Abstract
Purpose
To assess the value of magnetic resonance (MR) imaging in determining the site of origin of newly diagnosed uterine cancer (corpus vs cervix) when clinical and/or histologic evaluation is indeterminate.
Materials and Methods
The Institutional Review Board approved and waived informed consent for this HIPAA-compliant study of 59 women (median age, 59 years; range, 28–84 years) who underwent pelvic MR imaging to determine the anatomic origin of uterine cancer. Two radiologists independently retrospectively assessed all MR imaging studies. In 48 patients who underwent hysterectomy, surgical pathologic findings were the reference standard, and overall test yields and diagnostic likelihood ratios were measured. Accuracy in detecting invasion of adjacent structures was also calculated. For the remaining patients, imaging and biopsy findings are presented descriptively.
Results
At hysterectomy, 32 patients had uterine corpus cancer and 16 had cervical cancer. Overall test yields for reader 1 and reader 2 were 0.85 and 0.88, respectively. When a reader attributed a tumor’s origin to either the uterine corpus or cervix, the odds of the tumor originating from that site were 4.80–6.35 times greater than they would have been if no other information were available. Accuracy levels in detecting invasion of the myometrium, cervical stroma, parametria and/or adnexae, and vagina, respectively, were 72%, 69%, 74%, and 85% for reader 1 and 78%, 77%, 76%, and 85% for reader 2.
Conclusion
MR imaging is useful for determining the anatomic origin of uterine cancer and provides helpful information regarding invasion of adjacent structures.
© RSNA, 2011
Introduction
The uterus is the most common site of cancer in the female genital tract. Approximately 55 000 new cases of uterine cancer are expected in the United States in 2010, of which approximately 43 000 will be uterine corpus cancers and 12 000 will be uterine cervical cancers (1). Despite a common embryological origin and direct anatomic contiguity, cancers arising from the uterine corpus and those arising from the uterine cervix have different biologic behavior and clinical and epidemiologic features. In most cases, the anatomic site of origin of uterine cancer can be readily identified through findings from clinical examination and histologic evaluation of biopsy specimens. However, in some cases, it is difficult to confidently establish the origin of a tumor for reasons that may include difficulties with the biopsy specimens (eg, absence of precursor lesions, unusual morphologic patterns, mixed-type histologic findings, inadequate samples, or discordant readings at different institutions) or unexpected clinical findings (eg, when the suspected cancer histologic finding and site do not correlate with known risk factors or physical examination findings). The frequency with which this issue arises has not, to our knowledge, been reported in the literature, and although most of the obstacles to correct identification of the cancer site can be overcome after examining the hysterectomy specimen, making this differentiation preoperatively is critical, as it has substantial implications for patient management. Most uterine corpus carcinomas are simultaneously staged and treated with simple hysterectomy and bilateral salpingo-oophorectomy. In contrast, surgery in cervical cancer is usually limited to simple hysterectomy for carcinoma in situ and radical hysterectomy for early-stage invasive carcinomas, while advanced disease (with parametrial, lower vaginal, pelvic sidewall, rectal, or bladder invasion) is treated with a combination of chemo- and radiation therapy. Conservative treatment approaches are also an option for carefully selected patients with corpus or cervical cancer, particularly for those wishing to preserve their fertility. Though findings in the literature (2) suggest that magnetic resonance (MR) imaging may be a useful problem-solving tool in this clinical scenario, to our knowledge, there are no published studies that objectively evaluate its role. Thus, the purpose of our study was to assess the value of MR imaging in distinguishing the anatomic site of origin of newly diagnosed uterine cancer (corpus vs cervix) when clinical and/or histologic evaluation are indeterminate.
Materials and Methods
Subjects
Our study consisted of a retrospective single-institution analysis. The study complied with the Health Insurance Portability and Accountability Act and was approved by our Institutional Review Board, with a waiver of informed consent. The patient inclusion criterion for our study was pretreatment pelvic MR imaging performed owing to uncertainty regarding the anatomic site of origin of newly diagnosed uterine cancer, as stated in the MR imaging request received from the referring gynecologic surgeon. The uncertainty in all cases derived from histologic and clinical findings. This uncertainty involved difficulties with biopsy reports (eg, absence of precursor lesions, unusual morphologic patterns, mixed-type histologic findings, inadequate samples, or discordant readings at different institutions) and/or unexpected clinical findings (eg, when the suspected cancer histologic findings and site did not correlate with known risk factors or physical examination findings). The patients had no earlier imaging examinations showing problems, and previous imaging had no role in patient selection.
A search of hospital records data from January 2000 to December 2009 yielded 1811 patients who received treatment for uterine cancer (corpus, n = 1447; cervix, n = 364); 59 of these patients met the inclusion criterion and, thus, were included in our study. Forty-eight of the 59 patients underwent treatment with hysterectomy within 6 months after the MR imaging and received no other form of treatment (including hormones, chemotherapy, or radiation therapy) between the MR imaging and the hysterectomy. In the other 11 patients, surgery was not performed owing to advanced disease, lack of indication, presence of contraindications for surgery (including comorbidities), or patient preference. The patients’ median age was 59 years (range, 28–84 years). In the patients who underwent hysterectomy, the median interval between MR imaging and surgery was 13 days (range, 0–179 days).
