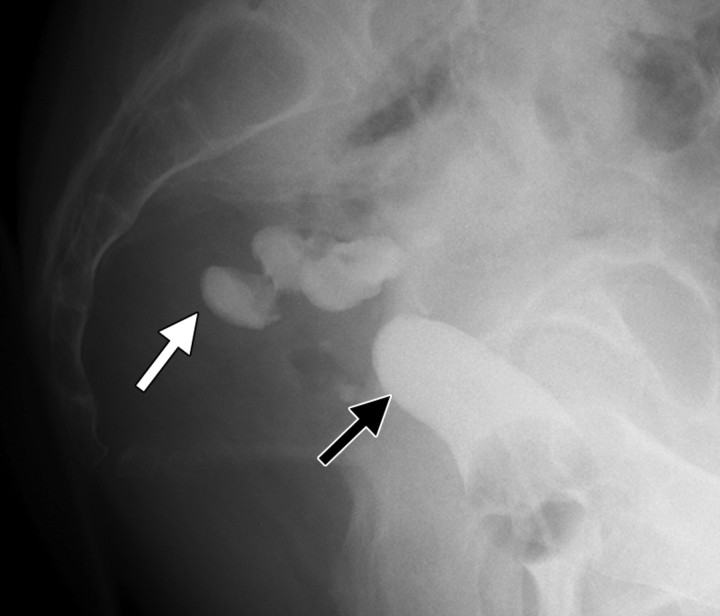
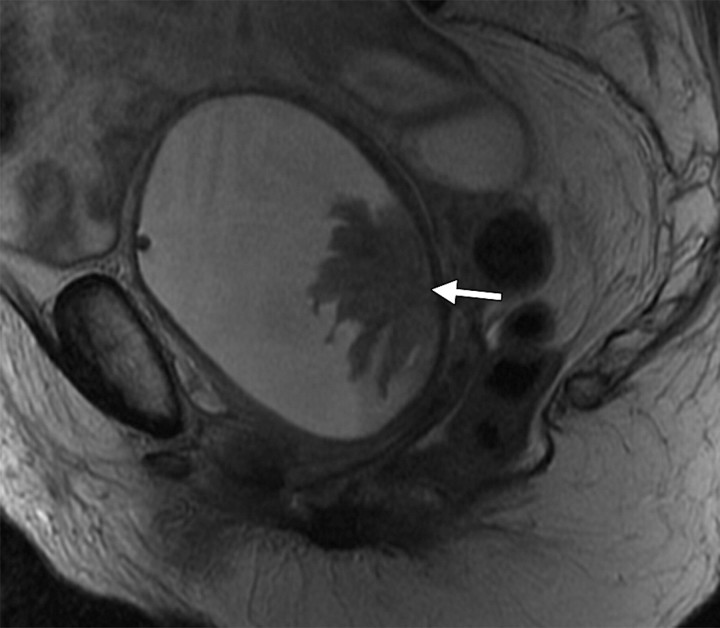
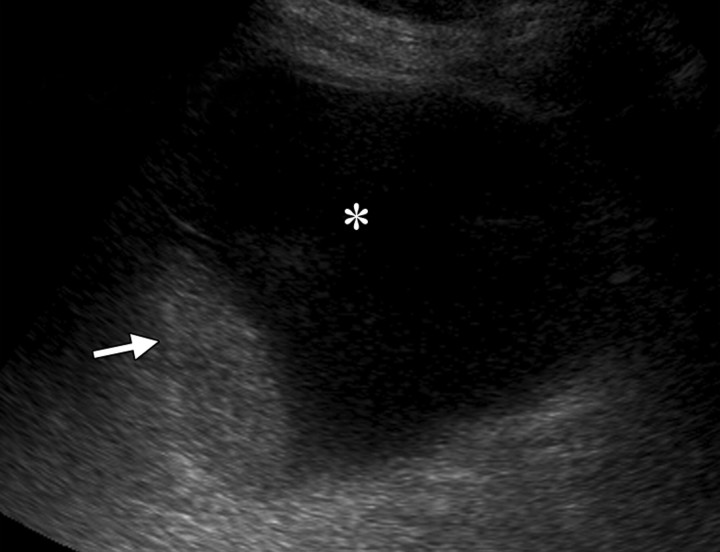
Recurrent stage IIB cervical carcinoma in a 72-year-old woman who had previously undergone external beam radiation therapy. The patient presented to a local hospital with pelvic pain and fever. (a) Transabdominal US image shows a distended uterus (arrow) posterior to a fluid-filled bladder (white *). The contents of the uterus (black *) are of intermediate and heterogeneous echogenicity, a finding that suggests that the contents are complex in nature and therefore consistent with pus. T = (possible) tumor. (b) Sagittal T2-weighted MR image shows a distended fluid-filled uterus containing an air-fluid level (arrowhead) that is the result of recurrent cervical carcinoma (arrow) obstructing the internal os.
Figure 4a.
Figure 4b.
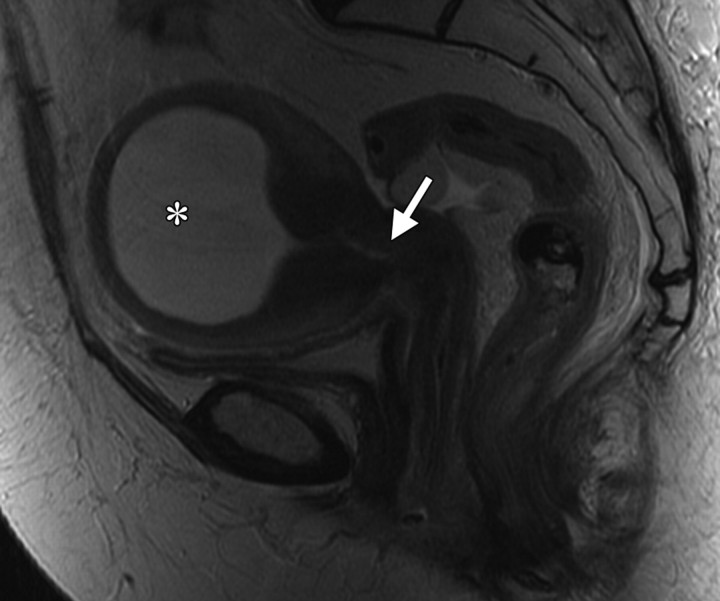
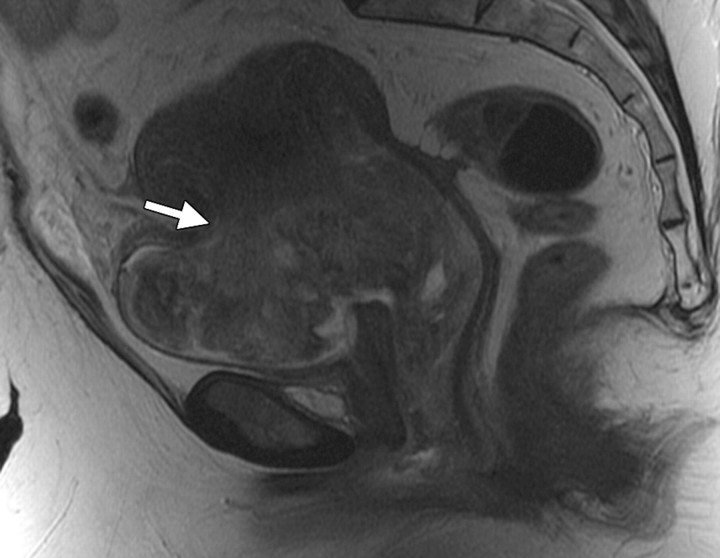
Figure 3.
