Abstract
In providing a new tool for imaging inflammation, the study by MacKenzie et al may enable more effective treatment plans for inflammatory arthritis by allowing early, noninvasive monitoring of response to therapy.
Summary
Sensitive new imaging methods for diagnosing inflammatory arthritis and monitoring its treatment would be beneficial, particularly for patients with rheumatoid arthritis. MacKenzie et al (1) have demonstrated a new technique for characterizing inflammation with hyperpolarized carbon 13 (13C)–pyruvate metabolic magnetic resonance (MR) imaging. They found alterations in the conversion from pyruvate to lactate that indicate the presence of inflammatory arthritis in animal models. There is promising clinical potential for hyperpolarized MR imaging with 13C-pyruvate, which is endogenous and has a low toxicity profile, as well as other 13C-labeled agents, and the first clinical trial with this technique is currently under way in patients with prostate cancer (2). The challenges for clinical translation include contrast agent generation, rapid MR imaging techniques, and accurate quantification methods.
The Setting
The detection and monitoring of inflammatory arthritis, which is commonly caused by rheumatoid arthritis, is currently limited to methods such as tissue sampling or anatomic imaging that are either invasive or do not directly measure cellular activity. Several promising molecular imaging approaches have been proposed but are still in relatively early stages of clinical development (3). Conventional hydrogen 1 (1H) MR imaging is superior to conventional radiography but does not show metabolic changes of inflammatory disease.
MR spectroscopic imaging with injectable hyperpolarized 13C agents is an emerging technique that has shown promise for metabolic imaging in vivo, with demonstrated scientific and potential clinical value (2,4,5). Conventional MR imaging methods provide primarily anatomic information, whereas this technique provides direct functional measures of cellular metabolism. In this issue of Radiology, MacKenzie et al (1) showed that inflammation can be detected by monitoring the conversion from 13C-pyruvate to 13C-lactate with use of hyperpolarized MR spectroscopy.
The Science
The MR signal is proportional to the nuclear spin polarization, which is on the order of 0.0001%–0.0005% under typical equilibrium conditions. Hyperpolarization methods can temporarily increase the spin polarization to be on the order of 10%, creating increases of more than 10 000 in MR signal. 13C-pyruvate metabolic imaging uses the dynamic nuclear polarization hyperpolarization method and a rapid dissolution technique to create an injectable hyperpolarized agent (4,5). In dynamic nuclear polarization, a mixture comprised of the molecule of interest and a stable radical is irradiated with microwaves at very low temperatures (~1 K) in a magnetic field (3–5 T). Under these conditions, the electron spins of the radical are more than 90% polarized, and the microwave energy transfers their polarization to the nuclei. After approximately 30–90 minutes of irradiation, the frozen sample is rapidly dissolved to create a hyperpolarized liquid. The liquid signal enhancement decays with the molecular T1 constant as the nuclei return to their equilibrium polarization. Imaging or spectroscopic acquisitions also deplete the enhancement owing to subsequent T2 relaxation. This narrow imaging window necessitates a rapid turnaround between hyperpolarized agent generation and agent delivery as well as fast MR spectroscopy acquisition methods. After injection, there is virtually no background 13C signal because this isotope has a low natural abundance, comprising 1.1% of all carbon atoms, compared with a nearly 100% abundance of 1H, and its lower gyromagnetic ratio results in an equilibrium spin polarization that is approximately four times smaller than that with 1H.
The most promising and most used molecule thus far has been 13C-pyruvate because it can readily be hyperpolarized, its signal decays relatively slowly (T1 ≈ 60 seconds in solution and 30 seconds in vivo), it is endogenous, it has a low toxicity profile, and, most important, its metabolic conversion to 13C-lactate has been shown to help differentiate normal from diseased tissues in preclinical animal models. The first clinical trial of hyperpolarized 13C-pyruvate MR spectroscopy in patients with prostate cancer is currently under way, with encouraging initial results (2). To date, hyperpolarized MR imaging has been investigated primarily for cancer metabolic imaging; however, it has also been applied to angiography, cardiac metabolic imaging, pH imaging, and more by using 13C-pyruvate as well as other agents (2).