MR Imaging Technique
MR imaging was performed by using a 1.5-T system (Signa; GE Medical Systems, Milwaukee, Wis). A body coil was used for excitation, and a pelvic phased-array coil was used for signal reception. Owing to the length of the study period, the MR imaging parameters varied slightly among patients as per the standard clinical protocols in place at our institution at the time each examination was performed. However, all involved a combination of axial, axial oblique, coronal, coronal oblique, and sagittal T2-weighted fast spin-echo images (typical parameters: repetition time msec/effective echo time msec, 4000–6500/100; bandwidth, 16 kHz; section thickness, 5 mm; intersection gap, 1 mm; field of view, 16–20 cm), axial T1-weighted images (typical parameters: 500/10; bandwidth, 15 kHz; section thickness, 5 mm; intersection gap, 1 mm; field of view, 28–36 cm), and three-dimensional spoiled gradient-recalled images (typical parameters: flip angle, 12°; bandwidth, 62.5 kHz; section thickness, 3 mm with no intersection gap; field of view, 32–36 cm) obtained before and 1, 2, and 3 minutes after the administration of intravenous gadopentetate dimeglumine (Magnevist; Berlex Laboratories, Montville, NJ). The contrast material was administered at a dose of 0.1 mmol per kilogram of body weight with a bolus injection rate of 2 mL/sec by using an automatic injector (Medrad, Warrendale, Pa) and was followed by a 20-mL saline bolus injection.
Data Collection
Two readers independently evaluated all the MR images by using a picture archiving and communication system workstation (Centricity; GE Medical Systems). One of the readers (H.A.V.) was a radiology fellow with a special interest in genitourinary imaging who had read approximately 150 female pelvis MR examinations prior to our study. The second reader (O.A.) was part of a dedicated team of genitourinary radiologists involved in the clinical care of all gynecologic oncology patients at our institution and had 6 years of experience in interpreting female pelvic MR imaging. Both readers were blinded to the patients’ clinical, histologic, and imaging findings other than the fact that the indication for the pelvic MR imaging was suspected or confirmed uterine cancer of uncertain anatomic origin.
Each reader assigned an overall suspicion score for the probability of the anatomic site of origin of the uterine cancer for each patient on a five-point index scale (1 = definitely corpus cancer, 2 = probably corpus cancer, 3 = indeterminate, 4 = probably cervical cancer, 5 = definitely cervical cancer). If a tumor extended across the corpus and cervix or if more than one tumor was present, the site of origin was assessed subjectively by establishing the location where the tumor appeared to be centered and the location of the bulk (>50%) of the tumor. If the reader could not establish a location on the basis of these criteria or if no tumor was visible, a score of 3 (indeterminate) was recorded. If a score of 1 or 2 was given (probably or definitely corpus cancer), the reader was asked to assess the depth of myometrial invasion by the tumor (<50% vs ≥50% of the myometrial thickness). If a score of 4 or 5 was given (probably or definitely cervical cancer), the reader was asked to record the presence of endometrial extension of the primary tumor. In addition, regardless of the origin of the tumor, the readers recorded the presence of invasion of the following structures: myometrium, cervical stroma, parametrium and/or adnexae, vagina, and pelvic sidewall.
Statistical Analysis
Measurements of accuracy were performed by analyzing the data from the 48 patients who underwent hysterectomy, with the pathologic analysis of the full surgical specimen serving as the reference standard. The data for the 11 patients who did not undergo surgery is presented in a descriptive sense only and was not taken into consideration for the accuracy calculations. The MR imaging overall diagnostic impression was indexed as a three-level result of corpus, cervical, or indeterminate cancer by combining definite and probable disease. For a test with indeterminate results, the conventional estimates of sensitivity and specificity for a two-level test result would be biased. Hence, accuracy was measured through overall test yields, conditional probabilities, and odds of a certain test result in favor of a disease. The overall test yield was calculated along with the corresponding 95% confidence interval. The conditional probability, a concept similar to conventional sensitivity, was defined as the probability, P, of a reader attributing a tumor’s origin, D, to one location (corpus vs cervix) on the basis of MR imaging information, MRD+, when the reference standard showed a tumor in that same location, PathD+. This quantity was denoted as P(MRD+|PathD+). Noticing that pathologic corpus cancer, corp, and cervical cancer, cerv, are mutually exclusive in this cohort, P(MRcorp+|Pathcorp+) = P(MRcerv−|Pathcerv−) and vice versa for the opposite diagnosis, where + indicates a positive diagnosis and − indicates a negative diagnosis. The concept of the right-hand side of the previous equation was the same as the conventional specificity.
In addition, for each reader, we estimated the positive and negative diagnostic likelihood ratios (DLRs) (3,4). The DLRs quantify the change in information about tumor origin that we had after the radiologist reading the MR images specified the origin. The positive DLR for a certain tumor origin (corpus or cervix) was defined as P(MRD+|PathD+)/P(MRD+|PathD−). It was the ratio between the likelihood of an MR imaging finding of a particular tumor origin conditional on a pathologic finding of the same tumor origin and the likelihood of an MR imaging finding of a particular tumor origin conditional on a pathologic finding of a different tumor origin. Similarly, the negative DLR for a specific tumor origin was defined as P(MRD−|PathD+)/P(MRD−|PathD−), or the ratio between the likelihood of MR imaging not indicating a particular tumor origin conditional on a pathologic finding specifying that particular tumor origin and the likelihood of MR imaging not indicating a particular tumor origin conditional on a pathologic finding not indicating that particular tumor origin. The number of patients in whom at least one of the two readers incorrectly established the primary site of cancer was recorded, and the MR images of these patients were retrospectively reviewed by both readers in consensus to attempt to identify possible causes for errors.
We also determined the accuracy for each reader in detecting the depth of myometrial invasion (<50% vs ≥50% of myometrial thickness) in patients with corpus cancer and the presence of parametrial invasion in patients with cervical cancer. Finally, the sensitivity, specificity, and weighted κ statistic were also calculated by using Fleiss-Cohen weights (5) of MR imaging for detecting invasion of the myometrium, cervical stroma, parametrium and/or adnexae, vagina, and pelvic sidewall regardless of the original tumor site. The scale used for interpretation of weighted κ statistics was as follows: slight agreement, 0–0.20; fair agreement, 0.21–0.40; moderate agreement, 0.41–0.60; substantial agreement, 0.61–0.80; and almost perfect agreement, 0.81–0.99.
