Abstract
Aims
To assess faecal calprotectin [Fcal] levels before and after therapeutic de-escalation, to predict clinical relapse in patients with inflammatory bowel disease [IBD].
Methods
From a prospectively maintained database, we enrolled 160 IBD patients [112 Crohn’s disease/48 ulcerative colitis] in clinical remission, with Fcal measured within 8 weeks before therapeutic de-escalation. Clinical relapse was defined using the Harvey-Bradshaw index or Simple Clinical Colitis Activity Index.
Results
Using a receiver operating characteristic [ROC] curve, Fcal >100 µg/g was the best threshold to predict clinical relapse after therapeutic de-escalation (area under the curve [AUC] = 0.84). In multivariate analysis, clinical remission >6 months before therapeutic de-escalation (hazard ratio [HR] = 0.57 [0.33–0.99]; p = 0.044) was associated with decreased risk of relapse, whereas current steroid medication ( = 1.67[1.00–2.79]; p <0.0001) was a risk factor. Fcal >100 µg/g was predictive of clinical relapse (HR = 3.96 [2.47–6.35]; p < 0.0001) in the whole cohort but also in patients receiving anti-tumour necrosis factor [TNF] agents [n = 85 patients; p <0.0001], anti-integrins [n = 32; p = 0.003], or no biologics [n = 43; p = 0.049], or attempting to discontinue steroids [n = 37; p = 0.001]. One patient [1/98] and seven patients [7/88, 8.0%] with baseline Fcal <100 µg/g relapsed within 3 months and 6 months after therapeutic de-escalation, respectively. A total of 74 Fcal measurements were performed in 52 patients after therapeutic de-escalation. Monitoring Fcal >200 µg/g [ROC curve with AUC = 0.96] was highly predictive of clinical relapse in multivariate analysis ([HR = 31.8 [3.5–289.4], p = 0.002). Only two relapses [2/45, 4.4%] occurred within 6 months while Fcal <200 µg/g.
Conclusions
Fcal level is highly accurate to predict and monitor the risk of relapse after therapeutic de-escalation in IBD patients and could be used in daily practice.
Keywords: Inflammatory bowel disease, faecal calprotectin, therapeutic de-escalation
1. Introduction
Inflammatory bowel diseases [IBD], including Crohn’s disease [CD] and ulcerative colitis [UC], are chronic, progressive, and disabling conditions.1 The natural history of these diseases may lead to cumulative bowel damage including fistulae, abscesses, and strictures in CD2 and dysmotility and colorectal neoplasm in UC.3–5 To alter the natural course of IBD, the therapeutic objectives have recently evolved from clinical remission to objective endpoints such as endoscopic mucosal healing.6–11 Besides, the tight control of objectively measured gastrointestinal inflammation, referred as the treat-to-target strategy, is now recommended in the management of IBD patients.6,12 However, repeated surveillance colonoscopies may be burdensome for patients,13 leading to the development of more convenient tools to monitor IBD patients. Among them, faecal calprotectin [Fcal] level has been shown to be a reliable surrogate marker of endoscopic activity14–19 and an independent predictor of clinical relapse in IBD patients achieving clinical remission during maintenance therapy.20–25
Therapeutic de-escalation is a rising question in IBD management. As IBDs are chronic and incurable diseases, selected patients are usually treated with sustained maintenance therapies. The prolonged use of such drugs increases the risk of drug-related complications26,27 and highly impacts on the health care system, due to increased direct and indirect costs.28–31 The best definition of the therapeutic target and a proactive management would enable safe therapeutic de-escalation in selected patients. De-escalation is an attractive option to limit safety concerns, encourage treatment adherence, and alleviate the economic burden on the health care system. However, it may expose IBD patients to high risk of relapse, ranging from 50% to 75%, depending on concomitant therapies.32 In the case of relapse, up to 20% of the patients did not respond to previous treatment,33 highlighting the need to select patients who could benefit from therapeutic de-escalation and to tightly monitor them. Many factors have been investigated as predictors of relapse after therapeutic de-escalation,32 but the role of Fcal in this specific situation remains poorly investigated and was limited to patients who discontinued infliximab.21,34 In this study, we aimed to assess the performance of Fcal level before therapeutic de-escalation as predictor of clinical relapse, and to evaluate the role of serial measurements of Fcal to predict clinical relapse after therapeutic de-escalation.
2. Methods
2.1. Ethical consideration
This study was performed in accordance with the Declaration of Helsinki, Good Clinical Practice, and applicable regulatory requirements. The study was approved by the Institutional Review Board [IRB17-0559].
2.2. Patients
From a prospectively maintained database [Genesys, IRB protocol 15573A], we identified all patients with established diagnosis of IBD who had been seen at the University of Chicago Medicine Inflammatory Bowel Disease Center andhave had an Fcal measurement. We enrolled all IBD patients older than 18 years, who were in clinical remission, with Fcal level assessed within 8 weeks before therapeutic de-escalation [mean time between Fcal measurement and de-escalation: 19.4 +/- 31.6 days]. All the patients had a follow-up longer than 3 months. Clinical characteristics including Harvey-Bradshaw index [HBI] and Simple Clinical Colitis Activity Index [SCCAI], as well as current medications, were retrieved from the prospectively maintained database.
2.3. Outcomes definitions
Clinical remission was defined as HBI ≤4 for CD patients, and SCCAI ≤2 with stool frequency subscore ≤1 and bleeding subscore ≤1 for UC patients. Therapeutic de-escalation was defined as one of the following conditions: decrease of dose; or increase of interval between two infusions/injections; or medication discontinuation; or replacement by a ‘lower’ medication (5-aminosalicylic acid [ASA] <thiopurines or methotrexate <biologics). In case of multiple concomitant drugs, these criteria applied to at least one IBD medication without intensification of another IBD therapy. Clinical relapse meant re-appearance of clinical manifestation [HBI >4] leading to: therapeutic intensification, hospitalisation, or CD-related surgery for CD patients; and re-appearance of clinical manifestation [SCCAI >2 with subscore >1 for at least one item among stool frequency and rectal bleeding] leading to medication intensification, hospitalization, or colectomy for UC patients.
2.4. Faecal calprotectin measurement
Calprotectin was measured, as routinely performed in our IBD centre, using a quantitative enzyme immunoassay test [Genova Diagnostics, Asheville, North Carolina]. Laboratory personnel, who were blinded from the current clinical activity of the patients, performed the analyses. The lower and the upper limits of detection for calprotectin were 15.6 and 2500 µg/g, respectively. Consequently, all calprotectin levels <15.6 and >2500 µg/g were considered as equal to 15.6 and 2500 µg/g, respectively. The results were expressed as µg/g.
2.5. Data management and statistical analysis
Statistical analysis was performed using Stata 13 software [StataCorp, College Station, TX]. The tests were two-sided, with a type I error set at a = 0.05. Subject’s characteristics were presented as mean ± standard deviation [SD] or median [interquartile range] for continuous data [assumption of normality assessed using the Shapiro-Wilk test], and as the number of patients and associated percentages for categorical parameters. Concerning the censored data, estimates were constructed using the Kaplan-Meier method. The log-rank test was used in a univariate analysis to test the prognostic value of patient characteristics for the occurrence of an event. For quantitative data (e.g., level of Fcal, level of C-reactive protein [CRP], or duration of clinical remission before therapeutic de-escalation), we performed ROC curves [when appropriate taking into account repeated measures] to determine the best threshold to predict the risk of relapse, fixed according to clinical relevance and usual indexes reported in literature.35,36 We used a statistics model that took into account the fact that several measurements came from the same patient and that there were variable numbers of measures at different time-points. Then, marginal Cox proportional hazards regression for repeated measures [with subject as random effect due to several measures for level of Fcal] was used to investigate associated factors in a multivariate situation, by backward and forward stepwise analysis of the factors considered significant in univariate analysis [entered the model if p <0.10] and according to clinically relevant parameters [especially the interval between therapeutic de-escalation and Fcal testing during the monitoring phase]. The proportional hazard hypothesis was verified using Schoenfeld’s test and plotting residuals. The interactions between prognostic factors were also tested. Results were expressed as HRs and 95% CI.
3. Results
3.1. Baseline characteristics of the patients
Overall, 160 patients were included in this study. The median interval between therapeutic de-escalation and clinical relapse or last follow-up was 45 [18.0–79.2] weeks. The baseline characteristics of these patients are detailed in Table 1. The types of therapeutic de-escalation are detailed in Figure 1.
Table 1.
