Abstract
The external environment has traditionally been considered as the primary driver of animal life history (LH). Recent research suggests that animals' internal state is also involved, especially in forming LH behavioural phenotypes. The present study investigated how these two factors interact in formulating LH in humans. Based on a longitudinal sample of 1223 adolescents in nine countries, the results show that harsh and unpredictable environments and adverse internal states in childhood are each uniquely associated with fast LH behavioural profiles consisting of aggression, impulsivity, and risk-taking in adolescence. The external environment and internal state each strengthened the LH association of the other, but overall the external environment was more predictive of LH than was the internal state. These findings suggest that individuals rely on a multitude and consistency of sensory information in more decisively calibrating LH and behavioural strategies.
Keywords: adolescents, harsh and unpredictable environment, internal body state, fast life-history behavioural profiles
1. Introduction
It has been widely observed that external environments shape life histories (LH) and LH-related traits [1–4]. For example, in eight populations of the tropical poecilid (Brachyraphis episcopi) from rivers along the Panama Canal, fish living downstream with high predatory risks adopt faster LH by exhibiting greater boldness and aggression than conspecifics living upstream under lower predation pressures [5]. Also, Bonnet macaque (Macaca radiata) mothers randomly assigned to unpredictable food conditions, compared with those assigned to predictable foraging conditions, engage in less affiliative mutual grooming, are more aggressive towards other adults, and are less responsive to their offspring, all characteristics of faster LH [6,7]. In rural human adolescents separated from their parents, childhood environmental harshness and unpredictability, represented by parental separation, family chaos, and unstable life events, is associated with fast LH and related behaviours, such as risk-taking, externalizing, and academic underperformance [8,9].
In addition to the external environment, evidence also suggests that animals develop profoundly different LH biobehavioural profiles depending on aspects of their internal or somatic state. For example, precocial breeding by young females increases substantially among Tasmanian devils (Sarcophilus harrisii) infected with a deadly facial cancer [10], marine snails (Cerithidea califomica) mature significantly earlier when infected with several trematoda parasites [11], and the common fruit fly (Drosophila melanogaster) has a significantly shortened developmental time from egg to adult when infected with picornavirus [12]. In human populations, data from the 1970 British birth cohort show that girls who suffered chronic illnesses in childhood had sex and gave birth earlier than their healthy age cohorts, while the incidence of chronic disease was unrelated to measures of environmental stress [13]. Similarly reported in the public health literature, adolescents suffering from chronic medical conditions subsequently exhibit earlier, more active, or riskier sexual activities, as well as other risky behaviours, such as smoking, drug use, and antisocial acts, that are all characteristic of faster LH [14–17]. Finally, behavioural evidence from healthy populations shows that both self-reported history of vulnerability to illness and laboratory measures of inflammation are positively correlated with fast LH behavioural profiles consisting of impulsivity, present focus, and difficulty in delaying gratification [18,19].
The theoretical rationale underpinning these observations follows that, in allocating limited bioenergy among different energy consumption demands, trade-offs are made that fundamentally respond to mortality cues either from external environments or from internal states or both [1,2,4,20]. This process is also known as predictive adaptive responses, wherein evolved mechanisms guide the subsequent development of biobehavioural phenotypes to match an organism's external environments or internal states [21,22]. Various bio-energetic trade-offs can be summarized into those allocated for reproduction or early reproduction and those allocated for somatic development and maintenance, including parenting or the development of the next generation. Relevant external and internal cues are primarily concerned with mortality and morbidity. In external environments, leading causes of mortality and morbidity beyond individuals' survival effort include extrinsic risks, such as predation, disease, and introspecific violence. Their high occurrence and high variability, known as environmental harshness and unpredictability [1], promote fast LH. Facing environmental harshness and unpredictability, animals accelerate growth and development and reproduce faster and more plentifully before mortality and morbidity strike. In safer environments, animals prioritize somatic development and maintenance over mating and reproduction. Slow LH strategists follow an affiliative, mutualistic and more other-centred, future-oriented sociality that aims for long-term coexistence with conspecifics, whereas fast LH strategists follow aggressive, antagonistic, and impulsive and opportunistic social strategies willing to take risks and ready to attend to immediate survival needs with reduced concern for future conspecific cooperation [9,23–25]. Through biobehavioural manifestations, LH is thus shaped by and responds to external environments.
Animals likewise respond to internal cues by adopting the same biobehavioural strategies. Aspects of the internal or somatic state, such as body size, energy reserves, immune functioning, quality of cell-repair mechanisms, and microbiotic conditions, determine the optimal behaviour an animal should adopt to maximize its evolutionary fitness [26–31]. Animals in poor physical condition who face direct mortality–morbidity threats internally should calibrate their LH accordingly to prioritize immediate survival and reproduction over development and long-term fitness pay-offs, and they should adopt antagonistic–opportunistic social strategies to achieve short-term reproductive goals. Those in sound physical condition that signal likely survival into the future with uncompromised life expectancy and longevity would make strategic decisions to forgo early reproduction and short-term rewards and focus on development and affiliative–cooperative sociality to achieve long-term fitness goals. Compared to external processes, internal processes may more accurately calibrate LH because mortality cues from an organism's own body carry more fidelity in forecasting future conditions than external signals of an environment that may change during an organism's lifespan [20,22,32].
Empirical studies implicating either the internal or the external influence of LH had remained as separate and different lines of research until recently when researchers started to investigate the two factors in the same organisms in part to test and develop the internal and external predictive adaptive response models. Using structural equation modelling, Hartman et al. [33] examined external adversity, indicated by risk factors such as maternal harshness, income disadvantage, household and employment transitions, and other factors, and internal state, represented by general health status and healthy body mass index (BMI) change, both calculated across years, in relation to fast LH consisting of risky behaviour, problematic functioning, and age of menarche [33]. They formulated a mediation model where internal state mediates the association between external adversity and fast LH, and external adversity has a direct effect on both fast LH and internal state. Their results, based on a large longitudinal US American sample, showed that external adversity and internal state each uniquely predicted fast LH in the expected directions. Three other studies tested almost identical mediation models and yielded similar findings, viz. that external and internal factors both predicted LH [34–36].