Results
Of the 48 patients who had surgery for the treatment of uterine cancer within 6 months of MR imaging, 32 patients had uterine corpus cancer and 16 patients had uterine cervical cancer (Table 1, Figs 1–3). The presence of three cases of squamous cell carcinoma in the study group was particularly interesting given that these are almost always cervical in origin. On careful review of the pathologic findings, one of the patients had repeated endometrial biopsies showing squamous cell carcinoma with normal cervical curettage and cone biopsies. This patient’s final postoperative diagnosis was the extremely rare form of primary squamous cell carcinoma of the corpus. In the second case, an endometrial curettage performed at an outside institution had been reported as adenosquamous carcinoma of the cervix. Review of the slides at our institution favored adenocarcinoma but could not establish whether it was cervical or corpus in origin owing to absence of precursor lesions (immunohistochemistry was not performed). The final histologic sample showed squamous cell carcinoma of the cervix. In the third case, although the biopsy was reported as “scant malignant cells, probable squamous cell carcinoma,” the patient was a postmenopausal woman with vaginal bleeding and an enlarged uterus at clinical examination. In all three cases, the referring gynecologic surgeon felt unsure about the exact origin of the primary tumor, and the MR imaging was performed.
Table 1.
Distribution of Histologic Subtypes by Anatomic Site of Origin Determined from Hysterectomy Specimens
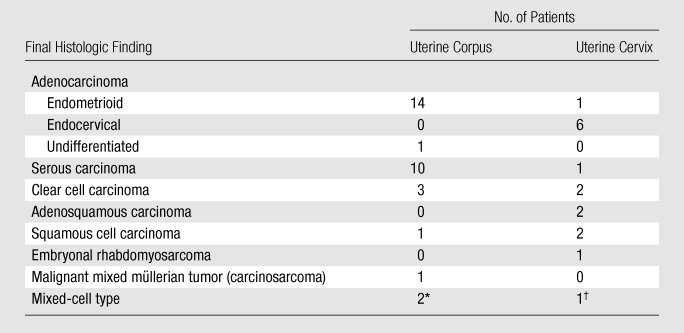
Mixed serous and clear cell carcinoma (n = 1) and mixed serous, clear cell, and endometrioid adenocarcinoma (n = 1).
Mixed squamous and adenocarcinoma.
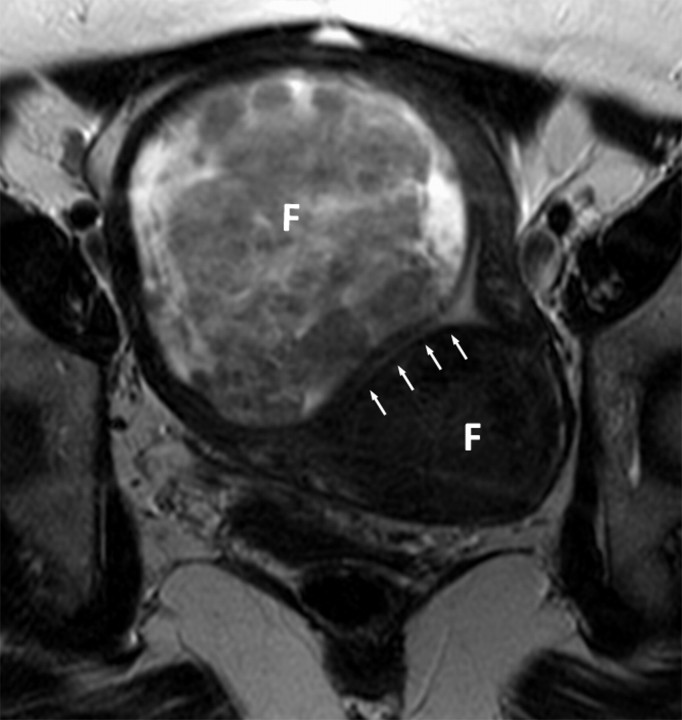
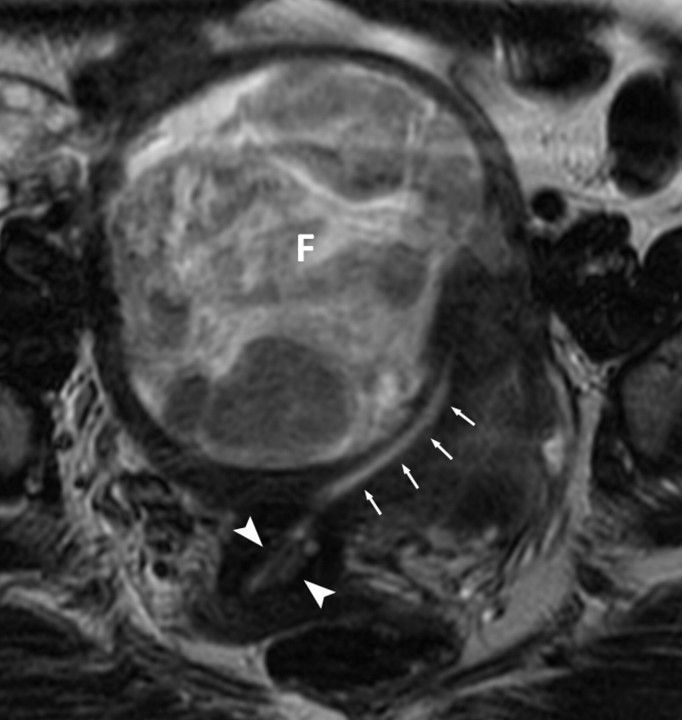
Figure 1a:
(a, b) Oblique coronal T2-weighted MR images (6317/106) of a 48-year-old woman with adenocarcinoma cells of indeterminate primary origin at cervix biopsy. A grossly enlarged uterus was noted clinically, raising suspicion of a corpus tumor with cervical extension. MR images show large fibroids (F) compressing the endometrial cavity. Endometrial stripe (arrows) was normal in thickness (4 mm) and signal intensity. A cervix tumor (arrowheads) was identified as an area of intermediate signal intensity extending into cervical stroma. Final pathologic diagnosis from the hysterectomy specimen was a stage T1b1 endocervical adenocarcinoma.
