Abstract
While vector-borne parasite transmission often operates via generalist-feeding vectors facilitating cross-species transmission in host communities, theory describing the relationship between host species diversity and parasite invasion in these systems is underdeveloped. Host community composition and abundance vary across space and time, generating opportunities for parasite invasion. To explore how host community variation can modify parasite invasion potential, we develop a model for vector-borne parasite transmission dynamics that includes a host community of arbitrary richness and species' abundance. To compare invasion potential across communities, we calculate the community basic reproductive ratio of the parasite. We compare communities comprising a set of host species to their subsets, which allows for flexible scenario building including the introduction of novel host species and species loss. We allow vector abundance to scale with, or be independent of, community size, capturing regulation by feeding opportunities and non-host effects such as limited oviposition sites. Motivated by equivocal data relating host species competency to abundance, we characterize plausible host communities via phenomenological relationships between host species abundance and competency. We identify an underappreciated mechanism whereby changes to communities simultaneously alter average competency and the vector to host ratio and demonstrate that the interaction can profoundly influence invasion potential.
Keywords: species richness, community ecology, infectious diseases, parasite invasion, vector-borne parasites, host competency
1. Introduction
The gain or loss of species in a community and changes in the abundance of member species are all predicted to affect generalist parasites that require sufficient transmission to sustain themselves [1–3]. Across space, landscape heterogeneity may admit or exclude certain species as a direct result of either environmental conditions or species interactions, including competition [4]. A gradual change in species composition of mammal communities distributed in space can often lead to patterns of nestedness [5,6]. Over time, communities can also gradually change their composition by processes including species invasion [7] and community disassembly in response to habitat degradation [8,9], both of which may alter the abundance of established community members. These examples motivate a general aim to describe changes in parasite invasion potential across host communities and their subsets.
Among the generalist parasites, vector-borne parasites are of particular interest. First, systems involving transmission by arthropod vectors, e.g. tick-borne Lyme disease [10,11] and mosquito-borne West Nile virus [12,13], have provided striking examples where reduced host species diversity is associated with increased transmission potential to humans. Second, because transmission is by generalist-feeding vectors, cross-species transmission is less impacted by potentially infrequent interspecific contact [14], i.e. different host species are reasonably well mixed in terms of transmission. Lastly, host community dynamics may directly influence vector population sizes introducing additional mechanisms relating to vector to host ratios that impact parasite invasion [15]. For example, if vectors are primarily limited by non-host factors, such as the availability of oviposition sites [16], then vector population sizes may remain constant while the host community size changes. Conversely, if vectors are limited by blood meals [17], then the vector population size may scale with the host community size.
While Lyme disease and West Nile virus demonstrate the potential for host species diversity to limit the transmission of generalist parasites, it is a conditional phenomenon only expected when host species vary in their quality to a parasite, when higher quality hosts tend to occur in species-poor communities and when lower quality hosts disrupt transmission by regulating populations of higher quality hosts, the population of vectors or host–parasite encounter rates [18]. Across disease systems, evidence for this ‘dilution effect’ is equivocal [19,20] and has led to a call for a more mechanistic framework to clarify conditions whereby parasites benefit from reduced host species diversity [19,21]. While several modelling studies have collectively begun to answer this call [14,22,23], few have explicitly included vectors, with studies limited to tractable, two host species systems [24] or simulation-based approaches that can handle greater levels of species diversity [25]. Given the natural and anthropogenically catalysed changes in host communities involving spatial heterogeneity, species invasions and community disassembly, a mechanistic understanding of comparative vector-borne parasite invasion potential into host communities and their subsets may potentially explain and predict a large number of wildlife disease events and concomitant zoonotic risk.
One of the challenges in developing mechanistic models of generalist parasite transmission is the limited knowledge of host species competency, defined as the ability to maintain and transmit parasites [26] and how competency relates to natural population abundance and tendency to occur in communities of low species richness. Typically, there is a negative relationship between species body mass and population size [27], which can determine competency in two ways. First, small-bodied, highly abundant species tend to exhibit life-history traits that correlate with low investment in immunity [28,29]. Second, parasites are more likely to adapt to abundant host resources [28]. Both of these arguments lead to a prediction that species with naturally abundant populations are relatively competent. However, a negative relationship between body mass and competency is not ubiquitous [30], and in some cases, species identity is an important predictor of competence over any trait [31]. Furthermore, some studies have found that following community disassembly, remnant communities comprise species of intermediate body size [32]. Consequently, we adopt a flexible approach that allows positive, negative or no correlation between population abundance and competency, consistent with studies suggesting that a range of mechanisms and associations probably occur in nature [22].
Recognizing the common pattern of nested communities, we develop a model for a vector-borne parasite in a host community and derive an inequality relating parasite invasion potential, the community parasite basic reproductive number (R0) [2,22,33], in the full community to the equivalent measure in a subset. This inequality allows conditions to be identified where parasite invasion potential is higher in either the community or the subset. Note that we do not assume that the parasite is evolving. If it were, competition between parasite strains in invaded communities becomes relevant [34]. Rather, we study changes in R0 owing to both host community composition and the relative abundance of hosts and vectors, assuming that the community is yet to be challenged with the parasite. We select a susceptible-infected-susceptible (SIS) model that describes a broad class of parasites that do not confer lifelong immunity [35]. Our version includes an explicit vector population, an arbitrary number of host species and demographic dynamics of host and vector populations. Assumptions about the community subset are minimal; provided that the new community is less species rich, the remaining species can be of equal, greater or lesser abundance compared to their abundance in the full community. We allow the vector population to either scale with or remain independent of community size, reflecting situations where vectors are limited or unlimited by blood meals.