These studies established external environment and internal state as unique predictors of LH, illustrating an additive relation between the two factors. An interesting and important next step in this line of research is to determine whether their relation is also multiplicative or how the two factors interact in calibrating LH. It is apparent from the literature that harsh and unpredictable environments and adverse internal states both accelerate fast LH by cuing mortality–morbidity threats, either through perceptual input from the external environment or through interoception of the internal state. If external input is consistent with the internal signal in cuing mortality as, for example, when one witnesses conspecific casualties from famine or disease in the external environment while internally experiencing physical fatigue as, for example, from low glucose or high inflammation, the external and internal conditions should each more decisively shape fast LH. If the two types of sensory information carry different signals about mortality threats, as when witnessing the same external mortality while maintaining gastrointestinal consummation, uncompromised immunocompetence, or homeostatic equilibrium, both factors will become more muted in predicting LH, and LH will become less decisively calibrated. Because the external environment is a cause of animal health status [32], as also evidenced by the four mediation models reviewed earlier, the two signals should be consistent in cuing mortality–morbidity and in reinforcing fast–slow LH development in the same direction. However, part of the internal state also represents individual genetics, gene–environment interplay, including differential susceptibility to both external and internal cues [4,37,38], and other idiosyncrasies (e.g. one singularly escaped or encountered extrinsic risks by chance) that may provide interoceptive input uncorrelated with the external sensory processing of mortality. Uncorrelated sensory inputs will then calibrate indecisive LH, yielding the observation that elements of external or internal conditions do not invariably predict LH (e.g. [39,40]).
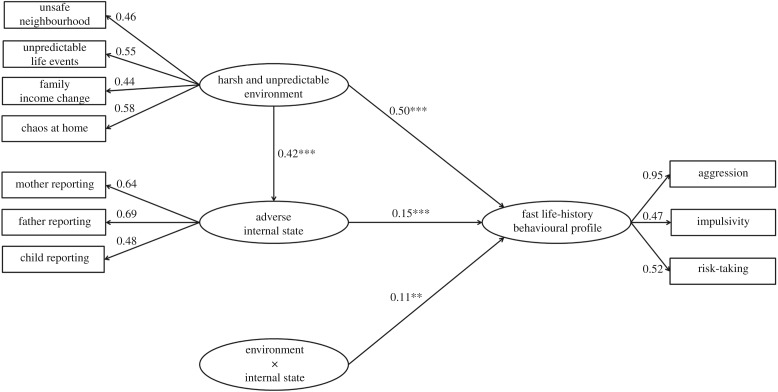
The purpose of the present study was to pursue the next-step question about the extent to which external environments and internal body states carry the same information regarding the direction of fast–slow LH plasticity and the extent to which these two factors reinforce each other in moulding LH development. We tested the prediction that, under high internal adversity signalling mortality threats, environmental harshness and unpredictability in signalling the same mortality conditions would be more predictive of fast LH than at lower levels of internal adversity. Similarly, we hypothesized that adverse internal states would more strongly predict fast LH when the environment was harsh and unpredictable than when it was benign. In testing these hypotheses, we developed an interaction model, which did not replace but integrated the mediation model employed in existing studies (e.g. [33]). Figure 1 displays the integrated mediation–interaction model (see [41] for a statistical exposition of such models). Based on a longitudinal sample of 1223 adolescents in nine countries, we examined harsh and unpredictable external environments and adverse internal states, both measured in childhood, in relation to fast LH behavioural profiles consisting of aggression, impulsivity, and risk-taking all obtained in adolescence. We tested the main effects of external environment and internal state, respectively, and the interaction between the two factors, in relation to fast LH behavioural profiles. Focusing on the interaction effect, we predicted a significant ordinal interaction suggesting that external environment and internal state would be mutually reinforcing in predicting LH.
Figure 1.
External harsh and unpredictable environment and adverse internal state and their interaction in relation to fast LH behavioural profiles.
2. Methods
(a). Participants
The sample consisted of 1223 adolescents (51% girls), their mothers (n = 1169), and their fathers (n = 959) drawn from 10 sites of nine countries: Shanghai, China (n = 102), Medellín, Colombia (n = 100), Naples, Italy (n = 97), Rome, Italy (n = 103), Zarqa, Jordan (n = 114), Kisumu, Kenya (n = 99), Manila, Philippines (n = 105), Trollhättan/Vänersborg, Sweden (n = 104), Chiang Mai, Thailand (n = 120), and Durham, NC, USA (n = 101 European Americans, n = 94 African Americans, n = 84 Latin Americans). The children were 10 years of age on average (M = 10.28 years, s.d. = 0.66) at Time 1 of the present study and were 15 years of age on average (M = 14.67; s.d. = 0.89) at Time 5 of the present study. Participants were recruited from primary schools serving high-, middle-, and low-income families in the approximate proportions these income groups were represented in the local population. These sampling procedures yielded samples with economic diversity that is comparable across sites. Primary school education was compulsory when we initially recruited participants, who were subsequently followed individually within or away from the school systems. Retention rates differed across sites but were overall high, with 87% of the initial sample at Time 1 of the present study continuing with the study 5.5 years later. Families who continued in the study did not differ in initial household income levels from families who attrited, F1,1081 = 1.181, p = 0.277. Families who provided complete data also did not differ from the initial sample with respect to child gender, parents' marital status, or mothers' and fathers’ education.
(b). Procedures
Data for the present study were drawn from separate interviews conducted with a child and the two parents, respectively. Measures used in the interviews were administered in the predominant language of each country, following forward- and back-translation by translators fluent in English and the target language and after group discussions to resolve any linguistic, semantic, and cultural ambiguities that arose during translation. All the measures used in the present study were statistically tested for and met measurement invariance across sites (see [42]). Interviews lasted 1.5–2 h at each of the five times of data collection. Family members were interviewed separately to ensure privacy. Adult participants were given financial compensation, children were given small gifts or monetary compensation, and families were entered into draws for prizes. Prior to the interviews, mothers and fathers provided written informed consent, and children provided assent. Procedures for the project were approved by the Duke University Institutional Review Board (IRB, protocol number 2032) as well as by university IRBs in all participating sites.
(c). Harsh and unpredictable environment
We used four measures to assess childhood harsh and unpredictable environment. Three of the four measures were all taken at Times 1 and 2 of the present study when the children were 10 and 11 years old on average; one measure was obtained at Time 4. These four measures are described below.
(i). Unsafe neighbourhood
At Time 1, mothers and children, respectively, reported on six items that measure the perceived safety and liveability of a neighbourhood on a four-point scale ranging from 0, ‘almost never true’, to 3, ‘almost always true’ (e.g. ‘There are a lot of drugs and gangs in my neighbourhood’, ‘My neighbourhood is a nice place to live’ (reverse coded), and ‘I feel scared in my neighbourhood’) [43]. The items were coded or recoded in the direction of unsafe neighbourhood. Internal consistency reliability estimates were 0.83 for mother reporting and 0.73 for child reporting. The correlation between the two ratings was 0.41. For the structural equation modelling and other analyses reported later, the average of the two ratings was used as an indicator of the harsh and unpredictable environment construct.