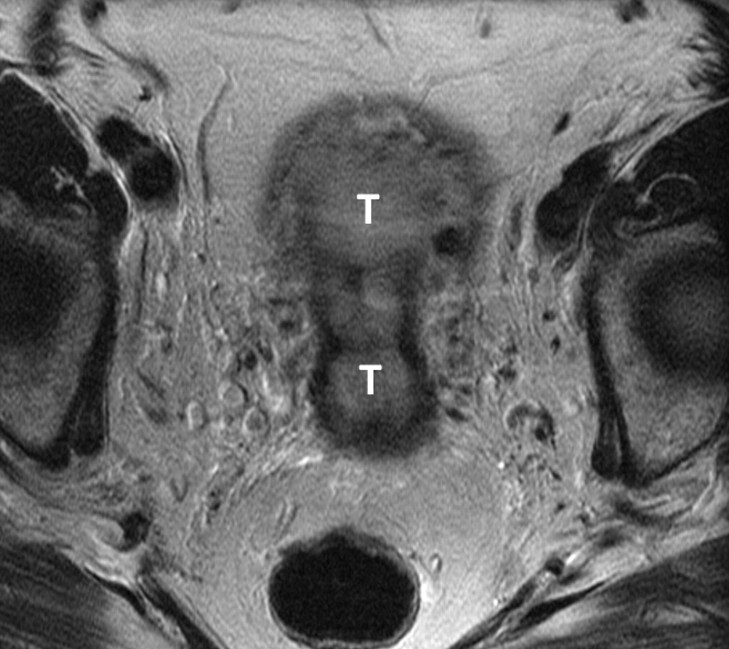
Figure 3a:
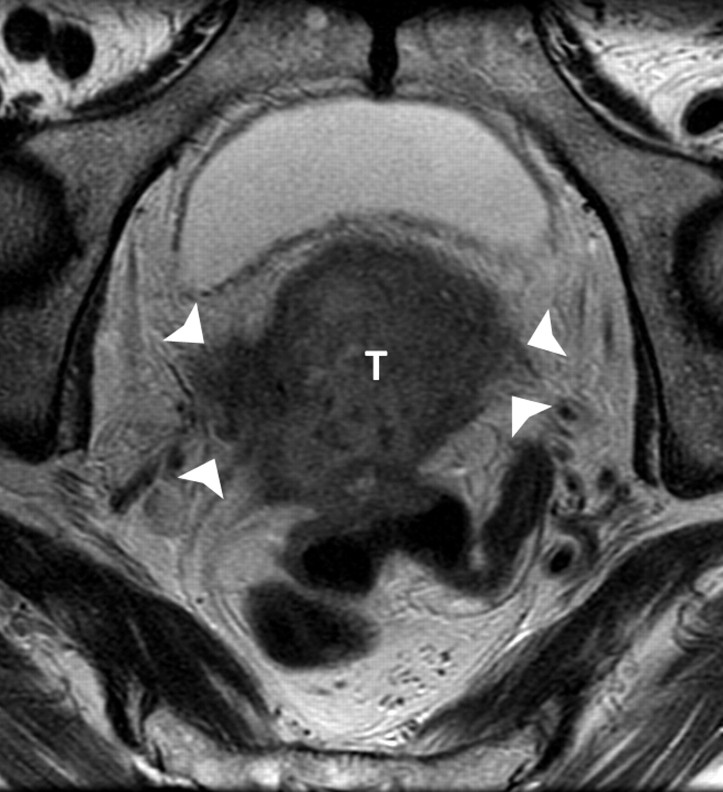
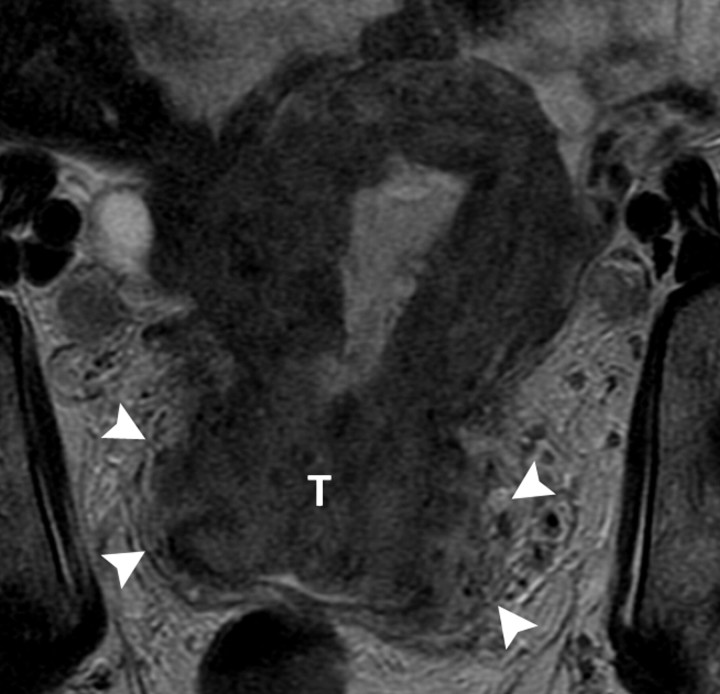
(a) Sagittal and (b) axial T2-weighted MR images (6200/108) of a 67-year-old woman with cervical biopsy show poorly differentiated carcinoma. Differential diagnosis was adenocarcinoma versus adenosquamous carcinoma. MR images show tumors (T) of intermediate signal intensity in both endometrial cavity and cervix. It was not possible to determine the primary origin on the basis of MR imaging appearance. Final pathologic diagnosis from the hysterectomy specimen was papillary serous carcinoma of the endometrium with deep myometrial and cervical stromal involvement.
