Abstract
Maternal and fetal sources of thyroid hormone are important for the development of many organ systems. Thyroid hormone deficiency causes variable intellectual disability and hearing impairment in mouse and man, but the basis for this variation is not clear. To explore this variation we studied two thyroid hormone-deficient mouse mutants with mutations in pituitary-specific transcription factors, POU1F1 and PROP1, that render them unable to produce thyroid stimulating hormone. DW/J-Pou1f1dwldw mice have profound deafness and both neurosensory and conductive hearing impairment, while DF/B-Prop1df/df mice have modest elevations in hearing thresholds consistent with developmental delay, eventually achieving normal hearing ability. The thyroid glands of Pou1f1 mutants are more severely affected than those of Prop1df/df mice, and they produce less thyroglobulin during the neonatal period critical for establishing hearing. We previously crossed DW/J-Pou1f1dw/+ and Cast/Ei mice and mapped a major locus on Chromosome 2 that protects against hypothyroidism-induced hearing impairment in Pou1f1dw/dw mice: Modifier of dw hearing (Mdwh). Here we refine that map with additional animals and genotypes, and we conduct novel mapping with a DW/J-Pou1f1dw/+ and 129/P2 cross that reveals 129/P2 mice also have a protective Mdwh locus. Using DNA sequencing of DW/J and DF/B strains, we determined that the genes important for thyroid gland function within Mdwh vary in amino acid sequence between strains that are susceptible or resistant to hypothyroidism-induced hearing impairment. These results suggest that the variable effects of congenital hypothyroidism on the development of hearing ability are attributable to genetic variation in postnatal thyroid gland folliculogenesis and function.
Keywords: genetic modifier, Mdwh, POU1F1, PROP1, thyroglobulin, thyroid hormone
Introduction
Congenital hearing impairment affects nearly 1 in every 1000 live births and is the most common birth defect in developed nations (White 2004). Hearing impairment affects speech and language development, the education of children, and career opportunities later in life. Hypothyroidism during pregnancy and in newborns can cause hearing loss in humans and in rodents, if not promptly identified and treated (Brucker-Davis et al. 1996; Glinoer and Delange 2000; Knipper et al. 2000; Vanderschueren-Lodeweyckx et al. 1983). Individual susceptibility to hypothyroidism-induced hearing impairment is highly variable, and the genetic factors that influence susceptibility are not known (Karolyi et al. 2007; Napiontek et al. 2004). Genetic background significantly affects phenotype in many mouse models of human diseases, including hearing impairment (Hamilton and Yu 2012; Ikeda et al. 2002; Johnson et al. 2006; Kousi and Katsanis 2015; Yan and Liu 2010). The mouse offers a powerful system for identifying complex genetic interactions in hearing development and disease because of the availability of numerous strains of mice with different genetic backgrounds (Niu et al. 2008). It is clear that genetic factors affect the severity of hearing impairment in humans, but it is much more difficult to identify genetic interaction in humans even when large pedigrees are available (Kallman et al. 2008). Due to the conservation of genes among mammals, the identity of candidate modifier genes in humans can be predicted from studies in model organisms such as mice. In order to realize the potential of the genome project to predict disease risk and severity for humans, we need to know more about gene-gene interactions that ultimately dictate the individual’s phenotype.
We studied two genetic models of severe hypothyroidism and the effects of genetic background on susceptibility to hearing deficits (Karolyi et al. 2007). DF/B-Prop1df/df (p.S83P) and DW/J-Pou1f1dw/dw (p.W251C) mice have loss-of-function mutations in two pituitary-specific transcription factors that result in arrested pituitary cell specification early in development and the inability to produce TSH, growth hormone, and prolactin, causing developmental delay, severe hypothyroidism, and growth insufficiency (Li et al. 1990; Sornson et al. 1996). PROP1 and POU1F1 regulate two sequential steps in pituitary differentiation, and one of the primary roles of Prop1 is to activate expression of Pou1f1. The pituitary hormone phenotypes of the two mutant mice are indistiguishable. Surprisingly, the severity of the hearing impairment in the two mutants is strikingly different despite their identical effects on pituitary hormone production. DF/B-Prop1df/df mutants have a mild hearing deficit of ~15 dB elevation in thresholds at 6 wk and normal hearing by 12 wk (Fang et al. 2012; Karolyi et al. 2007). In contrast, DW/J-Pou1f1dw/dw mutants are profoundly deaf at all ages tested (~65 dB elevation) (Fang et al. 2012; Karolyi et al. 2007). When these genetic backgrounds are mixed, the hearing impairment of the two mutants is indistinguishable (~30 dB threshold elevation). These genetic background effects are not maternal effects, but affect the fetus directly, because transfer of DF/B-Prop1dfldf and DW/J-Pou1f1dw/dw fertilized eggs to surrogate mothers did not alter the severity of the hearing impairment (Fang et al. 2012). The hearing impairment of both mutants is rescued by thyroid hormone supplementation initiated during pregnancy and continued during postnatal development, proving that thyroid hormone deficiency is linked to the hearing problems.
Thyroid hormone (thyroxine or 3,5,3’,5’-tetraiodothyronine) is necessary for development of hearing and many other body functions including fetal brain development, growth, metabolism, and regulation of body temperature (Brent 2012; van der Spek et al. 2017). Thyroid hormone is synthesized in the follicular cells of the thyroid gland from the protein precursor thyroglobulin (TG). Thyroglobulin is released into the lumen of the follicle, the colloid, where iodine is added to the tyrosine residues of the protein and subsequently oxidized by the action of thyroid peroxidase (TPO). The follicular cell takes up the modified thyroglobulin by endocytosis, and proteolyzes it to produce thyroxine (T4) and triiodothyronine (T3), which are released into the bloodstream in response to stimulation by pituitary thyroid stimulating hormone (TSH). TSH binds the TSH receptor on thyroid follicular cells and stimulates the endocytosis of the colloid, processing of thyroglobulin, and release of T4 into the bloodstream. In addition to its role in regulating thyroid gland function, pituitary TSH is important for stimulating thyroid gland growth and folliculogenesis at 18–20 wks of gestation in human fetuses and in mice after birth (Postiglione et al. 2002). Cells throughout the body take up T4 by active transport, and it is processed into the more biologically active T3 (triiodothyronine) by type 2 deiodinase (DIO2) (Bernal et al. 2015). Both T3 and T4 are inactivated by the deiodinase DIO3. T3 binds thyroid hormone receptors (THRA and THRB) and alters gene expression.
Pleiotropic effects of hypothyroidism on normal auditory development have been demonstrated in several rodent models (Christ et al. 2004; Knipper et al. 2000; Li et al. 1999; Richter et al. 2010; Sprenkle et al. 2001). Thyroid hormone also affects auditory pathways in the brain [reviewed in (Mustapha et al. 2009; Ng et al. 2013)]. The inner ear defects range from immature cochlear morphology, tectorial membrane abnormalities, inner and outer hair cell defects, reduced expression and function of potassium channels, hair cell loss, and impaired oto-acoustic emissions, and they have been thoroughly examined in Prop1 and Pou1f1 mutants (Fang et al. 2012; Mustapha et al. 2009; Sundaresan et al. 2016). Middle ear defects can include ossicular malformations and defects in middle ear cavitation, but these have not been explored in either Prop1 or Pou1f1 mutants. Many of these abnormalities can be corrected by thyroxine treatment during hearing maturation, although the period of sensitivity to hormone is transient (Knipper et al. 2000; Uziel 1986; Uziel et al. 1983). If hypothyroidism is not corrected promptly, permanent intellectual disability and deafness can result. In contrast, later TH replacement can correct growth insufficiency. In the auditory system, thyroid hormone controls the differentiation of the cochlea during the critical postnatal period between birth and the onset of hearing at 2 wks in rodents [reviewed in (Forrest et al. 2002; Ng et al. 2013)]. In humans, this window occurs largely during fetal development (Moore and Linthicum 2007).
Both genetic and environmental factors can cause hypothyroidism and hearing impairment. For example, environmental iodine deficiency can result in combined maternal and fetal hypothyroidism during pregnancy (Berbel et al. 2007). Genetic defects in thyroid hormone synthesis and action lead to primary hypothyroidism and varying levels of deafness in humans and mice. The variability results from several factors: the specific gene that is mutated, the timing of thyroid hormone deficiency during development, the effects of modifying genes, and the environment. Three relevant examples are illustrative of this. First, siblings with Pendred syndrome who are homozygous for the common SLC26A4 p.T416P allele vary in the severity and progression of deafness (Napiontek et al. 2004). Second, humans with mutations in THRB that reduce T3 binding affinity and disrupt interactions with transcriptional cofactors have variably penetrant hearing impairment. Notably, only 21% of patients with THRB loss of function have hearing loss (Brucker-Davis et al. 1996). Third, Thrb−/−; Thra−/− mice lack all known thyroid hormone receptors and are deaf due to numerous defects in cochlear physiology and morphology (Rusch et al. 2001). Initial studies of these mutants were carried out on mixed genetic backgrounds. Generation of congenic lines of these mutants on the C57BL/6J strain background reduced individual variability in hearing impairment. Thus, the incidence and severity of deafness due to hypothyroidism can be influenced by both genes and environment.
We previously mapped the effects of genetic background on hypothyroidism induced hearing impairment (Fang et al. 2011). We crossed Mus musculus musculus DW/J-Pou1f1dw/+ with wild type mice of the distantly related subspecies Mus musculus castaneus (CAST/EiJ). Forty-one F2 mice with extreme differences in auditory brain stem responses were genotyped with 90 microsatellite markers. We identified a modifier locus on mouse Chromosome 2 that protects against hearing impairment, which we designated as Mdwh (Modifier of dw hearing). This 20 Mb region of Chr 2 contained one known modifier of hearing, the gene encoding the microtubule-associated protein 1a (MTAP1A). However, additional genetic crosses with the AKR strain demonstrated that protective alleles of Mtap1a were neither necessary nor sufficient for normal hearing in Pou1f1dw/dw mice. We expect that future identification of the protective modifier gene(s) in mice could enhance our understanding of the mechanisms that underlie susceptibility to hypothyroidism-induced hearing impairment in humans. We report additional genetic and phenotypic analyis and the identification of candidate genes in the critical region of Chr 2 that may protect against hypothyroidism-induced deafness by influencing thyroid gland function.
Materials and Methods
Mice and genotyping
All mice were housed in a 12-h light, 12-h dark cycle in ventilated cages with unlimited access to tap water and Purina 5020 chow. All procedures were conducted in accordance with the principles and procedures outlined in the National Institutes of Health Guidelines of the Care and Use of Experimental Animals and approved by our Institutional Animal Care and Use Committee. Ames dwarf mice (Prop1df/df) were originally obtained from Dr. A. Bartke (Southern Illinois University, Carbondale, IL) from a non-inbred stock (DF/B). Snell dwarf mice (Pou1f1dw/dw) were originally obtained from The Jackson Laboratory from an inbred stock (DW/J) (Bar Harbor, ME). Separate colonies of DF/B-Prop1df/+ and DW/J-Pou1f1dw/+ mice have been maintained at the University of Michigan through heterozygous matings. CAST/EiJ inbred mice were from The Jackson Laboratory, and 129P2lOlaHsd inbred mice were from Harlan Sprague Dawley Inc. Crosses were conducted to produce (DWlJ-Pou1f1dw × CASTlEiJ) F2 hybrids and (DW/J-Pou1f1dw × 129P2lOlaHsd) F2 hybrids. The Prop1df p.S83P and Pou1f1dw p.W251C mutations were genotyped as described (Douglas et al. 2001; Gage et al. 1996). Both male and female mice were used.