We apply the R0 inequality to three cases. The first compares the simplest host community of two species to its subset of one species and is used to clarify how components of host competence, host species abundance and vector population size interact to determine how a decrease in host species richness affects parasite invasion potential. Next, we consider an invasion of a multi-species community by a novel host species. An invasive species may be of low competence because local parasites that may invade the community, i.e. are in contiguous host communities but not yet in the focal community, are not pre-adapted to the invasive species [36]. Alternatively, invasive species may be of high competence because invasive potential may correlate with those same life-history traits of small-bodied animals that render them parasite-competent [37]. Furthermore, the invasive species may attain high or low abundance in the invaded community [38] and may be most strongly competing with, and negatively affecting the population size of, relatively dominant established species or rare species [39]. Our broad aim is to establish which types of biological invasions are likely to promote the potential invasion of a generalist parasite. Finally, we consider a community disassembly scenario where the focus is on characterizing how the relative competency and abundance of early departing species affect the remnant community in terms of its potential to be challenged by a novel parasite. We pay particular attention to the relative changes in the host community and vector population sizes, as this has been predicted from theory to strongly impact parasite invasion into a naive host community [15].
We find that the abundance response of a vector population to host community dynamics is critical in understanding the fate of the parasite. When host species do not regulate each other's abundance, invasion potential is predicted to decline in species-rich communities, provided that the vector population size does not increase with host community size. This is most pronounced if a host species added to the community attains a high abundance and is of relatively low competence. Conversely, additional host species that render the host community relatively small, such as those in strong competition with pre-existing host species, can promote parasite invasion, especially if the novel species is of high competence. Consequently, invasive species that attain low abundance in novel communities and suppress the population size of either abundant resident host species or low competence host species can enhance parasite transmission, provided the vector population size remains similar to pre-invasion levels. In cases where vector abundance tracks host abundance, parasites instead benefit from the invasion of abundant, competent species. During host community disassembly, potential for invasion is predicted to increase most significantly when remnant host species do not increase their abundance and are competent for transmission, and when the vector population does not decline during disassembly.
2. Methods
We model a host community comprising an arbitrary number of host species (X) and a single vector species using an SIS model framework for transmission dynamics (equation (2.1)). A simple form for demography is used in which susceptible individuals of host species i (Si) are born at constant rate λi and experience a natural per capita mortality rate µi. These demographic processes define the disease-free equilibrium population size as . The host community disease-free equilibrium population size is the sum of the individual species abundances The vector population is modelled with similar demography, represented by parameters λV, µV and KV with state variables SV and IV. We chose to use this simple formulation for demography as all subsequent analyses only use the disease-free equilibrium value, defined as Ki. Specifically, we are asking if the change in the community composition of a parasite-free community affects the potential for future parasite invasion. Consequently, provided the demographic construction results in a disease-free equilibrium, the specific mechanisms involved (i.e. fecundity, survivorship, linear and nonlinear) will not influence these results.
Vectors bite hosts at rate b0, and it is assumed that the bite rate is distributed equally across hosts species (b0/C). When infected vectors bite susceptible hosts (Si), successful transmission of the parasite occurs with probability τiV. Susceptible hosts transition to the infected class at the rate , where individuals recover at per capita rate γi or die at per capita rate µi. Susceptible vectors become infected at the rate (τVi is the probability of successful transmission to the vector from infected host i) and infected vectors die at a per capita rate µV:
| 2.1 |
The next-generation matrix method allows for the calculation of a multi-species community R0 when the model includes more than one infectious class [2,14,35] and results in equation (2.2) for the model specified by equation (2.1):
| 2.2 |
This expression is written in abbreviated form using two composite parameters: the relative abundance of host species i () and the host competency of species i (), where a host species is competent owing to low recovery or mortality rates, Γi = γi + μi, and high probability of passing and acquiring the parasite during transmission opportunities (τ parameters). While we assumed no disease-induced mortality in the system, this could be encapsulated in the composite host parameter Γi and could be included as additional loss for infected vectors. Importantly, while mortality in susceptible and infected classes is different in the above case, it does not affect disease-free equilibrium analyses, which would proceed as presented but with numerically higher mortality rates.
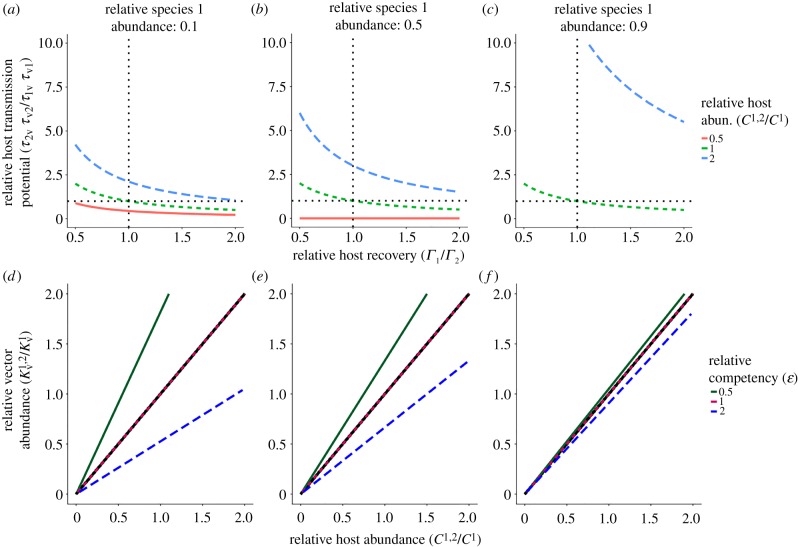
We compared a two host and single-host community using the inequality describing when parasite invasion potential into the single species community is higher than in the two species community . This allows demarcation of regions where as a function of parameters , pi, τ and Γi from which we generated two sets of parameter spaces to investigate the change in invasion potential with: (i) the relative competency of host traits and (ii) the relative abundance of host and vector communities. To investigate the effect of host traits, the proportion of hosts belonging to species 1 (p1∈{0.1, 0.5, 0.9}), the relative host community abundance and the relative vector abundance were held constant. Relative transmission probability was varied between [0,10] and relative recovery was varied between (0,2]. To investigate the influence of the relative abundance of host and vector communities, the relative abundance of host species 1 (p1∈{0.1, 0.5, 0.9}) and the relative competency of the second host species (; ε∈{0.5, 1.0, 2.0}) were fixed while the relative host community abundance and the relative vector abundance were varied between (0,2]. For each space, we plotted the line that defines when R0 remains constant. Regions on either side of the line are when R0 increases or decreases following the addition of host species 2.
