Abstract
Evidence suggests an association between autonomic nervous system (ANS) function and atrial fibrillation (AF) development. We sought to examine the association of baseline resting heart rate (RHR) and short-term heart rate variability (HRV) as surrogates of (ANS) with incident AF in individuals without prior cardiovascular disease. A total of 6261 participants of the Multi Ethnic Study of Atherosclerosis (MESA) who were free of AF and diagnosed cardiovascular disease were enrolled. Three standard 10-second, 12-lead electrocardiograms were used to measure RHR, the standard deviation of normal-to-normal intervals (SDNN) and the root mean square of successive differences in RR intervals (RMSSD). Cox proportional hazards models adjusted for demographics, atrioventricular nodal agents, and known cardiovascular risk factors were used to examine the association of baseline RHR, and log transformed SDNN and RMSDD with incident AF. Over a mean follow-up of 11.3 ± 3.7 years, 754 (12%) participants developed AF. Spline curve analysis revealed a non-linear association between RHR, HRV and incident AF. In fully adjusted models higher (but not lower) baseline resting heart rate (RHR >76 beats/min) was associated with incident AF (HR: 1.48 95% CI: 1.18-1.86). Additionally, lower values of RMSDD and SDNN and higher values of RMSDD were independently associated with incident AF. In conclusion, cardiac ANS dysregulation indicated as higher RHR and lower HRV is associated with incident AF independent of known cardiovascular risk factors.
Keywords: Atrial fibrillation, resting heart rate, heart rate variability, autonomic nervous system
Introduction
Several observations suggest a role for autonomic nervous system (ANS) in atrial fibrillation (AF) pathogenesis. These studies have used orthostatic hypotension, resting heart rate (RHR) and heart rate variability (HRV) as surrogates of autonomic tone.1-3 Inconsistent results have been reported regarding the association of RHR and HRV with new onset AF in the general population. An association between a higher RHR4 or a lower resting 5 or exercise heart rate6 with incident AF has been reported. Also, low HRV was associated with incident AF independent of cardiovascular risk factors in two studies.5, 7 While in another study baseline HRV was not different between participants who developed AF and those who did not.8 In this study we sought to examine the association between baseline RHR and short term HRV with incident AF in a multi-ethnic population free of any clinical cardiovascular disease at baseline.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) protocol has been described in detail.9 Briefly, between July 2000 and August 2002, 6814 individuals were recruited from 6 U.S. communities in California, Illinois, New York, North Carolina, Maryland, and Minnesota. The participants were between 45 and 84 years of age and were from 4 different self-reported racial/ethnic backgrounds (white, African-American, Hispanic and Chinese). At baseline, all participants were free of any clinically apparent cardiovascular disease. The institutional review boards of each of the 6 participating field sites approved the study, and all participants gave written informed consent. For this study, participants with baseline diagnosis of AF, and those on antiarrhythmic medications were excluded.
Trained technicians obtained three consecutive 10-second 12-lead electrocardiograms using GE MAC 1200 electrocardiographs based on standardized procedures. All ECGs were obtained after at least 5 minutes of resting and in the fasting state to negate dietary influences on recordings. Most (92%) of the ECGs were obtained in the morning. ECGs were then transmitted electronically to a central ECG reading center, which was blinded to all clinical and personal details of the participants. All ECGs were automatically processed, after visual inspection for technical errors and inadequate quality, using the 2001 version of the GE Marquette 12-SL program (GE, Milwaukee, Wisconsin, USA). To assess ANS function, following variables were measured from baseline (MESA Visit 1) ECGs using the average values of three obtained ECG tracings: 1) Resting heart rate (RHR); 2) standard deviation of normal-to-normal RR intervals (SDNN); and 3) root mean square of successive differences in normal-to-normal RR intervals (RMSDD). As part of the automated measurement of HRV, the beat before and the beat after premature atrial contractions were excluded from HRV measurements. Premature atrial contractions were detected by visual inspection or automatically by software. Also, if the number of PACs exceeded 50%, the whole ECG was excluded from the HRV analysis. The validity of the HRV calculation method was previously verified on a subset of 264 ECGs. Prior work also has shown high correlations between 10-sec and 6-min measures with a correlation coefficient of 0.76 (95% CI: 0.68–0.82) for SDNN and 0.82 (95% CI: 0.75–0.86) for RMSSD when 2-3 ECGs were used.10
The methods of covariate measurements, data collection, and follow up in MESA have been explained in details previously.9 Incident AF during follow-up was identified using a combination of MESA hospitalization surveillance, follow-up study ECGs in MESA Visit 5 (2010-2012), and for participants enrolled in fee-for-service Medicare, from inpatient, outpatient, and physician claims. An International Classification of Diseases, Ninth Revision diagnosis code of 427.31 (atrial fibrillation) or 427.31 (atrial flutter) in any position was considered evidence of AF. AF hospitalizations associated with open cardiac surgery were excluded. If the first AF claim occurred before the baseline MESA exam, the participant was considered to have prevalent AF and was excluded from the analysis.