Tissue Preparation and Histology
For middle ear histology, mice were euthanized at 3 weeks and 4 months of age. The middle ear was dissected and fixed in 4% paraformaldehyde for 2 hours. The middle ears were washed 3 times for 5’ each in 1X Phosphate Buffered Saline (PBS) and then placed in 10% ethylenediaminetetraacetic acid (EDTA) overnight at 4° C. The next day they were washed again 3 times for 5’ each in 1X PBS. Thyroid glands were dissected from postnatal day 9 (P9) mice and fixed in 4% paraformaldehyde for 1 hour and then washed 3 times for 5’ each in 1X PBS. Both middle ear and thyroid glands were dehydrated to 70% ethanol, and embedded in a Tissue Tek VIP Paraffin tissue processing machine. Sections at 6 μm thickness from at least 3 different animals of each age and genotype were analyzed by hematoxylin and eosin (H&E) staining and 4’,6-diamidino-2-phenylindole(DAPI) staining. H&E staining was performed as described previously (Mortensen et al. 2015). The H&E images were captured using a Leica DMRB fluorescent microscope.
For bullae histology, 3-month old mice were euthanized, heads were removed and skinned manually, and the remaining skull tissues were removed by dermestid beetles (Geister et al. 2015). Bullae images were captured with a Leica dissecting microscope.
Thyroid follicle measurement
H&E stained thyroid sections were used for quantifying the number of follicles with colloid per area of thyroid tissue. Three sections were analyzed from the middle of the gland that were 5 sections apart (n=3 for each genotype). The area of the thyroid was measured using the Image J software (National Center for Biotechnology Information), and the number of follicles with colloid was counted. The range of follicle numbers counted was as follows: DF/B-Prop1+/+ controls (97 to 189 follicles), DF/B-Prop1df/df mutants (49 to 110), DW/J-Pou1f1+/+ controls (60 to 116), and DW/J-Pou1f1dw/dw mutants (30 to 54). The average number of follicles with detectable colloid per thyroid area was calculated. Significance was determined using one-way ANOVA.
Real Time PCR
Thyroid glands were dissected from P9 mice and stored in RNALater at −20°C. Thyroids were collected from DF/B-Prop1+/+, DF/B-Prop1df/df, DW/J-Pou1f1+/+, and DW/J-Pou1f1dw/dw mice (n=8 for each genotype). RNA was isolated and DNase I treated using the protocols provided in the RNAqueous Micro kit (Life Technologies). The amount and quality of RNA was determined with the NanoDrop 2000 Spectrophometer (Thermo Scientific). The Superscript II reverse transcription system from Invitrogen (Life Technologies) was used to generate cDNA. Specifically, RNA and oligo dT primers were denatured at 70°C for 10 minutes and then placed on ice. 100 mM DTT, 10 mM dNTPs, 5X first strand buffer and 1 U Superscript II were added to the RNA primer mix. Samples were incubated at 42°C for 50 minutes, then heat-inactivated for 10 minutes at 70°C. Samples without reverse transcriptase were included as negative controls. Quantitative PCR (qPCR) was carried out using Taqman gene expression assays for Tg (Mm01200340_m1), Dio1 (Mm00839358_m1), Tpo (Mm00456355_m1), and Gapdh (4308313). The qPCR reaction was set up in triplicate with 5 ng cDNA per reaction using Taqman Universal PCR Master Mix and default run parameters on the ABI 7500 Real-Time PCR instrument. Results were expressed as average CT normalized to Gapdh. Triplicates containing the Master Mix and water with the Taqman primer sets were used as negative controls. All reagents and instruments were obtained from Life Technologies. Significance was determined using an ordinary one-way ANOVA.
Quantitative PCR (qPCR) was carried out on liver cDNA using Taqman gene expression assays for Thrsp (Mm01273967_m1) and Gapdh. The qPCR reaction was carried out in triplicate with 80 ng of cDNA per reaction, using same reagents, run parameters, and machine as previously described (Vesper et al. 2006) (Livak and Schmittgen 2001). Among T3 injected mice, the fold change in expression of DF/B-Prop1+/+ controls was compared to DF/B-Prop1df/df mice, and DW/J-Pou1f1+/+ values were compared to DW/J-Pou1f1dw/dw, and the calculations were performed as described previously (Brinkmeier et al. 2009).
Assessment of response to exogenous thyroid hormone
Two-month old DF/B-Prop1+/+ (n=5), DF/B-Prop1df/df (n=5), DW/J-Pou1f1+/+ (n=5), and DW/J-Pou1f1dw/dw (n=4) mice were injected with 4 μg of T3 (3,3’,5-Triiodo-L-thyronine sodium salt, Sigma, catalog # T6397) per g body weight on two consecutive days. On the third day, livers from the injected mice and non-injected control mice of the same genotypes were collected and stored in RNALater at −20°C. RNA was isolated using the RNAqueous 4PCR RNA isolation kit from Ambion (AM1914).
Total T4 Analysis
Eight P9 and 9 adult (~2 mo) mice of each of the following genotypes, DF/B-Prop1+/+, DF/B-Prop1df/df, DW/J-Pou1f1+/+, and DW/J-Pou1f1dw/dw, were euthanized with CO2 and blood was collected by cardiac puncture. After clotting at room temperature for 30 minutes, the blood was centrifuged at 8000 x g for 10 min. 25 ul of serum was analyzed using the Total Thyroxine (T4) Enzyme Immunoassay Test Kit (07BC-1007, MP Biomedicals, Ohio, USA).
Auditory brainstem response
The auditory brain stem response test was used to assess hearing thresholds of the mutant mice (Fang et al. 2011; Karolyi et al. 2007). A total of 183 Pou1f1dw/dw F2 mice from a (DW/J-Pou1f1dw/+ × CAST/EiJ)F1 intercross and 128 Pou1f1dw/dw F2 mice from a (DW/J-Pou1f1dw/+ × 129P2/OlaHsd)F1 intercross were tested. For the (DW/J-Pou1f1dw/+ x Cast/Ei)F1 intercross, the frequencies of tone bursts presented to the mice were click and 8, 16, 32 kHz at the Jackson laboratory and 10 and 20 kHz at the University of Michigan. Both 10 and 20 kHz frequencies of tone bursts were presented to the (DW/J-Pou1f1dw/+ × 129P2lOlaHsd)F1 intercross mice.
Genome Scan
Genotyping services were provided by the Sequencing Core at the University of Michigan using a commercially available Medium Density Mouse Linkage Panel (Illumina, San Diego, CA) to genotype 1,449 SNPs on 183 (DW/J-Pou1f1dw × CAST/EiJ) Pou1f1dw/dw F2 mice and 128 (DW/J-Pou1f1dw × 129P2/OlaHsd) Pou1f1dw/dw F2 mice. The panel carries approximately 3 SNPs per 5 Mb interval across the genome. ABR thresholds for 10 and 20 kHz stimuli were evaluated as quantitative traits, and QTL linkage analysis was performed using PLINK v1.07, which is a free, open-source whole genome association analysis toolset (Purcell et al. 2007). 1359 of the SNPs are located on Chr 1–19, and these were considered in the analysis. Individual samples that failed to type at more than 10% of the loci were excluded from the analysis (N=1 for 129P2 F2 and N=8 for CAST/Ei F2). Loci that failed to type on >5% of the samples were excluded from the analysis (N=66 for 129P2 and N=133 for CAST/Ei). Loci that were not informative between DW/J and 129P2 (N=629) or between DW/J and CAST/Ei (N=876) were also excluded. Thus, 664 and 876 informative SNPs were utilized in the analysis of the 129P2 and CAST/Ei crosses respectively. The threshold P value was calculated using the Bonferroni method (p=0.05lnumber of informative markers). The critical interval was defined by subtracting 1.5 from the LOD score for the peak SNP.
DNA sequencing
Candidate genes for Mdwh were selected based on relevance to thyroid biology and reported DNA sequence variation between the susceptible C57BL/6J strain and resistant 129P2 strain (Table S1). To verify the sequences, primers were designed to amplify exons of candidate genes from the DW/J and DF/B strains, and amplification products were subjected to Sanger sequencing (Table S2).
Results
DW/J-Pou1f1dw/dw mutants have poor middle ear development
Patients with resistance to thyroid hormone have both conductive and sensorineural deafness (Brucker-Davis et al. 1996). The development of the cochlea had been extensively compared in the hypothyroid DF/B-Prop1df/df and DW/J-Pou1f1dw/dw mutants, and the results demonstrated that the DW/J-Pou1f1dw/dw mutants had essentially every defective feature found in other hypothyroid mice, while the DF/B-Prop1df/df mice had none of them despite the lack of TSH and detectable thyroid hormone (Fang et al. 2012; Mustapha et al. 2009; Sundaresan et al. 2016). This led us to hypothesize that the protective locus in DF/B-Prop1df/df mice has a global impact on most thyroid hormone-dependent processes, with the exception of body growth. To test whether this predicted protection is true for conductive hearing impairment, we examined the development of the middle ear. The critical role of thyroid hormone in development of the middle ear has been demonstrated by elegant studies in three other mutant mice: the congenitally hypothyroid Tshr −/− mutants, TR-deficient Thra1−/−;Thrb−/− double mutants, and dominant-negative TRαl transgenics (Cordas et al. 2012). Thyroid hormone is necessary for cavitation of the middle ear space by clearing mesenchymal tissue to create an operational air-filled cavity, which is necessary for sound conduction and normal development of hearing (Jaskoll and Maderson 1978; Richter et al. 2010). It is also necessary for proper development of the ossicles. In mice, mesenchyme begins clearing at P6 and normally clears from the area surrounding the middle ear ossicles by P14, allowing free movement of the ossicles.
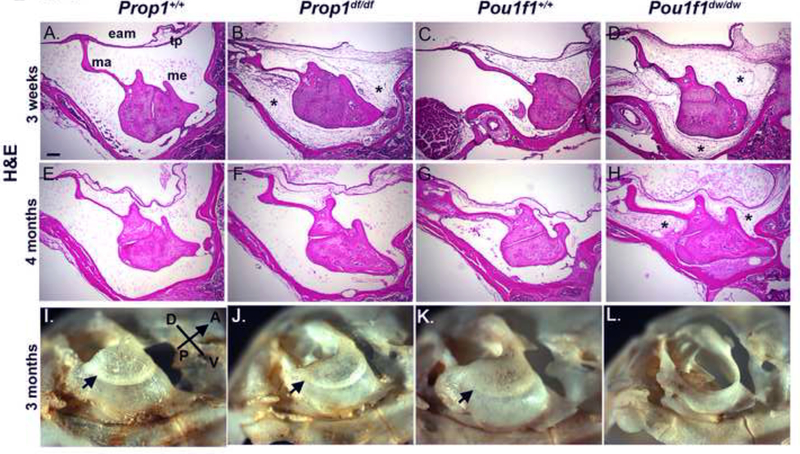
We observed complete mesenchymal clearing in 3 wk Prop1 +/+ and Pou1f1+/+ mice using H&E staining of sections (Fig. 1A and 1C), but both the Prop1df/df and Pou1f1dw/dw (Fig. 1B and 1D) mutants abnormally retain mesenchymal cells at this age, consistent with developmental delay due to hypothyroidism and elevated hearing thresholds in both mutants at this age (Karolyi et al. 2007). DAPI staining on adjacent sections confirms that the retained material in the mutants is nucleated cellular debris and that material in the space of normal middle ears is acellular (Supplemental Fig. 1). By 4 months, the mesenchyme is essentially cleared in the Prop1df/df (Fig. 1F) and appears similar to wild type DF/B and DW/J (Fig. 1E, G). The Pou1f1dw/dw mutants, however, continue to retain mesenchyme in the middle ear cavity (Fig. 1H), which could interfere with conductive hearing properties. DAPI staining confirmed these conclusions as well (Supplemental Fig. 1).