We generalized this approach to establish conditions for decreased parasite invasion potential in host communities of any species richness using equation (2.2) to compare R0 for a community to its subset. The following two scenarios were developed to examine how predictable changes in community composition following invasion or disassembly determine R0. In each scenario, we used two versions of the vector-borne transmission model: one where vector abundance changes by the same proportion as the host community abundance (vector tracking) and one where the vector population abundance remains constant (vector constant).
In the invasion scenario, we defined a resident host community of three host species in which each distinct species was either of high (100), medium (50) and low abundance (25). The competence of the species (ψ) was high (1.0), medium (0.5) or low (0.25). Three potential relationships between host abundance and competency were implemented: a positive relationship where the most abundant species was the most competent, no relationship between competency and abundance (neutral) where each species had the same competency (ψ = 0.5), and a negative relationship where the most abundant species was the least competent. In the pre-existing community, the vector population had an abundance of 250 individuals.
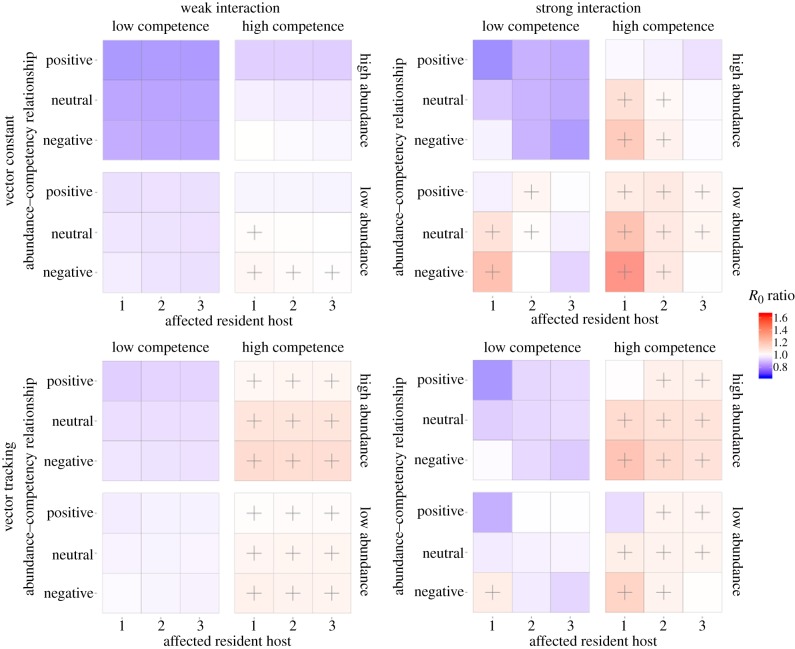
We assumed novel hosts invaded the community at either a high (100 individuals) or low (25) abundance and had a high (ψ = 1.0) or low (ψ = 0.25) competency. The invader also influenced the abundance of one resident species (with effect denoted as α∈[0, 1]). When α = 0, the invader species drove the targeted species locally extinct. When α = 1, the invader species had no effect on the abundance of the target species. For our simulations, we selected one level representing a weak interaction (α = 0.9, population reduced by 10%) and one representing a strong interaction (α = 0.1, 90% reduction). For each invaded community, R0 was calculated and compared to the R0 for the uninvaded community.
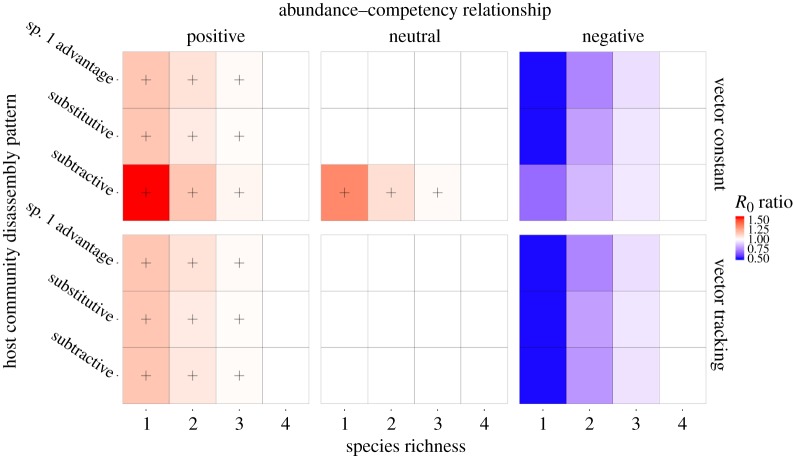
For community disassembly, we generated a host community of four host species, with abundances 100, 50, 25 and 5 and competencies 1.0, 0.5, 0.25 and 0.05. We implemented three disassembly rules. For the subtractive rule, we assumed that the abundance of each species did not change with species richness (parameter C in equation (2.2)). For the substitutive rule, when a species is removed from the community, the number of removed individuals was equally distributed and added to the abundance of the remaining species abundances. Under the Species 1 Advantage rule, when a species is removed from the community, the number of individuals of the removed species is added to the most abundant species. In each step of the simulation, we sequentially removed the host species with the lowest abundance in the original community ending with the single, most abundant host species. Following the removal of a species, the R0 of the community was compared to the respective R0 for the original host community. In the disassembly scenarios, the competency for the neutral abundance–competency relationship was the average competency from positive and negative relationships (ψi = 0.45). The vector population had an abundance of 250 individuals in the original community.
