Abstract
Cells that express FcεRIα, including mast cells, basophils, and plasmacytoid dendritic cells (pDCs), are regulated by IgE binding to FcεRIα. Omalizumab binds IgE and prevents its engagement with FcεRIα, thereby downregulating its expression and modulating cell function. Because these cells are implicated in the pathobiology of many allergic and immunologic diseases, as well as host defense mechanisms, it is unsurprising that omalizumab studies continue yielding biologic insights and treatment break-throughs for many diseases. Several recent updates in the biology and use of omalizumab will be presented here, and others will be summarized in Table I, highlighting available biomarker-based personalized approaches.
Keywords: IgE, FcεRIα, mAb, biologic therapy, allergy, antiviral immunity, asthma, chronic urticaria, food allergy, immunotherapy
ANTIVIRAL EFFECTS
One exciting contribution from recent studies on omalizumab has been the demonstration that omalizumab can ameliorate the inadequate antiviral response observed in patients with allergic asthma. Children with severe asthma are more susceptible to virus-induced asthma exacerbations, particularly those with higher serum IgE levels.1,2 This relationship has been postulated to be due to impaired interferon responses to viruses in patients with allergic asthma based on cell-based studies using peripheral blood–derived pDCs coincubated with viruses. Furthermore, these studies demonstrated a counterregulatory mechanism between FcεRIα and Toll-like receptor 7 (TLR7; an important receptor for sensing viruses and mounting innate immune responses), whereby their protein expression is inversely proportional.3,4
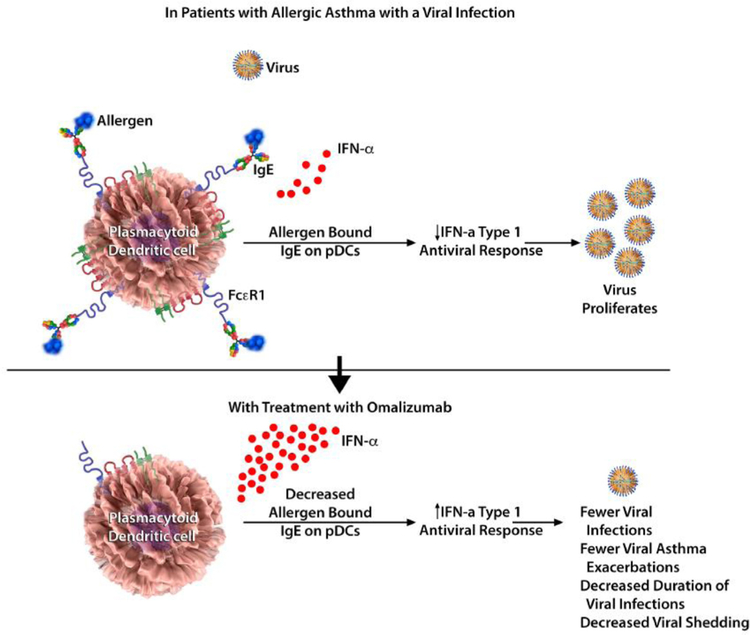
These observations have now been expanded by 2 recent studies using biospecimens and data from the Preventative Omalizumab or Step-up Therapy for Severe Fall Exacerbations (PROSE) study, which examined whether preventive administration of omalizumab could dampen the seasonal increase in asthma exacerbations seen in children.5 First, these studies revealed that omalizumab decreased the duration of human rhinovirus (HRV) infections, viral shedding, and risk of HRV-related illnesses compared with guideline-driven care alone.6 Second, omalizumab attenuated pDC FcεRIα protein expression while simultaneously augmenting pDC IFN-α responses to HRV and influenza virus.7 Together, these findings provide direct evidence that blocking IgE decreases susceptibility to respiratory viral illnesses through enhanced IFN-α responses in pDCs (Fig 1).5–7 These findings can provide a mechanistic explanation to the results from the Inner-City Anti-IgE Therapy for Asthma study, in which omalizumab inhibited seasonal increases in asthma exacerbations thought to be caused by viral infections.8
FIG 1.
