Abstract
Many species use social interactions to cope with challenges in their environment and a growing number of studies show that individuals which are well-connected to their group have higher fitness than socially isolated individuals. However, there are many ways to be ‘well-connected’ and it is unclear which aspects of sociality drive fitness benefits. Being well-connected can be conceptualized in four main ways: individuals can be socially integrated by engaging in a high rate of social behaviour or having many partners; they can have strong and stable connections to favoured partners; they can indirectly connect to the broader group structure; or directly engage in a high rate of beneficial behaviours, such as grooming. In this study, we use survival models and long-term data in adult female rhesus macaques (Macaca mulatta) to compare the fitness outcomes of multiple measures of social connectedness. Females that maintained strong connections to favoured partners had the highest relative survival probability, as did females well-integrated owing to forming many weak connections. We found no survival benefits to being structurally well-connected or engaging in high rates of grooming. Being well-connected to favoured partners could provide fitness benefits by, for example, increasing the efficacy of coordinated or mutualistic behaviours.
Keywords: sociality, fitness, social structure, survival, group living, Macaca mulatta
1. Introduction
Social relationships are a fundamental component of group life. Individuals often interact or associate with others in an affiliative or non-agonistic manner, and these interactions can have fitness consequences. For example, well-connected humans and other animals can live longer and produce more offspring than less well-connected individuals (e.g. [1–3]; table 1). Yet despite their apparent importance to biological success, the routes by which social connections impact fitness—how and why social connections are beneficial—remains unclear.
Table 1.
Summary of the different ways individuals can be well-connected in their social networks and the proposed fitness benefits of social connectedness.
| way to be well connected | proposed benefits | predictions. fitness benefits greatest for individuals… | associated with fitness benefits in… (for example) |
|---|---|---|---|
| (i) social integration | results in lowered aggression, increased tolerance | • that spend the most time interacting with others • with the greatest number of partners • with many weak connections |
yellow baboons (Papio cynocephalus) [4]; feral horses (Equus ferus) [5]; rhesus macaques (Macaca mulatta) [6]; chamca baboons (Papio ursinus) [7] |
| (ii) dyadic connectedness | improves behavioural coordination and cooperation, ensures investments are returned | • with many strong connections • with strong connections to their most important social partners • with stable connections to their most important social partners |
Assamese macaques (Macaca assamensi) [8]; greater ani (Crotophaga major) [9]; chacma baboons [10] |
| (iii) structural connectedness | improves access to information or social influence | • that are more indirectly connected | house finches (Haemorhous mexicanus) [11]; rhesus macaques [12]; killer whales (Orcinus orca) [13]; chacma baboons [14] |
| (iv) direct connectedness | results in directly beneficial outcomes, e.g. grooming removes parasites | • that receive a lot of grooming | meerkats (Suricata suricatta) [15]; gidgee skinks (Egernia stokesii) [16]; Japanese macaques (Macaca fuscata) [17] |
Critical to uncovering the means by which social connections are beneficial is an understanding of what it means for individuals to be ‘well-connected’ [18]. Sociality is multi-dimensional in nature [19,20] and there are many ways for group-living animals to connect to others. For example, an individual might be well-connected in one sense because they have a large number of social partners, but poorly connected in another sense if their partners are all from the same subgroup. By deconstructing sociality into its different dimensions, we can pinpoint the specific types of social connections that are linked to fitness and, as a result, begin to identify the function (or functions) of being well-connected.
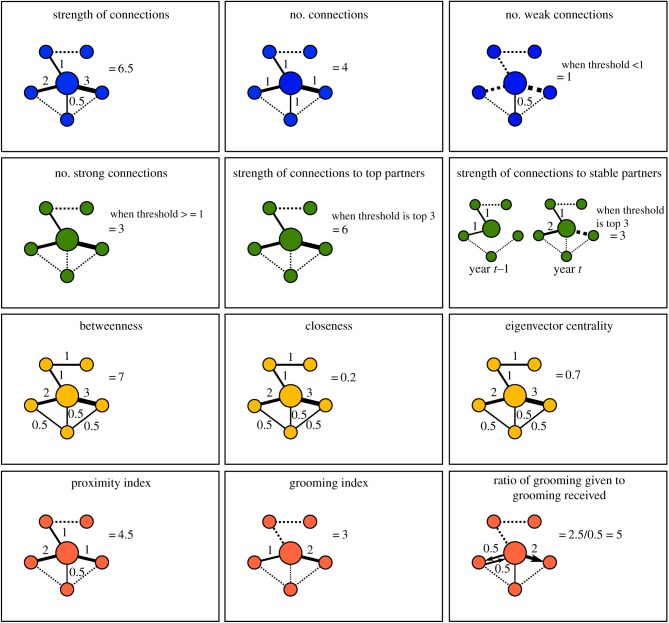
There are four main ways that connectedness has been conceptualized. We describe each here along with the proposed mechanisms by which each might be beneficial. For ease of understanding, we have named the four types of social connectedness as follows: (i) social integration; (ii) dyadic connectedness; (iii) structural connectedness; and (iv) direct connectedness (figure 1).
Figure 1.
Hypothetical network demonstrating how the same social connections were deconstructed in this study. Nodes represent individuals and lines between nodes represent a social connection. The width of lines increases as the strength of the connection between a pair of nodes increases. The large central node shows a focal individual but analyses were conducted using all individuals simultaneously. Solid lines show connections used to calculate a given measure of connectedness, dashed lines show connections not relevant to a given measure. Blue nodes (top row) represent measures of social integration: where we expect fitness benefits to be greatest for individuals spending more time socializing or with more social partners or with many social connections. Green nodes (second row) are measures of dyadic connectedness: with highest fitness predicted for females with many strong connections or strong connections to their most important and consistent partners. Yellow (third row) nodes show measures of structural connectedness where individuals with higher indirect connectedness are predicted to have higher fitness. Pink nodes (bottom row) are measures of direct connectedness: females' receiving more grooming or in proximity to others more often are predicted to have higher fitness. Social interactions in the context of this paper include grooming and spatial proximity represented as a dyadic sociality index, which differs from the direct connectedness measures (pink nodes) where social interactions are derived separately for proximity and grooming. (Online version in colour.)
