Abstract
Objective:
To determine the effects of omitting meal time insulin on arterial stiffness in children with type 1 diabetes.
Research design and methods:
In this prospective, randomized, crossover study, radial artery tonometry and augmentation index adjusted to heart rate 75 (AI75) were used to measure arterial stiffness. Children were randomized to receive or omit premeal insulin and completed the crossover portion of the study on a subsequent day. AI75 was determined when fasting, 1, and 2 h postmeal.
Results:
When comparing change in AI75 from baseline to 1 h and baseline to 2 h, when subjects received premeal insulin vs. no insulin, AI75 was 4.5 units lower at 1 h (p = 0.011, 95% CI:1.1 lower to 8 lower) and 4.3 units lower at 2 h (p = 0.062, 95% CI: 0.2 higher to 8.9 lower) (n = 40).
Conclusions:
Arterial stiffness is decreased by premeal insulin in children with type 1 diabetes.
Keywords: arterial stiffness, bolus insulin, CVD risk, meal insulin
Children with type 1 diabetes have increased arterial stiffness when compared with children without diabetes (1–5). In adolescents without diabetic complications, increased arterial stiffness and endothelial dysfunction are associated with increased right and left ventricular diastolic dysfunction (6). Notably, the SEARCH study showed that arterial stiffness was associated with type 1 diabetes, male sex, and mean arterial pressure but not glycated hemoglobin (HbA1c) (5). Similarly, our own studies of arterial stiffness have failed to demonstrate correlations between HbA1c and arterial stiffness (1). While the modern era of intensive glycemic control has brought about reductions in microvascular complications, reductions in macrovascular disease remain elusive (7).
Macrovascular disease in type 1 diabetes is probably mediated by both the independent and synergistic effects of insulin deficiency and hyperglycemia on vascular function. Vascular tone is maintained by a delicate balance between vasodilation and vasoconstriction. Insulin promotes vasodilation by directly stimulating the endothelial nitric oxide (NO) synthase pathway on endothelial cells (8). In addition, acute hyperglycemia reduces NO availability through the formation of advanced glycation end-products (8). As such, the combination of acute hyperglycemia and insulin deficiency frequently experienced by adolescents (and adults) who forget to provide adequate meal bolus insulin may, in part, explain the increased rate of macrovascular events noted in the type 1 diabetes population.
Despite the known effects of food intake and insulin exposure on endothelial function in adults, nearly all previous studies of vascular function in children have been performed in the fasting state. As such, little is known regarding arterial stiffness in children with type 1 diabetes and the acute effects of meal consumption, insulin administration and postmeal hyperglycemia. Given the lack of association with arterial stiffness and HbA1c, we hypothesized that the omission of meal time insulin, a common practice in children with type 1 diabetes, would result in acute increases in arterial stiffness, while provision of premeal insulin would result in acute decrements in arterial stiffness.
Research design and methods
A prospective, randomized, crossover design was used. Study subjects were recruited from the University of Florida diabetes clinic and the Florida Camp for Children and Youth with Diabetes. Subjects had type 1 diabetes for at least 1 yr, were using a basal-bolus insulin regimen, and were not taking oral hypoglycemic agents or antihypertensive drugs.
After an overnight fast, height was measured with a stadiometer (Seca 213®, Hamburg, Germany) weight was measured on an anolog scale (Tanita© HA520, Arlington Heights, IL, USA) and a digital oscillometric brachial cuff (©Panasonic EW3109, Beijing, China) was used to assess blood pressure. If fasting glucose was between 65 and 200 mg/dL subjects were randomized to receive premeal insulin (per their typical carbohydrate ratio) or no premeal insulin with completion of the opposite randomization on a subsequent day within 3 months. Radial artery tonometry was performed using the SphygmoCor VX version 7.01 (AtCor Medical, Sydney, Australia) to measure augmentation index corrected to a heart rate of 75 (AI75) as previously described (1). Both AI75 and blood glucose (One Touch Mini Meter, LifeScan© Inc.) were measured while fasting, 1 h, and 2 h after the subject consumed a meal replacement drink (Boost® High Protein, Nestlé©, 237 mL). The subject’s most recent HbA1c was recorded and each subject self-reported weekly exercise in minutes. The study was approved by the Institutional Review Board of the University of Florida.