(ii). Unpredictable life events
At Time 1, mothers reported on the Social Readjustment Rating Scale [44], containing 10 negative and unpredictable life events that happened in the last 2 years in the family to which the child was likely to be exposed (e.g. ‘severe and/or frequent illness’, ‘accidents and/or injuries’, and ‘death of other important person’). The 10 items were averaged to create another indicator of harsh and unpredictable environments. The internal consistency reliability estimate was 0.61.
(iii). Family income change
At Times 1 and 2, mothers assessed how much in the last 12 months the household's annual income had changed and indicated the change on a five-point scale (1, decreased a lot (more than 25%); 2, decreased a little bit (between 5 and 25%); 3, did not change at all or it did not significantly change (less than 5%); 4, increased a little bit (between 5 and 25%); 5, increased a lot (more than 25%)). The rating was reverse coded so that higher numbers indicated income decrease. The two ratings over 2 years were averaged to form the final variable as the third indicator of environmental harshness and unpredictability. Internal consistency reliability estimate based on the two ratings was 0.42, and the correlation between the two ratings was 0.27.
(iv). Chaos at home
At Time 4, using a five-point scale ranging from 1, ‘definitely untrue’, to 5, ‘definitely true’, mothers and children responded to a six-item measure of confusion, chaos, and disorder at home (Confusion, Hubbub and Order Scale; e.g. ‘It's a real zoo in our home’, ‘The atmosphere in our home is calm’ (reverse coded), and ‘You can't hear yourself think in our home’) [45]. Internal consistency reliability estimates were 0.60 for mothers and 0.57 for children. The correlation between the two ratings was 0.37. The average of the two ratings formed the final indicator of the harsh and unpredictable environment construct.
(d). Adverse internal state
Adverse internal state was measured at Times 1 and 2. We used the Somatic Complaints Scale of the Child Behavior Checklist (CBCL) [46] to measure adverse internal state. Translated into 90 languages, CBCL is the most widely used report form in identifying children's problem behaviour in both research and clinical practice. The somatic complaints scale of the CBCL has strong validity support as a measure of compromised internal states. For example, the scale is either significantly correlated with symptoms of a disease or can reliably separate sufferers of a disease from healthy age cohorts. For the age range of the present study, we found the said discriminant validity evidence with asthma [47], diabetes [48], recurrent abdominal pain [49], inflammation and oxidative stress [50], inflammatory bowel diseases [51], and spinal bifida [52]. Additional meta-analyses provide similar validity evidence either in a specific disease (e.g. epilepsy, [53]) or across a range of chronic illnesses [54]. In these reports, externalizing and internalizing scales of the CBCL are also correlated with disease status, a finding that is itself consistent with our LH predictions. However, the correlations involving the somatic complaints scale are of a much larger magnitude that is up to two to three times of those involving externalizing and internalizing scales, further supporting the validity of the somatic complaints scale. Fathers and mothers, respectively, reported about their children, and the children self-reported on 10 somatic complaints using a three-point scale ranging from 0, ‘never’, to 2, ‘often’ to register the frequency of each complaint (e.g. ‘stomach aches and cramps’, ‘overtired’, ‘dizzy’, and ‘physical problem with no known medical cause’). We used the average of 2 years to capture health status consistency over time. Internal consistency reliability estimates based on the 2-year averages were 0.78, 0.80, and 0.82 for father, mother, and child reporting, respectively. We used the ratings from fathers, mothers, and children to form three indicators of the adverse internal state construct.
(e). Fast life history behavioural profile
At Time 5, the final year of the study when the children were 15 years old on average, we measured the following three aspects of a fast LH behavioural profile.
(i). Aggression
Fathers and mothers, respectively, described their adolescent children and the adolescents self-reported, all using 30 relevant items making up the Aggressive Behavior Scale of the CBCL (e.g. ‘physically attack people’, ‘screams a lot’, and ‘threatens people’). A three-point response format ranging from 0, ‘never’, to 2, ‘often’ registers the frequency an adolescent engaged in each of these behaviours. Internal consistency reliability estimates were 0.89, 0.89, and 0.86 for father, mother, and child reporting, respectively. The average of the three ratings formed an indicator of the fast LH behavioural profile construct.
(ii). Impulsivity
The adolescents were given the 34-item Barratt Impulsiveness Scale (e.g. ‘I act on the spur of the moment’; ‘it is hard for me to think about two things at the same time’) [55]. The items were rated on a four-point scale ranging from 1, ‘never true’, to 4, ‘always true’. The internal consistency reliability estimate was 0.73. This is the second indicator of the fast LH construct.
(iii). Risk-taking
We used an adapted version of a self-report measure of risk-taking [56,57]. There are nine scenarios involving risky behaviour: smoking cigarettes, drinking alcohol, vandalizing property, going to dangerous places, riding in cars with drunk drivers, having unprotected sex, stealing from stores, engaging in gang fights, and using weapons to threaten someone. The adolescents were asked four questions on each scenario, rated on a four-point scale based on the following questions: ‘How often did you do the activity?’ (1, ‘never’, 4, ‘five or more times’); ‘To what extent are you at risk of something bad happening?’ (1, ‘very much’, 4, ‘not at all’); ‘How would you compare the benefits of this activity with the risks?’ (1, ‘the risks are far greater than the benefits’, 4, ‘the benefits are far greater than the risks'); ‘If something bad happened because of this activity, how serious would it be?’ (1, ‘not at all serious’, 4, ‘very serious’). A summary of the four ratings for nine scenarios formed the risk-taking variable, with a higher score indicating a greater degree of risky behaviour. The internal consistency reliability estimate was 0.88. This is the final indicator of the fast LH behavioural profile construct.
(f). Data analysis
There is a conceptual, but not computational, distinction between an interaction model and a moderation model [58]. In an interaction model, the two concerning variables are both defined as predictor variables and the estimated interaction effect represents the prediction of either of the two predictors conditional on the values of the other predictor. In a moderation model, the two variables are distinctively designated as a predictor and a moderator and the estimated moderating effect represents the prediction of the predictor conditional on the values of the moderator. In the moderation model, a simple slope of only one conditional prediction is plotted for the predictor variable conditional on the moderator variable, but in the interaction model, simple slopes of conditional prediction can be plotted for both predictor variables conditional on each other. We used the interaction model to determine the extent to which harsh and unpredictable environment and adverse internal state reinforced each other in predicting fast LH.