Socially integrated individuals are those that engage in a high frequency of interactions with others and/or interact with a large number of partners (figure 1 and table 1). Measures of social integration are blind to the identity of social partners; individuals with a given rate of interaction are considered equivalent, regardless of whether they interact with a single individual or 10 individuals. Socially integrated individuals can also have a large number of weak (infrequent or transient) social partners [21]. Social integration has been proposed to be beneficial because it leads to social tolerance, increasing an individual's access to contested resources or spatial locations, minimizing their chances of injury or death owing to aggression [22–24].
For dyadic connectedness, the identity of social partners is important and social relationships are built up over a series of interactions with particular individuals (figure 1 and table 1). Dyadic connections might be considered analogous to friendships in humans [25,26]. Measures of dyadic connectedness rely on inferring an individual's most frequent or consistent partners (figure 1). Frequent and consistent engagement with the same partner may be beneficial because it increases the efficacy of coordinated behaviours [27,28] as well as opportunities for mutualism or reciprocal exchange of behavioural services [29,30].
Structural connectedness is based on indirect (i.e. with a partner's partners) as well as direct connections, capturing the wider pattern of relationships between all group members (figure 1 and table 1). Measures of structural connectedness include metrics commonly used in social network analysis, such as betweenness and closeness, the benefits of which may include an increased chance of learning new information from others (e.g. [31]), increased access to resources (e.g. [32]), enhanced likelihood of being alerted to the presence of a predator (e.g. [33]), and greater travelling and foraging efficiency (e.g. [34]).
Direct connectedness refers to scenarios where being well-connected is not necessarily about the properties of the social connections themselves, but is instead about the interactions involved in forming those connections (figure 1 and table 1). Grooming, for example, removes parasites [35] and is a common behaviour in many birds and mammals. Reduced parasite burdens could lead to decreased mortality of individuals who are groomed by others the most [36] regardless of the number or identity of their partners or of their position in the broader social structure. Similarly, maintaining spatial proximity to others may provide enhanced protection from predators or increased hunting success [37].
Studies have revealed fitness correlates for each of these four types of social connectedness in a taxonomically broad range of species (table 1). But distinguishing between the proposed ways that sociality contributes to fitness requires studies that evaluate the relationship between fitness and the different types of social connectedness in tandem. To our knowledge, no study to date has evaluated all four types of connectedness in a single study system. Here, we deconstruct the relationship between social connectedness and survival in a long-lived and highly social primate. Although a growing number of studies have linked social connections to the health (e.g. [2,3,38]) and reproduction (e.g. [39]) of individuals, longevity is also a major contributor to fitness, especially in female mammals where limited variation in reproductive rates results in longevity being the main predictor of lifetime reproductive success [40]. However, studies of the relationship between longevity and social connectedness are rare owing to a scarcity of datasets with sufficiently large numbers of individuals with known survival outcomes. In this study, we take advantage of data in a large number of (n = 319) adult females from a free-living population of rhesus macaques (Macaca mulatta) that has been studied for 80 years [41] to test the relationship between measures of the four different types of social connectedness and survival.
2. Methods
(a). Study subjects and behavioural data
We undertook this study on rhesus macaques inhabiting the island of Cayo Santiago, Puerto Rico. The population consists of approximately 500 adults living in 6–9 mixed-sex social groups. The animals are descendants of 409 Indian-origin rhesus macaques introduced in 1938. Subjects were mature adult females, greater than or equal to 6 years old [42]. There is no regular medical intervention and the major causes of death at this provisioned and predator-free site are disease and injury [43]. This population, therefore, allows us to investigate the fitness benefits of social connections in the absence of starvation and predator-driven mortality [6]. The expected lifespan for a female that reached adulthood in this study was 20 years (95% confidence interval 19–22), with a maximum observed lifespan of 28 years.
We collected behavioural data on 319 adult females between the years of 2010–2017, resulting in 754 macaque years. Behavioural data were collected on an average of two study groups each year: group F 2010–2017; group HH 2014; group KK 2015; group R 2015–2016; group S 2011; group V 2015–2016. Of our subjects, 34 died during the study (electronic supplementary material, table S1). We collected behavioural data using 10 min focal animal samples [44]. We selected animals in a pseudo-randomized order balanced within days and years, resulting in roughly the same number of observations per subject per year. We recorded the duration and direction of grooming and identities of all adult social partners. We included only interactions between adult females in analyses. Juveniles' interactions are influenced by their lack of independence from their mothers, while female–male interactions tend to be concentrated in the breeding season, making it difficult to isolate social processes from sexual ones. To establish spatial association (hereafter, spatial proximity), we recorded the identities of all adult females found within 2 m of a study subject (but not touching or grooming them) at three evenly-spaced intervals throughout a focal animal sample. Female rhesus macaques have a strict dominance hierarchy with maternal rank inheritance [42]. For each female, dominance rank was established in a given year based on observed submissive, win-loss, interactions [45].
(b). Quantifying social connectedness
As with previous studies (e.g. [4,7,10,12,46,47]), we used grooming and spatial proximity as indicators of social connections. We calculated a dyadic composite sociality index—DSI [20]—which represents the relative rate at which a pair of individuals (i and j) engage in behaviour x, relative to the mean rate of occurrence of that behaviour by all subjects in their group in a given year (eqn 1; [20]). For grooming, x represents the duration (seconds) of grooming given and received between a pair of animals. For proximity, x represents the number of times a pair of females were in proximity to one another relative to the number of times they were observed but were not in proximity to one another. As DSI is scaled by the mean rate of behaviour, DSI values are relative to within-group social opportunities, which allows comparisons of individuals from groups with divergent group sizes (electronic supplementary material, table S1) or gregariousness, and avoids potentially confounding within-group differences as individual effects [20].
We calculated the DSI between all pairs of females in a group in any given year. This allowed us to represent each female's level of connectedness relative to the group and year in which she lived. DSI forms the basis of all measures of social connectedness, acting in social network terms as the network ‘edge’. Our measures of connectedness either limit the social connections used or slightly alter the calculation of DSI (figure 1). Measures of social connectedness are described in detail below (see also the electronic supplementary material, figure S1).
(c). Social integration
We measured social integration in three ways.
-
(i)
Strength of connections. The overall strength of an individual's connections is a measure of social effort relative to other group members: i.e. how frequently an individual engages in social activity regardless of the identity of their social partners. This is calculated as the sum of all an individual's DSIs: their composite sociality index (CSI) and is equivalent to weighted degree in social network analysis.
-
(ii)
Number of connections. A count of the number of different individuals a subject interacts with, equivalent to ‘degree’ in social network analysis.