Statistical methods
The primary comparison utilized the two sample method for crossover studies. For each order, we computed Y, half the paired differences in changes from period baseline to the end of the period, irrespective of ordering. The difference between the sample means of the Y’s for ordering AB and ordering BA is an unbiased estimate of the arithmetic means of the treatment effects in periods one and two, regardless of imbalance in randomization, period effects, or treatment by period interaction. To allow for unequal population variances, we used the Satterthwaite corrected t-test. The sample sizes are sufficient for the central limit theorem to apply, making this an assumption-free analysis. Correlations were obtained within the insulin period between the changes in AI75 at both 1 and 2 h with period baseline variables.
Results
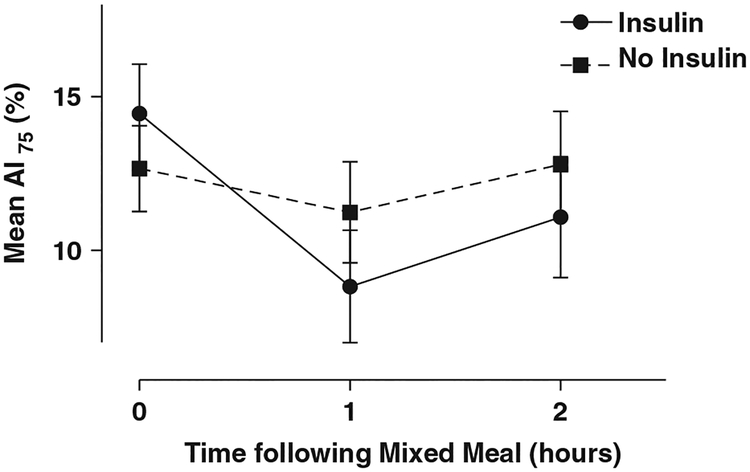
Of the 60 subjects enrolled (32 female), 40 subjects completed the crossover study. A total of 48 of the enrollees were Caucasian, 8 were Hispanic and 4 were African American. Mean age was 12.5 yr, length of diagnosis was 5.2 yr, and HbA1c was 7.9% (63 mmol/mol) (Table 1). Fasting glucose average for both periods was 138 mg/dL (7.7 mmol/L). The average postmeal glucose was lower when insulin was given [226 mg/dL (12.6 mmol/L) at 1 h and 257 mg/dL (14.3 mmol/L) at 2 h] compared with insulin omission [272 mg/dL (15.1 mmol/L) at 1 h and 330 mg/dL (18.3 mmol/L) at 2 h]. The average AI75 was lower when insulin was given compared with insulin omission (Figs 1 and 2). With the exception of arriving glucose level (where p = 0.04 (R = 0.33), only at hour 1, a weak association), none of the baseline covariates had a significant correlation with the change in AI75 in the insulin period whether at 1 or 2 h.
Table 1.
Characteristics of subjects
| N | Mean | Standard deviation | |
|---|---|---|---|
| Age (yr) | 60 | 12.5 | 2.13 |
| Duration of diabetes (yr) | 59 | 5.2 | 3.41 |
| HbA1c-% (mmol/mol) | 51 | 7.86 (63) | 0.96 |
| Baseline Al75 | 52 | 13.4 | 10.4 |
| Height - cm (percentile, z-score) | 52 | 154 (53.7, 0.62) | 9.67 |
| Weight - kg (percentile, z-score) | 52 | 49.4 (60.7, 0.51) | 12.6 |
| BMI - kg/m2 (percentile, z-score) | 52 | 20.6 (61.2, 0.51) | 3.88 |
| Systolic blood pressure (mmHg) | 52 | 114 | 9.02 |
| Diastolic blood pressure (mmHg) | 52 | 69 | 9.28 |
| Heart rate (bpm) | 51 | 86 | 10.5 |
| Weekly exercise (min) | 49 | 380 | 389 |
AI75, augmentation index corrected to a heart rate of 75; BMI, body mass index; HbA1c, glycated hemoglobin.
Fig. 1.
Change in augmentation index adjusted to heart rate 75 (AI75) with and without premeal insulin. The raw data showing mean AI75 for subjects receiving insulin or omitting insulin are shown over time following a standard mixed meal. Error bars show standard error.
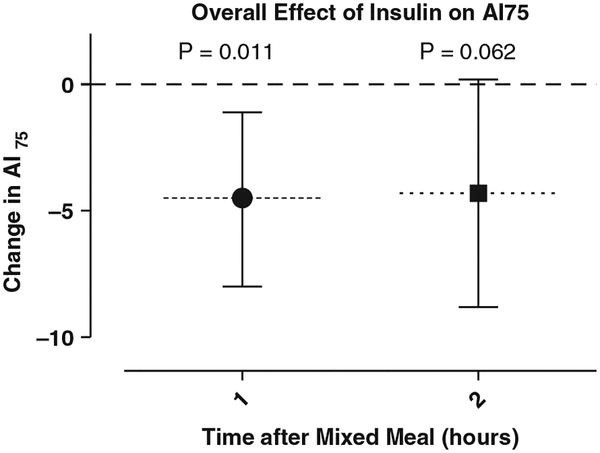
Fig. 2.
