Abstract
Background
Osseointegrated prosthetic implants are biocompatible metal devices that are inserted into the residual bone to integrate with the bone and attach to the external prosthesis, eliminating the need for socket prostheses and the problems that may accompany their use. We conducted a health technology assessment of osseointegrated prosthetic implants, compared with conventional socket prostheses, for people with lower-limb amputation who experience chronic problems with their prosthetic socket, leading to prosthesis intolerance and reduced mobility. Our analysis included an evaluation of effectiveness, safety, cost-effectiveness, the budget impact of publicly funding osseointegrated prosthetic implants, and patient preferences and values.
Methods
We performed a systematic literature search of the clinical evidence on the safety and effectiveness of the latest iterations of three implant systems: the Osseointegrated Prostheses for the Rehabilitation of Amputees (OPRA) Implant System, the Endo-Exo-Femur-Prosthesis, and the Osseointegration Group of Australia–Osseointegration Prosthetic Limb (OGAP-OPL). We assessed the risk of bias of individual studies and determined the quality of the body of evidence according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. We performed a systematic economic literature search and conducted a cost–utility analysis with a lifetime horizon from a public payer perspective. We also analyzed the net budget impact of publicly funding osseointegrated prosthetic implants in Ontario. To contextualize the potential value of osseointegrated prosthetic implants, we spoke with people with lower-limb amputations.
Results
We included nine studies in the clinical evidence review. All studies included patients with above-the-knee amputation who underwent two-stage surgery and mostly had short-term follow-up. With osseointegrated prosthetic implants, scores for functional outcomes improved significantly as measured by 6-Minute Walk Test (6MWT), Timed Up and Go (TUG) test, and Questionnaire for Persons with a Transfemoral Amputation (Q-TFA). The scores for quality of life measured by SF-36 showed significant improvement in the physical component summary but a nonsignificant decline for the mental component summary. The most frequently seen adverse event was superficial infection, occurring in about half of patients in some studies. Deep or bone infection was a serious adverse event, with variable rates among the studies depending on the length of follow-up. The treatment of deep or bone infection required long-term antibiotic use, surgical debridement, revision surgery, and implant extraction in some cases. Other adverse events included femoral bone fracture, implant breakage, issues with extramedullary parts that required replacement, and implant removal. Our assessment of the quality of the clinical evidence according to the GRADE criteria found low certainty in terms of improvement in functional outcomes, low certainty for quality of life, and high certainty of an increase in adverse events; all findings compared receiving an osseointegrated prosthetic implant with not receiving an osseointegrated prosthetic implant.
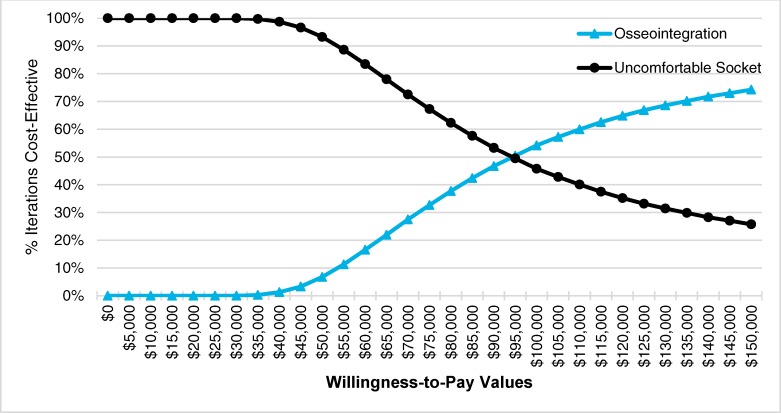
In our economic model, osseointegrated prosthetic implants were found to be more effective and more expensive than having people remain users of an uncomfortable socket prosthesis. Our best estimate of the incremental cost-effectiveness ratio (ICER) for osseointegration, compared with an uncomfortable socket, was $94,987 per quality-adjusted life-year (QALY) gained. The probability of osseointegration being cost-effective was 54.2% at a willingness-to-pay value of $100,000 per QALY gained. The annual net budget impact of publicly funding osseointegrated prosthetic implants in Ontario over the next 5 years, for a small population of eligible candidates, would range from $1.5 million in year 1 to $0.6 million in year 5, for a 5-year total of $5.3 million.
We interviewed 13 people with a lower-limb amputation; nine had experience with both a conventional socket prosthesis and an osseointegrated prosthetic implant, three had experience with a conventional socket prosthesis only, and one had only recently undergone amputation and had not yet chosen a prosthesis. People who had received an osseointegrated prosthetic implant said they had better mobility and quality of life than before receiving this implant but had concerns about the ongoing risk of infection and potential for problems with implant maintenance. People using a conventional socket prosthesis said cost was the only factor preventing them from undergoing an osseointegration procedure.
Conclusions
In the studies included in the clinical evidence review, most people who received osseointegrated prosthetic implants were followed for only a few years. Studies showed that functional outcomes and physical ability improved with osseointegrated prosthetic implants (GRADE: Low), but there was uncertainty about the impact of these implants on people's emotional health (GRADE: Low). Osseointegrated prosthetic implants can lead to serious adverse events such as bone infection and bone fracture in some patients, which may require additional surgeries (GRADE: High). The reference case of the primary economic evaluation represented a conservative estimate of cost-effectiveness and found osseointegration may be cost-effective, but there is a large degree of uncertainty given parameter uncertainty and the need to use proxy costs. Scenario analyses explored potential variations in approaches to modelling and parameter selection. Qualitative interviews with people with a lower-limb amputation and caregivers underscored the challenges of conventional socket prostheses, but cost remains an important barrier to pursuing osseointegrated prosthetic implantation.
OBJECTIVE
This health technology assessment evaluates the effectiveness, safety, and cost-effectiveness of osseointegrated prosthetic implants, compared with conventional socket prostheses, for people with a lower-limb amputation. It also evaluates the budget impact of publicly funding osseointegrated prosthetic implants for people with a lower-limb amputation and the experiences, preferences, and values of people with a lower-limb amputation.
BACKGROUND
Health Condition
The loss of a limb is a traumatic life event that can dramatically affect quality of life for amputees and their families. In Canada, diabetes is the main cause of lower-limb amputations (65.4%), followed by other vascular diseases and infection (25.6%), trauma (6%), cancer (1.8%), and congenital defects (0.6%).1 Above-the-knee (transfemoral) and below-the-knee (transtibial) amputations are performed in 23.9% and 30.9%, respectively, of all lower-limb amputations.1
In Canada, 44,430 lower-limb amputations were performed between April 1, 2006, and March 31, 2012, including 16,724 (37.7%) in Ontario.1 The mean age of people with these amputations was 65.7 years, and 68.8% were males. Most (54.9%) occurred among people 50 to 74 years old; 31% were in people 75 years of age or older; and 14.1% were performed in people younger than 50.
Clinical Need, Target Population, and Current Treatment Options
Following lower-limb amputation, most people are fitted with a customized prosthesis (also called a prosthetic limb) to regain mobility and independence. The prosthetic limb attaches to the residual limb (the remaining natural leg) with the aid of a socket, which fits around the residual limb. The socket is secured by a suspension system.
The effectiveness and comfort of a lower-limb prosthesis depend largely on how well the socket fits onto the residual limb. An uncomfortable socket can cause blisters, cysts, dermatitis, or skin breakdown, making it painful to walk. It can also alter the distribution of load (body weight) on the residual limb, which can affect the person's balance and contribute to falls. Fluctuations in the volume of the residual limb is another major concern for people who use a prosthesis. Loss of residual limb volume can lead to improper distribution of body weight and poor fit of the socket. As the residual limb shrinks, the socket may compress tissue that is not meant to support body weight, potentially leading to skin breakdown.2
People with lower-limb amputation often develop musculoskeletal pathologies as secondary complications. If the socket becomes loose or unstable, the person may compensate by walking in abnormal ways, but this tendency places greater forces on the intact limb, causing degenerative arthritis, joint pain, and back pain. Gailey et al3 found that the risk of degenerative joint disease and back pain increases after amputation, with higher prevalence in people with above-the-knee than below-the-knee amputation. A Swedish study investigated problems with the socket prosthesis experienced by people with unilateral above-the-knee amputation due to nonvascular causes. Common problems were pain (51%), back pain (47%), pain in the other leg (46%), sores or skin irritation (62%), inability to walk quickly (59%), inability to walk in woods and fields (61%), heat and sweating in the socket (72%), and phantom pain (48%).4
Trained prosthetists (professionals who fit artificial limbs) can help in solving many of the problems with the socket by using technological solutions to make adjustments and alter the distribution of stress on the residual limb. They can check whether load is evenly distributed across the residual limb and determine if the person has problems with gait (their manner of walking). Prescription of socket prostheses is a multidisciplinary effort that involves professionals such as prosthetists, physiatrists (specialists in rehabilitation medicine), and physiotherapists in making decisions about fabricating personalized sockets that are functional and convenient.
Some people may continue to experience issues with their socket, which can limit the use of their prosthesis, even after all adjustments have been made. These people may consider undergoing surgery to receive an osseointegrated prosthetic implant. Based on clinical expert opinion (Nancy Dudek, MD, e-mail communication, November 2018), we estimate that about 7 above-the-knee amputations in Ontario would be eligible for osseointegrated prosthetic implant surgery.
Health Technology Under Review
Osseointegrated prosthetic implants are biocompatible metal devices that are surgically inserted into the remaining bone to connect the bone to an artificial limb. The term osseointegration refers to a strong connection between the living bone and the outer surface of the implant so that the two act as one component, similar to some other orthopedic procedures such as total hip replacement. For people with lower-limb amputation, osseointegrated prosthetic implants have some biomechanical advantages over the socket prosthesis. The primary advantage is that an implant allows forces (such as from walking) to be transferred directly to the bone, whereas in a socket prosthesis these forces are also transferred to the soft tissue.
One disadvantage of the osseointegrated prosthetic implant is the risk of infection. Soft-tissue integration at the skin–implant interface relies on the formation of a seal to prevent bacteria from adhering to the junction. Lack of a seal increases susceptibility to infection. Infection may affect only the superficial soft tissue, but it can also involve deeper structures and cause osteitis and osteomyelitis (infections in the bone).
A bone of good quality is the most important factor in promoting osseointegration.
Osseointegration typically occurs in the bone as long as it is not prevented from ingrowth, which may result, for example, from chemotherapy and some medications. When osseointegration does not occur, resulting in implant loosening, the intramedullary component can be removed and replaced with a new implant. This is called a revision surgery; during this surgery, a small amount of cortical bone is removed from the inner cortical wall.
Osseointegrated prosthetic implants are not currently recommended for people whose amputation was due to a vascular cause such as diabetes, which can compromise the osseointegration process or the ability to control infection. People with vascular amputations are generally older and have multiple health issues, whereas those with amputations due to trauma, tumour, or congenital malformation tend to be younger at the time of amputation.5
Earlier approaches to the osseointegrated procedure and earlier versions of the implants had issues related to high rates of infection and implant failure.6 Recent advances in implant design and modifications in surgical techniques have reduced these concerns and improved outcomes of osseointegration procedures. We reviewed the following osseointegrated prosthetic implants with published evidence on their safety and effectiveness, and we evaluated only the newest iterations of these implants:
Osseointegrated Prostheses for the Rehabilitation of Amputees (OPRA) Implant System, Integrum AB, Mölndal, Sweden
ESKA Endo-Exo Femur-Prosthesis, ESKA Orthopaedic, Lübeck, Germany; also known as Integral Leg Prosthesis (ILP)
Osseointegration Group of Australia–Osseointegration Prosthetic Limb (OGAP-OPL), Permedica, s.p.a, Milan, Italy
The current method of implantation often involves two surgery sessions, separated by a period of time, both under general anesthesia. More recently, a single-stage procedure, in which the two operations are performed during a single surgical session, has occurred in some centres, and the results have been published as abstracts.8,8 One abstract suggested that patients need optimal bone quality and good compliance to be eligible for the single-stage surgery.8
So far, clinical data have been published only for the two-stage surgery. The recommended time between the first and the second surgery varies among the implant systems, as does the time when the patient is allowed to fully load the prosthesis6,9,10; procedure and recovery times also take into account the individual patient and the quality of their residual bone.
Osseointegrated Prostheses for the Rehabilitation of Amputees (OPRA) Implant System
The idea for and design of an osseointegrated prosthetic implant for limb prostheses were based on the successful experiments with osseointegrated dental implants pioneered by Per-Ingvar Branemark.11 The first human experiment of a lower-limb implant was performed in Sweden in 1990 in a bilateral above-the-knee amputee.12 The treatment protocol, including surgical and rehabilitation procedures, was standardized in 1999, and clinical investigation for the OPRA protocol started in Sweden the same year.13 Today, the OPRA implant has been used in above-the-knee amputees for more than 20 years and is available in at least 12 countries.12 The OPRA implant is manufactured by Swedish manufacturer Integrum, which is partnering with Ottobock for distribution of prosthetic connection elements related to Integrum's OPRA implant system.
The system has three main components: a fixture that is implanted into the femoral bone, an abutment that is press-fit (fastened through friction) into the distal end of the fixture, and an abutment screw. The abutment attaches the fixture to the prosthetic limb. During the first surgery, the fixture piece is inserted into the femoral bone. The fixture is a metal rod with an outer surface threaded like a screw. The screw shape increases the surface area between the implant and the bone to promote osseointegration and enhance stability. The implant gradually integrates into the bone as the bone undergoes its natural process of remodelling.
The two stages of surgery are separated by approximately 6 months to allow osseointegration to take place. However, 6 months is considered the healing period for patients with the most unsuitable bone, and it can be shorter in patients with good-quality bone.8 During the healing period, the fixture remains unloaded to allow osseointegration to take place, but patients are allowed to use their socket prosthesis.8 During the second stage, the abutment is attached to the distal end of the fixture and secured with the abutment screw. The surgeon also refashions soft tissue during this procedure to reduce the risk of infection.
Since its introduction, the OPRA implant system has undergone several design changes, including enhancements in material from commercially pure titanium to the medical-grade titanium alloy, Ti6Al4V. The surgical technique has also improved to address the problem of distal bone resorption and to reduce the risk of infection in the bone–fixture interface.12 To avoid the risk of fracture, a safety device was added between the abutment and the limb prosthesis. In case of excessive load, the safety device automatically releases the connection with the external prosthesis.12
Endo-Exo-Femur-Prosthesis (Integral Leg Prosthesis, ILP)
The ILP implant was designed by Staubach and Grundei14 and first produced in Germany in 1999.9 It was first used in an 18-year-old man with above-the-knee amputation.14 Since its introduction, the implant has gone through several design iterations. Today, it is manufactured by ESKA Orthopaedic, located in Lübeck, Germany, and is also available in Netherlands and Australia. The implant is made of a cobalt-chromium-molybdenum alloy, sealed with a titaniumniobium layer, and has a press-fit design that encourages bone to grow on the surface of the implant.
This implant requires two surgery sessions separated by 6 to 8 weeks.9 In the first stage, the implant stem is press-fit into the femoral bone. In the second stage, the bridge component is attached. After the second surgery, care by a prosthetist starts and the patient strengthens the skeleton and muscles by gradually increasing the load on the prosthesis. Approximately 4 to 6 weeks after the second surgery, full weight-bearing is allowed if the patient has no other medical issues.6,9
Osseointegration Group of Australia–Osseointegration Prosthetic Limb (OGAP-OPL)
The OGAP-OPL was developed in Australia in 2013 by Al Muderis and his colleagues. Its design is similar to the ILP, with a press-fit design. The differences between the ILP and the OGAP-OPL include a material change to titanium (Ti6Al4V), the introduction of 1-mm sharp longitudinal splines at the proximal end of the implant (closer to the centre of the body), and a plasma-sprayed rough titanium coating, instead of the microporous surface.12 The two-stage procedure is separated by 4 to 6 weeks. A single-stage protocol, known as OGAAP-2, has been developed but no evidence on its safety and effectiveness is currently available through full-text publication.
Other Systems in Development
Several other osseointegrated prosthetic implant systems are currently at the stage of experiment in humans:
Intraosseous Transcutaneous Amputation Prosthesis (ITAP), Stanmore Implants Worldwide, Waterford, United Kingdom
Percutaneous Osseointegrated Prosthesis (POP), DJO Global, Austin, Texas, United States
Compress, Zimmer Biomet, Warsaw, Indiana, United States
The Keep Walking Advanced, Tequir S.L., Valencia, Spain
Regulatory Information
None of the osseointegrated prosthetic implants has received approval from Health Canada (email communication, February 2019). However, Health Canada's Special Access Programme may, on request, authorize a physician to use a medical device that has not been approved for sale in Canada. Two osseointegration prosthetic implantation procedures have been performed in Canada through the Health Canada Special Access Programme (Robert E. Turcotte, MD, email communication, November 2018).
The OPRA implant system has been granted the European certification mark, CE, in Europe for 18 years and was approved by the US Food and Drug Administration (FDA) in July 2015.15 The FDA has approved the implant for patients with above-the-knee amputation due to trauma or cancer who cannot use or have problems using a conventional socket prosthesis. The FDA has approved the OPRA implant system based on two surgical stages, and their approval was for use only with the prosthetic components manufactured by Ottobock. Use of other than the specified manufactured components is considered off-label use of the device.15
The FDA lists the following as contraindications to using the OPRA implant:
Incomplete skeletal growth
Atypical skeletal anatomy that may affect treatment with OPRA
When the patient would have less than 2 mm of remaining cortical bone available around the implant, if implanted
Osteoporosis
Age older than 65 years or younger than 22 years
Body weight more than 220 pounds, including the prosthesis
Pregnancy
When the patient is not expected to be able to comply with the treatment and follow-up requirements
Severe peripheral vascular disease
Diabetic mellitus with complications
Skin disorders involving the residual extremity
Neuropathy or neuropathic disease and severe phantom pain
Active infection or dormant bacteria
The FDA considers osseointegrated prosthetic implants to be Class III devices, which require the highest degree of control to ensure the device is safe and effective.16 Post-approval requirements include yearly reports from the manufacturer.
The other two osseointegrated prosthetic implants (ILP and OGAP-OPL) are in use outside of the United States and have not yet been approved by the FDA.
Ontario Context
Currently, osseointegration surgery is not offered in Ontario. In 2018, a surgeon in a private multidisciplinary clinic in Montreal performed the first osseointegrated prosthetic implant surgery in Canada, using devices made available through Health Canada's Special Access Programme (Robert E. Turcotte, MD, email communication, November 2018).
Orthotics Prosthetics Canada, a national professional organization, has reported that, as of November 2017, 26 Canadian amputees have undergone osseointegrated prosthetic implant surgery abroad.17 Countries in which the surgery is performed include Sweden, Germany, the Netherlands, Australia, and the United States. A few Ontarians with above-the-knee amputation have travelled to Australia to receive their implant, either privately or through the Out-of-Country Prior Approval Program of the Ontario Health Insurance Plan (OHIP). The cost of the procedure for each person was about $100,000 AUD (approximately $95,000 CAD).
Expert Consultation
We consulted with experts in the specialty areas of orthopedic surgery, physical medicine and rehabilitation, physiotherapy, and prosthetics to help inform our understanding of aspects of the health technology and our methodologies and to contextualize the evidence.
PROSPERO Registration
This health technology assessment has been registered in PROSPERO, the international prospective register of systematic reviews (CRD 42018102032), available at https://www.crd.york.ac.uk/PROSPERO.
CLINICAL EVIDENCE
Research Question
What are the clinical effectiveness and safety of osseointegrated prosthetic implants, compared with conventional socket prostheses, for people with lower-limb amputation who have chronic problems using a socket prosthesis?
Methods
Clinical Literature Search
We performed a clinical literature search on June 5, 2018, to retrieve studies published from database inception until the search date. We used the Ovid interface to search the following databases: MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, the Health Technology Assessment database, and the National Health Service Economic Evaluation Database (NHS EED).
A medical librarian developed the search strategies using controlled vocabulary (e.g., Medical Subject Headings) and relevant keywords. The final search strategy was peer-reviewed using the PRESS Checklist.18
We created database auto-alerts in MEDLINE and Embase and monitored them for the duration of the assessment period. We also performed a targeted grey literature search of health technology assessment agency websites as well as clinical trial and systematic review registries. See Appendix 1 for our literature search strategies, including all search terms.
Eligibility Criteria
Studies
Inclusion Criteria
English-language full-text publications
Studies published from inception until the search date
Randomized controlled trials, cohort studies, cross-sectional studies, and case series investigating safety and effectiveness of osseointegrated prosthetic implants
Exclusion Criteria
Studies that did not report any of the outcomes of interest for this review
Abstracts, case reports, editorials, commentaries, narrative reviews, letters
Animal and in vitro studies
Participants
Adults with lower-limb amputation due to nonvascular causes who have problems with the use of socket prosthesis or cannot use a conventional socket prosthesis
Interventions
Osseointegrated prosthetic implants for lower-limb amputation with published evidence on the safety and effectiveness of the most recent iteration of the device
Osseointegrated prosthetic implants not combined with total hip replacement and total knee replacement
Outcome Measures
Functional Outcomes and Health-Related Quality of Life
6-Minute Walk Test (6MWT)
Timed Up and Go (TUG)
Amputee Mobility Predictor (AMP)
Range of motion
Questionnaire for Persons with a Transfemoral Amputation (Q-TFA)
36-Item Short Form Health Survey (SF-36)
Harm Outcomes
Superficial infection
Deep infection
Bone infection
Bone fracture
Implant removal
Reimplantation
Intramedullary breakage
Extramedullary mechanical issues
Noninfectious soft tissue and bone complications
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts using the DistillerSR management software and then obtained the full texts of studies that appeared eligible for review according to the inclusion criteria. A single reviewer then examined the full-text articles and selected studies eligible for inclusion. The reviewer also examined reference lists for any additional relevant studies not identified through the search.