Continuous variables are presented as mean ± SD. Categorical data are presented as numbers and percentages. The measures of HRV were log transformed to normalize the distribution. Baseline characteristics were compared among participants with and without incident AF using the chi-square test and Student t test where appropriate. To provide detailed analyses of the dose-response relationship of RHR and HRV variables and incident AF, we modeled the variables with restricted quadratic splines with knots at the 10th, 50th and 90th percentiles of their distribution. Restricted cubic splines were also used to examine if the associations of RHR and HRV with incident AF are nonlinear. Multivariable Cox proportional hazards models with incremental adjustments were used to determine the association of RHR and HRV with incident AF. Covariates entered in models were chosen based on their association with incident AF in the present analyses and in published data. Model 1 was adjusted for basic demographic characteristics (age, sex, race/ethnicity, and educational levels). Model 2 included covariates in Model 1 plus body mass index, smoking status (never, former, current), hypertension, high-density and low-density lipoprotein cholesterol levels, diabetes mellitus status, alcohol consumption (never, former, current), beta blocker and/or calcium channel blocker use, and beta agonist and/or anticholinergic use. Proportional hazards assumptions were checked using Schoenfeld residuals. Results are presented as hazard ratios (HR) with 95% confidence intervals (CI). Statistical analyses were performed using STATA software (Version 14.1, STATA Corp, Texas, U.S.A.).
Results
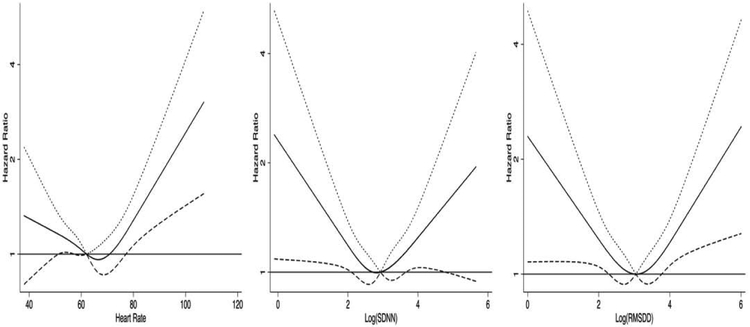
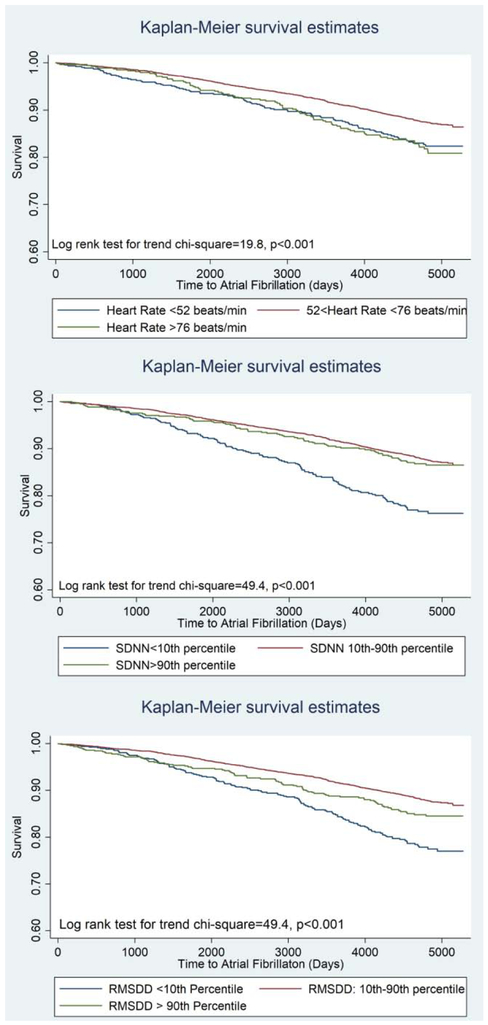
After excluding participants with prevalent AF at baseline, and those taking antiarrhythmic medications, 6261 individuals had baseline measures of heart rate and HRV. Over a mean follow-up of 11.3 ± 3.7 years, 754 (12%) individuals with incident AF were identified. Baseline characteristics of participants with and without incident AF are summarized in table 1. The P Values for quadratic term for RHR, and HRV were <0.05 rejecting linear associations. The multivariable-adjusted restricted cubic spline curves (figure 1) also suggested non-linear associations. Therefore, to compare the associations of high or low RHR and HRV with incident AF we categorized participants based on having RHR and HRV measures of <10th percentile, 10th-90th percentile and >90th percentile. Hazard ratios of the multivariable associations of categories of RHR and HRV and incident AF are shown in table 2. In Model 1, when adjusted for age, sex, race/ethnicity and educational status, having a RHR <52bpm (<10th percentile) or >76 bpm (>90th percentile) compared to 52-56 bpm (10th -90th percentile) were associated with higher incident