Omalizumab and the antiviral response in pDCs. Omalizumab blocks IgE and enhances IFN-α responses in pDCs, thereby decreasing susceptibility to viral respiratory tract illness (based on results from the PROSE study).5–7
In contrast, a recent study showed that mepolizumab administration (which blocks IL-5, the main driver of eosinophilic inflammation) did not abrogate lung function decreases provoked by HRV challenge in participants with mild asthma.9 This suggests that enhancement of the antiviral response is specific to blocking IgE and not cytokines important in eosinophilic inflammation. Still, these recent studies raise new questions on allergy-antiviral immunity interactions and the counterregulatory relationship between FcεRIα and TLR7. Unexpectedly, omalizumab decreased TLR7 protein expression,7 increases of which were presumed to be one mechanism that promoted greater IFN-α production. Whether type 1 interferons other than IFN-α (eg, IFN-β1) or IFN-γ (also deficient in patients with allergic asthma) are also upregulated and responsible for the enhanced viral clearance observed with omalizumab treatment remains unknown. Future omalizumab studies might identify intermediaries downstream of FcεRIα that could be targeted to further improve or restore interferon responses in patients vulnerable to viral respiratory tract infections.
RELATIONSHIP WITH EOSINOPHILS
Another interesting asthma-related finding has been the association between asthma exacerbation reductions and peripheral blood eosinophil counts.10 In patients with blood eosinophil counts of greater than 300 or greater than 400 cells/µL, the reduction in exacerbations noted with omalizumab was 67% and 74%, respectively. Interestingly, data from this and the Prospective Observational Study to Evaluate Predictors of Clinical Effectiveness in Response to Omalizumab (PROSPERO) study suggest that exacerbation reductions relative to blood eosinophil counts are largely driven by the placebo arm because placebo-assigned participants with high blood eosinophil counts had substantially greater asthma exacerbation rates compared with placebo-assigned participants with low blood eosinophil counts.E1
URTICARIA
Omalizumab has transformed the treatment of chronic urticaria (CU) since US Food and Drug Administration approval for this indication in 2014. The majority of patients with CU experience substantial benefit from omalizumab, but not all are complete responders, and some are nonresponders. Recent small studies have identified several response biomarkers. The serum total IgE ratio of levels obtained before and 4 weeks after omalizumab therapy initiation demonstrated an area under the curve of 0.95, with ratios of less than 1.9 having both a sensitivity and a specificity of 93% to predict nonresponse to omalizumab (n = 96).E2 Baseline serum total IgE levels (threshold, <43 kU/L) alone showed a 95% negative predictive value for response. Others have also reported on low baseline serum IgE levels as negatively predictive of response.E3 Conversely, greater baseline basophil FcεRIα expression was 100% sensitive and 72% specific for responsiveness to omalizumab.E4 Importantly, this assay discriminated well between responders and nonresponders; a mean fluorescence intensity threshold of 5000 for FcεRIα by using flow cytometry completely distinguished responders from nonresponders.
Sera from patients with CU, which activates basophils (increased CD203c levels, as determined by using flow cytometry), associates with nonresponsiveness to omalizumab.E5 Serum IL-31 levels were found to decrease with omalizumab treatment and not placebo, but baseline IL-31 levels did not correlate with CU disease activity indexes and did not distinguish responders from nonresponders.E6 A large (n = 470) retrospective study showed no association between baseline D-dimer levels and omalizumab’s therapeutic response,E7 contradicting prior reports of this biomarker’s predictive ability.E8
Improved patient selection is needed, and optimal duration of omalizumab therapy remains undetermined. Furthermore, its therapeutic mechanism remains unclear. Indeed, omalizumab response times in patients with CU seem slower in patients with autoantibodies,E9 suggesting that variations in pathogenic mechanisms (IgE-mediated vs IgG-mediated FcεRIα activation) likely underlie differences in response times.
Finally, omalizumab’s performance on several physical urticarias has been recently reviewed, with the strongest data available for symptomatic dermographism, cold-induced urticaria, and solar urticaria.E10
OTHER DISORDERS
Omalizumab has been shown to be beneficial in patients with various other disorders. Omalizumab facilitates immunotherapy to inhalant, food, and Hymenoptera venom allergens. A recent phase 2 randomized, double-blind, placebo-controlled trial of children with peanut allergy undergoing oral immunotherapy showed that omalizumab administration allowed for tolerance of 2000 mg of peanut on discontinuation of immunotherapy more often than placebo (23/27 [74%] vs 1/8 [13%], P <.01).E11 Participants assigned to omalizumab were also less likely to experience adverse reactions to peanut immunotherapy. Omalizumab has also been shown to improve the safety of oral immunotherapy to other food allergens administered alone or in combination.E12 Considering the high burden of disease posed by food allergy and the important role food desensitizations will likely play in clinical practice, omalizumab will probably be frequently used to improve safety and efficacy with oral immunotherapy.