Data Extraction
We extracted relevant data using a data form to collect information about the following:
Source (i.e., author, year, country, number of participants, number of implants, number of lower-limb amputations, age, gender, reason for amputation)
Methods (i.e., study design, study period, duration of follow-up)
-
Outcomes:
-
–
Functional outcomes and health-related quality of life (i.e., 6MWT, TUG, AMP, ROM, Q-TFA, SF-36)
-
–
Adverse events (i.e., superficial infection, deep infection, bone infection, bone fracture, implant removal, reimplantation, intramedullary breakage, extramedullary mechanical issues, noninfectious soft tissue and bone complications), time points at which adverse events occurred and intervention for treatment of adverse events
-
–
Risk of bias
Statistical Analysis
Performing a meta-analysis by pooling data for each outcome was not possible because of substantial variation in duration of follow-up, implant systems, and treatment protocols among the studies. Instead, we did a qualitative synthesis of the included studies, summarizing outcomes in tables. We compared functional and quality-of-life outcomes where reported for before and after surgery, stratifying outcomes by implant type and treatment protocol. For harm outcomes, we used the reported data to calculate the proportion of patients who experienced each type of adverse event.
Critical Appraisal of Evidence
We assessed risk of bias using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool.19 We evaluated the quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Handbook.20 The body of evidence was assessed based on the following considerations: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The overall rating reflects our certainty in the evidence.
Results
Literature Search
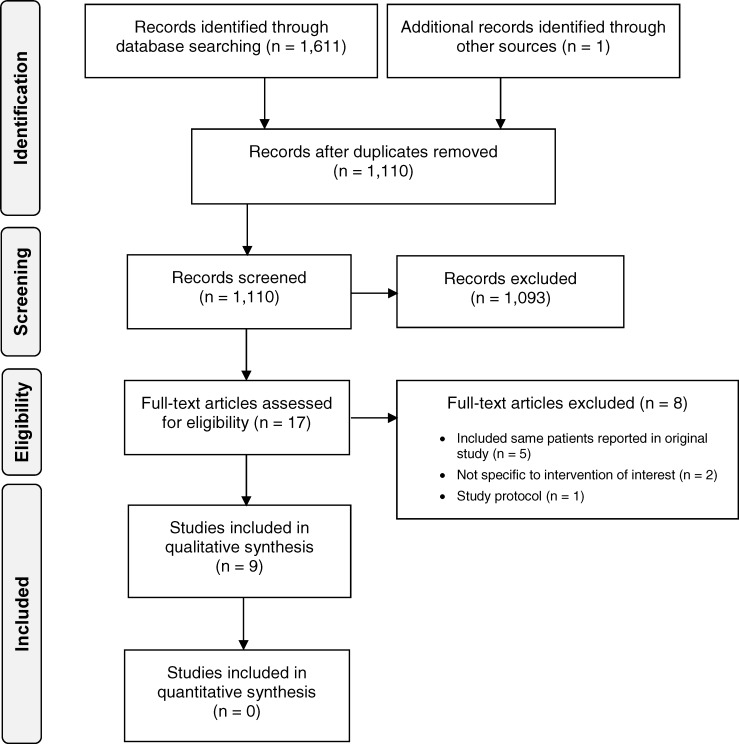
The clinical literature search yielded 1,110 citations published from database inception until June 5, 2018, after removing duplicates. We identified one additional study from the grey literature search. No relevant health technology assessments or systematic reviews were identified. Previous systematic reviews either did not report on both safety and effectiveness,21,22 included all types of implant,23 or had different inclusion criteria from ours.24 In addition, most previous systematic reviews included some studies with the same patient populations. From the 17 studies we reviewed, two reported on a device out of scope for this review, one was a protocol for an ongoing study,7 and five included the same patients reported in other studies and did not report new outcomes.9,13,25–27 (See Appendix 2 for a list of studies excluded after full-text review.) Therefore, nine studies met the inclusion criteria. Figure 1 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the clinical literature search.
Figure 1: PRISMA Flow Diagram—Clinical Search Strategy.
Source: Adapted from Moher et al.28
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Characteristics of Included Studies
Nine studies met our eligibility criteria. Table 1 shows study design and patient characteristics for all studies included in this review. Four studies from Sweden reported on the OPRA (Osseointegrated Prostheses for the Rehabilitation of Amputees) implant system.10,29–31 Three studies—one each from Germany, Netherlands, and Australia—reported on the ILP (Integrated Leg Prosthesis) system.6,32,33 One study from Australia reported on patients who received either the ILP or OGAP-OPL implant (Osseointegration Group of Australia–Osseointegration Prosthetic Limb),34 and another study reported on patients who received the OGAP-OPL implant in Australia.35
Table 1:
Characteristics of Included Studies, by Implant System
| Author, Year | Country | Study Design | Study Period | Patients (Implants), N | Bilateral Amputation, N (%) | Age, Mean (Range), Y | Male, N (%) | Reason for Amputation, N (%) | Reported Outcomes | Follow-Up, Mean (Range), Y |
|---|---|---|---|---|---|---|---|---|---|---|
| OPRA Implant System | ||||||||||
| Tillander et al, 201031,a | Sweden | Prospective case series | Jan 2005–Jun 2005 | 39 (45) TFA: 32 (33) TTA: 1 (1) Arm: 6 (11) |
TFA: 1 (3) | 49.3 (28–74) | 21 (54) | Trauma/tumour: 39 (100) | Risk of infection | 3 (NR) |
| Branemark et al, 201410 | Sweden | Prospective case series: single centre | 1999–2007 | 51 TFA (55); consecutive patients | 6 (11.8) | 44 (20–65) | 28 (55) | Trauma: 33 (65) Tumour: 12 (24) Other: 6 (11) |
Q-TFA SF-36 Implant survival Adverse events |
2 (for all) |
| Tillander et al, 201730,b | Sweden | Retrospective case series | May 1990–Jan 2010 | 96 TFA (102); 28% were treated before standardized OPRA protocol | 6 (6.3) | 43.5 (19–65) | 60 (62.5) | Trauma: 71 (70) Tumour: 20 (19) Ischemia: 5 (5) Infection: 5 (5) Other: 1 (1) |
Risk of osteomyelitis Extraction due to osteomyelitis |
7.9 (1.5–19.6) |
| Hagberg, 201829 | Sweden | Retrospective case series | 1990–2015 | 12 TFA (22) | 12 (100) | 35 (19–62) | 9 (75) | Trauma: 12 (100) | Prosthetic use Adverse events | Median: 7 (1–20) |
| ILP | ||||||||||
| van de Meent et al, 201333 | Netherlands | Prospective case series | May 2009–May 2011 | 22 TFA (22) | 1 (4.5) | 46.5 (23–67) SD 10.7 | 18 (82) | Trauma: 20 Tumour: 2 |
Q-TFA 6MWT TUG |
1 (for all) |
| Juhnke et al, 20156 | Germany | Retrospective case series | Jan 2009–Dec 2013 | 39 TFA (42) | 3 (7.7) | 45 (24–76) SD 12 | 31 (79) | Trauma, 28 (72) Tumour: 2 (5) Infection: 2 (5) Other: 7 (18) |
Adverse events | 2.7 (0.08–4.9) |
| Al Muderis et al, 201632,c | Australia, Netherlands | Prospective case series | May 2009–May 2013 | 86 TFA (91): Australia: 44 Netherlands: 42 | 5 (5.8): Australia: 3 Netherlands: 2 | 48 (25–81) SD 14 | 65 (76) | Trauma: 65 (76) Tumour: 11 (13) Infection: 8 (9) Other: 2 (2) |
Adverse events | Median: 2.8 (2–6) |
| ILP and OGAP-OPL | ||||||||||
| Al Muderis et al, 201634,d | Australia | Prospective case series | Mar 2011–Jun 2014 | 50 TFA (50) | 0 (0) | 48.4 (24–73) | 34 (68) | Trauma: 35 (70) Infection: 5 (10) Other: 10 (20) |
Q-TFA SF-36 6MWT TUG Adverse events |
1.8 (NR) after S1 |
| OGAP-OPL | ||||||||||
| Al Muderis et al, 201735,e | Australia | Retrospective case series | Dec 2013–Nov 2014 | 22 TFA (22) | 0 (0) | 46.2 (20–67) | 17 (77) | Trauma: 16 Neoplasia: 4 Infection: 2 |
Q-TFA 6MWT TUG Adverse events |
1.2 (0.8–2.5) |
Abbreviations: ILP, Integral Leg Prosthesis; NR, not reported; OGAP-OPL, Osseointegration Group of Australia–Osseointegration Prosthetic Limb; OPRA, Osseointegrated Prostheses for the Rehabilitation of Amputees; Q-TFA, Questionnaire for Persons with a Transfemoral Amputation; S1, stage-1 surgery; SD, standard deviation; SF-36, 36-Item Short Form Health Survey; TFA, transfemoral (above-the-knee) amputation; TTA, transtibial (below-the-knee) amputation; TUG, Timed Up and Go Test; Y, years; 6MWT, 6-Minute Walk Test.
Recruited patients who attended the osseointegration clinic for regular and emergency visits during a 6-month period and followed them for a mean of 3 years.
Includes patients before the OPRA protocol, during the study of the OPRA implant with OPRA protocol,10 and after that. The study reported long-term risk of osteomyelitis.
Includes patients from the study by van de Meent et al33 but the two studies reported different outcomes.
Overlapped patients who received ILP in Australia.32
Overlapped patients who received OGAP-OPL in a previous study.34
All published studies reported on two-stage surgery in patients with above-the-knee amputation. We did not find any studies on single-stage surgery. Also, we did not identify any study that investigated the outcomes of osseointegrated prosthetic implants in below-the-knee amputation; only one study included one patient with below-the-knee amputation.31
Four studies had a retrospective design,6,29,35,30 and, in five studies, data were collected prospectively.10,31–34 The mean or median duration of follow-up ranged from 1 year to 3 years among most studies. One study had a follow-up of 7.9 years but only reported on the risk of osteomyelitis.30 In each study, more than two-thirds of patients had amputation due to trauma. Tumour was the second most frequent cause of amputation.
Two Australian studies included only patients with unilateral amputation,34,35 and one Swedish study included only patients with bilateral amputation.29 The remaining studies included some patients with bilateral amputation, ranging from 3%31 to 11.8%.10 Since bilateral amputation could have influenced the outcomes, due to patients having an additional restrictive condition, we presented the results of the study on bilateral amputation in a separate table.
Methodologic Quality of the Included Studies
Appendix 3 shows the results of our assessment of risk of bias using the ROBINS-I instrument and the strength of the evidence according to the GRADE criteria for each outcome. Three studies showed risk of bias. One study33 did not report all subscales of the Q-TFA, and another34 did not report all subscales of the Q-TFA and SF-36. In another study,35 selection of participants was not clearly reported, and functional outcomes and quality of life were shown only in graphs (Appendix 3, Table A1).
Functional Outcomes
Two studies reported scores for the 6-Minute Walk Test and the Timed Up and Go test.33,34 Both studies reported significant improvement in scores (Table 2). The 6MWT, which measures the distance a person can walk in a 6-minute period, has been shown to reliably measure functional capacity in various populations, including amputees.36,37 The TUG test is a measure of function that correlates with balance and risk of fall. It is a reliable, valid test for quantifying functional mobility and may also be useful in following clinical change over time. The test is quick (it measures the time a person takes to rise from a chair, walk 3 metres, walk back, and sit down), requires no special equipment or training, and is easily included as part of the routine medical examination.38 Originally created to test basic mobility skills of frail elderly people, the TUG test has also been used to measure function in other populations, including people with arthritis, stroke, vertigo, and lower-limb amputation. The TUG test is interpreted as follows:
Table 2:
Functional Outcomes—6-Minute Walk Test and Timed Up and Go
| Author, Year | Follow-Up, Years | 6MWT Score, Mean (SD), Metres | TUG Score, Mean (SD), Seconds |
|---|---|---|---|
| van de Meent et al, 201333 | 1 | Preoperative: 321 (28) Follow-up: 423 (21) P = .002 |
Preoperative: 15.1 (2.1) Follow-up: 8.1 (0.7) P = .002 |
| Al Muderis et al, 201634 | Mean: 1.8 (after S1) |
Wheelchair user Preoperative: NR Follow-up: 411 (31.44) P = NR |
Wheelchair user Preoperative: NR Follow-up: 9.0 (0.56) P = NR |
| Prosthesis user | Prosthesis user | ||
| Preoperative: 281 (93) | Preoperative: 14.59 (5.94) | ||
| Postoperative: 419 (133) | Postoperative: 8.74 (2.81) | ||
| P < .001 | P < .01 |
Abbreviations: 6MWT, 6-Minute Walk Test; NR, not reported; S1, stage-1 surgery; SD, standard deviation; TUG, Timed Up and Go.
≤ 10 seconds = normal
≤ 20 seconds = good mobility, can go out alone, mobile without a gait aid
< 30 seconds = problems, cannot go outside alone, requires a gait aid
No studies reported on Amputee Mobility Predictor. However, one study reported on K-levels based on previously reported AMP scores; K-level improved in 60% of patients and was unchanged in 40% (P = .001).34 K-levels are a rating system used by the US Medicare health insurance program to indicate the extent of a person's disability and their potential for rehabilitation. The K-levels system has a rating from 0 (no potential to walk independently, even with a prosthesis) to 4 (exceeds basic ambulation skills).36 This system has often been used in the literature to validate various outcome measures, such as the AMP.
The AMP instrument is a reliable, valid measure for the assessment of mobility in people with lower-limb amputation, with or without using a prosthesis (AMPPRO and AMPnoPRO, respectively). It can therefore be used before prosthetic fitting to predict functional ability after prosthetic fitting. For bilateral above-the-knee amputees, only the AMPPRO can be used because it is not physically possible for them to perform the AMPnoPRO.
Three studies reported data from the Questionnaire for Persons with a Transfemoral Amputation, a self-reported outcome measure designed for above-the-knee amputees (Table 3). The Q-TFA reflects four domains: prosthetic use, mobility, problems, and global health.39 It was developed to study outcomes in nonelderly people who received an osseointegrated prosthetic implant after previously using a socket prosthesis. Prosthetic use is calculated as the number of days per week the person normally wears their prosthesis, multiplied by the number of hours it is used each day. A score of 100 means the prosthesis is used 7 days a week for more than 15 hours per day. The mobility score is the average of three subscores: capability, use of walking aid, and walking habits. A score of 100 indicates the best possible prosthetic mobility. The problem score measures specific problems related to the amputation and prosthesis and their impact on quality of life; a higher score indicators more serious problems (unlike the other domains). The global health score measures the person's perception of their functional ability, any problems with the prosthesis, and their overall circumstances. A score of 100 indicates the best possible overall situation.
Table 3:
Functional Outcomes—Questionnaire for Persons with Transfemoral Amputation
| Author, Year | Follow-Up, Years | Prosthetic Usea | Mobility | Problemsb | Global Health | Overall Situation, N (%) |
|---|---|---|---|---|---|---|
| OPRA Implant System | ||||||
| Branemark et al, 201410 | 2 (all patients) |
Baseline score Mean (range): 47 (0–100) Mean change (range) 32 (−100 to 100) P < .001 |
Baseline score Mean (range): 52 (0–82) Mean change (range) 18 (−29 to 48) P < .001 |
Baseline score Mean (range): 44 (5–77) Mean change (range): −27 (−59 to 7) P < .001 |
Baseline score Mean (range): 38 (0–92) Mean change (range) 39 (0 to 92) P < .001 |
Baseline Extremely poor/poor: 20 (39) Average: 17 (33) Extremely good/good: 14 (28) At 2 years Improved: 31 (69) No change: 11 (24) Declined: 3 (7) |
| ILP | ||||||
| van de Meent et al, 201333 | 1 |
Mean hours per week (SD) Socket: 56 (7.9) Osseointegrated prosthetic implant: 101 (2.4) P < .001 |
NR | NR |
Mean scores (SD) Socket: 39 (4.7) Osseointegrated prosthetic implant: 63 (5.3) P = .001 |
NR |
| ILP and OGAP-OPL | ||||||
| Al Muderis et al, 201634 | Mean: 1.8 (after S1) | NR | NR | NR | Preoperative: 47.82 (17.28) Follow-up: 83.52 (18.04) P < .001 |
NR |
Abbreviations: ILP, Integral Leg Prosthesis; NR, not reported; OGAP-OPL, Osseointegration Group of Australia–Osseointegration Prosthetic Limb; OPRA, Osseointegrated Prostheses for the Rehabilitation of Amputees; S1, stage-1 surgery.
Prosthetic use score of 0 means that patient is not using a prosthesis and, therefore, the other Q-TFA scores cannot be calculated.
For problem domain, lower score indicates fewer problems related to amputation or prosthesis.
Branemark et al10 reported scores for all domains of Q-TFA. Before surgery to receive an osseointegrated prosthetic implant, only 57% of patients used their prosthesis daily, but at a 2-year follow-up, that increased to 89%.10 The mean score for prosthetic use increased from 47 (of possible 100) before surgery to 79 at 2-year follow-up. Other measures of Q-TFA also improved significantly. Van de Meent et al33 reported that prosthetic use improved from 56 hours to 101 hours per week, and the global scores also significantly improved. They did not report the other domains of Q-TFA. Al Muderis et al34 only reported scores for global health, which showed a significant improvement. None of the studies reported on changes in range of motion.
Our assessment using the GRADE criteria was that there is low certainty of improvement with respect to functional outcomes.
Quality of Life
Two studies reported data on the SF-36 health survey, a generic measure of quality of life.11,35 This tool has eight subscales: four measure physical health (physical functioning, role functioning–physical, bodily pain, general health) and four measure mental and psychological health (vitality, social functioning, role functioning–emotional, mental health). The results are also captured in two summary measures: the physical component summary (PCS) and the mental component summary (MCS). In each scale, values run between 0 and 100. A higher score indicates better physical or mental health.
Branemark et al10 reported on all subscales of the SF-36 regardless of whether the patient had a unilateral or bilateral amputation. The improvement in quality of life after osseointegration was significant for the domains of physical functioning and role functioning–physical, but there was no significant improvement for other subscales. The mean scores for the mental component summary declined by 3 points, but this result was not statistically significant. Al Muderis et al34 reported only on the physical component summary, which showed a significant improvement, but did not report scores for any of the mental health components of the survey (Table 4).
Table 4:
Functional Outcomes—36-Item Short-Form Health Survey
| Author, Year | Physical Functioning | Role Physical | Bodily Pain | General Health | Vitality | Social Functioning | Role Emotional | Mental Health | PCS | MCS |
|---|---|---|---|---|---|---|---|---|---|---|
| OPRA Implant System | ||||||||||
| Branemark et al, 201410,a | Baseline: 35 (0–85) |
Baseline: 41 (0–100) |
Baseline: 55 (10–100) |
Baseline: 78 (37–100) |
Baseline: 60 (15–90) |
Baseline: 78 (13–100) |
Baseline: 75 (0–100) |
Baseline: 74 (4–100) |
Baseline: 74 (4–100) |
Baseline: 53 (19–69) |
| Change: 23 (−23 to 75) |
Change: 22 (−50 to 100) |
Change: 6 (−61 to 59) |
Change: –1 (−42 to 40) |
Change: 3 (−70 to 45) |
Change: 1 (−100 to 63) |
Change: 0 (0 to 100) |
Change: 2 (−76 to 40) |
Change: 2 (−76 to 40) |
Change: –3 (−44 to 22) |
|
| (n = 45) | (n = 44) | (n = 45) | (n = 45) | (n = 45) | (n = 45) | (n = 44) | (n = 45) | (n = 44) | (n = 44) | |
| P < .001 | P < .001 | NS | NS | NS | NS | NS | NS | P < .001 | NS | |
| ILP and OGAP-OPL | ||||||||||
| Al Muderis et al, 201634,b | NR | NR | NR | NR | NR | NR | NR | NR | Baseline: 37.09 (9.54) Follow-up: 47.29 (9.33) P < .001 |
NR |
Abbreviations: ILP, Integral Leg Prosthesis; MCS, mental component summary; NR, not reported; NS, not significant; OGAP-OPL, Osseointegration Group of Australia–Osseointegration Prosthetic Limb; OPRA, Osseointegrated Prostheses for the Rehabilitation of Amputees; PCS, physical component summary.
Outcomes reported as mean scores (range).
Outcomes reported as mean scores (standard deviation).
Our assessment using the GRADE criteria was that there is low certainty of improvement with respect to quality-of-life outcomes.
Harm Outcomes
Most studies reported harm outcomes. Two studies reported only on infection outcomes.30,31 Superficial infections involving soft tissue were the most frequently observed adverse event and were typically treated with antibiotics. Deep infection involving the bone was less frequent but required surgical intervention in all cases. In the studies from Australia, infection was graded in 5 levels in: grade 0 (no infection), grade 1 (mild soft tissue infection, responded to oral antibiotics, grade 2 (severe soft tissue infection, required intravenous antibiotics), grade 3 (bony infection, required surgical debridement), and grade 4 (implant failure, required implant removal).34
Superficial Infections
Table 5 shows detailed outcomes for superficial infection. Branemark et al10 followed 51 consecutive patients who received the OPRA implant system for 2 years after the second surgery. Fifty-five percent had one or more superficial infections during the study period.
Table 5:
Harm Outcomes—Superficial Infections and Related Treatments
| Author, Year | Follow-Up, Years | Patients With Infection, N (%) | Incidencea | Treatment | |
|---|---|---|---|---|---|
| Antibiotic Use, N (%) | Other Interventions, N (%) | ||||
| OPRA Implant System | |||||
| Branemark et al, 201410 | 2 for all patients | 28 (55) | 41 | Oral, 10 days: 20 (39) Prolonged treatment: 4 (8) |
Hospital admission: 4 (8) |
| ILP | |||||
| Juhnke et al, 20156 | Mean: 2.7 (range, 0.08–4.9) |
0 (0) | – | – | – |
| Al Muderis et al, 201632,b | Median: 2.8 (range, 2–6) |
Mild: 23 (27) Severe: 1 (1) |
43 | Oral: 23 (27) IV: 1 (1) |
NR |
| ILP and OGAP-OPL | |||||
| Al Muderis et al, 201634,c | Mean: 1.8 after S1 | 18 (36) Mild: 13 (26) Severe: 5 (10) |
Oral: 13 (26) IV: 5 (10) |
Refashioning surgery: 10 (20) |
|
| OGAP-OPL | |||||
| Al Muderis et al, 201735,d | Mean: 1.2 after S1 | 10 (45.5) | 15 | Oral: 12 (54.5) IV: 3 (13.6) |
Refashioning surgery: 6 (27) |
Abbreviations: ILP, Integral Leg Prosthesis; IV, intravenous; NR, not reported; OGAP-OPL, Osseointegration Group of Australia–Osseointegration Prosthetic Limb; OPRA, Osseointegrated Prostheses for the Rehabilitation of Amputees; S1, stage-1 surgery.