AF. In fully adjusted models the association of RHR and incident AF was attenuated, however, remained significant for RHR>76 bpm (HR: 1.38 95% CI: 1.09-1.75). Lower (<10th percentile) SDNN (log transformed) and RMSDD (log transformed) and higher RMSDD (>90th percentile) were associated with higher hazards of incident AF. In fully adjusted models, lower values of SDNN and both lower and higher values of RMSDD remained associated with incident AF (Table 2). In fully adjusted models, in addition to RHR and HRV measures, age (HR: 1.09; CI: 1.08-1.10), male sex (HR: 1.5; CI:1.3-1.9), active smoking (HR:1.8; CI:1.4-2.3), BMI (HR: 1.04; CI:1.02-1.06), history of HTN (HR: 1.23; CI: 1.06-1.45) remained associated with incident AF. Additionally, compared to white race, black and Hispanic race/ethnicities were associated with lower incident AF (HR: 0.59 CI: 0.48-0.72 and HR: 0.66 CI: 0.53-0.83 for black and Hispanic race/ethnicity respectively). Figure 2 shows Kaplan-Meier analyses graphs for incident AF based on categories of RHR and HRV.
Table 1.
Baseline characteristic of participants with incident AF and no AF.
| Variable | Atrial Fibrillation | P Value | |
|---|---|---|---|
| No (N=5507) | Yes (N=754) | ||
| Age (years) | 60.7±10.0 | 69.0±7.8 | <0.001 |
| Men | 2504(45.5%) | 404(53.6%) | <0.001 |
| White | 2025(36.8%) | 348(46.15%) | |
| Chinese | 660(11.9%) | 100(13.3%) | |
| Black | 1554(28.0%) | 166(22.0%) | |
| Hispanic | 1278(23.2%) | 140(18.6%) | |
| Body mass index(kg/m2) | 28.3±5.5 | 28.5±5.6 | 0.498 |
| Cholesterol (mg/dl) | |||
| Total | 194.9±35.8 | 191.1±35.3 | 0.006 |
| LDL | 117.9±31.4 | 113.8±31.6 | <0.001 |
| HDL | 50.9±14.7 | 51.4±15.4 | 0.352 |
| Triglyceride | 132.5±90.4 | 132.1±90.6 | 0.922 |
| Cigarette smoker | 0.001 | ||
| Never | 2801(51.0%) | 344(45.7%) | |
| Former | 1960(35.7%) | 323(42.9%) | |
| Current | 729(13.3%) | 86(11.4%) | |
| Diabetes Mellitus | 654(11.9%) | 126(16.7%) | <0.001 |
| Blood Pressure (mmHg) | |||
| Systolic | 125.3±21.1 | 133.8±21.9 | <0.001 |
| Diastolic | 71.9±10.2 | 72.5±10.2 | 0.176 |
| Alcohol use | 0.064 | ||
| Never | 1134(20.8%) | 155(20.6%) | |
| Former | 1290(23.6%) | 206(27.4%) | |
| Current | 3039(55.6%) | 391(52.0%) | |
| Antihypertensive use | 1714(31.1%) | 329(43.6%) | <0.001 |
| Betablocker use | 433(7.9%) | 108(14.3%) | <0.001 |
| Calcium Channel Blocker use | 617(11.2%) | 131(17.4%) | <0.001 |
| Anticholinergic or Beta Agonist Use | 28(0.5%) | 14(1.9%) | <0.001 |
| Resting heart rate (bpm) | 63.1±9.4 | 63.2±10.4 | 0.870 |
| Log (SDNN) | 2.95±0.61 | 2.82±0.69 | <0.001 |
| Log (RMSDD) | 3.06±0.66 | 2.95±0.78 | <0.001 |
LDL: low density lipoprotein; HDL: high density lipoprotein; SDNN: standard deviation of normal-to-normal intervals; RMSDD: root mean square of successive differences in RR intervals
Figure 1. Association of incident atrial fibrillation with resting heart rate and heart rate variability parameters.
