Abstract
Oral immunotherapy (OIT) has reproducibly demonstrated successful desensitization in food-allergic subjects completing clinical trials and, in some studies, sustained unresponsiveness. These clinical outcomes have been associated with characteristic modifications in the allergen-specific immune response, but a detailed synthesis of OIT’s mechanisms of action is lacking. In this Rostrum, we review the current evidence regarding the human immune response to OIT, explore possible mechanisms, and identify knowledge gaps for future research.
Keywords: oral immunotherapy, immune mechanisms, desensitization, sustained unresponsiveness, basophils, T cells, B cells, mast cells, antibodies
Introduction
The mechanisms of action of oral immunotherapy (OIT) remain poorly understood, with the literature comprised of primarily descriptive peripheral blood studies in human patients. While the stromal and immune cells in the mucosa of the gastrointestinal (GI) tract, its associated secondary lymphoid structures, the GI microbiome, et cetera are likely to critically influence human food allergy, the role of these structures in the mechanisms of OIT remains obscure given the inability to routinely sample these structures in humans. While much can be learned from animal model systems, the knowledge gained is inherently limited by experimental conditions that do not resemble human food allergy. Despite these obstacles, the application of new technologies is enhancing our current understanding of the abundance and diversity of OIT’s impact on immune cell subsets. Our aim in this Rostrum is to review what is known about clinically relevant OIT mechanisms, and we have chosen to focus primarily on human studies, supplementing them with data from animals where appropriate. We have organized our approach sequentially, in an attempt to outline the temporal changes from baseline during the OIT treatment protocol.
The primary clinical objective of most OIT programs for food allergy is to induce a desensitized state in the individual, defined here as a temporary increase in the threshold reactivity to the allergen such that clinical protection from accidental ingestion may be achieved. This occurs through continuously stimulating the immune system with sub-threshold daily doses of allergen and then gradually escalating the dose level over time to reach a target maintenance dose. The oral route of administration may take advantage of the unique set of immune cells and pathways involved in the induction of oral tolerance. Protocols vary in their approaches to the initial dose escalation phase, but they consistently begin OIT with low doses (e.g. ≤5 mg of allergenic protein) and generally increase the doses by 25–100% at a periodic interval until the target maintenance dose is reached or a dose-limiting toxicity (DLT) occurs. Holding, reducing, or terminating dosing is occasionally required during this period of treatment due to allergic symptoms caused by the daily dose, as participants transition from allergen avoidance pre-OIT to steadily progressive exposures. It is this period of transition that we will refer to in this paper as the “initiation phase,” to describe the mechanistic changes occurring during initial exposures. Clinical studies repeatedly have shown that the majority of OIT subjects in clinical trials will have adverse events related to dosing, usually mild to moderate in severity, and that they are more common during initiation, lessening in frequency over time (1–3). In approximately 15–20% of subjects, more severe symptoms and/or DLT can occur, and while clinical co-factors have been identified for systemic reactions, the biological basis (i.e., the “endotype”) that explains this phenotype has not been elucidated. The repeated engagement of allergen-specific IgE on mast cells and basophils, which in many participants can lead to the elicitation of some symptoms, may also contribute to OIT’s mode of action, which later engages regulatory pathways that aim to control allergic inflammation through effector cell suppression and antibody production (e.g. the “modified TH2 response”), but the optimal relationship of excitation and inhibition is not well understood.
As OIT participants progress through the dose escalation, the initial initiation phase of the desensitization process gives way to a consolidation phase. In this phase, the clinical benefit of the regimen is preserved through maintenance dosing (i.e., no further escalation), and effector cells remain stably suppressed. Lymphocytes and their products (cytokines and antibodies) are modulated further, culminating in some participants in a result known as “sustained unresponsiveness (SU)” a persistent state of elevated allergen threshold in the absence of daily dosing. The mechanistic changes associated with SU will be discussed in this section, followed by some selected key knowledge gaps that serve as future research needs in this field.
Initiation Phase
Mast Cells and Basophils
At baseline, the mast cells and basophils of OIT participants express the high-affinity IgE receptor FcεRI on their cell surface, are primed with allergen-specific IgE, and are the major effector cells of IgE-mediated allergic reactions to foods, owing to their granule contents. These primed effector cells are rapidly activated by a signaling cascade through FcεRI signaling when untreated food-allergic individuals accidentally and occasionally encounter allergen in supra-threshold amounts (Figure 1). However, the steady sub-threshold dosing used in OIT trials for peanut, egg, and milk allergies have consistently demonstrated significantly decreased skin prick test (SPT) wheal size and basophil activation (as measured by upregulation of CD63 and/or CD203c) in response to the antigen used for OIT, (4–11) and this effect likely accounts for the initial desensitization seen clinically. Suppression of these effector cell responses occurs within the first few months of OIT and therefore may be linked to escalating antigen dose. It is important to note that this desensitization occurs in the absence of a decrease in sIgE, and often during the period of time that sIgE is actually increasing from baseline (5, 11). This finding, across several studies, implies that desensitization of mast cells and basophils does not rely on decreased sIgE as its underlying mechanism. However, decreased IgE via omalizumab treatment prior to initiating OIT allows for much higher doses of antigen to be safely given in the initial escalation phase (12, 13). The effects of anti-IgE therapy on reducing circulating IgE and down-regulating the FcεRI levels on basophils may explain this finding. Therefore, while low sIgE levels are not requisite for desensitization, removal of IgE by omalizumab allows for a rapid escalation in antigen dose.