Figure 1. Conductive hearing loss contributes to hearing impairment in Pou1f1 mutant mice.
Sections of the middle ear were stained with hematoxylin and eosin (H&E) and photographed in brightfield or dark field, respectively, at 100x. The scale bar is 200 microns. Samples were taken from Prop1+/+ (A, E), Prop1df/df (B, F), Pou1f1+/+ (C, G), and Pou1f1dw/dw (D, H) mice at 3 wks (A-D) and 4 mo (E-H). The external auditory meatus (eam), tympanic membrane (TP), middle ear cavity (mec) and malleus (ma) structures are indicated. Retained mesenchyme is indicated with an asterisk. Bullae collected from 3 mo old Prop1+/+ (I), Prop1df/df (J), Pou1f1+/+ (K), and Pou1f1dw/dw (L) mice were exposed to dermestid beetles to visualize the bone structure. The sulcus tympanicus, or tympanic ring, is indicated with an arrowhead.
The middle ear ossicles are encased within the auditory bulla, a structure that forms from the fusion of multiple intramembranous ossifications (Hanken and Thorogood 1993). The formation of the auditory bulla in conjunction with cavitation results in an enclosed air space, through which sound vibrations are carried. The bulla is also the seat of the tympanic membrane. Therefore, development of the bulla is critical for hearing. The patterning of the auditory bulla is complete in mice by P9, and it continues to increase in size until P21 (Huangfu and Saunders 1983). At 3 months of age, some defects are evident in the auditory bullae of both the Prop1df/df (Fig. 1J) and the Pou1f1dwldw (Fig. 1L) mutants, compared wtih wild type littermates (Fig. 1I, 1K). The Pou1f1dw/dw mutant auditory bulla is much more severely affected than the Prop1df/df mutant, including lack of the tympanic ring, which likely contributes to conductive hearing impairment in Pou1f1dw/dw mutant.
Thyroid hormone and its cellular actions in Prop1df/df and Pou1dw/dw mutants
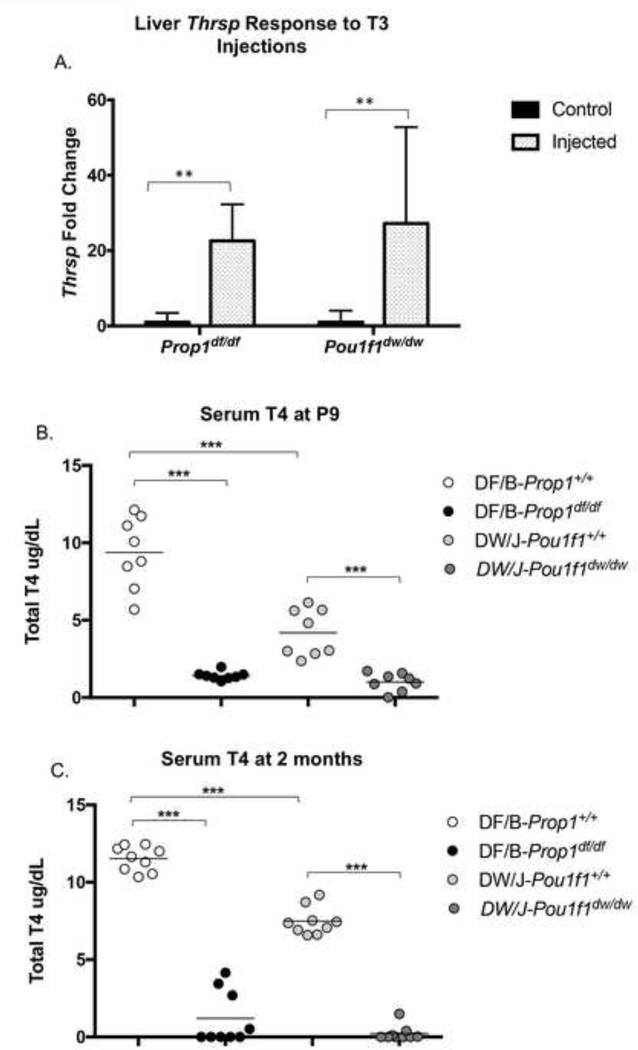
Given the diversity of features that are more severely affected in susceptible hypothyroid mutant strains relative to resistant ones, we considered the possibility that there might be a global difference in thyroid hormone responsiveness between strains affecting regulatory or coding sequences for thyroid hormone transporters, receptors, co-factors or other aspects of the thyroid hormone-mediated regulation of gene expression. If this were the case, then the DW/J strain might respond more poorly to exogenous thyroid hormone administration than the DF/B strain. To test this we examined expression of Spot 14, also called Thrsp (thyroid hormone responsive protein), a gene for which expression is highly induced by thyroid hormone in the liver (Jump et al. 1984; Seelig et al. 1981). We injected 2-month old Prop1df/df and Pou1f1dwldw mutant mice and their wild type littermates with maximal stimulating doses of triiodothyronine (T3) on 2 consecutive days and collected livers 24 hr after the last injection. Non-injected mice served as controls. We determined Thrsp and Gapdh expression levels using qRT-PCR. As expected, Thrsp expression was substantially lower in the non-injected Prop1df/df and Pou1f1dw/dw mutants compared to their non-injected, normal littermates (38-fold and 33-fold, respectively), consistent with the hypothyroid status of the mutants (data not shown). The Prop1+/+ and Pou1f1+/+ wild type mice responded to T3 injection with 2 to 2.6 fold increase in Thrsp expression compared with the non-injected controls, respectively. T3 injection caused both mutants to increase liver Thrsp expression levels markedly over those of non-injected mutants. A 23-fold increase was observed in Prop1df/df mice and 27-fold in Pou1f1dw/dw mice (Fig. 2A). Using ANOVA we determined that the level of induction of Thrsp by T3 injection is not significantly different between the mutants. Thus, the strain backgrounds of Prop1df/df and Pou1f1dw/dw mutants permit equivalent, robust responses of liver gene expression to exogenous thyroid hormone.
Figure 2. Hypothyroid Prop1 and Pou1f1 mutants mount strong responses to exogenous thyroid hormone, and their strain backgrounds have different circulating levels of thyroid hormone.
A. RT-PCR was used to measure expression levels of the thyroid hormone responsive gene Trsp in the livers of Prop1df/df and Pou1f1dw/dw control and thyroid hormone injected mice relative to Gapdh. ** indicates p<0.01. B. Serum T4 levels were measured in 9 day old Prop1+/+, Prop1df/df, Prop1+/+, and Pou1f1dw/dw mice. *** indicates p<0.001 C. Serum T4 levels were measured in adult, ~2 mo old animals of the same four genotypes.
The critical period for thyroid hormone action in the development of rodent hearing is within the first 2 weeks after birth [reviewed in (Ng et al. 2013)]. Accordingly, we measured the serum levels of T4 in Prop1df/df, Pou1f1dw/dw, and wild type littermates at P9 to determine whether there were differences in circulating thyroid hormone during this critical developmental stage. The T4 levels in the Pou1f1+/+ wild type mice were 2.2 fold lower than the Prop1+/+ wild types (4.2±1.5 vs. 9.4±2.3) (Fig. 2B), confirming significant strain differences in circulating thyroid hormone levels. The T4 levels in both the Prop1df/df mutants (1.42±0.27) and Pou1f1dw/dw mutants (1.01±0.58) were substantially lower than their normal littermates. These values were essentially at the lower limit of detection by the assay. The DF/B and DW/J strain differences in serum T4 levels persisted in adult mice, and the T4 levels in both mutants were near the limit of detection (Fig. 2C).
Thyroid gland folliculogenesis is poorer in Pou1f1dw/dw than Prop1df/df neonates
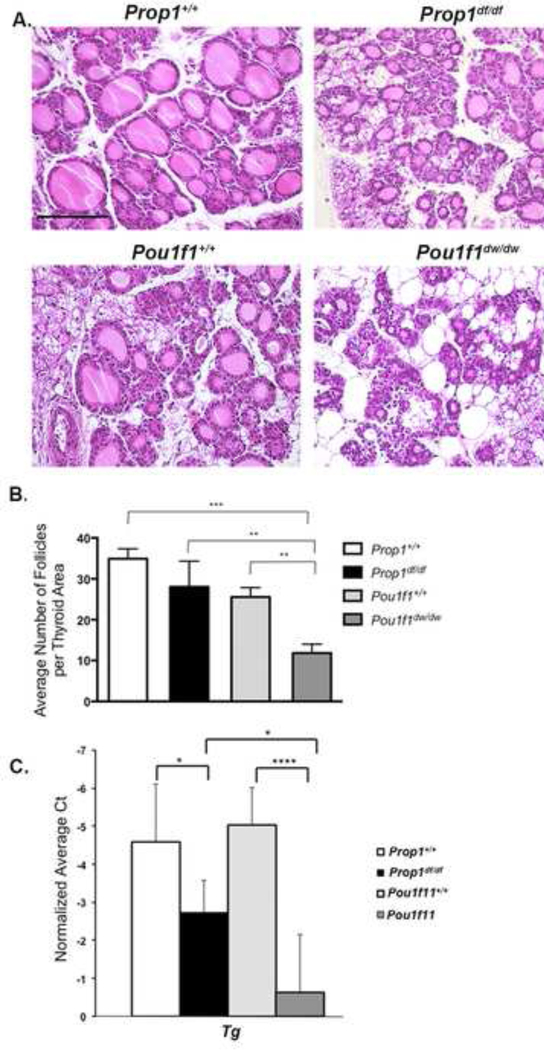
To determine whether the lower serum levels of T4 in the normal neonates of the DW/J strain relative to the DF/B strain arose from differences in thyroid gland folliculogenesis or function, we characterized the thyroid follicles in hypothyroid mutants and wild type mice on these two genetic backgrounds using H&E staining of sections from P9 animals. Normal DF/B mice appeared to have more large follicles than normal DW/J mice, and both mutants have smaller and less well-developed follicles than their normal littermates (Fig. 3A). The young Pou1f1dw/dw mutants appeared to have much less well developed follicles and more fatty infiltrate relative to Prop1df/df mutants. We quantified the differences in the number of follicles with colloid per area of thyroid tissue using ImageJ software and a one-way ANOVA (Fig. 3B). The Pou1f1dw/dw mutants had significantly fewer follicles with developed colloid per thyroid area (11.8 ± 2.2) compared to Prop1df/df mutants (28.0 ± 6.3), or wild type mice of either strain (34.9 ± 2.5 for DF/B and 25.5 ± 2.3 for DW/J). It is noteworthy that these values in Prop1df/df mutants are in the same range as the DW/J wild type mice. There was no significant difference between the values of any of the other genotypes.
Figure 3. Thyroid development and function is strongly influenced by strain background and pituitary dysfunction.
A. Thyroid glands of P9 Prop1+/+, Prop1df/df, Pou1f1+/+, and Pou1f1dw/dw mice were sectioned and stained with hematoxylin and eosin and photographed at 200x. The scale bar is 400 microns. B. The average number of thyroid follicles per area were quantified. ** indicates p<0.01 and *** indicates p<0.001. C. Thyroid gland RNA was prepared from P9 Prop1+/+, Prop1df/df, Pou1f1+/+, and Pou1f1dwldw mice and thyroglobulin (Tg) transcripts were measured by RT-PCR and normalized to Gapdh. * indicates p<0.05 and **** indicates p<0.0001.