In a traditional interaction or moderation model, the two concerning variables are correlated with unspecified causal directions. In our interaction model, presented in figure 1, external environment was specified as a cause of internal state, making our baseline model a mediation model rather than a traditional correlation model. Testing moderation or interaction in a mediation model requires special manual computations mainly for estimating conditional indirect effects or predictions [41,59]. We employed these computations. Because our model was based on latent constructs rather than directly observed variables, we conducted structural equation modelling using Mplus 7.0 [60], and we used full information maximum-likelihood estimation to treat missing data [61]. We first computed an interaction construct by multiplying indicators of each of the two concerning constructs, harsh and unpredictable environment and adverse internal state, using the Mplus default approach rather than randomly pairing indicators and multiplying them manually [62]. This default approach does not provide the usual goodness-of-fit statistics [63,64]. Data fitness of an interaction model is assessed relative to that of the baseline model by computing the difference of the log-likelihood values of the two models (D = −2 × [(log-likelihood for the main effect model) − (log-likelihood for the interaction model)], [58]). D follows χ2 distribution with DF being the difference in the number of estimated parameters between the two models, which, in the present case, was 1.
3. Results
Table 1 presents means, standard deviations, and zero-order correlation coefficients of all the indicator variables used in the study. These are based on multi-informant (mothers, fathers, and children) and longitudinal (5.5 years) observations. The correlation coefficients were statistically significant and mostly substantial considering expected attenuation by the multi-source and cross-lag data. Within each construct, the indicators were positively and statistically significantly correlated. Across constructs, clusters of indicators were correlated with one another in the expected directions. For example, the cluster of environmental indicators and the cluster of internal state indicators were each positively and significantly correlated with the cluster of indicators measuring fast LH behavioural profiles. These intercorrelations provided the foundation to test the structural interaction between external environment and internal state constructs.
Table 1.
Correlations, means, and standard deviations of variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| harsh and unpredictable environment | ||||||||||
| 1. Unsafe neighbourhood | — | |||||||||
| 2. Unpredictable life events | 0.29*** | — | ||||||||
| 3. Family income change | 0.13*** | 0.21*** | — | |||||||
| 4. Chaos at home | 0.09** | 0.14*** | 0.15*** | — | ||||||
| adverse internal state | ||||||||||
| 5. Mother reporting | 0.12*** | 0.25** | 0.21*** | 0.17*** | — | |||||
| 6. Father reporting | 0.09** | 0.18*** | 0.17*** | 0.15*** | 0.49*** | — | ||||
| 7. Child reporting | 0.11*** | 0.21*** | 0.23*** | 0.15*** | 0.29*** | 0.30** | — | |||
| fast life-history behavioural profile | ||||||||||
| 8. Aggression | 0.23*** | 0.25*** | 0.28** | 0.25*** | 0.29*** | 0.27*** | 0.19*** | — | ||
| 9. Impulsivity | 0.10** | 0.10** | 0.19*** | 0.17*** | 0.08* | 0.07* | 0.15*** | 0.16*** | — | |
| 10. Risk-taking | 0.14*** | 0.16*** | 0.19*** | 0.14*** | 0.13*** | 0.13*** | 0.07* | 0.18*** | 0.16*** | — |
| mean | 0.69 | 0.14 | 2.80 | 2.22 | 0.19 | 0.15 | 0.39 | 8.37 | 2.08 | 2.06 |
| s.d. | 0.42 | 0.17 | 0.79 | 0.53 | 0.22 | 0.19 | 0.30 | 5.47 | 0.36 | 0.18 |
*p < 0.05, **p < 0.01, ***p < 0.001.
We first tested the mediation model as the baseline model. The goodness-of-fit statistics (χ2/d.f. = 7.32, Comparative Fit Index = 0.94, Tucker–Lewis Index = 0.91, Root Mean Square Residual = 0.077, Standardized Root Mean Square Residual = 0.095) of the model taken together were adequate according to the minimum standard [65,66]. We then included the computed interaction construct in the model. The log-likelihood for the mediation or baseline model was −6312.88 and that for the integrated mediation–interaction model was −6301.70, D = 22.36, p < 0.001, indicating that the mediation–interaction model showed statistically significant improvement in data fitness over the mediation model not containing the interaction term.
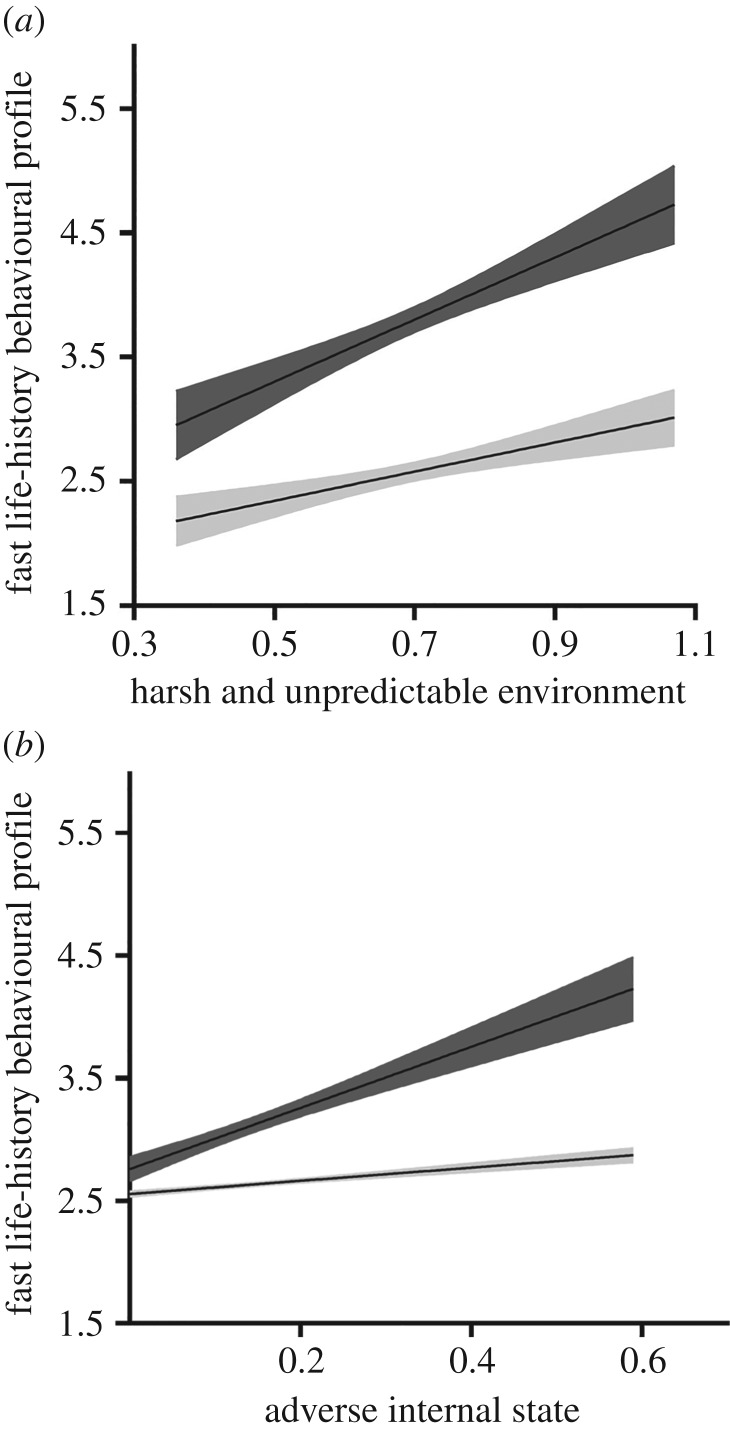
As shown in figure 1, parameter estimates of the mediation–interaction model including the interaction term were all statistically significant and adequate. Most of the factor loadings exceeded 0.50 with an average of 0.58, suggesting adequate measurement models. The structural model was consistent with our LH theorizing. Harsh and unpredictable environment (β = 0.50, p < 0.001) and adverse internal state (β = 0.15, p < 0.001) were each uniquely longitudinally associated with a fast LH behavioural profile after controlling the linear prediction of internal state by external environment. Both predictors were statistically significant, but the external environment was the stronger predictor of fast LH than internal state. More importantly, the interaction term was statistically significant representing an ordinal direction (β = 0.11, p < 0.01) as hypothesized. The conditional direct longitudinal prediction of LH by external harsh and unpredictable environment at higher levels of adverse internal state (+1 s.d.) was more robust (β = 0.69, p < 0.001) compared to the conditional direct association (β = 0.32, p < 0.001) at lower levels of internal state (−1 s.d.). Both conditional predictions were statistically significant. Similarly, at higher (+1 s.d.) compared to lower levels (−1 s.d.) of harsh and unpredictable environment, adverse internal state was a significant (β = 0.26, p < 0.001) versus non-significant (β = −0.03, n.s.) longitudinal predictor of fast LH. These conditional effects were obtained after controlling the presumed causal relation of harsh and unpredictable environment to adverse internal state (the mediation model). Finally, the conditional indirect prediction by harsh and unpredictable environment of LH through the mediation of adverse internal state was slightly attenuated (β = 0.075, p < 0.001) at higher values of adverse internal state (+1 s.d.) compared to the same conditional indirect effect (β = 0.089, p < 0.001) corresponding to lower levels of adverse internal states (−1 s.d.). Relevant to the present study, we plotted the two sets of conditional direct predictions of LH in figure 2.