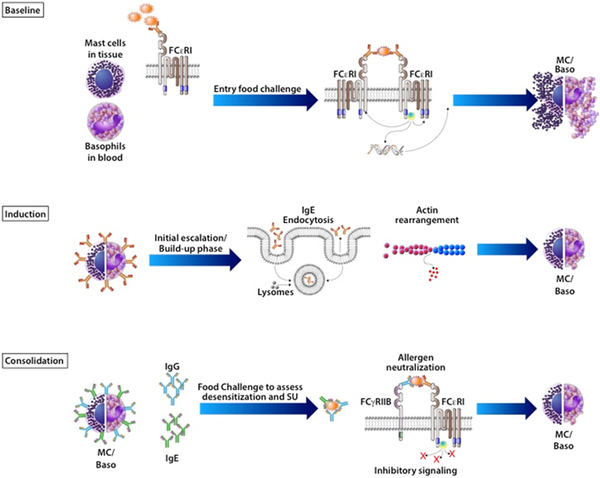
Figure 1: Modulation of mast cells and basophils during OIT.
At baseline, allergic individuals’ mast cells and basophils are decorated with allergen-specific IgE bound to cell surface FcεRI receptors. Upon antigen exposure (e.g. entry food challenge or accidental exposure), IgE molecules are cross-linked leading to degranulation and subsequent manifestation of allergic symptoms. During the initiation phase of OIT, repeated exposures to low dose antigen leads to direct effects on mast cells and basophils, including IgE endocytosis and actin rearrangement, rendering these effector cells hyporesponsive to allergen. As OIT continues and higher doses of antigen are administered, the production of allergen-specific IgG in the consolidation phase plays an important role and can lead to further, potentially long-lived inhibitory mechanisms, seen clinically as sustained unresponsiveness. In particular, circulating allergen-specific IgG can neutralize allergen such that IgE are not crosslinked on effector cells, whereas IgG bound to cell surface FcɣRIIb can induce inhibitory signaling with IgE and IgG crosslinking, thus preventing degranulation.
More detailed mechanistic studies of effector cells have tended to focus on basophils, which are easier targets given their circulation in peripheral blood, compared to tissue-resident, long-lived mast cells. It should be noted that there are key differences between basophils and mast cells and their functional equivalence should not be assumed. Interestingly, OIT appears to inhibit the entire IgE-signaling pathway in basophil activation assays as polyclonal anti-IgE and egg allergen responses on basophils are decreased with peanut OIT, pointing to a peanut-nonspecific mechanism (14). Given the technical difficulty in studying cellular mechanisms and inaccessibility of mucosal tissues in humans undergoing OIT, findings from orally-induced desensitization mouse models may provide important further mechanistic insights; however, there is a relative scarcity of literature from OIT models. Mouse models of rapid desensitization, along with supporting cellular studies, indicate that short-term desensitization is induced by inhibiting calcium flux and remodeling of actin through repeated stimulation of sIgE on mast cells (15), while another report demonstrates that endocytosis of surface-bound IgE is critical for mast cell desensitization (16) (Figure 1). A model of oral desensitization in egg-allergic mice demonstrated that allergic mice can be rendered non-reactive to oral egg challenge (17). However, these mice reacted when given an intraperitoneal injection of egg antigen, indicating that effector cells could still respond vigorously to antigen in the bloodstream. This study hints at the role of local effects, presumably on mast cells in the GI tract, that prevent allergic reactions on oral challenge, and emphasize the temporal changes that occur during OIT. Within weeks of starting dosing, we hypothesize that the effector cell suppression is likely to be predominantly mediated by the intrinsic responses of those effector cells to repeated low-level allergen exposure, consistent with in vitro studies of desensitization (15, 16). Early antibody responses, which are just beginning to change at this time, may also contribute. Peripheral allergen-specific antibodies and B cells also emerge within weeks of beginning OIT (18), likely interacting with T cells, and these concerted regulatory actions ultimately lead to further changes in antibody repertoire that interact with and can suppress basophil responses through multiple pathways (19, 20) late in the initiation phase and into consolidation, to be discussed in greater detail below.
T cells
In allergic individuals, T cell activation drives the main effector phases of allergy, including eosinophil activation and B cell production of allergen-specific IgE. This takes place primarily through a TH2-biased response pathway initiated by epithelial-derived soluble mediators, such as thymic stromal lymphopoietin (TSLP), IL-25 and IL-33 (21). Conversely, regulatory T cells (Tregs) and/or CD4+ T cells able to produce the anti-inflammatory interleukin-10 (IL-10) are generally considered to be significant contributors to the induction and maintenance of peripheral tolerance to allergen; regulatory B cells may also contribute IL-10 (22, 23). With antigen-specific TH2 cells at the core of the allergic process in atopic individuals, changes in the magnitude and polarization of allergen-specific CD4+ T cells are likely to be a key component to the effectiveness of OIT, driven by the duration and dose of antigen exposure.