Cervical os stenosis in a 59-year-old woman who had undergone chemotherapy–radiation therapy for stage IIB cervical carcinoma. Sagittal T2-weighted MR image obtained 5 months after treatment shows distention of the uterine cavity (*) secondary to cervical os stenosis (arrow).
Ovaries
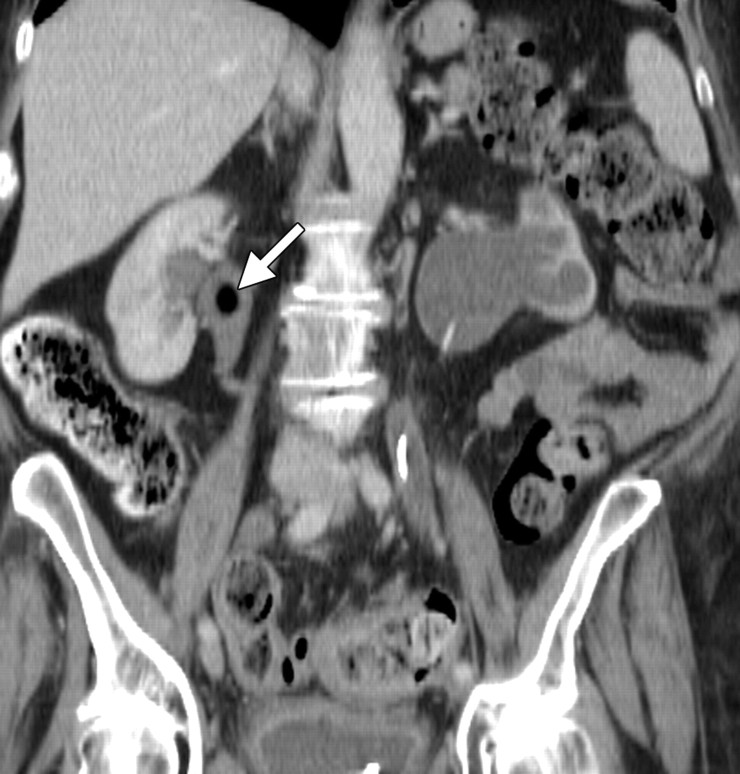
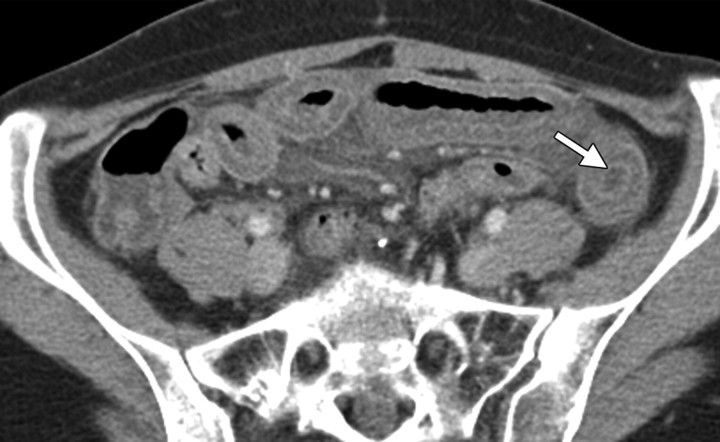
The ovaries decrease in size and signal intensity with loss of physiologic follicles beginning 6 months after radiation treatment (5). In premenopausal patients undergoing pelvic radiation therapy, the function of the ovaries may be maintained by surgically repositioning them outside the radiation field with no increase in risk for primary or secondary malignancy (18). This procedure is termed ovarian transposition, with the ovaries most commonly repositioned laterally within the pelvis, in the lower or intraabdominal paracolic gutters (Fig 5), or anterior to the psoas muscles (19).
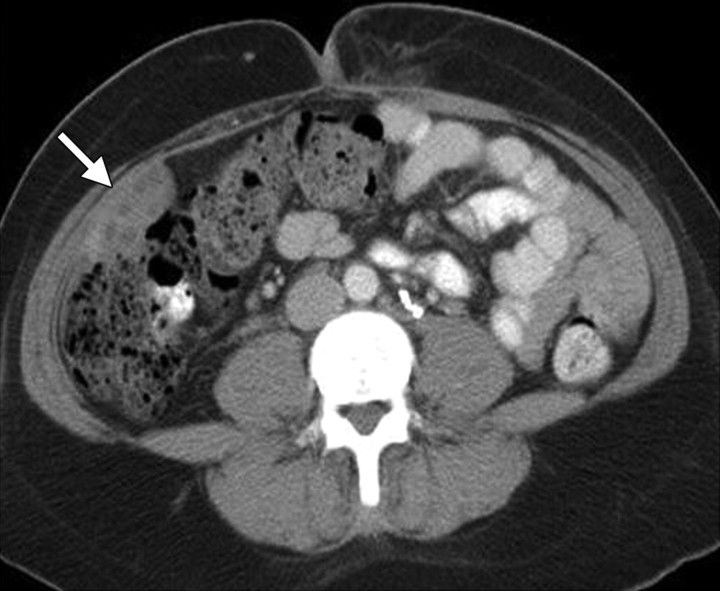
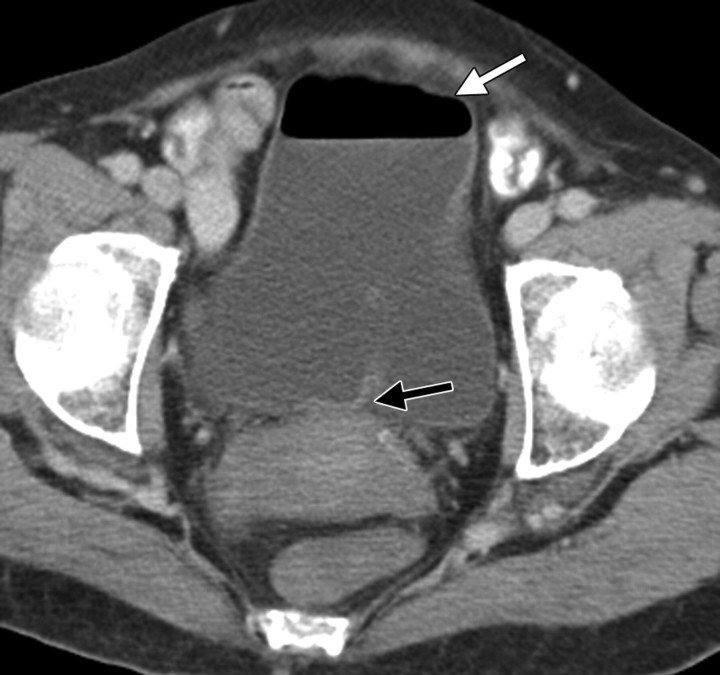
Figure 5.