Baseline characteristics of the 160 patients with inflammatory bowel diseases enrolled in this study. Results of univariate and multivariate analyses investigating the predictors of clinical relapse after therapeutic de-escalation.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| n = 160 patients | HR [95% CI] | p-Value | HR [95% CI] | p-Value | |
| Age at diagnosis mean ± sd | 25.9 ± 12.7 years | 1.0 [0.98–1.02] | 0.98 | ||
| Age at baseline mean ± sd | 36.0 ± 16.3 years | 1.0 [0.98–1.01] | 0.73 | ||
| Male gender n [%] | 81 [50.6%] | 0.95 [0.62–1.44] | 0.80 | ||
| Smoking habits | |||||
| Current smoker | 10 [6.3%] | Reference | |||
| Former smoker | 30 [18.7%] | 1.65 [0.56–4.88] | 0.36 | ||
| Non-smoker | 120 [75.0%] | 1.51 [0.55–4.17] | 0.42 | ||
| Disease duration | 9.7 ± 10.0 years | 0.99 [0.97–1.01] | 0.55 | ||
| IBD subtype | 1.31 [0.84–2.06] | 0.22 | |||
| Crohn’s disease n [%] | 112 [70.0%] | ||||
| Ulcerative colitis n [%] | 48 [30.0%] | ||||
| Montreal classification | |||||
| Location | |||||
| L1 n [%] | 37/112 [33.0%] | Reference | |||
| L2 n [%] | 24/112 [21.4%] | 1.26 [0.62–2.57] | 0.52 | ||
| L3 n [%] | 51/112 [45.5%] | 1.30 [0.71–2.38] | 0.40 | ||
| L4 n [%] | 19/112 [17.0%] | 0.85 [0.40–1.80] | 0.66 | ||
| Behaviour | |||||
| B1 n [%] | 52/112 [46.4%] | Reference | |||
| B2 n [%] | 32/112 [28.6%] | 1.10 [0.59–2.05] | 0.76 | ||
| B3 n [%] | 28/112 [25.0%] | 1.24 [0.67–2.32] | 0.48 | ||
| Extent | 0.69 | ||||
| E1 n [%] | 6/48 [12.5%] | Reference | |||
| E2 n [%] | 12/48 [25.0%] | 0.62 [0.19–2.04] | 0.43 | ||
| E3 n [%] | 30/48 [62.5%] | 0.67 [0.24–1.86] | 0.45 | ||
| Perianal lesions n [%] | 18/112 [16.4%] | 1.60 [0.83–3.09] | 0.16 | ||
| Previous intestinal resection n [%] | 48/112 [42.9%] | 1.27 [0.76–2.11] | 0.36 | ||
| HBI mean ± sd | 1.9 ± 1.5 | 1.46 [1.21–1.76] | <0.0001 | ||
| HBI ≤4 n [%] | 112/112 [100.0%] | - | - | ||
| SCCAI mean ± sd | 0.7 ± 0.9 | 0.018 | |||
| SCCAI ≤2 n [%] | 48/48 [100.0%] | - | - | ||
| SCCAI = 0 or HBI ≤2 | 100 [62.5%] | 3.05 [1.98–4.69] | <0.0001 | 1.02 [0.62–1.68] | 0.93 |
| Duration of clinical remission at baseline mean ± sd | 8.5 ± 14.8 months | 0.97 [0.95–0.99] | 0.016 | ||
| Duration of clinical remission at baseline >6 months, n [%] | 57 [35.6%] | 0.34 [0.21–0.57] | <0.0001 | 0.57 [0.33–0.99] | 0.044 |
| CRP mean ± sd | 6.6 ± 16.5 | 1.01 [1.00–1.02] | 0.037 | ||
| CRP > 5.0 g/L n [%] | 25/148 [16.9%] | 2.40 [1.50–3.85] | <0.0001 | 1.44 [0.81–2.59] | 0.22 |
| Faecal calprotectin mean ± sd | 233.9 ± 404.8 | 1.00 [1.00-1.00] | <0.0001 | ||
| Faecal calprotectin >100 µg/g n [%] | 62 [38.8%] | 5.55 [3.59–8.61] | <0.0001 | 3.96 [2.47–6.35] | <0.0001 |
| Medications at baseline | |||||
| Biologics | 117 [73.1%] | 1.27 [0.78–2.06] | 0.33 | ||
| Anti-TNF agents | 85 [53.1%] | 1.08 [0.71–1.64] | 0.72 | ||
| Infliximab | 38 [23.8%] | 1.10 [0.68–1.79] | 0.69 | ||
| Adalimumab | 42 [26.3%] | 1.07 [0.67–7.72] | 0.77 | ||
| Golimumab | 1 [0.6%] | - | NA | ||
| Certolizumab pegol | 4 [2.5%] | 0.32 [0.04–2.30] | 0.23 | ||
| Anti-integrins | 32 [20.0%] | 1.18 [0.69–2.02] | 0.52 | ||
| Vedolizumab | 31 [19.4%] | 1.12 [0.66–1.91] | 0.66 | ||
| Natalizumab | 1 [0.6%] | - | NA | ||
| Immunosuppressant therapies | 109 [68.1%] | 0.63 [0.41–0.97] | 0.036 | 0.82 [0.52–1.31] | 0.40 |
| Azathioprine | 42 [26.3%] | 0.83 [0.51–1.36] | 0.46 | ||
| 6-mercaptopurine | 18 [11.3%] | 0.71 [0.33–1.55] | 0.39 | ||
| Methotrexate | 48 [30.0%] | 0.81 [0.51–1.30] | 0.38 | ||
| Tacrolimus | 2 [1.3%] | NA | |||
| Cyclosporin | 1 [0.6%] | NA | |||
| Steroids | 40 [25.0%] | 2.75 [1.77–4.29] | <0.0001 | 1.67 [1.00–2.79] | 0.048 |
| Prednisone or prednisolone | 25 [15.6%] | 2.95 [1.80–4.83] | <0.0001 | ||
| Budesonide | 14 [8.8%] | 1.81 [0.96–3.41] | 0.061 | ||
| Budesonide MMX | 4 [2.5%] | 0.62 [0.08–4.50] | 0.63 | ||
| 5-ASA | 36 [22.5%] | 1.16 [0.68–1.82] | 0.66 |
NA, not applicable; sd, standard deviation; n, number; TNF, tumuor necrosis factor; IBD, inflammatory bowel disease; HBI, Harvey-Bradshaw index; SCCAI, Simple Clinical Colitis Activity Index; HR, hazard ratio; CI, confidence interval; CRP, C-reactive protein; MMX, multimatrix; 5-ASA, 5-aminosalicylic acid. Statistically significant values are given in boldface.
Figure 1.
Flow chart summarising the types of therapeutic de-escalation among the 160 patients with inflammatory bowel disease enrolled in this study.
3.2. Baseline level of faecal calprotectin as predictor of clinical relapse after therapeutic de-escalation
3.2.1. Overall population
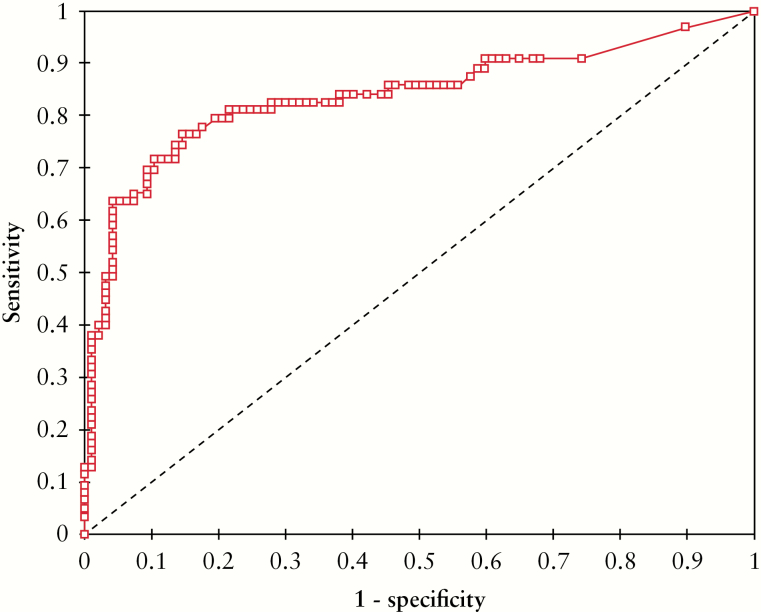
The mean levels of Fcal and CRP were 233.9 ± 404.8 µg/g and 6.6 ± 16.5 g/L, respectively. The mean duration of clinical remission before therapeutic de-escalation was 8.5 ± 14.8 months, with 57 patients [35.7%] with a clinical remission longer than 6 months. The level of Fcal before therapeutic de-escalation was inversely associated with the time of relapse [p <0.0001]. Using a ROC curve, we determined that an Fcal level >100 µg/g was the best threshold to predict the risk of clinical relapse within 1 year after therapeutic de-escalation [Figure 2] (area under the curve [AUC] = 0.84, sensitivity = 0.76, specificity = 0.86, positive predictive value [PPV] = 0.77, negative predictive value [NPV] = 0.85, accuracy = 0.82).
Figure 2.
Receiving operator curve illustrating the performances of faecal calprotectin level before therapeutic de-escalation, to predict the risk of relapse within 1 year.
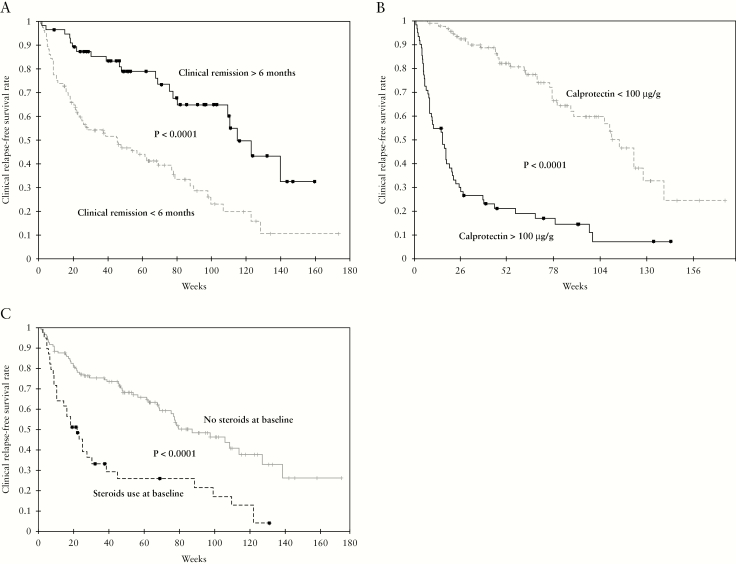
In univariate analysis [Table 1], lower clinical score [HBI ≤2 or SCCAI = 0] [p <0.0001], clinical remission longer than 6 months before therapeutic de-escalation [p <0.0001] [Figure 3A], and receiving immunosuppressant therapies [p = 0.036] were protective factors of clinical relapse. In contrast, CRP level >5 g/L [p <0.0001], Fcal level >100 µg/g [p <0.0001] [Figure 3B], and steroids usage at the time of therapeutic de-escalation [p <0.0001] [Figure 3C] were associated with higher risk of clinical relapse. The median interval between therapeutic de-escalation and clinical relapse was 16.0 [6.1–38.7] weeks in patients with Fcal level above 100 µg/g compared with 114.7 [69.1–139.7] weeks in those with Fcal level below 100 µg/g [p <0.0001]. In the subgroup of 98 patients with Fcal level below 100 µg/g, 35 experienced clinical relapse and the cumulative relapse-free survival rates were 97.9%, 92%, and 80.7% at, respectively, 12 weeks, 26 weeks, and 52 weeks following therapeutic de-escalation. In comparison, among the 62 patients with Fcal >100 µg/g at baseline, 53 experienced a relapse during the entire follow-up and the cumulative relapse-free survival rates were 54.8%, 28.3%, and 19.0% after 12 weeks, 26 weeks, and 52 weeks, respectively, [p <0.0001]. Among the 98 patients [61.3%] who had Fcal level <100 µg/g before therapeutic de-escalation, only one patient [1.0%] experienced relapse within 3 months [8.7 weeks after de-escalation] and seven patients relapsed within 6 months [7/88, 8.0%]. Given these findings, we suggest performing the first Fcal surveillance 3 months after therapeutic de-escalation.