The solid lines indicate multivariable-adjusted hazard ratios for AF as a function of resting heart rate (left), log transformed SDNN (middle) and log transformed RMSDD (right) using restricted quadratic splines. The dotted and dashed lines delineate the upper and lower 95% confidence intervals respectively. The horizontal line indicates a hazard ratio of 1. The models are adjusted for age, sex, ethnicity/race, educational level, smoking status, hypertension, high-density and low-density lipoproteins, diabetes mellitus, alcohol consumption, beta agonist and or anticholinergic use, beta-blocker use, and calcium channel blocker use.
Table 2.
Association of resting heart rate and heart rate variability variables with incident AF.
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Resting heart rate | ||||||
| <10th percentile | 1.26 | 1.01-1.57 | 0.040 | 1.22 | 0.98-1.54 | 0.072 |
| 10th -90th percentile | reference | |||||
| >90th percentile | 1.48 | 1.18-1.86 | 0.001 | 1.38 | 1.09-1.75 | 0.008 |
| SDNN* | ||||||
| <10th percentile | 1.31 | 1.06-1.57 | 0.010 | 1.22 | 1.01-1.49 | 0.050 |
| 10th -90th percentile | reference | |||||
| >90th percentile | 1.18 | 0.93-1.51 | 0.175 | 1.17 | 0.92-1.50 | 0.198 |
| RMSDD* | ||||||
| <10th percentile | 1.31 | 1.08-1.59 | 0.005 | 1.27 | 1.04-1.55 | 0.017 |
| 10th-90th percentile | reference | |||||
| >90th percentile | 1.38 | 1.09-1.74 | 0.006 | 1.36 | 1.08-1.71 | 0.009 |
Model 1 is adjusted for age, gender, race and educational level. Model 2 is additionally adjusted for body mass index, smoking status, hypertension, high-density and low-density lipoproteins, diabetes mellitus, alcohol consumption, beta agonist/anticholinergic use, beta-blocker use, and calcium channel blocker use.
SDNN: standard deviation of normal-to-normal intervals; RMSDD: root mean square of successive differences in RR intervals
Log Transformed
Figure 2.
Kaplan-Meier analysis showing the event free survival of participants based on cut off values of resting heart rate (top), SDNN (middle) and RMSD (bottom).
To assess the differences in relationship of RHR and HRV with AF by age, gender, and ethnicity, we included an interaction term between these variables and RHR and HRV measures. The sub-analysis revealed no interaction between age, gender and ethnicity and RHR and HRV measures and incident AF.
Discussion
In this multi-ethnic population-based study on individuals without baseline clinical cardiovascular disease, we found an association of lower and higher HRV and higher RHR with incident AF independent of demographics, known cardiovascular risk factors and medications affecting heart rate. Our findings suggest that high baseline sympathetic tone (measured as higher RHR) and poor heart rate modulation (measured as low HRV) are associated with higher risk of incident AF.