Some patients experienced more than one episode of infection.
Includes van de Meent et al.33
Overlapped patients who received ILP in Australia.32
Overlapped patients who received OGAP-OPL in a previous study.34
Van de Meent et al33 reported that 36% of patients who received a third-generation ILP implant in the Netherlands had mild soft tissue infection. The study by Juhnke et al,6 conducted in Germany, reported no superficial infections for the third-generation ILP implant. Another study of the third-generation ILP implant that combined the experience of two centres (Australia and Netherlands) found that 24 patients (28%) had at least one superficial infection (median follow-up, 2.8 years).32
A study that investigated the outcomes of both ILP and OGAP-OPL implants in Australia reported that 18 patients (36%) developed superficial infection, 5 cases of which were severe.34 In another study, in which 22 patients received OGAP-OPL implant in Australia, 10 patients (45.5%) developed superficial infection in a mean of 1.2 years.35 Most were treated with oral antibiotics, but in three cases (13.6%), the infection was severe and required intravenous antibiotics. Refashioning surgery (surgery performed on soft tissue) to control the infection was performed in two studies.34,35
Deep Infections and Bone Infections
Deep and bone infections were serious complications that required both antibiotics and surgical intervention in all cases. Table 6 presents details about these infections from the included studies. When treatment was not effective in controlling the infection, the osseointegrated prosthetic implant was removed.
Table 6:
Harm Outcomes—Deep or Bone Infections and Related Treatments
| Author, Year | Follow-Up, Years | Patients With Infection, N (%) | Time of Occurrence | Treatment | |
|---|---|---|---|---|---|
| Antibiotic, N (%) | Surgical Intervention, N (%) | ||||
| OPRA Implant System | |||||
| Tillander et al, 201031,a | Mean: 3 | 6 (18) | Mean: 2.8 years (SD, 1.3) after S2 |
4 (12.5) Prolonged antibiotic: 2 (6) |
Revision: 2 (6) Debridement: 1 (3) Extraction: 1 (3) |
| Branemark et al, 201410 | 2 for all patients | Had signs of infection: 2 (4) Only had positive culture: 2 (4) |
Immediately after S1 to 42 days after S2 | 4 (8) | Extraction: 1 (2) |
| Tillander et al, 201730 | Mean: 7.9 (range 1.5–19.6) |
Osteomyelitis Overall: 16 (17) Before OPRA protocol: 7/27 (26) During and after OPRA protocol: 9/69 (13) Osteitis 6 (6) |
Osteomyelitis Median: 2.6 years (range 0.3–13.8) Osteitis Diagnosed ≥ 5 years after implantation |
Osteomyelitis All received Osteitis Mean number of courses: 21.5 (range 10–30) |
Osteomyelitis Extraction: 10 (10) Reimplant: 1 (1) |
| ILP | |||||
| Juhnke et al, 20156 | Mean: 2.7 (range 0.08–4.9) | 0 (0) | – | – | – |
| Al Muderis et al, 201632 | Median: 2.8 (range 2–6) | Abscess formation: 4 (5) | NR | NR | Surgical debridement: 4 (5) |
| ILP and OGAP-OPL | |||||
| Al Muderis et al 201634,b | Mean: 1.8 after S1 | 3 (6) | NR | NR | Surgical debridement: 3 (6) |
| OGAP-OPL | |||||
| Al Muderis et al 201735,c | Mean: 1.2 after S1 | 0 (0) | – | – | – |
Abbreviations: ILP, Integral Leg Prosthesis; NR, not reported; OGAP-OPL, Osseointegration Group of Australia–Osseointegration Prosthetic Limb; OPRA, Osseointegrated Prostheses for the Rehabilitation of Amputees; S1, stage-1 surgery; S2, stage-2 surgery; SD, standard deviation.
Study followed patients with osseointegrated prosthetic implants who attended the osseointegration outpatient clinic in Sweden.
Overlapped patients who received ILP in Australia.32
Overlapped patients who received OGAP-OPL in a previous study.34
In the study of the OPRA implant by Branemark et al,10 4 patients (8%) developed deep infection during a 2-year follow-up period. Two of these patients did not have signs of infection, but samples of soft tissues taken during surgery showed positive culture, for Escherichia coli in one patient and Pseudomonas aeruginosa in the other. These cases were treated with antibiotics for 5 and 6 months. The deep infection in one implant led to loosening of the implant, which was removed 6 months after the second-stage surgery.
Studies with longer follow-up showed that the risk of deep infection continued over time. Tillander et al, who retrospectively analyzed data on patients treated with the OPRA implant system in Sweden, reported that, in a mean follow-up of 7.9 years after surgery, 9 of 69 patients (13%) who had received an osseointegrated prosthetic implant during and after the OPRA rehabilitation protocol developed osteomyelitis. This study also showed that the 10-year risk of osteomyelitis among all patients who received an osseointegrated prosthetic implant since 1990 (i.e. before, during, and after the OPRA rehabilitation protocol) was 20% (95% confidence interval [CI] 12%–33%) and the 10-year risk that infection would lead to implant extraction was 9% (95% CI 4%–20%).30 In another study, Tillander et al31 prospectively followed 39 patients with arm and/or leg amputation due to trauma or neoplasia who were fitted with 45 upper- and lower-limb osseointegrated prosthetic implants a mean of 4.7 years earlier. Some patients had their surgery before the OPRA protocol was established. Thirty-three of the implants were femoral, including one patient who received bilateral implants. During 3 years of follow-up, six patients (18%) who had undergone transfemoral amputation developed a deep infection. These results may not be representative of all patients who received osseointegrated prosthetic implants, as the study included only patients who attended the authors' clinic and, therefore, has the potential for selection bias.
No deep infection was reported for ILP implants in the German study by Juhnke et al.6 A single surgeon performed all the surgeries, and the mean duration of follow-up was 2.7 years. However, in the Australia–Netherlands study of the ILP, 4 patients (5%) developed an abscess, in a median follow-up of 2.8 years.32 All were treated with antibiotics and surgical debridement. Al Muderis et al34 reported that 6% of patients who received ILP or OGAP-OPL in Australia developed deep infection in a mean follow-up of 1.8 years.
Bone Fracture
In the study that investigated the OPRA implant, no periprosthetic fractures (around the implant) occurred during the 2-year study period.10 Four patients (8%) had fracture in locations other than femoral bone. For the ILP implant, all fractures were located in femoral bone. One study reported 2 periprosthetic fractures (5% of patients), which occurred about 2.5 to 3 years after implantation,6 and another study reported 3 (3.5%) femoral fractures in a mean follow-up of 2.8 years but time of occurrence was not reported.32 The Australian study that investigated outcomes of both ILP and OGAP-OPL reported 4 (8%) periprosthetic fractures in a mean follow-up of 1.8 years but time of occurrence was not reported.34 The study on OGAP-OPL alone did not observe any fractures within its one year of follow-up35 (Table 7).
Table 7:
Harm Outcomes—Bone Fracture and Anatomical Location
| Author, Year | Follow-Up, Years | Femoral Fracture, N (%) | Other Locations, N (%) |
|---|---|---|---|
| OPRA Implant System | |||
| Branemark et al, 201410 | 2 for all patients | 0 (0) | Ipsilateral hip: 3 (6) Below elbow: 1 (2) Vertebral compression fracture: 1 (2) |
| ILP | |||
| Juhnke et al, 20156 | Mean: 2.7 (range 0.08–4.9) | 2 (5) | NR |
| Al Muderis et al, 201632 | Median: 2.8 (range 2–6) | 3 (3.5) | NR |
| ILP and OGAP-OPL | |||
| Al Muderis et al, 201634,a | Mean 1.8 after S1 | 4 (8) | NR |
| OGAP-OPL | |||
| Al Muderis et al, 201735,b | Mean: 1.2 after S1 | 0 (0) | NR |
Abbreviations: ILP, Integral Leg Prosthesis; NR, not reported; OGAP-OPL, Osseointegration Group of Australia–Osseointegration Prosthetic Limb; OPRA, Osseointegrated Prostheses for the Rehabilitation of Amputees; S1, stage-1 surgery.
Overlapped patients who received ILP in Australia.32
Overlapped patients who received OGAP-OPL in a previous study.34
Implant Removal
Reasons for implant removal (explantation) included infection, failed osseointegration, implant breakage, and fatigue failure (damage to the device due to repeated loading and unloading). Overall, among the studies with 1 to 3 years of follow-up, implants were removed in 3.6% of patients, and lack of osseointegration was the cause in half of these cases. Branemark et al10 reported that 3 implants were removed during 2 years of follow-up. The authors reported the cumulative implant survival, accounting for one explantation that occurred after the study ended, as 92% (95% CI 80%–97%).10
Table 8 shows details of explantations reported by these studies.
Table 8:
Harm Outcomes—Implant Removal
| Follow-Up, Years | Implant Removal | ||
|---|---|---|---|
| N (%) | Reason, Time of Occurrence | ||
| OPRA Implant System | |||
| Tillander et al, 201031 | Mean: 3a | 1 (3) | Deep infection |
| Branemark et al, 201410 | 2 for all | 3 (5.8) | Deep infection: 1 at 6 months Failed integration: 2 at 1.3 and 1.7 months |
| ILP | |||
| Juhnke et al, 20156 | Mean: 2.7 (range 0.08–4.9) |
1 (2.6) | Failed integration |
| Al Muderis et al, 201632 | Median: 2.8 (range 2–6) |
3 (3.5) | Failed integration: 1 Breakage of implant: 2 at 42 and 47 months after S1 |
| ILP and OGAP-OPL | |||
| Al Muderis et al, 201632,b | Mean: 1.8 after S1 | 2 (4) | Failed integration: 1 at 2 years Fatigue failure: 1 at 3.5 years |
| OGAP-OPL | |||
| Al Muderis et al 201735,c | Mean: 1.2 after S1 | 0 (0) | – |
Abbreviations: ILP, Integral Leg Prosthesis; OGAP-OPL, Osseointegration Group of Australia–Osseointegration Prosthetic Limb; OPRA, Osseointegrated Prostheses for the Rehabilitation of Amputees; S1, stage-1 surgery.
Study followed patients with osseointegrated prosthetic implants who attended the osseointegration outpatient clinic in Sweden.
Overlapped patients who received ILP in Australia.32
Overlapped patients who received OGAP-OPL in a previous study.34
Breakage of Implant Components
Three studies reported breakage of intramedullary or extramedullary components of the osseointegrated prosthetic implant systems (Table 9).6,10,32 In the study by Branemark et al,10 no intramedullary breakage occurred during a 2-year follow-up. Four patients (8%) required exchange of abutment or abutment screws. Since breakage of the intramedullary implant requires a major surgical procedure, the system was designed to ensure that, in case of excessive loads, the abutment and the abutment screws fracture before the implant.12 The external components are easier to replace than the implant. The study by Juhnke et al6 reported no intramedullary or extramedullary breakage. In the two-centre study from Australia and Netherlands,32 2 patients (2.3%) had implant breakage and 25 (29%) had breakage of the safety device that is added to the system to avoid the risk of fracture in case of excessive load.
Table 9:
Harm Outcomes—Breakage of Implant Components
| Author, Year | Follow-Up, Years | Implant Breakage, N (%) | Issues With Extramedullary Parts, N (%) |
|---|---|---|---|
| OPRA Implant System | |||
| Branemark et al, 201410 | 2 for all patients | 0 (0) | Changing the abutment or its screws: 4 (8) (9 events; 6 occurred in 1 patient) |
| ILP | |||
| Juhnke et al, 20156 | Mean: 2.7 (range 0.08–4.9) | 0 (0) | 0 (0) |
| Al Muderis et al, 201632 | Median: 2.8 (range 2–6) | 2 (2.3) | Breakage of safety parts: 25 (29) (30 events) |
| ILP and OGAP-OPL | |||
| Al Muderis et al, 201634,a | Mean: 1.8 after S1 | NR | NR |
| OGAP-OPL | |||
| Al Muderis et al, 201735,b | Mean: 1.2 after S1 | 0 (0) | NR |
Abbreviations: ILP, Integral Leg Prosthesis; NR, not reported; OGAP-OPL, Osseointegration Group of Australia–Osseointegration Prosthetic Limb; OPRA, Osseointegrated Prostheses for the Rehabilitation of Amputees; S1, stage-1 surgery.
Overlapped patients who received ILP in Australia.32
Overlapped patients who received OGAP-OPL in a previous study.34
Noninfectious Soft-Tissue and Bone Complications
Two studies reported on noninfectious soft-tissue and bone complications (Table 10).6,32 It was not clear if such complications occurred in other studies or if the authors did not consider reporting them.
Table 10:
Harm Outcomes—Noninfectious Soft-Tissue and Bone Complications
| Author, Year | Follow-Up, Years | Other Soft-Tissue/Bone Adverse Events, N (%) | Treatment |
|---|---|---|---|
| Juhnke et al, 20156 | Mean: 2.7 (range 0.08–4.9) |
Excess granulation tissue at stoma: 1 | Removed granulations |
| Al Muderis et al, 201632 | Median: 2.8 (range 2–6) |
Hypertrophic bone formation: 9 (10) | NR |
| Redundant soft tissue: 14 (16); 23 events | Excised redundant soft tissues | ||
| Hypergranulation at stoma: 17 (20); 22 events | Treated with chemical cauterization | ||
| Rounding and resorption of distal femoral cortex: 17 (20) | NR |
Abbreviations: NR, not reported.
Our assessment using the GRADE criteria was that there is high certainty of an increase in harms in comparison to not receiving an osseointegrated prosthetic implant.
Outcomes in Study on Bilateral Amputees
Hagberg et al29 studied the outcomes of the OPRA implant in 12 patients with bilateral above-the-knee amputation who received implants between 1990 and 2015. Two patients received their implants before the OPRA rehabilitation protocol was standardized in 1999. Ten of the 12 patients received implants for both limbs. The median follow-up time was 7 years (range 1–20 years).
Before osseointegration surgery, 9 patients used prostheses to various degrees; 3 of the 9 patients had very limited use. All 9 prosthetic users had problems with their prosthesis. At the latest postsurgical follow-up, 11 patients used their limb prostheses and one stopped using the prosthesis due to other health-related problems. The prosthetic use scores (calculated as number of days per week times number of hours per day) increased to 90 or more in 4 patients. During standing and walking, 5 patients had no pain, 4 had a small amount of pain, and 2 had a moderate amount of pain. Seven patients reported no prosthetic sitting problem and 3 had small problems (1 answer was missing).
Table 11 shows harm outcomes related to osseointegrated prosthetic implants in patients with bilateral amputation reported in this single study.29 Following implant surgery, the incidence of superficial infection in these patients was relatively higher than in the other studies on unilateral amputation (see Table 5). Ten of the 12 patients (83%) had at least one superficial infection. Superficial infections were treated by administration of oral or intravenous antibiotics. Deep infection occurred in only one patient (8%), three years after the second-stage surgery. Treatment of deep infection in this patient involved seven months of antibiotic use and two surgical revisions. One patient had two implants removed due to progressive and chronic infection 20 years after osseointegration surgery. Two patients (17%) had fracture of the femoral bone after falls. The time of occurrence is not reported. Exchange of implant parts occurred in 8 patients (67%).
Table 11:
Harm Outcomes in Patients With Bilateral Amputation
| Author, Year | Follow-Up, Years | Superficial Infection, N (%) | Deep or Bone Infection, N (%) | Fracture, N (%) | Explant, N (%) | Reimplant, N (%) | Issues With Extramedullary Parts, N (%) |
|---|---|---|---|---|---|---|---|
| Hagberg et al, 201829 | Median: 7 | 10 (83%) | 1 (8) | 2 (17) | 2 implants in 1 patient | 0 (0) | 8 (67) |
Five-Year Follow-Up Data
One study10 identified in this health technology assessment published 5-year follow-up data40 after we had completed the initial draft of this report. Here, we describe these results. At 5 years, the functional and quality-of-life outcomes were similar to those reported at 2 years, while the incidence of infections increased.
In this study,40 5-year outcomes of 51 patients who had received the OPRA implant were compared with both preoperative and 2-year follow-up data. Forty-five patients had received a unilateral transfemoral amputation, and six had received a bilateral transfemoral amputation, of which four were treated bilaterally (i.e., they received an osseointegrated implant in both legs). At 5 years, 11 patients were withdrawn from the study (two died for reasons unrelated to the implant, four had their implant removed about 2 years after surgery, and five were lost to follow-up). Therefore, 5-year follow-up data were available for 40 patients.
An analysis of the 5-year data showed a statistically significant improvement in scores for domains of the Q-TFA and two domains of the SF-36 when comparing 5-year scores with preoperative scores (Tables 12 and 13). At baseline, 29 of 42 patients (69%) used their prosthesis for at least 13 hours per day; at the 5-year follow-up, 28 of 40 patients (70%) used their prosthesis for at least 13 hours per day. Analyses comparing the 2-year and 5-year follow-ups showed no significant differences in the Q-TFA and SF-36 scores.
Table 12:
Functional Outcomes—Questionnaire for People With Transfemoral Amputation
| Author, Year | Follow-Up, Years | Prosthetic Usea | Mobility | Problemsb | Global Health | Overall Situation, N (%) |
|---|---|---|---|---|---|---|
| OPRA Implant System | ||||||
| Branemark et al, 201840 | 5 | Change from baseline | Change from baseline | Change from baseline | Change from baseline | Not reported |
| Mean (SD): 38 (34.2) | Mean (SD): 20 (20.4) | Mean (SD): −28 (16.9) | Mean (SD): 38 (23.2) | |||
| Median (range): 29 (−10 to 100) | Median (range): 17 (−24 to 78) | Median (range): −31 (−57 to 2) | Median (range): 33 (8 to 100) | |||
| (n = 40) | (n = 34) | (n = 34) | (n = 34) | |||
| P < .0001 | P < .0001 | P < .0001 | P < .0001 | |||
Abbreviation: SD, standard deviation.
A prosthetic use score of 0 means that the patient is not using a prosthesis and, therefore, the other Q-TFA scores cannot be determined.
For the problem domain, scores are reversed, meaning that lower scores indicate fewer problems related to the amputation or prosthesis.
Table 13:
Functional Outcomes—36-Item Short-Form Health Survey
| Author, Year | Physical Functioning | Role Physical | Bodily Pain | General Health | Vitality | Social Functioning | Role Emotional | Mental Health | PCS | MCS |
|---|---|---|---|---|---|---|---|---|---|---|
| OPRA Implant System | ||||||||||
| Branemark et al, 201840 | Change from baseline: | Change from baseline: | Change from baseline: | Change from baseline: | Change from baseline: | Change from baseline: | Change from baseline: | Change from baseline: | Change from baseline: | Change from baseline: |
| Mean (SD): 28 (23.1) | Mean (SD): 19 (47.9) | Mean (SD): 4 (30.7) | Mean (SD): 3 (22.7) | Mean (SD): 3 (22.1) | Mean (SD): 1 (31.8) | Mean (SD): −1 (42.9) | Mean (SD): 1 (22.2) | Mean (SD): 10 (9.9) | Mean (SD): 4 (14.4) | |
| Median (range): 25 (−15 to 85) | Median (range): 0 (−100 to 100) | Median (range): 6 (−69 to 90) | Median (range): 3 (−55 to 5) | Median (range): 0 (−55 to 50) | Median (range): 0 (−63 to 75) | Median (range): 0 (−100 to 100) | Median (range): 2 (−56 to 52) | Median (range): 11 (−11 to 37) | Median (range): −0.5 (−35.7 to 20.0) | |
| (n = 40) | (n = 39) | (n = 40) | (n = 40) | (n = 40) | (n = 40) | (n = 39) | (n = 40) | (n = 39) | (n = 39) | |
| P < .0001 | P = 0.020 | P = 0.45 | P = 0.31 | P = 0.35 | P = 0.96 | P = 1.00 | P = 0.56 | P < .0001 | P = 0.22 | |
Abbreviations: ILP, Integral Leg Prosthesis; MCS, mental component summary; NR, not reported; NS, not significant; OGAP-OPL, Osseointegration Group of Australia–Osseointegration Prosthetic Limb; OPRA, Osseointegrated Prostheses for the Rehabilitation of Amputees; PCS, physical component summary.
The risk of superficial infection increased from 55% at the 2-year follow-up to 67% at the 5-year follow-up. The risk of deep or bone infection increased from 8% at the 2-year follow-up to 22% at the 5-year follow-up (Table 14).
Table 14:
Harm Outcomes—Infections and Related Treatments
| Author, Year | Follow-Up, Years | Patients With Infection, N (%) | Incidence | Treatment | |
|---|---|---|---|---|---|
| Antibiotic, N (%)a | Surgical Intervention, N (%) | ||||
| OPRA Implant System | |||||
| Branemark et al, 201840 | 5 | Superficial infection: 34 (67) | 70 | 10 days: 18 (35%) Longer period: 16 (31%) | 1 implant loosening and explantation |
| Deep infection: 11 (22) | 14 | 9 treated with oral antibiotics for a mean time of 5 mo | |||
| 1 resulted in implant loosening | |||||
| 1 did not resolve at the 5-y follow-up | |||||
Percentages were calculated based on intention to treat.
At the 5-year follow-up, 43 mechanical complications had occurred in 15 patients, requiring replacement of the damaged parts. Four implants were removed, and three patients required stump revision. The cumulative implant survival rate at 5 years was 92%, and the revision-free survival rate was reported as 45%.
Ongoing Studies
We are aware of the following ongoing studies that have potential relevance to this report:
Al Muderis M, Lu W, Tetsworth K, Bosley B, Li JJ. Single-stage osseointegrated reconstruction and rehabilitation of lower limb amputees: the Osseointegration Group of Australia Accelerated Protocol-2 (OGAAP-2) for a prospective cohort study. BMJ Open. 2017;7(3):e01350810
Leijendekkers RA, Staal JB, van Hinte G, Frolke JP, van de Meent H, Atsma F, et al. Long-term outcomes following lower extremity press-fit bone-anchored prosthesis surgery: a 5-year longitudinal study protocol. BMC Musculoskelet Disord. 2016;17(1):48440
Discussion
Given that follow-up in the studies was between 1 and 5 years, we could not assess outcomes beyond these time points for osseointegrated prosthetic implants for people with a lower-limb amputation. The included studies showed that the implants improve functional outcomes as measured by the 6MWT and TUG test. Functional outcomes, as measured by the condition-specific Q-TFA, also improved.