-
(iii)
Number of weak connections. Classifying connections as ‘weak’ requires a threshold value above which a connection is considered ‘strong’ and below which it is considered ‘weak’ [7,48]. Previous studies have used a threshold DSI of 1 as the boundary between strong and weak connections because 1 is the mean DSI in any population when pairs of animals that do and do not (DSI = 0) interact are considered (e.g. [7,48]). However, by including connections that are not present, this approach has the potential to categorize many connections as strong and few as weak. Indeed, there was too little variation in the number of weak connections using this approach to perform a reliable test of survival outcomes with our data. There were no clear discontinuities or cut-points in the distribution of DSI values to use as an intuitive threshold to distinguish strong and weak connections (electronic supplementary material, figure S2). There were also no clear biological reasons that a particular threshold value should be chosen. We therefore explored a range of thresholds, using fixed percentages of a group's DSIs as the ‘weak’ threshold, whereby the lowest 90%, 80%, 70%, 60%, 50%, 40%, 30%, 20% and 10% of DSI values in the population were considered weak. For example, under a 50% threshold, half of all connections were considered weak, whereas under a 40% threshold two-fifths of the connections were considered weak, and so on (electronic supplementary material, figure S3). At higher threshold values the ‘number of weak connections' measure approximates the ‘number of connections’ measure. For each threshold, we counted each subject's number of weak connections (electronic supplementary material, table S2) and used this value as the fixed effect in a survival model.
(d). Dyadic connectedness
We measured dyadic connectedness in three ways:
-
(i)
Number of strong dyadic connections. A count of the number of different individuals with whom a subject shared a ‘strong’ connection [7,48]. As for weak connections, classifying connections as ‘strong’ required a threshold above which a connection is considered ‘strong’ and below which it is considered ‘weak’ [7,48]. We used variable thresholds that defined the top 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80% and 90% of connections in the population were considered strong (electronic supplementary material, table S3). It is important to note that these are not the inverse of the weak connections measures (i.e. defining 10% of connections as weak will not give the same result as defining 90% as strong) because weak connections are defined as being below the threshold, while strong connections are defined as being above the threshold (electronic supplementary material, figure S3). The distribution of values for weak and strong measures do not therefore overlap.
-
(ii)
Strength of dyadic connections to ‘top’ partners. The frequency of interactions between an individual and their most frequent partners. Previous studies have typically summed the DSIs between a subject and their partners that fall within the subject's top (strongest) three DSI values [10,49,50]. We followed this procedure, varying the number of partners considered ‘top’ from 1 to 10 (few individuals in our study had more than 10 social partners). Females were only included in an analysis if they had the number of top partners under consideration in that year (i.e. for the top eight partners threshold all females included had at least eight partners).
-
(iii)
Strength of dyadic connections to stable partners. The frequency of interaction between a subject and its preferred partners that were consistent over time (figure 1). We calculated a female's total DSI to stable partners that: (i) had a DSI > 0 (i.e. any social partner); (ii) were within her top three DSI values [49–51]; and, (iii) were in the top 50% of her DSI values. Partner stability was only evaluated in group F because this was the only group with data across at least three consecutive years. To be included in the analysis for the top three stable partners (ii) or top 50% of partners (iii) a female must have had at least three or two partners, respectively.
(e). Structural connectedness
We quantified structural connectedness using social network metrics of indirect connectedness. A social network integrates individual social interactions into a representation of the social structure of the population [19]. An individual's position within the social structure of the whole group can then be quantified. We used three social network metrics that are among the most commonly used and have been previously correlated with fitness in social species: betweenness, closeness and eigenvector centrality (table 1). Betweenness is the number of shortest paths between all pairs of individuals that pass through a particular individual [19]. Individuals with a high betweenness connect subgroups within a population and can influence the transfer of items, e.g. information, through a network [52]. Closeness is the inverse of the average number of paths from a given individual to all others in a network [19]. An individual with high closeness can be connected to all others in a short number of steps and can, for example, disseminate a new piece of information throughout the network quickly. Eigenvector centrality is a measure of the quality of an individual's partners. Individuals with high eigenvector centrality have partners who themselves are well-connected [53]. All network metrics were calculated as their weighted version, where the weight of a social connection was the DSI. As DSI is a relative measure, weights are comparable between years and groups. Individuals without any social partners (n = 4) could not be included in this analysis.
(f). Direct connectedness
To test whether specific types of interactions, and in particular the amount of grooming individuals received from others, predicted survival, we re-calculated DSI values to include only one type of interaction, resulting in a ‘grooming sociality index’ and a ‘proximity sociality index’. An individual's grooming-CSI and proximity-CSI were calculated by summing the grooming and proximity DSIs for that individual. We also separated grooming based on its direction, and calculated a ‘grooming given’ index and a ‘grooming received’ index. We used the ratio of the grooming received index to the grooming given index to isolate the impact of receiving grooming from giving grooming to the greatest extent possible (i.e. separate analyses could result in significant relationships with survival for both the rate of giving and the rate of receiving grooming owing to autocorrelation between these terms). Females were only included in this ratio analysis if they were observed both giving and receiving grooming in a given year.
(g). Quantifying mortality
Parentage (maternal from 1956, paternal from 1992) and dates of birth and death (where applicable) are known for all Cayo Santiago animals [41]. Dates of birth and death are typically known to within a few days. For each subject in each year (n = 754), we established their age and survival status (number of deaths = 34), which we defined as whether or not they survived through a given year of study.
(h). Analyses
We used extended Cox proportional hazards (Cox PH) models to determine how an individual's instantaneous risk of death varied with their level of social connectedness. An individual's level of social connectedness can vary from year to year—extended Cox PH models allow for the use of these time-dependent covariates [54]. All connectedness measures were normalized to between 0 and 10 by dividing each value by the maximum value for that group and multiplying by 10 (the multiplication is to scale hazards to an easily understandable range). The number of mortality events in our data precluded including multiple variables in analyses and the use of model selection. To limit problems with over-parameterization and autocorrelation of variables (electronic supplementary material figure S1), we included a single variable per model and compared across models using a concordance analysis. Mortality data are time-linked: individuals in a dataset die in a known order, e.g. individual A died before individual B. Concordance determines the proportion of times that a model correctly predicts the order of death of all pairs of individuals in a dataset [55]. We used concordance as a measure of how well the parameters included in a model reflected real-world processes. We also investigated the relationship between survival and: (i) group size, (ii) dominance rank, and (iii) hours an individual was observed, each of which is a potentially important correlate of survival in this system [6,42,56], independently of our measures of social connectedness.