Stage IB cervical carcinoma in a 25-year-old woman. CT scan obtained after intravenous contrast material administration shows transposition and fixation of the right ovary (arrow) in the right paracolic gutter. The ovaries are most commonly repositioned laterally within the pelvis, in the lower or intraabdominal paracolic gutters, or anterior to the psoas muscles.
Transposed ovaries can be mistaken for peritoneal disease because of their abnormal anatomic location, usually in the paracolic gutters. Careful interrogation for the presence of follicles is helpful in avoiding this pitfall.
Gastrointestinal Tract
Increased signal intensity of the rectal and sigmoid submucosa on T2-weighted images and increased perirectal space due to fatty deposition are normal findings after radiation therapy (20).
Urologic System
The bladder is the most radiosensitive organ of the urinary system. The bladder wall becomes edematous and symmetrically thickened. At MR imaging, the earliest changes of the bullous mucosal edema manifest with high signal intensity on T2-weighted images, whereas the bladder wall is of normal thickness (<5 mm). More severe changes are seen in radiation cystitis (see “Urologic Complications”).
Bones
Following radiation therapy, fatty change in the bone marrow typically appears as well-defined areas of high signal intensity on T1-weighted images. These changes can occur as early as 2 weeks following radiation therapy and may persist for years (21). The fatty conversion that occurs within the radiation field usually produces very well-demarcated signal intensity changes (Fig 6). Occasionally, however, during conversion from hemopoietic to fatty marrow, patchy differences in signal intensity may mimic metastatic bone disease. Later changes such as osteopenia result from a diffuse decrease in bone density. Focal areas of demineralization are difficult to distinguish from lytic metastases if they occur within the radiation field (22). Radiation-induced osteitis and remodeling occur, resulting in areas of sclerosis and mottling.
Fatty conversion of bone marrow in a 44-year-old woman who had undergone chemotherapy–radiation therapy for stage IIB cervical carcinoma. (a) Axial T1-weighted MR image obtained 6 months after therapy shows increased signal intensity of the sacral bone marrow (arrows) caused by conversion to fat. (b) Sagittal T1-weighted MR image shows a well-demarcated change in signal intensity at the level of the L4 vertebral body (arrow), with increased signal intensity in the bone marrow below this level.
Figure 6a.
Figure 6b.
Complications in the Abdomen and Pelvis
Fistulas
Radiation therapy can cause fibrosis, loss of soft-tissue planes, and, in some patients, necrosis, leading to the formation of fistulas in the pelvis. Fistulas are late complications of radiation therapy and most commonly occur between the bladder and the vagina or rectum (Figs 7, 8) but can also involve the small bowel. In addition, it is important to consider the possibility that a fistulous tract has been caused by recurrent disease invading adjacent organs. At CT, it can be difficult to directly visualize a fistulous tract, and it is important to recognize indirect signs of fistula. These signs include the presence of air or oral contrast material in the bladder and focal thickening of the bladder or bowel wall (23). Use of rectal contrast material for imaging enteric fistulas and of additional excretory phase imaging for ureteral or vesical fistulas should always be considered. Three-dimensional reconstruction of the CT data should also be standard practice to optimize visualization, but because complex fistulas are difficult to visualize at CT (Fig 7), MR imaging is the investigation of choice (23–25). In a study by Healy et al (24), MR imaging successfully demonstrated 10 of 11 vaginal fistulas (later confirmed at examination performed with the patient anesthetized), with the false-negative finding occurring in a patient with an epithelialized tract. At MR imaging, the sagittal plane is optimal for the detection of fistulas. With heavily T2-weighted sequences, the fistulous tract may best be identified as a high-signal-intensity finding.
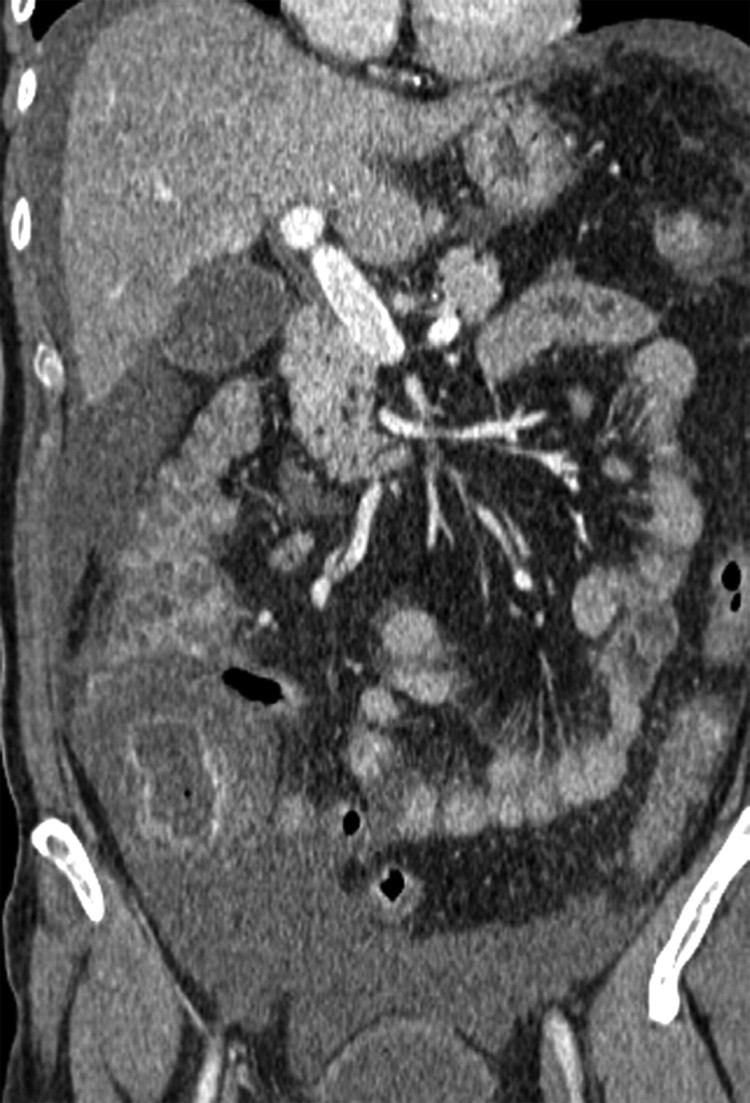
Radiation-induced fistula in a 60-year-old woman with recurrent stage IB endometrial carcinoma who had undergone hysterectomy. Left-sided hydronephrosis due to pelvic recurrence was treated with ureteric stent placement, adjuvant chemotherapy, and radical radiation therapy. Routine follow-up CT and MR imaging were performed 4 months after treatment. (a) Coronal CT scan shows a left ureteric stent in situ. Gas is seen within the proximal right ureter (arrow) consistent with a fistula. (b) Sagittal T2-weighted MR image shows a low-signal-intensity area (white *) consistent with air and a high-signal-intensity area (black *) consistent with vesical fluid, findings that are indicative of a fistula. (c) Corresponding sagittal T1-weighted MR image obtained following intravenous contrast material administration shows a fistulous tract connecting the bladder to the vagina (arrow) and the vagina to the rectum (arrowhead). (d) Sagittal spot image from a vaginographic examination performed in a different patient with the instillation of water-soluble contrast material into the vagina (black arrow) via a urinary catheter shows a fistula containing contrast material within the sigmoid colon (white arrow).