Figure 3.
Kaplan-Meier survival curves showing the impact of clinical remission longer than 6 months [A], faecal calprotectin level above 100 µg/g [B], and steroids use at baseline [C] on the risk of clinical relapse in 160 IBD patients after therapeutic de-escalation.
In multivariate analysis, clinical remission longer than 6 months before therapeutic de-escalation (HR = 0.57 [0.33–0.99]; p = 0.044) was associated with decreased risk of clinical relapse, and Fcal level >100 µg/g (HR = 3.96 [2.47–6.35]; p <0.0001) and steroids usage at the time of therapeutic de-escalation (HR = 1.67 [1.00–2.79]; p <0.0001) were risk factors of clinical relapse in IBD patients after therapeutic de-escalation [Table 1].
3.2.2. IBD patients treated with anti-TNF agents
The baseline characteristics of the 85 IBD patients treated with anti-TNF agents are detailed in Table 2. In univariate analysis [Table 2], a lower clinical score [HBI ≤2 or SCCAI = 0] [p = 0.014] and a clinical remission longer than 6 months before therapeutic de-escalation [p = 0.022] were associated with decreased risk of clinical relapse after therapeutic de-escalation. We also observed a non-significant trend toward lower risk of relapse among patients who discontinued immunosuppressant therapy [p = 0.055]. In contrast, CRP level >5 g/L [p = 0.01], Fcal level >100 µg/g [p < 0.0001] [Supplementary Figure S1, available as Supplementary data at ECCO-JCC online], higher infliximab trough levels [p = 0.026], and steroids usage at the time of therapeutic de-escalation [p = 0.021] were associated with higher risk of clinical relapse. We did not observe any impact of adalimumab drug concentration [p = 0.25] or 6-TGN levels [p = 0.83] on the risk of relapse.
Table 2.
Baseline characteristics of the 85 patients with inflammatory bowel diseases treated with anti-TNF agents in our cohort. Results of univariate and multivariate analyses investigating the predictors of clinical relapse after therapeutic de-escalation.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| n = 85 patients | HR [95% CI] | p-Value | HR [95% CI] | p-Value | |
| Age at diagnosis mean ± sd | 24.2 ± 11.3 years | 0.98 [0.96–1.01] | 0.30 | ||
| Age at baseline mean ± sd | 33.5 ± 14.0 years | 0.99 [0.97–1.01] | 0.46 | ||
| Male gender n [%] | 45 [52.9%] | 0.55 | |||
| Smoking habits | |||||
| Current smoker | 5 [5.9%] | Reference | |||
| Former smoker | 18 [21.1%] | 0.92 [0.20–4.27] | 0.92 | ||
| Non-smoker | 62 [73.0%] | 1.11 [0.26–4.66] | 0.89 | ||
| Disease duration | 8.8 ± 8.9 years | 1.00 [0.97–1.04] | 0.92 | ||
| IBD subtype | 1.54 [0.80–2.98] | 0.19 | |||
| Crohn’s disease n [%] | 68 [80.0%] | ||||
| Ulcerative colitis n [%] | 17 [20.0%] | ||||
| Montreal classification | |||||
| Location | |||||
| L1 n [%] | 24/68 [35.3%] | Reference | |||
| L2 n [%] | 13/68 [19.1%] | 1.27 [0.49–3.27] | 0.62 | ||
| L3 n [%] | 31/68 [45.6%] | 1.62 [0.76–3.43] | 0.21 | ||
| L4 n [%] | 11/68 [16.2%] | 0.85 [0.33–2.20] | 0.74 | ||
| Behaviour | 0.76 | ||||
| B1 n [%] | 31/68 [45.6%] | Reference | |||
| B2 n [%] | 21/68 [30.9%] | 1.04 [0.47–2.30] | 0.93 | ||
| B3 n [%] | 16/68 [23.5%] | 1.33 [0.60–2.94] | 0.48 | ||
| Extent | 0.58 | ||||
| E1 n [%] | 2/17 [11.8%] | Reference | |||
| E2 n [%] | 5/17 [29.4%] | 0.49 [0.09–3.00] | 0.44 | ||
| E3 n [%] | 10/17 [58.8%] | 0.43 [0.08–2.24] | 0.32 | ||
| Perianal lesions n [%] | 9/68 13.2%] | 1.56 [0.60–4.05] | 0.35 | ||
| Previous intestinal resection n [%] | 30/68 [44.1%] | 1.37 [0.71–2.64] | 0.34 | ||
| HBI ≤4 n [%] | 68/68 [100.0%] | - | NA | ||
| SCCAI ≤2 n [%] | 17/17 [100%] | - | NA | ||
| SCCAI >0 or HBI >2 | 31 [36.5%] | 2.02 [1.14–3.59] | 0.014 | 1.14 [0.57–2.28] | 0.72 |
| Duration of clinical remission at baseline mean ± sd | 6.7 ± 12.2 months | 0.99 [0.96–1.02] | 0.51 | ||
| Duration of clinical remission at baseline >6 months n [%] | 26 [30.6%] | 1.45 [0.22–0.91] | 0.022 | 0.65 [0.30–1.44] | 0.29 |
| CRP mean ± sd | 6.1 ± 13.6 | 1.00 [0.99–1.02] | 0.22 | ||
| CRP >5.0 g/L n [%] | 16 [18.8%] | 1.80 [1.04–3.44] | 0.01 | 2.49 [1.26–4.93] | 0.009 |
| Faecal calprotectin >100 µg/g n [%] | 33 [38.8%] | 8.5 [4.34–16.57] | <0.0001 | 7.08 [3.22–15.53] | <0.0001 |
| Medications at baseline | |||||
| Anti-TNF medications | |||||
| Infliximab | 38 [44.7%] | 1.08 [0.61–1.91] | 0.78 | ||
| Infliximab trough level median [IQR] [n = 25] | 11.0 [5.9–25.0] | 2.81 [1.12–9.32] | 0.026 | 2.26 [0.62–8.21] | 0.22 |
| Infliximab trough level >11 | 13/25 [52.0%] | 3.31 [1.12–13.56] | 0.07 | ||
| Antibodies anti-infliximab positive | 1/25 [4.0%] | NA | |||
| Adalimumab | 42 [49.4%] | 1.02 [0.58–1.80] | 0.94 | ||
| Adalimumab drug concentration median [IQR] [n = 23] | 9.2 [6.4–13.9] | 1.16 [0.78–4.24] | 0.25 | ||
| Adalimumab drug concentration >9.2 | 13/23 [56.5%] | 1.08 [0.67–1.97] | 0.61 | ||
| Antibodies anti-adalimumab positive | 2/23 [8.7%] | - | NA | ||
| Golimumab | 1 [1.2%] | - | NA | ||
| Certolizumab pegol | 4 [4.7%] | 0.30 [0.04–2.20] | NA | ||
| Immunosuppressant therapies | 62 [72.9%] | 0.74 [0.39–1.39] | 0.34 | ||
| Azathioprine | 25 [29.4%] | 0.99 [0.53–1.87] | 0.99 | ||
| 6-mercaptopurin | 5 [5.9%] | 0.84 [0.20–3.50] | 0.81 | ||
| 6-TGN concentration [n = 16] | 199.6 ± 126.0 | 0.83 | |||
| Methotrexate | 32 [37.6%] | 0.81 [0.45–1.46] | 0.48 | ||
| Steroids | 16 [18.8%] | 2.13 [1.10–4.14] | 0.021 | 2.17 [0.97–4.84] | 0.058 |
| 5-ASA | 8 [9.4%] | 0.83 [0.33–2.12] | 0.70 | ||
| Type of therapeutic de-escalation | |||||
| Anti-TNF discontinuation | 22 [25.9%] | 1.2 [0.65–2.26] | 0.55 | ||
| Immunosuppressants discontinuation | 35 [41.2%] | 0.56 [0.30–1.02] | 0.055 | 1.02 [0.46–2.23] | 0.97 |
| 5-ASA discontinuation | 1 [1.2%] | - | NA | ||
| Decrease of anti-TNF dose | 3 [3.5%] | 0.67 [0.16–2.83] | 0.59 | ||
| Increase of anti-TNF interval | 8 [9.4%] | 1.12 [0.40–3.17] | 0.82 | ||
| Steroids discontinuation | 5 [5.9%] | 1.89[0.47–5.72] | 0.50 | ||
| Budesonide discontinuation | 6 [7.0%] | 1.33 [0.47–3.72] | 0.58 | ||
| Decrease of immunosuppressants dose | 5 [5.9%] | 0.63 [0.15–2.63] | 0.52 |
NA, not applicable; sd, standard deviation; n, number; TNF, tumour necrosis factor; IBD, inflammatory bowel disease; HBI, HarveyBradshaw index; SCCAI, Simple Clinical Colitis Activity Index; HR, hazard ratio; CI, confidence interval; 6-TGN, 6-thioguanines; CRP, C-reactive protein; 5-ASA, 5-aminosalicylic acid; IQR, interquartile range. Statistically significant values are given in boldface.