To account for the inherent lack of independence in our relational data we created null models from 1000 permutations of individual identity (‘node-label permutations’: [19,57]). Each permuted dataset had the same structure of social connections as the observed data, but the identities of the animals to which those connections belong were randomized. For each permuted dataset, we derived our measures of social connectedness and ran Cox PH models to establish the relationship between connectedness and survival. p-values were calculated from the number of times the test statistic from our observed data was greater (or less) than the test statistic in the null models [58]. p-values for analyses without social interaction variables (and therefore without relational non-independence), e.g. group size, were taken from the Cox model without permutation. To account for multiple comparisons of the same data, we adjusted p-values using the Benjamini–Hochberg method [59]. Unadjusted p-values are reported in the electronic supplementary material, table S4.
Analyses were undertaken in R using the dplyr, stringr, survival, sna, igraph, lme4 and ggplot2 packages.
3. Results
Female rhesus macaques had a mean (±s.d.) of 7.96 (±6.26) social connections and a mean CSI (±s.d.) of 47.55 (±43.13). There was a wide distribution of connection strengths. For example, in group F in 2012, the mean DSI was 8.35 (±9.28) but the weakest connection had a DSI of 0.46 and the strongest a DSI of 51.24 (complete distributions are shown in the electronic supplementary material, figure S2).
(a). Social integration and survival
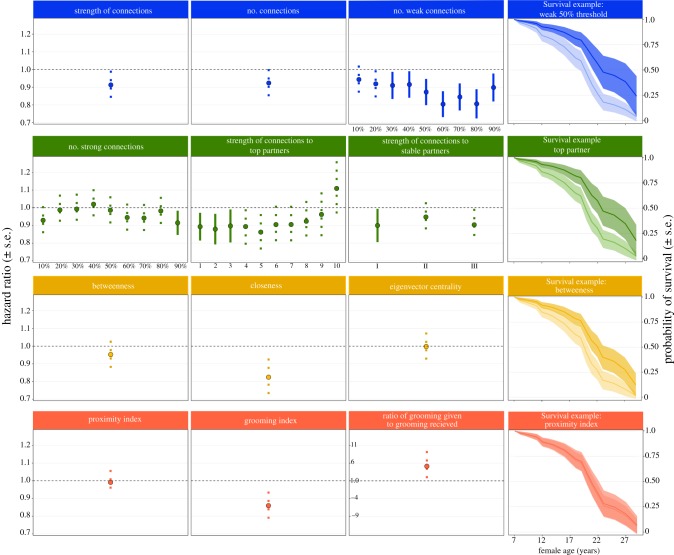
Neither an individual's strength of connections with other adult females nor her number of adult female partners were significant predictors of mortality risk (strength of connections: hazard (Haz.) = 0.91 ± 0.08, z = −0.93, n = 754, e = 34, p = 0.138; number of connections: Haz. = 0.92 ± 0.08, z = −0.79, n = 754, e = 34, p = 0.131; figure 2). However, a female's number of weak connections was a significant predictor of survival for all thresholds where ‘weak’ connections included more than 20% of connections in the population (figure 2; electronic supplementary material, table S2). Females with a greater number of weak connections typically lived longer than those with fewer weakly connected partners.
Figure 2.
The relationships between different measures of social connectedness and mortality hazard (hazard ± s.e.) of adult female rhesus macaques. The first three columns (from left) show the mortality hazard under each measure of connectedness used in this study. Hazards of 1 indicate no change in survival in relation to social connectedness, while hazards of less than 1 indicate models where mortality decreases (and the probability of survival increases) as social connectedness increases. Solid error bars indicate measures that significantly predicted survival. Dashed error bars indicate measures did not significantly predict survival. Colours indicate the type of connectedness measure: blue (top row) are social integration, green (second row) are dyadic connectedness, yellow (third row) are structural connectedness and pink (bottom row) are direct connectedness. For ‘strong connections’ and ‘weak connections’, x-axis labels indicate the proportion of connections in the population considered ‘strong’ or ‘weak’. In ‘top partners’, x-axis labels indicate the number of partners considered to be ‘top’. In ‘stable partners’ x-axis labels indicate the definition of stability used: I is any partner, II is top 3 partners and III is a top-50% of all partners. In contrast to the other measures, ratio of grooming given to grooming received does not show the changing mortality hazard as ‘connectedness’ increases, it instead represents a ratio. The y-axis in this plot is expanded to accommodate its divergent scale. ‘Survival examples’ (furthest right column) show an example of the relationship between age and survival probability for one of the measures used under each type of connectedness measure. Curves show the predicted survival probability for individuals with low (lighter colour; 10th quartile of observed values) and high (darker colour; 90th quartile of observed values) connectedness. (Online version in colour.)
(b). Dyadic connectedness and survival
A female's number of strong dyadic connections was a significant predictor of survival when the strongest 90% of connections (i.e. almost all connections in the population) were considered ‘strong’ (Haz. = 0.91 ± 0.07, z = −1.32, n = 754, e = 34, p = 0.008; figure 2). There was no relationship between a female's number of strong connections and her probability of survival under all other ‘strong’ connections thresholds (figure 2; electronic supplementary material, table S3).
There was a significant relationship between the strength of a female's connections to her most frequent partners and her probability of survival. Females with stronger relationships with their top 1–3 partners had a greater probability of survival than those with weaker connections to those top partners (figure 2; electronic supplementary material, table S5). The strength of a female's social connections to her top 4–10 partners did not predict survival (figure 2; electronic supplementary material, tables S5 and S4). It is important to note that as the number of top partners increases, the proportion of an individual's total CSI that value represents increases, and the strength of connections to top partners begins to approximate total strength of connections (electronic supplementary material figure S4).
There was a significant positive relationship between the strength of a female's connections to partners that were stable and her probability of surviving when all stable partners were considered (Haz. = 0.90 ± 0.09, n = 469, e = 24, z = −1.17, p = 0.031; figure 2). No such relationship was found when stable partners only included a female's top three partners (Haz. = 0.94 ± 0.08, n = 467, e = 24, z = −0.75, p = 0.437; figure 2) or the strongest 50% of partners (Haz. = 0.90 ± 0.09, n = 458, e = 24, z = −1.11, p = 0.218; figure 2).