3. Results
Comparing a two species community with a single species community, the resulting equality (equation (3.1)) represents the scenarios when R0 remains constant:
| 3.1 |
For the parasite to have increased invasion potential in the more speciose community, the second host species needs to be more competent for the parasite in either transmission or infectious period so long as relative community size remains the same or increases (figure 1a–c). However, if community size decreases, then the parasite can benefit even when the additional host species is poorly competent. Furthermore, in larger community sizes (blue lines in figure 1a–c), the second host species must compensate for its rarity by increased competence in terms of relative transmission and infectious period for the parasite to benefit.
Figure 1.
Parameter spaces where lines separate regions of increased and decreased parasite invasion potential (R0) following the addition of a second host species. Each column represents the relative species 1 abundance. (a–c) The parameter space is defined by relative host recovery and relative host transmission potential determined from equation (3.1). Regions above and to the right of each of the lines define scenarios where parasite invasion is promoted in the more speciose community, whereas invasion is hindered in the complementary region. Each line colour represents the change in host community abundance following the addition of a second host species. The dotted vertical and horizontal black lines are reference lines denoting where the two host species have equal components of competency. (d–f) The parameter space is defined by relative host community abundance and relative vector population abundance determined from equation (3.2). Regions above and to the left of each line define scenarios where parasite invasion is promoted in the more speciose community, whereas invasion potential decreases in the complementary region. Each line colour represents the relative competency of the second host species compared to the first. The solid black line is where the vector population increases proportionally with community abundance. (Online version in colour.)
To further investigate changes in host and vector abundance, we used equation (3.3), which describes the conditions for constant R0 across the two communities as a function of the relative abundance of the vector community :
| 3.2 |
Parasite invasion may be promoted following the addition of a second host species, provided the relative vector abundance exceeds the relative host community abundance (figure 1d–f). However, following the addition of a less competent host, the vector population abundance must change by a much higher proportion than the host community for the parasite to benefit. When the additional host is more competent, vector abundance can decrease proportionally more than the host community and the parasite can still benefit. Should both host species have the same competency, the parasite will benefit simply when there are more vectors per host. When the community is dominated by species 1, the competency of additional host species 2 plays a lesser role in determining if the parasite benefits (shown by the angular spread of lines in figure 1d–f).
The following inequality describes conditions for increased R0 when the host community (Y) loses a set of hosts (Y–X):
| 3.3 |
For invasion potential to increase, the species-poor community should consist of highly competent species, which is especially true when these competent species are disproportionately represented in the community. Parasite invasion is promoted owing to reduced vector to host ratios (K/C terms), but these component effects do not guarantee an increase in R0; rather, their combined effect ultimately determines if the parasite will benefit in the lower richness community.
A highly competent invader that strongly regulates other host species and itself reaches a low abundance enhances parasite transmission if the vector population remains constant (figure 2; top-right panel: high competence–low abundance). However, should the vector population track the community size, the effect is much reduced, and even reversed if the host species impacted by the invader is abundant and competent (figure 2; bottom-right panel: high competence–low abundance). A highly competent invader will have no effect on parasite invasion potential or can even reduce it if the invader has a negligible regulatory effect on the abundance of other species in the community and the vector population remains constant (figure 2; top-left panels: high competence). This effect is much stronger if the invader is of low competence, when R0 is reduced regardless of the response of the vector population (figure 2; left-hand panels: low competence). A low competence invader with strong interspecific regulation will dramatically reduce transmission should it invade at high abundance (figure 2; top-right panel: low competence–high abundance). By contrast, parasite invasion can be promoted if a low competence, low abundance invader suppresses a low competence, high abundance resident host (figure 2; right-hand panels: low competence–low abundance).
Figure 2.
Heatmaps detailing the change in parasite invasion potential (R0) following invasion by a novel host species. Each column represents the strength of interaction the invading host has on a resident host species. Each row is the response the vector population has to changes in host community abundance. For each combination, there are four heatmaps, where the columns and rows represent the competence and abundance of the invading host species. The x-axis of each heatmap shows the identity of the resident host species that the invading host regulates. Affected resident host 1 is always the most abundant species and 3 the least abundant. The y-axis represents the abundance–competency relationship of the uninvaded community. Cell colour represents the R0 ratio of the invaded community (four species) to the uninvaded community (three). Red colours (and ‘+’ symbol when R0 ratio >1.01) identify scenarios where the invaded community benefits the parasite and blue colours represent the opposite. (Online version in colour.)
There are also interesting scenarios where the resulting R0 is determined by a single factor. For example, when a highly competent invader joins a community at a high abundance and weakly interacts with resident hosts, the regulation of the vector population determines if R0 increases or decreases (figure 2; left-hand panels: high competence–high abundance). For invasion potential to increase, the vector population must track the host community so that bites per host remain constant, but the community competence increases. The abundance–competency relationship determines the change in R0 when a competent and abundant invader strongly regulates a host and there is a constant vector population (figure 2; top-right panel: top right). For R0 to increase, the invading host must strongly regulate low competence, abundant hosts, thereby increasing host community competence offsetting the decrease in bites per host (figure 2; right-hand panels: negative abundance–competency relationship). However, the change in community competence cannot sufficiently offset the decrease in bites per host for R0 to increase when the invader regulates low abundance, low competence hosts (figure 2; right-hand panels: positive abundance–competency relationship).