In multivariate analysis [Table 2], CRP level >5 g/L (HR = 2.49 [1.26–4.93]; p = 0.009) and Fcal level >100 µg/g (HR = 7.08 [3.22–15.53]; p < 0.0001) were associated with higher risk of clinical relapse after therapeutic de-escalation in patients treated with anti-TNF therapy. We observed also the same trend with steroids usage at the time of therapeutic de-escalation (HR = 2.17 [0.97–4.84]; p = 0.058), but it did not reach the statistical significance. The other factors, which were tested in the multivariate analysis, were not associated with the risk of relapse [see Table 2].
3.2.3. IBD patients treated with integrins antagonists
Overall, 32 IBD patients were treated with integrins antagonists. Their characteristics are given in Table 3. In univariate analysis, a lower clinical score [HBI ≤2 or SCCAI = 0] [p = 0.001] and a clinical remission longer than 6 months before therapeutic de-escalation [p = 0.015] were protective factors of clinical relapse. In contrast, Fcal level >100 µg/g [p <0.0001] [Supplementary Figure S2, available as Supplementary data at ECCO-JCC online], and steroids usage at the time of therapeutic de-escalation [p = 0.013] were associated with higher risk of clinical relapse. In multivariate analysis, Fcal level >100 µg/g was the only factor associated with an increased risk of clinical relapse after therapeutic de-escalation in patients treated with anti-integrins (HR = 15.68 [2.49–98.54], p = 0.003) [Table 3].
Table 3.
Baseline characteristics of the 32 patients with inflammatory bowel diseases treated with integrins antagonists in our cohort. Results of univariate and multivariate analyses investigating the predictors of clinical relapse after therapeutic de-escalation.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| n = 32 patients | HR [95% CI] | p-Value | HR [95% CI] | p-Value | |
| Age at diagnosis mean ± sd | 28.0 ± 14.2 years | 0.99 [0.95–1.02] | 0.48 | ||
| Age at baseline mean ± sd | 40.9 ± 17.9 years | 0.98 [0.96–1.01] | 0.21 | ||
| Male gender n [%] | 16 [50.0%] | 0.59 | |||
| Smoking habits | 0.49 | ||||
| Current smoker | 3 [9.4%] | Reference | |||
| Former smoker | 6 [18.8%] | 1.81 [0.19–17.04] | 0.60 | ||
| Non-smoker | 23 [71.8%] | 2.78 [0.36–21.51] | 0.33 | ||
| Disease duration | 12.4 ± 12.7 years | 0.98 [0.94–1.02] | 0.34 | ||
| IBD subtype | 2.51 [0.87–7.28] | 0.079 | |||
| Crohn’s disease n [%] | 23 [71.8%] | ||||
| Ulcerative colitis n [%] | 9 [28.2%] | ||||
| Montreal classification | |||||
| Location | |||||
| L1 n [%] | 6/23 [26.1%] | Reference | |||
| L2 n [%] | 4/23 [17.4%] | 1.22 [0.20–7.63] | 0.83 | ||
| L3 n [%] | 13/23 [56.5%] | 1.08 [0.28–4.19] | 0.91 | ||
| L4 n [%] | 4/23 [17.4%] | 1.52 [0.32–7.35] | 0.60 | ||
| Behaviour | 0.54 | ||||
| B1 n [%] | 10/23 [43.5%] | Reference | |||
| B2 n [%] | 6/23 [26.1%] | 0.15 [0.02–1.30] | 0.086 | ||
| B3 n [%] | 7/23 [30.4%] | 1.70 [0.48–6.03] | 0.411 | ||
| Extent | 0.61 | ||||
| E1 n [%] | 3/9 [33.3%] | - | |||
| E2 n [%] | 2/9 [22.2%] | - | |||
| vE3 n [%] | 4/9 [45.5%] | - | |||
| Perianal lesions n [%] | 6/23 [26.1%] | 1.26 [0.33–4.90] | 0.73 | ||
| SCCAI>0 or HBI > 2 | 16 [50.0%] | 6.98 [1.95–24.90] | 0.001 | 1.92 [0.42–8.75] | 0.40 |
| Duration of clinical remission at baseline >6 months n, % | 6 [18.8%] | 0.11 [0.01–0.93] | 0.015 | 0.19 [0.02–1.86] | 0.15 |
| CRP > 5.0 g/L n [%] | 4/27 [14.8%] | 2.35 [0.78–7.11] | 0.12 | ||
| Faecal calprotectin > 100 µg/g n [%] | 15 [46.9%] | 19.00 [4.08–88.81] | <0.0001 | 15.68 [2.49–98.54] | 0.003 |
| Medications at baseline | |||||
| Anti-integrins medication | |||||
| Vedolizumab | 31 [96.9%] | - | |||
| Natalizumab | 1 [3.1%] | NA | |||
| Vedolizumab infusion interval | 31[96.9%] | 0.62 | |||
| Every 8 weeks | 26/31 [83.9%] | - | |||
| Every 6 weeks | 1/31 [3.2%] | NA | |||
| Every 4 weeks | 4/31 [12.9%] | NA | |||
| Immunosuppressant therapies | 20 [62.5%] | 0.74 [0.29–1.90] | 0.52 | ||
| Azathioprine | 7 [21.9%] | 0.43 [0.10–1.89] | 0.23 | ||
| 6-mercaptopurin | 2 [6.3%] | 1.14 [0.18–10.78] | 0.74 | ||
| Methotrexate | 10 [31.3%] | 1.04 [0.36–3.02] | 0.93 | ||
| Tacrolimus | 2 [6.3%] | - | 0.28 | ||
| Cyclosporin | 1 [3.1%] | - | NA | ||
| Steroids | 14 [43.7%] | 3.25 [1.19–8.90] | 0.013 | 1.36 [0.35–5.23] | 0.66 |
| 5-ASA | 8 [25.0%] | 2.10 [0.76–5.82] | 0.14 | ||
| Type of therapeutic de-escalation | |||||
| Anti-integrins discontinuation | 3 [9.4%] | 0.48 [0.06–3.69] | 0.47 | ||
| Immunosuppressants discontinuation | 11 [34.4%] | 0.62 [0.20–1.93] | 0.40 | ||
| 5-ASA discontinuation | 4 [12.5%] | 1.09 [0.25–4.83] | 0.90 | ||
| Steroids discontinuation | 8 [25.0%] | 1.55 [0.57–4.19] | 0.37 | ||
| Steroids tapering | 4 [12.5%] | 1.91 [0.61–6.04] | 0.23 | ||
| Budesonide discontinuation | 1 [3.1%] | - | NA | ||
| Decrease of Immunosuppressants dose | 1 [3.1%] | - | NA |
NA: not applicable; sd: standard deviation; n: number; TNF: tumor necrosis factor; IBD: inflammatory bowel disease; HBI: Harvey-Bradshaw index; SCCAI: simple clinical colitis activity index; HR: hazard ratio; CI: confidence interval; CRP: C-reactive protein. Statistically significant values are given in boldface.
3.2.4. IBD patients treated without biologics
In this cohort, 43 patients did not receive any biologics at the time of therapeutic de-escalation. The baseline characteristics of this subgroup are detailed in Table 4. In univariate analysis [Table 2], a lower clinical score [HBI ≤2 or SCCAI = 0], a clinical remission longer than 6 months before therapeutic de-escalation [p = 0.001], older age at baseline [p < 0.001], and shorter disease duration [p = 0.009] were protective factors of clinical relapse. In contrast, Fcal level >100 µg/g [p = 0.025], higher clinical score [HBI or SCCAI] [p <0.001], and steroids usage at the time of therapeutic de-escalation [p = 0.001] were associated with higher risk of clinical relapse [Table 4]. In multivariate analysis, a clinical remission longer than 6 months before therapeutic de-escalation was associated with decreased risk of relapse (HR = 0.17 [0.03–0.86]; p = 0.032). In contrast, Fcal level >100 µg/g (4.21 [1.01–17.63]; p = 0.049) and higher clinical score [HBI >2 or SCCAI>0] (3.87 [1.12–13.40]; p = 0.032) were risk factors of relapse after therapeutic de-escalation in the subgroup of patients who did not receive any biologics [Figure S3, available as Supplementary data at ECCO-JCC online].
Table 4.