(c). Structural connectedness and survival
No measure of structural connectedness was significantly related to mortality risk (betweenness: Haz. = 0.95 ± 0.08, n = 750, e = 33, z = −0.64, p = 0.403; closeness: Haz. = 0.82 ± 0.11, n = 750, e = 33, z = −1.53, p = 0.260; eigenvector centrality: Haz. = 1.0 ± 0.08, n = 750, e = 33, z = −0.03, p = 0.276; figure 2).
(d). Direct connectedness and survival
There was no relationship between survival and the amount of time females spent in proximity to others (Haz. = 0.99 ± 0.07, n = 754, e = 34, z = −0.86, p = 0.142), engaged in grooming (Haz. = 0.86 ± 0.08, n = 754, e = 34, z = −1.47, p = 0.0.247), or the ratio at which females gave and received grooming (received to given, Haz. = 5.13 ± 4.31, n = 673, e = 28, z = 1.92, p = 0.414; given to received, Haz. = 2.03 ± 2.81, n = 673, e = 28, z = 0.6, p = 0.121).
(e). Concordance
There was little variance in the concordance of the models (electronic supplementary material, figure S5), suggesting no model better explained the mortality patterns in the data than any other.
(f). Other variables and survival
We found no relationship between group size (Haz. = 0.84 ± 0.07, n = 924, e = 42, z = −1.82, p = 0.695), dominance rank (high versus low: Haz. = 0.87 ± 0.42, n = 871, e = 34, z = −0.26, p = 0.782; high versus medium: Haz. = 1.19 ± 0.56, n = 871, e = 34, z = 0.39, p = 0.712) or hours observed (Haz. = 1.00 ± 0.01, n = 924, e = 42, z = 0.39, p = 0.149) and survival. Similarly, group identity did not significantly predict survival (electronic supplementary material, table S5).
4. Discussion
By quantifying the relationship between survival and four of the most common operational definitions of social connectedness in a single system, this study highlights the fact that being ‘well-connected’ is multi-faceted in nature and provides evidence that some aspects of sociality represent more straightforward routes to biological success than others. In particular, we found support for a relationship between survival and dyadic connectedness: adult female rhesus macaques that frequently interacted with their top partners and that had partners that were stable over time were more likely to survive than females which interacted less often with their preferred and stable partners. However, we found no relationship between a female's number of strong connections and her probability of survival. For dyadic connections, at least, it appeared as though quality was more important than quantity. We also found some support for a relationship between social integration and survival: females that had a large number of weak connections experienced a lower mortality hazard. Other predictions of the social integration hypothesis were not supported, and there was little evidence that being structurally or directly well-connected resulted in survival benefits.
Our results add to previous studies linking the quality of dyadic relationships with positive fitness outcomes in social animals (table 1). In this study, rhesus macaque females with the strongest connections to their top partner had an 11% higher probability of survival than females that were less well-connected to their top partner. Repeatedly interacting with the same small number of individuals may facilitate the emergence and maintenance of cooperative relationships, whereby partners exchange behavioural services, such as grooming and coalitionary support, and where the consistency of partner identity may improve coordination of those behaviours and deter cheating [60,61].
Consistent and frequent partners may also result in benefits related to mutual social tolerance. In despotic, hierarchical, societies, like those of many female Old World primates, tolerated access to necessary resources, including food and space, may be beneficial to individuals [62–64]. Repeated and stable partnerships may initially arise because of shared needs or preferences amongst pairs of individuals. For example, individuals with similar metabolisms, thermoregulatory needs, or preferences for certain foods, may repeatedly find themselves attempting to access the same resource [65,66]. If alliances between pairs of individuals result in tolerance of that pair when accessing a resource, combined with mutualistic joint defence of that resource against competing groupmates, repeated and stable relationships may emerge. This scenario relies on relative stability in resource availability and in individual differences in needs and preferences. Individuals living outside of those conditions may have little need for stable partners, and may therefore exhibit a divergent relationship between dyadic connectedness and fitness [22,23,30]. In these species, a more flexible and generalized strategy of connectedness—via, for example, social integration—may be a better strategy for coping with the challenges of group-living.
In addition to dyadic connectedness, we found that some aspects of social integration predicted survival in this study; the number of weak connections a female maintained was linked to her mortality hazard. Wide social tolerance derived from these connections may allow a female to feed without disturbance or avoid harassment in a greater number of settings than females with fewer weak connections. Similar to the results presented here, blue monkeys (Cercopithecus mitis) survival has been shown to be positively associated with both strong-consistent connections and weak-inconsistent connections [51]. In the current population of rhesus macaques, measures of social integration have been positively linked to reproductive output [12] and proxies of social integration (family size) have been linked to survival [6]. Interestingly, correlations (electronic supplementary material, figure S1) and principal component analysis (electronic supplementary material, figure S6) suggest that dyadic connectedness measures and social integration measures are negatively associated in this population. That is, females with strong dyadic connectedness tend to have weak social integration. Taken together, these results may suggest that both dyadic connectedness and social integration can provide fitness benefits (albeit perhaps of different types) within the same system.
There was quantitative and qualitative variation in the relationship between survival and a female's number of strong connections, and between survival and number of weak connections depending on the threshold used to define connections as strong or weak. Choice of the threshold can, therefore, have important implications for the conclusions reached by a study, and we suggest that thresholds either be based on features of the data or behaviour of study species. More generally, connectedness is an individual effect. Defining connections as strong or weak at the population level and then calculating connectedness at the individual level may not best represent the salient features of the social environment experienced by individuals. This is highlighted by our contrasting results for number of strong connections and strength of connection to top associates (which is a measure defined at the individual level).
We found no evidence of a relationship between an individual's position in the broader social network and their probability of surviving. Individuals that are well-connected to their broader social worlds have been suggested to benefit from being among the first to receive useful information when it enters the system. For example, in resident-ecotype killer whales, indirect network position predicts male survival, potentially because well-positioned males are more likely to receive information about the presence and location of resources [13]. The rhesus macaques in our study were provisioned at regular intervals and predictable locations and have no predators. The opportunities for individuals to gain survival benefits from social information in this population may, therefore, be limited. Although information about the social environment such as mating opportunities, changes in group membership or dominance rank, are probably important for the success of these animals, the benefits of this information might be more tightly born out in terms of reproductive success [12] and less so in terms of survival.