Omalizumab has shown effectiveness in patients with nasal polyps,E13 and phase 3 studies for this indication are under way. Many studies have demonstrated therapeutic benefit for omalizumab in seasonalE14 and perennialE15 allergic rhinitis. Small case series show positive therapeutic effects for patients with idiopathic anaphylaxis,E16 mast cell activation disorders,E17 allergic bronchopulmonary aspergillosis,E18 atopic dermatitis,E19 eosinophilic gastrointestinal disorders,E20 nonallergic asthma,E21 and asthma–chronic obstructive pulmonary disease overlap.E22 There is conflicting evidence on whether omalizumab can facilitate aspirin desensitization in patients with aspirin-sensitive asthma.E23,E24
Cost ($10,000–$70,000/year [minimum-maximum dose]) largely prevents omalizumab from wider use for a broader range of allergic, nonallergic, and immunologic conditions, although most analyses in asthma and CU cohorts have considered it cost-effective when targeted to select groups.E25,E26 Considering the importance of IgE and cells that bear IgE receptors, omalizumab will likely continue gaining prominence in the treatment of disorders beyond allergic asthma and CU.
TABLE I.
Omalizumab and its uses
| Indications by quality of supportive evidence | Findings | References |
|---|---|---|
| High (eg, large, double-blind, placebo-controlled clinical trials or meta-analyses) | ||
| Allergic asthma | Reduction in asthma exacerbations | Busse et al. J Allergy Clin Immunol 2001;108:184–90 |
| CU | Reduction in symptoms in patients whose symptoms were uncontrolled by antihistamines | Maurer et al. N Engl J Med 2013;368:924–35 |
| Allergic rhinitis | Improvement in rhinitis symptoms and quality of life | Casale et al. JAMA 2001;286:2956–67 |
| Moderate (eg, small clinical trials) | ||
| Facilitation of oral food allergen immunotherapy | Facilitation of peanut oral immunotherapy | MacGinnitie et al. J Allergy Clin Immunol 2017;139: 873–81 |
| Facilitation of subcutaneous immunotherapy to aeroallergens | Improvement in safety of rush immunotherapy to ragweed | Casale et al. J Allergy Clin Immunol 2006;117:134–40 |
| Nonallergic asthma | Improvement in lung function and reduction of bronchial mucosal IgE cells | Pillai et al. Eur Respir J 2016;48:1593–1601 |
| Nasal polyposis | Reduction in endoscopic and radiographic polyp scores | Gevaert et al. J Allergy Clin Immunol 2013;131:110–6 |
| ABPA | Reduction in exacerbations | Voskamp et al. J Allergy Clin Immunol Pract 2015;3:192–9 |
| Low (eg, case series or reports; anecdotal, retrospective, conflicting evidence) | ||
| Mast cell activation syndrome | Reduction in anaphylactic episodes and skin symptoms | Broesby-Olsen et al. Allergy 2018;73:230–8 |
| Idiopathic anaphylaxis | Reduction in anaphylactic episodes | Warrier et al. Ann Allergy Asthma Immunol 2009;102: 257–8 |
| Atopic dermatitis | Conflicting responses to therapy | Belloni et al. J Allergy Clin Immunol 2007;120:1223–5 |
| ACO | Improvement in asthma control and QoL | Maltby et al. Chest 2017;151:78–89 |
| EoE | Clinical and histologic improvement in a minority of patients | Loizou et al. PLoS One 2015;10:e0113483 |
| AERD | Conflicting evidence on whether omalizumab can facilitate aspirin desensitization |
Lang et al. Ann Allergy Asthma Immunol 2018;121:98–104 Waldram et al. J Allergy Clin Immunol 2018;141:250–6 |
ABPA, Allergic bronchopulmonary aspergillosis; ACO, asthma-COPD overlap; AERD, aspirin-exacerbated respiratory disease; COPD, chronic obstructive pulmonary disease; EoE, eosinophilic esophagitis; QoL, quality of life
Acknowledgments
Supported by grants K23AI125785 (to J.C.C.) and R01HL116849 (to T.B.C.) and by generous contributions by the Culverhouse family fund (to J.C.C. and T.B.C.).
T. B. Casale was involved in several clinical trials using omalizumab, including industry-sponsored trials, and he reports that his university employer has received grants and consulting fees from Genentech and Novartis.
Footnotes
Disclosure of potential conflict of interest: J. C. Cardet declares that he has no relevant conflicts of interest.