Figure 1b:
(a, b) Oblique coronal T2-weighted MR images (6317/106) of a 48-year-old woman with adenocarcinoma cells of indeterminate primary origin at cervix biopsy. A grossly enlarged uterus was noted clinically, raising suspicion of a corpus tumor with cervical extension. MR images show large fibroids (F) compressing the endometrial cavity. Endometrial stripe (arrows) was normal in thickness (4 mm) and signal intensity. A cervix tumor (arrowheads) was identified as an area of intermediate signal intensity extending into cervical stroma. Final pathologic diagnosis from the hysterectomy specimen was a stage T1b1 endocervical adenocarcinoma.
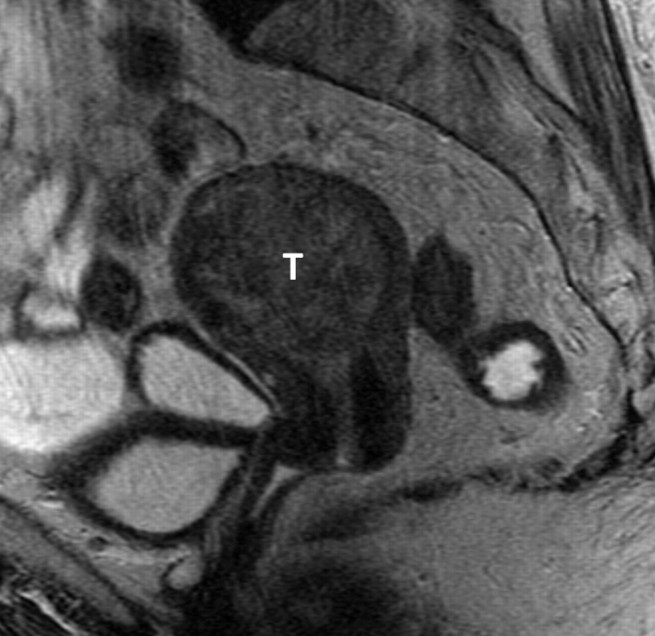
Figure 2:
Sagittal T2-weighted MR image (5616/102) of an 83-year-old woman. Endometrial and endocervical biopsies showed clear cell carcinoma. The absence of identifiable normal tissue precluded differentiation between cervical and endometrial origin. MR image shows a large tumor (T) of intermediate signal intensity expanding the endometrial cavity. Final pathologic diagnosis from the hysterectomy specimen was a clear cell carcinoma arising from the corpus.
Figure 3b:
(a) Sagittal and (b) axial T2-weighted MR images (6200/108) of a 67-year-old woman with cervical biopsy show poorly differentiated carcinoma. Differential diagnosis was adenocarcinoma versus adenosquamous carcinoma. MR images show tumors (T) of intermediate signal intensity in both endometrial cavity and cervix. It was not possible to determine the primary origin on the basis of MR imaging appearance. Final pathologic diagnosis from the hysterectomy specimen was papillary serous carcinoma of the endometrium with deep myometrial and cervical stromal involvement.
The overall test yield was 0.85 for reader 1 and 0.88 for reader 2 (Table 2). The conditional probabilities for readers 1 and 2, respectively, were 0.85 and 0.82 for corpus cancer and 0.87 and 0.86 for cervical cancer. The positive DLRs for readers 1 and 2, respectively, were 6.35 and 5.75 for corpus cancer and 5.63 and 4.80 for cervical cancer, meaning that when a reader attributed a tumor’s origin to either the uterine corpus or cervix based on MR imaging, the odds of the tumor originating from that site were 4.80–6.35 times greater than they would have been if no other information about the tumor were available. Overall, agreement between the readers was substantial (weighted κ = 0.68). Reader 1 determined the primary site of cancer incorrectly in four patients with primary uterine corpus cancer and two with primary cervical cancer; reader 2 determined the primary cancer site incorrectly in five patients with primary uterine corpus cancer and two with primary cervical cancer. In the two patients with cervical cancer that were incorrectly recorded by both readers, retrospective review demonstrated small masses in the endometrial cavity on MR images, which were described as polyps in the pathology report, while no imaging abnormality was identified in the cervix. Cervical adenocarcinoma (stage IB1 [cancer confined to the cervix and smaller than 4 cm]) was diagnosed at pathologic analysis. The causes of errors for the patients with corpus cancer were nonspecific signal changes in the cervix on MR images that may have represented postbiopsy debris, since no cervical abnormalities were reported in the pathology report, and no MR imaging abnormality in the corpus; multiple masses in the cervix and corpus; and a subtle endometrial lesion with an atypical enhancement pattern, similar to that of adjacent myometrium, that was interpreted as a probable benign polyp. For each reader, no specific cause of error could be determined in one patient.
Table 2.