Consistent with increased production of related specific-IgE commonly observed during the initiation phase of OIT (5, 7, 10, 11, 24), the first low-dose exposures to allergen may not only reinforce the pathogenic TH2 cell effector responses but may also create an inhibitory milieu that hampers establishment of Tregs (Figure 3 – Wambre). Data from various models inform these concepts. For instance, IL-4 production has been shown to cause TH2 functional activities to become resistant to Treg-mediated suppression and to antagonize the post-thymic development of Foxp3(+) Treg cells (25–27). Subsequent increasing doses of allergen exposure during escalation are associated with a decrease in TH2 cell activity and in clonal expansion (28) and with increased frequency of IL-10 producing CD4+ T cells (29). This in turn leads to the production of allergen-specific IgG4 antibody that could attenuate IgE-mediated allergic symptoms (30) and may create a milieu that suppresses de novo generation of pathogenic TH2 cells. However, at this stage, a high frequency of allergen-specific CD4+ T cells is still present (Figure 3 – Wambre). Therefore, this could explain why the clinical benefit of OIT may be lost or significantly decreased when dosing is interrupted or discontinued at this point. One possible mechanism to explain and integrate all these results into a cohesive schema is that chronic stimulation of allergen-specific TH2 cells, during the initial initiation phase of OIT, may culminate in a counter-regulatory immune response which consists of pathogenic TH2 cells driven to an anergic, regulatory-like phenotype transiently preventing allergic symptoms through the production of suppressive factor(s) like IL-10. However, solid evidence for induction of allergen-specific Tregs in humans mediating T-cell tolerance through IL-10 or other means during current OIT protocols remains elusive. Though the suppression of TH2 cytokine production has been observed in OIT subjects, multiple groups have examined Foxp3(+) Tregs, with inconsistent results. During this phase, if treatment is not continued long enough, the initial pathogenic properties of allergen-specific TH2 cells may gradually recover, consistent with transient clinical benefits. This idea is supported by work demonstrating that during chronic inflammation, IL-10-producing TH2 cells (which fulfill the criteria of inducible Tregs), can arise directly from non-suppressive TH2 cells once a specific threshold of activation is achieved (31). In support of the presence of anergic T cells, a recent study showed that allergen-specific CD4+ T cells expand during OIT and shift towards an anergic TH2 cell phenotype (28).
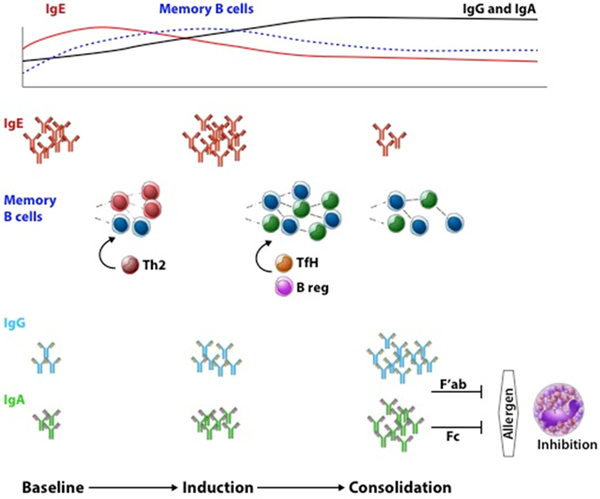
Figure 3. Humoral mechanisms of OIT.
The diverse pool of allergen-specific IgE antibodies are a marker of food allergies in affected individuals at baseline. On antigenic re-exposure in the form of OIT, allergen-specific memory B cells are reactivated to undergo somatic hypermutation and affinity maturation. During the induction phase, these memory cell responses contribute to plasma cells that will promote the rise in functional allergen-specific IgG and IgA responses. On the other hand, pathogenic Th2 cells, on re-activation by these low allergen exposures, may in part drive allergen-specific IgG memory B cells to IgE producing cells, hence transiently increasing allergen-specific IgE. During the consolidation phase, TfH and regulatory B cell compartments may drive memory B cell responses. In turn, the continued rise in titers of diversified, affinity matured allergen-specific IgG and IgA result in persistent suppression of allergic effector cells and lasting efficacy of OIT.
Antigen specific B cells and their antibodies: Bridging the Initiation and Consolidation Phases
High affinity specific antibody is a hallmark of an adaptive immune response, and is a characteristic of IgE-mediated hypersensitivity. IgE-mediated food allergy is driven by allergen-specific IgE antibodies; and the association between food challenge outcomes, and circulating levels of allergen-specific IgE, as well as specific-to-total IgE ratios is well known (32). Qualitative aspects of allergen-specific IgE, such as clonality, epitope specificities, and post-translational modifications, may play a decisive role in the allergic immune response. Linear epitope analysis of allergen-specific IgE in allergic patient sera has revealed not only variability in the number of bound epitopes, but also a positive association with reaction severity during food challenge outcomes (33, 34). Our understanding of how the level of allergen-specific IgE, the ratio of specific-to-total IgE, the clonality and the affinity of specific IgE, can impact effector cell degranulation (35), has been further expanded by data from in vitro model systems using basophils sensitized with recombinant allergen-specific IgE antibodies.
The study of antigen-specific B cells has provided new insights into how the clonal contribution of these cells may be important in the humoral response to peanut OIT. An early, transient population of rare circulating antigen-specific memory and plasmablast B cells can be identified early in the initiation phase of peanut OIT, by using an Ara h 2 fluorescent multimer (18). New techniques to isolate and clone recombinant allergen-specific antibodies induced during peanut OIT have proven highly informative. The majority of the allergen specific antibodies from OIT subjects bind to conformational epitopes (36); this observation is also supported by phage display analysis of sera (37). Interestingly, even though antibody repertoires are considered to be highly individual, selection of homologous Ara h 2 specific antibody clones in the repertoires of multiple patients has been observed during OIT. It remains to be proven whether these homologous clones recognize the same epitope, as would be expected, or have unique functional significance such as in suppressing IgE-dependent reactivity (18).