Measures of direct connectedness were also not important predictors of survival in female rhesus macaques: neither a greater amount of time spent in proximity to others, engaged in grooming, nor the relative amount of grooming received were associated with increased probability of survival. In some primate species, grooming rates have been linked to lower parasite loads (e.g. [35]). Our findings suggest that the benefits of sociality are not directly derived from the behaviours involved in sociality, at least in this population. This interpretation aligns with suggestions that relationships are a commodity or resource that are promoted and maintained in some social animals.
Other social factors not considered in detail here are also likely to influence mortality. Dominance rank has been shown to be an important predictor of fitness and health (e.g. [10]) and a source of variation in social behaviour [67]) in primates, including in rhesus macaques [6,42,68]. Dominance rank did not significantly predict survival when evaluated as a term on its own and it was therefore not included as a main effect in subsequent models. Dominance rank was also not included as an interaction term with social connectedness because of concerns of overfitting. The analyses—in essence—represent the fitness consequences of sociality in females of ‘average’ rank. Including the interaction between connectedness and rank in future analyses may reveal important subtleties in the relationship between sociality and fitness. It is conceivable, for example, that the importance of social connectedness differs for females of high and low rank, though it should be noted that including rank has increased the observed benefits of sociality in this study system [6]. Further analyses based on longer observations and increased sample sizes would be needed to reveal how rank, and other behavioural and ecological constraints, influence the relationship between connectedness and longevity.
Overall, the results presented here demonstrate the value of understanding what exactly is meant by being ‘socially well-connected’. Although ‘sociality’ and ‘connectedness’ are useful catch-all terms, the methods used to measure them can influence results revealed and the conclusions reached. We have highlighted how different aspects of sociality can result in different biological conclusions. Future work in other species is needed to understand the generality of the conclusions reached here. Testing whether different conceptualizations of being well-connected are related to proxies of fitness other than survival, such as reproductive success, are also required, as are studies investigating how different aspects of connectedness interact in other systems.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the Caribbean Primate Research Center (CPRC) for the permission to undertake research on Cayo Santiago, along with Bonn Aure, Jacqueline Buhl, Aparna Chandrashekar, Joel Glick, Josue Negron, Glorienelle Perez, Daniel Phillips and many interns who assisted in behavioural data collection. We thank Elizabeth Maldonado for assistance with the CPRC database, and the CRAB group in Exeter for helpful comments and discussion. We also thank the editor and reviewers for their useful comments and suggestions.
Ethics
Collection of field data and use of the Cayo Santiago long-term database were approved by the Animal Care and Use Committee of the University of Puerto Rico (A6850108) and by the Ethics Committee for the School of Psychology, University of Exeter.
Data accessibility
Anonymized data are included as electronic supplementary material.
Authors' contributions
S.E. and L.J.N.B. conceived the study in consultation with N.S.-M. S.E. performed the analysis in discussion with L.J.N.B. on data collected by L.J.N.B. and A.R.-L. S.E. and L.J.N.B. drafted the paper with input from N.S.-M. and M.L.P. All authors approved the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by NIMH grant nos R01-MH096875 and R01-MH089484 to M.L.P. and L.J.N.B., and by a Leverhulme Trust Early Career Fellowship to L.J.N.B. The CPRC is supported by grant no. 2P40OD012217 from the National Center for Research Resources and the Office of Research Infrastructure Programs of the National Institutes of Health.
References
- 1.Silk JB. 2012. The adaptive value of sociality. In The evolution of primate societies (eds Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB), pp. 552–564. Chicago, IL: University of Chicago Press. [Google Scholar]
- 2.Steptoe A, Shankar A, Demakakos P, Wardle J. 2013. Social isolation, loneliness, and all-cause mortality in older men and women. Proc. Natl Acad. Sci. USA 110, 5797–5801. ( 10.1073/pnas.1219686110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holt-Lunstad J. 2017. The potential public health relevance of social isolation and loneliness: prevalence, epidemiology, and risk factors. Public Policy Aging Rep. 27, 127–130. ( 10.1093/ppar/prx030) [DOI] [Google Scholar]
- 4.Archie EA, Tung J, Clark M, Altmann J, Alberts SC. 2014. Social affiliation matters: both same-sex and opposite-sex relationships predict survival in wild female baboons. Proc. R. Soc. B 281, 20141261 ( 10.1098/rspb.2014.1261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nuñez CMV, Adelman JS, Rubenstein DI. 2015. Sociality increases juvenile survival after a catastrophic event in the feral horse (Equus caballus). Behav. Ecol. 26, 138–147. ( 10.1093/beheco/aru163) [DOI] [Google Scholar]
- 6.Brent LJN, Ruiz-Lambides A, Platt ML. 2017. Family network size and survival across the lifespan of female macaques. Proc. R. Soc. B 284, 20170515. ( 10.1098/rspb.2017.0515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McFarland R, Murphy D, Lusseau D, Henzi SP, Parker JL, Pollet TV, Barrett L. 2017. The ‘strength of weak ties' among female baboons: fitness-related benefits of social bonds. Anim. Behav. 126, 101–106. ( 10.1016/j.anbehav.2017.02.002) [DOI] [Google Scholar]
- 8.Schülke O, Bhagavatula J, Vigilant L, Ostner J. 2010. Social bonds enhance reproductive success in male macaques. Curr. Biol. 20, 2207–2210. ( 10.1016/j.cub.2010.10.058) [DOI] [PubMed] [Google Scholar]