The desire for a more active lifestyle may motivate amputees to undergo osseointegration surgery. Although studies found improvements in quality of life for physical domains measured by the SF-36 survey, the observed changes in mental health scores were either negative (statistically nonsignificant)10 or not reported.34 Serious adverse events were not rare. One study reported a 12.8% rate of reoperation.6 A retrospective study with a mean follow-up of 7.9 years reported that 13% of patients developed osteomyelitis. Five-year data reported after the initial draft of this report was published indicated that 22% of patients developed deep infection.40 Across the studies, the occurrence of bone infection, fracture, implant loosening, and implant breakage were serious complications requiring additional surgeries. Overall, implant removal occurred in 11 of 287 patients (3.8%). Of all explantations that occurred during a follow-up of 1 to 5 years, the reason for implant removal was loosening in 64%, infection in 18%, and implant breakage in 18%. The cumulative survival of the implant at 2 years and at 5 years was reported as 92%, which is below the 10-year survival of total hip arthroplasty procedures performed during the learning phase of the technique. The estimated 10-year implant survival after total hip arthroplasty in patients operated on between 1982 and 1992 was reported as 96%.41
The three greatest concerns with osseointegrated prosthetic implants are deep or bone infection, lack of osseointegration, and bone fracture. Superficial infections around the implant site are common and, although treatable, can create a serious issue if the infection reaches the deeper layers of tissue or the host bone. Protecting bone health in this context may depend heavily on human factors such as the patient's ability to follow instructions for wound hygiene to reduce the risk of infection.
The quality of bone around the implant affects the process of osseointegration, as well as the risk of bone fracture in the years after surgery. Inadequate bone mineral density compromises osseointegration, leading to loosening of the implant and implant extraction. After amputation of a lower limb, patients may walk less, which puts them at risk of decreased bone density in their residual femoral bone. Prolonged unloading, particularly among above-the-knee amputees, can cause significant bone loss in the hip and distal bone of the residual limb, putting people at increased risk for osteoporosis and fragility fractures in the hip.42 One of the studies we reviewed reported that three of four post-transplant fractures occurred in patients who had previously used a wheelchair exclusively and had severe osteoporosis.34 For the well-being of people considering an osseointegrated prosthetic implant, professionals commonly investigate the quality of the bone before recommending osseointegration surgery. By the nature of their condition, above-the-knee amputees have an additional risk of fall and fracture, particularly if they try to walk a short distance without using their prosthesis. Fractures may also occur with socket prostheses.
One critical aspect of the technology that affects the success of the intervention is the manner in which mechanical stresses are transferred from the implant to the bone, as these implants must withstand considerable loads. The stability of the implant around the bone depends on how stress is distributed and how loads are transferred. Loads are transferred from the external prosthesis to the extramedullary parts, then to the implant, and finally to the bone. The rates of mechanical failure were very different in the studies we reviewed, which may be related to differences in implant design and in patients' levels of participation in high-impact activities. Implant breakage requires major surgeries to remove and replace the implant. Exchanging the extramedullary parts is much easier; it requires considerably shorter surgery, and some parts can be replaced without general anesthesia.
Strengths and Limitations
This systematic review adds to the existing body of knowledge. Previous systematic reviews either did not report on both safety and effectiveness,21,22 included all types of implants,23 or had different inclusion criteria.24 In addition, most previous systematic reviews included some studies with the same patient populations. We considered the latest iteration of each implant for this evaluation to avoid including designs no longer in clinical use. Other strengths of the current study are a focus on above-the-knee amputation, reporting outcomes for both safety and effectiveness of the osseointegrated prosthetic implants, and presenting the available data separately, by device, for clarity.
One limitation of this review is that most of the included studies had a mean follow-up of 1 to 5 years; as such, we were unable to make conclusions about effectiveness beyond these time points. In addition, the studies did not report outcomes after explantation and/or reimplantation. Another limitation is that some studies had overlapping patient data for some outcomes, and some did not completely report some outcomes. The most complete and useful data for this review were from the study by Branemark et al.10 The fixed duration of follow-up in that study was more appropriate than the mean or median duration of follow-up that other studies reported, often with a broad range, which indicates that some patients had minimal contribution to the results. Studies that retrospectively performed a chart review may have underestimated the complication rates, as some issues may have been treated by other practitioners. The only published study on bilateral amputation had a very small sample size and included surgeries before and after the standardized OPRA rehabilitation protocol, which makes it difficult to assess outcomes in these patients.
Conclusions
In studies of osseointegrated prosthetic implants for lower-limb amputation, most patients were followed for only 1 to 5 years, a fairly short follow-up to evaluate this technique. The quality of evidence, assessed using the GRADE criteria, was low for functional outcomes and quality life and high for adverse events. Studies with short-term follow-up showed that patients' functional outcomes improved with osseointegrated prosthetic implants, but their emotional health did not improve. Osseointegrated prosthetic implants can lead to serious adverse events such as bone infection and fracture, which may require additional surgeries and negatively impact emotional health. However, chronic problems with socket fit and limited function can also impact mental health and quality of life for amputees using conventional prostheses.
ECONOMIC EVIDENCE
Research Question
What is the cost-effectiveness of osseointegrated prosthetic implants compared with conventional socket prostheses in treating people with lower-limb amputation?
Methods
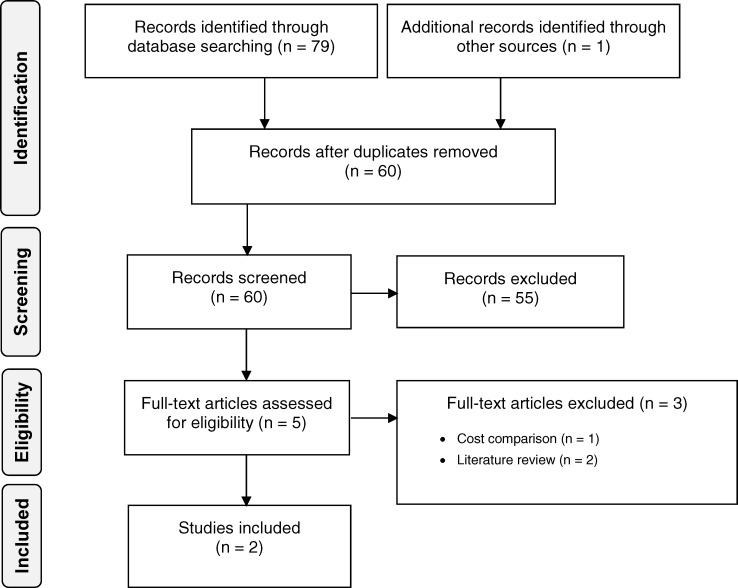
Economic Literature Search
We performed an economic literature search on June 5, 2018, to retrieve studies published from database inception until the search date. To retrieve relevant studies, we developed a search using the clinical search strategy with an economic and costing filter applied.
We created database auto-alerts in MEDLINE and Embase and monitored them for the duration of the assessment period. We performed a targeted grey literature search of health technology assessment agency websites, clinical trial and systematic review registries, and the Tufts Cost-Effectiveness Analysis Registry. See Clinical Literature Search, above, for further details on methods used. See Appendix 1 for our literature search strategies, including all search terms.
Eligibility Criteria
Studies
Inclusion Criteria
English-language full-text publications
Studies published from inception to June 5, 2018
Studies on osseointegrated prosthetic implants for lower-limb amputation
Cost–utility, cost-effectiveness, or cost–benefit studies
Exclusion Criteria
Narrative reviews, letters/editorials, case reports, commentaries, abstracts, posters, unpublished studies, cost estimate/comparison studies
Outcome Measures
Costs
Quality-adjusted life-years (QALYs)
Incremental cost and incremental effectiveness
Incremental cost-effectiveness ratio (ICER)
Literature Screening
A single reviewer reviewed titles and abstracts, and, for those studies likely to meet the eligibility criteria, we obtained full-text articles and performed further assessment for eligibility.
Data Extraction
We extracted relevant data on the following:
Source (i.e., first author, country, year)
Population
Interventions and comparator
Outcomes (i.e., health outcomes, costs, and incremental cost-effectiveness ratio)
Study Applicability
We determined the usefulness of each identified study for decision-making by applying a modified quality appraisal checklist for economic evaluations originally developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom to inform the development of NICE's clinical guidelines.43 We retained questions from the NICE checklist related to study applicability and modified the wording of the questions to remove references to guidelines and to make it Ontario-specific. A summary of the number of studies judged to be directly applicable, partially applicable, or not applicable to the research question is presented.
Results
Literature Search
The economic literature search yielded 60 citations published from database inception until June 5, 2018, after removing duplicates. We identified 2 studies (both cost–utility analyses) that met our inclusion criteria. We hand-searched the reference lists of the included studies to identify other relevant studies but did not find any additional citations. Figure 2 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the economic literature search.
Figure 2: PRISMA Flow Diagram—Economic Search Strategy.
Source: Adapted from Moher et al.28
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Overview of Included Economic Studies
Table 15 provides a summary of the two included studies.
Table 15:
Results of Economic Literature Review—Summary
| Name, Year, Country of Publication | Analytic Technique, Study Design, Perspective, Time Horizon | Population | Intervention, Comparator | Results | ||
|---|---|---|---|---|---|---|
| Health Outcomes | Costs | Cost-Effectiveness | ||||
| Frossard et al, 2017, Australia44 | Cost–utility analysis Model informed by a retrospective individual case-controlled observation study No discounting Australian state prosthetic care provider perspective 6-year time horizon |
Individuals with above-the-knee amputation Mean age 55 years 88% male |
Osseointegrated prosthesis Conventional socket prosthesis |
Total QALYs at 6 years:
|
2016/17 Australian dollars ($) Individual yearly:
|
ICER: $16,632 per QALY gained (range −$25,700 to $53,500) |
| Hansson et al, 2018, Sweden45 | Cost–utility analysis Model informed by prospective clinical study No discounting Swedish health care perspective 20-year time horizon |
Individuals with a unilateral above-the-knee amputation Mean age 44 years 43% male |
Osseointegrated prosthesis Conventional socket prosthesis |
Total QALYs at 20 years:
|
2009 Euros (€) Individual overall:
|
ICER: €83,374 per QALY gained |
Abbreviations: ICER, incremental cost-effectiveness ratio, QALY, quality-adjusted life-year.
Frossard et al44 conducted a cost–utility analysis of osseointegrated prosthetic implants (which they referred to as bone-anchored protheses) compared to conventional socket prostheses in a population of people with an above-the-knee amputation. They used Swedish health care administrative data and surveys from a prosthetic facility to determine per-patient prosthesis costs over a 6-year period for each intervention, while utility estimates were taken from the literature and multiplied over the 6 years to obtain differences in QALYs. The analysis used the perspective of the Australian state prosthetic care provider, and did not use discounting, as “the largest portion of the overall costs occurs in [the] first and third years when [prosthetic] knees and feet are supplied.”44 The ICER (2016/17 Australian dollars) between bone-anchored prostheses and socket prostheses was $16,632 per QALY gained. Bone-anchored protheses were cost-saving in 19% of participants and cost-effective in 88% of participants. Scenario analyses using different utility sources found varied ICERs, from $13,740 per QALY gained to $21,066 per QALY gained. Typical probabilistic sensitivity analyses were not conducted; instead, the authors evaluated only the total cost through a correlation coefficient calculated from the relationship between overall cost and potential confounders (e.g., age, height, mass, body mass index, age at amputation, age at treatment). Then they constructed a variable corresponding to relative typical cost over the total cost, to determine the level of uncertainty of the cost information.
Hansson et al45 carried out a cost–utility analysis of osseointegrated prosthetic implants compared to conventional socket prostheses in a population with above-the-knee amputation. The authors constructed a Markov model, and clinical inputs were mainly informed by a 2-year prospective clinical study.10 Cost inputs were derived from hospital data, literature, and expert opinion, while utility data were taken from a study by Hagberg et al.26 The analysis was modelled from the Swedish health care perspective over a 20-year period. The authors did not use discounting; they stated the costs being incurred were one-off early in the treatment. The ICER (2009 euros) for osseointegrated prosthetic implants was €83,374 per QALY gained compared with socket prostheses. The probability of osseointegrated prosthetic implants being cost-effective was 0.40 for a willingness-to-pay value of €48,000. The authors performed several analyses of uncertainty, such as one-way sensitivity analyses, probabilistic sensitivity analyses, and alternative scenario analyses. In one-way sensitivity analyses, the parameters with the most influence on cost-effectiveness were changes in the utility values and the monthly cost of the prosthesis, for both the socket and osseointegration cohorts. The model was also sensitive to the time horizon: a lower time horizon led to a higher ICER (i.e., 15-year, 5-year, and 1-year ICERs of €98,519, €243,322, and €2,578,563, respectively).
Applicability of the Included Studies
Appendix 5, Table A3, provides the results of the applicability checklist for economic evaluations applied to the two included studies. We deemed both studies to be partially applicable to the research question.
Discussion
The economic evidence review identified two studies with differing methodological approaches to evaluating the cost-effectiveness of osseointegrated prosthetic implants compared to conventional socket prostheses for lower-limb amputees. The first study calculated costs by summing patient costs from an administrative database over a 6-year period, while the second used a Markov model and unique health states to simulate costs and effects over time. The studies also differed in their conclusions. Frossard et al44 found osseointegrated prosthetic implants were either cost-saving or cost-effective for 19% and 88% of participants, respectively, while Hansson et al45 found osseointegrated prosthetic implants had an ICER of €83,374 per QALY gained and the probability of it being cost-effective was very low: 40% at a willingness-to-pay value of €48,000.
Although commonly used, the discounting of costs and outcomes were not included in either study. Since osseointegrated prosthetic implants for lower-limb amputees are estimated to maintain their effectiveness throughout the person's lifetime, and the majority of costs are assumed to occur in the first year, the omission of discounting could artificially inflate the reported ICER.
There appeared to be several methodological limitations in how the costing of osseointegrated prosthetic implants was conducted in the reviewed articles. In Frossard et al44 the costing data were specific to labour and parts billed by the prosthetist and orthotist services. Not included were the costs of surgical implantation, hospital stay, rehabilitation, complications, medical professional fees, and drugs. These omissions result in a significantly underestimated ICER when considered from a health care payer perspective. The study by Hansson et al45 provided a better estimate of all costs associated with osseointegration; however some costs, such as the cost of a deep infection, appeared small (i.e., €850), despite the severity of such events and the treatment required.
Neither study incorporated disutilities for complications. Utility values have been published on users of conventional socket prostheses prior to receiving an osseointegrated prosthetic implant, and on osseointegrated prosthesis users at 1 and 2 years postsurgery,10,13,26 but some complications, such as soft-tissue refashioning, may not be captured by these health states given their relatively short duration and time to recovery. If literature is available, the inclusion of disutilities for complications could provide a more comprehensive picture of the cost-effectiveness of osseointegrated prostheses compared with conventional socket prostheses.
Conclusions
The economic literature review identified two economic evaluations of osseointegrated prosthetic implants compared with conventional socket prostheses for people with lower-limb amputation. Overall, results were mixed. The ICER varied from $16,632 AUD per QALY gained (2016/17 AUD) when accounting for only prosthetic services, to €83,374 per QALY gained (2009 EUR) when the full care pathway was included, from implantation to rehabilitation and complications. Given their differing perspectives, cost inputs, and methodological approaches, both studies were considered partially applicable to the Ontario context; key limitations included a lack of Canadian or Ontario-specific costs and the use of a perspective other than a Canadian provincial health care system such as that of the Ontario Ministry of Health and Long-Term Care.
PRIMARY ECONOMIC EVALUATION
Although the published economic evaluations identified in the economic literature review addressed the interventions of interest, they did not use Canadian costs, nor did the authors take a Canadian perspective. Owing to these limitations, we conducted a primary economic evaluation using Ontario-specific cost inputs and clinical care pathways.
Research Question
Within the context of the Ontario Ministry of Health and Long-Term Care, what is the cost-effectiveness of osseointegrated prosthetic implants compared with conventional socket prostheses to treat people with lower-limb amputation who have chronic problems using a socket prosthesis?
Methods
The information presented in this report follows the reporting standards set out by the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Statement.46
Analysis
We conducted a reference case analysis and sensitivity analyses. Our reference case analysis adhered to the Canadian Agency for Drugs and Technologies in Health (CADTH) guidelines when appropriate and represents the analysis with the most likely set of input parameters and model assumptions.47 Our sensitivity analyses explored how the results are affected by varying input parameters and model assumptions.
We conducted a cost–utility analysis to determine the costs and health outcomes (i.e., quality-adjusted life-years [QALYs]) associated with each treatment strategy. We chose this type of analysis because utility inputs are available and a generic outcome measure such as QALYs allows decision-makers to make comparisons across different conditions and interventions. The outcomes reported are total costs and total QALYs for each treatment, and incremental cost per QALY gained compared to the next most effective strategy. For this analysis, incremental costs and QALYs are key outcomes considered by decision-makers, while total costs and QALYs of treatment options are informative measures for decision-makers.
Target Population
This model evaluated a population of individuals with a lower-limb amputation, not due to diabetes or severe vascular disease, with a medical history of issues related to the use of a conventional socket prosthesis that resulted in an uncomfortable prosthesis fit and difficulty walking. Additionally, patients had to meet distinct eligibility criteria specific to receiving the osseointegrated prosthetic implant, such as having reached full skeletal maturity, not currently undergoing chemotherapy, and not taking corticosteroid or immunosuppressant drugs. For the full list of clinical requirements, see Appendix 6, Table A4.
The model's population characteristics were based on sample-size weighted averages from observational studies used to inform the clinical and state-transition parameters of the model (Appendix 6, Table A5).24 The population was 46 years old on average at the time of receiving their osseointegrated prosthetic implant and consisted of 70% males and 30% females. Clinical experts validated these characteristics as representative of the Canadian population (Nancy Dudek, MD; Richard Jenkinson, MD; Dan Mead, CP(c); Amanda Mayo, MD; John Murnaghan, MD; email communications, August to November 2018). We also assumed everyone in the model had a unilateral above-the-knee amputation, which represented the majority of osseointegrated prosthetic implants and allowed us to standardize and simplify costing.
Perspective
We conducted this analysis from the perspective of the Ontario Ministry of Health and Long-Term Care.
Intervention and Comparator
As described in the Background section of this report, unlike a conventional leg prosthesis that uses a specially fitted cup-like shell (socket) that fits over the remaining portion of the residual leg, osseointegrated prosthetic implants are inserted into an amputee's remaining bone to connect the bone to an external prosthetic limb. The implant systems include an intramedullary component that integrates with the bone, and a bridging connector (also called an abutment) that connects from one side to the intramedullary implant and from the other side to the prosthetic limb. Traditionally, the treatment requires two operations: the first inserts the intramedullary implant, and the second, performed 6 to 9 months later, creates a percutaneous skin opening allowing for abutment attachment and prosthesis fitting.7 However, the duration between surgeries has been reduced in more recent publications, with the overall time between surgeries being approximately 6 to 8 weeks.32 Recently, a group in Australia has published a protocol describing a single-stage surgery for osseointegration that they have been conducting since April 2014.7 Despite this development, no published papers are available on the effectiveness of single-stage osseointegration, outside of a protocol from the OGAAP-2 (Osseointegration Group of Australia Accelerated Protocol-2) cohort study.7
For this economic evaluation, we evaluated the cost-effectiveness of both two-stage and single-stage osseointegration surgery. However, given the data available, we compared the two-stage procedure to conventional socket prostheses in the reference case, and evaluated the single-stage procedure in a scenario analysis. Despite the absence of published literature on single-stage surgery, it appears to be a technological innovation, as the first osseointegration surgery conducted in Canada was a single-stage surgery performed in Montreal in March 2018. In modeling single-stage surgery in a scenario analysis, given the absence of published evidence, we assumed complication rates and effectiveness equal to the two-stage procedure; therefore, we pooled two-stage data and used them as estimates for the single-stage model inputs.
To further refine the scope of this analysis, we also excluded certain variations of osseointegrated prosthetic implants. For example, we did not cost implants combined with total hip replacement and total knee replacement, due to a lack of available data. Furthermore, this analysis excluded customized implants not commercially available, also due to a lack of available data. Table 16 summarizes the interventions evaluated in the economic model.
Table 16:
Health Intervention and Comparator Evaluated in the Primary Economic Model
| Intervention | Comparator | Patient Population | Outcomes |
|---|---|---|---|
| Two-stage osseointegration surgery for prosthetic implant | Conventional socket prosthesis | People with unilateral above-the-knee amputation who have an uncomfortable socket prosthesis and difficulty walking | Costs QALYs ICER |
Abbreviations: ICER, incremental cost-effectiveness ratios; QALY, quality-adjusted life-year.
Discounting and Time Horizon
In accordance with the CADTH guidelines, we applied an annual discount rate of 1.5% to both costs and QALYs after the model's first year.47 We also explored different discount rates of 0%, 3%, and 5% in the sensitivity analysis.
We used a lifelong time horizon in the reference case analysis, given the chronic nature of the health condition and intervention, thus capturing all health effects and costs relevant to the decision problem. We also conducted sensitivity analyses around the time horizon, including a 10-year time horizon, which approximates the longest average follow-up recorded in an observational study on osseointegrated prosthetic implants.30 Additionally, we used time horizons from other cost–utility analyses of 6 years and 20 years in sensitivity analyses.44,45
Main Assumptions
The model's main assumptions were as follows:
All patients in both cohorts have a unilateral above-the-knee amputation
Both health-related quality of life and complications attributed to having an osseointegrated prosthetic implant are not affected by choice of external prosthetic components
Patients use existing prosthesis components and do not purchase new components following the implant procedure, aside from the implant system's components (i.e., connector from the implant system to the prosthetic knee)
Replacement costs for external prosthetic components are equal for both cohorts (including the external connector in the implant system)
Mortality does not differ between cohorts
Utility gains observed in the literature over 2 years remain constant over the model duration
If an implant is extracted and then reimplanted, new components are purchased for the reimplantation, and patients whose implant is extracted have the same utility until reimplantation as someone using an uncomfortable socket prosthesis
Annual prosthesis maintenance fees by a prosthetist are equal between cohorts
Model Structure/Structure of the Analysis
We modified a previously published Markov decision-analytic model structure from Hansson et al45 to estimate the long-term clinical and economic outcomes of osseointegrated prosthetic implants for lower-limb amputees. The cycle length was 1 year, because patients are usually monitored annually by a physiatrist (Nancy Dudek, MD; Amanda Mayo, MD; John Murnaghan, MD; email communications, August to November 2018). We applied a half-cycle correction on all health-state transitions. The model was built using TreeAge Pro 201848 and repeated cycling until the time horizon was reached.