When a community disassembles, parasite invasion is most likely promoted when it is the more competent host species that remain in a species-poor community (positive abundance–competency relationship), and if there is a subtractive disassembly pattern. This is because the remnant community is of higher average competency compared with the original community, but the remnant community size is relatively small, increasing the ratio of vectors to hosts (figure 3; top-left panel). However, if the vector population size scales with host community size, the disassembly rule (species 1 advantage, substitutive or subtractive) has little influence on changes in R0 (figure 3; bottom panels). If abundant, late-leaving host species are of poor competence, parasite invasion is hindered during community disassembly (figure 3; right-hand panels), but this effect is diminished if the community size shrinks (subtractive disassembly) and the vector population size does not track community size (vector constant), because reductions in average competency are partly offset by increases in vector to host ratios. Under most other assumptions, particularly those that assume no relationship between host competency and natural abundance, parasite invasion potential is relatively invariant across stages of community disassembly.
Figure 3.
Heatmaps detailing the change in parasite invasion potential (R0) following host community disassembly from a full community with four host species to a single species population. Each column represents the abundance–competency relationship. Each row is the response the vector population has to changes in host abundance. The x-axis of each heatmap is the species richness of the community. The y-axis is the pattern of host community assembly. Each cell colour is the R0 ratio of the community with the specified richness (x-axis) to the community with all four host species. Red colours (and ‘+’ symbol when R0 ratio >1.01) identify scenarios where the invaded community benefits the parasite and blue colours represent the opposite. (Online version in colour.)
4. Discussion
Across time and space, host community composition can vary owing to a variety of mechanisms, often resulting in a nested host community structure [5,6]. Vector-borne parasites present the opportunity to better understand how parasite invasion potential responds to changes in community composition as vectors feed on multiple species and often carry generalist parasites, but they require special consideration. First, we needed to define how the vector population responds to changes in the host community. Second, community competence may change via changes in the relative abundance of the member species, including loss of species as well as the addition of new host species. Our approach allowed us to navigate these complexities and derive an expression for the reproductive ratio of the parasite in an arbitrary host community in which the vector population size may scale with, or be independent of, the host community size. We have demonstrated that these two considerations of parasite invasion can act synergistically or antagonistically.
Echoing the equivocal findings in support of dilution effects across disease systems [19,20], our results identify mechanisms that demonstrate the conditional nature of this expectation. For example, although it is intuitive to expect that the addition of a more competent species to a community would lead to an increase in parasite invasion potential, our results collectively show that if the vector to host ratio decreases following this addition, then invasion potential can decrease even though average competency increases. This underscores the importance of evaluating what regulates vector abundance. For example, habitat constraints may limit abundance by restricting oviposition site availability [40], and climate can impact abundance via effects on development, survivorship and fecundity [41]. Alternatively, vector populations may be primarily limited by the availability of blood meals [42]. There is evidence that many vector species capable of transmitting zoonotic parasites are habitat- and host-generalists, which may cause these species to be sensitive to changes in host community size [42,43]. Furthermore, environmental drivers may influence both the host community and vector population simultaneously, creating a novel environment for the parasite in terms of average competency and vector to host ratios [44].
The straightforward assumption that the bites of a vector population are distributed proportionally among component host species allows us to retain tractability when comparing communities that vary in composition, abundance and competency. However, the approach we present can be extended to include vector feeding preference [45]. Feeding preferences may manifest in several ways and have been shown to impact transmission in both West Nile virus [46] and Lyme disease systems [42]. In the West Nile virus system, feeding preference for American robins, an important reservoir species for the virus, can increase the number of infected vectors [31,46]. Furthermore, infection status of individuals may influence their attractiveness to a vector. Birds infected with the avian malaria parasite Plasmodium relictum have been shown to be more attractive to susceptible vectors [47]. Infection could also alter host behaviour such that vectors can more easily feed [48], potentially promoting parasite invasion. Furthermore, the vectors themselves may represent communities of several species with distinct demography, phenology and biting preferences that could be included in bespoke models tailored to a specific system [45,49].
Our results illustrate that the relationship between competence and abundance can be critical in determining how parasite invasion responds to changes in the parasite-free host community, and point to the importance of establishing these relationships in the host species of the generalist parasite being studied. Often, host species with fast life-history traits, i.e. species that often have a large number of offspring and are highly abundant, are also more competent in terms of parasite transmission, including in several multi-host vector-borne disease systems [28,30]. There are at least two explanations for this trend. First, parasites may be pre-adapted to abundant host species in the community, as the parasite is likely to have encountered individuals of those species in neighbouring communities [42,43,50]. Second, species of this type have been found to invest less in key aspects of immunity compared to reproduction, rapid growth and development [51]. Therefore, life-history data for a host species may allow the prediction of competency. For example, invasive species often are able to reach high abundances but may not be competent for parasite transmission, as the parasite may instead be adapted to native host species [36], which are likely to be present in the contiguous communities where the parasite originates. Alternatively, more generalist parasites may benefit from invasive species owing to the congruence between invader traits and fast pace of life traits [52].