Overall effect of insulin on augmentation index adjusted to heart rate 75 (AI75). The primary statistical comparison for this study was the difference of the mean AI75 over time. When compared with insulin omission, provision of premeal insulin was associated with a significant decline in AI75 1 h postmixed meal (difference of mean AI75 of −4.5, p = 0.011). Premeal insulin continued to provide for a relative reduction on AI75 at 2 h postmixed meal (difference of mean AI75 of −4.3, p = 0.062).
When comparing the change of AI75 from baseline to 1 h, AI75 was 4.5 units lower under the effects of premeal insulin when compared with the same subjects who omitted premeal insulin [p = 0.011, 95% CI (1.1 to 8 lower)]. Similarly, the mean change of AI75 from baseline to 2 h was 4.3 units lower under the effects of premeal insulin versus no insulin [p = 0.062, 95%CI (0.2 higher to 8.9 lower)] (Table 2).
Table 2.
Difference of mean AI75 with premeal insulin versus no premeal insulin
| Time after insulin (h) | Difference of mean Al75 | 95% Cl | p-value |
|---|---|---|---|
| 1 | −4.5 | −1.1 to −7.97 | 0.011 |
| 2 | −4.3 | −8.9 to 0.2 | 0.062 |
AI75, augmentation index corrected to a heart rate of 75; CI, confidence intervals.
Conclusions
Our group has repeatedly demonstrated that children and adolescents with type 1 diabetes have arterial stiffness when compared with controls. This study expands on previous observations by demonstrating that arterial stiffness, as measured by radial tonometry, is decreased by premeal insulin in children with type 1 diabetes. Given the observation that fasting arterial stiffness is consistently higher in children with type 1 diabetes than in controls, the acute effects of bolus insulin on postmeal arterial function may inform efforts to modify macrovascular disease risk.
In our study there was no association between glucose and the change in arterial stiffness at 1 or 2 h postmeal, regardless of whether insulin was received or omitted. In addition, there were no other baseline covariates that impacted change in arterial stiffness. These observations argue against acute effects of hyperglycemia as an explanation for postmeal vascular dysfunction and further support the concept that insulin plays the more critical role in mediating postmeal vascular response.
Previous studies evaluating arterial stiffness in relation to glycemic control have shown that: (1) those with recent onset of diabetes do not have increased arterial stiffness; (2) children with type 1 diabetes for at least 1 yr have stiffer arteries compared to children without diabetes; and (3) arterial stiffness is not correlated with HbA1c (1, 5, 9). These observations are consistent with our findings that arterial stiffness may be more related to omission of meal time insulin rather than the chronic or even acute effects of hyperglycemia.
In terms of mechanistic explanations for our observations, it is well known that the binding of insulin to its receptor on the endothelium leads to the activation of the eNOS pathway and vasodilation. As such, insulin omission or deficiency should impair this physiology. Nevertheless, it remains unclear why children receiving basal insulin have fasting arterial stiffness despite normal glycemia and conversely why arterial stiffness is reduced following meal time insulin despite post meal hyperglycemia. That said, hyperglycemia increases the formation of advanced glycation end products which inhibit NO and impair endothelial function (8). In addition, oxidative stress because of chronic hyperglycemia has been implicated in endothelial dysfunction (10). We must also consider that postmeal hypertriglyceridemia and high free fatty acid levels could affect arterial stiffness. Previous studies of children with type 1 diabetes have been performed in fasting children. We have shown that children with type 1 diabetes have a less proatherogenic lipoprotein profile than controls despite increased arterial stiffness (1, 11). In addition, small studies of young healthy adult males who received a high-fat meal did show transient impairment of brachial artery vasodilation (12, 13). Thus, the lack of lipid studies obtained in the postmeal state is a limitation of our study.
Because the majority of this study was completed in a diabetes camp setting, there are additional limitations worth noting. Namely, the potential effects of improved insulin sensitivity on arterial stiffness (given the increased physical activity associated with camp), the lack of data regarding pubertal staging and family history of cardiovascular disease (14, 15). In addition, we must note the lack of data on changes in arterial stiffness following meals in children without diabetes. Despite these limitations, the data provides strong support for larger confirmatory studies designed to account for other co-variates that may or may not affect arterial function in children with and without diabetes.