Accuracy of MR Imaging for Determination of Site of Cancer Origin
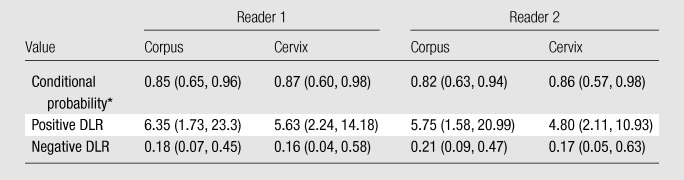
Note.—Data in parentheses are 95% confidence intervals. Overall test yield was 0.85 (95% confidence interval: 0.72, 0.94) for reader 1 and 0.88 (95% confidence interval: 0.75, 0.95) for reader 2.
Conditional probability is the probability of a reader attributing a tumor’s origin to one location (corpus vs cervix) on the basis of MR images when the reference standard shows a tumor in that same location.
Among the 32 patients with corpus cancer, the depth of myometrial invasion (<50% vs ≥50% of myometrial thickness) was correctly identified in 26 (81%) by reader 1 and 25 (78%) by reader 2. Among the 16 patients with cervical cancer, the presence of parametrial invasion was correctly recorded in 12 (75%) by reader 1 and 13 (81%) by reader 2. Readers 1 and 2 made false-negative diagnoses of parametrial invasion in four and three patients, respectively. Neither reader made a false-positive diagnosis of parametrial invasion.
Accuracy levels in detecting invasion of the myometrium, cervical stroma, parametria and/or adnexae, and vagina regardless of the primary cancer site, respectively, were 72%, 69%, 74%, and 85% for reader 1 and 78%, 77%, 76%, and 85% for reader 2. Sensitivities and specificities for the detection of myometrial, cervical stromal, parametria and/or adnexal, and vaginal invasion are provided in Table 3, along with the corresponding 95% confidence intervals and weighted κ statistics. Of note, neither pathologic nor MR imaging readings noted any instances of pelvic sidewall involvement.
Table 3.
Sensitivity and Specificity for Detecting Involvement of Adjacent Structures with MR Imaging Regardless of Primary Cancer Site
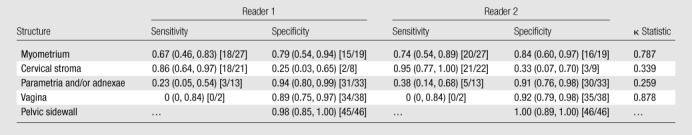
Note.—Data in parentheses are 95% confidence intervals, and those in brackets are the numbers used to calculate the values.
Of the 11 patients who did not have surgery, seven were felt to have advanced cervical cancer on the basis of clinical examination and imaging and, thus, were treated with radiation therapy and/or chemotherapy. Both readers identified the presumptive correct site of origin (cervix) and advanced disease features in all seven of these patients (Fig 4). In addition, two patients with metastatic endometrial cancer (as identified by both readers on MR images), one patient with cervical involvement from primary peritoneal carcinoma, and one patient with primary lung adenocarcinoma metastatic to the cervix were deemed unfit for surgery. In the patient with cervical metastasis from lung cancer, both readers mistakenly identified the metastasis as a primary cervical tumor. In the patient with primary peritoneal carcinoma, one reader correctly established that the primary cancer site was neither endometrial nor cervical.
Figure 4a:
(a) Axial (5017/108) and (b) coronal (4000/109) T2-weighted MR images of a 76-year-old woman presenting with postmenopausal bleeding. Cervical mass biopsy showed poorly differentiated adenocarcinoma of indeterminate origin. MR images show a large cervical tumor (T) with gross bilateral parametrial extension (arrowheads). Clinical examination was also suspicious for parametrial extension. On the basis of clinical and imaging findings, the patient was diagnosed with a stage IIB cervical cancer and treated with chemo- and radiation therapy.
Figure 4b:
(a) Axial (5017/108) and (b) coronal (4000/109) T2-weighted MR images of a 76-year-old woman presenting with postmenopausal bleeding. Cervical mass biopsy showed poorly differentiated adenocarcinoma of indeterminate origin. MR images show a large cervical tumor (T) with gross bilateral parametrial extension (arrowheads). Clinical examination was also suspicious for parametrial extension. On the basis of clinical and imaging findings, the patient was diagnosed with a stage IIB cervical cancer and treated with chemo- and radiation therapy.
Discussion
MR imaging has been shown to be a useful and cost-effective tool for the assessment of both uterine corpus and cervical cancers (6,7). However, its role in the primary diagnosis of uterine malignancies is limited, as most uterine cancers can be readily and accurately diagnosed through a combination of clinical and histologic evaluation. Patients with uterine corpus cancer typically present with postmenopausal bleeding. The most common histologic subtype found at endometrial biopsy or dilatation and curettage is endometrioid adenocarcinoma. On the other hand, cervical cancer may be diagnosed through routine cervical smears or biopsy of clinically suspicious lesions, and the most common histologic subtype is squamous cell carcinoma, although the incidence of endocervical adenocarcinomas is rising, particularly in the Western world (8). Other much less common histologic subtypes include serous, clear cell, sarcomas, and mixed-cell type tumors (8,9). Although the frequency and distribution of each histologic subtype varies between the corpus and cervix, ultimately almost all histologic subtypes may be present in either site. We assessed the value of MR imaging in the small but important subset of cases in which the site of origin of uterine cancer is uncertain (3.25% of all uterine cancers at our institution). We found that the two readers achieved overall accuracy levels of 0.85 and 0.88 in attributing the cancer origin to the corpus or cervix by using MR imaging. We also showed that, when a radiologist attributed a tumor’s origin to either the uterine corpus or cervix on the basis of MR images, the odds of the tumor originating from that site were about six times greater than they would have been if no other information about the tumor were available. These results indicate that MR imaging may help establish the site of cancer in these patients, contributing incremental value to clinical and histologic evaluation.