- 9.Riehl C, Strong MJ. 2018. Stable social relationships between unrelated females increase individual fitness in a cooperative bird. Proc. R. Soc. B 285, 20180130 ( 10.1098/rspb.2018.0130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. 2010. Strong and consistent social bonds enhance the longevity of female baboons. Curr. Biol. 20, 1359–1361. ( 10.1016/j.cub.2010.05.067) [DOI] [PubMed] [Google Scholar]
- 11.Oh KP, Badyaev AV. 2010. Structure of social networks in a passerine bird: consequences for sexual selection and the evolution of mating strategies. Am. Nat. 176, E80–E89. ( 10.1086/655216) [DOI] [PubMed] [Google Scholar]
- 12.Brent LJN, Heilbronner SR, Horvath JE, Gonzalez-Martinez J, Ruiz-Lambides A, Robinson AG, Pate Skene JH, Platt ML. 2013. Genetic origins of social networks in rhesus macaques. Sci. Rep. 3, 1–8. ( 10.1038/srep01042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellis S, Franks DW, Nattrass S, Cant MA, Weiss MN, Giles D, Balcomb KC, Croft DP. 2017. Mortality risk and social network position in resident killer whales: sex differences and the importance of resource abundance. Proc. R. Soc. B 284, 20171313 ( 10.1098/rspb.2017.1313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheney DL, Silk JB, Seyfarth RM. 2016. Network connections, dyadc bonds and fitness in wild female baboons. R. Soc. Open Sci. 3, 160255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drewe JA. 2010. Who infects whom? Social networks and tuberculosis transmission in wild meerkats. Proc. R. Soc. B 277, 633–642. ( 10.1098/rspb.2009.1775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godfrey SS, Bull CM, James R, Murray K. 2009. Network structure and parasite transmission in a group living lizard, the gidgee skink, Egernia stokesii. Behav. Ecol. Sociobiol. 63, 1045–1056. ( 10.1007/s00265-009-0730-9) [DOI] [Google Scholar]
- 17.Duboscq J, Romano V, Sueur C, Macintosh AJJ. 2016. Network centrality and seasonality interact to predict lice load in a social primate. Sci. Rep. 6, 1–13. ( 10.1038/srep22095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostner J, Schülke O. 2018. Linking sociality to fitness in primates: a call for mechanisms. Adv. Study Behav. 50, 127–175. ( 10.1016/bs.asb.2017.12.001) [DOI] [Google Scholar]
- 19.Whitehead H. 2008. Analyzing animal soceities: quantative methods for vertebrate social analysis. Chicago, IL: University of Chicago Press. [Google Scholar]
- 20.Silk JB, Cheney DL, Seyfarth R. 2013. A practical guide to the study of social relationships. Evol. Anthropol. 22, 213–225. ( 10.1002/evan.21367) [DOI] [PubMed] [Google Scholar]
- 21.Granovetter M. 1973. The strength of weak ties. Am. J. Sociol. 78, 1360–1380. ( 10.1086/225469) [DOI] [Google Scholar]
- 22.Barrett L, Henzi SP, Weingrill T, Lycett JE, Hill RA. 1999. Market forces predict grooming reciprocity in female baboons. Proc. R. Soc. B 266, 665 ( 10.1098/rspb.1999.0687) [DOI] [Google Scholar]
- 23.Henzi SP, Barrett L. 2007. Coexistence in female-bonded primate groups. Adv. Study Behav. 37, 43–81. ( 10.1016/S0065-3454(07)37002-2) [DOI] [Google Scholar]
- 24.Mcfarland R, Fuller A, Hetem RS, Mitchell D, Maloney SK, Henzi SP, Barrett L. 2015. Social integration confers thermal benefits in a gregarious primate. J. Anim. Ecol. 84, 871–878. ( 10.1111/1365-2656.12329) [DOI] [PubMed] [Google Scholar]
- 25.Brent LJN, Chang SWC, Gariépy JF, Platt ML. 2014. The neuroethology of friendship. Ann. N. Y. Acad. Sci. 1316, 1–17. ( 10.1111/nyas.12315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hruschka DJ. 2010. Friendship: development, ecology, and evolution of a social relationship. Berkeley, CA: Univeristy of California Press. [Google Scholar]
- 27.Dunbar RIM, Shultz S. 2010. Bondedness and sociality. Behaviour 147, 775–803. ( 10.1163/000579510X501151) [DOI] [Google Scholar]
- 28.Croft DP, James R, Thomas POR, Hathaway C, Mawdsley D, Laland KN, Krause J. 2006. Social structure and co-operative interactions in a wild population of guppies (Poecilia reticulata). Behav. Ecol. Sociobiol. 59, 644–650. ( 10.1007/s00265-005-0091-y) [DOI] [Google Scholar]
- 29.Schino G, Aureli F. 2017. Reciprocity in group-living animals: partner control versus partner choice. Biol. Rev. 92, 665–672. ( 10.1111/brv.12248) [DOI] [PubMed] [Google Scholar]
- 30.Gilby IC. 2012. Cooperation among non-kin: reciprocity, markets and mutualism. In The evolution of primate societies (eds Mitani JC, Call J, Kappeler PM, Palmobit RA, Silk JB), pp. 514–530. Chicago, IL: University of Chicago Press. [Google Scholar]
- 31.Aplin LM, Farine DR, Morand-Ferron J, Sheldon BC. 2012. Social networks predict patch discovery in a wild population of songbirds. Proc. R. Soc. B 279, 4199–4205. ( 10.1098/rspb.2012.1591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellis S, Franks DW, Robinson EJH. 2017. Ecological consequences of colony structure in dynamic ant nest networks. Ecol. Evol. 7, 1170–1180. ( 10.1002/ece3.2749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heathcote RJP, Darden SK, Franks DW, Ramnarine IW, Croft DP. 2017. Fear of predation drives stable and differentiated social relationships in guppies. Sci. Rep. 7, 1–10. ( 10.1038/s41598-016-0028-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cook Z, Franks DW, Robinson EJH. 2013. Exploration versus exploitation in polydomous ant colonies. J. Theor. Biol. 323, 49–56. ( 10.1016/j.jtbi.2013.01.022) [DOI] [PubMed] [Google Scholar]
- 35.Akinyi MY, Tung J, Jeneby M, Patel NB, Altmann J, Alberts SC. 2013. Role of grooming in reducing tick load in wild baboons (Papio cynocephalus). Anim. Behav. 85, 559–568. ( 10.1016/j.anbehav.2012.12.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Godfrey SS. 2013. Networks and the ecology of parasite transmission: a framework for wildlife parasitology. Int. J. Parasitol. Parasites Wildl. 2, 235–245. ( 10.1016/j.ijppaw.2013.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krause J, Ruxton GD. 2002. Living in groups. Oxford, UK: Oxford University Press. [Google Scholar]