The model included 5 health states:
Uncomfortable socket prosthesis: patients living with an uncomfortable socket fit who are prone to continued complications (only for socket users)
Implant surgery and recovery: patients who have surgery to receive an osseointegrated prosthetic implant, after which the prothesis is fitted and they undergo rehabilitation (year 1 of osseointegration cohort)
Osseointegrated prosthesis: patients who have successfully completed surgery and rehabilitation and have full use of their prosthesis (year 2 and onward of osseointegration cohort)
Implant extraction: patients whose osseointegrated prosthetic implant is surgically removed due to complications (e.g., failed osseointegration, fatigue failure, implant breakage)
Reimplant: patients who are reimplanted after an implant extraction and go through a shortened care pathway (compared to the “implant surgery and recovery” health state)
The socket cohort began in the “uncomfortable socket prosthesis” health state and remained in this state for the model duration. While in this state, patients were at risk for stump revision, a complication that results in a costly hospital stay.
The osseointegration cohort began in the “implant surgery and recovery” health state, where they underwent surgery and rehabilitation. Patients could then transition to either “osseointegrated prosthesis” or “implant extraction.” In the “osseointegrated prosthesis” health state, patients had full use of their prosthesis but were still at risk of transitioning to “implant extraction.” Anytime they were in the “implant surgery and recovery” and “osseointegrated prosthesis” health states, patients were at risk for complications specific to osseointegration, which included soft-tissue problems, fractures, superficial infections, and deep infections. These complications had distinct costs assigned to their occurrence. We assumed that patients in the “implant extraction” health state would not be susceptible to implant-related complications, given that the implant was extracted (no complications were identified in the literature specific to implant extraction). In the “implant extraction” health state, the implant failed and was removed. Some patients then transitioned to the “reimplant” health state to have the implant reinserted, but a proportion who were no longer suitable for osseointegration permanently returned to their original socket prosthesis and remained in the “uncomfortable socket prosthesis” health state. In that state, as previously mentioned, patients were at risk for stump revisions, a complication specific to socket prosthesis users.
Expert consultants advised that the “implant extraction” health state could be treated similarly to joint arthroplasty infections: the infected prosthesis is removed during an initial debridement, a temporary prosthesis is fitted, and patients are treated with intravenous (IV) antibiotics and then considered for reimplantation (Richard Jenkinson, MD; Amanda Mayo, MD; John Murnaghan, MD; Nancy Dudek, MD; Wade Gofton, MD; email communications, August to November 2018). This process, although specific to an extraction due to infection, was estimated to take 6 to 12 months. Although other causes have been identified for reimplantation, our model assumed this process would take 12 months (i.e., the model's cycle length). This assumption provided a conservative estimate of the QALY gain in the osseointegration cohort, penalizing implant extractions.
Figure 3 presents a diagram of the two Markov models and transitions within the osseointegration and socket cohorts.
Figure 3: Simplified Model Structure.
Notes: This model structure was modified from Hansson et al, 2018.45
The “M” with a circle indicates the beginning of a Markov model.
Socket complication substates included stump revision.
Osseointegration complications substates included soft-tissue refashioning, fracture, superficial infection, and deep infection.
All health states had a probability of moving to a death state (not shown), derived through an age- and sex-specific general mortality rate.
In our economic evaluation, we considered the impact of costs and quality of life associated with both treatments. We included adverse events that are severe, expensive to treat, or have a large impact on patients' health-related quality of life (e.g., infection, soft-tissue refashioning, implant extraction, fracture, stump revision). We excluded adverse events that have a negligible impact on health effects or resources (e.g., skin rash, blisters, cysts). Other complications considered for conventional socket prostheses included pressure ulcers, neuromas, fungal infections, and mechanical limb pain, but we could not find incidence rates for these complications. Nevertheless, we assumed these chronic conditions were represented by the published utility value for users of an uncomfortable socket prosthesis. Despite the lack of incidence rates, the complication rate may be similar between the socket and osseointegration cohorts, as it has been stated that in osseointegration patients, “infection and irritation of the soft tissue in the skin penetration area are common during the first 2 years.”27 Regardless, if the previously published stump revision rates are not inclusive to pressure ulcers and neuromas, the economic model may be underestimating the total cost of the socket prosthesis cohort.
Clinical Outcomes and Utility Parameters
Health State and Event Occurrences
Table 17 summarizes transitions between health states in the model, derived from pooled rates. All transitions not explicitly mentioned in the table but found in Figure 3 (i.e., osseointegrated prosthesis and reimplant) are calculated as the complement of the sum of the other branch probability, which is calculated by subtracting the probability found in Table 17 from the value 1.00.
Table 17:
Model Health State Transitions
| Model Parameters | Probability of Outcome | Distribution | Reference |
|---|---|---|---|
| Implant extraction | 0.01848 | Beta ∼ (22, 1312) | Hagberg, 201829; Branemark et al, 201410; Tilander et al, 201031; Tilander et al, 201730; Al Muderis et al, 201632; Al Muderis et al, 201634 |
| Permanent implant extraction (person returns to using socket) | 0.6880 | Fixeda | Hagberg, 201829; Branemark et al, 201410; Tilander et al, 201031; Tilander et al, 201730; Al Muderis et al, 201632; Juhnke et al, 20156; Al Muderis et al, 201634 |
Due to a lack of data, measures of variability could not be estimated around the point estimate.
Table 18 contains the rates from multiple sources for complications in both cohorts. Due to a lack of data, we could not derive the risk of fractures for patients with an uncomfortable socket, but we included fractures in the model as they were expected to have a greater cost in the osseointegration cohort compared with the socket cohort. Given this potentially conservative estimate for osseointegrated prosthetic implants, we conducted a sensitivity analysis that excluded fractures from the model in the event the risk of fracture is similar between cohorts and the incremental cost difference for treatment is marginal. Treatment pathways for deep infections involved either IV antibiotics alone, IV antibiotics and debridement (with variations in whether the debridement was conducted as inpatient or day surgery). Unfortunately, the rates found in the literature did not differentiate between these pathways, so the model conservatively assumed that patients received IV antibiotics and debridement in an inpatient setting. We tested this assumption in scenario analyses, where we assumed that IV antibiotics were administered in a home-care setting. Given the lack of data on complications for users of conventional socket prostheses, specifically in a population with nonvascular amputations, only stump revisions were included as a complication for the socket cohort.
Table 18:
Rate of Complication Occurrence
| Model Parameter | Probability of Outcome | Distribution | Reference |
|---|---|---|---|
| Osseointegration cohort | |||
| Superficial infection | 0.2261 | Beta ∼ (133, 442) | Hagberg, 201829; Branemark et al, 201410; Tilander et al, 201031; Al Muderis et al, 201632; Al Muderis et al, 201634 |
| Deep infection | 0.03303 | Beta ∼ (41, 1293) | Hagberg, 201829; Branemark et al, 201410; Tilander et al, 201031; Tilander et al, 201730; Al Muderis et al, 201632; Al Muderis et al, 201634 |
| Soft-tissue refashioning | 0.1865 | Beta ∼ (55, 206) | Al Muderis et al, 201632; Al Muderis et al, 201634 |
| Fracture | 0.02762 | Beta ∼ (12, 446) | Hagberg, 201829; Branemark et al, 201410; Al Muderis et al, 201632; Al Muderis et al, 201634 |
| Socket cohort | |||
| Stump revision | 0.02608 | Fixeda | Hansson et al, 201845 |
Due to a lack of data, measures of variability could not be estimated around the point estimate.
Mortality
The model assumed equal mortality rates for the two cohorts. Although the broad cohort of conventional socket prosthesis users has a higher mortality rate, this is in part due to vascular comorbidities such as diabetes and heart disease, which would preclude an individual from meeting eligibility criteria for an osseointegrated prosthetic implant (see Appendix 6, Table A4). However, because most osseointegration procedures are conducted several years postamputation, and our model compared a hypothetical population of the same patients who either remained as socket prosthesis users or converted to osseointegrated prosthetic implants, we assumed the survival rate was comparable for both treatments. Given that the average age of the target population for osseointegrated prosthetic implants is 46 years old and the procedure has strict eligibility criteria, this created a subpopulation of relatively healthy individuals; therefore, we used age- and sex-specific mortality rates from the Ontario general population.49 Clinical experts verified this assumption because inputs for the alternative method—mortality rates or hazard ratios specific to a population with nonvascular amputation—were not available in the literature (Richard Jenkinson, MD; Nancy Dudek, MD; Amanda Mayo, MD; John Murnaghan, MD; email: communications, August to November 2018).
Intervention Utilities
We performed a targeted literature search in MEDLINE for utility values on June 11, 2018, for studies published from inception to the search date. We based the search on the clinical search strategy with a methodological filter applied to limit retrieval to health state utility values.50 A second utilities search was conducted on June 27, 2018, to retrieve studies on leg prosthetics using the same filter. See Appendix 1 for our literature search strategies, including all search terms.
Utilities represent a person's preference for certain health outcomes, such as being able to walk. These are often measured on a scale of 0 (death) to 1 (full health). Table 19 summarizes utility data specific to each health state. All studies evaluating individuals' quality of life before and after two-stage osseointegration used the 36-Item Short Form Health Survey (SF-36). To obtain utilities, the studies converted SF-36 values to the SF-6D, a six-domain version of the survey. In cases where only the mean SF-36 domain scores were available, we obtained the utility value by using equation 1 in an SF-36 to SF-6D map/crosswalk published by Ara et al.51 Hagberg et al26 was the only study reporting a direct utility value using the SF-6D, and we therefore chose it for the reference case analysis. Utilities from other sources that used a crosswalk were used in scenario analyses. In the reference case, we conducted a probabilistic analysis and used a beta distribution around the values of the mean and standard error.
Table 19:
Utilities Used in the Economic Model
| Health State | Utility | Standard Error | Reference |
|---|---|---|---|
| Osseo surgery and recovery | 0.682 | 0.014 | Hagberg et al, 201426 |
| Osseointegrated prosthesis | 0.692 | 0.017 | Hagberg et al, 201426 |
| Uncomfortable socket prosthesis | 0.653 | 0.015 | Hagberg et al, 201426 |
| Reimplant | 0.682 | 0.014 | Assume value of implant surgery and recovery |
| Implant extraction | 0.653 | 0.015 | Assume value of uncomfortable socket prosthesis |
Cost Parameters
We included all relevant costs that individuals incurred both prior to and following surgery for osseointegrated prosthetic implants. The costs consisted of the following:
Diagnostic tests
Professional fees
Hospitalization costs
Cost of the device
Cost of managing adverse events
All costs were reported in 2018 Canadian dollars. We obtained cost inputs from standard Ontario sources and published literature. The fees for professional visits, procedures and consultations were obtained from the Ontario Schedule of Benefits for Physician Services. Hospitalization costs were obtained from the Ontario Case Costing Initiative (OCCI). Diagnostic and laboratory fees were obtained from the Ontario Schedule of Benefits for Laboratory Services.
Due to the comparative nature of this analysis, we used several costing assumptions to simplify the analysis. We did not include the cost of replacing prosthesis components over time, because we assumed patients in both cohorts used the same external components. Since all individuals receiving osseointegrated prosthetic implants were prior socket users, we excluded the cost of a socket prosthesis device, because this cost would be incurred prior to the implant procedure. Costs of screening to determine patients' eligibility for implants and training costs for surgeons were assumed to be out of scope and were not included. We did not evaluate bilateral osseointegrated prosthetic implants compared to bilateral socket prostheses due to a lack of utility data specific to bilateral amputees and the assumption that the results of a single implant would provide a valid approximation of the results for bilateral implants. We did not include minor procedures along the patient pathway (e.g., staple removal at 3 to 4 weeks postsurgery), due to a lack of costing data and to simplify the model. Personal support workers and home care between the two stages of implant surgery were not costed, because clinical experts indicated that home care would be necessary only in the event of rare complications with wound healing that would require long-term dressings, which would also likely result in implant failure (James Waddell, MD; Amanda Mayo, MD; John Murnaghan, MD; Wade Gofton, MD; email communications, September to November 2018). Finally, we did not account for differences in maintenance costs in the reference case analysis, such as the number of visits to a prosthetist as outlined by Haggstrom et al,52 due to both insufficient patient-level data and data on the average cost per prosthetist visit. However, we did conduct a scenario analysis to test this assumption.
Table 20 presents itemized costs for the “implant surgery and recovery” health state, which includes patients who undergo a two-stage osseointegration surgery and rehabilitation. Costs include the implant system, diagnostic testing for screening eligibility, professional service fees during hospitalization, inpatient hospital costs (including rehabilitation), prosthetist services, and outpatient care. As this procedure has not been conducted in Ontario and there are no specific billing codes, we estimated the costs using proxies informed by expert opinion (Nancy Dudek, MD; Richard Jenkinson, MD; Dan Mead, CP(c); Amanda Mayo, MD; James Waddell, MD; Wade Gofton, MD; email communications, August 2018). As shown in Table 20, the cost of the implant system was derived from a surgical group in Montreal who conducted the first osseointegrated prosthetic implant surgery in Canada (Natalie Habra, MD, email communication, June 2018). After expert consultation, we assumed that postsurgical rehabilitation was provided in an inpatient setting, due to the anticipated need for nursing support with dressing changes and the intensive rehabilitation process (Nancy Dudek, MD, email communication, August 2018). Furthermore, given the specialized nature of the surgery for a low annual volume of patients, we assumed the surgery would be conducted in a select number of specialty hospitals across the province, which would make outpatient rehabilitation inequitable for patients travelling long distances for the procedure. Finally, prosthetist fees could not be directly estimated using the Limb Prostheses (Conventional) Product Manual53 from the province's Assistive Device Program (ADP), so we used hourly rates for clinical ($183.29/hour) and technical ($126.22/hour) prosthetic services in Ontario (as described in the product manual under “modifications not listed”).54 This information, alongside the estimated time for services required by patients with osseointegrated prosthetic implants described in Frossard et al,55 provided a cost estimate for prosthetic services. The model incorporated only 75% of the total cost of prosthetic services; the ADP requires patients to cover the remaining 25%, and, because the model took the perspective of the Ministry of Health and Long-Term Care, we excluded out-of-pocket expenses.
Table 20:
Health State Costs—Implant Surgery and Recovery
| Variable | Unit Cost, $ | Duration/Quantity | Total Cost, $ | Standard Errora | Reference |
|---|---|---|---|---|---|
| Device cost | |||||
| Internal OGAP-OPLb | 21,500 | 1 | 21,500 | Montreal surgeryc | |
| External OGAP-OPLd | 11,000–15,000 | 1 | 15,000 | Montreal surgeryc | |
| Presurgery diagnostice | |||||
| X-ray of femur | 31 | 1 | 31 | SoB X223 | |
| Bone density survey, CT | 103 | 1 | 103 | SoB X155 | |
| Professional feesf | |||||
| Presurgery consultation | |||||
| Surgeon | 160 | 1 | 160 | SoB A935 | |
| Surgeon | 52 | 1 | 52 | SoB A066 | |
| Physiatrist | 173 | 1 | 346 | SoB A315 | |
| Physiatrist | 74 | 1 | 74 | SoB A313 | |
| Surgery, stage 1 | |||||
| Surgical | 1,304 | NA | 1,304 | SoB R241 | |
| Assistant | 12 | 8 BU & 12 TU | 241 | SoB R241 | |
| Anesthesia | 15 | 15 BU & 14 TU | 435 | SoB R241 | |
| Postsurgery, surgeon consultation | 43 | 1 | 43 | SoB A063 | |
| Surgery, stage 2 | |||||
| Surgical | 477 | NA | 477 | SoB R074 | |
| Assistant | 12 | 6 BU & 4 TU | 120 | SoB R074 | |
| Anesthesia | 15 | 7 BU & 4 TU | 165 | SoB R074 | |
| Postsurgery, surgeon consultation | 43 | 1 | 43 | SoB A063 | |
| Rehabilitation | |||||
| Medical reassessment | 65 | 1 | 65 | SoB C314 | |
| Team management | 39 | 14 | 546 | SoB H312 | |
| Rehab counsel | 77 | 2 | 154 | SoB H313 | |
| Case conference | 31 | 2 | 62 | SoB K121 | |
| Discharge summary | 59 | 1 | 59 | SoB C124 | |
| Inpatient stayg | 32,477 | 23.2 days | 32,477 | 3,101 | OCCI, CMG 180 |
| Prosthetic fittingh | |||||
| Consultation after surgeries | $183.29/hour | 2 hours | 275 | Frossard et al, 201755 | |
| Prefitting of definitive limb | $126.22/hour | 1 hours | 94 | Frossard et al, 201755 | |
| Fitting of definitive limb | $183.29/hour | 10 hours | 1,374 | Frossard et al, 201755 | |
| Outpatient care | |||||
| Physiotherapy | $312 per episode of care | 1 | 312 | Ministry of Health and Long-Term Carei | |
| Surgeon follow-up | 24 | 2 | 48 | SoB A064 | |
| Yearly physiatrist follow-up | 74 | 1 | 74 | SoB A313 | |
Abbreviations: BU, basic units; CMG, case mix group; CT, computed tomography; NA, not applicable; OGAP-OPL, The Osseointegration Group of Australia Osseointegration Prosthetic Limb; OCCI, Ontario Case Costing Initiative; Rehab, rehabilitation; SoB, Ontario Physician Schedule of Benefits; TU, time units.
Table 20 notes continued on the next page.
Table 20 notes continued from the previous page.
Gamma distributions were used in the probabilistic reference case analysis using both the mean and standard error. In cases where a standard error is not provided, the values were assumed to be fixed.
Indicates internal components of the system, including the implant and dual cone.
Natalie Habra, MD, email communication, June 8, 2018.
Indicates external components of the system, including the taper sleeve, screw, bushing, and connector.
Diagnostic procedures excluded indirect costs (i.e., overhead expenses relating to the running of hospitals, such as administration, finance, human resources, plant operations, etc.) and only included direct costs (i.e., costs directly related to the provision of care to the patient, including nursing, diagnostic imaging, pharmacy, and laboratory costs).
Basic and time units were multiplied by either the anesthesiologist unit fee ($15.01) or the assistant fee ($12.04) to calculate total cost.
Derived from the Ontario Case Costing Initiative, using inpatient records from the Ottawa Hospital, a hospital with a specialized musculoskeletal rehabilitation program that provides inpatient beds to amputees. This stay includes both a patient's postoperative and rehabilitation stay.
As this analysis took the perspective of the Ministry of Health and Long-Term Care, total costs assumed only 75% coverage through the Assistive Device Program, the current cost to the ministry.
Ministry of Health and Long-Term Care, Clinic-based physiotherapy [Internet]. Cited July 2, 2018 from: http://www.health.gov.on.ca/en/pro/programs/physio/physio_pro_doctors_nurses.aspx.
Fee codes (in order of appearance in table):
SoB X223: Lower extremities, femur including one joint, three or more views
SoB X155: Dual-energy x-ray absorptiometry (DXA) by axial technique only, subsequent test, high-risk patient
SoB A935: General/orthopedic surgery, special surgical consultation
SoB A066: Repeat consultation (orthopedic surgery's general listings)
SoB A315: Consultation (physical medicine and rehabilitation's general listings)
SoB A313: Medical specific assessment (physical medicine and rehabilitation's general listings)
SoB R241: Revision total arthroplasty hip, one or both components (acetabular or femoral)
SoB A063: Specific assessment (orthopedic surgery's general listings)
SoB R074: Skin-flaps – rotations, transpositions, Z-plasties
SoB C314: Medical specific reassessment (physical medicine and rehabilitation)
SoB H312: Team management in a rehabilitation unit (first 12 weeks)
SoB H313: Rehabilitation counselling (physical medicine and rehabilitation)
SoB K121: Hospital inpatient case conference (family practice and practice in general)
SoB C124: Subsequent visits by the most responsible physician (day of discharge)
CMG 180: Amputation of limb except hand/foot
SoB A604: Partial assessment (orthopedic surgery's general listings)
Table 21 contains the costs components of the remaining model health states (i.e., osseointegrated prosthesis, implant extraction, reimplant, uncomfortable socket prosthesis). In both the “osseointegrated prosthesis” and “uncomfortable socket prosthesis” health states, we assumed patients would have a yearly physiatrist check-up. However, when costing physiatrist visits for complications, we assumed the socket cohort had 4 annual visits, while the osseointegration cohort had their physiatrist visits built into the model whenever a complication occurred (Nancy Dudek, MD, email communication, August 2018). Fixing the number of physiatrist visits for socket-related complications was necessary as few complication rates were available in the literature to inform the model, yet experts indicated this population would be frequently seen for active problems such as socket pain and cysts (Nancy Dudek, MD; James Waddell, MD; email communications, August to September 2018). For the “reimplant” health state, we assumed that inpatient costs and prosthetic fitting would both cost 25% less than the initial implantation. Expert consultation indicated that rehabilitation following reimplantation would go through the same progressive weight-bearing following a repeat surgery, but there would be no need for additional gait training once full weight-bearing was achieved, as the patient would have already learned how to optimize their walking pattern (Nancy Dudek, MD, email communication, August 2018).