Baseline characteristics of the 43 patients with inflammatory bowel diseases who were not receiving any biologics in our cohort. Results of univariate and multivariate analyses investigating the predictors of clinical relapse after therapeutic de-escalation.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| N = 43 patients | HR [95% CI] | P-value | HR [95% CI] | P-value | |
| Age at diagnosis mean ± sd | 27.5 ± 14.2 years | 1.02 [0.99–1.05] | 0.10 | ||
| Age at baseline mean ± sd | 37.4 ± 18.6 years | 1.01 [0.99–1.04] | < 0.001 | 1.05 [1.03–1.20] | 0.13 |
| Male gender n [%] | 25 [58.1%] | 0.31 | |||
| Smoking habits | 0.053 | ||||
| Current smoker | 2 [4.7%] | Reference | |||
| Former smoker | 6 [14.0%] | 4.6 [0.52–40.52] | 0.17 | ||
| Non-smoker | 35 [81.4%] | 1.43 [0.19–10.96] | 0.73 | ||
| Disease duration | 9.5 ± 9.7 years | 0.95 [0.90–1.01] | 0.009 | 0.91 [0.82–1.01] | 0.087 |
| IBD subtype | 1.03 [0.45–2.39] | 0.93 | |||
| Crohn’s disease n [%] | 21 [48.8%] | ||||
| Ulcerative colitis n [%] | 22 [51.2%] | ||||
| Montreal classification | |||||
| Location | 0.60 | ||||
| L1 n [%] | 7/21 [33.3%] | Reference | |||
| L2 n [%] | 7/21 [33.3%] | 1.04 [0.24–4.47] | 0.95 | ||
| L3 n [%] | 7/21 [33.3%] | 0.52 [0.10–2.70] | 0.43 | ||
| L4 n [%] | 4/21 [19.0%] | 0.44 [0.05–3.49] | 0.43 | ||
| Behaviour | 0.11 | ||||
| B1 n [%] | 11/21 [52.4%] | Reference | |||
| B2 n [%] | 5/21 [23.8%] | 22.97 [2.49–211.63] | 0.006 | ||
| B3 n [%] | 5/21 [23.8%] | 0.38 [0.04–3.27] | 0.38 | ||
| Extent | 0.53 | ||||
| E1 n [%] | 1/22 [4.5%] | Reference | |||
| E2 n [%] | 5/22 [22.7%] | 0.25 [0.02–3.04] | |||
| E3 n [%] | 16/22 [72.7%] | 0.38 [0.05–3.25] | |||
| SCCAI>0 or HBI > 2 | 13 [30.2%] | 4.75 [1.93–11.69] | <0.001 | 3.87 [1.12–13.40] | 0.032 |
| Duration of clinical remission at baseline >6 months n, % | 25 [58.1%] | 0.26 [0.11–0.67] | 0.001 | 0.17 [0.03–0.86] | 0.032 |
| CRP > 5.0 g/L n [%] | 5 [11.6%] | 4.22 [1.68–10.58] | 0.26 | ||
| Faecal calprotectin > 100 µg/g n [%] | 14 [32.6%] | 2.55 [1.09–5.95] | 0.025 | 4.21 [1.01–17.63] | 0.049 |
| Medications at baseline | |||||
| Immunosuppressant therapies | 27 [62.8%] | 0.46 [0.20–1.06] | 0.062 | ||
| Azathioprine | 10 [23.3%] | 0.86 [0.32–2.36] | 0.78 | ||
| 6-mercaptopurin | 11 [25.6%] | 0.68 [0.23–2.04] | 0.50 | ||
| 6-TGN concentration [n = 12] | 266.8 ± 127.5 | 0.29 | |||
| Methotrexate | 6 [14.0%] | 0.27 [0.04–2.01] | 0.17 | ||
| Steroids | 9 [20.9%] | 4.45 [1.78–11.17] | 0.001 | 2.85 [0.64–12.87] | 0.17 |
| 5-ASA | 20 [46.5%] | 1.20 [0.52–2.79] | 0.66 | ||
| Type of therapeutic de-escalation | |||||
| Immunosuppressants discontinuation | 19 [44.2%] | 0.75 [0.32–1.80] | 0.53 | ||
| Decrease of Immunosuppressants dose | 4 [9.3%] | 0.25 [0.034–1.91] | 0.15 | ||
| 5-ASA discontinuation | 4 [9.3%] | 1.09 [0.25–4.76] | 0.90 | ||
| Decrease of 5ASA dose | 8 [18.6%] | 0.69 [0.23–2.06] | 0.50 | ||
| Steroids tapering | 2 [4.7%] | 7.99 [0.59–40.09] | 0.13 | ||
| Steroids discontinuation | 2 [4.7%] | 2.07 [0.48–8.98] | 0.32 | ||
| Budesonide discontinuation | 6 [14.0%] | 3.05 [0.95–9.76] | 0.11 |
NA: not applicable; sd: standard deviation; n: number; TNF: tumor necrosis factor; IBD: inflammatory bowel disease; HBI: Harvey-Bradshaw index; SCCAI: simple clinical colitis activity index; HR: hazard ratio; CI: confidence interval; CRP: C-reactive protein. Statistically significant values are given in boldface.
3.2.5. IBD patients attempting to discontinue steroids
In our cohort, 37 IBD patients attempted to discontinue oral steroids. Demographics of this subgroup are mentioned in Table 5. The mean duration of clinical remission was 1.5 ± 3.6 months at the time of therapeutic de-escalation. Overall, 25 patients received prednisone or prednisolone [67.6%] with a mean dose of 31.0 ± 13.6 mg. In addition, 11 [29.7%] and four [10.8%] patients were treated with budesonide and budesonide MMX [multimatrix], respectively. Most of the patients received concomitant medications besides steroids, such as anti-TNF agents [n = 15, 40.5%], integrins antagonists [n = 13, 35.1%], thiopurines [n = 9, 24.3%], methotrexate [n = 12, 32.4%], or 5-ASA [n = 5, 13.5%].
Table 5.
Baseline characteristics of the 37 patients with inflammatory bowel diseases attempting to discontinue steroids in this study. Results of univariate and multivariate analyses investigating the predictors of steroids dependency.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| N = 37 patients | HR [95% CI] | p-value | HR [95% CI] | p-value | |
| Age at diagnosis mean ± sd | 27.0 ± 14.9 years | 0.99 [0.96–1.02] | 0.48 | ||
| Age at baseline mean ± sd | 37.6 ± 19.0 years | 0.99 [0.97–1.01] | 0.29 | ||
| Female gender n [%] | 18 [48.6%] | 0.06 | 0.42 [0.19–0.93] | 0.033 | |
| Smoking habits | 0.12 | ||||
| Current smoker | 2 [5.4%] | Reference | |||
| Former smoker | 9 [24.3%] | 0.24 [0.04–1.32] | 0.10 | ||
| Non-smoker | 26 [70.3%] | 0.60 [0.14–2.64] | 0.50 | ||
| Disease duration | 10.0 ± 10.8 years | 0.37 | |||
| IBD subtype | 1.37 [0.60–3.10] | 0.45 | |||
| Crohn’s disease n [%] | 26 [70.3%] | ||||
| Ulcerative colitis n [%] | 11 [29.7%] | ||||
| Montreal classification | |||||
| Location | 0.35 | ||||
| L1 n [%] | 9/26 [34.6%] | Reference | |||
| L2 n [%] | 4/26 [15.4%] | 0.35 [0.07–1.66] | 0.19 | ||
| L3 n [%] | 13/26 [50.0%] | 0.63 [0.23–1.73] | 0.37 | ||
| L4 n [%] | 2/26 [7.7%] | 1.93 [0.43–8.65] | 0.39 | ||
| Behaviour | 0.57 | ||||
| B1 n [%] | 11/26 [42.3%] | Reference | |||
| B2 n [%] | 8/26 [30.8%] | 1.51 [0.55–4.13] | 0.42 | ||
| B3 n [%] | 7/26 [26.9%] | 1.64 [0.52–5.9] | 0.40 | ||
| Extent | 0.66 | ||||
| E1 n [%] | 2/11 [18.2%] | Reference | |||
| E2 n [%] | 3/11 [27.3%] | 1.35 [0.21–8.71] | 0.75 | ||
| E3 n [%] | 6/11 [54.5%] | 0.96 [0.17–5.41] | 0.96 | ||
| Perianal lesions n [%] | 7/26 [26.9%] | 1.22 [0.42–3.53] | 0.70 | ||
| Prior intestinal resection n [%] | 11/26 [42.3%] | 1.65 [0.70–3.94] | 0.22 | ||
| SCCAI = 0 or HBI ≤ 2 | 23 [62.2%] | 4.81 [1.74–13.32] | 0.001 | 1.72 [0.52–5.66] | 0.375 |
| Duration of clinical remission at baseline mean ± sd | 1.5 ± 3.6 months | 0.98 [0.96–1.02] | 0.13 | ||
| Duration of clinical remission at baseline >6 months n, % | 4 [10.8%] | 0.49 [0.16–1.48] | 0.17 | ||
| CRP > 5.0 g/L n [%] | 8 [21.6%] | 1.79 [0.77–4.11] | 0.16 | ||
| Faecal calprotectin > 100 µg/g n [%] | 15 [40.5%] | 7.52 [2.78–20.32] | <0.0001 | 7.43 [2.31–23.92] | 0.001 |
| durin | |||||
| Biologics | 28 [75.7%] | 0.84 | |||
| Anti-TNF agents | 15 [40.5%] | 1.39 [0.66–2.96] | 0.38 | ||
| Infliximab | 4 [10.8%] | 1.84 [0.63–5.39] | 0.25 | ||
| Adalimumab | 10 [27.0%] | 0.93 [0.39–2.20] | 0.87 | ||
| Golimumab | 1 [2.7%] | - | NA | ||
| Anti-integrins | 13 [35.1%] | 0.76 [0.34–1.69] | 0.50 | ||
| Vedolizumab | 12 [32.4%] | 0.64 [0.28–1.48] | 0.47 | ||
| Natalizumab | 1 [2.7%] | - | NA | ||
| Immunosuppressant therapies | 21 [56.8%] | 1.19 [0.56–2.56] | 0.64 | ||
| Azathioprine | 7 [18.9%] | 1.46 [0.58–3.67] | 0.41 | ||
| 6-mercaptopurin | 2 [5.4%] | - | 0.11 | ||
| Methotrexate | 12 [32.4%] | 1.38 [0.63–3.01] | 0.40 | ||
| Steroids | |||||
| Prednisone or prednisolone | 25 [67.6%] | 1.44 [0.63–3.30] | 0.38 | ||
| Prednisone or prednisolone posology [mg] | 31.0 ± 13.6 | 0.46 | |||
| Budesonide | 11 [29.7%] | 1.01 [0.46–2.25] | 0.97 | ||
| Budesonide MMX | 4 [10.8%] | 0.23 [0.03–1.72] | 0.12 | ||
| 5-ASA | 5 [13.5%] | 1.70 [0.63–4.60] | 0.28 |
NA: not applicable; sd: standard deviation; n: number; TNF: tumor necrosis factor; IBD: inflammatory bowel disease; HBI: Harvey-Bradshaw index; SCCAI: simple clinical colitis activity index; HR: hazard ratio; CI: confidence interval; CRP: C-reactive protein. Statistically significant values are given in boldface.