Figure 8a.
Figure 8b.
Figure 8c.
Figure 8d.
Figure 7.
Fistula in a 78-year-old woman who had undergone chemotherapy–radiation therapy for stage IIB cervical carcinoma 3 years earlier and presented with pneumaturia. CT scan obtained following intravenous contrast material administration shows indirect evidence of a fistula, with gas in the urinary bladder (white arrow) and, possibly, a fistulous tract (black arrow).
The fistulous tract may also be better visualized on T1-weighted images obtained following the administration of intravenous gadolinium-based contrast material (26). Water-soluble enema examination, cystography, and vaginography may also be helpful in demonstrating the fistula.
As mentioned earlier, recurrent disease that invades adjacent organs can also cause a fistulous tract (Fig 9). Therefore, careful evaluation for the presence of a soft-tissue mass or other features of malignancy such as lymphadenopathy should routinely be performed.
Figure 9.
Fistula in a 39-year-old woman who had undergone chemotherapy–radiation therapy for stage IIB cervical carcinoma 3 years earlier. The patient presented with passage of debris in the urine. Sagittal T2-weighted MR image shows a large, heterogeneous mass invading the bladder, resulting in the formation of a fistulous tract (arrow) between the bladder and the uterine cavity.
Gastrointestinal Complications
Gastrointestinal complications following radiation therapy are common because the gastrointestinal tract is sensitive to the effects of radiation, with 75% of patients complaining of acute diarrhea and gastrointestinal symptoms. Chronic radiation injury to the colon and rectum is dose dependent and occurs in 1%–5% of patients treated with 45–55 Gy of external beam radiation (27). The rectum and sigmoid colon sustain acute damage to the actively proliferating mucosa, resulting in proctitis and colitis.
At CT, there is uniform thickening and enhancement of the bowel wall, which can progress to areas of stricture. At MR imaging, the findings are similar, with bowel wall enhancement on contrast-enhanced T1-weighted images. Furthermore, there is loss of definition of the muscle layers on T1-weighted images. The small bowel is more radiosensitive than the large bowel but is usually subject to lower doses of radiation due to its increased mobility. Enteritis of the small bowel typically involves the more fixed terminal ileum and manifests as bowel wall thickening and submucosal edema at CT (Fig 10). Chronic changes such as fibrosis can lead to stenotic segments, which may in turn cause small bowel obstruction (22).
Enteritis in a 27-year-old woman who had undergone total abdominal hysterectomy, chemotherapy, and radiation therapy for metastatic small cell adenocarcinoma of the cervix. (a) Follow-up CT scan obtained after the administration of intravenous and oral contrast material shows a thick-walled sigmoid colon and rectum, as well as free fluid in the pelvis. (b) CT scan from the same examination shows an abnormal thick-walled small bowel with enhancing mucosa (arrow), abnormal findings that are consistent with enteritis but do not indicate a malignant small bowel process.
Figure 10a.
Figure 10b.
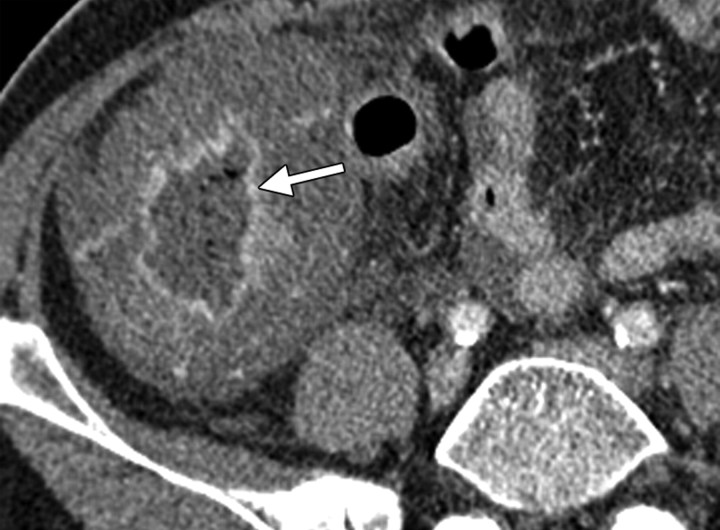
Immunosuppression occurring after chemotherapy can cause neutropenic colitis (typhlitis). This condition typically affects the cecum and ascending colon, resulting in segmental circumferential bowel wall thickening with surrounding fat stranding and free fluid (Fig 11). A small amount of ascites commonly occurs following chemotherapy but has no clinical implications as an isolated finding. However, the presence of peritoneal deposits in addition to ascites is very suspicious for disease recurrence. Patients with radiation-induced changes can be asymptomatic, whereas patients with typhlitis are typically symptomatic and present with lower abdominal pain, guarding, and fever. CT is the examination of choice and clearly demonstrates the extent of typhlitis and the presence of transmural necrosis, which may ultimately lead to perforation (Fig 10) (28).
Typhlitis in a 54-year-old woman who had undergone total abdominal hysterectomy and bilateral salpingo-oophorectomy and chemotherapy for stage IIIC serous papillary carcinoma of the ovary. The patient presented with severe abdominal pain and fever. (a) Axial CT scan obtained following intravenous contrast material administration shows gross thickening of the cecal wall with mucosal enhancement (arrow), findings that are consistent with typhlitis. (b) Coronal CT scan shows less extensive involvement of the ascending colon, which has a thickened wall with a “thumbprint” appearance. There is surrounding free fluid in the right flank and pelvis.
Figure 11a.
Figure 11b.