Table 21:
Health State Costs—Other Health States
| Variable | Unit Cost, $ | Duration/Quantity | Total Cost, $ | Standard Errora | Reference |
|---|---|---|---|---|---|
| Osseointegrated prosthesis | |||||
| Yearly physiatrist check-up | 74 | 1 | 74 | SoB A313 | |
| Implant extraction | |||||
| Professional feesb | |||||
| Surgical | 1,304 | NA | 1,304 | SoB R241 | |
| Assistant | 12 | 8 BU & 12 TU | 241 | SoB R241 | |
| Anesthesia | 15 | 15 BU & 14 TU | 435 | SoB R241 | |
| Inpatient stay | 14,979 | 7.7 days | 14,979 | 413 | OCCI – CMG 317 |
| Reimplant | |||||
| Device cost | |||||
| Internal OGAP-OPLc | 21,500 | 1 | 21,500 | Montreal surgeryd | |
| External OGAP-OPLe | 11,000–15,000 | 1 | 15,000 | Montreal surgeryd | |
| Professional fees | |||||
| Surgery | |||||
| Surgical | 1,304 | NA | 1,304 | SoB R241 | |
| Assistant | 12 | 8 BU & 12 TU | 241 | SoB R241 | |
| Anesthesia | 15 | 15 BU & 14 TU | 435 | SoB R241 | |
| Postsurgery, surgeon consultation | 43 | 1 | 43 | SoB A063 | |
| Rehabilitation | |||||
| Medical reassessment | 65 | 1 | 65 | SoB C314 | |
| Team management | 39 | 14 | 546 | SoB H312 | |
| Rehab counsel | 77 | 2 | 154 | SoB H313 | |
| Case conference | 31 | 2 | 62 | SoB K121 | |
| Discharge summary | 59 | 1 | 59 | SoB C124 | |
| Inpatient stayf | 32,477 | NA | 24,357 | 2,326 | Assume 25% reduction from initial implant surgery |
| Prosthetic fittingg | |||||
| Fitting of definitive limb | $183.29/hour | 10 hours | 1,030 | Assume 25% reduction from initial implant surgery | |
| Outpatient care | |||||
| Physiotherapy | $312 per episode of care | 1 | 312 | Ministry of Health and Long-Term Careh | |
| Surgeon follow-up | 24 | 2 | 48 | SoB A064 | |
| Yearly physiatrist follow-up | 74 | 1 | 74 | SoB A313 | |
| Uncomfortable socket prosthesis | |||||
| Yearly physiatrist check-up | 74 | 1 | 74 | SoB A313 | |
| Physiatrist visit for complication | 71 | 4 | 142 | SoB A311 | |
Abbreviations: BU, basic units; CMG, case mix group; CT, computed tomography; NA, not applicable; OGAP-OPL, The Osseointegration Group of Australia Osseointegration Prosthetic Limb; OCCI, Ontario Case Costing Initiative; Rehab, rehabilitation; SoB, Ontario Physician Schedule of Benefits; TU, time units.
Gamma distributions were used in the probabilistic reference case analysis using both the mean and standard error. In cases where a standard error is not provided, the values were assumed to be fixed.
Basic and time units were multiplied by either the anesthesiologist unit fee ($15.01) or the assistant fee ($12.04) to calculate total cost.
Table 21 notes continued on the next page.
Table 21 notes continued from the previous page.
Indicates internal components of the system, including the implant and dual cone.
Natalie Habra, MD, email communication, June 8, 2018.
Indicates external components of the system, including the taper sleeve, screw, bushing, and connector.
Derived from the Ontario Case Costing Initiative, using inpatient records from the Ottawa Hospital, a hospital with a specialized musculoskeletal rehabilitation program that provides inpatient beds to amputees. This stay includes both a patient's postoperative and rehabilitation stay.
As this analysis took the perspective of the Ministry of Health and Long-Term Care, total costs assumed only 75% coverage through the Assistive Device Program, the current cost to the ministry.
Ministry of Health and Long-Term Care, Clinic-based physiotherapy [Internet]. Cited July 2, 2018 from: http://www.health.gov.on.ca/en/pro/programs/physio/physio_pro_doctors_nurses.aspx
Fee codes (in order of appearance in table):
SoB A313: Medical specific assessment (physical medicine and rehabilitation)
SoB R241: Revision total arthroplasty hip, one or both components (acetabular or femoral)
CMG 317: Revised hip replacement without infection
SoB A063: Specific assessment (orthopedic surgery's general listings)
SoB C314: Medical specific reassessment (physical medicine and rehabilitation)
SoB H312: Team management in a rehabilitation unit (first 12 weeks)
SoB H313: Rehabilitation counselling (physical medicine and rehabilitation)
SoB K121: Hospital inpatient case conference (family practice and practice in general)
SoB C124: Subsequent visits by the most responsible physician (day of discharge)
SoB A604: Partial assessment (orthopedic surgery's general listings)
SoB: A311: Complex medical specific reassessment
Table 22 describes costs for complications. We assumed all complications except superficial infections were treated in an inpatient setting. Femoral fractures can be subdivided into fractures requiring fixation (stable stem) and complex fractures requiring fixation and implant revision (unstable stem). As the rates of femoral fracture related to osseointegrated prosthetic implants reported in the literature did not differentiate between stable and unstable stems, we assumed the rate for implant revision would include fractures requiring both fixation and implant revision; therefore, femoral fractures were costed as requiring only fixation (i.e., for people with these fractures, an implant revision is unnecessary). For superficial infections, we assumed patients would visit their physiatrist and receive a prescription for antibiotics. Patients with a deep infection would be admitted to a hospital and undergo a debridement procedure.
Table 22:
Complication Costs
| Variable | Unit Cost, $ | Duration/Quantity | Total Cost, $ | Standard Errora | Reference |
|---|---|---|---|---|---|
| Osseointegration complications | |||||
| Soft-tissue refashioning | |||||
| Physiatrist visit for complication | 71 | 1 | 71 | SoB A311 | |
| Professional fees | |||||
| Surgical | 325 | NA | 326 | SoB R242 | |
| Assistant | 12 | 6 BU & 8 TU | 168 | SoB R242 | |
| Anesthesia | 15 | 7 BU & 8 TU | 225 | SoB R242 | |
| Inpatient stay | 8,313 | 4.6 days | 8,313 | 1,034 | OCCI – CCI 1VD80LA |
| Femoral fracture | |||||
| Professional fees | |||||
| Surgical | 493.8 | NA | 494 | SoB F096 | |
| Assistant | 12 | 6 BU & 14 TU | 240 | SoB F096 | |
| Anesthesia | 15 | 8 BU & 17 TU | 375 | SoB F096 | |
| Inpatient stay | 12,141 | 7.6 days | 12,141 | 493 | OCCI – ICD S72300 |
| Superficial infection | |||||
| Physiatrist visit for complication | 71 | 1 | 71 | SoB A311 | |
| Deep infection | |||||
| Physiatrist visit for complication | 71 | 1 | 71 | SoB A311 | |
| Professional fees | |||||
| Surgical | 470 | NA | 470 | SoB R423 | |
| Assistant | 12 | 6 BU & 8 TU | 168 | SoB R423 | |
| Anesthesia | 15 | 7 BU & 8 TU | 225 | SoB R423 | |
| Inpatient stay | 14,535 | 9.9 days | 14,535 | 1,270 | OCCI – ICD T8463 |
| Socket complications | |||||
| Stump revision | |||||
| Physiatrist visit for complication | 71 | 1 | 71 | SoB A311 | |
| Professional fees | – | – | 719 | Assume soft tissue value | |
| Inpatient stay | – | – | 8,313 | 1,034 | Assume soft tissue value |
Abbreviations: BU, base units; CCI, Canadian Classification of Interventions; ICD, International Classification of Diseases; OCCI, Ontario Case Costing Initiative; NA, not applicable; SoB, Ontario Physician Schedule of Benefits; TU, time units.
Gamma distributions were used in the probabilistic reference case analysis using both the mean and standard error. In cases where a standard error is not provided, the values were assumed to be fixed.
Fee codes (in order of appearance in table):
SoB A311: Complex medical specific reassessment
SoB R242: Femur, incision and drainage (bone)
CCI 1VD80LA: Repair, muscles of hip and thigh, using open approach and apposition (suture, staple) (includes fascioplasty)
SoB F096: Fracture, closed reduction, open reduction
ICD S72300: Fracture of shaft of femur, closed
SoB R423: Synovectomy/debridement
ICD T8463: Infection and inflammatory reaction due to internal fixation device of femur
Analysis
For the reference case analysis, we performed a probabilistic analysis to determine the mean incremental cost and mean incremental QALYs, and we calculated the incremental cost-effectiveness ratio (ICER) for an osseointegrated prosthesis compared with an uncomfortable socket prosthesis. We performed a probabilistic sensitivity analysis by running 5,000 Monte Carlo simulations to capture parameter uncertainty. When possible, we specified distributions around each estimate, using the mean and standard deviation. Costs were characterized by gamma distributions, and probabilities and utilities were characterized by beta distributions.
In addition to the reference case results described, we present the impact of uncertainty and variability through a cost-effectiveness acceptability curve.
We validated our economic evaluation by verifying the TreeAge model and its inputs, communicating with clinical experts to ensure the model had face validity, and cross-validating the results with previously published economic evaluations addressing similar decision problems.
Deterministic Sensitivity Analysis
We conducted deterministic sensitivity analyses to assess how sensitive our reference case results were to specific parameters. In the one-way sensitivity analyses, we varied specific model variables (e.g., transitional probabilities, costs, utilities) and recorded and presented their impact on the results in a tornado diagram. Details of these analyses and the specific parameters varied are presented in Appendix 6, Table A6.
Scenario Analyses
As described in Table 23, we conducted several scenario analyses. Given that osseointegrated prosthetic implant surgery has yet to be performed in Ontario, these scenario analyses tested not only different input parameters, but also some of the assumptions required to estimate Ontario-specific costs. For each scenario, we recalculated the mean incremental costs and QALYs for each treatment, along with the ICER. All scenarios were performed probabilistically unless otherwise stated. Appendix 6, Tables A7 and A8, provide a full list of input parameters.
Table 23:
Scenario Analyses
| Parameter | Parameter/Assumption in Reference Case | Parameter/Assumption in Scenario Analysis |
|---|---|---|
| Time horizon | Lifetime | 6 years, 10 years, 20 years |
| Discount rate | 1.5% | 0%, 3%, 5% |
| Discount implant system price | 0% | 10%, 25%, 50% |
| Additional outpatient physiotherapy | Included | Excluded |
| Yearly maintenance costs by a prosthetist | Excluded | Included |
| Deep infection | Inpatient | Outpatient |
| Complication: femoral fractures | Included | Excluded |
| Complication: mechanical part replacement | Excluded | Included |
| Stump revision rate | Hospital data from Hansson et al, 201845 | Assume same as soft tissue refashioning in osseointegration cohort |
| Event rates | Pooled rates | Time-varying rates10 |
| Disutilities | Excluded | Included |
| Utilities | Hagberg et al, 201426 | Hagberg et al, 200813; Branemark et al, 201410 |
| Surgery type | Two-stage surgery | Single-stage surgery |
Time Horizon
We varied the time horizon based on previously published studies. A time horizon of 10 years (mean 7.9 years) approximates the longest average follow-up recorded in an observational study on osseointegrated prosthetic implants,30 while time horizons of 6 years and 20 years represent inputs used in other cost–utility analyses.44,45
Discounting
In accordance with CADTH guidelines, to evaluate the impact of uncertainty in the discount rate, we incorporated discount rates of 0% and 3% per year, along with an additional analysis at 5%, which corresponds to discount rates recommended in previous CADTH guidelines.47
Costs
Device costs were taken from the first known lower-limb osseointegration procedure conducted in Canada (Natalie Habra, MD, email communication, June 2018). There may be potential for the cost of the device to be negotiated lower in future, given the anticipated volume of surgeries.
As a conservative assumption, the model currently incorporates the costs of both inpatient and outpatient rehabilitation after implant surgery. However, due to the intensive inpatient rehabilitation process, it is possible that patients may not require additional rehabilitation services in the outpatient setting. Therefore, we explored excluding it in a scenario analysis.
We modelled differences in maintenance cost in the scenario analysis, such as the number of visits to a prosthetist, as outlined by Haggstrom et al,52 by assuming 7 visits to a prosthetist per year for a socket user and 3 visits for an implant user. The costs were calculated by using the hourly fee for a prosthetist in Ontario ($183.29/hour) and assuming each adjustment session takes 1 hour for an implant user and 2 hours for a socket user (Nancy Dudek, MD, email communication, August 2018).
As identified in the clinical evidence section of this report, a deep infection required surgical intervention in all cases in the literature. Our reference case assumed that patients with deep infection stayed in hospital an average of 9.9 days for postoperative care, but clinical experts indicated that IV antibiotics could be administered in an outpatient or home care setting (Nancy Dudek, MD; Wade Gofton, MD; email communications, November 2018). Therefore, a scenario analysis considered this possibility, and based on the results of Wolter et al,56 we assumed home care expenses would be half the cost of an inpatient stay.
Event Rates
The reference case included costs for femoral fractures but only for the osseointegration cohort, due to insufficient data on the rate of femoral fractures among socket prosthesis users. But that assumption may overestimate the costs for patients with osseointegrated prosthetic implants if the fracture rate in a socket cohort is not close to zero. Therefore, in a scenario analysis, we excluded fractures to consider the possibility that the two cohorts have similar risk of fracture and that the incremental cost difference for treatment is marginal.
The reference case excluded mechanical complications due to varied reporting in the literature and a lack of specific information as to which implant parts broke or were exchanged. The scenario analysis conservatively assumed all external components required replacement.
The reference case parameter for stump revision rates came from a previously published cost– utility analysis that cited rates from hospital data.45 Furthermore, we were unable to determine a measure of uncertainty around the estimate. Therefore, in a scenario analysis we used the soft tissue refashioning rate in the osseointegration cohort for the stump revision rate in the socket cohort.
We pooled event rates for the reference case, as the available studies were of equally low quality and reported varying event rates. In a scenario analysis, we used time-varying event rates from Branemark et al.10 Expert opinion informed us that the risk for complications (e.g., superficial and deep infection), although always present, may be higher immediately following surgery (James Waddell, MD, email communication, September 2018). We calculated time-varying rates for 0 to 12 months postsurgery and 12 to 24 months postsurgery. Rates at 0 to 12 months were assigned to the health states of “implant surgery and recovery” and “reimplant,” while rates at 12 to 24 months were assigned to the health state “osseointegrated prosthesis.”
Disutilities
We excluded disutilities from the reference case as they used measurement methods (i.e., EuroQol [EQ-5D] and Assessment of Quality of life [AQoL] instruments) that differed from the rest of the core model utility values, which were derived from the SF-6D survey. In a scenario analysis, we used disutilities found in the literature for fractures and deep infections. In the model, when a complication occurred in a health state, the state was assigned a disutility value representative of the amount of time individuals would be affected by the complication. The resulting QALYs incurred were calculated as the utility value of the current health state, minus the utility value of the complication multiplied by the duration of the complication (representing the disutility). For example, to calculate the change in QALYs for an individual who is in the “osseointegrated prosthesis” health state and who has a fracture during one model cycle, 0.692 would be subtracted by the result of 0.120 ∗ 0.5 (with 0.5 representing 6 of 12 months), which would result in a QALY of 0.632.26,57 We also tested alternative utility values for the health states in additional scenario analyses.
Surgery Type
Finally, we conducted a scenario analysis where all implant surgeries were single-stage instead of two-stage. We estimated that compared to two-stage surgery, single-stage surgeries would have lower costs, as they would have reduced inpatient stays and would not require multiple operating room days to complete the procedure. As there is currently no published literature evaluating single-stage osseointegrated prosthetic implant surgery for lower-limb amputees, the model assumed the same clinical effectiveness, utility gains, and complication rates as two-stage surgery.
Generalizability
The findings of this economic analysis cannot be generalized to all patients with a lower-limb amputation. They may, however, be used to guide decision-making about the specific patient populations addressed in the studies investigated by Health Quality Ontario.
Results
Reference Case Analysis
Table 24 presents results from the reference case analysis. Over a lifetime horizon, the osseointegration cohort had an average total cost of $101,166 and 19.12 QALYs per person. Compared with an uncomfortable socket prosthesis, an osseointegrated prosthetic implant has an incremental cost of $84,559 and an incremental effect of 0.890 QALYs. The reference case ICER for an osseointegrated prosthetic implant compared with an uncomfortable socket prosthesis is $94,987 per QALY gained.
Table 24:
Reference Case Analysis Results
| Strategy | Average Total Costs, $ (95% CI) | Incremental Cost, $a (95% CI) | Average Total Effects, QALYs (95% CI) | Incremental Effect, QALYsb (95% CI) | ICER, $ |
|---|---|---|---|---|---|
| Uncomfortable socket prosthesis | 16,607.11 (15,227–18,139) | – | 18.2318 (17.38–19.05) |
– | – |
| Osseointegrated prosthetic implant | 101,166.88 (90,315–113,193) | 84,559.77 (73,611–96,703) | 19.1220 (18.35–19.87) | 0.8902 (−0.12 to 1.91) | 94,987.49 |
Abbreviation: CI, confidence interval; QALYs, quality-adjusted life-years; ICER, incremental cost-effectiveness ratio.
Incremental cost = average cost (osseointegration) – average cost (uncomfortable socket).
Incremental effect = average effect (osseointegration) – average effect (uncomfortable socket).
The cost-effectiveness acceptability curve presented in Figure 4 shows the probability of both interventions (osseointegrated prosthetic implants and uncomfortable socket prostheses) being cost-effective across a range of willingness-to-pay values. At a willingness-to-pay value of $100,000 per QALY, the probability of osseointegrated prosthetic implants being cost-effective was 54.17%. As the willingness-to-pay value crossed $40,000 per QALY and continued to rise, the probability of implants (the more costly strategy) being cost-effective also rose. Appendix 6, Figure A1, presents an incremental cost-effectiveness scatterplot for the reference case results.
Figure 4: Cost-Effectiveness Acceptability Curve—Osseointegrated Prosthetic Implant vs. Uncomfortable Socket Prosthesis.
Sensitivity Analysis
One-Way Sensitivity Analyses
Figure 5 presents the results of the one-way sensitivity analyses through a tornado diagram. The ICER was highly sensitive to five variables: the utilities for osseointegrated prosthesis and uncomfortable socket prosthesis, time horizon, implant extraction rate, and stump revision rate. When the utility input for the “osseointegrated prosthesis” health state (reference case = 0.692) was set at 0.726 (high estimate), the ICER dropped to $51,620 per QALY gained, but rose to $653,555 per QALY gained if the utility input was 0.658 (low estimate). When the model time horizon dropped below a lifetime horizon to only 5 years, the ICER rose to a peak of $265,581 per QALY gained. As the probability of implant extraction rose to 0.0581, the ICER increased to $154,899 per QALY gained, but if the implant extraction rate dropped to 0.0057 the resulting ICER was $78,062 per QALY gained. Finally, if the stump revision rate in socket prosthesis users increased to 0.186 (a rate similar to the soft-tissue refashioning in the osseointegration cohort) the ICER dropped to $57,076 per QALY gained.
Figure 5: Tornado Diagram—Osseointegrated Prosthetic Implant vs. Uncomfortable Socket Prosthesis.
Abbreviations: ICER, incremental cost-effectiveness ratio; Misc, miscellaneous input; osseo, osseointegration; Prob, probability.
Scenario Analyses
Table 25 presents the results of all scenario analyses, previously described (see Table 23). They show a wide range of ICER values based on variations in model inputs and model assumptions. At a willingness-to-pay value of $100,000 per QALY, the probability of osseointegrated prosthetic implants being cost-effective in the reasonable best scenario was 92.2% and in the reasonable worst scenario was 35.1%.
Table 25:
Scenario Analysis Results
| Parameter | Incremental Cost, $a | Incremental Effectb | ICER, $ |
|---|---|---|---|
| Time horizon | |||
| 6 years | 48,575.62 | 0.207506204 | 234,092.37 |
| 10 years | 55,184.04 | 0.330084604 | 167,181.51 |
| 20 years | 68,475.95 | 0.578449714 | 118,378.40 |
| Discount rate | |||
| 0% | 98,298.95 | 1.157704883 | 84,908.47 |
| 3% | 75,493.78 | 0.718649876 | 105,049.46 |
| 5% | 67,195.98 | 0.555546203 | 120,954.81 |
| Discount implant system price | |||
| 10% | 82,642.94 | 0.885869181 | 93,290.23 |
| 25% | 79,194.06 | 0.894915578 | 88,493.33 |
| 50% | 73,618.27 | 0.889871027 | 82,729.15 |
| Outpatient physiotherapy excluded | 84,696.61 | 0.889031972 | 95,268.35 |
| Yearly prosthetist maintenance included | 55,830.01 | 0.882939573 | 63,231.97 |
| Deep infection, outpatient postsurgical care | 80,880.17 | 0.887204644 | 91,162.93 |
| Complications | |||
| No femoral fractures | 80,073.21 | 0.891213462 | 89,847.39 |
| Mechanical part replacement included | 101,061.04 | 0.891483839 | 113,362.72 |
| Stump revision rate | 45,519.44 | 0.89056097 | 51,113.22 |
| Time-varying event rates | 45,204.25 | 0.883428266 | 51,169.12 |
| Disutilities included | 84,778.20 | 0.80889623 | 104,807.27 |
| Utilities | |||
| Hagberg et al, 200813 | 84,768.21 | 1.324919 | 63,979.92 |
| Branemark et al, 201410 | 84,751.93 | 0.896983532 | 94,485.49 |
| Single-stage surgery | 84,354.86 | 0.889765366 | 94,805.73 |
| Reasonable best and worst scenarios | |||
| Reasonable bestc | 13,597.10 | 0.893327498 | 15,220.74 |
| Reasonable worstd | 101,060.54 | 0.814835036 | 124,025.77 |
Abbreviation: ICER, incremental cost-effectiveness ratio.
Incremental cost = average cost (osseointegration) – average cost (uncomfortable socket).
Incremental effect = average effect (osseointegration) – average effect (uncomfortable socket).
Reasonable best scenario includes the following changes to the reference case: a 10% reduction in osseointegrated prosthetic implant cost, inclusion of prosthesis maintenance costs (yearly visits to prosthetist for adjustments), use of single-stage implant surgery, and time-varying rates of complications.
Reasonable worst scenario includes the following changes to the reference case: inclusion of mechanical part replacement and disutilities for complications (fractures, deep tissue infection).
Discussion
The results of the analysis indicated that, in eligible patients, an osseointegrated prosthetic implant is more costly and more effective than continuing to use an uncomfortable socket prosthesis. The reference case analysis showed osseointegrated prosthetic implants, when compared to an uncomfortable socket prosthesis, had an ICER of $94,987 per QALY gained, which corresponds to a 54.2% probability of being cost-effective at a willingness-to-pay value of $100,000 per QALY. As willingness-to-pay values increased, the probability of implants being cost-effective increased to 74.2% at $150,000 per QALY.