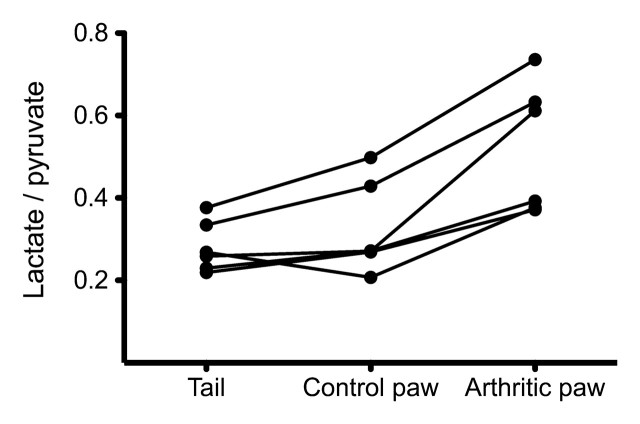
MacKenzie et al (1) have shown, in a rat model, that 13C-pyruvate can be used for imaging inflammation. The inflammatory response, induced in one hind paw, was validated by using joint thickness measurements, visual assessment, and histologic findings. Hyperpolarized MR studies were performed at the peak of this response. These studies used two-dimensional MR spectroscopy performed with 2.5 × 2.5-mm in-plane resolution and 10-mm-thick sections and initiated 20 seconds after the start of hyperpolarized 13C-pyruvate injection. In these studies, the inflamed paws showed significantly higher lactate conversion than did the control paws and tail, as quantified by the lactate-to-pyruvate and lactate-to–total carbon ratios. They also observed 13C-alanine in the control paws but not in inflamed paws. The authors hypothesize that the observed increase in pyruvate metabolism is due to a hypoxic and acidodic environment in the inflamed joints.
The Practice
Clinical use: MR imaging with hyperpolarized 13C-pyruvate has the potential for detecting and monitoring inflammatory arthritis noninvasively. Monitoring metabolism with this technique has also shown promise for other applications, particularly cancer imaging. Preclinical studies have demonstrated early detection, prediction of disease severity, and monitoring of response to therapy in models of prostate, brain, and liver cancer. Myocardial ischemia and other metabolic diseases (eg, type 1 diabetes) have also been detected and monitored in preclinical settings. The recent progress of clinical trials in patients with prostate cancer are encouraging in bringing this technique closer to clinical availability, and the detection of inflammation adds to the promising applications that will help drive the proliferation of this technology.
Future opportunities: In providing a new tool for imaging inflammation, the study by Mackenzie et al (1) may enable more effective treatment plans for inflammatory arthritis by allowing early, noninvasive monitoring of the response to therapy. There are many other promising hyperpolarized agents that currently include markers for perfusion (urea), necrosis (fumarate), pH (bicarbonate), and glucose metabolism (fructose). Agents can also be hyperpolarized concurrently (6), allowing for simultaneous imaging of multiple compounds that may provide a more comprehensive and diagnostic hyperpolarized MR study.
Challenges: The creation of injectable hyperpolarized agents requires specialized equipment, including a cryostat, located near an MR unit, and sterile 13C-enriched molecules. After agent generation, the decay of the hyperpolarization signal enhancement necessitates that injection, metabolic conversion, and imaging must be done within several minutes. Further regulatory approval will also be required for future clinical studies and for agents other than pyruvate.
Quantification of hyperpolarized MR spectroscopy can be challenging because of the high experimental and biologic variability, as shown by the broad interanimal range of lactate-to-pryuvate ratio (1). The lactate-to-pyruvate ratio is sensitive to the timing of imaging, agent delivery, and in vivo concentration. A contralateral or healthy reference tissue was used in the study by Mackenzie et al; however, this may not always be available in clinical examinations. A reliable quantification should also account for blood flow, which can be measured by using a dynamic imaging approach.
Footnotes
See also the article by MacKenzie et al.
Disclosures of Potential Conflicts of Interest: P.E.Z.L. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: institution received a grant or grants are pending from GE Healthcare; received money for patents (planned, pending, or issued) from GE Healthcare, Phillips, and Siemens; institution received money for patents (planned, pending, or issued) from GE Healthcare, Phillips, and Siemens. Other relationships: none to disclose. G.E.G. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: receives money for consultancy from Arthrocare, Zimmer, and ISTO; institution received grant or grants are pending from GE Healthcare; receives royalties from Elsevier. Other relationships: none to disclose.
References
- 1.MacKenzie JD, Yen YF, Mayer D, Tropp JS, Hurd RE, Spielman DM. Detection of inflammatory arthritis by using hyperpolarized 13C-pyurvate with MR imaging and spectroscopy. Radiology 2011;259(2):414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurhanewicz J, Vigneron DB, Brindle K, et al. Analysis of cancer metabolism by imaging hyperpolarized nuclei: prospects for translation to clinical research. Neoplasia 2011;13(2):81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswal S, Resnick DL, Hoffman JM, Gambhir SS. Molecular imaging: integration of molecular imaging into the musculoskeletal imaging practice. Radiology 2007;244(3):651–671. [DOI] [PubMed] [Google Scholar]
- 4.Ardenkjaer-Larsen JH, Fridlund B, Gram A, et al. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A 2003;100(18):10158–10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golman K, Ardenkjaer-Larsen JH, Petersson JS, Mansson S, Leunbach I. Molecular imaging with endogenous substances. Proc Natl Acad Sci U S A 2003;100(18):10435–10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson DM, Keshari KR, Larson PEZ, et al. Multi-compound polarization by DNP allows simultaneous assessment of multiple enzymatic activities in vivo. J Magn Reson 2010;205(1):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]