To investigate whether the under-developed thyroid follicles of Pou1f1dw/dw mutants were associated with reduced expression of genes indicative of thyroid gland function (Amendola et al. 2005), we used qRT-PCR to measure expression of thyroglobulin (Tg), iodothyronine deiodinase type 1 (Dio1), and thyroperoxidase (Tpo) in RNA from thyroid glands of Prop1df/df, Pouf11dw/dw, and wild type littermates at P9. All three genes were expressed at significantly lower levels in the mutants compared to wild type mice, which is consistent with expectations for severely hypothyroid animals (Fig. 3C and Supplemental Fig. 2). Tg expression was 4.3 fold lower in the Pou1dw/dw mutants compared to Prop1df/df mutants. This finding is compelling since thyroglobulin is the most abundant protein in follicular cells and is the critical precursor in thyroid hormone biogenesis (Di Jeso and Arvan 2016; Salvatore and Edelhoch 1973). Therefore, reduced Tg expression is consistent with the histological findings of reduced thyroid folliculogenesis and function in Pou1f1dw/dw mutants compared to Prop1df/df mutants during the period critical for developing hearing. We found no significant difference in the expression of Dio1 and Tpo between the Prop1df/df and Pou1f1dw/dw mutant mice (Supplemental Fig. 2).
A region of mouse Chr 2 from CAST/Ei and 129P2 strains protects Pou1f1dwldw mutants against hypothyroidism-induced hearing impairment
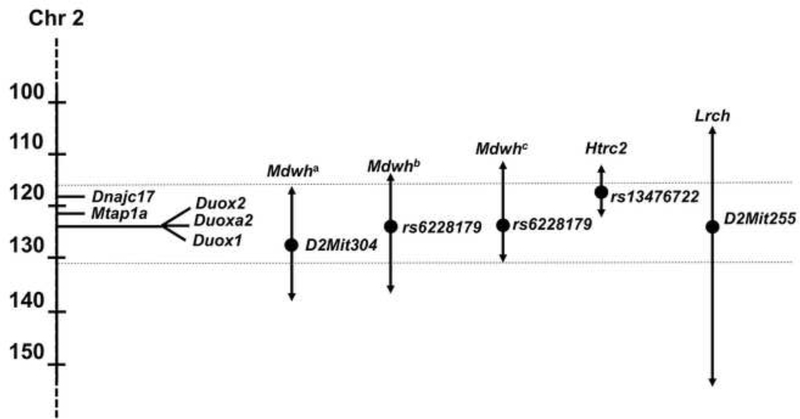
Our previous genetic mapping studies placed the Mdwh critical region between ~118 and 138 Mb on Chr 2 and suggested that the DW/J background is more similar to C57 substrains than other inbred strains (Fang et al. 2011). This analysis used 90 microsatellite markers to analyze forty-one F2 mutant progeny with the most extreme differences in auditory brain stem responses to 16 kHz sounds, and a follow-up screen was done with 196 F2 mutant progeny and eight microsatellite markers on Chr 2. The lead marker in that study was D2Mit304 located at 128 Mb. In an effort to narrow the critical region, we conducted a higher resolution genome wide association study (GWAS) with 196 mutant F2 progeny from the (DW/J-Pou1f1dw/+ × CAST/EiJ) F1 intercross using a mouse medium density linkage panel with 876 informative SNPs (Fig. 4A). The association between hearing threshold and SNP genotypes was evaluated with the PLINK whole genome data analysis software. Loci with LOD scores above 4.24 are considered statistically significant using the Bonferroni method (p=0.05/number of informative markers). The lead SNP for Mdwh on Chr 2 was rs6228179 at bp position 123 Mb (GRC m38) with a LOD of 9.13. The 1.5 LOD confidence interval was between 114 and 137 Mb. An additional locus on Chr 5 reached significance, rs3705458 with LOD=4.41, suggesting additional genetic complexity in protecting against hypothyroidism-induced hearing impairment.
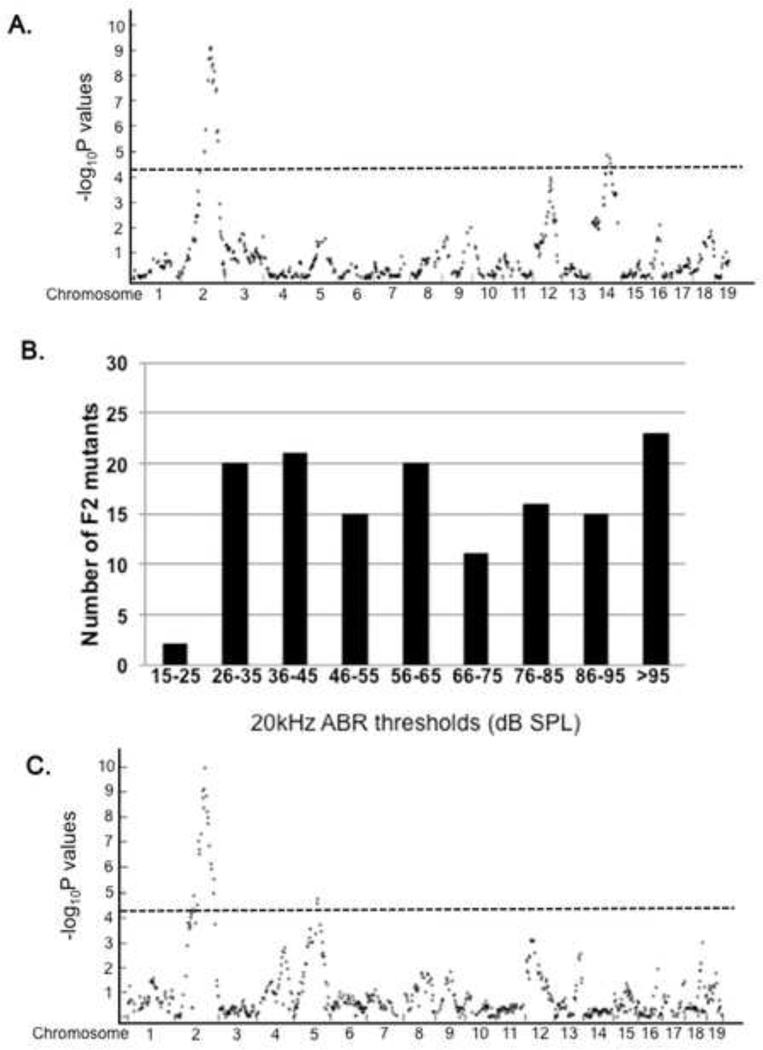
Figure 4. Genetic mapping of hearing as a quantitative trait reveals the same region of Chr 2 in CAST/Ei and 129P2 strains modifies dw hearing.
A. A GWAS was carried out to assess the association of hearing ability with SNP typing of mutants from an F1(CAST/Ei wild type × DW/J-Pou1f1dw/+) × F1 cross. Each dot represents the -log10P value for an individual, informative SNP on each chromosome. The dotted line indicates the baseline for significant association. B. The hearing of Pou1f1dw/dw mutants from an F1(129P2 wild type × DW/J-Pou1f1dw/+) × F1 cross was assessed by auditory brain stem response (ABR) to 20kHz tone and the threshold sound pressure level (SPL) recorded in dB. The number of mutants with thresholds in the indicated ranges were recorded. C. A similar GWAS was carried out to assess the association of hearing ability with SNP typing of mutants from an F1(129P2 wild type × DW/J-Pou1f1dw/+) × F1 cross. Each dot represents the -log10P value for an individual, informative SNP on each chromosome. The dotted line indicates the baseline for significant association.
Recombination rates differ significantly between inbred strains. SNP panels facilitate genetic mapping between closely related strains which may have less suppression of recombination than more distantly related strains (Dumont and Payseur 2011). Therefore, we carried out a second cross to narrow the critical interval using the good hearing strain 129P2/OlaHsd, which is more closely related to DW/J than CAST/EiJ, 23.3% SNP variation vs. 43.6% (Table 1). We conducted genetic mapping in 128 mutant F2 progeny from a (DW/J-Pou1f1dw/+ X 129P2/OlaHsd) F1 intercross. These mice exhibited a broadly distributed range of hearing thresholds (Fig. 4B). Approximately 15% of the progeny had excellent hearing, with ABR thresholds of 35 dB or less at 20kHz. The nearly continuous distribution of hearing thresholds for these mutant progeny contrasts with the bimodal distribution observed in (DW/J x CAST/Ei)F2 mutant mice.
Table 1.
SNP variation between DW/J and common inbred mouse strains
| Strains | # variable SNPs | % variation |
|---|---|---|
| DW/J vs. C57BL/6J | 47 | 0.68 |
| DW/J vs. AKR/J | 1514 | 21.97 |
| DW/J vs. BALB/cJ | 1521 | 22.08 |
| DW/J vs. 129P2 | 1605 | 23.30 |
| DW/J vs. DBA/2J | 1824 | 26.47 |
| DW/J vs. CBA/J | 1986 | 28.82 |
| DW/J vs. C3H/HeJ | 2232 | 32.39 |
| DW/J vs. CAST/Ei | 3004 | 43.60 |
We conducted a GWAS with the mouse medium-density linkage panel described above. 664 SNPs were informative (Fig. 4C). Loci with LOD scores above 4.12 are considered significant according to the Bonferroni method. The GWAS data from 20 kHz ABR thresholds identified the same major modifier locus on Chr 2 with the same lead SNP rs6228179 as the CAST/EiJ cross, and a LOD of 9.09. The 1.5 LOD confidence intervals were positioned at 112–137 Mb. A peak on Chr 14 achieved significance, rs61543475, LOD 4.87, suggesting that additional loci contribute to the protected phenotype in this strain, and that the additional modifiers are different than for CAST/Ei. Similar results were obtained in a GWAS analysis of the 10 kHz ABR thresholds (data not shown).
These studies demonstrate that the major modifier on Chr 2 is conserved across several strains. However, higher resolution mapping did not substantially narrow the critical interval, even with the more closely related strain. This result suggested that there could be more than one gene within the non-recombinant interval that contributes to the quantitative trait.
Analysis of candidate genes in the Chr 2 critical region
The phenotypic comparison of susceptible and protected strains suggested that thyroid folliculogenesis and/or function could be the process that underlies their difference in auditory development among hypothyroid strains. This is consistent with the independent mapping of a modifier of hypothyroidism, hypothyroid related chr 2 or Htrc2, to a region that overlaps with Mdwh (Fig. 5) (Amendola et al. 2005; Amendola et al. 2010). We identified seven candidate genes within the Mdwh critical region that are relevant to thyroid biology and contain potentially damaging variation between the C57BL/6J (which is closely related to DW/J) and 129P2 strains (Table 2) (Keane et al. 2011). Two to six missense variants were detected between susceptible (C57BL/6J) and resistant (129P2) strains for each of these genes. These include the closely linked dual oxidase genes Duox1 and Duox2, and the dual oxidase maturation factor Duoxa2 that map within a 68 kb interval. Another candidate gene located 3.1 Mb proximal, Dnajc17, encodes a type III heat-shock protein-40 involved in splicing, which has been suggested as a candidate for Htrc2 (Pascarella et al. 2018). This gene is hypothesized to be the modifier of thyroid gland development and function in Nkx2.1, Pax8 double heterozygotes and affects Tg transcription in cell culture (Amendola et al. 2010). Less compelling candidates include three genes involved in thyroid cancer: the spindle checkpoint gene Bub1b, the notch ligand Dll4, and the P53 binding protein Trp53bp1.
Figure 5. Multiple modifiers of thyroid gland folliculogenesis map on Chr 2.
The 1.5 LOD interval for Mdwh was originally mapped with microsatellite markers in mutant progeny of a (DW/J-Pou1f1dw/+ × CAST/Ei)F1 × F1 intercross (Mdwh1) with the lead marker D2Mit304 (1128331784). A GWAS analysis of this cross (Mdwh2) and a similar intercross with 129P2 (Mdwh3) refined the location and identified the same lead SNP in each case, rs6228179 (123293026). The lead candidate genes for Mdwh are DNAjc17 and the cluster containing Duox2, Duoxa2, and Duox1. The Htrc2 locus was mapped to rs12476722, with Dnajc17 as the lead candidate, and Lrch to D2Mit255, completely overlapping Mdwh.