Urologic Complications
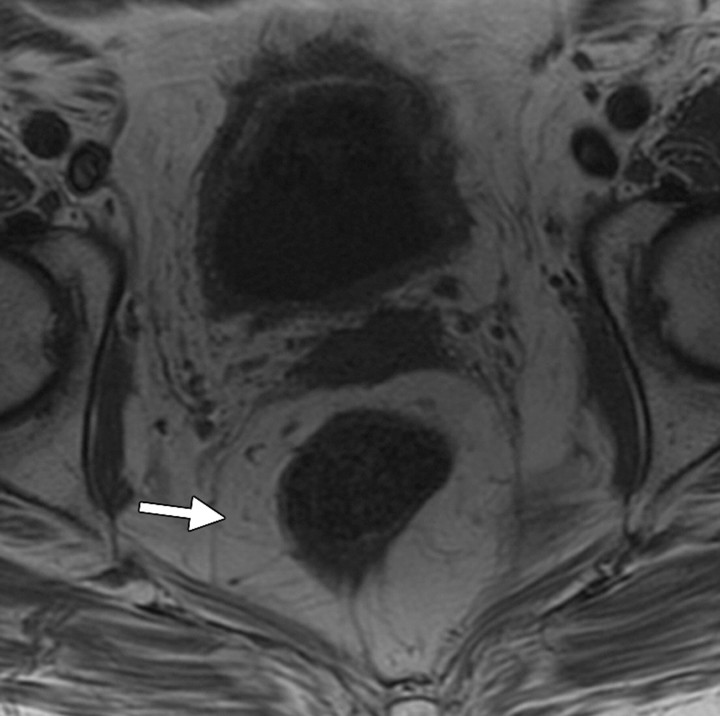
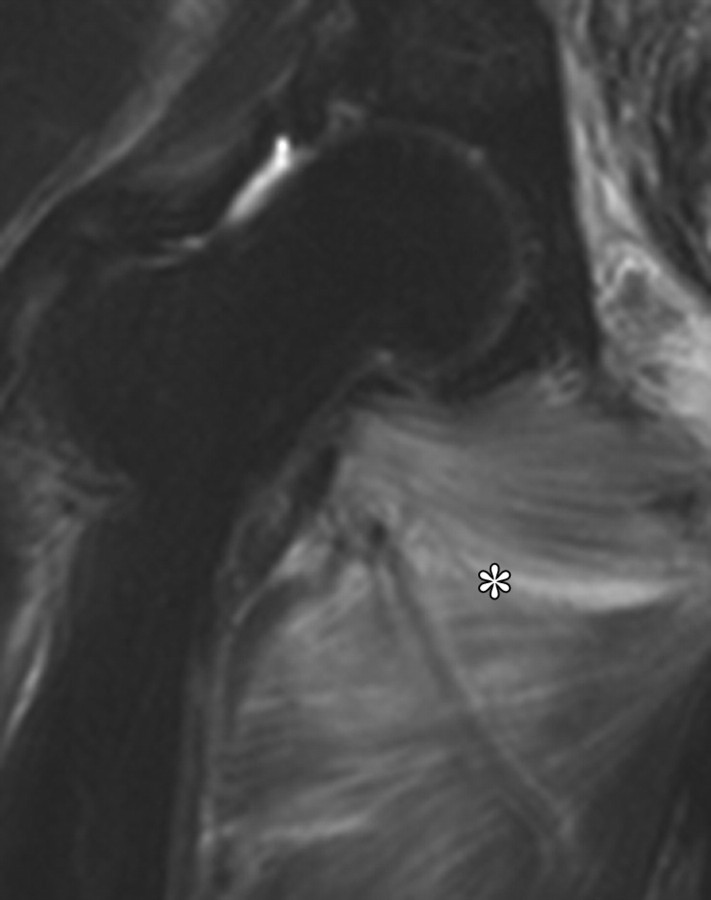
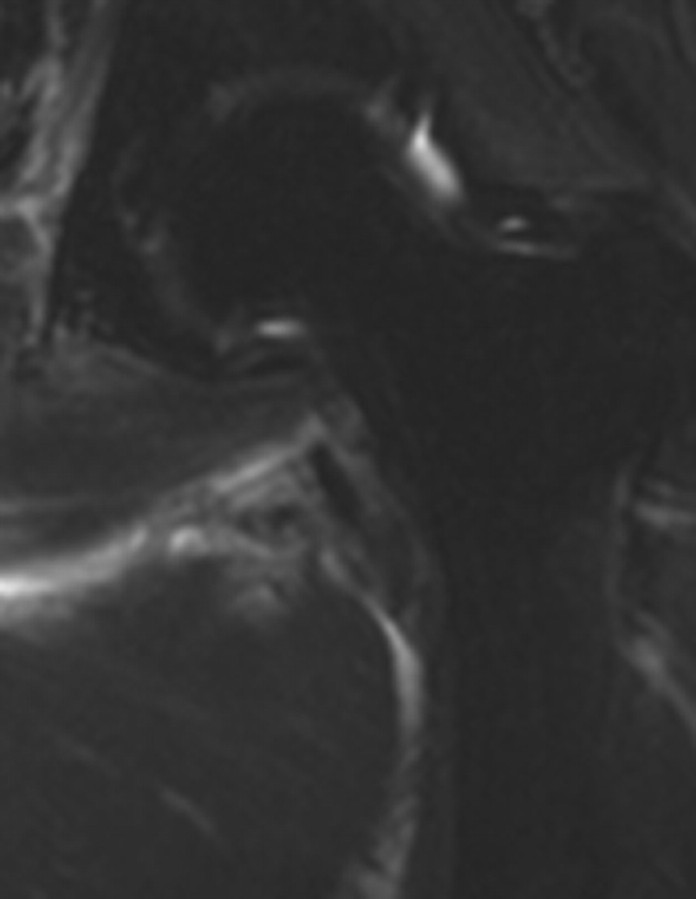
Lower doses of radiation (30-Gy external beam therapy over a 4-week period) can cause mild cystitis, with higher doses of up to 70 Gy causing longer-term effects. Radiation cystitis occurs in up to 12% of cases and is dose dependent (22). The appearance of bladder wall thickening at CT and MR imaging is often worse than the clinical symptoms. Severe changes result in bladder wall thickening manifesting as high signal intensity in the outer layer of the bladder on T2-weighted MR images and as enhancement of the mucosal layer on contrast-enhanced T1-weighted images (Fig 12). Hemorrhage and necrosis can occur, causing hematuria and clot formation (Fig 13). In patients with hematuria, initial investigations often include US, which can be very helpful in demonstrating the mobility of clot compared with the tumor (Fig 14). In the chronic phase, the bladder has a small volume and cannot be fully distended because of fibrosis, which appears as a thin band of low signal intensity on the inner aspect of the bladder (29). Perforation of the bladder is a recognized late complication of radiation therapy that occurs only rarely but is life threatening (30).