The rise in the frequency of memory B cells and plasmablasts during the initiation phase of peanut OIT coincides with the increase in Ara h 2-specific IgG4 antibodies, as well as total Ara h 2-specific IgG and IgA, suggesting that these cells may have a clonal contribution to the functionally suppressive antibodies post-peanut OIT (Figure 2). This suggestion is further supported by the induction of new Ara h 2-specific IgG4 recognizing linear epitopes post-peanut OIT (38) as well as the observation of increased somatic hypermutation in a clonal lineage of IgG4 (36). These changes, in the context of effector cell suppression by allergen-specific IgG4 (19), suggest that the reactivation of memory response and development of new allergen-specific antibodies may contribute to post-OIT sustained unresponsiveness. However, the relevance of the newly emergent clones, and even their isotype, to clinical outcomes in peanut OIT remains the subject of investigation.
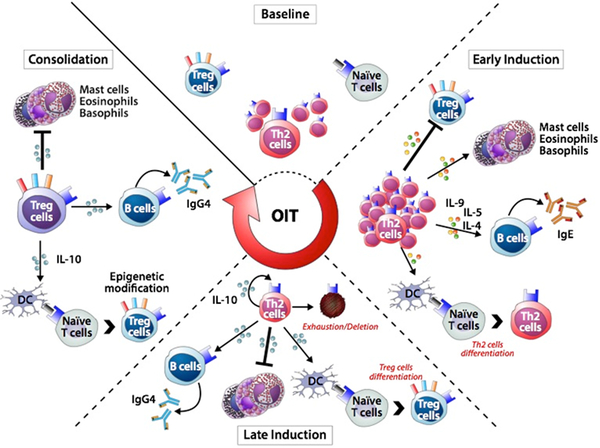
Figure 2. Sequential immune mechanisms of OIT.
At baseline, pro-allergic TH2 (TH2A) cells are at the core of the allergic process in food-allergic individuals. During the early initiation phase of OIT, the first low-dose exposures to food allergen reinforce the pathogenic effector responses, increasing pro-inflammatory cells and B cells pathogenic activities, while creating an inhibitory milieu that hampers early establishment of Treg cells. Subsequent chronic stimulation of allergen-specific TH2 cells with increasing doses of OIT rapidly culminate in a counter-regulatory immune response to prevent excessive effector responses. These in turn drive a desensitization state via decrease in TH2A cell activity, IL-10 production and change in the IgE/IgG4 ratio. At this point, the clinical benefit of OIT may be significantly decreased when dosing is interrupted or discontinued. Consolidation phase of OIT arises once a specific threshold of activation is achieved and triggers selective T cells exhaustion/deletion skewing effector responses away from the pro-allergic TH2 response. Prolonged continuous antigenic stimulation during maintenance OIT may also have other direct consequences associated with sustained unresponsiveness, enhancing epigenetic modifications at the Foxp3 locus during Treg differentiation mechanisms.
Consolidation Phase
Changes in antibody and effector cell responses during the consolidation phase of OIT are likely associated with significant and stable changes at the T cell level. This might be due in part to selective exhaustion/deletion of allergen-specific TH2 cells induced by persistent higher dose allergen exposure, allowing concurrent regulatory immune responses to emerge slowly during the consolidation/maintenance phase of OIT (Figure 3 – Wambre). Allergen-specific TH2 cells have recently been shown to represent a phenotypically distinct TH2 subpopulation confined to atopic individuals, and they display greater adverse activity relative to conventional TH2 cells (39). This pro-allergic TH2 subset, denoted as the TH2A cell subset, is characterized by stable co-expression of CRTH2, CD161 and IL-33R and low expression of CD45RB, CD27 and Bcl-2, consistent with cells that are highly sensitive to activation-induced cell death (40). Furthermore, ex vivo analysis of the peanut specific TH2A responses in the peripheral blood of patients during the course of OIT demonstrated that elimination of allergen-reactive TH2 cells from the periphery was associated with clinical benefit. This is consistent with the notion that skewing allergen-specific effector T cells away from the pro-allergic TH2 response could facilitate other protective changes and may be a causative mechanism for the clinical benefits during OIT.
Regarding antibodies, it is now well established that allergen-specific IgG, and particularly IgG4, levels are increased within a few months after starting OIT, often increased greater than 10-fold from baseline, and these remain elevated even after many years of OIT (4–6, 9, 11). The induction of peanut-specific IgG during OIT has been linked to suppression of allergic effector cells by two mechanisms, suggesting a gradual temporal convergence in suppressive mechanisms involving the humoral and effector cell responses (Figure 1). The first postulated mechanism is that allergen-specific IgG may block allergen-IgE interactions, thus sequestering the allergen (41). Functional blocking antibodies correlate with clinical outcomes in subcutaneous immunotherapy (41). Not only do peanut-specific IgG4 antibodies rise during the course of peanut OIT, but IgG4 from post-peanut OIT sera can suppress peanut-stimulated basophil and mast cell activation (19). The second hypothesis highlights the Fc portion of IgG. Human basophil suppression by post-OIT IgG has been shown to be mediated by interactions through the inhibitory receptor FcγRIIb, as shown in murine models of food allergy (20, 42). Blocking FcɣRIIb with a monoclonal antibody prevented inhibition of basophil degranulation, indicating that specific IgG binds this inhibitory receptor and prevents antigen-driven activation by inhibitory signaling. The interactions of antibodies with inhibitory Fc receptors may be influenced by antibody Fc subtypes (e.g., sIgG1, sIgG2, and sIgG3 are all increased during peanut OIT (20)), as well as by post-translational modifications, such as glycosylation. The increase in allergen-specific IgG4 may be related to IL-10 production from T regulatory cells or B regulatory cells, as has been shown in other forms of immunotherapy (30, 43).