However, sensitivity analyses indicated these results were largely influenced by both parameter uncertainty and key assumptions. One input to note is the probability of stump revisions in the uncomfortable socket cohort. This was the only complication evaluated for the socket cohort in our model, and given a lack of evidence in the published literature, we used a probability estimate taken from hospital data from a previous cost–utility analysis for our reference case.45 This probability estimate was the lowest complication in the model (0.026) and was slightly less common than infrequent complications in the osseointegration cohort such as fractures (0.027) and deep infections (0.033). The reference case estimate may be conservative, given that stump revisions often treat a wide variety of conditions related to an uncomfortable socket prosthesis, such as bone spikes, soft tissue–socket interface problems, neuromas, and infections.58 A one-way sensitivity tested this by increasing the probability to the same value as osseointegration's soft tissue refashioning (0.18), and the ICER decreased to $45,519 per QALY gained.
Osseointegrated prosthetic implants are a novel technology in Ontario, and estimating costs required that we use proxy values, validated by clinical experts. Owing to the uncertainty this creates, we designed the model's reference case to conservatively estimate the cost-effectiveness of osseointegration. For example, the reference case did not include the cost of prosthetist visits for prosthesis maintenance and adjustment, even though these costs appear to be a key driver of cost-effectiveness in osseointegrated prosthetic implants. This decision was due to a lack of data to inform the cost differences between cohorts. Ontario has not developed fee codes for prosthetist care for amputees using an osseointegrated prosthetic implant; instead, we calculated these fees using an hourly rate for clinical and technical prosthetist services, which is typically used for cases where there are modifications not listed; for billing, these services require approval from the ministry's Assistive Devices Program. Although a previous publication found that implant users visit a prosthetist less frequently than conventional socket users (7 vs. 3 visits per year), the cost of each visit was difficult to estimate as it is specific to the purpose of the visit.52 Based on expert feedback, we expected the average cost of a prosthetist appointment would be lower for implant users because the soft tissue–socket interface is eliminated (Dan Mead, CP(c), email communication, August 2018). To estimate costs, we assumed each adjustment would take 1 hour for an implant user and 2 hours for a socket user (Nancy Dudek, MD, email communication, August 2018). Despite published data on the number of maintenance visits, we excluded prosthetic maintenance fees from the reference case due to the uncertainty around the true cost difference between cohorts.
We used scenario analyses to test various model assumptions. Creating a “reasonable best scenario” and “reasonable worst scenario” helps to better understand how the model assumptions and variability of the inputs impact cost-effectiveness when combined. Both best and worst reasonable scenarios excluded changes to the time horizon, discount rate, and utilities, and instead focused on other “reasonable” inputs. These analyses excluded alternative utility values from Branemark et al10 and Hagberg et al13 because those studies mapped health-related quality of life scores to derive utilities, specifically from mean SF-36 domain scores to SF-6D utility values. This technique, although useful if there are data limitations, is less precise and accurate than utility values directly elicited from a SF-6D questionnaire.
The ICER obtained from the reasonable best scenario was heavily influenced by the inclusion of prosthetist maintenance costs and time-varying complication rates. Unlike in the reference case, the reasonable best scenario used time-varying rates from a single study, instead of an average weighted rate from multiple studies. In that single study, no cases of soft tissue refashioning occurred, and there were no cases of deep infection after the first year. When modelling the time-varying complication rates, we used the event rate in the second year (zero) for all subsequent years. As there would be no cases of deep infection after the first year, compared to the reference case this approach would underestimate the costs of deep infections over a lifetime horizon. Based on these limitations, we did not use time-varying complication rates in the reference case. However, the single study informing these rates does suggest that rates of superficial and deep infection are lower in the second year after osseointegration surgery.10 If complication rates do decrease after the first year, our reference case model with its constant rates may overestimate the occurrence and costs of complications over a patient's lifetime. Other inputs used in the reasonable best scenario include a reduction in the device cost for osseointegrated prosthetic implants, as the cost in the reference case was based on a cost reported by a surgeon performing a single surgery. As previously discussed, another input, single-stage vs. two-stage osseointegration surgery, is a newer development that could reduce operating room costs but as yet has no published evidence to inform its effectiveness and complication rates.
The reasonable worst scenario included two key inputs that were excluded from the reference case due to insufficient evidence to properly inform their estimates. The first, the inclusion of mechanical part replacement, was derived from studies that did not specify the implant parts that required replacement, so we conservatively assumed that all external parts were replaced. The second, the inclusion of disutility values for complications (i.e., fractures and deep infections), was not specific to lower-limb amputees and was based on values from instruments other than the model's SF-6D values (i.e., EuroQol 5-Dimension [EQ-5D] and Assessment of Quality of Life [AQoL]). Further research is needed to refine these important model inputs, which influence the cost-effectiveness of osseointegrated prosthetic implants for lower-limb amputees.
Model Considerations
In our economic evaluation, we assessed osseointegrated prosthetic implants as a therapeutic class to inform recommendations about public funding the intervention. We did not differentiate between manufacturers, primarily due to a lack of manufacturer-specific data that could inform all model inputs. However, it should be noted that the majority of publications used to inform this analysis were from the OPRA and ILP systems. There is uncertainty around whether the longevity of the implant differs among the systems currently available and around whether implant design leads to different challenges in the event of surgical removal and reimplantation.
The model assumed that rehabilitation of patients undergoing osseointegration surgery took place in an inpatient setting. Despite costing more than care in an outpatient setting, an inpatient rehabilitation stay likely best represents both the clinical and Ontario health care context. A lengthy, intensive rehabilitation process is required as patients progress through a gradual, stepped approach to weight-bearing on the prosthetic limb.29 Clinical experts indicated that, if the program is implemented, it should be restricted to a select number of specialty health centres with interdisciplinary teams of surgeons, physiatrists, nurses, and physiotherapists. This approach would promote optimal surgical performance by ensuring surgeons receive an adequate annual volume of osseointegration surgeries (Wade Gofton, MD, email communication, August 2018). Given this advice, we assumed that patients from across the province would travel to these centres to receive care and that a lengthy outpatient rehabilitation would be a burden to all but those living nearby. Inpatient rehabilitation was also anticipated so that wound care and medications could be actively managed by nursing staff after surgery and into early rehabilitation (Nancy Dudek, MD, email communication, August 2018).
To simplify economic costing, this model assumed external components were similar for socket and osseointegrated prostheses. Therefore, incremental costs and utility gains attributed to these components were excluded from the analysis. If future data become available, this assumption should be tested further, because another analysis by Haggstrom et al52 suggested that osseointegrated prostheses may be more costly due to the use of more advanced prosthetic components (e.g., microprocessor knees).
We did not use a societal perspective in this analysis due to insufficient data on the target population. However, osseointegrated prosthetic implants may provide unique benefits, such as allowing a person to go back to work and no longer require disability support because their mobility has improved, which may lead to financial savings from a societal perspective.
Comparison With Other Studies
Our study had distinct differences from two previous cost-effectiveness analyses. The first, by Frossard et al,44 found an ICER of $16,632 per QALY gained, but the study only included prosthetic costs from a dataset at a single prosthetic facility. Specifically, it did not include costs of surgery, rehabilitation, and complications. The authors used utility values similar to those in our reference case, but the overall QALY calculation was simplified as they used neither a Markov model nor other forms of decision-modelling. As the study pulled costs from their real-world dataset, the time horizon was only 6 years to reflect the length of follow-up in the dataset.
Hansson et al45 found an ICER of €83,374 per QALY gained, and based their complications and health state transitions primarily on a single study by Branemark et al.10 This ICER was higher than what was reported in our reference case, but this difference is likely due to the 20-year time horizon, as our scenario with that time horizon found a similar ICER of $123,112 per QALY gained. The authors stated that they chose this time horizon for their reference case as the technology is relatively new and they are uncertain how long patients will benefit from it. However, they do report being aware of 8 patients from previous studies who have passed 15 years of follow-up.45 The cost-effectiveness analysis by Hansson et al45 was similar to our work in that their model was sensitive to changes in the interventions' utility values, and we both used similar utility values and clinical complications. Of note, both Frossard et al44 and Hansson et al45 differed from our analysis in that they did not use discounting, with one stating that costs were not discounted because the majority of costs occurred in the first and third years, when prosthetic knees and feet were supplied.44
Strengths and Limitations
Our primary economic evaluation has several strengths. It is the first analysis to estimate the economic value in Canada of osseointegrated prosthetic implants for eligible lower-limb amputees. The study used Ontario-specific costs wherever possible, and Canadian costs for the implant system. Our analysis is also the first to include disutilities for complications related to the osseointegration surgery and implant (deep infections and fractures), as well as other analyses such as time-varying rates to inform event transitions. Another strength is the use of pooled rates for health-state transitions and complications to capture parameter variability across the published literature. Additionally, cost estimates were informed by a multidisciplinary team of medical professionals involved in the care pathway, including surgeons, physiatrists, physiotherapists, and prosthetists.
Our analysis also has limitations. As the osseointegration procedure is not currently being conducted in Ontario, the Schedule of Benefits has no physician fee codes to inform precise estimates of surgical costs and inpatient stays. In the absence of costing data, we used proxies validated by clinical experts. We also used simplifying assumptions in the absence of established clinical practices in Ontario for patients with osseointegrated prosthetic implants. There are potential areas for additional costs that we did not evaluate, such as additional prosthetist adjustments after femoral fractures in implant users or prosthetic adjustments to alter socket fit following stump revisions in conventional prosthesis users. As described earlier, the data on users of uncomfortable socket prostheses are limited. As a result, we could not cost additional issues identified by experts: scar revisions, wound care, superficial infections, material and suspension system costs for prosthetist socket adjustment and replacement, and (if we included a societal perspective) out-of-pocket costs for antibiotics, antifungals, and dressing supplies (Nancy Dudek, MD; Wade Gofton, MD; email communications, November 2018). Another potential limitation is the applicability of the literature to our comparator: patients with an uncomfortable socket prosthesis. Previously published literature did not always specify the comparator in this way, so these studies may include patients with a comfortable socket fit and a preference to switch to an osseointegrated prosthetic implant. Therefore, given the pre-post study design commonly used in this literature, the rates of complications in our uncomfortable socket cohort may have been higher if we were able to strictly enforce this population in our model inputs.
Conclusions
Our economic analysis found that, in patients with a lower-limb amputation and eligible for osseointegration surgery, osseointegrated prosthetic implants had higher costs and greater QALYs gained compared with those living with an uncomfortable socket prosthesis. Results were sensitive to parameter uncertainties and model assumptions. The ICER for the osseointegrated prosthesis compared to an uncomfortable socket prosthesis was $94,987 per QALY gained in the reference case. There was a high degree of uncertainty in the reference case, in which the probability of osseointegration being cost-effective was 54.2% at a willingness-to-pay value of $100,000 per QALY gained.
BUDGET IMPACT ANALYSIS
Research Question
What is the potential 5-year budget impact for the Ontario Ministry of Health and Long-Term Care of publicly funding osseointegrated prosthetic implants for people with a lower-limb amputation who have chronic problems using a conventional socket prosthesis?
Methods
Analytic Framework
We estimated the budget impact of publicly funding osseointegrated prosthetic implants using the cost difference between two scenarios: (1) current clinical practice without public funding for osseointegrated prosthetic implants (the current scenario) and (2) anticipated clinical practice with public funding for osseointegrated prosthetic implants for patients with an uncomfortable socket prosthesis (the new scenario).
We conducted a reference case analysis and sensitivity analyses. Our reference case analysis represents the analysis with the most likely set of input parameters and model assumptions. Our sensitivity analyses explored how the results are affected by varying input parameters and model assumptions. Although the ministry previously funded osseointegrated prosthetic implants through the Out-of-Country (OOC) Prior Approval Program at the Ministry of Health and Long-term Care, this funding has since stopped. For our current scenario, we did not select the previous funding by the OOC Prior Approval Program because that funding was only provided for single-stage surgeries and only covered surgery expenses, not the entire care pathway. Therefore, using the same population but two hypothetical cohorts, this analysis compared the cost of publicly funding osseointegrated prosthetic implants versus health services for people who remained users of an uncomfortable socket prosthesis.
Key Assumptions
All patients in both cohorts had a unilateral above-the-knee amputation
Patients used their existing prosthesis components and did not purchase new components following implant surgery, aside from the implant system's components (i.e., connector from the implant to the artificial knee)
Costs for the replacement of external prosthetic components (including the osseointegration external connector) were equal between cohorts
New components were purchased in the event of an implant being reimplanted after failure
The cost of prosthetist services to maintain the prosthesis were equal between cohorts
Target Population
The target population was people eligible for an osseointegrated prosthetic implant, with key criteria being that they had a nonvascular above-the-knee amputation and chronic problems achieving a comfortable fit with a socket prosthesis (see Appendix 6, Table A4, for additional criteria). In Ontario, the incidence of nonvascular lower-limb amputation is approximately 289 people per year (Table 26). An estimated 24% of these amputations are above the knee, and complications related to using a socket prosthesis are common. One Swedish survey on a population with above-the-knee amputations reported a high prevalence of problems related to the use of socket prostheses, with 72% having symptomatic heat and sweating of the stump, 62% reporting sores or skin irritation from the socket, 61% with interferences to mobility, and 51% with pain in the stump when standing or walking.7 However, not all of those reported complications would necessarily lead to socket intolerance (Amanda Mayo, MD, email communication, November 2018).
Table 26:
Target Population Estimates
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
|---|---|---|---|---|---|
| Incidence of nonvascular lower-limb amputees, na | 289 | 293 | 297 | 301 | 305 |
| Estimated number of above-the-knee amputees, nb | 69 | 70 | 71 | 72 | 73 |
| Estimated number eligible for osseointegration, nc | 37 | 7 | 7 | 7 | 7 |
| Number of osseointegration surgeries conducted, nd | 20 | 20 | 11 | 7 | 7 |
Incidence rate of nonvascular lower-limb amputations in Canada was approximately 2 per 100,000 individuals, and we used the Ontario population projections from the Ontario Ministry of Finance. Includes all levels of lower-limb amputation such as femoral, tibial, partial foot, and ankle disarticulation.1,59
Assuming 24% of all nonvascular lower-limb amputees are above-the-knee (Nancy Dudek, MD, email communication, November 2018).
Assuming 10% of above- and below-the-knee amputees are eligible for osseointegration. Year 1 includes the yearly incidence plus the estimated prevalence of 30 patients. Estimates were informed by a clinical expert (Nancy Dudek, MD, email communication, November 2018).
Based on a capacity of 20 surgeries per year conducted by one specialty hospital.
Given that eligibility for osseointegrated prosthetic implants is highly selective, requiring more than socket-related problems, only a certain percentage of nonvascular amputees would be candidates for this procedure. As data on this specific subset are unavailable, we estimated the target population using clinical expert opinion (Nancy Dudek, MD, email communication, November 2018) and tested this estimate through sensitivity analyses. For the reference case, we estimated a yearly incidence of approximately 7 above-the-knee amputees who would be eligible for an osseointegrated prosthetic implant, and in the first year an additional 30 people, representing the prevalent cases. To account for population growth, we applied the same year-over-year rise in nonvascular lower-limb amputees to the estimated yearly incidence but, due to the small numbers involved, this did not increase the number of people eligible over 5 years.
We then determined the maximum number of procedures that could be conducted, based on expert opinion about current system capacity and resource constraints (Nancy Dudek, MD, November 2018; Wade Gofton, MD; email communications, November 2018). We assumed all surgeries would take place at one centre, representing a capacity of 50 surgeries and 20 inpatient rehabilitation patients per year. As both surgery and rehabilitation are required, we used the lower capacity, resulting in an estimate of 20 patients treated per year. Based on the estimates in Table 26, this capacity would exceed the number of eligible patients, allowing for an additional 35 patients, in total, in years 3 to 5.
Resources and Costs
For each cohort, we obtained the cost per patient from our primary economic evaluation (Appendix 7, Table A9). We used annual undiscounted costs for five years from the base case analysis of the primary economic evaluation. These included resource use and costs related to medical devices, surgery and rehabilitation, prosthetic fitting, and adverse events. We have provided a detailed description of these costs in the methods section of the primary economic evaluation. All costs are reported in 2018 Canadian dollars.
Analysis
We estimated the net budget impact of funding osseointegrated prosthetic implants as the cost difference between two scenarios: the current scenario and the new scenario. We calculated the annual costs for 2018 by multiplying the volume of patients in year 1 (see Table 26) by the first-year treatment costs. We calculated annual costs for subsequent years using the ongoing costs of year 1 patients and costs of volumes of patients expected in respective years.
In addition to the reference case, we analyzed several other scenarios:
In two scenarios, we varied the estimated target population up or down by 25% to provide a rough range around the reference case estimate
Another scenario used the total incidence of nonvascular above-the-knee amputees as the target population. This scenario assumes a significant increase to the target population and represents the budget impact if all nonvascular above-the-knee amputees received osseointegrated prosthetic implants
We calculated the budget impact of including both above- and below-the-knee amputees with chronic socket problems that were eligible for osseointegration
Another scenario provided a conservative estimate of market expansion at a yearly rate of 5%. This scenario assumes that some patients may want an osseointegrated prosthesis for its perceived higher performance, even if they don't have chronic problems with a conventional socket prosthesis
Finally, we used cohort-specific costs from the best case (i.e., most cost-effective) and worst case (i.e., most conservative) scenarios in our primary economic evaluation (Appendix 7, Table A9)
No scenario analysis capped the number of annual procedures based on hospital capacity and resource constraints. In all scenarios, all eligible patients (prevalent and incident) were treated in year 1. Appendix 7, Table A10, provides estimates of the target populations for all scenario analyses.
Results
Reference Case
Table 27 presents the results of the reference case. The estimated net budget impact of publicly funding osseointegrated prosthetic implants compared with services for people with uncomfortable socket prostheses ranged from $1.5 million in the first year to $650,000 in the fifth year. Over the 5 years, the net budget impact would be approximately $5.3 million.
Table 27:
Budget Impact Analysis—Reference Case Results
| Budget Impact, $a | ||||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | 5-Year Total | |
| Current scenario | 11,900 | 23,779 | 30,277 | 34,380 | 38,469 | 138,805 |
| New scenario | 1,547,920 | 1,593,748 | 949,267 | 670,536 | 688,834 | 5,450,304 |
| Net budget impact | 1,536,019 | 1,569,969 | 918,989 | 636,156 | 650,365 | 5,311,498 |
In 2018 Canadian dollars.
Scenario Analyses
Table 28 presents results from the scenario analyses. When the estimated population size was varied up or down by 25%, the 5-year net budget impact went as high as $6.7 million and as low as $4.0 million. When we applied a 5% annual expansion rate to the number of people eligible for osseointegrated prosthetic implants, the 5-year net total rose to $10.5 million. If the entire Ontario population of nonvascular above-the-knee amputees were treated (regardless of the criteria for osseointegrated prosthetic implants), the yearly net budget impact would range from $5.3 million in year 1 to $6.1 million in year 5, with a net 5-year total of $28.6 million. When we included both above- and below-the-knee nonvascular amputees eligible for the osseointegration surgery, the yearly net budget impact ranged from $5.0 million in year 1 to $1.4 million in year 5, with a net 5-year total of $10.6 million. Finally, applying the costs from the best- and worst-case scenarios, as described in the primary economic evaluation, had a 5-year total net budget impact of $4.4 million and $5.5 million, respectively.
Table 28:
Budget Impact Analysis—Scenario Analyses Results
| Budget Impact, $a | ||||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | 5-Year Total | |
| Increase population size by 25% | ||||||
| New scenario | 3,573,028 | 786,568 | 830,646 | 861,233 | 891,223 | 6,942,698 |
| Net budget impact | 3,545,558 | 753,916 | 792,753 | 818,047 | 842,699 | 6,752,972 |
| Decrease population size by 25% | ||||||
| New scenario | 2,143,817 | 471,941 | 498,388 | 516,740 | 534,734 | 4,165,619 |
| Net budget impact | 2,127,335 | 452,350 | 475,652 | 490,828 | 505,619 | 4,051,783 |
| Include all nonvascular above-the-knee amputees | ||||||
| New scenario | 5,365,424 | 5,605,128 | 5,863,925 | 6,117,730 | 6,366,271 | 29,318,478 |
| Net budget impact | 5,324,175 | 5,522,084 | 5,738,589 | 5,949,654 | 6,155,060 | 28,689,562 |
| Include all nonvascular above-the-knee and below-the-knee amputees eligible for osseointegrationb | ||||||
| New scenario | 5,077,020 | 1,375,723 | 1,449,590 | 1,505,178 | 1,559,664 | 10,967,175 |
| Net budget impact | 5,037,988 | 1,327,343 | 1,391,754 | 1,437,792 | 1,482,644 | 10,677,521 |
| Expand annual number of eligible amputees by 5% | ||||||
| New scenario | 2,858,422 | 1,733,269 | 1,899,029 | 2,066,568 | 2,241,935 | 10,799,223 |
| Net budget impact | 2,836,447 | 1,698,660 | 1,851,003 | 2,004,310 | 2,164,599 | 10,555,017 |
| Apply reasonable best scenario costs from primary economic evaluation | ||||||
| New scenario | 2,680,830 | 547,003 | 573,210 | 589,351 | 605,173 | 4,995,567 |
| Net budget impact | 2,597,930 | 448,458 | 458,843 | 459,003 | 458,708 | 4,422,943 |
| Apply reasonable worst scenario costs from primary economic evaluation | ||||||
| New scenario | 2,884,649 | 659,995 | 699,887 | 728,984 | 757,594 | 5,731,109 |
| Net budget impact | 2,862,673 | 633,873 | 669,573 | 694,435 | 718,775 | 5,579,329 |
In 2018 Canadian dollars.
Assuming 54% of all nonvascular lower-limb amputees in Table 26 were above-the-knee (24%) and below-the knee (30%), 10% of amputees had chronic socket-related problems, and a prevalence of 50 (30 above-the-knee and 20 below-the-knee) amputees were eligible for an osseointegrated prosthetic implant. Estimates were informed by a clinical expert (Nancy Dudek, MD, email communication, November 2018).
Discussion
In the reference case analysis, the net budget impact of publicly funding osseointegrated prosthetic implants for a defined population, compared with the health system cost of amputees' continued use of an uncomfortable socket prosthesis, ranged from approximately $1.5 million in the first year to $0.6 million in the fifth year, with a total net budget impact of $5.3 million over a 5-year period.