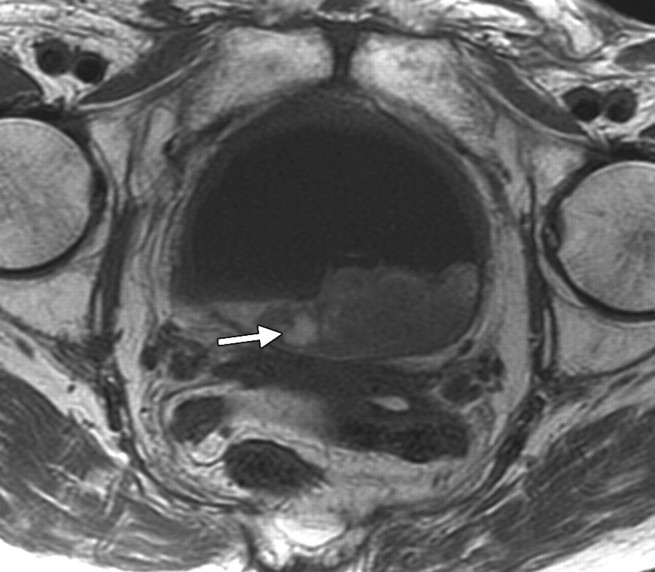
Radiation cystitis in a 72-year-old woman who had undergone total abdominal hysterectomy and radiation therapy for stage IC endometrial carcinoma. Axial follow-up T1-weighted (a) and fat-saturated T2-weighted (b) MR images demonstrate a small-volume urinary bladder with a thickened wall. Increased perirectal space due to fat deposition from radiation therapy is clearly seen on the T1-weighted image (arrow in a). The outer layer of the bladder has high signal intensity on the T2-weighted image.
Figure 12a.
Figure 12b.
Hematoma in a 75-year-old woman who had undergone chemotherapy–radiation therapy for stage IIB cervical carcinoma. (a) Sagittal T2-weighted MR image shows a frondlike abnormality at the base of the bladder (arrow) consistent with a hematoma. However, transitional cell carcinoma of the bladder can also have a frondlike appearance, and biopsy is required to distinguish between a hematoma and a malignant process. (b) Axial T1-weighted MR image shows increased signal intensity within the clot (arrow), a finding that represents hemorrhage.
Figure 13a.
Figure 13b.
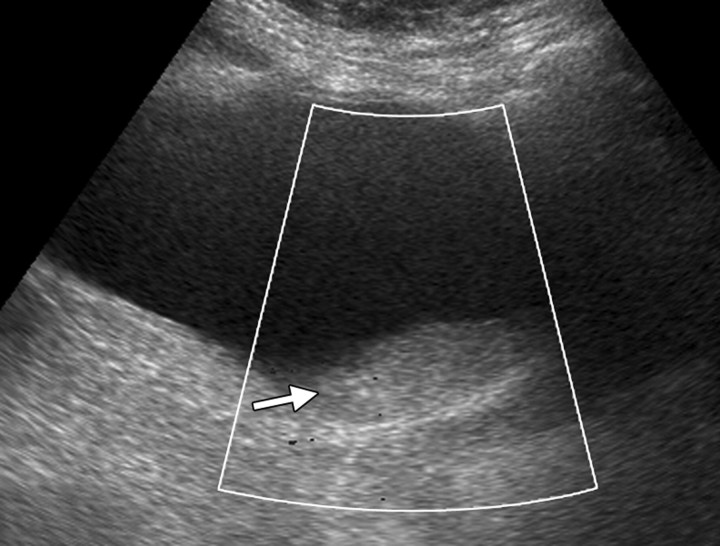
Mobile clot in a 51-year-old woman who had undergone radiation therapy for cervical carcinoma. The patient presented with frank hematuria. (a) Transabdominal Doppler US image (shown in black and white), obtained with the patient supine, demonstrates an echogenic filling defect (arrow) in the urinary bladder. No flow was evident. (b) US image obtained with the patient lying on her right side shows an echogenic mass (arrow) in a dependent position within the urinary bladder (*), a finding that is consistent with a mobile clot.
Figure 14a.
Figure 14b.
The distal ureters are less commonly affected by pelvic irradiation, but their involvement may cause stricture formation, resulting in hydroureter and, ultimately, hydronephrosis. This can be a pitfall in the reporting of follow-up CT and MR imaging findings in these patients because parametrial disease can also affect the distal ureters, resulting in hydronephrosis. A ureteric stricture from radiation treatment is smoothly tapered and can be clearly visualized on delayed CT scans obtained after the administration of intravenous contrast material, which opacifies the ureters. Stricture formation is also a late complication of radiation treatment, occurring several months or even years after completion of treatment (20).
Because hydronephrosis may also be caused by recurrent parametrial disease, careful evaluation of the parametria for the presence of tumor should routinely be performed.
Musculoskeletal Complications
Radiation affects the cellular bone, resulting in a spectrum of changes. The earliest change seen at MR imaging is replacement of the irradiated marrow by fat, which occurs in 90% of patients by 8 weeks following radiation treatment (31).
Ultimately, insufficiency fractures caused by normal stress on abnormal bone occur, with the most common sites being the sacrum and pubic rami (Figs 15, 16). Nonunion and aseptic necrosis are known complications. Although these complications can be seen at conventional radiography, which is the first line of investigation in symptomatic patients, they are often detected incidentally at follow-up CT or MR imaging. At CT, breach of the cortex is best observed with bone window settings. The changes may be seen earlier at MR imaging, with high signal intensity representing bone marrow edema on T2-weighted images and the fracture line having low signal intensity on T1-weighted images (Fig 16). The appearance of symmetric changes should not prompt an alternative diagnosis, since insufficiency fractures are commonly symmetric (29). Bone scintigraphy is sensitive for the detection of fractures, with the characteristic H-shaped pattern of increased radionuclide uptake occurring across the sacrum and representing bilateral fractures of the sacral ala (32).