More recently, serum Ara h 2-specific IgG and IgA have also been shown to increase in peanut OIT (18). These antibodies may play a blocking role (44) or may have a deeper role in disease pathogenesis and treatment. Alternatively, the rise of specific IgA during OIT may point to a mucosal origin of allergen-specific B cells which may ultimately shape the allergen-specific B cell repertoire (45). For example, significant increases in IgA and IgA2 were found in egg OIT, and these may contribute to effector cell suppression (46).
Sustained Unresponsiveness
Sustained unresponsiveness (SU) is a relatively new and loosely defined term referring to the durability of the clinical effect following cessation of the dosing protocol. The term SU was coined to differentiate this post-immunotherapy outcome from true immunological tolerance, which is regarded as the default state in healthy individuals, and can be naturally re-established when food allergies spontaneously resolve (e.g., “outgrowing” egg or milk allergy). Nonetheless, while SU probably differs from true tolerance, it is a significant clinical achievement, allowing more flexible consumption of the previously allergenic food in its natural forms. There are two main explanations for SU: simple desensitization occurring after extended maintenance treatment, such that the elimination kinetics of the effect are prolonged, or an intermediate phase change that is neither simple desensitization nor full tolerance.
Our understanding of SU in OIT, and its association with the humoral response, has been recently significantly strengthened through the development of novel tools and methodologies. There may be a pre-existing bias, within the adaptive response of those who do not develop SU, for the propagation of allergen-specific IgE. For example, SU has been associated with lower quantities of pre-treatment peanut- and milk-specific IgE (9, 10, 47), while the importance of diversity and clonality in persistent and severe food allergy (33, 34) suggests that qualitative differences may also exist. Whether this is due to IgE-switched memory B cells or another compartment such as IgG-switched memory B cells, TH2, or T follicular helper cells, is still unknown. The induction of antibodies directed against new linear epitopes and an oligoclonal allergen-specific memory B cells with somatically hypermutated antibodies suggests that OIT modulates the B cell repertoire (Figure 2). While we can speculate that these post-OIT allergen-specific antibodies in SU may effectively suppress allergen effector cells by antigen sequestration (19) or by engagement of inhibitory Fc receptors (20), SU may be more related to the longevity of the induced B cell memory response or to novel immunomodulatory functions of allergen-specific antibodies.
As previously discussed, this effector cell suppression occurs rapidly with the continuous administration of antigen, but SPTs rarely become negative and some activation is seen in basophil assays even after many months or years of therapy. This implies that once OIT is stopped the cells may become increasingly responsive to antigen. Indeed, clinical desensitization resulting from OIT can be short-lived, with a large percentage of subjects regaining allergic reactivity within 2 weeks after stopping OIT (4, 7, 9, 24), and in some cases as soon as 1 week after stopping OIT (6). In these cases, it appears that the suppressive effects on mast cells and basophils are transient and these effector cells will become reactive once antigen administration is stopped. Studies have demonstrated a return in SPT and basophil responses in subjects who failed an oral food challenge (OFC) several weeks after stopping OIT. However, the opposite was seen in subjects achieving SU, where SPT and basophil activation remained suppressed (9, 48). Importantly, a study of egg OIT demonstrated that longer treatment regimens led to a higher proportion of subjects developing sustained unresponsiveness (49), possibly due to further reduction in sIgE, or to increasing sIgG4 or sIgA, or more permanent changes in mast cell signaling pathways.
Prolonged continuous antigenic stimulation during maintenance treatment may also have other direct consequences on CD4+ T cells, enhancing epigenetic mechanisms that have been associated with SU (8). The “disease induction model” (50), proposes that the presence of a pathogenic CD4+ T cell subset with distinct phenotypic and functional properties might be sufficient for the pathogenesis of an immune-mediated disease regardless of the balance of other T helper subsets. Similarly, it is possible that current OIT protocols may target allergen-specific TH2A cells in a stepwise way, including T-cell exhaustion followed by T-cell deletion, to restore a hypo-responsive state to allergen. This is consistent with previous studies suggesting that allergen-specific T cells may represent a suitable therapeutic target during OIT.
Conclusions / Future Directions
There has been significant progress in understanding how OIT suppresses mast cell and basophil reactivity, while newer methodologic approaches are beginning to uncover the roles of T and B cells in OIT-induced immunomodulation. However, several key knowledge gaps remain. We need to understand specifically how the effector cells are desensitized at the molecular level, as this could lead to targeted therapies for food allergies. We need to know whether the basophil activation assay can be used as a biomarker to reliably determine a state of allergy before treatment, and then to monitor desensitization and/or SU as outcomes of treatment. It was recently demonstrated that basophil activation assays can predict allergy vs sensitization and can eliminate the need for OFCs in some individuals (51). In addition, it is of paramount importance to have a better understanding of the cellular changes that associate with different clinical outcomes during or after OIT; e.g., it is not known why some subjects achieve partial or full desensitization whereas others achieve SU. For example, is there a change in the signaling pathways through the FcεRI that re-emerges on cessation, or does sIgE and/or FcεRI-density increase, leading some subjects to become reactive again? SU likely requires concerted coordination of the adaptive response to delete pathogenic TH2A cells and induce protective and functionally suppressive allergen-specific clonal memory B cell responses to suppress effector cell responses for long-lasting clinical efficacy of OIT, but the relative importance of these mechanisms and their kinetics need further study. It will require the endophenotyping of larger numbers of subjects to do this. Ultimately, this work should lead to the development of a reliable biomarker assay, or group of assays, for diagnosis and/or treatment response monitoring, which will facilitate widespread OIT implementation.