Figure 2.
(a) Simple slopes and 95% confidence bands from fast LH's regression on external environment at 1 s.d. above (darkened) and 1 s.d. below (light) the mean of internal state. (b) Simple slopes and 95% confidence bands from fast LH's regression on internal state at 1 s.d. above (darkened) and 1 s.d. below (light) the mean of external environment.
4. Discussion
Existing research has shown, in separate studies, that both external environments and internal body states affect animal LH. Investigating these two factors in the same individuals, recent studies have demonstrated unique LH predictions by the two predictors (e.g. [33]). Findings of the present study add to the literature by showing the two factors interact in reinforcing LH calibration in the same direction. Childhood harsh and unpredictable environments and adverse internal states were each uniquely associated with fast LH behavioural profiles in adolescence, and each factor strengthened the LH association of the other factor. Specifically, at higher, compared to lower, levels of internal adversity, external harshness and unpredictability was more predictive of fast LH. Similarly, adverse internal state was predictive of fast LH when the external environment was harsh and unpredictable but not when it was benign. These conditional predictions suggest that external environments and internal body states likely reinforce the same cues about mortality–morbidity in accelerating fast LH. In the present study, we obtained information about childhood environmental harshness and unpredictability and adverse internal state at approximately the same time, 3 years before measuring LH profiles, therefore establishing longitudinal, unconditional, and conditional associations with or predictions of LH from the external and internal predictors. Additionally, external environment was more strongly associated with fast LH than was internal state as evidenced both by the two main effects (β = 0.50 versus 0.15) and the four conditional associations (figure 2). External environment may be an underlying cause of both fast LH behavioural profiles and internal body states [1] and, when this common variance was statistically accounted for in the present study by integrating the mediation modelling of the relation of external environment to internal state, LH prediction by the internal but not the external predictor was attenuated. These comprehensive findings support the conclusion that external environment has a stronger impact in shaping LH than internal state. Nonetheless, the internal state is still predictive of a fast LH behavioural profile overall and when external harshness and unpredictability are severe rather than benign.
Traditional LH theory emphasizes the environment as the sole actor in activating and formulating animal LH. Specifically, the mortality-causing harshness and unpredictability, as well as resource conditions (which were not investigated in the present study and have rarely been studied by other human LH research [8]), cause physiological (e.g. endocrine and homeostasis) and psychological tuning (e.g. cognitive and behaviour) that is oriented either towards growth and development (slow LH) or mating and reproduction (fast LH). Recent research has introduced animal internal state as a complementary actor in forming LH strategies [13,22,67]. In the light of the present findings, external environment and internal body state should not be distinguished as categorically separate drivers of LH. The two can be regarded as representing different and unique sensory inputs. For humans, the external environment is sensorially processed mainly as visual, auditory, and tactile data, whereas internal state is processed mainly as interoceptive including visceroceptive information. Humans and other animals processing more and more consistent sensory information should calibrate LH more decisively than those having less or less consistent information. Such is the implication of the present findings about the significant ordinal interaction effect. In the human case, for example, a child who constantly feels pains in her stomach while witnessing adult neighbours falling ill to a strange parasite should be more readily set on a faster LH path compared to the one who receives only one but not both sensory inputs or who receives two inconsistent inputs by experiencing external mortality but internal homeostasis or vice-versa.
A proviso to the present findings and discussion is that, like most of the existing human LH literature (e.g. [68]), the external environment operationalized in the present study was mostly individually specific, representing a person's unique home and family environment rather than a larger ecology shared with other conspecifics and thus increasing seeming consistency between external and internal conditions in impacting LH. This is not necessarily a limitation of the present study but, rather, represents the reality of LH research on contemporary human participants. Because humans have long mastered the external environment [69], most ecology-wide variables, such as mortality, pandemic and even intraspecific conflict that are traditionally defined in the species-general LH literature as extrinsic risks beyond individuals' survival effort, operate exactly depending on modern human individuals’ survival abilities or, more precisely, resource conditions. Thus, they do not fully satisfy the definition of extrinsic risks [70]. Because of these difficulties, individual-level variables, such as chaos at home, family misfunctioning, and unpredictable life events, have been substituted as proxies of extrinsic risks in human LH research (e.g. [40]). Another related weakness is using multi-informant reporting to measure internal states, as well as external environments, because individual differences in interoception may potentially be correlated with LH (e.g. [71]) and may in general confound the effects of body conditions. However, cognitive assessment of both internal and external information may become integral parts of human LH calibration. An appreciation of these human-specific conditions is needed in applying species-general models to study human LH. The present study represents such an appreciation and one of the first attempts to our knowledge to investigate how external environments and internal states interact in calibrating human LH behavioural profiles.