To chart the general mechanisms influencing parasite invasion potential in host communities, we assume that competency is constant for a given species, but it may not be a fixed trait of a species. Interspecific interactions with other hosts, and non-hosts, in the community can alter the ability of a host to acquire and transmit a parasite [53,54]. For example, competitive interactions may reduce the availability of resources resulting in decreased body condition of the host [55], which could affect the infectious period of the host, positively via a reduced parasite clearance rate or negatively via a reduced survivorship. Spatially aggregated resources may reduce competency by increasing host condition but could augment transmission via host aggregation [56]. However, in contrast to directly transmitted parasites, vector-borne parasite invasion will be largely determined by the resulting vector to host ratios in such aggregated host communities. Beyond competition, predatory interactions may also influence host competency. The presence of a predator may not only reduce encounter rates between hosts and vectors through changes in host behaviour [53] but may also induce stress on the host, which could compromise immune function [57]. These examples emphasize the importance of considering host competency as a dynamic trait that can be influenced by community composition. Future work may investigate how trophic structure can induce a range of density- and trait-mediated effects on vector-borne parasite invasion potential.
Host community composition can vary across space and time via a variety of mechanisms and has been shown to influence parasite invasion. We have demonstrated that community abundance and competence, and the ratio of vectors to hosts may vary simultaneously, creating a rich, but tractable, set of consequences for parasite invasion. Notably, our results emphasize that parasites do not consistently benefit from invading lower richness communities, but rather the interplay between average competency in the host community and the vector to host ratio is key to understanding transmission potential. Our modelling approach also enables predictions of how parasite invasion potential will change as a result of commonly observed phenomena, such as biological invasions and community disassembly, provided we understand how species regulate abundance [14], that we can anticipate how vector populations will change as a result of community dynamics and we have estimates of the competency of the host species in the community.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank John Roquet for insightful discussion when developing the analytical results. We also thank John Drake, Richard Hall, David Vasquez, Annakate Schatz and Thomas Strayer for providing friendly reviews of the manuscript.
Data accessibility
R code generating figures 1–3 is provided online as electronic supplementary material at https://dx.doi.org/10.6084/m9.figshare.c.4544411 [58].
Authors' contribution
J.E.V. and A.W.P. conceived and designed the study. J.E.V. developed the model, and derived analytical results and performed the simulations. J.E.V. and A.W.P. interpreted model results. J.E.V. wrote the first draft of the manuscript and both authors contributed substantially to revisions.
Competing interests
We declare that we have no competing interests.
Funding
A.W.P. was supported by the National Science Foundation under grant no. NSF DEB 1754255.
References
- 1.Holt RD, Dobson AP, Begon M, Bowers RG, Schauber EM. 2003. Parasite establishment in host communities. Ecol. Lett. 6, 837–842. ( 10.1046/j.1461-0248.2003.00501.x) [DOI] [Google Scholar]
- 2.Dobson A. 2004. Population dynamics of pathogens with multiple host species. Am. Nat. 164(Suppl 5), S64–S78. ( 10.1086/424681) [DOI] [PubMed] [Google Scholar]
- 3.Fenton A, Pedersen AB. 2005. Community epidemiology framework for classifying disease threats. Emerg. Infect. Dis. 11, 1815–1821. ( 10.3201/eid1112.050306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kraft NJB, Adler PB, Godoy O, James EC, Fuller S, Levine JM. 2015. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 29, 592–599. ( 10.1111/1365-2435.12345) [DOI] [Google Scholar]
- 5.Lynam AJ, Billick I. 1999. Differential responses of small mammals to fragmentation in a Thailand tropical forest. Biol. Conserv. 91, 191–200. ( 10.1016/S0006-3207(99)00082-8) [DOI] [Google Scholar]
- 6.Young HS, et al. 2017. Interacting effects of land use and climate on rodent-borne pathogens in central Kenya. Phil. Trans. R. Soc. Lond. B 372, 20160116 ( 10.1098/rstb.2016.0116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clout MN, Russell JC. 2008. The invasion ecology of mammals: a global perspective. Wildl. Res. 35, 180–184. ( 10.1071/WR07091) [DOI] [Google Scholar]
- 8.Lomolino MV, Perault DR. 2000. Assembly and disassembly of mammal communities in a fragmented temperate rain forest. Ecology 81, 1517–1532. ( 10.1890/0012-9658(2000)081[1517:AADOMC]2.0.CO;2) [DOI] [Google Scholar]
- 9.Lindo Z, Whiteley J, Gonzalez A. 2012. Traits explain community disassembly and trophic contraction following experimental environmental change. Glob. Chang. Biol. 18, 2448–2457. ( 10.1111/j.1365-2486.2012.02725.x) [DOI] [Google Scholar]
- 10.LoGiudice K, Duerr STK, Newhouse MJ, Schmidt KA, Killilea ME, Ostfeld RS. 2008. Impact of host community composition on Lyme disease risk. Ecology 89, 2841–2849. ( 10.1890/07-1047.1) [DOI] [PubMed] [Google Scholar]