RHR and HRV have been used as indirect measures of ANS function. Higher RHR suggests a more dominant sympathetic tone, while a lower RHR shows a high basal vagal tone. On the other hand, high HRV shows a balanced modulation between sympathetic and parasympathetic nervous systems at physiologic levels. However, the interpretation of low HRV is more complicated. Variation in heart rate during breathing (high frequency HRV) is mostly a function of parasympathetic modulation, while HRV during the day and sleep (low frequency HRV) is attributable to sympathetic modulation.11 In a study on 29 healthy individuals, HRV increased with higher levels of parasympathetic effect, but there was a plateau level after which higher levels of parasympathetic activation resulted in a decrease in HRV.12Inconsistent results have been reported regarding the association of RHR and incident AF. In a large population based study on 309,540 Norwegian men and women, every 10 beats/min decrease in RHR was associated with 26% and 15% increase in the risk of AF incidence in men and women, respectively.13 A relatively recent publication from ARIC showed lower RHR and HRV being associated with AF.5 In contrast, a study on 281,451 primary care patients found a U-shaped association between RHR and incident AF.11 In our study we found an association between higher RHR and incident AF. This may be a sign of dominant sympathetic tone, which has been shown to modify atrial electrophysiological properties in people with cardiac disease.14 A tendency towards higher sympathetic tone has been reported before AF episodes during sleep or postoperative AF episodes.15, 16 However, in 2 other studies a combination of primary increase in adrenergic tone followed by an acute shift towards vagal predominance were observed.1, 17 These observations suggest a competition of high sympathetic and parasympathetic tones prior to AF episodes. Inconsistent population-based reports also exist regarding the association of HRV and incident AF. In the Framingham Heart Study, despite a trend towards an association between lower SDNN and incident AF, the association was attenuated after adjusting for traditional cardiovascular risk factors.8 In contrast, two other population based studies showed an independent association between lower HRV and incident AF.5, 7 Although we did not have the data on low and high frequency changes in HRV, we also found an association between lower HRV and incident AF independent of known cardiovascular risk factors. Additionally, in our study higher measures of RMSDD, but not SDNN, was also associated with incident AF.
Multiple risk factors such as pressure or volume overload, inflammation, or autonomic changes can work as potential mediators to create a favorable atrial substrate for AF development.18 However, traditional cardiovascular risk factors can only explain AF development in half of the cases.19Therefore many studies have looked at other contributing factors to AF development such as ANS regulation. Vagal nerve stimulation is shown to shorten the atrial effective refractory period and facilitate AF induction.20 While AF is vagally mediated in patients with idiopathic AF, in patients with cardiovascular disease, sympathetic tone plays a greater role in AF initiation.14 Cardiac ANS is also affected after pulmonary vein isolation performed for treatment of AF.21 Therefore, modulation of the ANS through targeting ganglionated plexi or anterior pericardial fat pad removal has been proposed as an adjunct for AF treatment.22, 23
Despite several reports on direct contribution of autonomic regulation to AF initiation, a causal relationship is not proven. Another possible explanation of such associations is shared risk factors affecting ANS and atrial substrate at the same time. Previous studies have shown an association between known cardiovascular risk factors such as active smoking, hypertension, and diabetes with lower HRV.24-26The same risk factors are also associated with atrial fibrosis, which is the final common atrial substrate for AF development.18, 27 In our study, those who developed AF had more cardiovascular risk factors such as older age, hypertension and diabetes diagnosis, and positive smoking history. Whether these risk factors contribute directly to autonomic dysfunction and also the degree of their contribution is not known.
A few limitations should be addressed. We used only time-domain, and not frequency-domain, HRV parameters. In addition, the HRV variables were extracted from standard ECGs and not prolonged ECG data. While use of short-term, time-domain HRV parameters could be seen as a limitation, given its feasibility, it increases the clinical applicability of our findings. In our study incident AF was identified based on inpatient and outpatient diagnosis codes and MESA follow up visits, this may underestimate AF diagnosis as many of AF cases are asymptomatic and do not require hospitalization. However, a validation sub-study on 45 MESA participants with the diagnosis of AF based on hospital discharge codes confirms the diagnosis of AF in 93% of hospitalizations. 28 Additionally, based on a systematic review using information from 16 unique studies, a large proportion of prevalent AF cases identified by ICD-9 code were valid (positive predictive value 70%-96%, median 89%). 29
In conclusion, we found an independent association of high RHR and low or high HRV, as surrogates of ANS function, with new onset AF in a multi-ethnic population. Whether frequent rhythm surveillance in this population would be beneficial for early diagnoses of AF or whether modulation of ANS will decrease the risk of future AF needs further studies.