Supplementary Material
Ethics
Procedures for the project were approved by the Duke University Institutional Review Board (IRB, protocol no. 2032) as well as by university IRBs in all participating sites.
Data accessibility
Data of this study are available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.nvx0k6dn7.
Authors' contributions
L.C. and H.J.L. conceptualized and wrote the paper; J.E.L., M.H.B., L.S., K.A.D., and K.D.-D. commented on drafts of the paper; A.T.S. and H.J.L. managed and analysed the data; D.B., C.P., L.P.A., S.T., S.Y., B.-B. C., E.S., P.O., S.M.A.-H., L.D.G., and L.M.U.T., respectively, collected the longitudinal data across 10 cities, 9 countries.
Competing interests
We declare we have no competing interests.
Funding
This research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant no. RO1-HD054805) and Fogarty International Center (grant no. RO3-TW008141). This research was also supported by National Institute on Drug Abuse (NIDA) (grant no. P30 DA023026), the Intramural Research Program of the NIH/NICHD, USA, and an International Research Fellowship at the Institute for Fiscal Studies (IFS), London, UK, funded by the European Research Council (ERC) under the Horizon 2020 research and innovation programme (grant agreement no. 695300-HKADeC-ERC-2015-AdG).
References
- 1.Del Giudice M. In press Rethinking the fast-slow continuum of individual differences. Evol. Hum. Behav. ( 10.31234/osf.io/4uhz8) [DOI] [Google Scholar]
- 2.Del Giudice M, Gangestad SW, Kaplan HS. 2015. Life history theory and evolutionary psychology. In The handbook of evolutionary psychology, vol. 1: foundations, 2nd edn (ed. Buss DM.), pp. 88–114. New York, NY: Wiley. [Google Scholar]
- 3.Ellis BJ, Figueredo AJ, Brumbach BH, Schlomer GL. 2009. Fundamental dimensions of environmental risk. Hum. Nat. 20, 204–268. ( 10.1007/s12110-009-9063-7) [DOI] [PubMed] [Google Scholar]
- 4.Ellis BJ, Del Giudice M. 2019. Developmental adaptation to stress: an evolutionary perspective. Annu. Rev. Psychol. 70, 111–139. ( 10.1146/annurev-psych-122216-011732) [DOI] [PubMed] [Google Scholar]
- 5.Brown C, Jones F, Braithwaite V. 2005. In situ examination of boldness–shyness traits in the tropical poeciliid, Brachyraphis episcopi. Anim. Behav. 70, 1003–1009. ( 10.1016/j.anbehav.2004.12.022) [DOI] [Google Scholar]
- 6.Rosenblum LA, Andrews MW. 1994. Influences of environmental demand on maternal behavior and infant development. Acta Paediatr. 83, 57–63. ( 10.1111/j.1651-2227.1994.tb13266.x) [DOI] [PubMed] [Google Scholar]
- 7.Rosenblum LA, Paully GS. 1984. The effects of varying environmental demands on maternal and infant behavior. Child Dev. 55, 305 ( 10.2307/1129854) [DOI] [PubMed] [Google Scholar]
- 8.Chang L, Lu HJ. 2018. Resource and extrinsic risk in defining fast life histories of rural Chinese left-behind children. Evol. Hum. Behav. 39, 59–66. ( 10.1016/j.evolhumbehav.2017.10.003) [DOI] [Google Scholar]
- 9.Lu HJ, Chang L. 2019. Aggression and risk-taking as adaptive implementations of fast life history strategy. Dev. Sci. 22, e12827 ( 10.1111/desc.12827) [DOI] [PubMed] [Google Scholar]
- 10.Jones ME, Cockburn A, Hamede R, Hawkins C, Hesterman H, Lachish S, Mann D, McCallum H, Pemberton D. 2008. Life-history change in disease-ravaged Tasmanian devil populations. Proc. Natl Acad. Sci. USA 105, 10 023–10 027. ( 10.1073/pnas.0711236105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lafferty KD. 1993. The marine snail. Cerithidea californica, matures at smaller sizes where parasitism is high. Oikos 68, 3 ( 10.2307/3545303) [DOI] [Google Scholar]
- 12.Gomariz-Zilber E, Thomas-Orillard M. 1993. Drosophila C virus and Drosophila hosts: a good association in various environments. J. Evol. Biol. 6, 677–689. ( 10.1046/j.1420-9101.1993.6050677.x) [DOI] [Google Scholar]
- 13.Waynforth D. 2012. Life-history theory, chronic childhood illness and the timing of first reproduction in a British birth cohort. Proc. R. Soc. B. 279, 2998–3002. ( 10.1098/rspb.2012.0220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alderman EM, Lauby JL, Coupey SM. 1995. Problem behaviors in inner-city adolescents with chronic illness. J. Dev. Behav. Pediatr. 16, 339–344. ( 10.1097/00004703-199510000-00005) [DOI] [PubMed] [Google Scholar]
- 15.Miauton L, Narring F, Michaud P-A. 2003. Chronic illness, life style and emotional health in adolescence: results of a cross-sectional survey on the health of 15–20-year-olds in Switzerland. Eur. J. Pediatr. 162, 682–689. ( 10.1007/s00431-003-1179-x) [DOI] [PubMed] [Google Scholar]
- 16.Nylander C, Seidel C, Tindberg Y. 2013. The triply troubled teenager—chronic conditions associated with fewer protective factors and clustered risk behaviours. Acta Paediatr. 103, 194–200. ( 10.1111/apa.12461) [DOI] [PubMed] [Google Scholar]
- 17.Suris J-C, Parera N. 2005. Sex, drugs and chronic illness: health behaviours among chronically ill youth. Eur. J. Public. Health. 15, 484–488. ( 10.1093/eurpub/cki001) [DOI] [PubMed] [Google Scholar]
- 18.Gassen J, et al. 2019. Inflammation predicts decision-making characterized by impulsivity, present focus, and an inability to delay gratification. Sci. Rep. 9, 4928 ( 10.1038/s41598-019-41437-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prokosch ML, Hill SE. 2015. Live fast if you're going to die young: decision making shifts as a function of history of infection. Paper presented at the Inaugural Meeting for the International Society for Evolution, Medicine, and Public Health, Tempe, Arizona.