- 38.Balasubramaniam KN, Beisner BA, Hubbard JA, Vandeleest JJ, Atwill ER, McCowan B. 2019. Affiliation and disease risk: social networks mediate gut microbial transmission among rhesus macaques. Anim. Behav. 151, 131–143. ( 10.1016/j.anbehav.2019.03.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cameron EZ, Setsaas TH, Linklater WL. 2009. Social bonds between unrelated females increase reproductive success in feral horses. Proc. Natl Acad. Sci. USA 106, 13 850–13 853. ( 10.1073/pnas.0900639106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clutton-Brock TH. 1988. Reproductive success: studies of individual variation in contrasting breeding systems. Chicago, IL: Univeristy of Chicago Press. [Google Scholar]
- 41.Rawlings R, Kessler M. 1986. The Cayo Santiago macaques: history, behaviour and biology. New York, NY: State University of New York Press. [Google Scholar]
- 42.Blomquist GE, Sade DS, Berard JD. 2011. Rank-related fitness differences and their demographic pathways in semi-free-ranging rhesus macaques (Macaca mulatta). Int. J. Primatol. 32, 193–208. ( 10.1007/s10764-010-9461-z) [DOI] [Google Scholar]
- 43.Widdig A, et al. 2016. Genetic studies on the Cayo Santiago rhesus macaques: a review of 40 years of research. Am. J. Primatol. 78, 44–62. ( 10.1002/ajp.22424) [DOI] [PubMed] [Google Scholar]
- 44.Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour 49, 227–267. ( 10.1163/156853974X00534) [DOI] [PubMed] [Google Scholar]
- 45.Brent LJN. 2010. Investigating the causes and consequences of sociality in adult female rhesus macaques using a social network approach. London, UK: University of Roehampton. [Google Scholar]
- 46.Silk JB, Alberts SC, Altmann J. 2003. Social bonds of female baboons enhance infant survival. Science 302, 1231–1235. ( 10.1126/science.1088580) [DOI] [PubMed] [Google Scholar]
- 47.Silk JB, Altmann J, Alberts SC. 2006. Social relationships among adult female baboons (Papio cynocephalus) I. Variation in the strength of social bonds. Behav. Ecol. Sociobiol. 61, 183–195. ( 10.1007/s00265-006-0249-2) [DOI] [Google Scholar]
- 48.Silk JB, Seyfarth RM, Cheney DL. 2018. Quality versus quantity: do weak bonds enhance the fitness of female baboons? Anim. Behav. 140, 207–211. ( 10.1016/j.anbehav.2018.04.013) [DOI] [Google Scholar]
- 49.Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. 2009. The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proc. R. Soc. B 276, 3099–3104. ( 10.1098/rspb.2009.0681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silk JB, Alberts SC, Altmann J. 2006. Social relationships among adult female baboons (Papio cynocephalus) II. Variation in the quality and stability of social bonds. Behav. Ecol. Sociobiol. 61, 197–204. ( 10.1007/s00265-006-0250-9) [DOI] [Google Scholar]
- 51.Thompson NA, Cords M. 2018. Stronger social bonds do not always predict greater longevity in a gregarious primate. Ecol. Evol. 8, 1604–1614. ( 10.1002/ece3.3781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freeman L. 1977. A set of measures of centrality based on betweenness. Sociometry 40, 35–41. ( 10.2307/3033543) [DOI] [Google Scholar]
- 53.Wasserman S, Faust K. 1994. Social network analysis: methods and applications. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 54.Kleinbaum DG, Klein M. 2013. Survival analysis: a self learning text, 3rd edn Berlin, Germany: Springer. [Google Scholar]
- 55.Newson RB. 2010. Comparing the predictive powers of survival models using Harrell's C or Somers’ D. Stata J. 10, 339–358. ( 10.1177/1536867X1001000303) [DOI] [Google Scholar]
- 56.Silk JB. 2007. The adaptive value of sociality in mammalian groups. Phil. Trans. R. Soc. 362, 539–559. ( 10.1098/rstb.2006.1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farine DR, Whitehead H. 2015. Constructing, conducting and interpreting animal social network analysis. J. Anim. Ecol. 84, 1144–1163. ( 10.1111/1365-2656.12418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruxton GD, Neuhäuser M. 2013. Improving the reporting of p-values generated by randomization methods. Methods Ecol. Evol. 4, 1033–1036. ( 10.1111/2041-210X.12102) [DOI] [Google Scholar]
- 59.Benjamini Y, Hochbery Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 57, 289–300. [Google Scholar]
- 60.Seyfarth RM. 1977. A model of social grooming among adult female monkeys. J. Theor. Biol. 65, 671–698. ( 10.1016/0022-5193(77)90015-7) [DOI] [PubMed] [Google Scholar]
- 61.Cheney DL, Moscovice LR, Heesen M, Mundry R, Seyfarth RM. 2010. Contingent cooperation between wild female baboons. Proc. Natl Acad. Sci. USA 107, 9562–9566. ( 10.1073/pnas.1001862107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mayagoitia LM, Santillan-Doherty AM, Lopez-Vergara L, Mondragon-Ceballos R. 1993. Affiliation tactics prior to a period of competition in captive groups of stumptail macaques. Ethol. Ecol. Evol. 5, 435–446. [Google Scholar]
- 63.de Waal FBM. 1997. The chimpanzee's service economy: food for grooming. Evol. Hum. Behav. 18, 375–386. ( 10.1016/S1090-5138(97)00085-8) [DOI] [Google Scholar]
- 64.Ventura R, Majolo B, Koyama N, Hardie S, Schino G. 2006. Reciprocation and interchange in wild Japanese macaques: grooming, cofeeding and agonistic support. Am. J. Primatol. 68, 1138–1149. ( 10.1002/ajp.20314) [DOI] [PubMed] [Google Scholar]
- 65.McPherson M, Smith-Lovin L, Cook JM. 2002. Birds of a feather: homophily in social networks. Annu. Rev. Sociol. 27, 415–444. ( 10.1146/annurev.soc.27.1.415) [DOI] [Google Scholar]
- 66.Christakis NA, Fowler JH. 2007. The spread of obesity in a large social network over 32 years. N. Engl. J. Med. 357, 370–379. ( 10.1056/NEJMsa066082) [DOI] [PubMed] [Google Scholar]
- 67.Schülke O, Ostner J. 2012. Ecological and social influences of sociality. In The evolution of primate societies (eds Mitani JC, Call J, Kappeler PM, Palmobit RA, Silk JB), pp. 195–219. Chicago, IL: University of Chicago Press. [Google Scholar]
- 68.Vandeleest JJ, Beisner BA, Hannibal DL, Nathman AC, Capitanio JP, Hsieh F, Atwill ER, McCowan B. 2016. Decoupling social status and status certainty effects on health in macaques: a network approach. PeerJ 2016, 1–25. ( 10.7717/peerj.2394) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data are included as electronic supplementary material.