In conclusion, our study documents the beneficial effects meal time insulin provides in reducing arterial stiffness in children with type 1 diabetes. Additional studies of arterial stiffness in children with type 1 diabetes are warranted. Specifically, efforts to examine the relationships between insulin dosage, timing of premeal insulin, meal content, and acute change in arterial stiffness will help to further our understanding of assessing macrovascular risk in children with type 1 diabetes.
Acknowledgements
We would like to thank the Florida Camp for Children and Youth with Diabetes (FCCYD) and the University of Florida for allowing patient recruitment and Dr. Arlan Rosenbloom, Professor Emeritus of the University of Florida, for his editorial assistance.
We would like to thank the Diabetes Action Research and Education Foundation for their support in our study. This work was also supported in part by the NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1 TR000064 and NIH T35 NRSA Short-term Research Training Grant.
Footnotes
Conflicts of interest
The authors have no conflicts of interests to disclose.
References
- 1.Haller MJ, Samyn M, Nichols WW et al. Radial artery tonometry demonstrates arterial stiffness in children with type 1 diabetes. Diabetes Care 2004: 27: 2911–7. [DOI] [PubMed] [Google Scholar]
- 2.Zineh I, Beitelshees AL, Haller MJ. NOS3 polymorphisms are associated with arterial stiffness in children with type 1 diabetes. Diabetes Care 2007: 30: 689–93. [DOI] [PubMed] [Google Scholar]
- 3.Haller MJ, Stein JM, Shuster JJ et al. Pediatric Atorvastatin in Diabetes Trial (PADIT): a pilot study to determine the effect of atorvastatin on arterial stiffness and endothelial function in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2009: 22: 65–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palombo C, Kozakova M, Morizzo C et al. Circulating endothelial progenitor cells and large artery structure and function in young subjects with uncomplicated type 1 diabetes. Cardiovasc Diabetol 2011: 10: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urbina EM, Wadwa RP, Davis C et al. Prevalence of increased arterial stiffness in children with type 1 diabetes mellitus differs by measurement site and sex: the SEARCH for Diabetes in Youth Study. J Pediatr 2010: 156: 731–737.e1. [DOI] [PubMed] [Google Scholar]
- 6.Ciftel M, Ertug H, Parlak M, Akcurin G, Kardelen F. Investigation of endothelial dysfunction and arterial stiffness in children with type 1 diabetes mellitus and the association with diastolic dysfunction. Diab Vasc Dis Res 2014: 11: 19–25. [DOI] [PubMed] [Google Scholar]
- 7.Gubitosi-Klug RA. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: summary and future directions. Diabetes Care 2014: 37: 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartge MM, Unger T, Kintscher U. The endothelium and vascular inflammation in diabetes. Diab Vasc Dis Res 2007: 4: 84–8. [DOI] [PubMed] [Google Scholar]
- 9.Yu MC, Lo FS, Yu MK, Huang WH, Lee F. Arterial stiffness is not increased in teens with early uncomplicated type 1 diabetes mellitus. Eur J Pediatr 2012: 171: 855–8. [DOI] [PubMed] [Google Scholar]
- 10.Ceriello A, Kumar S, Piconi L, Esposito K, Giugliano D. Simultaneous control of hyperglycemia and oxidative stress normalizes endothelial function in type 1 diabetes. Diabetes Care 2007: 30: 649–54. [DOI] [PubMed] [Google Scholar]
- 11.Gallo LM, Silverstein JH, Shuster JJ, Haller MJ. Arterial stiffness, lipoprotein particle size, and lipoprotein particle concentration in children with type 1 diabetes. J Pediatr Endocrinol Metab 2010: 23: 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol 1997: 79: 350–4. [DOI] [PubMed] [Google Scholar]
- 13.Marchesi S, Lupattelli G, Schillaci G et al. Impaired flow-mediated vasoactivity during post-prandial phase in young healthy men. Atherosclerosis 2000: 153: 397–402. [DOI] [PubMed] [Google Scholar]
- 14.Fuchsjager-Mayrl G, Pleiner J, Wiesinger GF et al. Exercise training improves vascular endothelial function in patients with type 1 diabetes. Diabetes Care 2002: 25: 1795–801. [DOI] [PubMed] [Google Scholar]
- 15.Mason NJ, Jenkins AJ, Best JD, Rowley KG. Exercise frequency and arterial compliance in non-diabetic and type 1 diabetic individuals. Eur J Cardiovasc Prev Rehabil 2006: 13: 598–603. [DOI] [PubMed] [Google Scholar]