- 20.Nettle D, Bateson M. 2015. Adaptive developmental plasticity: what is it, how can we recognize it and when can it evolve? Proc. R. Soc. B 282, 20151005 ( 10.1098/rspb.2015.1005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gluckman PD, Hanson MA, Spencer HG. 2005. Predictive adaptive responses and human evolution. Trends. Ecol. Evol. 20, 527–533. ( 10.1016/j.tree.2005.08.001) [DOI] [PubMed] [Google Scholar]
- 22.Nettle D, Frankenhuis WE, Rickard IJ. 2013. The evolution of predictive adaptive responses in human life history. Proc. R. Soc. B 280, 20131343 ( 10.1098/rspb.2013.1343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Figueredo AJ, Jacobs WJ. 2010. Aggression, risk-taking, and alternative life history strategies: the behavioral ecology of social deviance. In Bio-psycho-social perspectives on interpersonal violence (eds Frias-Armenta M, Corral-Verdugo V), pp. 3–28. Hauppauge, NY: NOVA Science Publishers. [Google Scholar]
- 24.Figueredo AJ, et al. 2018. Intimate partner violence, interpersonal aggression, and life history strategy. Evol. Behav. Sci. 12, 1–31. ( 10.1037/ebs0000101) [DOI] [Google Scholar]
- 25.Zhu N, Hawk ST, Chang L. 2018. Living slow and being moral. Hum. Nat. 29, 186–209. ( 10.1007/s12110-018-9313-7) [DOI] [PubMed] [Google Scholar]
- 26.Belsky J, Shalev I. 2016. Contextual adversity, telomere erosion, pubertal development, and health: two models of accelerated aging, or one? Dev. Psychopathol. 28, 1367–1383. ( 10.1017/s0954579416000900) [DOI] [PubMed] [Google Scholar]
- 27.Hartman S, Sayler K, Belsky J. 2019. Prenatal stress enhances postnatal plasticity: the role of microbiota. Dev. Psychobiol. 61, 729–738. ( 10.1002/dev.21816) [DOI] [PubMed] [Google Scholar]
- 28.Hooks KB, Konsman JP, O'Malley MA. 2019. Microbiota-gut-brain research: a critical analysis. Behav. Brain. Sci. 42, e60: 1–53. ( 10.1017/S0140525X18002133) [DOI] [PubMed] [Google Scholar]
- 29.Monaghan P. 2014. Organismal stress, telomeres and life histories. J. Exp. Biol. 217, 57–66. ( 10.1242/jeb.090043) [DOI] [PubMed] [Google Scholar]
- 30.Selman C, Blount JD, Nussey DH, Speakman JR. 2012. Oxidative damage, ageing, and life-history evolution: where now? Trends Ecol. Evol. 27, 570–577. ( 10.1016/j.tree.2012.06.006) [DOI] [PubMed] [Google Scholar]
- 31.Wells JCK. 2012. Obesity as malnutrition: the role of capitalism in the obesity global epidemic. Am. J. Hum. Biol. 24, 261–276. ( 10.1002/ajhb.22253) [DOI] [PubMed] [Google Scholar]
- 32.Rickard IJ, Frankenhuis WE, Nettle D. 2014. Why are childhood family factors associated with timing of maturation? A role for internal prediction. Perspect. Psychol. Sci. 9, 3–15. ( 10.1177/1745691613513467) [DOI] [PubMed] [Google Scholar]
- 33.Hartman S, Li Z, Nettle D, Belsky J. 2017. External-environmental and internal-health early life predictors of adolescent development. Dev. Psychopathol. 29, 1839–1849. ( 10.1017/s0954579417001432) [DOI] [PubMed] [Google Scholar]
- 34.Belsky J, Ruttle PL, Boyce WT, Armstrong JM, Essex MJ. 2015. Early adversity, elevated stress physiology, accelerated sexual maturation, and poor health in females. Dev. Psychol. 51, 816–822. ( 10.1037/dev0000017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brumbach BH, Figueredo AJ, Ellis BJ. 2009. Effects of harsh and unpredictable environments in adolescence on development of life history strategies. Hum. Nat. 20, 25–51. ( 10.1007/s12110-009-9059-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellis BJ, Essex MJ. 2007. Family environments, adrenarche, and sexual maturation: a longitudinal test of a life history model. Child Dev. 78, 1799–1817. ( 10.1111/j.1467-8624.2007.01092.x) [DOI] [PubMed] [Google Scholar]
- 37.Belsky J, Pluess M. 2009. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol. Bull. 135, 885–908. ( 10.1037/a0017376) [DOI] [PubMed] [Google Scholar]
- 38.Del Giudice M. 2016. Differential susceptibility to the environment: are developmental models compatible with the evidence from twin studies? Dev. Psychol. 52, 1330–1339. ( 10.1037/dev0000153) [DOI] [PubMed] [Google Scholar]
- 39.Petersen MB, Aarøe L. 2015. Birth weight and social trust in adulthood. Psychol. Sci. 26, 1681–1692. ( 10.1177/0956797615595622) [DOI] [PubMed] [Google Scholar]
- 40.Simpson JA, Griskevicius V, Kuo SI-C, Sung S, Collins WA. 2012. Evolution, stress, and sensitive periods: the influence of unpredictability in early versus late childhood on sex and risky behavior. Dev. Psychol. 48, 674–686. ( 10.1037/a0027293) [DOI] [PubMed] [Google Scholar]
- 41.Hayes AF. 2015. An index and test of linear moderated mediation. Multivariate Behav. Res. 50, 1–22. ( 10.1080/00273171.2014.962683) [DOI] [PubMed] [Google Scholar]
- 42.Chang L, et al. 2019. Environmental harshness and unpredictability, life history, and social and academic behavior of adolescents in nine countries. Dev. Psychol. 55, 890–903. ( 10.1037/dev0000655) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murray C, Greenberg MT. 2006. Examining the importance of social relationships and social contexts in the lives of children with high-incidence disabilities. J. Spec. Educ. 39, 220–233. ( 10.1177/00224669060390040301) [DOI] [Google Scholar]
- 44.Holmes TH, Rahe RH. 1967. The social readjustment rating scale. J. Psychosom. Res. 11, 213–218. ( 10.1016/0022-3999(67)90010-4) [DOI] [PubMed] [Google Scholar]
- 45.Matheny AP Jr, Wachs TD, Ludwig JL, Phillips K. 1995. Bringing order out of chaos: psychometric characteristics of the confusion, hubbub, and order scale. J. Appl. Dev. Psychol. 16, 429–444. ( 10.1016/0193-3973(95)90028-4) [DOI] [Google Scholar]