Table 2.
Genes in Mdwh region with genetic variation and relevance to thyroid biology
| Mouse Gene Symbol |
Gene Name | Function | Variants 129P2 vs. DW/J and B6 |
Location | Disease | Human Gene Symbol |
|---|---|---|---|---|---|---|
| Bublb | Spindle assembly checkpoint | Overexpressed in thyroid carcinoma | 6 missense | 118,598,211-118,641,592 | colorectal cancer,variegated aneuploidy syndrome 1 | BUB1B |
| DNAjc17 | DNAJ(Hsp40) homolog, subfamily C, member 17 | Expressed in thyroid bud | 6 Missense1 | 119,172,500-119,208,795 | homozygous lethal in mice | DNAJB1 |
| Dll4 | Delta-like 4 | Notch pathway, plays a role in thyroid carcinogenesis and angiogenesis | 2 missense | 119,325,784-119,335,666 | heterozygous partial lethal, homozygous embryonic lethal in mice | DLL4 |
| Trp53bp1 | Transformation related protein 53 binding protein 1 | Nuclear Trp53BP1 foci in thyroid cancer | 3 missense | 121,267,312-121,271,407 | growth retardation, immunodeficiency, thymic lymphoma in mice | TP53BP1 |
| Duox2 | Dual oxidase 2 | A component of the thyroid H2O2 generator critical for thyroid hormone synthesis | 5 missense | 122,280,437-122,298,165 | congenital hypothyroidism, low T4, high TSH, hearing impaired | DUOX2 |
| Duoxa1 | Dual oxidase maturation factor 1 | Required for targeting of functional DUOX enzymes to the cell surface | 3 missense | 122,303,549-122,313,730 | severe hypothyroidism with growth retardation | DUOXA1 |
| Duox1 | Dual oxidase 1 | Main source of hydrogen peroxide in rat thyroid cell line PCCl3 | 3 missense | 122,315,672-122,347,972 | unknown | DUOX1 |
Highly conserved p.Phe273Tyr allele is implicated in altered DNAJC17 activity.
We used Sanger sequencing to determine the predicted missense variation and stop gain in each of these genes in the DW/J and DF/B strains relative to the previously reported values for C57/BL6J and 129/P2 (Tables 3 and S1). In all cases the DW/J strain had the same variant as the C57BL/6J strain, and in most cases the 129P2 and DF/B alleles were the same as each other and matched the predominate allele amongst inbred strains that have been sequenced (Table 3). We used phyloP, GERP (genomic evolutionary rate profiling), and SIFT (sorting intolerant from tolerant) to evaluate the conservation of each of the changes between DW/J and 129/P2 (Cooper et al. 2005; Kumar et al. 2009; Pollard et al. 2010). In PhyloP analysis, sites predicted to be conserved have positive scores, and those predicted to be fast-evolving have negative scores. The absolute values of the scores represent -log p-values under a null hypothesis of neutral evolution. Each of these genes has some variants with strongly positive phyloP scores except Dll4 and Duoxl. GERP scores that are positive indicate that a deficit of substitutions relative to neutrality and suggest evolutionary constraint. Each of the candidate genes has variants with positive scores greater than 1 except Trp53bp1. SIFT scores that are below 0.05 are considered likely damaging. Bublb, Trp53bp1, and Duoxal have variants that are likely damaging according to SIFT. Thus, all candidate genes have variants predicted to be deleterious by one of the three prediction programs. These programs suggest variants that are most likely to be affected by the variation in DW/J relative to 129P2 and DF/B, but functional studies are required as follow up.
Table 3.
Genetic Variation in candidate gene for Mdwh and evaluation of function.
| Gene | Position | dbSNP | Amino Acid B6>129 Mouse |
Amino Acid Human |
PhyloP | GERP | SIFT | DW and B6 |
129P2 | DF/B | B6/129 allele count1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bublb | 118612729 | rs3144766 | Gly240Ser | Gly | 1.512 | 2.36 | 0.46 | G | A | A | 0/16 |
| 118615032 | rs3149979 | Thr278Pro | Ala | −0.049 | 0.959 | 0.97 | A | C | C | 0/26 | |
| 118615135 | rs3149980 | Ala312Val | Leu | 0.094 | 1.82 | 0.35 | C | T | T | 0/16 | |
| 118630960 | rs27423851 | Glu664Asp | Asp | 1.55 | −6.63 | - | G | C | C | 0/26 | |
| 118631761 | rs27423850 | Asp705Asn | Asn | 0.50 | 3.61 | 0.7 | G | A | A | 0/26 | |
| 118631824 | rs252392177 | Leu726Ile | Leu | 1.29 | 1.15 | 0.01 | T | A | A | 0/14 | |
| Dnajc17 | 119172660 | rs8279388 | Phe273Tyr3 | Tyr | 1.51 | 1.92 | 1.0 | A | T | T | 0/26 |
| 119179372 | rs49263590 | Gly258Ser | Ser | −0.17 | 2.53 | 1.0 | C | T | T | 0/26 | |
| 119180535 | rs27453586 | Glu184Asp | Glu | 0.25 | 3.04 | 0.24 | C | A | A | 0/26 | |
| 119180966 | rs51167341 | Arg161Lys | Lys | 1.24 | 2.9 | 1.0 | C | T | T | 0/26 | |
| 119180967 | rs46745469 | Arg161Gly | Lys | 1.24 | 5.33 | 0.17 | T | C | C | 0/26 | |
| 119183622 | rs27453572 | Gln152Arg | Gln | 1.55 | 3.27 | 0.7 | T | C | C | 0/26 | |
| Dll4 | 119332155 | rs27423750 | Gln419Leu | Leu | 0.13 | 3.58 | 0.61 | A | T | T | 0/26 |
| 119332778 | rs27423747 | Gly627Ser | Thy | −0.054 | 1.72 | 1.0 | G | A | A | 0/26 | |
| Trp53bp1 | 121216105 | rs27454672 | Lys1071Asn | His | −0.10 | 0.866 | 0.47–0.63 | T | G | G | 0/27 |
| 121243989 | rs27439904 | Ala427Thr | Thr | 0.0088 | −3.11 | 0.55–0.68 | C | T | T | 0/26 | |
| 121244066 | rs29674073 | Val401Ala | Glu | 1.32 | −10.4 | 0.02–0.07 | A | G | G | 0/28 | |
| Duox2 | 122280988 | rs217232017 | Cys1410Arg | His | 1.66 | −4.91 | 0.61 | A | G | G | 0/26 |
| 122291506 | rs27453362 | His627Arg | His | 1.56 | 0.412 | 0.46 | T | C | C | 0/27 | |
| 122292682 | rs32841329 | Asp495Val | Asp | 0.28 | 4.34 | - | A | T | A4 | 0/11 | |
| 122294092 | rs27453345 | Asn378Ser | Asn | 1.27 | 5.36 | 0.36 | T | C | C | 0/26 | |
| 122296719 | rs219434625 | His 159Pro/Arg | Pro | 1.56 | 2.14 | 0.2/1.0 | A | C/G | C | 0/12 | |
| Duoxa1 | 122303818 | rs47922057 | Ile318Thr | Ile | 1.41 | 5.43 | 0.0 | A | G | G | 0/26 |
| 122303917 | rs27453307 | Gln285Leu | Gln | 1.75 | 4.17 | 0.68–0.71 | T | A | A | 0/26 | |
| 122305560 | rs29816247 | Leu112Phe | n.a. | −2.84 | 4.18 | 0.0–0.3 | G | A | A | 0/26 | |
| Duox1 | 122319515 | rs236906605 | His129Arg | Pro | −0.30 | 0.712 | 0.47 | A | G | A | 0/11 |
| 122319587 | rs229793548 | Arg153His/Pro | Pro | −0.13 | 2.59 | 0.12 | G | A/C | C | 0/3 | |
| 122326535 | rs27453258 | Ala561Glu | Lys | −0.48 | −7.12 | 1 | C | A | C | 0/11 | |
mouse strains with alleles matching B6/# matching 129P2.
Grey highlighting indicates variants more likely to be damaging.
This highly conserved amino acid alters DNAJC17 activity.
Underlining reveals DF/B alleles that are the same as b6.
Discussion
Genetic disorders are frequently classified as either rare, “simple Mendelian” diseases or common, complex diseases. This classification over-simplifies the frequent observation that related individuals with the same Mendelian mutation often have different clinical presentations that range from relatively high functioning to very disabled. This variability in clinical features is attributable to both environmental and genetic factors. An excellent example are the genetic modifiers that have been found to influence the presentation of the autosomal recessive mutation in CFTR that causes cystic fibrosis in Caucasian populations (Corvol et al. 2015; Furlan et al. 2016; Sofia et al. 2016; Strug et al. 2016). Mouse models provide the means to detect interacting pathways with strong modifying effects on phenotype, and they have been used to identify over 280 modifier loci (Hamilton and Yu 2012). The mouse has been particularly informative in identifying genetic modifiers of hearing impairment, including age related hearing loss (Johnson et al. 2006). Two spectacular examples bear mention. First, the microtubule associated protein Mtap1a is a modifier of the hearing impairment in Tubby mice, but it does not affect the obesity or retinal phenotypes, and the effect of the amino acid alterations is only evident in the context of the Tubby mutant allele (Ikeda et al. 2002). Second, cadherin 23 (Cdh23) is a modifier of the hearing of deaf waddler mutants, which have a defective calcium transporting membrane protein ATP2B2, and hearing loss allele of the adhesion G-protein coupled receptor V1 gene, Adgv1frings (Johnson et al. 2005; Noben-Trauth et al. 2003; Noben-Trauth et al. 1997). The sensitive variant Cdh23753A also contributes to age-related hearing loss. These mouse studies accurately predicted similar genetic interactions in humans, in which alleles of the ATP2B2 gene influence hearing impairment caused by mutations in CDH23 (DFNB12) and MYO6 (DFNB37) (Schultz et al. 2005). We previously reported mapping a modifier of hypothyroidism-induced hearing impairment in Pou1f1dw mice, Mdwh, to Chr 2, and we demonstrated that it acts intrinsically in the fetus (Fang et al. 2012; Fang et al. 2011). Here we demonstrate that poor thyroid gland folliculogenesis during the critical period for developing hearing is associated with susceptibility to hypothyroidism-induced hearing impairment (Table 4), and we present four strong candidate genes: Dnajc17 and the closely linked Duox1, Duox2 and Duoxa2 genes.
Table 4.