REFERENCES
- 1.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol 2015;16: 45–56. [DOI] [PubMed] [Google Scholar]
- 2.Kantor DB, Stenquist N, McDonald MC, Schultz BJ, Hauptman M, Small-wood CD, et al. Rhinovirus and serum IgE are associated with acute asthma exacerbation severity in children. J Allergy Clin Immunol 2016;138: 1467–71.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gill MA, Bajwa G, George TA, Dong CC, Dougherty II, Jiang N, et al. Counter-regulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol 2010;184:5999–6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durrani SR, Montville DJ, Pratt AS, Sahu S, DeVries MK, Rajamanickam V, et al. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol 2012; 130:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ Jr, Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol 2015;136:1476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esquivel A, Busse WW, Calatroni A, Togias AG, Grindle KG, Bochkov YA, et al. Effects of omalizumab on rhinovirus infections, illnesses, and exacerbations of asthma. Am J Respir Crit Care Med 2017;196:985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill MA, Liu AH, Calatroni A, Krouse RZ, Shao B, Schiltz A, et al. Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J Allergy Clin Immunol 2018;141:1735–43.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med 2011;364:1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabogal Pineros YS, Bal SM, van de Pol MA, Dierdorp BS, Dekker T, Dijkhuis A, et al. Anti-IL5 in Mild Asthma Alters Rhinovirus-Induced Macrophage, B Cell and Neutrophil Responses (MATERIAL): a placebo-controlled, double-blind study. Am J Respir Crit Care Med 2019;199:508–17. [DOI] [PubMed] [Google Scholar]
- 10.Casale TB, Luskin AT, Busse W, Zeiger RS, Trzaskoma B, Yang M, et al. Omalizumab effectiveness by biomarker status in patients with asthma: evidence From PROSPERO, a prospective real-world study. J Allergy Clin Immunol Pract 2019; 7:156064.e1. [DOI] [PubMed] [Google Scholar]
- E1.Casale TB, Chipps BE, Rosen K, Trzaskoma B, Haselkorn T, Omachi TA, et al. Response to omalizumab using patient enrichment criteria from trials of novel biologics in asthma. Allergy 2018;73:490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Ertas R, Ozyurt K, Atasoy M, Hawro T, Maurer M. The clinical response to omalizumab in chronic spontaneous urticaria patients is linked to and predicted by IgE levels and their change. Allergy 2018;73:705–12. [DOI] [PubMed] [Google Scholar]
- E3.Straesser MD, Oliver E, Palacios T, Kyin T, Patrie J, Borish L, et al. Serum IgE as an immunological marker to predict response to omalizumab treatment in symptomatic chronic urticaria. J Allergy Clin Immunol Pract 2018;6:1386–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Deza G, Bertolin-Colilla M, Pujol RM, Curto-Barredo L, Soto D, Garcia M, et al. Basophil FcεRI expression in chronic spontaneous urticaria: a potential immunological predictor of response to omalizumab therapy. Acta Derm Venereol 2017; 97:698–704. [DOI] [PubMed] [Google Scholar]
- E5.Palacios T, Stillman L, Borish L, Lawrence M. Lack of basophil CD203c-upregulating activity as an immunological marker to predict response to treatment with omalizumab in patients with symptomatic chronic urticaria. J Allergy Clin Immunol Pract 2016;4:529–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Altrichter S, Hawro T, Hänel K, Czaja K, Lüscher B, Maurer M, et al. Successful omalizumab treatment in chronic spontaneous urticaria is associated with lowering of serum IL-31 levels. J Eur Acad Dermatol Venereol 2016; 30:454–5. [DOI] [PubMed] [Google Scholar]
- E7.Marzano AV, Genovese G, Casazza G, Fierro MT, Dapavo P, Crimi N, et al. Predictors of response to omalizumab and relapse in chronic spontaneous urticaria: a study of 470 patients. J Eur Acad Dermatol Venereol 2018. [Epub ahead of print]. [DOI] [PubMed]
- E8.Asero R, Marzano AV, Ferrucci S, Cugno M. Elevated baseline D-dimer plasma levels are associated with a prompt response to omalizumab in patients with severe CSU. J Allergy Clin Immunol Pract 2017;5:1740–2. [DOI] [PubMed] [Google Scholar]