For many years, mechanistic studies investigating the effect of OIT on B cells and CD4+ T cells have been hampered by the absence of adequately sensitive approaches that directly assess immunological changes within these rare allergen-specific cell populations. Hence the need for a comprehensive understanding of the targeted CD4+ T-cell population, which is critical to designing more effective immunotherapy. However, new technologies like polychromatic flow cytometry, mass cytometry, and transcriptional profiling have been applied to the study of food allergy patients and are now making it possible to characterize these and other cells with unprecedented resolution (52–54). As with other routes of allergen-specific immunotherapy, OIT may alter the T cell responses via multiple parallel or overlapping mechanisms, including exhaustion/deletion of pro-allergic T cell responses (immune disease induction model), the switch in T cell effector immune responses (immune deviation model), or the induction of concurrent immune-regulating T cells (immune regulation model). More research is needed to develop a more unified understanding of which of these T cell mechanisms – or others yet undiscovered – are operative in the short- and long-term outcomes during OIT. Data from such studies should inform rational strategies to enhance OIT by combining it with immune modulating strategies (e.g. monoclonal antibodies) that can either induce a counter-regulatory immune response or block de novo generation of pro-allergic TH2 cells, leading to improved safety and durable clinical benefit. On the B cell side, emerging data suggest that we may be approaching a new era of antibody-directed enhancement for OIT, through modulating the antibodies produced during therapy to induce long-lasting clinical tolerance. The next generation of antibody-directed vaccine efforts may involve careful shaping of the antibody repertoire, either using antigen-specific B cell modulation (55) or sequential vaccination strategies, such as used in HIV vaccine trials to drive the generation of protective antibodies (56).
Finally, local immune mechanisms in the gut associated with OIT remain to be further investigated, most particularly with respect to the factors involved in antigen uptake and response at the site of administration, such as epithelial cells, innate lymphoid cells, and local microbial factors. Emerging evidence suggests that dendritic cells may play a role in OIT outcomes (8, 57), and this is a key area requiring more investigation. We look forward to future studies that will fill in these and other knowledge gaps and lead us to a better a unified understanding of the complex interplay of molecular, cellular, and humoral changes that occurs during and after OIT.
Acknowledgements
We are indebted to Wesley Burks, William Kwok, and Wayne Shreffler for their careful review of the manuscript and helpful suggestions.
Funding: This work did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors.
Abbreviations:
- CD
cluster of differentiation
- CRTH2
chemoattractant receptor-homologous molecule expressed on TH2 cells
- DLT
dose-limiting toxicity
- Ig
immunoglobulin
- IL
interleukin
- OFC
oral food challenge
- OIT
oral immunotherapy
- SPT
skin prick test
- SU
sustained unresponsiveness
- TH2
T helper 2
- TSLP
thymic stromal lymphopoetin
References
- 1.Wood RA. Food allergen immunotherapy: Current status and prospects for the future. J Allergy Clin Immunol. 2016;137(4):973–82. [DOI] [PubMed] [Google Scholar]
- 2.Vazquez-Ortiz M, Turner PJ. Improving the safety of oral immunotherapy for food allergy. Pediatr Allergy Immunol. 2016;27(2):117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virkud YV, Burks AW, Steele PH, Edwards LJ, Berglund JP, Jones SM, et al. Novel baseline predictors of adverse events during oral immunotherapy in children with peanut allergy. J Allergy Clin Immunol. 2017;139(3):882–8 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367(3):233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124(2):292–300, e1–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012;129(2):448–55 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narisety SD, Frischmeyer-Guerrerio PA, Keet CA, Gorelik M, Schroeder J, Hamilton RG, et al. A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. The Journal of allergy and clinical immunology. 2015;135(5):1275–82 e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J Allergy Clin Immunol. 2014;133(2):500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014;133(2):468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vickery BP, Berglund JP, Burk CM, Fine JP, Kim EH, Kim JI, et al. Early oral immunotherapy in peanut-allergic preschool children is safe and highly effective. J Allergy Clin Immunol. 2017;139(1):173–81 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127(3):654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacGinnitie AJ, Rachid R, Gragg H, Little SV, Lakin P, Cianferoni A, et al. Omalizumab facilitates rapid oral desensitization for peanut allergy. J Allergy Clin Immunol. 2017;139(3):873–81 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nadeau KC, Schneider LC, Hoyte L, Borras I, Umetsu DT. Rapid oral desensitization in combination with omalizumab therapy in patients with cow’s milk allergy. J Allergy Clin Immunol. 