In univariate analysis, female gender [p = 0.06] and a lower clinical score [HBI ≤2 or SCCAI = 0] [p = 0.001] were protective factors, whereas Fcal level >100 µg/g [p <0.0001] was a risk factor of relapse when attempting to discontinue steroids [Supplementary Figure S4, available as Supplementary data at ECCO-JCC online]. In multivariate analysis, female gender (HR = 0.42 [0.19–0.93]; p = 0.033) was associated with decreased risk of relapse, and Fcal level >100 µg/g (HR = 7.43 [2.31–23.92]; p = 0.001) was the only risk factor of clinical relapse in patients attempting to discontinue steroids [Table 5].
3.2.6. Sensitivity analysis in patients with ulcerative colitis
The baseline characteristics of the 48 patients with UC are detailed in Table 1. In univariate analysis, Fcal level >100 µg/g (HR = 5.58 [2.42–12.83], p < 0.0001), SCCAI = 1 or 2 vs SCCAI = 0 (HR = 2.95 [1.33–6.54]; p = 0.008) and steroids usage at the time of therapeutic de-escalation (HR = 4.04 [1.68–9.72]; p = 0.001) were associated with higher risk of clinical relapse. In contrast, a clinical remission longer than 6 months before therapeutic de-escalation (HR = 0.29 [0.12–0.70]; p = 0.004) decreased the risk of clinical relapse. In multivariate analysis, Fcal level >100µg/g was the only predictor for clinical relapse in patients with UC (HR = 4.15 [1.70–10.15]; p = 0.002).
3.2.7. Sensitivity analysis in patients with Crohn’s disease
The characteristics of the 112 patients with CD at the time of therapeutic de-escalation are mentioned in Table 1. In univariate analysis, steroids usage at the time of therapeutic de-escalation (HR = 2.65 [1.56–4.51]; p < 0.001), HBI >2 (HR = 3.07 [1.82–5.18]; p <0.0001), Fcal level >100 µg/g (HR = 5.81 [3.41–9.90]; p < 0.0001), and CRP level >5 mg/L (HR = 2.56 [1.44–4.56]; p = 0.001) were associated with a higher risk of relapse. In contrast, clinical remission longer than 6 months before therapeutic de-escalation (HR = 0.37 [0.20–0.67]; p = 0.001) was associated with a lower risk of clinical relapse.
In multivariate analysis, Fcal level >100µg/g was the only predictor for clinical relapse in patients with CD (HR = 3.82 [2.08–7.02]; p < 0.0001). In contrast, we did not find any significant relationship between the risk of clinical relapse and the following factors included in the statistical model: clinical remission longer than 6 months before therapeutic de-escalation (HR = 0.65 [0.33–1.27]; p = 0.21); perianal lesions (HR = 2.00 [0.96–4.21];, p = 0.066), HBI >2 (HR = 1.73 [0.96–3.12]; p = 0.067), and CRP level >5 mg/L (HR = 1.75 [0.92–3.32]; p = 0.09).
3.3. Repeated measurements of faecal calprotectin after therapeutic de-escalation as predictor of clinical relapse
In our cohort, 52 IBD patients had at least one Fcal testing during the follow-up after therapeutic de-escalation (Table 6). These patients were treated with one or several medications among anti-TNF agents [25/52, 48.1%], integrins antagonists [10/52, 19.2%], thiopurines [19/52, 36.5%], methotrexate [13/52, 25.0%], steroids [9/52, 17.3%], or 5-ASA [12/52, 23.1%]. Among the patients treated with biologics, 13 patients discontinued biologics, 11 stopped immunosuppressant therapies, two decreased biologics dose, one increased interval between infusions, five decreased the dose of immunosuppressant therapies, and three attempted to stop steroids. In patients receiving no biologics, the types of de-escalation were discontinuation [n = 2] or dose decrease [n = 8] of immunosuppressant medication, discontinuation [n = 3] or dose decrease [n = 1] of 5-ASA, or steroids weaning [n = 5] [several of them may apply for one patient].
Table 6.
Characteristics at the time of therapeutic de-escalation of the 52 patients with IBD with serial monitoring of faecal calprotectin.
| Age at baseline, mean ± sd | 38.2 ± 17.6 years |
| Female gender n [%] | 27 [51.9%] |
| Current smoker | 4 [7.7%] |
| Disease duration, mean ± sd | 9.9 ± 10.2 years |
| IBD subtype | |
| Crohn’s disease n [%] | 37 [71.2%] |
| Ulcerative colitis n [%] | 15 [28.8%] |
| Montreal classification | |
| Location | |
| L1 n [%] | 16/37 [43.2%] |
| L2 n [%] | 10/37 [27.0%] |
| L3 n [%] | 11/37 [29.7%] |
| L4 n [%] | 8/37 [21.7%] |
| Behaviour | |
| B1 n [%] | 17/37 [46.0%] |
| B2 n [%] | 13/37 [35.1%] |
| B3 n [%] | 7/37 [18.9%] |
| Extent | |
| E1 n [%] | 3/15 [20.0%] |
| E2 n [%] | 4/15 [26.7%] |
| E3 n [%] | 8/15 [53.3%] |
| Perianal lesions n [%] | 4/37 [10.8%] |
| Previous intestinal resection n [%] | 15/37 [40.5%] |
| Faecal calprotectin >100 µg/g n [%] | 13 [25.0%] |
| Baseline level of faecal calprotectin, mean ± sd | 89.1 ± 147.2 µg/g |
IBD, inflammatory bowel disease; sd, standard deviation; n, number.
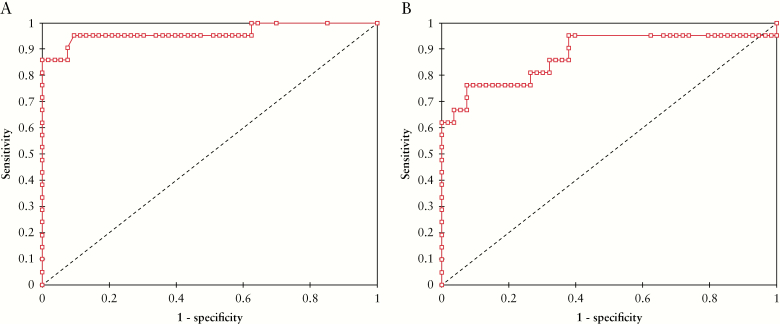
These 52 patients underwent a total of 74 calprotectin measurements performed during the surveillance following therapeutic de-escalation [mean level = 182.3 ± 366.6 µg/g]. The mean interval between therapeutic de-escalation and these measurements was 40 ± 28.3 weeks. Using a ROC curve [Figure 4A], we determined that Fcal level >200 µg/g was the best threshold to avoid clinical relapse within 12 months [highest NPV] in IBD patients monitored after therapeutic de-escalation [AUC = 0.96, sensitivity = 0.93, specificity = 0.93, PPV = 0.78, NPV = 0.98]. A level of Fcal >400 µg demonstrated the highest PPV to predict clinical relapse within 12 months (sensitivity = 0.53 [0.27–0.79], specificity = 1.00 [0.63–1.00], PPV = 1.00 [0.63–1.00], NPV = 0.89 [0.79–0.96]) and should then lead to therapeutic intensification. We also found that an increase of Fcal level [ΔFcal] compared with baseline [i.e., the value of Fcal at the time of therapeutic de-escalation] >+100 µg/g was the best variation to predict relapse within 12 months [AUC = 0.80, sensitivity = 0.67, specificity = 0.88, PPV = 0.59, NPV = 0.91] [Figure 4B]. Using a Kaplan-Meier curve, we observed that Fcal level >200 µg/g [Figure 5], >400 µg/g, and ΔFcal >+100 µg/g were associated with higher risk of relapse [p <0.0001 for both]. We also investigated whether Fcal level >200 µg/g and/or ΔFcal >+100 µg/g could be of additional value, but we failed to show any superiority compared with Fcal level >200 µg/g or >400 µg/g alone in predicting clinical relapse [data not shown]. In univariate analysis, we also observed that a lower clinical score [HBI ≤2 or SCCAI = 0] [p <0.001], a clinical remission longer than 6 months before calprotectin testing [p <0.0001], and receiving immunosuppressant therapies [p = 0.002] were protective factors of clinical relapse.
Figure 4.
Receiving operator curve illustrating the performances of faecal calprotectin level [4] and faecal calprotectin variation [B] compared with baseline, during the monitoring after therapeutic de-escalation to predict the risk of relapse within s6 months.
Figure 5.