- 11.Ostfeld RS, Keesing F. 2000. Biodiversity and disease risk: the case of Lyme disease. Conserv. Biol. 14, 722–728. ( 10.1046/j.1523-1739.2000.99014.x) [DOI] [Google Scholar]
- 12.Ezenwa VO, Godsey MS, King RJ, Guptill SC. 2006. Avian diversity and West Nile virus: testing associations between biodiversity and infectious disease risk. Proc. R. Soc. B 273, 109–117. ( 10.1098/rspb.2005.3284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allan BF, et al. 2009. Ecological correlates of risk and incidence of West Nile virus in the United States. Oecologia 158, 699–708. ( 10.1007/s00442-008-1169-9) [DOI] [PubMed] [Google Scholar]
- 14.O'Regan SM, Vinson JE, Park AW. 2015. Interspecific contact and competition may affect the strength and direction of disease-diversity relationships for directly transmitted microparasites. Am. Nat. 186, 480–494. ( 10.1086/682721) [DOI] [PubMed] [Google Scholar]
- 15.Smith DL, Battle KE, Hay SI, Barker CM, Scott TW, McKenzie FE. 2012. Ross, Macdonald, and a theory for the dynamics and control of mosquito-transmitted pathogens. PLoS Pathog. 8, e1002588 ( 10.1371/journal.ppat.1002588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Day JF. 2016. Mosquito oviposition behavior and vector control. Insects 7, 65 ( 10.3390/insects7040065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayoh MN, et al. 2010. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar. J. 9, 62 ( 10.1186/1475-2875-9-62) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostfeld RS, Keesing F. 2012. Effects of host diversity on infectious disease. Annu. Rev. Ecol. Evol. Syst. 43, 157–182. ( 10.1146/annurev-ecolsys-102710-145022) [DOI] [Google Scholar]
- 19.Civitello DJ, et al. 2015. Biodiversity inhibits parasites: broad evidence for the dilution effect. Proc. Natl Acad. Sci. USA 112, 8667–8671. ( 10.1073/pnas.1506279112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salkeld DJ, Padgett KA, Jones JH. 2013. A meta-analysis suggesting that the relationship between biodiversity and risk of zoonotic pathogen transmission is idiosyncratic. Ecol. Lett. 16, 679–686. ( 10.1111/ele.12101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson PTJ, Ostfeld RS, Keesing F. 2015. Frontiers in research on biodiversity and disease. Ecol. Lett. 18, 1119–1133. ( 10.1111/ele.12479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joseph MB, Mihaljevic JR, Orlofske SA, Paull SH. 2013. Does life history mediate changing disease risk when communities disassemble? Ecol. Lett. 16, 1405–1412. ( 10.1111/ele.12180) [DOI] [PubMed] [Google Scholar]
- 23.Faust CL, Dobson AP, Gottdenker N, Bloomfield LSP, McCallum HI, Gillespie TR, Diuk-Wasser M, Plowright RK. 2017. Null expectations for disease dynamics in shrinking habitat: dilution or amplification? Phil. Trans. R. Soc. B 372, 20160173 ( 10.1098/rstb.2016.0173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller E, Huppert A. 2013. The effects of host diversity on vector-borne disease: the conditions under which diversity will amplify or dilute the disease risk. PLOS ONE 8, e80279 ( 10.1371/journal.pone.0080279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roche B, Rohani P, Dobson AP, Guégan J-F. 2013. The impact of community organization on vector-borne pathogens. Am. Nat. 181, 1–11. ( 10.1086/668591) [DOI] [PubMed] [Google Scholar]
- 26.Johnson PTJ, Preston DL, Hoverman JT, Richgels KLD. 2013. Biodiversity decreases disease through predictable changes in host community competence. Nature 494, 230–233. ( 10.1038/nature11883) [DOI] [PubMed] [Google Scholar]
- 27.White EP, Ernest SKM, Kerkhoff AJ, Enquist BJ. 2007. Relationships between body size and abundance in ecology. Trends Ecol. Evol. 22, 323–330. ( 10.1016/j.tree.2007.03.007) [DOI] [PubMed] [Google Scholar]
- 28.Ostfeld RS, Levi T, Jolles AE, Martin LB, Hosseini PR, Keesing F. 2014. Life history and demographic drivers of reservoir competence for three tick-borne zoonotic pathogens. PLOS ONE 9, e107387 ( 10.1371/journal.pone.0107387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keesing F, et al. 2010. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468, 647–652. ( 10.1038/nature09575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang ZYX, de Boer WF, van Langevelde F, Olson V, Blackburn TM, Prins HHT. 2013. Species' life-history traits explain interspecific variation in reservoir competence: a possible mechanism underlying the dilution effect. PLOS ONE 8, e54341 ( 10.1371/journal.pone.0054341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. 2006. Host heterogeneity dominates West Nile virus transmission. Proc. R. Soc. B 273, 2327–2333. ( 10.1098/rspb.2006.3575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okie JG, Brown JH. 2009. Niches, body sizes, and the disassembly of mammal communities on the Sunda Shelf islands. Proc. Natl Acad. Sci. USA 106(Suppl 2), 19 679–19 684. ( 10.1073/pnas.0901654106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen LJS, Brown VL, Jonsson CB, Klein SL, Laverty SM, Magwedere K, Owen JC, van den Driessche P. 2012. Mathematical modeling of viral zoonoses in wildlife. Nat. Resour. Model. 25, 5–51. ( 10.1111/j.1939-7445.2011.00104.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lion S, Metz JAJ. 2018. Beyond R0 maximisation: on pathogen evolution and environmental dimensions. Trends Ecol. Evol. 33, 458–473. ( 10.1016/j.tree.2018.02.004) [DOI] [PubMed] [Google Scholar]
- 35.Keeling MJ, Rohani P. 2008. Modeling infectious diseases in humans and animals. Princeton, NJ: Princeton University Press. [Google Scholar]