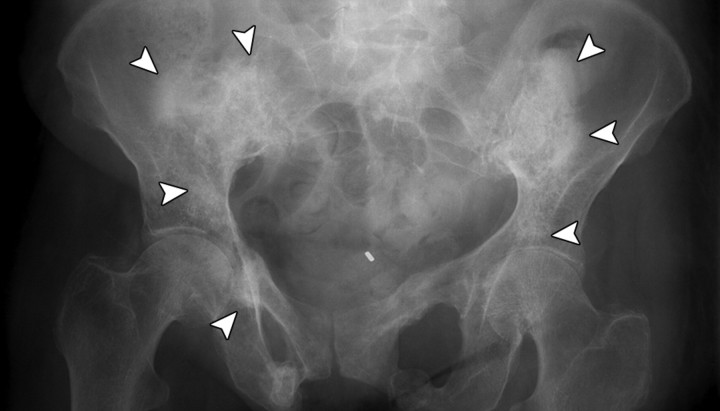
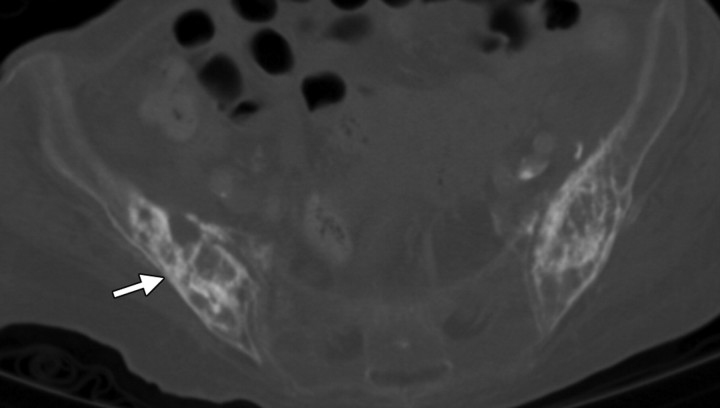
Insufficiency fractures in a 58-year-old woman who had undergone chemotherapy–radiation therapy for stage IIA cervical carcinoma. (a) Pelvic radiograph shows mottling and sclerosis of the bones in the radiation field (arrowheads), as well as bilateral fractures of the pubic rami. (b) Corresponding CT scan shows abnormal iliac bone texture (arrow) with osteopenia of the sacrum.
Figure 15a.
Figure 15b.
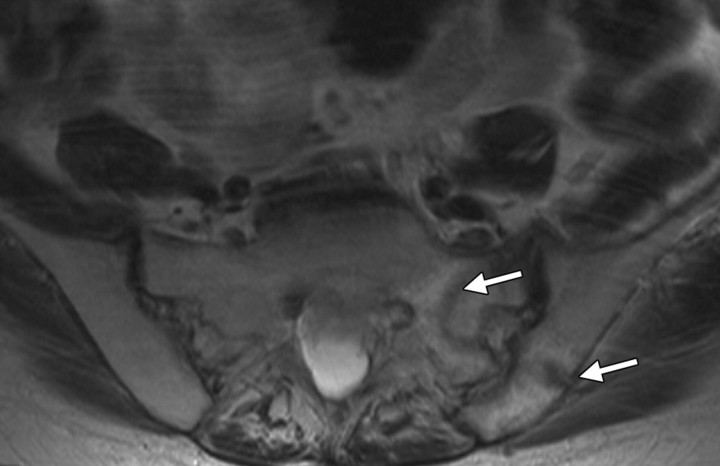
Insufficiency fractures in the same patient as in Figure 8. Axial T1-weighted MR images obtained 3 years after the end of treatment show insufficiency fractures of the sacrum and ilium (arrows in a) and pubic rami (arrow in b), the most commonly affected sites. The fractures are seen as hypointense lines. Nonunion is a common complication of pubic ramus fractures.
Figure 16a.
Figure 16b.
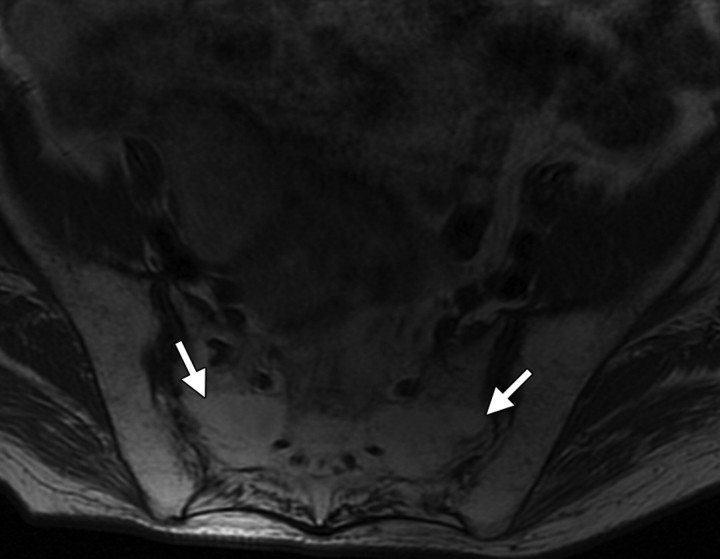
Myositis of striated muscles within the radiation field can occur, manifesting with high signal intensity on T2-weighted images (Fig 17) and demonstrating enhancement following intravenous contrast material administration (5).
Myositis in a 69-year-old woman who underwent radiation therapy for stage IIB cervical carcinoma. Coronal T2-weighted MR images of the right (a) and left (b) adductor muscles demonstrate high signal intensity within the striated muscles, a finding that is consistent with myositis. The hyperintensity is more prominent on the right side (* in a).
Figure 17a.
Figure 17b.
Conclusions
Interpretation of the posttreatment appearances of the female pelvis can be difficult, with these appearances as well as complications mimicking malignant disease and vice versa. A basic understanding of the findings commonly seen after chemotherapy and radiation therapy helps in making the correct interpretation and avoiding possible pitfalls. Radiologists should be familiar with the common immediate and long-term posttreatment appearances of the pelvis, including those of both the organ containing the tumor and the adjacent organs; complications that are specifically related to the therapy; and differentiation of these findings from recurrent tumor.
Footnotes
Presented as an education exhibit at the 2009 RSNA Annual Meeting.
For this CME activity, the authors, editors, and reviewers have no relevant relationships to disclose.