- 46.Achenbach TM. 1991. Integrative guide for the 1991 CBCL 14-18, YSR, and TRF profiles. Burlington, VT: University of Vermont, Department of Psychiatry. [Google Scholar]
- 47.Gupta S, Crawford SG, Mitchell I. 2006. Screening children with asthma for psychosocial adjustment problems: a tool for health care professionals. J. Asthma 43, 543–548. ( 10.1080/02770900600857267) [DOI] [PubMed] [Google Scholar]
- 48.Holmes CS, Respess D, Greer T, Frentz J. 1998. Behavior problems in children with diabetes: disentangling possible scoring confounds on the Child Behavior Checklist. J. Pediatr. Psychol. 23, 179–185. ( 10.1093/jpepsy/23.3.179) [DOI] [PubMed] [Google Scholar]
- 49.Dufton LM, Dunn MJ, Compas BE. 2008. Anxiety and somatic complaints in children with recurrent abdominal pain and anxiety disorders. J. Pediatr. Psychol. 34, 176–186. ( 10.1093/jpepsy/jsn064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cunha GR, et al. 2016. Inflammation, neurotrophism and oxidative stress and childhood psychopathology in a large community sample. Acta. Psychiatr. Scand. 133, 122–132. ( 10.1111/acps.12453) [DOI] [PubMed] [Google Scholar]
- 51.Väistö T, Aronen ET, Simola P, Ashorn M, Kolho K-L. 2010. Psychosocial symptoms and competence among adolescents with inflammatory bowel disease and their peers. Inflamm. Bowel Dis. 16, 27–35. ( 10.1002/ibd.21002) [DOI] [PubMed] [Google Scholar]
- 52.Friedman D, Bryant FB, Holmbeck GN. 2007. Testing the factorial invariance of the CBCL somatic complaints scale as a measure of internalizing symptoms for children with and without chronic illness. J. Pediatr. Psychol. 32, 512–516. ( 10.1093/jpepsy/jsl051) [DOI] [PubMed] [Google Scholar]
- 53.Rodenburg R, Stams GJ, Meijer AM, Aldenkamp AP, Dekovic M. 2005. Psychopathology in children with epilepsy: a meta-analysis. J. Pediatr. Psychol. 30, 453–468. ( 10.1093/jpepsy/jsi071) [DOI] [PubMed] [Google Scholar]
- 54.Pinquart M, Shen Y. 2011. Behavior problems in children and adolescents with chronic physical illness: a meta-analysis. J. Pediatr. Psychol. 36, 1003–1016. ( 10.1093/jpepsy/jsr042) [DOI] [PubMed] [Google Scholar]
- 55.Patton JH, Stanford MS, Barratt ES. 1995. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 51, 768–774. () [DOI] [PubMed] [Google Scholar]
- 56.Benthin A, Slovic P, Severson H. 1993. A psychometric study of adolescent risk perception. J. Adolesc. 16, 153–168. ( 10.1006/jado.1993.1014) [DOI] [PubMed] [Google Scholar]
- 57.Galvan A, Hare T, Voss H, Glover G, Casey BJ. 2007. Risk-taking and the adolescent brain: who is at risk? Dev. Sci. 10, F8–F14. ( 10.1111/j.1467-7687.2006.00579.x) [DOI] [PubMed] [Google Scholar]
- 58.Marsh HW, Hau KT, Wen Z, Nagengast B, Morin AJS. 2013. Moderation. In The Oxford handbook of quantitative methods, vol. 2 (ed. Little TD.), pp. 361–386. New York, NY: Oxford University Press. [Google Scholar]
- 59.Preacher KJ, Rucker DD, Hayes AF. 2007. Addressing moderated mediation hypotheses: theory, methods, and prescriptions. Multivariate Behav. Res. 42, 185–227. ( 10.1080/00273170701341316) [DOI] [PubMed] [Google Scholar]
- 60.Muthén LK, Muthén BO. 1998-2012 Mplus user's guide, 7th edn Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- 61.Schafer JL, Graham JW. 2002. Missing data: our view of the state of the art. Psychol. Methods 7, 147–177. ( 10.1037/1082-989x.7.2.147) [DOI] [PubMed] [Google Scholar]
- 62.Marsh HW, Wen Z, Hau K-T. 2004. Structural equation models of latent interactions: evaluation of alternative estimation strategies and indicator construction. Psychol. Methods 9, 275–300. ( 10.1037/1082-989x.9.3.275) [DOI] [PubMed] [Google Scholar]
- 63.Maslowsky J, Jager J, Hemken D. 2014. Estimating and interpreting latent variable interactions. Int. J. Behav. Dev. 39, 87–96. ( 10.1177/0165025414552301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muthén BO. 2012. Latent variable interactions. See http://www.statmodel.com/download/LV%20Interaction.pdf.
- 65.Hu L, Bentler PM. 1999. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct. Equ. Modeling 6, 1–55. ( 10.1080/10705519909540118) [DOI] [Google Scholar]
- 66.Schreiber JB, Nora A, Stage FK, Barlow EA, King J. 2006. Reporting structural equation modeling and confirmatory factor analysis results: a review. J. Educ. Res. 99, 323–338. ( 10.3200/joer.99.6.323-338) [DOI] [Google Scholar]
- 67.Hill SE, Boehm GW, Prokosch ML. 2016. Vulnerability to disease as a predictor of faster life history strategies. Adapt. Hum. Behav. Physiol. 2, 116–133. ( 10.1007/s40750-015-0040-6) [DOI] [Google Scholar]
- 68.Doom JR, Vanzomeren-Dohm AA, Simpson JA. 2015. Early unpredictability predicts increased adolescent externalizing behaviors and substance use: a life history perspective. Dev. Psychopathol. 28, 1505–1516. ( 10.1017/s0954579415001169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alexander RD. 1990. How did humans evolve? Reflections on the uniquely unique species. In Museum of zoology special publication No. 1, pp 1–38. Ann Arbor, MI: The University of Michigan. [Google Scholar]
- 70.Chang L, Lu HJ. 2017. Environmental risk. In Encyclopedia of evolutionary psychological science (eds Shackelford TK, Weekes–Shackelford V), pp. 1–8. Berlin, Germany: Springer International Publishing. [Google Scholar]
- 71.Proffitt LRP, Hill SE. 2018. Unpredictability, body awareness, and eating in the absence of hunger: a cognitive schemas approach. Health Psychol. 37, 691–699. ( 10.1037/hea0000634) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data of this study are available from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.nvx0k6dn7.