2011;127(6):1622–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thyagarajan A, Jones SM, Calatroni A, Pons L, Kulis M, Woo CS, et al. Evidence of pathway-specific basophil anergy induced by peanut oral immunotherapy in peanut-allergic children. Clin Exp Allergy. 2012;42(8):1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ang WX, Church AM, Kulis M, Choi HW, Burks AW, Abraham SN. Mast cell desensitization inhibits calcium flux and aberrantly remodels actin. J Clin Invest. 2016;126(11):4103–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oka T, Rios EJ, Tsai M, Kalesnikoff J, Galli SJ. Rapid desensitization induces internalization of antigen-specific IgE on mouse mast cells. J Allergy Clin Immunol. 2013;132(4):922–32 e1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonard SA, Martos G, Wang W, Nowak-Wegrzyn A, Berin MC. Oral immunotherapy induces local protective mechanisms in the gastrointestinal mucosa. J Allergy Clin Immunol. 2012;129(6):1579–87 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patil SU, Ogunniyi AO, Calatroni A, Tadigotla VR, Ruiter B, Ma A, et al. Peanut oral immunotherapy transiently expands circulating Ara h 2-specific B cells with a homologous repertoire in unrelated subjects. J Allergy Clin Immunol. 2015;136(1):125–34.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos AF, James LK, Bahnson HT, Shamji MH, Couto-Francisco NC, Islam S, et al. IgG4 inhibits peanut-induced basophil and mast cell activation in peanut-tolerant children sensitized to peanut major allergens. J Allergy Clin Immunol. 2015;135(5):1249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burton OT, Logsdon SL, Zhou JS, Medina-Tamayo J, Abdel-Gadir A, Noval Rivas M, et al. Oral immunotherapy induces IgG antibodies that act through FcgammaRIIb to suppress IgE-mediated hypersensitivity. J Allergy Clin Immunol. 2014;134(6):1310–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han H, Roan F, Ziegler SF. The atopic march: current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunol Rev. 2017;278(1):116–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. 2016;16(12):751–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van de Veen W, Stanic B, Wirz OF, Jansen K, Globinska A, Akdis M. Role of regulatory B cells in immune tolerance to allergens and beyond. J Allergy Clin Immunol. 2016;138(3):654–65. [DOI] [PubMed] [Google Scholar]
- 24.Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, de Oliveira LC, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010;126(1):83–91 e1. [DOI] [PubMed] [Google Scholar]
- 25.Noval Rivas M, Burton OT, Oettgen HC, Chatila T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J Allergy Clin Immunol. 2016;138(3):801–11 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantel PY, Kuipers H, Boyman O, Rhyner C, Ouaked N, Ruckert B, et al. GATA3-driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol. 2007;5(12):e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hadjur S, Bruno L, Hertweck A, Cobb BS, Taylor B, Fisher AG, et al. IL4 blockade of inducible regulatory T cell differentiation: the role of Th2 cells, Gata3 and PU.1. Immunol Lett. 2009;122(1):37–43. [DOI] [PubMed] [Google Scholar]
- 28.Ryan JF, Hovde R, Glanville J, Lyu SC, Ji X, Gupta S, et al. Successful immunotherapy induces previously unidentified allergen-specific CD4+ T-cell subsets. Proc Natl Acad Sci U S A. 2016;113(9):E1286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mobs C, Ipsen H, Mayer L, Slotosch C, Petersen A, Wurtzen PA, et al. Birch pollen immunotherapy results in long-term loss of Bet v 1-specific TH2 responses, transient TR1 activation, and synthesis of IgE-blocking antibodies. J Allergy Clin Immunol. 2012;130(5):1108–16 e6. [DOI] [PubMed] [Google Scholar]
- 30.van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Sollner S, Akdis DG, et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. The Journal of allergy and clinical immunology. 2013;131(4):1204–12. [DOI] [PubMed] [Google Scholar]
- 31.Altin JA, Goodnow CC, Cook MC. IL-10+ CTLA-4+ Th2 inhibitory cells form in a Foxp3-independent, IL-2-dependent manner from Th2 effectors during chronic inflammation. J Immunol. 2012;188(11):5478–88. [DOI] [PubMed] [Google Scholar]
- 32.Ballmer-Weber BK, Lidholm J, Fernandez-Rivas M, Seneviratne S, Hanschmann KM, Vogel L, et al. IgE recognition patterns in peanut allergy are age dependent: perspectives of the EuroPrevall study. Allergy. 2015;70(4):391–407. [DOI] [PubMed] [Google Scholar]
- 33.Flinterman AE, Knol EF, Lencer DA, Bardina L, den Hartog Jager CF, Lin J, et al. Peanut epitopes for IgE and IgG4 in peanut-sensitized children in relation to severity of peanut allergy. J Allergy Clin Immunol. 2008;121(3):737–43 e10. [DOI] [PubMed] [Google Scholar]
- 34.Shreffler WG, Lencer DA, Bardina L, Sampson HA. IgE and IgG4 epitope mapping by microarray immunoassay reveals the diversity of immune response to the peanut allergen, Ara h 2. The Journal of allergy and clinical immunology. 2005;116(4):893–9. [DOI] [PubMed] [Google Scholar]
- 35.Christensen LH, Holm J, Lund G, Riise E, Lund K. Several distinct properties of the IgE repertoire determine effector cell degranulation in response to allergen challenge. J Allergy Clin Immunol. 2008;122(2):298–304. [DOI] [PubMed] [Google Scholar]