References
- 1.Kehoe S. Treatments for gynaecological cancers. Best Pract Res Clin Obstet Gynaecol 2006;20(6):985–1000. [DOI] [PubMed] [Google Scholar]
- 2.Funt SA, Hricak H, Abu-Rustum N, Mazumdar M, Felderman H, Chi DS. Role of CT in the management of recurrent ovarian cancer. AJR Am J Roentgenol 2004;182(2):393–398. [DOI] [PubMed] [Google Scholar]
- 3.Kinkel K, Ariche M, Tardivon AA, et al. Differentiation between recurrent tumor and benign conditions after treatment of gynecologic pelvic carcinoma: value of dynamic contrast-enhanced subtraction MR imaging. Radiology 1997;204(1):55–63. [DOI] [PubMed] [Google Scholar]
- 4.Delaney G, Jacob S, Barton M. Estimation of an optimal radiotherapy utilization rate for gynecologic carcinoma. I. Malignancies of the cervix, ovary, vagina and vulva. Cancer 2004;101(4):671–681. [DOI] [PubMed] [Google Scholar]
- 5.Hricak H, Akin O, Sala E, Ascher S, Levine D, Reinhold C. Diagnostic imaging: gynecology. Salt Lake City, Utah: Amirsys, 2006. [Google Scholar]
- 6.Barnes EA, Thomas G, Ackerman I, et al. Prospective comparison of clinical and computed tomography assessment in detecting uterine perforation with intracavitary brachytherapy for carcinoma of the cervix. Int J Gynecol Cancer 2007;17(4):821–826. [DOI] [PubMed] [Google Scholar]
- 7.Viswanathan AN. The Frank Ellis memorial lecture: the use of three-dimensional imaging in gynaecological radiation therapy. Clin Oncol (R Coll Radiol) 2008;20(1):1–5. [DOI] [PubMed] [Google Scholar]
- 8.Georg P, Georg D, Hillbrand M, Kirisits C, Pötter R. Factors influencing bowel sparing in intensity modulated whole pelvic radiotherapy for gynaecological malignancies. Radiother Oncol 2006;80(1):19–26. [DOI] [PubMed] [Google Scholar]
- 9.Portelance L, Chao KS, Grigsby PW, Bennet H, Low D. Intensity-modulated radiation therapy (IMRT) reduces small bowel, rectum, and bladder doses in patients with cervical cancer receiving pelvic and para-aortic irradiation. Int J Radiat Oncol Biol Phys 2001;51(1):261–266. [DOI] [PubMed] [Google Scholar]
- 10.Roeske JC, Lujan A, Rotmensch J, Waggoner SE, Yamada D, Mundt AJ. Intensity-modulated whole pelvic radiation therapy in patients with gynecologic malignancies. Int J Radiat Oncol Biol Phys 2000;48(5):1613–1621. [DOI] [PubMed] [Google Scholar]
- 11.Mark RJ, Poen J, Tran LM, Fu YS, Heaps J, Parker RG. Postirradiation sarcoma of the gynecologic tract: a report of 13 cases and a discussion of the risk of radiation-induced gynecologic malignancies. Am J Clin Oncol 1996;19(1):59–64. [DOI] [PubMed] [Google Scholar]
- 12.Vincens E, Balleyguier C, Rey A, et al. Accuracy of magnetic resonance imaging in predicting residual disease in patients treated for stage IB2/II cervical carcinoma with chemoradiation therapy: correlation of radiologic findings with surgicopathologic results. Cancer 2008;113(8):2158–2165. [DOI] [PubMed] [Google Scholar]
- 13.Flueckiger F, Ebner F, Poschauko H, Tamussino K, Einspieler R, Ranner G. Cervical cancer: serial MR imaging before and after primary radiation therapy—a 2-year follow-up study. Radiology 1992;184(1):89–93. [DOI] [PubMed] [Google Scholar]
- 14.Hricak H, Swift PS, Campos Z, Quivey JM, Gildengorin V, Göranson H. Irradiation of the cervix uteri: value of unenhanced and contrast-enhanced MR imaging. Radiology 1993;189(2):381–388. [DOI] [PubMed] [Google Scholar]
- 15.Weber TM, Sostman HD, Spritzer CE, et al. Cervical carcinoma: determination of recurrent tumor extent versus radiation changes with MR imaging. Radiology 1995;194(1):135–139. [DOI] [PubMed] [Google Scholar]
- 16.Arrivé L, Chang YC, Hricak H, Brescia RJ, Auffermann W, Quivey JM. Radiation-induced uterine changes: MR imaging. Radiology 1989;170(1 Pt 1): 55–58. [DOI] [PubMed] [Google Scholar]
- 17.De Graef M, Karam R, Juhan V, Daclin PY, Maubon AJ, Rouanet JP. High signals in the uterine cervix on T2-weighted MRI sequences. Eur Radiol 2003; 13(1):118–126. [DOI] [PubMed] [Google Scholar]
- 18.Feeney DD, Moore DH, Look KY, Stehman FB, Sutton GP. The fate of the ovaries after radical hysterectomy and ovarian transposition. Gynecol Oncol 1995;56(1):3–7. [DOI] [PubMed] [Google Scholar]
- 19.Sella T, Mironov S, Hricak H. Imaging of transposed ovaries in patients with cervical carcinoma. AJR Am J Roentgenol 2005;184(5):1602–1610. [DOI] [PubMed] [Google Scholar]
- 20.Capps GW, Fulcher AS, Szucs RA, Turner MA. Imaging features of radiation-induced changes in the abdomen. RadioGraphics 1997;17(6):1455–1473. [DOI] [PubMed] [Google Scholar]
- 21.Yankelevitz DF, Henschke CI, Knapp PH, Nisce L, Yi Y, Cahill P. Effect of radiation therapy on thoracic and lumbar bone marrow: evaluation with MR imaging. AJR Am J Roentgenol 1991;157(1):87–92. [DOI] [PubMed] [Google Scholar]
- 22.Iyer RB, Jhingran A, Sawaf H, Libshitz HI. Imaging findings after radiotherapy to the pelvis. AJR Am J Roentgenol 2001;177(5):1083–1089. [DOI] [PubMed] [Google Scholar]
- 23.Narayanan P, Nobbenhuis M, Reynolds KM, Sahdev A, Reznek RH, Rockall AG. Fistulas in malignant gynecologic disease: etiology, imaging, and management. RadioGraphics 2009;29(4):1073–1083. [DOI] [PubMed] [Google Scholar]
- 24.Healy JC, Phillips RR, Reznek RH, Crawford RA, Armstrong P, Shepherd JH. The MR appearance of vaginal fistulas. AJR Am J Roentgenol 1996;167(6): 1487–1489. [DOI] [PubMed] [Google Scholar]
- 25.Outwater E, Schiebler ML. Pelvic fistulas: findings on MR images. AJR Am J Roentgenol 1993;160(2):327–330. [DOI] [PubMed] [Google Scholar]
- 26.Semelka RC, Hricak H, Kim B, et al. Pelvic fistulas: appearances on MR images. Abdom Imaging 1997;22(1):91–95. [DOI] [PubMed] [Google Scholar]
- 27.Donner CS. Pathophysiology and therapy of chronic radiation-induced injury to the colon. Dig Dis 1998; 16(4):253–261. [DOI] [PubMed] [Google Scholar]
- 28.Hoeffel C, Crema MD, Belkacem A, et al. Multi–detector row CT: spectrum of diseases involving the ileocecal area. RadioGraphics 2006;26(5):1373–1390. [DOI] [PubMed] [Google Scholar]
- 29.Sugimura K, Okizuka H. Postsurgical pelvis: treatment follow-up. Radiol Clin North Am 2002;40(3):659–680, viii. [DOI] [PubMed] [Google Scholar]
- 30.Addar MH, Stuart GC, Nation JG, Shumsky AG. Spontaneous rupture of the urinary bladder: a late complication of radiotherapy—case report and review of the literature. Gynecol Oncol 1996;62(2):314–316. [DOI] [PubMed] [Google Scholar]
- 31.Blomlie V, Rofstad EK, Skjønsberg A, Tverå K, Lien HH. Female pelvic bone marrow: serial MR imaging before, during, and after radiation therapy. Radiology 1995;194(2):537–543. [DOI] [PubMed] [Google Scholar]
- 32.Tai P, Hammond A, Dyk JV, et al. Pelvic fractures following irradiation of endometrial and vaginal cancers: a case series and review of literature. Radiother Oncol 2000;56(1):23–28. [DOI] [PubMed] [Google Scholar]