Summary of evidence for variation in thyroid gland folliculogenesis and function affecting hypothyroidism-induced hearing impairment
| Phenotype | Strain and genotype | |||
|---|---|---|---|---|
| DF/B +/+ | DF/B df/df | DW/J +/+ | DW/J dw/dw | |
| Strain sensitivity | resistant | resistant | sensitive | sensitive |
| Pituitary hormones | normal | TSH, GH, PRL deficient | normal | TSH, GH, PRL deficient |
| Response to T3 | 2 fold | 23 fold | 2.6 fold | 27 fold |
| ABR 1 | ~28 | ~40 | ~38 | >100 |
| Adult inner ear | normal | normal | normal | Multiple abnormalities |
| Adult middle ear | normal | normal | normal | Multiple abnormalities |
| Thyroid follicles/area | 35 ± 3 | 28 ± 6 | 26 ± 2 | 12 ± 2 |
| Thyroglobulin mRNA 2 | − 4.6 ± 1.5 | − 2.7 ± 0.9 | − 5.0 ± 1.0 | − 0.63 ± 1.5 |
| Thyroid hormone | 9.4 ± 2.3 | 1.4 ± 0.27 | 4.2 ± 1.5 | 1.0 ± 0.58 |
Auditory brain stem response thresholds in dB SPL to 10 kHz in 6 wk old mice
Normalized average Ct value from qRT-PCR
We present the first analysis of middle ear development in Prop1df/df and Pou1f1dw/dw. We observed persistent mesenchyme in both Prop1df/df and Pou1f1dw/dw mutants at 3 wk of age, consistent with elevated hearing thresholds at that time (Karolyi et al. 2007), and the mesenchyme persisted through 4 mo of age in Pou1f1dw/dw mutants, consistent with earlier reports of Pou1f1dw/dw mutants (Marovitz et al. 1968). In contrast, the mesenchyme was completely cleared by 4 mo in Prop1df/df mutants, consistent with their acquisition of normal hearing by that time (Fang et al. 2012). Persistent mesenchyme is thought to predispose to middle ear infections, which can influence learning and speech. Genetic and infectious disease models of otitis media have been studied in mice (Hardisty et al. 2003; Rye et al. 2011). We did not observe any difference in otitis media in hypothyroid mice that are susceptible or resistant to hearing impairment. The persistent developmental delay in bone maturation in Pou1f1dw/dw mutants also extended to the auditory bullae, in which the tympanic ring failed to develop. We conclude that conductive hearing impairment contributes to the developmental delay in hearing acquisition of Prop1df/df mutants and to the persistent, profound deafness in Pou1f1dw/dw mutants. These findings are consistent with observations in thyroid stimulating hormone receptor mutant mice (Tshr1−/−) and thyroid hormone receptor deficient mice that are doubly mutant for both receptors (Thra1−/−, Thrabr-−/−). Both have defects in inner ear and middle ear development including persistent retention of mesenchyme surrounding the ossicles, enlarged ossicles, and delayed ossification (Cordas et al. 2012).
We hypothesized that the susceptibility to hypothyroidism-induced hearing loss could result from a systemic difference in thyroid hormone production, cellular transport, bioactivation or degradation, or in coactivators and corepressors that facilitate thyroid hormone receptor mediated changes in transcription (Ng et al. 2013). This idea is supported by the pleiotropic effects of thyroid hormone deficiency on inner and middle ear development in susceptible strains and the relatively normal development of these structures in hypothyroid, resistant strains [(Fang et al. 2012; Fang et al. 2011; Mustapha et al. 2009), and this report]. However, we found no difference in the ability of susceptible and resistant strains to respond to exogenous thyroid hormone by rapid and robust activation of thyroid hormone responsive protein (THRSP or Spot14) expression in the liver. The liver utilizes a variety of thyroid hormone transporters that are also expressed in cochlear development (MCT8, MCT10, LAT1) (Engels et al. 2015). This suggests that thyroid hormone receptors, auxiliary nuclear factors, and thyroid transporters are unlikely candidates for Mdwh.
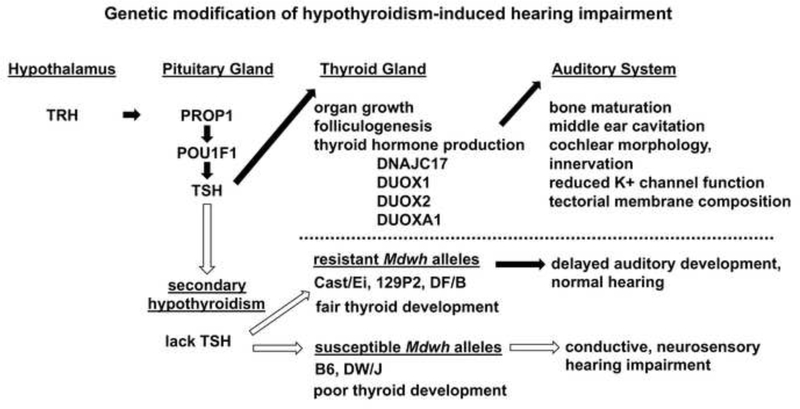
The thyroid gland begins to develop follicles even in the absence of pituitary thyroid stimulating hormone signaling, and the effect of TSH deficiency on thyroid gland development becomes evident in early neonatal life in mice (Beamer et al. 1981; Postiglione et al. 2002). The circulating levels of thyroid hormone in adult mice varies widely among inbred strains (Center for Genome Dynamics). We detected striking differences (~2 fold) between the circulating levels of T4 in normal mice from DF/B and DW/J strains near the normal time for onset of hearing (P9). We were unable to detect significant levels of circulating thyroid hormone in either Prop1df/df or Pou1f1dw/dw mice at this time or in adult mice. However, the thyroid glands of Pou1f1dw/dw mice clearly had a paucity of functional follicles, as evidenced by the lack of colloid, and thyroglobulin transcription was reduced ~ 10 fold relative to normal littermates. The number of follicles with colloid per thyroid tissue area was not significantly different in Prop1df/df mutants from either wild type strain. Taken together these data strongly support the idea that the DW/J strain poorly supports thyroid gland development in the absence of pituitary stimulation, while the DF/B strain supports significant thyroid gland function (Fig. 6).
Figure 6. Genetic modification of hypothyroidism-induced hearing impairment.
Hypothalamic production of TRH stimulates the pituitary gland to produce TSH. The transcription factor PROP1 is necessary to activate expression of the transcription factor POU1F1, and both are necessary for development of the cells that produce TSH. Pituitary TSH stimulates thyroid gland growth, folliculogenesis and thyroid hormone production in the postnatal period. DUOX1, DUOX2, and DUOXA1 are necessary for thyroid hormone production from the thyroglobulin precursor protein. DNAJC17 is expressed early in thyroid bud development and is a candidate gene for regulation of thyroglobulin expression. In rodents the critical period for thyroid hormone stimulation of brain and auditory system development is the first two weeks after birth. Prop1 and Pou1f1 mutations cause severe TSH deficiency. We propose that the variable effects of TSH deficiency on hearing are caused by genetic modifiers that influence thyroid gland function in the early neonatal period. Dnajc17, Duox1, Duox2, and Duoxa1 are candidate genes for the resistant Mdwh alleles that allow for better thyroid gland development and function in the absence of pituitary stimulation.
We mapped Mdwh in two inbred strains to the same location on Chr 2. There are several candidate genes for differential regulation of thyroid gland function within the Mdwh critical region. Among the most compelling of these are the genes involved in thyroid hormone biogenesis: Duox1, Duox2, Duoxa2, and Dnajc17, each of which have likely deleterious amino acid substitutions in the susceptible DW/J strain relative to the resistant 129P2 and DF/B strains. The enzymes encoded by Duox1 and Duox2 are critical for generating the hydrogen peroxide required by thyroid peroxidase for incorporation of iodine into thyroglobulin, a crucial step in thyroid hormone production. Defects in DUOX2 gene expression cause congenital hypothyroidism and hearing impairment in mouse and man, with a high level of phenotypic variability (Fugazzola et al. 2011; Johnson et al. 2007; Zheng et al. 2017). Mutations in dual oxidase maturation factor, DUOXA2, also cause severe hypothyroidism in mouse and man (Grasberger et al. 2012; Vigone et al. 2005). The phenotypic consequences are likely to be influenced by genetic background and environmental factors (Weber et al. 2013). This maturation factor is a transmembrane protein in the endoplasmic reticulum that is essential for the deposition of DUOX2 at the plasma membrane (Grasberger and Refetoff 2006). It is intriguing that comprehensive screening for genes mutated in congenital hypothyroidism identified numerous cases of digenic inheritance involving DUOX2, TG, and TPO, providing proof of principle that genetic interaction in this pathway could be pathogenic (Nicholas et al. 2016). Other chromosomal regions contribute to the resistance to hypothyroidism induced hearing impairment, and more than one gene in the Mdwh region may contribute to poor thyroid gland development and deafness of Pou1f1dw/dw mice on the DW/J strain background. Additive effects of multiple genes within a quantitative trait locus have been observed in many genetic studies (Buchner and Nadeau 2015).
We suspect that the Mdwh candidate genes also modify other loci that cause congenital hypothyroidism (Fig. 5). For example, a locus designated Htrc2 (hypothyroidism-related chromosome 2), that confers resistance to congenital hypothyroidism caused by double heterozygosity for the transcription factors Nxk2.1 and Pax8, was mapped to a narrow interval between 112–121 Mb, near rs13476722, using only 52 doubly heterozygous animals (Amendola et al. 2010). The C57BL6/J (B6) genetic background is susceptible, whereas the 129/SvPasCrl (Sv) strain is resistant, and the effect is polygenic, involving a second locus on Chr 5. The authors considered Dnajc17 to be the most likely candidate gene for the Htrc2 locus on Chr 2 because susceptible mice have reduced levels of thyroglobulin during embryogenesis, and the B6 allele of Dnajc17 suppresses NKX2.1-mediated transcription of thyroglobulin more robustly than the 129 allele. Duox1, Duox2and Duoxa2are outside their critical region. In a second example, a locus for resistance to congenital hypothyroidism, Lrch, was mapped to Chr 2 between 104.0–154.3 Mb, with D2Mit255 as the lead SNP (Hosoda et al. 2012). This interval overlaps the Mdwh critical region. A 129-derived strain [129(+Ter)/SvJcI] has a resistant allele, and the DW and B6 strains have susceptible alleles that influence both body growth and the development of the thyroid gland in Tpst2grt mutants. These mutants have an inactivating mutation (p.H266Q) in the tyrosylprotein sulfotransferase 2 gene, which catalyzes the sulfation of the thyroid stimulating hormone receptor which is required for TSH signaling (Sasaki et al. 2007). The nature of this modifier is not known, but the authors considered numerous candidate genes on Chr 2 including Duox2 and Duoxa2 (candidates for Mdwh, Table 2) and Kcnip3, a Kv channel interacting protein. Dnajc17 could also be a candidate for Lrch, as it is for Htrc2 and Mdwh. However, we suspect that mutiple candidates in the Mdwh interval contribute to the resistance to hearing impairment in mice with hypopituitarism because we were unable to substantially narrow the critical interval even with large numbers of mutants or closely related strains.
Congenital hypothyroidism is a common birth defect, affecting ~1 in 3500 children (for reviews, see (Abu-Khudir et al. 2017; Ng et al. 2013; Stoppa-Vaucher et al. 2011)). It can be caused by several mechanisms, including thyroid gland dysgenesis, where the thyroid gland is missing, ectopic or severely underdeveloped, and dyshormonogenesis, which involves failure to produce adequate amounts of thyroid hormone. These problems have varied incidence by ethnicity. Early detection and treatment of newborns with hormone replacement therapy within the first two weeks of life is effective, and this occurs is most countries, avoiding permanent effects on intelligence and hearing (Bellman et al. 1996; Lazarus et al. 2012; Rovet and Daneman 2003; Rovet and Ehrlich 2000). Women with hypothyroidism need increased thyroid hormone replacement to meet the increased demands of pregnancy. Failure to manage this properly can have lasting effects on the child’s hearing, which has been observed in the case of POU1F1 p.R271W mutation (Cuevas et al. 2005; Haddow et al. 1999; Lavado-Autric et al. 2003; Pine-Twaddell et al. 2013). Our study has identified candidate genes and pathways that could contribute to human variation in susceptibility to hypothyroidism-induced hearing impairment and neurological deficits.
Supplementary Material
Acknowledgments
We thank the March of Dimes for funding (grant N018020 to SAC), the NIH (T32GM00754 and T32HG000040 to AZD), Stephen H. Hinshaw for access to the dermestid beetle colony at the University of Michigan Ruthven Museum, Dr. Robert H. Lyons, Jr. and members of the University of Michigan DNA Sequencing Core Facility for sequencing and genotyping, members of the Kresge Hearing Research Institute including Jennifer Benson, Lisa Kabala, and David F. Dolan for auditory brainstem response testing, and Jun Z. Li, David T. Burke, Ronald J. Koenig, and Miriam H. Meisler for helpful discussions.