- E9.Gericke J, Metz M, Ohanyan T, Weller K, Altrichter S, Skov PS, et al. Serum autoreactivity predicts time to response to omalizumab therapy in chronic spontaneous urticaria. J Allergy Clin Immunol 2017;139:1059–61.e1. [DOI] [PubMed] [Google Scholar]
- E10.Maurer M, Metz M, Brehler R, Hillen U, Jakob T, Mahler V, et al. Omalizumab treatment in patients with chronic inducible urticaria: a systematic review of published evidence. J Allergy Clin Immunol 2018;141:638–49. [DOI] [PubMed] [Google Scholar]
- E11.MacGinnitie AJ, Rachid R, Gragg H, Little SV, Lakin P, Cianferoni A, et al. Omalizumab facilitates rapid oral desensitization for peanut allergy. J Allergy Clin Immunol 2017;139(3):873–81.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E12.Wood RA, Kim JS, Lindblad R, Nadeau K, Henning AK, Dawson P, et al. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow’s milk allergy. J Allergy Clin Immunol 2016;137:1103–10.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E13.Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol 2013;131:110–6.e1. [DOI] [PubMed] [Google Scholar]
- E14.Casale TB, Condemi J, LaForce C, Nayak A, Rowe M, Watrous M, et al. Effect of omalizumab on symptoms of seasonal allergic rhinitis: a randomized controlled trial. JAMA 2001;286:2956–67. [DOI] [PubMed] [Google Scholar]
- E15.Chervinsky P, Casale T, Townley R, Tripathy I, Hedgecock S, Fowler-Taylor A, et al. Omalizumab, an anti-IgE antibody, in the treatment of adults and adolescents with perennial allergic rhinitis. Ann Allergy Asthma Immunol 2003;91:160–7. [DOI] [PubMed] [Google Scholar]
- E16.Warrier P, Casale TB. Omalizumab in idiopathic anaphylaxis. Ann Allergy Asthma Immunol 2009;102:257–8. [DOI] [PubMed] [Google Scholar]
- E17.Broesby-Olsen S, Vestergaard H, Mortz CG, Jensen B, Havelund T, Hermann AP, et al. Omalizumab prevents anaphylaxis and improves symptoms in systemic mastocytosis: efficacy and safety observations. Allergy 2018;73:230–8. [DOI] [PubMed] [Google Scholar]
- E18.Voskamp AL, Gillman A, Symons K, Sandrini A, Rolland JM, O’Hehir RE, et al. Clinical efficacy and immunologic effects of omalizumab in allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract 2015;3:192–9. [DOI] [PubMed] [Google Scholar]
- E19.Belloni B, Ziai M, Lim A, Lemercier B, Sbornik M, Weidinger S, et al. Low-dose anti-IgE therapy in patients with atopic eczema with high serum IgE levels. J Allergy Clin Immunol 2007;120:1223–5. [DOI] [PubMed] [Google Scholar]
- E20.Loizou D, Enav B, Komlodi-Pasztor E, Hider P, Kim-Chang J, Noonan L, et al. A pilot study of omalizumab in eosinophilic esophagitis. PLoS One 2015;10: e0113483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E21.Pillai P, Chan YC, Wu SY, Ohm-Laursen L, Thomas C, Durham SR, et al. Omalizumab reduces bronchial mucosal IgE and improves lung function in non-atopic asthma. Eur Respir J 2016;48:1593–601. [DOI] [PubMed] [Google Scholar]
- E22.Maltby S, Gibson PG, Powell H, McDonald VM. Omalizumab treatment response in a population with severe allergic asthma and overlapping COPD. Chest 2017;151:78–89. [DOI] [PubMed] [Google Scholar]
- E23.Lang DM, Aronica MA, Maierson ES, Wang XF, Vasas DC, Hazen SL. Omalizumab can inhibit respiratory reaction during aspirin desensitization. Ann Allergy Asthma Immunol 2018;121:98–104. [DOI] [PubMed] [Google Scholar]
- E24.Waldram J, Walters K, Ronald S, Woessner K, Waalen J, White A. Safety and outcomes of aspirin desensitization for aspirin-exacerbated respiratory disease: a single-center study. J Allergy Clin Immunol 2018;141:250–6. [DOI] [PubMed] [Google Scholar]
- E25.McQueen RB, Sheehan DN, Whittington MD, van Boven JFM, Campbell JD. Cost-effectiveness of biological asthma treatments: a systematic review and recommendations for future economic evaluations. Pharmacoeconomics 2018;36: 957–71. [DOI] [PubMed] [Google Scholar]
- E26.Kanters TA, Thio HB, Hakkaart L. Cost-effectiveness of omalizumab for the treatment of chronic spontaneous urticaria. Br J Dermatol 2018;179:702–8. [DOI] [PubMed] [Google Scholar]