- 36.Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM. 2003. Introduced species and their missing parasites. Nature 421, 628–630. ( 10.1038/nature01346) [DOI] [PubMed] [Google Scholar]
- 37.Duncan RP, Blackburn TM, Veltman CJ. 1999. Determinants of geographical range sizes: a test using introduced New Zealand birds. J. Anim. Ecol. 68, 963–975. ( 10.1046/j.1365-2656.1999.00344.x) [DOI] [Google Scholar]
- 38.Strayer DL, Eviner VT, Jeschke JM, Pace ML. 2006. Understanding the long-term effects of species invasions. Trends Ecol. Evol. 21, 645–651. ( 10.1016/j.tree.2006.07.007) [DOI] [PubMed] [Google Scholar]
- 39.Jeschke JM, et al. 2014. Defining the impact of non-native species. Conserv. Biol. 28, 1188–1194. ( 10.1111/cobi.12299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gottdenker NL, Streicker DG, Faust CL, Carroll CR. 2014. Anthropogenic land use change and infectious diseases: a review of the evidence. Ecohealth 11, 619–632. ( 10.1007/s10393-014-0941-z) [DOI] [PubMed] [Google Scholar]
- 41.Yang G-J, Brook BW, Whelan PI, Cleland S, Bradshaw CJA. 2008. Endogenous and exogenous factors controlling temporal abundance patterns of tropical mosquitoes. Ecol. Appl. 18, 2028–2040. ( 10.1890/07-1209.1) [DOI] [PubMed] [Google Scholar]
- 42.Keesing F, Brunner J, Duerr S, Killilea M, Logiudice K, Schmidt K, Vuong H, Ostfeld RS. 2009. Hosts as ecological traps for the vector of Lyme disease. Proc. R. Soc. B 276, 3911–3919. ( 10.1098/rspb.2009.1159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ostfeld R, Keesing F. 2000. The function of biodiversity in the ecology of vector-borne zoonotic diseases. Can. J. Zool. 78, 2061–2078. ( 10.1139/Cjz-78-12-2061) [DOI] [Google Scholar]
- 44.Gage KL, Burkot TR, Eisen RJ, Hayes EB. 2008. Climate and vectorborne diseases. Am. J. Prev. Med. 35, 436–450. ( 10.1016/j.amepre.2008.08.030) [DOI] [PubMed] [Google Scholar]
- 45.Takken W, Verhulst NO. 2013. Host preferences of blood-feeding mosquitoes. Annu. Rev. Entomol. 58, 433–453. ( 10.1146/annurev-ento-120811-153618) [DOI] [PubMed] [Google Scholar]
- 46.Simpson JE, Hurtado PJ, Medlock J, Molaei G, Andreadis TG, Galvani AP, Diuk-Wasser MA. 2012. Vector host-feeding preferences drive transmission of multi-host pathogens: West Nile virus as a model system. Proc. R. Soc. B 279, 925–933. ( 10.1098/rspb.2011.1282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cornet S, Nicot A, Rivero A, Gandon S. 2013. Malaria infection increases bird attractiveness to uninfected mosquitoes. Ecol. Lett. 16, 323–329. ( 10.1111/ele.12041) [DOI] [PubMed] [Google Scholar]
- 48.Day JF, Ebert KM, Edman JD. 1983. Feeding patterns of mosquitoes (Diptera: Culicidae) simultaneously exposed to malarious and healthy mice, including a method for separating blood meals from conspecific hosts. J. Med. Entomol. 20, 120–127. ( 10.1093/jmedent/20.2.120) [DOI] [PubMed] [Google Scholar]
- 49.Park AW, Cleveland CA, Dallas TA, Corn JL. 2016. Vector species richness increases haemorrhagic disease prevalence through functional diversity modulating the duration of seasonal transmission. Parasitology 143, 874–879. ( 10.1017/S0031182015000578) [DOI] [PubMed] [Google Scholar]
- 50.Johnson PTJ, Rohr JR, Hoverman JT, Kellermanns E, Bowerman J, Lunde KB. 2012. Living fast and dying of infection: host life history drives interspecific variation in infection and disease risk. Ecol. Lett. 15, 235–242. ( 10.1111/j.1461-0248.2011.01730.x) [DOI] [PubMed] [Google Scholar]
- 51.Hawley DM, Altizer SM. 2011. Disease ecology meets ecological immunology: understanding the links between organismal immunity and infection dynamics in natural populations. Funct. Ecol. 25, 48–60. ( 10.1111/j.1365-2435.2010.01753.x) [DOI] [Google Scholar]
- 52.Allen WL, Street SE, Capellini I. 2017. Fast life history traits promote invasion success in amphibians and reptiles. Ecol. Lett. 20, 222–230. ( 10.1111/ele.12728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keesing F, Holt RD, Ostfeld RS. 2006. Effects of species diversity on disease risk. Ecol. Lett. 9, 485–498. ( 10.1111/j.1461-0248.2006.00885.x) [DOI] [PubMed] [Google Scholar]
- 54.Barron DG, Gervasi SS, Pruitt JN, Martin LB. 2015. Behavioral competence: how host behaviors can interact to influence parasite transmission risk. Curr. Opin. Behav. Sci. 6, 35–40. ( 10.1016/j.cobeha.2015.08.002) [DOI] [Google Scholar]
- 55.Jessop TS, Smissen P, Scheelings F, Dempster T. 2012. Demographic and phenotypic effects of human mediated trophic subsidy on a large Australian lizard (Varanus varius): meal ticket or last supper? PLOS ONE 7, e34069 ( 10.1371/journal.pone.0034069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Becker DJ, Hall RJ. 2014. Too much of a good thing: resource provisioning alters infectious disease dynamics in wildlife. Biol. Lett. 10, 20140309 ( 10.1098/rsbl.2014.0309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Young HS, Dirzo R, Helgen KM, McCauley DJ, Nunn CL, Snyder P, Veblen KE, Zhao S, Ezenwa VO. 2016. Large wildlife removal drives immune defence increases in rodents. Funct. Ecol. 30, 799–807. ( 10.1111/1365-2435.12542) [DOI] [Google Scholar]
- 58.Vinson J, Park A. 2019. Vector-borne parasite invasion in communities across space and time ( 10.6084/m9.figshare.c.4544411) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Vinson J, Park A. 2019. Vector-borne parasite invasion in communities across space and time ( 10.6084/m9.figshare.c.4544411) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
R code generating figures 1–3 is provided online as electronic supplementary material at https://dx.doi.org/10.6084/m9.figshare.c.4544411 [58].