Funding: March of Dime Foundation (N018020) Sally A. Camper
National Institutes of Health (T32GM007544) Alexandre Z. Daly
National Institutes of Health (T32HG000040) Alexandre Z. Daly
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- Abu-Khudir R, Larrivee-Vanier S, Wasserman JD, Deladoey J (2017) Disorders of thyroid morphogenesis. Best Pract Res Clin Endocrinol Metab 31, 143–159 [DOI] [PubMed] [Google Scholar]
- Amendola E, De Luca P, Macchia PE, Terracciano D, Rosica A, Chiappetta G, Kimura S, Mansouri A, Affuso A, Arra C, Macchia V, Di Lauro R, De Felice M (2005) A mouse model demonstrates a multigenic origin of congenital hypothyroidism. Endocrinology 146, 5038–5047 [DOI] [PubMed] [Google Scholar]
- Amendola E, Sanges R, Galvan A, Dathan N, Manenti G, Ferrandino G, Alvino FM, Di Palma T, Scarfo M, Zannini M, Dragani TA, De Felice M, Di Lauro R (2010) A locus on mouse chromosome 2 is involved in susceptibility to congenital hypothyroidism and contains an essential gene expressed in thyroid. Endocrinology 151, 1948–1958 [DOI] [PubMed] [Google Scholar]
- Beamer WJ, Eicher EM, Maltais LJ, Southard JL (1981) Inherited primary hypothyroidism in mice. Science 212, 61–63 [DOI] [PubMed] [Google Scholar]
- Bellman SC, Davies A, Fuggle PW, Grant DB, Smith I (1996) Mild impairment of neuro-otological function in early treated congenital hypothyroidism. Arch Dis Child 74, 215–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbel P, Obregon MJ, Bernal J, Escobar del Rey F, Morreale de Escobar G (2007) Iodine supplementation during pregnancy: a public health challenge. Trends Endocrinol Metab 18, 338–343 [DOI] [PubMed] [Google Scholar]
- Bernal J, Guadano-Ferraz A, Morte B (2015) Thyroid hormone transporters--functions and clinical implications. Nat Rev Endocrinol 11, 506. [DOI] [PubMed] [Google Scholar]
- Brent GA (2012) Mechanisms of thyroid hormone action. J Clin Invest 122, 3035–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmeier ML, Davis SW, Carninci P, MacDonald JW, Kawai J, Ghosh D, Hayashizaki Y, Lyons RH, Camper SA (2009) Discovery of transcriptional regulators and signaling pathways in the developing pituitary gland by bioinformatic and genomic approaches. Genomics 93, 449–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucker-Davis F, Skarulis MC, Pikus A, Ishizawar D, Mastroianni MA, Koby M, Weintraub BD (1996) Prevalence and mechanisms of hearing loss in patients with resistance to thyroid hormone. J Clin Endocrinol Metab 81, 2768–2772 [DOI] [PubMed] [Google Scholar]
- Buchner DA, Nadeau JH (2015) Contrasting genetic architectures in different mouse reference populations used for studying complex traits. Genome Res 25, 775–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Genome Dynamics, Multi-system survey of mouse physiology in 72 inbred strains of mice (ANOVA-adjusted methodology) In MPD:CGDpheno1. Mouse Phenome Database web site The Jackson Laboratory, Bar Harbor, Maine USA. [Google Scholar]
- Christ S, Biebel UW, Hoidis S, Friedrichsen S, Bauer K, Smolders JW (2004) Hearing loss in athyroid pax8 knockout mice and effects of thyroxine substitution. Audiol Neurootol 9, 88–106 [DOI] [PubMed] [Google Scholar]
- Cooper GM, Stone EA, Asimenos G, Program NCS, Green ED, Batzoglou S, Sidow A (2005) Distribution and intensity of constraint in mammalian genomic sequence. Genome Res 15, 901–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordas EA, Ng L, Hernandez A, Kaneshige M, Cheng SY, Forrest D (2012) Thyroid hormone receptors control developmental maturation of the middle ear and the size of the ossicular bones. Endocrinology 153, 1548–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvol H, Blackman SM, Boelle PY, Gallins PJ, Pace RG, Stonebraker JR, Accurso FJ, Clement A, Collaco JM, Dang H, Dang AT, Franca A, Gong J, Guillot L, Keenan K, Li W, Lin F, Patrone MV, Raraigh KS, Sun L, Zhou YH, O’Neal WK, Sontag MK, Levy H, Durie PR, Rommens JM, Drumm ML, Wright FA, Strug LJ, Cutting GR, Knowles MR (2015) Genome-wide association meta-analysis identifies five modifier loci of lung disease severity in cystic fibrosis. Nat Commun 6, 8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas E, Auso E, Telefont M, Morreale de Escobar G, Sotelo C, Berbel P (2005) Transient maternal hypothyroxinemia at onset of corticogenesis alters tangential migration of medial ganglionic eminence-derived neurons. Eur J Neurosci 22, 541–551 [DOI] [PubMed] [Google Scholar]
- Di Jeso B, Arvan P (2016) Thyroglobulin From Molecular and Cellular Biology to Clinical Endocrinology. Endocr Rev 37, 2–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas KR, Brinkmeier ML, Kennell JA, Eswara P, Harrison TA, Patrianakos AI, Sprecher BS, Potok MA, Lyons RH, Jr., MacDougald OA, Camper SA(2001) Identification of members of the Wnt signaling pathway in the embryonic pituitary gland. Mamm Genome 12, 843–851 [DOI] [PubMed] [Google Scholar]
- Dumont BL, Payseur BA (2011) Genetic analysis of genome-scale recombination rate evolution in house mice. PLoS Genet 7, e1002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels K, Rakov H, Zwanziger D, Moeller LC, Homuth G, Kohrle J, Brix K, Fuhrer D (2015) Differences in Mouse Hepatic Thyroid Hormone Transporter Expression with Age and Hyperthyroidism. Eur Thyroid J 4, 81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Q, Giordimaina AM, Dolan DF, Camper SA, Mustapha M (2012) Genetic background of Prop1(df) mutants provides remarkable protection against hypothyroidism-induced hearing impairment. J Assoc Res Otolaryngol 13, 173–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Q, Longo-Guess C, Gagnon LH, Mortensen AH, Dolan DF, Camper SA, Johnson KR (2011) A modifier gene alleviates hypothyroidism-induced hearing impairment in Pou1f1dw dwarf mice. Genetics 189, 665–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest D, Reh TA, Rusch A (2002) Neurodevelopmental control by thyroid hormone receptors. Curr Opin Neurobiol 12, 49–56 [DOI] [PubMed] [Google Scholar]
- Fugazzola L, Muzza M, Weber G, Beck-Peccoz P, Persani L (2011) DUOXS defects: Genotype-phenotype correlations. Ann Endocrinol (Paris) 72, 82–86 [DOI] [PubMed] [Google Scholar]
- Furlan LL, Marson FA, Ribeiro JD, Bertuzzo CS, Salomao Junior JB, Souza DR (2016) IL8 gene as modifier of cystic fibrosis: unraveling the factors which influence clinical variability. Hum Genet 135, 881–894 [DOI] [PubMed] [Google Scholar]
- Gage PJ, Brinkmeier ML, Scarlett LM, Knapp LT, Camper SA, Mahon KA (1996) The Ames dwarf gene, df, is required early in pituitary ontogeny for the extinction of Rpx transcription and initiation of lineage-specific cell proliferation. Mol Endocrinol 10, 1570–1581 [DOI] [PubMed] [Google Scholar]
- Geister KA, Brinkmeier ML, Cheung LY, Wendt J, Oatley MJ, Burgess DL, Kozloff KM, Cavalcoli JD, Oatley JM, Camper SA (2015) LINE-1 Mediated Insertion into Pocla (Protein of Centriole 1 A) Causes Growth Insufficiency and Male Infertility in Mice. PLoS Genet 11, e1005569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinoer D, Delange F (2000) The potential repercussions of maternal, fetal, and neonatal hypothyroxinemia on the progeny. Thyroid 10, 871–887 [DOI] [PubMed] [Google Scholar]
- Grasberger H, De Deken X, Mayo OB, Raad H, Weiss M, Liao XH, Refetoff S (2012) Mice deficient in dual oxidase maturation factors are severely hypothyroid. Mol Endocrinol 26, 481–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasberger H, Refetoff S (2006) Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem 281, 18269–18272 [DOI] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O’Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ (1999) Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341, 549–555 [DOI] [PubMed] [Google Scholar]
- Hamilton BA, Yu BD (2012) Modifier genes and the plasticity of genetic networks in mice. PLoS Genet 8, e1002644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanken J, Thorogood P (1993) Evolution and development of the vertebrate skull: The role of pattern formation. Trends Ecol Evol 8, 9–15 [DOI] [PubMed] [Google Scholar]
- Hardisty RE, Erven A, Logan K, Morse S, Guionaud S, Sancho-Oliver S, Hunter AJ, Brown SD, Steel KP (2003) The deaf mouse mutant Jeff (Jf) is a single gene model of otitis media. J Assoc Res Otolaryngol 4, 130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda Y, Sasaki N, Kameda Y, Torigoe D, Agui T (2012) Identifying quantitative trait loci affecting resistance to congenital hypothyroidism in 129/SvJcl strain mice. PLoS One 7, e31035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu M, Saunders JC (1983) Auditory development in the mouse: structural maturation of the middle ear. J Morphol 176, 249–259 [DOI] [PubMed] [Google Scholar]
- Ikeda A, Zheng QY, Zuberi AR, Johnson KR, Naggert JK, Nishina PM (2002) Microtubule-associated protein 1A is a modifier of tubby hearing (moth1). Nat Genet 30, 401–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskoll TF, Maderson PF (1978) A histological study of the development of the avian middle ear and tympanum. Anat Rec 190, 177–199 [DOI] [PubMed] [Google Scholar]
- Johnson KR, Marden CC, Ward-Bailey P, Gagnon LH, Bronson RT, Donahue LR (2007) Congenital hypothyroidism, dwarfism, and hearing impairment caused by a missense mutation in the mouse dual oxidase 2 gene, Duox2. Mol Endocrinol 21, 1593–1602 [DOI] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Noben-Trauth K (2006) Strain background effects and genetic modifiers of hearing in mice. Brain Res 1091, 79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Weston MD, Ptacek LJ, Noben-Trauth K (2005) The Mass1frings mutation underlies early onset hearing impairment in BUB/BnJ mice, a model for the auditory pathology of Usher syndrome IIC. Genomics 85, 582–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jump DB, Narayan P, Towle H, Oppenheimer JH (1984) Rapid effects of triiodothyronine on hepatic gene expression. Hybridization analysis of tissue-specific triiodothyronine regulation of mRNAS14. J Biol Chem 259, 2789–2797 [PubMed] [Google Scholar]
- Kallman JC, Phillips JO, Bramhall NF, Kelly JP, Street VA (2008) In search of the DFNA11 myosin VIIA low- and mid-frequency auditory genetic modifier. Otol Neurotol 29, 860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolyi IJ, Dootz GA, Halsey K, Beyer L, Probst FJ, Johnson KR, Parlow AF, Raphael Y, Dolan DF, Camper SA (2007) Dietary thyroid hormone replacement ameliorates hearing deficits in hypothyroid mice. Mamm Genome 18, 596–608 [DOI] [PubMed] [Google Scholar]
- Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, Heger A, Agam A, Slater G, Goodson M, Furlotte NA, Eskin E, Nellaker C, Whitley H, Cleak J, Janowitz D, Hernandez-Pliego P, Edwards A, Belgard TG, Oliver PL, McIntyre RE, Bhomra A, Nicod J, Gan X, Yuan W, van der Weyden L, Steward CA, Bala S, Stalker J, Mott R, Durbin R, Jackson IJ, Czechanski A, Guerra-Assuncao JA, Donahue LR, Reinholdt LG, Payseur BA, Ponting CP, Birney E, Flint J, Adams DJ (2011) Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 477, 289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipper M, Zinn C, Maier H, Praetorius M, Rohbock K, Kopschall I, Zimmermann U (2000) Thyroid hormone deficiency before the onset of hearing causes irreversible damage to peripheral and central auditory systems. J Neurophysiol 83, 3101–3112 [DOI] [PubMed] [Google Scholar]
- Kousi M, Katsanis N (2015) Genetic modifiers and oligogenic inheritance. Cold Spring Harb Perspect Med 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Henikoff S, Ng PC (2009) Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 4, 1073–1081 [DOI] [PubMed] [Google Scholar]
- Lavado-Autric R, Auso E, Garcia-Velasco JV, Arufe Mdel C, Escobar del Rey F, Berbel P, Morreale de Escobar G (2003) Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. J Clin Invest 111, 1073–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus JH, Bestwick JP, Channon S, Paradice R, Maina A, Rees R, Chiusano E, John R, Guaraldo V, George LM, Perona M, Dall’Amico D, Parkes AB, Joomun M, Wald NJ (2012) Antenatal thyroid screening and childhood cognitive function. N Engl J Med 366, 493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Henley CM, O’Malley BW, Jr. (1999) Distortion product otoacoustic emissions and outer hair cell defects in the hyt/hyt mutant mouse. Hear Res 138, 65–72 [DOI] [PubMed] [Google Scholar]
- Li S, Crenshaw EB 3rd, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG (1990) Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene Pit-1. Nature 347, 528–533 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- Marovitz WF, Berryhill BH, Peterson RR (1968) Disruptions of bony labyrinth, ossicular chain and tympanic bullae in dwarf mice. Laryngoscope 78, 863–872 [DOI] [PubMed] [Google Scholar]
- Moore JK, Linthicum FH, Jr. (2007) The human auditory system: a timeline of development. Int J Audiol 46, 460–478 [DOI] [PubMed] [Google Scholar]
- Mortensen AH, Schade V, Lamonerie T, Camper SA (2015) Deletion of OTX2 in neural ectoderm delays anterior pituitary development. Hum Mol Genet 24, 939–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustapha M, Fang Q, Gong TW, Dolan DF, Raphael Y, Camper SA, Duncan RK (2009) Deafness and permanently reduced potassium channel gene expression and function in hypothyroid Pitldw mutants. J Neurosci 29, 1212–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napiontek U, Borck G, Muller-Forell W, Pfarr N, Bohnert A, Keilmann A, Pohlenz J (2004) Intrafamilial variability of the deafness and goiter phenotype in Pendred syndrome caused by a T416P mutation in the SLC26A4 gene. J Clin Endocrinol Metab 89, 5347–5351 [DOI] [PubMed] [Google Scholar]
- Ng L, Kelley MW, Forrest D (2013) Making sense with thyroid hormone-the role of T3 in auditory development. Nat Rev Endocrinol. 9, 296–307 [DOI] [PubMed] [Google Scholar]
- Nicholas AK, Serra EG, Cangul H, Alyaarubi S, Ullah I, Schoenmakers E, Deeb A, Habeb AM, Almaghamsi M, Peters C, Nathwani N, Aycan Z, Saglam H, Bober E, Dattani M, Shenoy S, Murray PG, Babiker A, Willemsen R, Thankamony A, Lyons G, Irwin R, Padidela R, Tharian K, Davies JH, Puthi V, Park SM, Massoud AF, Gregory JW, Albanese A, Pease-Gevers E, Martin H, Brugger K, Maher ER, Chatterjee VK, Anderson CA, Schoenmakers N (2016) Comprehensive Screening of Eight Known Causative Genes in Congenital Hypothyroidism With Gland-in-Situ. J Clin Endocrinol Metab 101, 4521–4531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H, Li X, Makmura L, Friedman RA (2008) Mapping of genetic modifiers of Eya1 ( bor/bor ) in CAST/EiJ and BALB/cJ that suppress cochlear aplasia and associated deafness. Mamm Genome 19, 634–639 [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR (2003) Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet 35, 21–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR, Nishina PM (1997) mdfw: a deafness susceptibility locus that interacts with deaf waddler (dfw). Genomics 44, 266–272 [DOI] [PubMed] [Google Scholar]
- Pascarella A, Ferrandino G, Credendino SC, Moccia C, D’Angelo F, Miranda B, D’Ambrosio C, Bielli P, Spadaro O, Ceccarelli M, Scaloni A, Sette C, De Felice M, De Vita G, Amendola E (2018) DNAJC17 is localized in nuclear speckles and interacts with splicing machinery components. Sci Rep 8, 7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine-Twaddell E, Romero CJ, Radovick S (2013) Vertical transmission of hypopituitarism: critical importance of appropriate interpretation of thyroid function tests and levothyroxine therapy during pregnancy. Thyroid 23, 892–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KS, Hubisz MJ, Rosenbloom KR, Siepel A (2010) Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res 20, 110–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postiglione MP, Parlato R, Rodriguez-Mallon A, Rosica A, Mithbaokar P, Maresca M, Marians RC, Davies TF, Zannini MS, De Felice M, Di Lauro R (2002) Role of the thyroid-stimulating hormone receptor signaling in development and differentiation of the thyroid gland. Proc Natl Acad Sci U S A 99, 15462–15467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81, 559–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CA, Amin S, Linden J, Dixon J, Dixon MJ, Tucker AS (2010) Defects in middle ear cavitation cause conductive hearing loss in the Tcof1 mutant mouse. Hum Mol Genet 19, 1551–1560 [DOI] [PubMed] [Google Scholar]
- Rovet J, Daneman D (2003) Congenital hypothyroidism: a review of current diagnostic and treatment practices in relation to neuropsychologic outcome. Paediatr Drugs 5, 141–149 [DOI] [PubMed] [Google Scholar]
- Rovet JF, Ehrlich R (2000) Psychoeducational outcome in children with early-treated congenital hypothyroidism. Pediatrics 105, 515–522 [DOI] [PubMed] [Google Scholar]
- Rusch A, Ng L, Goodyear R, Oliver D, Lisoukov I, Vennstrom B, Richardson G, Kelley MW, Forrest D (2001) Retardation of cochlear maturation and impaired hair cell function caused by deletion of all known thyroid hormone receptors. J Neurosci 21, 9792–9800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rye MS, Bhutta MF, Cheeseman MT, Burgner D, Blackwell JM, Brown SD, Jamieson SE (2011) Unraveling the genetics of otitis media: from mouse to human and back again. Mamm Genome 22, 66–82 [DOI] [PubMed] [Google Scholar]
- Salvatore G, Edelhoch H (1973) Chemistry and Biosynthesis of Thyroid Iodoproteins In Hormonal Proteins and Pepetides, Li CH, ed. New York, NY: Academic Press, pp 201–244 [Google Scholar]
- Sasaki N, Hosoda Y, Nagata A, Ding M, Cheng JM, Miyamoto T, Okano S, Asano A, Miyoshi I, Agui T (2007) A mutation in Tpst2 encoding tyrosylprotein sulfotransferase causes dwarfism associated with hypothyroidism. Mol Endocrinol 21, 1713–1721 [DOI] [PubMed] [Google Scholar]
- Schultz JM, Yang Y, Caride AJ, Filoteo AG, Penheiter AR, Lagziel A, Morell RJ, Mohiddin SA, Fananapazir L, Madeo AC, Penniston JT, Griffith AJ (2005) Modification of human hearing loss by plasma-membrane calcium pump PMCA2. N Engl J Med 352, 1557–1564 [DOI] [PubMed] [Google Scholar]
- Seelig S, Liaw C, Towle HC, Oppenheimer JH (1981) Thyroid hormone attenuates and augments hepatic gene expression at a pretranslational level. Proc Natl Acad Sci U S A 78, 4733–4737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofia VM, Da Sacco L, Surace C, Tomaiuolo AC, Genovese S, Grotta S, Gnazzo M, Petrocchi S, Ciocca L, Alghisi F, Montemitro E, Martemucci L, Elce A, Lucidi V, Castaldo G, Angioni A (2016) Extensive molecular analysis suggested the strong genetic heterogeneity of idiopathic chronic pancreatitis. Mol Med 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O’Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG (1996) Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature 384, 327–333 [DOI] [PubMed] [Google Scholar]
- Sprenkle PM, McGee J, Bertoni JM, Walsh EJ (2001) Consequences of hypothyroidism on auditory system function in Tshr mutant (hyt) mice. J Assoc Res Otolaryngol 2, 312–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppa-Vaucher S, Van Vliet G, Deladoey J(2011) Variation by ethnicity in the prevalence of congenital hypothyroidism due to thyroid dysgenesis. Thyroid 21, 13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strug LJ, Gonska T, He G, Keenan K, Ip W, Boelle PY, Lin F, Panjwani N, Gong J, Li W, Soave D, Xiao B, Tullis E, Rabin H, Parkins MD, Price A, Zuberbuhler PC, Corvol H, Ratjen F, Sun L, Bear CE, Rommens JM (2016) Cystic fibrosis gene modifier SLC26A9 modulates airway response to CFTR-directed therapeutics. Hum Mol Genet 25, 4590–4600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan S, Kong JH, Fang Q, Salles FT, Wangsawihardja F, Ricci AJ, Mustapha M (2016) Thyroid hormone is required for pruning, functioning and long-term maintenance of afferent inner hair cell synapses. Eur J Neurosci 43, 148–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uziel A (1986) Periods of sensitivity to thyroid hormone during the development of the organ of Corti. Acta Otolaryngol Suppl 429, 23–27 [DOI] [PubMed] [Google Scholar]
- Uziel A, Legrand C, Ohresser M, Marot M (1983) Maturational and degenerative processes in the organ of Corti after neonatal hypothyroidism. Hear Res 11, 203–218 [DOI] [PubMed] [Google Scholar]
- van der Spek AH, Fliers E, Boelen A (2017) The classic pathways of thyroid hormone metabolism. Mol Cell Endocrinol 458, 29–38 [DOI] [PubMed] [Google Scholar]
- Vanderschueren-Lodeweyckx M, Debruyne F, Dooms L, Eggermont E, Eeckels R (1983) Sensorineural hearing loss in sporadic congenital hypothyroidism. Arch Dis Child 58, 419–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesper AH, Raetzman LT, Camper SA (2006) Role of prophet of Pit1 (PROP1) in gonadotrope differentiation and puberty. Endocrinology 147, 1654–1663 [DOI] [PubMed] [Google Scholar]
- Vigone MC, Fugazzola L, Zamproni I, Passoni A, Di Candia S, Chiumello G, Persani L, Weber G (2005) Persistent mild hypothyroidism associated with novel sequence variants of the DUOX2 gene in two siblings. Hum Mutat 26, 395. [DOI] [PubMed] [Google Scholar]
- Weber G, Rabbiosi S, Zamproni I, Fugazzola L (2013) Genetic defects of hydrogen peroxide generation in the thyroid gland. J Endocrinol Invest 36, 261–266 [DOI] [PubMed] [Google Scholar]
- White KR (2004) Early hearing detection and intervention programs: opportunities for genetic services. Am J Med Genet A 130A, 29–36 [DOI] [PubMed] [Google Scholar]
- Yan D, Liu XZ (2010) Modifiers of hearing impairment in humans and mice. Curr Genomics 11, 269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Ma SG, Guo ML, Qiu YL, Yang LX (2017) Compound Heterozygous Mutations in the DUOX2lDUOXA2 Genes Cause Congenital Hypothyroidism. Yonsei Med J 58, 888–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.