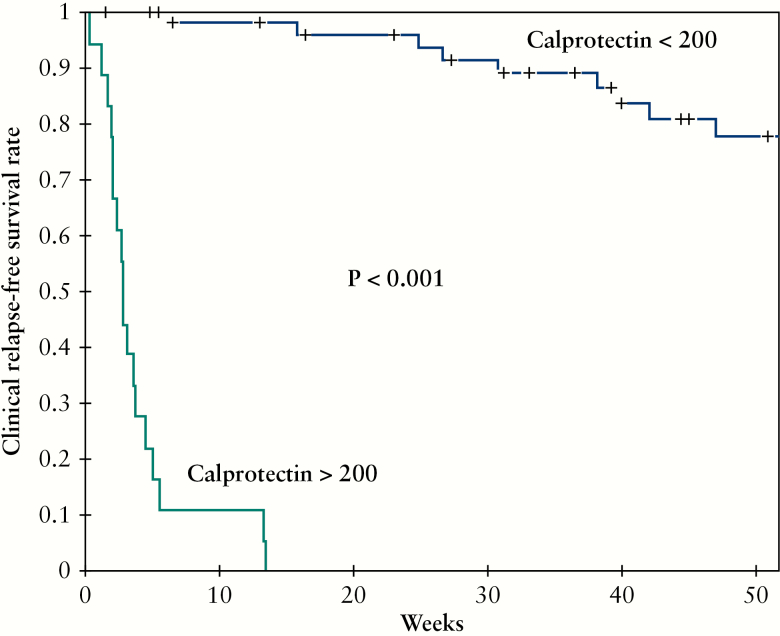
Kaplan-Meier survival curves showing the impact of surveillance of faecal calprotectin level above 200 µg/g on the risk of clinical relapse in IBD patients after therapeutic de-escalation.
The median interval between FCal testing during the follow-up and clinical relapse was 2.9 [2.0–4.4] weeks in patients with Fcal level above 200 µg/g compared with 87.4 [61.7–104.1] weeks in those with Fcal level below 200 µg/g [p <0.0001]. Among the 56 patients [75.7%] who had Fcal level <200 µg/g, only one patient [1.8%] experienced relapse within 3 months [6.2 weeks after calprotectin testing but presented with ΔFcal = +120 compared with baseline], and another one [2/45, 4.4%] relapsed within 6 months [15.1 weeks after calprotectin testing but presented with baseline Fcal value of 133 µg/g]. Overall, nine patients [8/33, 25.0%] had a clinical relapse within the first year following Fcal testing. Consequently, after the first surveillance measurement, Fcal should be tested every 6 months after therapeutic de-escalation.
In multivariate analysis, we found that Fcal level >200 µg/g during the monitoring after therapeutic de-escalation was highly predictive of clinical relapse (HR = 7.21 [2.49–20.89]; p < 0.001), whereas a clinical remission longer than 6 months before calprotectin testing was a protective factor (HR = 0.27 [0.13–0.57]; p = 0.001). None of the other factors was significantly associated with the risk of relapse: ΔFcal >+100 µg/g (HR = 2.45 [0.95–6.37]; p = 0.065), lower clinical score [HBI ≤2 or SCCAI = 0] (HR = 0.52 [0.26–1.02]; p = 0.06), or receiving immunosuppressant therapies (HR = 1.03 [0.52–2.04]; p = 0.93).
4. Discussion
In this large cohort from a referral tertiary centre, we reported that Fcal level >100 µg/g before therapeutic de-escalation was highly predictive of the risk of clinical relapse, regardless of current medication. In addition, serial monitoring of faecal calprotectin was very useful after therapeutic de-escalation, as a level >200 µg/g had a high negative predictive value to avoid clinical relapse and a level >400 µg/g a very strong positive predictive value for clinical relapse. Our results could also suggest that the first faecal calprotectin measurement should be performed 3 months after therapeutic de-escalation and then every 6 months.
We highlighted here the key role of Fcal in selecting IBD patients who could benefit from therapeutic de-escalation, regardless of the current therapies, including subgroups of patients receiving anti-TNF agents, integrins antagonists, or no biologics. We also reported that the duration of clinical remission before therapeutic de-escalation [>6 months] [HR = 0.57; p = 0.044] and steroids usage at the time of therapeutic de-escalation [HR = 1.67; p <0.0001] were associated with the risk of clinical relapse. These data are in line with a recent systematic review reporting all the potential risk factors of relapse related to baseline disease activity, prognostic features, and previous disease course.32 We investigated here a cohort of IBD patients in clinical remission, and only few of them with CD presented with previous perianal lesions [16.4%]. Our practice is to not de-escalate patients with perianal lesions. Earlier publications have reported that perianal involvement highly increases the risk of relapse in this situation.32 We also provided a clear definition of therapeutic de-escalation which reflects our clinical practice. Besides drug discontinuation, it included decrease of dose, increase of interval between two infusions/injections, and replacement by a ‘lower’ medication.
To date, only few data are available on the role of Fcal in patients with therapeutic de-escalation and focused on anti-TNF discontinuation.21,34 In the landmark STORI trial, it was observed that a baseline Fcal level ≥300 µg/g was associated with higher risk of relapse after discontinuing infliximab therapy in 115 CD patients receiving concomitant thiopurines (HR = 2.5 [1.1–5.8]; p = 0.04).21 In a retrospective cohort from the UK, including 166 patients with IBD [146 with CD and 20 with UC], the authors also found that Fcal level at drug withdrawal could be a reliable predictor for clinical relapse after anti-TNF discontinuation [HR 2.95 for >50 µg/g].34 These data are in line with ours, showing that baseline Fcal is useful to predict the risk of relapse even though we identified a different threshold [Fcal level ≥100 µg/g]. In the subgroup of patients with anti-TNF, we did not solely focus on anti-TNF discontinuation and we looked also at other types of de-escalation [see Figure 1]. The type of therapeutic de-escalation was not an independent risk factor for clinical relapse. According to our results, a case series enrolling 16 CD patients with deep remission [CRP ≤0.5 mg/dl, no endoscopic ulcer, and Fcal level <100 μg/g] reported a very low prevalence of relapse after increasing infliximab dose interval from every 8 to every 10 weeks.37 We also found that baseline CRP level [>5 mg/L] was associated with higher risk of relapse in our cohort, which has been already described by other authors.21,38,39
We investigated the impact of infliximab trough level and adalimumab drug concentration on the risk of relapse after therapeutic de-escalation. Whereas adalimumab drug concentration was not related to the risk of relapse, patients with higher infliximab trough level were more likely to experience clinical relapse in univariate analysis. However, our multivariate model did not confirm this trend. This specific result has to be taken with caution, as trough levels were not available for all the patients and the study was not specifically dedicated to answer this question. The STORI trial reported that infliximab trough level <2 at the time of de-escalation was associated with a decreased risk of relapse after discontinuing anti-TNF.21 In contrast, Drobne and colleagues showed that infliximab trough level >5 mg/L at the time of de-escalation was associated with a decreased risk of relapse after discontinuing immunomodulators.38 The predictive value of the infliximab trough level likely depends on the de-escalation strategy used: withdrawing the anti-TNF or stopping the co-treatment with immunosuppressive therapy.
We also investigated, for the first time, therapeutic de-escalation among patients treated with vedolizumab. Although the use of concomitant medication with vedolizumab remains questionable, and we analysed a small sample-size subgroup [n = 32] with heterogeneous type of de-escalation, we showed that baseline Fcal before therapeutic de-escalation was a very strong predictor of relapse in IBD patients receiving such therapy. We also looked at a subgroup of patients with no biologics. We found that Fcal level [>100 µg/g] was associated with the risk of relapse after therapeutic de-escalation. To our knowledge, it is the first time that the impact of Fcal has been underlined in patients who de-escalated immunosuppressants or 5-ASA therapies. It may be partly explained by the fact that the widespread use of Fcal measurements was more recent than most of the study on immunosuppressive therapies. We also observed that a deeper clinical remission [HBI ≤2 or SCCAI = 0] and a longer duration a clinical remission [>6 months] may prevent the risk of relapse in patients with no biologics attempting therapeutic de-escalation.
Steroids dependency is a major concern in IBD. Several potential predictors such as smoking habits, colonic location, non-fibrostenosing pattern, young age at diagnosis, or thrombocytosis have been identified so far, but their utility remains poor in daily practice.40,41 In the present work, we assessed the potential role of Fcal in predicting steroids dependency. We reported that Fcal level [>100 µg/g] was a risk factor of relapse in IBD patients attempting to discontinue steroids. This finding could be very helpful in clinical practice, but dedicated prospective study should be conducted to confirm these results. We also observed that female sex was associated with decreased risk of steroids dependency. Although we do not have any obvious explanation, it could be partly related to hormonal factors or better adherence to medical care.
In the present study, we showed that serial Fcal measurements could be very useful to monitor IBD patients after therapeutic de-escalation, with Fcal <200 µg/g [best NPV] or >400 µg/g [highest PPV] as best threshold. This cut-off value is close to those usually admitted for detecting endoscopic ulcerations.14,15 These data are also consistent with those reported by Molander and colleagues, indicating that Fcal >200 μg/g could predict relapse within 2–4 months after stopping anti-TNF agents.25 We recommend a cost-effective monitoring protocol, with the first Fcal measurement performed 3 months after therapeutic de-escalation and then every 6 months. As Fcal is not reimbursed by the different health care systems in most of the USA, our suggestion is based on the best ratio between risk of relapse and costs of avoidable examinations. In case of value lower than 200 µg/g, the same therapeutic regimen can safely be continued, whereas higher level of Fcal [>400 μg/g] should lead to a therapeutic intensification to avoid clinical relapse, as symptoms may reappear as shortly as within 1 month for most of the patients. When Fcal value ranged from 200 µg/g to 400 µg/g, performing an endoscopy could be of great value.
Our study presented with several strengths. It is the largest cohort reported so far allowing subgroup analyses. Besides, the data were retrieved from a prospectively maintained database. We also have to admit some limitations, such as the heterogeneity regarding the type of therapeutic de-escalation and the lack of data on anti-TNF drug concentration for some patients. In addition, endoscopic evaluation at the same time as Fcal measurement would have been of great interest.