Acknowledgments
We thank all the investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding: This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI), by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS, and by grant R01 HL127659 from NHLBI. This work was additionally supported by American Heart Association grant 16EIA26410001 (Alonso).
Footnotes
Disclaimer Statement
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bettoni M and Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation 2002;105:2753–2759. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal SK, Alonso A, Whelton SP, Soliman EZ, Rose KM, Chamberlain AM, Simpson RJ Jr., Coresh J and Heiss G. Orthostatic change in blood pressure and incidence of atrial fibrillation: results from a bi-ethnic population based study. PLoS One 2013;8:e79030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ko D, Preis SR, Lubitz SA, McManus DD, Vasan RS, Hamburg NM, Benjamin EJ and Mitchell GF. Relation of Orthostatic Hypotension With New-Onset Atrial Fibrillation (From the Framingham Heart Study). Am J Cardiol 2018;121:596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okin PM, Wachtell K, Kjeldsen SE, Julius S, Lindholm LH, Dahlof B, Hille DA, Nieminen MS, Edelman JM and Devereux RB. Incidence of atrial fibrillation in relation to changing heart rate over time in hypertensive patients: the LIFE study. Circ Arrhythm Electrophysiol 2008;1:337–343. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal SK, Norby FL, Whitsel EA, Soliman EZ, Chen LY, Loehr LR, Fuster V, Heiss G, Coresh J and Alonso A. Cardiac Autonomic Dysfunction and Incidence of Atrial Fibrillation: Results From 20 Years Follow-Up. J Am Coll Cardiol 2017;69:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grundvold I, Skretteberg PT, Liestol K, Erikssen G, Engeseth K, Gjesdal K, Kjeldsen SE, Arnesen H, Erikssen J and Bodegard J. Low heart rates predict incident atrial fibrillation in healthy middle-aged men. Circ Arrhythm Electrophysiol 2013;6:726–731. [DOI] [PubMed] [Google Scholar]
- 7.Perkiomaki J, Ukkola O, Kiviniemi A, Tulppo M, Ylitalo A, Kesaniemi YA and Huikuri H. Heart rate variability findings as a predictor of atrial fibrillation in middle-aged population. J Cardiovasc Electrophysiol 2014;25:719–724. [DOI] [PubMed] [Google Scholar]
- 8.Singh JP, Larson MG, Levy D, Evans JC, Tsuji H and Benjamin EJ. Is baseline autonomic tone associated with new onset atrial fibrillation?: Insights from the framingham heart study. Ann Noninvasive Electrocardiol 2004;9:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M and Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 10.Schroeder EB, Whitsel EA, Evans GW, Prineas RJ, Chambless LE and Heiss G. Repeatability of heart rate variability measures. J Electrocardiol 2004;37:163–172. [DOI] [PubMed] [Google Scholar]
- 11.Skov MW, Bachmann TN, Rasmussen PV, Olesen MS, Pietersen A, Graff C, Lind B, Struijk JJ, Kober L, Haunso S, Svendsen JH, Gerds TA, Holst AG and Nielsen JB. Association Between Heart Rate at Rest and Incident Atrial Fibrillation (from the Copenhagen Electrocardiographic Study). Am J Cardiol 2016;118:708–713. [DOI] [PubMed] [Google Scholar]
- 12.Goldberger JJ, Challapalli S, Tung R, Parker MA and Kadish AH. Relationship of heart rate variability to parasympathetic effect. Circulation 2001;103:1977–1983. [DOI] [PubMed] [Google Scholar]
- 13.Thelle DS, Selmer R, Gjesdal K, Sakshaug S, Jugessur A, Graff-Iversen S, Tverdal A and Nystad W. Resting heart rate and physical activity as risk factors for lone atrial fibrillation: a prospective study of 309,540 men and women. Heart 2013;99:1755–1760. [DOI] [PubMed] [Google Scholar]