Other techniques may also be useful in some patients presenting with this clinical scenario. In cases where the diagnostic uncertainty is the result of difficulty differentiating primary endometrioid adenocarcinoma of the corpus and primary endocervical adenocarcinomas on the basis of morphologic features at histologic analysis, the use of immunohistochemical panels has been found to be helpful. Primary endometrioid adenocarcinomas of the corpus are characterized by positive staining for vimentin and estrogen receptors and negative staining for carcinoembryogenic antigen. In contrast, primary endocervical adenocarcinomas are characterized by carcinoembryogenic antigen positivity and by negativity for vimentin and estrogen receptors (10–12). The accuracy for detecting the primary tumor origin by using such panels is reported at 0.65–0.80 (13). Immunohistochemical staining with p16, a sensitive surrogate for the presence of human papillomavirus infection, should also be added to the panel as strong positivity for this marker suggests an endocervical, rather than an endometrial, origin of an adenocarcinoma (14).
The primary role of MR imaging in gynecologic oncology is in delineating the extent of the disease. When used for this purpose, MR imaging has a reported accuracy of 85%–93% for endometrial cancer (15–19) and 75%–96% for cervical cancer (20–24). The critical staging parameter in uterine corpus cancer is the depth of myometrial invasion. When cancer invades 50% or more of the myometrial thickness (International Federation of Gynecology and Obstetrics [FIGO] stage IB), there is an increased incidence of lymph node metastases and a poorer prognosis. In our study, we accurately determined the depth of myometrial invasion according to the 2009 FIGO criteria (25) in around 80% of patients. For uterine cervix cancers, the most critical staging parameter is the presence of parametrial invasion (FIGO Stage IIB) (26). We also accurately established the presence of parametrial invasion in around 80% of patients with cervical cancer. It is worth pointing out that, owing to the use of hysterectomy specimens as the standard of reference, patients with cervical cancer who were felt to have advanced cervical cancer (FIGO stage IIB and above) on the basis of clinical examination and imaging were treated with radiation therapy and/or chemotherapy and were excluded from our accuracy assessments owing to the absence of a reliable standard of reference.
Finally, we analyzed MR imaging features of invasion of adjacent pelvic structures independent of the primary tumor site. In detecting myometrial, cervical stroma, and parametria and/or adnexae invasion, the readers’ accuracy ranged from 69% to 78%. No cases of pelvic sidewall invasion were present in this series; reader 1 falsely identified one instance of pelvic sidewall invasion, while reader 2 correctly established that all cases were negative for pelvic sidewall invasion. This shows that, even when MR imaging does not reveal the anatomic site of origin of uterine cancer, it may reveal important prognostic features that have implications for treatment selection and/or planning.
Our study had several limitations. First, it was a retrospective analysis of patients presenting over a 10-year period. Potential changes in the clinical, pathology, and imaging fields and the effect of these changes on our findings were not taken into account. Although the changes that occurred in MR imaging parameters over the course of the 10-year period as a result of the natural evolution of MR imaging technique were not substantial, their effect on the readers’ performance remains unknown. Second, one of the readers was part of a dedicated team of genitourinary radiologists involved in the clinical care of all gynecologic oncology patients at our institution, and although all readings were performed blinded to all clinical details, there may have been an element of recall bias. Third, although the median time between MR imaging and surgery was 13 days, a delay of up to 6 months between the MR imaging and surgery may have had an influence on our findings. Last, as mentioned earlier, patients who did not undergo hysterectomy were not eligible for accuracy assessments in our study.
In summary, our findings show that MR imaging can help in distinguishing the site of origin of uterine cancer when clinical and histologic findings are indeterminate. In addition, regardless of the site of the primary tumor, MR imaging may provide information regarding the extent of the disease and invasion of adjacent structures that has important prognostic and treatment planning implications.
Advance in Knowledge.
-
•.
MR imaging is useful for determining the anatomical site of origin of uterine cancer when clinical and/or histologic assessment is indeterminate, as two independent readers achieved overall test yields of 0.85 and 0.88 in attributing the cancer origin to the corpus or cervix with MR imaging.
Implication for Patient Care.
-
•.
The distinction between corpus or cervix origin is important for appropriate treatment planning, as most corpus carcinomas are simultaneously staged and treated with surgery (hysterectomy and bilateral salpingo-oophorectomy), while generally only early-stage cervical cancers are treated with surgery, and advanced cervical disease is treated with chemotherapy and radiation therapy.
Acknowledgments
We thank Ada Muellner, MS, for editing this manuscript.
Received June 8, 2010; revision requested July 22; revision received August 26; accepted October 7; final version accepted October 20.
Disclosures of Potential Conflicts of Interest: H.A.V. No potential conflicts of interest to disclose. O.A. No potential conflicts of interest to disclose. J.Z. No potential conflicts of interest to disclose. C.M. No potential conflicts of interest to disclose. R.S. No potential conflicts of interest to disclose. N.A. No potential conflicts of interest to disclose. R.R.B. No potential conflicts of interest to disclose. H.H. No potential conflicts of interest to disclose.