In conclusion, Fcal >100 µg/g before therapeutic de-escalation is highly predictive of the risk of clinical relapse in patients with IBD. Serial monitoring with Fcal could be performed 3 months after therapeutic de-escalation and then every 6 months. A value of Fcal <200 µg/g during the surveillance is highly predictive of absence of clinical relapse, whereas Fcal >400 µg/g should lead to therapeutic intensification due to the high risk of clinical relapse. These data should be confirmed in a dedicated prospective trial.
Funding
None.
Conflict of Interest
AB declare lecture fees for MSD, Abbvie, Ferring, Takeda, Vifor Pharma, Hospira, and consulting fees for Abbvie, Takeda, and Hospira. DTR declares consultant fees for Abbvie, Abgenomics, Allergan, Amgen, Celgene, Forward Pharma, Genentech/Roche, Janssen Pharmaceuticals, Merck, Miraca Life Sciences, Napo Pharmaceuticals, Pfizer, Salix Pharmaceuticals, Samsung Bioepis, Sandoz Pharmaceuticals, Shire, Takeda, and grant support from Abbvie, Genentech/Roche, Janssen Pharmaceuticals, Prometheus Laboratories, Shire, Takeda, and UCB Pharma. The other authors declare no conflict of interest related to this work
Supplementary Material
References
- 1. Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol 2010;105:289–97. [DOI] [PubMed] [Google Scholar]
- 2. Pariente B, Mary JY, Danese S, et al. Development of the Lémann index to assess digestive tract damage in patients with Crohn’s disease. Gastroenterology 2015;148:52–63.e3. [DOI] [PubMed] [Google Scholar]
- 3. Torres J, Billioud V, Sachar DB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis as a progressive disease: the forgotten evidence. Inflamm Bowel Dis 2012;18:1356–63. [DOI] [PubMed] [Google Scholar]
- 4. Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 2004;126:451–9. [DOI] [PubMed] [Google Scholar]
- 5. Gupta RB, Harpaz N, Itzkowitz S, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology 2007;133:1099–105; quiz 1340–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease [STRIDE]: determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110:1324–38. [DOI] [PubMed] [Google Scholar]
- 7. Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut 2014;63:88–95. [DOI] [PubMed] [Google Scholar]
- 8. Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis 2009;15:1295–301. [DOI] [PubMed] [Google Scholar]
- 9. Shah SC, Colombel JF, Sands BE, Narula N. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment Pharmacol Ther 2016;43:317–33. [DOI] [PubMed] [Google Scholar]
- 10. Baert F, Moortgat L, Van Assche G, et al. ; Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology 2010;138:463–8; quiz e10–1. [DOI] [PubMed] [Google Scholar]
- 11. Frøslie KF, Jahnsen J, Moum BA, Vatn MH; IBSEN Group Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology 2007;133:412–22. [DOI] [PubMed] [Google Scholar]
- 12. Bouguen G, Levesque BG, Feagan BG, et al. Treat to target: a proposed new paradigm for the management of Crohn’s disease. Clin Gastroenterol Hepatol 2015;13:1042–50.e2. [DOI] [PubMed] [Google Scholar]
- 13. Buisson A, Gonzalez F, Poullenot F, et al. ; ACCEPT study group Comparative acceptability and perceived clinical utility of monitoring tools: a nationwide survey of patients with inflammatory bowel disease. Inflamm Bowel Dis 2017;23:1425–33. [DOI] [PubMed] [Google Scholar]
- 14. D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012;18:2218–24. [DOI] [PubMed] [Google Scholar]
- 15. Goutorbe F, Goutte M, Minet-Quinard R, et al. Endoscopic factors influencing faecal calprotectin value in Crohn’s disease. J Crohns Colitis 2015;9:1113–9. [DOI] [PubMed] [Google Scholar]
- 16. Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease [SES-CD] than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol 2010;105:162–9. [DOI] [PubMed] [Google Scholar]
- 17. Sipponen T, Kärkkäinen P, Savilahti E, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther 2008;28:1221–9. [DOI] [PubMed] [Google Scholar]
- 18. Buisson A, Vazeille E, Minet-Quinard R, et al. Faecal chitinase 3-like 1 is a reliable marker as accurate as faecal calprotectin in detecting endoscopic activity in adult patients with inflammatory bowel diseases. Aliment Pharmacol Ther 2016;43:1069–79. [DOI] [PubMed] [Google Scholar]
- 19. Nancey S, Boschetti G, Moussata D, et al. Neopterin is a novel reliable fecal marker as accurate as calprotectin for predicting endoscopic disease activity in patients with inflammatory bowel diseases. Inflamm Bowel Dis 2013;19:1043–52. [DOI] [PubMed] [Google Scholar]
- 20. Mao R, Xiao YL, Gao X, et al. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm Bowel Dis 2012;18:1894–9. [DOI] [PubMed] [Google Scholar]
- 21. Louis E, Mary J-Y, Vernier-Massouille G, et al. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology 2012;142:63–70.e5; quiz e31. [DOI] [PubMed] [Google Scholar]
- 22. Costa F, Mumolo MG, Ceccarelli L, et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut 2005;54:364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Vos M, Louis EJ, Jahnsen J, et al. Consecutive fecal calprotectin measurements to predict relapse in patients with ulcerative colitis receiving infliximab maintenance therapy. Inflamm Bowel Dis 2013;19:2111–7. [DOI] [PubMed] [Google Scholar]
- 24. Ferreiro-Iglesias R, Barreiro-de Acosta M, Lorenzo-Gonzalez A, et al. Accuracy of consecutive fecal calprotectin measurements to predict relapse in inflammatory bowel disease patients under maintenance with anti-TNF therapy: a prospective longitudinal cohort study. J Clin Gastroenterol 2016;52:229––34.. [DOI] [PubMed] [Google Scholar]
- 25. Molander P, Färkkilä M, Ristimäki A, et al. Does faecal calprotectin predict short-term relapse after stopping TNFα-blocking agents in inflammatory bowel disease patients in deep remission? J Crohns Colitis 2015;9:33–40. [DOI] [PubMed] [Google Scholar]
- 26. Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol 2006;4:621–30. [DOI] [PubMed] [Google Scholar]
- 27. Lichtenstein GR, Rutgeerts P, Sandborn WJ, et al. A pooled analysis of infections, malignancy, and mortality in infliximab- and immunomodulator-treated adult patients with inflammatory bowel disease. Am J Gastroenterol 2012;107:1051–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park KT, Bass D. Inflammatory bowel disease-attributable costs and cost-effective strategies in the United States: a review. Inflamm Bowel Dis 2011;17:1603–9. [DOI] [PubMed] [Google Scholar]
- 29. Rubin DT. When should therapy for inflammatory bowel disease be stopped? Gastroenterol Hepatol [N Y] 2015;11:400–2. [PMC free article] [PubMed] [Google Scholar]
- 30. Park KT, Colletti RB, Rubin DT, Sharma BK, Thompson A, Krueger A. Health insurance paid costs and drivers of costs for patients with Crohn’s disease in the United States. Am J Gastroenterol 2016;111:15–23. [DOI] [PubMed] [Google Scholar]
- 31. Foram Mehta MS. Report: economic implications of inflammatory bowel disease and its management. Am J Manag Care 2016;22[Suppl 3]:551––60.. [PubMed] [Google Scholar]
- 32. Torres J, Boyapati RK, Kennedy NA, Louis E, Colombel JF, Satsangi J. Systematic review of effects of withdrawal of immunomodulators or biologic agents from patients with inflammatory bowel disease. Gastroenterology 2015;149:1716–30. [DOI] [PubMed] [Google Scholar]
- 33. Casanova MJ, Chaparro M, García-Sánchez V, et al. Evolution after anti-TNF discontinuation in patients with inflammatory bowel disease: a multicenter long-term follow-up study. Am J Gastroenterol 2017;112:120–31. [DOI] [PubMed] [Google Scholar]
- 34. Kennedy NA, Warner B, Johnston EL, et al. ; UK Anti-TNF withdrawal study group Relapse after withdrawal from anti-TNF therapy for inflammatory bowel disease: an observational study, plus systematic review and meta-analysis. Aliment Pharmacol Ther 2016;43:910–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–5. [DOI] [PubMed] [Google Scholar]
- 36. Liu X. Classification accuracy and cut point selection. Stat Med 2012;31:2676–86. [DOI] [PubMed] [Google Scholar]
- 37. Papamichael K, Karatzas P, Mantzaris GJ. De-escalation of infliximab maintenance therapy from 8- to 10-week dosing interval based on faecal calprotectin in patients with Crohn’s disease. J Crohns Colitis 2016;10:371–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Drobne D, Bossuyt P, Breynaert C, et al. Withdrawal of immunomodulators after co-treatment does not reduce trough level of infliximab in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2015;13:514–21.e4. [DOI] [PubMed] [Google Scholar]
- 39. Molnár T, Lakatos PL, Farkas K, et al. Predictors of relapse in patients with Crohn’s disease in remission after 1 year of biological therapy. Aliment Pharmacol Ther 2013;37:225–33. [DOI] [PubMed] [Google Scholar]
- 40. Franchimont DP, Louis E, Croes F, Belaiche J. Clinical pattern of corticosteroid dependent Crohn’s disease. Eur J Gastroenterol Hepatol 1998;10:821–5. [DOI] [PubMed] [Google Scholar]
- 41. Chow DK, Sung JJ, Tsoi KK, et al. Predictors of corticosteroid-dependent and corticosteroid-refractory inflammatory bowel disease: analysis of a Chinese cohort study. Aliment Pharmacol Ther 2009;29:843–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.