- 14.Lu Z, Scherlag BJ, Lin J, Niu G, Fung KM, Zhao L, Ghias M, Jackman WM, Lazzara R, Jiang H and Po SS. Atrial fibrillation begets atrial fibrillation: autonomic mechanism for atrial electrical remodeling induced by short-term rapid atrial pacing. Circ Arrhythm Electrophysiol 2008;1:184–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coccagna G, Capucci A, Bauleo S, Boriani G and Santarelli A. Paroxysmal atrial fibrillation in sleep. Sleep 1997;20:396–398. [DOI] [PubMed] [Google Scholar]
- 16.Dimmer C, Tavernier R, Gjorgov N, Van Nooten G, Clement DL and Jordaens L. Variations of autonomic tone preceding onset of atrial fibrillation after coronary artery bypass grafting. Am J Cardiol 1998;82:22–25. [DOI] [PubMed] [Google Scholar]
- 17.Amar D, Zhang H, Miodownik S and Kadish AH. Competing autonomic mechanisms precede the onset of postoperative atrial fibrillation. J Am Coll Cardiol 2003;42:1262–1268. [DOI] [PubMed] [Google Scholar]
- 18.Goldberger JJ, Arora R, Green D, Greenland P, Lee DC, Lloyd-Jones DM, Markl M, Ng J and Shah SJ. Evaluating the Atrial Myopathy Underlying Atrial Fibrillation: Identifying the Arrhythmogenic and Thrombogenic Substrate. Circulation 2015;132:278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, Konety S and Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011;123:1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirose M, Leatmanoratn Z, Laurita KR and Carlson MD. Partial vagal denervation increases vulnerability to vagally induced atrial fibrillation. J Cardiovasc Electrophysiol 2002;13:1272–1279. [DOI] [PubMed] [Google Scholar]
- 21.Miyanaga S, Yamane T, Date T, Tokuda M, Aramaki Y, Inada K, Shibayama K, Matsuo S, Miyazaki H, Abe K, Sugimoto K, Mochizuki S and Yoshimura M. Impact of pulmonary vein isolation on the autonomic modulation in patients with paroxysmal atrial fibrillation and prolonged sinus pauses. Europace 2009;11:576–581. [DOI] [PubMed] [Google Scholar]
- 22.Rajendran PS, Buch E and Shivkumar K. Marshaling the autonomic nervous system for treatment of atrial fibrillation. J Am Coll Cardiol 2014;63:1902–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S, Jing Y, Zhang J, Bian C, Zhang YU and Zhang X. Does Anterior Fat Pad Removal Reduce the Incidence of Atrial Fibrillation after CABG? A Meta-Analysis of Randomized Controlled Trials. Pacing Clin Electrophysiol 2015;38:1363–1368. [DOI] [PubMed] [Google Scholar]
- 24.Minami J, Ishimitsu T and Matsuoka H. Effects of smoking cessation on blood pressure and heart rate variability in habitual smokers. Hypertension 1999;33:586–590. [DOI] [PubMed] [Google Scholar]
- 25.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 1996;17:354–381. [PubMed] [Google Scholar]
- 26.Carnethon MR, Prineas RJ, Temprosa M, Zhang ZM, Uwaifo G, Molitch ME and Diabetes Prevention Program Research G. The association among autonomic nervous system function, incident diabetes, and intervention arm in the Diabetes Prevention Program. Diabetes Care 2006;29:914–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chrispin J, Ipek EG, Habibi M, Yang E, Spragg D, Marine JE, Ashikaga H, Rickard J, Berger RD, Zimmerman SL, Calkins H and Nazarian S. Clinical predictors of cardiac magnetic resonance late gadolinium enhancement in patients with atrial fibrillation. Europace 2017;19:371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patton KK, Heckbert SR, Alonso A, Bahrami H, Lima JA, Burke G and Kronmal RA. N-terminal pro-B-type natriuretic peptide as a predictor of incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis: the effects of age, sex and ethnicity. Heart 2013;99:1832–1836. [DOI] [PubMed] [Google Scholar]
- 29.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R and Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf 2012;21 Suppl 1:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]