Abbreviation:
- DLR
- diagnostic likelihood ratio
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300. [DOI] [PubMed] [Google Scholar]
- 2.Hricak H. Diagnostic imaging: gynecology. Salt Lake City, Utah: Amirsys, 2007. [Google Scholar]
- 3.Simel DL, Rennie D, Bossuyt PM. The STARD statement for reporting diagnostic accuracy studies: application to the history and physical examination. J Gen Intern Med 2008;23(6):768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simel DL, Feussner JR, DeLong ER, Matchar DB. Intermediate, indeterminate, and uninterpretable diagnostic test results. Med Decis Making 1987;7(2):107–114. [DOI] [PubMed] [Google Scholar]
- 5.Fleiss JL, Cohen J. The equivalence of weighted kappa and the intraclass correlation coefficient as measures of reliability. Educ Psychol Meas 1973;33(3):613–619. [Google Scholar]
- 6.Hardesty LA, Sumkin JH, Nath ME, et al. Use of preoperative MR imaging in the management of endometrial carcinoma: cost analysis. Radiology 2000;215(1):45–49. [DOI] [PubMed] [Google Scholar]
- 7.Hricak H, Powell CB, Yu KK, et al. Invasive cervical carcinoma: role of MR imaging in pretreatment work-up—cost minimization and diagnostic efficacy analysis. Radiology 1996;198(2):403–409. [DOI] [PubMed] [Google Scholar]
- 8.Silverberg SG, Ioffe OB. Pathology of cervical cancer. Cancer J 2003;9(5):335–347. [DOI] [PubMed] [Google Scholar]
- 9.Soslow RA, Oliva E. Uterine cancer: pathology. In: , Muggia F, Oliva E, eds. Uterine cancer: screening, diagnosis, and treatment. New York, NY: Humana, 2009; 51–86. [Google Scholar]
- 10.McCluggage WG, Sumathi VP, McBride HA, Patterson A. A panel of immunohistochemical stains, including carcinoembryonic antigen, vimentin, and estrogen receptor, aids the distinction between primary endometrial and endocervical adenocarcinomas. Int J Gynecol Pathol 2002;21(1):11–15. [DOI] [PubMed] [Google Scholar]
- 11.Castrillon DH, Lee KR, Nucci MR. Distinction between endometrial and endocervical adenocarcinoma: an immunohistochemical study. Int J Gynecol Pathol 2002;21(1):4–10. [DOI] [PubMed] [Google Scholar]
- 12.Kamoi S, AlJuboury MI, Akin MR, Silverberg SG. Immunohistochemical staining in the distinction between primary endometrial and endocervical adenocarcinomas: another viewpoint. Int J Gynecol Pathol 2002;21(3):217–223. [DOI] [PubMed] [Google Scholar]
- 13.Liao CL, Lee MY, Tyan YS, et al. Progesterone receptor does not improve the performance and test effectiveness of the conventional 3-marker panel, consisting of estrogen receptor, vimentin and carcinoembryonic antigen in distinguishing between primary endocervical and endometrial adenocarcinomas in a tissue microarray extension study. J Transl Med 2009;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCluggage WGF, Jenkins DF. p16 immunoreactivity may assist in the distinction between endometrial and endocervical adenocarcinoma. Int J Gynecol Pathol 2003;22(3):231–235. [DOI] [PubMed] [Google Scholar]
- 15.Manfredi R, Mirk P, Maresca G, et al. Local-regional staging of endometrial carcinoma: role of MR imaging in surgical planning. Radiology 2004;231(2):372–378. [DOI] [PubMed] [Google Scholar]
- 16.Hricak H, Stern JL, Fisher MR, Shapeero LG, Winkler ML, Lacey CG. Endometrial carcinoma staging by MR imaging. Radiology 1987;162(2):297–305. [DOI] [PubMed] [Google Scholar]
- 17.Hricak H, Rubinstein LV, Gherman GM, Karstaedt N. MR imaging evaluation of endometrial carcinoma: results of an NCI cooperative study. Radiology 1991;179(3):829–832. [DOI] [PubMed] [Google Scholar]
- 18.Barwick TD, Rockall AG, Barton DP, Sohaib SA. Imaging of endometrial adenocarcinoma. Clin Radiol 2006;61(7):545–555. [DOI] [PubMed] [Google Scholar]
- 19.Joja I, Asakawa M, Asakawa T, et al. Endometrial carcinoma: dynamic MRI with turbo-FLASH technique. J Comput Assist Tomogr 1996;20(6):878–887. [DOI] [PubMed] [Google Scholar]
- 20.Subak LL, Hricak H, Powell CB, Azizi L, Stern JL. Cervical carcinoma: computed tomography and magnetic resonance imaging for preoperative staging. Obstet Gynecol 1995;86(1):43–50. [DOI] [PubMed] [Google Scholar]
- 21.Hori M, Kim T, Murakami T, et al. Uterine cervical carcinoma: preoperative staging with 3.0-T MR imaging—comparison with 1.5-T MR imaging. Radiology 2009;251(1):96–104. [DOI] [PubMed] [Google Scholar]
- 22.Hricak H, Lacey CG, Sandles LG, Chang YC, Winkler ML, Stern JL. Invasive cervical carcinoma: comparison of MR imaging and surgical findings. Radiology 1988;166(3):623–631. [DOI] [PubMed] [Google Scholar]
- 23.Kim SH, Choi BI, Lee HP, et al. Uterine cervical carcinoma: comparison of CT and MR findings. Radiology 1990;175(1):45–51. [DOI] [PubMed] [Google Scholar]
- 24.Hricak H, Gatsonis C, Chi DS, et al. Role of imaging in pretreatment evaluation of early invasive cervical cancer: results of the intergroup study American College of Radiology Imaging Network 6651-Gynecologic Oncology Group 183. J Clin Oncol 2005;23(36):9329–9337. [DOI] [PubMed] [Google Scholar]
- 25.Creasman W. Revised. FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet 2009;105(2):109. [DOI] [PubMed] [Google Scholar]
- 26.Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. Int J Gynaecol Obstet 2009;105(2):107–108. [DOI] [PubMed] [Google Scholar]