- 36.Hoh RA, Joshi SA, Liu Y, Wang C, Roskin KM, Lee JY, et al. Single B-cell deconvolution of peanut-specific antibody responses in allergic patients. The Journal of allergy and clinical immunology. 2016;137(1):157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X, Negi SS, Liao S, Gao V, Braun W, Dreskin SC. Conformational IgE epitopes of peanut allergens Ara h 2 and Ara h 6. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2016;46(8):1120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vickery BP, Lin J, Kulis M, Fu Z, Steele PH, Jones SM, et al. Peanut oral immunotherapy modifies IgE and IgG4 responses to major peanut allergens. The Journal of allergy and clinical immunology. 2013;131(1):128–34 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wambre E, Bajzik V, DeLong JH, O’Brien K, Nguyen Q-A, Speake C, et al. A phenotypically and functionally distinct human TH2 cell subpopulation is associated with allergic disorders. Science Translational Medicine. 2017;9(401). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wambre E, DeLong JH, James EA, LaFond RE, Robinson D, Kwok WW. Differentiation stage determines pathologic and protective allergen-specific CD4+ T-cell outcomes during specific immunotherapy. J Allergy Clin Immunol. 2012;129(2):544–51, 51 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shamji MH, Ljorring C, Francis JN, Calderon MA, Larche M, Kimber I, et al. Functional rather than immunoreactive levels of IgG4 correlate closely with clinical response to grass pollen immunotherapy. Allergy. 2012;67(2):217–26. [DOI] [PubMed] [Google Scholar]
- 42.Burton OT, Tamayo JM, Stranks AJ, Koleoglou KJ, Oettgen HC. Allergen-specific IgG antibody signaling through FcgammaRIIb promotes food tolerance. 2017(1097–6825 (Electronic)). [DOI] [PMC free article] [PubMed]
- 43.Piconi S, Trabattoni D, Rainone V, Borgonovo L, Passerini S, Rizzardini G, et al. Immunological effects of sublingual immunotherapy: clinical efficacy is associated with modulation of programmed cell death ligand 1, IL-10, and IgG4. Journal of immunology. 2010;185(12):7723–30. [DOI] [PubMed] [Google Scholar]
- 44.Dodev TS, Bowen H, Shamji MH, Bax HJ, Beavil AJ, McDonnell JM, et al. Inhibition of allergen-dependent IgE activity by antibodies of the same specificity but different class. Allergy. 2015;70(6):720–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lemke A, Kraft M, Roth K, Riedel R, Lammerding D, Hauser AE. Long-lived plasma cells are generated in mucosal immune responses and contribute to the bone marrow plasma cell pool in mice. Mucosal immunology. 2016;9(1):83–97. [DOI] [PubMed] [Google Scholar]
- 46.Wright BL, Kulis M, Orgel KA, Burks AW, Dawson P, Henning AK, et al. Component-resolved analysis of IgA, IgE, and IgG4 during egg OIT identifies markers associated with sustained unresponsiveness. Allergy. 2016;71(11):1552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keet CA, Seopaul S, Knorr S, Narisety S, Skripak J, Wood RA. Long-term follow-up of oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gorelik M, Narisety SD, Guerrerio AL, Chichester KL, Keet CA, Bieneman AP, et al. Suppression of the immunologic response to peanut during immunotherapy is often transient. J Allergy Clin Immunol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones SM, Burks AW, Keet C, Vickery BP, Scurlock AM, Wood RA, et al. Long-term treatment with egg oral immunotherapy enhances sustained unresponsiveness that persists after cessation of therapy. J Allergy Clin Immunol. 2016;137(4):1117–27 e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakayama T, Hirahara K, Onodera A, Endo Y, Hosokawa H, Shinoda K, et al. Th2 Cells in Health and Disease. Annu Rev Immunol. 2017;35:53–84. [DOI] [PubMed] [Google Scholar]
- 51.Santos AF, Douiri A, Becares N, Wu SY, Stephens A, Radulovic S, et al. Basophil activation test discriminates between allergy and tolerance in peanut-sensitized children. J Allergy Clin Immunol. 2014;134(3):645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rust B, Wambre E. Human Immune Monitoring Techniques during Food Allergen Immunotherapy. Curr Allergy Asthma Rep. 2017;17(4):22. [DOI] [PubMed] [Google Scholar]
- 53.Kosoy R, Agashe C, Grishin A, Leung DY, Wood RA, Sicherer SH, et al. Transcriptional Profiling of Egg Allergy and Relationship to Disease Phenotype. PLoS One. 2016;11(10):e0163831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tordesillas L, Rahman AH, Hartmann BM, Sampson HA, Berin MC. Mass cytometry profiling the response of basophils and the complete peripheral blood compartment to peanut. J Allergy Clin Immunol. 2016;138(6):1741–4 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orgel KA, Duan S, Wright BL, Maleki SJ, Wolf JC, Vickery BP, et al. Exploiting CD22 on antigen-specific B cells to prevent allergy to the major peanut allergen Ara h 2. The Journal of allergy and clinical immunology. 2017;139(1):366–9 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kwong PD, Mascola JR, Nabel GJ. Broadly neutralizing antibodies and the search for an HIV-1 vaccine: the end of the beginning. Nature reviews Immunology. 2013;13(9):693–701. [DOI] [PubMed] [Google Scholar]
- 57.Frischmeyer-Guerrerio PA, Keet CA, Guerrerio AL, Chichester KL, Bieneman AP, Hamilton RG, et al. Modulation of dendritic cell innate and adaptive immune functions by oral and sublingual immunotherapy. Clin Immunol. 2014;155(1):47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]