As administrative data were unavailable on both the prevalence and incidence of eligible osseointegrated prosthetic implants, we used scenario analyses to evaluate alternative target population estimates. While osseointegrated prosthetic implants are currently indicated for a select patient population, there is the potential for expansion, specifically for young people with below-the-knee amputation who may find an osseointegrated prosthesis more comfortable, as well as functionally and cosmetically superior to a socket prosthesis (Richard Jenkinson, MD, email communication, August 2018). With this potential in mind, we conducted scenario analyses looking at (1) an annual 5% expansion in the number of eligible patients and (2) the inclusion of all nonvascular lower-limb amputees (above- or below-the-knee) estimated to be eligible for an osseointegrated prosthetic implant. Based on published literature, we are uncertain of the clinical effectiveness of osseointegrated prosthetic implants in below-the-knee amputees; our clinical evidence review for this report found only one published study that included a single patient with a below-the-knee amputation. If, however, this population were to become eligible for osseointegrated prosthetic implants, our reference case results would significantly underestimate the true net budget impact. To further explore this uncertainty in the size of the target population, we also conducted a scenario that included all nonvascular above-the-knee amputees, which tested the extreme possibility of a complete shift from socket prostheses to osseointegrated prosthetic implants. Of note, our scenarios evaluating all nonvascular amputees are likely underestimated; no data were available on the prevalence of nonvascular amputation in Ontario, so we relied on estimates by clinical experts. Other scenario analyses included the reasonable best and worst scenarios from our primary economic evaluation, providing alternative cost estimates for both the current and new scenarios in this budget impact analysis.
Strengths and Limitations
Our analysis had several strengths. Given the uncertainty around the target population, we were able to explore the budget impact of several alternative scenarios, ranging from various changes in the target population to changes in annual per-patient costs (informed through alternative primary economic models). Furthermore, through our model, we were able to estimate the annual capacity for osseointegration surgeries in Ontario, given current funding and resource availability. Our analysis assumed that one centre would conduct the osseointegration procedures and rehabilitation. As noted, over the 5-year period an additional 35 patients could be treated in that single centre. Because only about 7 patients per year are expected to be eligible for osseointegrated prosthetic implants, further discussion with surgeons may be important to understand the adequate volume of surgeries needed to ensure surgical proficiency, which may in turn have an impact on reducing complication rates. On the other hand, some of our alternative scenarios had larger target populations (see Appendix 7, Table A10), and if one of these were to reflect the true target population for this procedure, that may negate safety concerns related to surgical volume. In addition, if the scenario of a 5% annual expansion was found to accurately represent the target population for osseointegrated prosthetic implants, an additional surgical/rehabilitation centre may be needed.
Our analysis also had several limitations. Due to a lack of administrative data, there was uncertainty around the target population estimate used in the reference case, as noted above. Additionally, as described previously, the primary economic evaluation may conservatively estimate costs; therefore, the actual net budget impact may be lower than presented in the reference case and may instead approximate the lower best-case scenario or the higher worst-case scenario.
Conclusions
Our budget impact analysis indicates that publicly funding osseointegrated prosthetic implants for a small population of people with above-the-knee amputation who cannot tolerate a socket prosthesis and are eligible for osseointegration surgery may result in extra spending ranging from $1.5 million in year 1 to $0.6 million in year 5, for a 5-year total of $5.3 million.
PATIENT PREFERENCES AND VALUES
Objective
The objective of this analysis was to explore the underlying values, needs, preferences, priorities, and values of those who have lived experience with lower-limb amputation. The treatment focus was osseointegrated prosthetic implants versus conventional socket prostheses.
Background
Exploring patient preferences and values provides a unique source of information about people's experiences of a health condition and the health technologies or interventions used to manage or treat the health condition. It includes the impact of the condition and its treatment on the person with the health condition, their family and other caregivers, and the person's personal environment. Engagement also provides insights into how a health condition is managed by the province's health system.
Information shared from lived experience can also identify gaps or limitations in published research (e.g., outcomes important to those with lived experience that are not reflected in the literature).60–62 Additionally, lived experience can provide information and perspectives on the ethical and social values implications of health technologies or interventions.
Because the needs, preferences, priorities, and values of those with lived experience in Ontario are not often adequately explored in published literature, we speak directly with people who live with a given health condition, including those with experience with the intervention we are exploring.
Methods
Partnership Plan
The engagement plan for this health technology assessment focused on consultation to examine the experiences of people with a lower-limb amputation and those of their caregivers. We engaged people via telephone interviews and email.
We used a qualitative interview, as this method of engagement allowed us to explore the meaning of central themes in the experiences of people with a lower-limb amputation, as well as those of their families and caregivers.63 The sensitive nature of exploring people's experiences of a health condition and their quality of life are other factors that support our choice of an interview methodology.
Participant Outreach
We used an approach called purposive sampling,64–67 which involves actively reaching out to people with direct experience of the health condition and health technology or intervention being reviewed. We approached a variety of social media groups, including the Osseointegration Group of Canada and the Osseointegration Peer Support Group, to spread the word about this engagement activity and to contact people with lower-limb amputation, family members, and caregivers, including those with experience of osseointegrated prosthetic implants.
Inclusion Criteria
We sought to speak with people and their caregivers who have been actively managing lower-limb amputation by using different treatment options.
Exclusion Criteria
We did not set specific exclusion criteria.
Participants
For this project, we spoke with 13 adults with a lower-limb amputation (above or below the knee), as well as three family members or caregivers. Participants lived in Ontario, elsewhere in Canada, or outside Canada, were of different genders, and came from diverse socioeconomic backgrounds.
Nine participants had experience with both a conventional socket prosthesis and an osseointegrated prosthetic implant, three had experience with a socket prosthesis only, and one had only recently undergone amputation and had not yet chosen a prosthetic. Of the participants with osseointegrated prosthetic implants, six had undergone single-stage surgery to receive the implant and three had undergone two-stage surgery.
Approach
At the beginning of the interview, we explained the role of Health Quality Ontario, the purpose of this health technology assessment, the risks of participation, and how participants' personal health information would be protected. We gave this information to participants by email, verbally, and in a printed letter of information (Appendix 8). We obtained participants' verbal consent before starting the interview. With participants' consent, we audio-recorded and then transcribed the interviews.
Interviews lasted approximately 30 to 60 minutes. The interview was loosely structured and consisted of a series of open-ended questions. Questions were based on a list developed by the Health Technology Assessment International Interest Group on Patient and Citizen Involvement in Health Technology Assessment.68 Questions focused on the impact of lower-limb amputation on participants' quality of life, their experiences with treatment options, including osseointegrated prosthetic implants, and their perceptions of the benefits and limitations of osseointegrated prosthetic implants and socket prostheses. For family members and caregivers, questions also focused on the impact on themselves of the person's amputation and treatments. Appendices 9 and 10 reproduce our interview guides.
Data Extraction and Analysis
We used a modified version of a grounded-theory methodology to analyze interview transcripts. The grounded-theory approach allowed us to organize and compare information on experiences across participants. This method consists of a repetitive process of obtaining, documenting, and analyzing responses while simultaneously collecting, analyzing, and comparing information.69,70 We used the qualitative data analysis software program NVivo71 to identify and interpret patterns in the data. The patterns we identified allowed us to highlight the impact of lower-limb amputation and treatment options on the people with a lower-limb amputation, family members, and caregivers we interviewed.
Results
During the interviews, people with lower-limb amputation and their family members emphasized the struggle of living with very limited movement, managing their condition before and after receiving a prosthesis, and the impact of receiving a socket prosthesis and/or an osseointegrated prosthetic implant.
People who had received an osseointegrated prosthetic implant were able to compare it with their experience with a socket prosthesis. They had chosen to get the implant for various reasons, and they have continued to use it for one to three years. Many participants perceived advantages of the implant, including enhanced mobility, better balance, and fewer skin irritations compared to a socket prosthesis. Some said the implant made it easier for them to do simple tasks such making a bed or walking to the bathroom, as well as more complex tasks such as working an eight-hour shift or hiking for a longer period.
Participants also identified challenges to receiving an osseointegrated prosthetic implant, including barriers to accessing the surgery, skin infections after the surgery, and concerns about maintaining their device over time.
Impact of Lower-Limb Amputation
All participants had experience of lower-limb amputation through a traumatic event. All said the decision to amputate was their only option; for some, not amputating could have led to other significant health risks.
Participants described the immense impact of lower-limb amputation in their day-to-day lives, prior to receiving a prosthesis. They discussed their struggles in managing their condition and adjusting to being an amputee.
They recalled the initial confusion and frustration as to how they would be able to move or walk again or what would be the next steps to this new and complicated adjustment:
Now the whole thing changes. How am I going to walk? How am I going to do this?
You're in the rehabilitation for twice a day, for an hour and half. You knew you will be okay to a point but didn't know what the future actually was.
For some people, their limited mobility had an impact on their career; they had to either take time off or give up their line of work altogether:
I worked in concrete. It's a challenging career that I had to give up.
I was off work for five and a half months, when was told to take off a year.
He lost his livelihood. He could not work at the work he worked at for all his life … He had his own business going. He had to give that up.
Some people noted the impact on others who cared for them after their surgery:
Mum takes care of me each day, it's wearing her down each day.
Family caregivers also expressed frustration and sadness about the traumatic event that their loved one had to go through, and about how it had impacted their personal and professional lives:
Our whole life was cancelled, everything was cancelled. I had my own business going and I had to give that up.
I felt it was my duty to get her into all appointments … My life changed quite drastically… I have trained for 28 to 40 years [to do a certain kind of work] … and had to cancel assignments, and people become worried that I won't be able to accept assignments … of course very stressful.
Conventional Socket Prosthesis
In Ontario, the currently available treatment for lower-limb amputation is a socket prosthesis. All but one of the people interviewed had experience with a socket prosthesis.
Process of Receiving the Socket Prosthesis
Participants were connected to a prosthetist for an initial fitting, once they had recovered from amputation surgery. Recovery time varied, and could mean living for some time without any prosthesis:
My husband was told that I will be in the hospital for 3 months [following the amputation]. I was airlifted [from the site of the accident to a nearby hospital], spent 3 weeks there. I begged the chief nurse and director… to take me back [to the local hospital] … It took a long time to heal … so I was not fitted until early November and did not start walking until mid-November [5.5 months from the time of the accident].
Not all patients received the same level of support for finding a socket prosthesis. One noted the lack of information they were provided and described their struggle to get a good fit:
In terms of getting a socket, it was very rough at first. I was a fresh amputee. I had no idea who, what, where, how on anything, on what to do, on how to get hooked up with a prosthesis, or even where to start looking. So, the first guy I saw, I was like, “You can help me”, [but] he eventually gave up and said “I couldn't do anything more for you.” I was in so much pain and couldn't get that fit. Found another prosthetist, it wasn't good, he pretty much told me, when I would say I'm in pain, he would say, “No, you don't, it's all in your head.” Reconnected with a friend [who] recommended a prosthetist. It was night and day: made the socket, if something wasn't working he didn't tell me I was wrong, [he] made it work for me.
Benefits of the Socket Prosthesis
Some interviewees identified that having a socket prosthesis did help them regain some mobility and the ability to do day-to-day tasks, even though they felt limited. Many felt it was their only option to start walking again, after their amputation:
I have three children. I haven't had any sort of real interaction with them, doing things with them has been limited for so long … If I get the prosthetic, I can get going on my feet again.
Barriers to Receiving the Socket Prosthesis
Cost
Cost was not seen as a major barrier to receiving the socket prosthesis. All the Ontario participants received their socket prostheses through the province's Assistive Devices Program (ADP), and those living in other provinces received coverage based on local requirements. Some people were able to get full coverage, but others had to pay part of the cost out of pocket:
ADP Ontario, they cover 75%, my insurance company covers 80% of the balance of that, so I end up usually paying 20% … So if the socket is $10,000, $11,000 … I end up paying about $2,500 … It is a financial burden, yes, very much so … especially with all these sockets [maintenance and replacements]. I have to sit down with my prosthetist and say, “Every time we do this, it's going to cost me $2,500, and I'm going to run out of money.”
Some people said the costs of frequently adjusting their socket, and replacing it when it is no longer functional, can eventually add up to the point that they may be better off getting an implant instead.
Access
In terms of access, amputees were often required to travel to their prosthetist, either by themselves or with the help of a caregiver. These visits were necessary for the prosthetist to make adjustments or change the socket when it was no longer functional:
It is my understanding that above-knee amputees are generally difficult to fit with a socket, and I personally was going back for adjustments and new test sockets on a very regular basis, every couple of weeks for over a year.
Limitations of the Socket Prosthesis
Most participants expressed gratitude for having a socket and being able to walk. However, some also outlined the physical and emotional challenges they had faced, from the first few days of receiving a socket through the years that followed. Physical limitations of the device described by participants include difficulties with putting on the socket and keeping it on, skin irritation and pain, and the inability to sit or walk for a long period. These physical limitations had negative emotional and social consequences for participants and caregivers alike.
Some participants had moved on to using an osseointegrated prosthetic implant, while some had remained with the socket prosthesis.
Putting on the Socket
One of the main limitations participants described was the challenge of putting on the socket. The process can take some time, as the socket may have different components and it may take several tries to create the suction necessary to keep the socket in place:
I wear a liner … the liner locks into the socket. Putting the liner on can be a pain in the butt … if it doesn't line up with the lock, you have to sit down and start over again.
It took 10 minutes or more to take off or put on a customized socket … I was into 13 sockets before I went into a permanent socket [to get the right fit and suction].
Some interviewees expressed concern that the time it takes to put on the socket could put them at risk. In the event of an emergency, they may not be able to move quickly and would require some sort of assistance:
If there was an emergency, there were times [I worried about] how long it took for me to get out of the house … put on my liner, squeeze your leg into the
Keeping Suction on the Socket
The most common challenge participants mentioned was keeping the socket's suction intact. If the suction releases, it becomes difficult to walk or perform day-to-day tasks:
You would sit down and feel the suction loosen. You got to stand up to pump it back in.
Every time you sat down [or] hopped into the car, you would always lose suction, [and] if you didn't deal with it right away, it would slip off more … and when it would slip off, [if] you were wearing pants, [you would] have to drop down the trousers…[and] slip it back on [the socket].
Skin Irritation and Pain
Participants reported commonly experiencing various kinds of skin irritations caused by the socket not fitting properly, losing suction, or being worn too long. They noted this was not only uncomfortable, but could curtail their day-to-day activities, as the skin issues could develop into infection, blisters and open sores:
Lots of rubbing issues, lots of sweat issues, lots of skin issues, ingrown hair and that would produce boils … [I would] have to wait on it until it heals [to wear the socket again].
Because of the [poor] fit, I would end up with getting blisters all over the place … Before I got the infection, I wouldn't wear my leg, just walk with crutches.
Open sores, didn't matter what we did … I was getting worse and worse and doing less and less. Towards the eighth year, especially last year, I was on the couch [most of the day]. By the time I got up and put my socket on and made my bed, I was done for the day.
Most participants also shared their frustration around the pain they experienced from wearing a socket. The onset of pain differed, depending on various factors, including people's condition before and after the amputation, how long they wore the socket, and how active they were:
It was OK at first. For the first or second year it wasn't too bad, I think because I probably still had my strength, my muscle strength, [I was] still quite active … [But] time kind of went on, and it was getting worse and worse. [It became] more difficult to do things and walk on my socket.
I couldn't do anything, couldn't go grocery shopping, even hanging onto a cart was just too painful. I couldn't do it. It was too painful.
I've gone through I don't know how many test sockets because of the pain … trying to figure out something to eliminate the pain. I think it has been five to six sockets over the past 7 years.
[I] took about a year to walk in the socket … [but then] in couple of days, [I] broke through the scar [from the amputation surgery]. I would need to wait another 2 or 3 months for the scar to heal again… [After starting to use the socket again] it was painful, it hurt, it wasn't comfortable, it didn't bring me back to any quality of life.
Difficulty Walking With the Socket
For all participants who had used a socket, it initially gave them the ability to walk again. Over the years, however, some participants said an uncomfortable socket made it hard for them to walk or stand for long, or to walk on slippery or uneven surfaces:
I have been called the 10-minute man: 10 minutes on, 10 minutes off. Do activities for 10 minutes, sit down for 10 to 15 minutes, settle down, go ahead and do it again … I feel bad at times. Let's say we wanted to go to … the farmers' market, somewhere out in the country. I can't walk around: 10 minutes, [and] I got to find a place to sit … Basically [I] don't do heck a lot unless I can go walk to where we are going, and sit.
Walking was a real problem especially in the snow. I would walk with a cane, but sometimes if I go ahead without it, there is a risk of falling … During the winter, we didn't do anything. My wife and I would stay home … basically stayed inside.
Emotional, Social, and Economic Impacts
Due to the physical limitations of the socket prosthesis, participants experienced negative emotional, social, and economic impacts. Some described feeling irritated and alone. Some reported their relationships had changed and they would interact less often with others:
[I became] more and more frustrated with my pain, my socket, and [my] communication skills … You snap at people, you are on edge a lot more … My friends had to back away from me as I was becoming more miserable.
One family member noted that the time it took to put on the socket limited her husband's ability to take care of their newborn child. This left her burdened with additional tasks to take care of the baby and the household:
Once we had our first child, it became more of a prominent issue … because he could not really help out with the baby as much as he would have liked to or as much as I would have appreciated some more help … Getting up at night with the baby … [it was a] 5-minute process to wake up and go and get his leg ready … [He] had a suction sleeve so it took forever to get it properly suctioned.
Amputees and caregivers reported they had become more isolated and stopped participating in what had once been usual activities:
By the time the evening comes, we don't go out because he is so sore … Everything is such an effort now … It takes so long to take everything off and put the new stuff on, by dinner time we don't go out at night.
Going on a holiday, most of the time I would decline [to do things]… like I was budgeting, you know. I can maybe walk the pier but no I can't, you know, we're going to need a wheelchair. You guys go, I'll sit here and wait … that sort of thing.
For some, the loss of mobility also affected their employment:
Eventually [I] went back to work, switched from the on-the-site job—I was a plumber—to an office job … so I could sit all day. It wasn't very comfortable … Most days [I] would take my socket off … [and] when I needed to get up, I would put it on and use it, and [then get] back to the chair and take it off again.
I kept getting ulcers. In fact, I was a bus driver … [but] they wouldn't hire me because I couldn't drive on certain dates … so I lost my job with the city … That was a big impact there.
Osseointegrated Prosthetic Implants
In this section, we provide some comparison of patients' experiences with the single-stage and two-stage surgeries, as well their overall experience with osseointegrated prosthetic implants.
Process of Receiving an Osseointegrated Prosthetic Implant
Participants had to go through a lengthy process to get their osseointegrated prosthetic implant and then, after surgery, to train themselves to start walking with the new prosthesis. As noted, they all had to travel outside Canada to receive the implant.
For single-stage surgery, participants travelled out of country for about a month, which included time for surgery, recovery, and rehabilitation. Most chose single-stage surgery for the convenience of having the entire procedure done at one time and lessening the time they would take from work. Cost was another factor; some said they would not be able to afford a second round of the procedure, as the first was quite expensive:
I was aware of the two-stage surgery but was not offered it. I would not have had time to go [out of country] twice to have that done. I work full-time in a busy job.
Knowing what I'm experiencing now with osseointegration, I would do any stage whatsoever. This procedure is amazing, and I can't say enough positive things about it.
Participants who underwent two-stage surgery were required to travel out of country twice, for two surgeries at two different times, allowing for the osseointegration process and recovery time in between. Participants felt that this longer process was necessary for the bone and implant to fully integrate, and they believed it would make the implant stronger and less prone to breaking:
The surgeon believes the integrated time between first stage and second stage is vital for the bone to really lock into the mesh of the implant.
I have seen several of those [implants via single-stage surgery], and the penetration [the integration of bone around the rod] is not quite like what I have.
Benefits of the Osseointegrated Prosthetic Implant
Comparing it with the socket prosthesis, people who had received an osseointegrated prosthetic implant reported experiencing better mobility, less skin irritation and pain, and, as a result, less stress and frustration. They reported positive changes in their own and their caregivers' lives, including going back to work, spending more time with loved ones, and doing day-to-day tasks with more ease.
Better Mobility
Participants said the implant allowed them to walk better and for a longer periods, without needing to rest until the pain subsided, as they had with the socket prosthesis:
[On the] very first day I was standing … standing on my leg two weeks after surgery, I had walked better than ever before.
My mobility is almost unlimited now … I don't have to plan out if I will be able to sit down.
[I] can go where I want … [I have] driven out on my own for 7 hours … I could have never done that before … I could never hold them before (grandchildren)… I can pick up what I want. I can walk everywhere. Go wherever I want.
With improved mobility, some participants said they felt more comfortable doing simple, common tasks and even sleeping with the prosthesis on:
Simple little things … I go to bed, I never take my leg off … To get up to even go to the bathroom in the night, before I would just bum it to the bathroom, then hoist myself up on to the toilet, and whereas now I just get out of bed and go to the bathroom like anybody else.
We have gone and done so much stuff, even something as simple as walking down the road to the park … He couldn't even walk to the park, or we walked to the park [and] he would have had to sit down … Now he can go to the park, push them [the kids] on the swings, go down the slide … It's really nice.
Less Skin Irritation and Pain
Participants also reported that the pain they had experienced with the socket prosthesis had decreased or disappeared once they received the implant and recovered from the surgery:
Before, I would come home, even after shopping from the mall, I literally just want to sit down and get my socket off. And now I don't even think about it, I have no pain, I just don't think about it.
All the pain is gone, no more skin pain, no more scar breakdown, all my lower back pain is gone … My muscles grew back, glute evened out, hips are back to being symmetrical
… Lower back pain is gone … I don't need any drugs or medical services for my pain, it is nonexistent now.
Before, my leg [with the socket prosthesis], if it ever hurt, it's my back that gets sore … Now I can stand all day at work for eight hours.
Positive Outlook
Some participants perceived the implant as a normal leg, which contributed to the positive impact it had on their quality of life:
I just came back from [a vacation], and I was able to go for long hikes … [I] said to [my spouse], “This is so life-changing,” because [it] was hot, and [it] was so lovely to have the breeze around my leg … [It] feels like I have my normal leg back.
It feels like you can almost feel what your walking on. It just feels like you have got your leg back.
It feels like a leg, feels like a body part.
Barriers to the Osseointegrated Prosthetic Implant
Despite the benefits, participants also discussed the substantial cost involved in getting an osseointegrated prosthetic implant and the challenges in accessing this treatment. Both issues had an impact on their mental health and quality of life, and for their family or caregivers.
Cost of Treatment
The biggest barrier experienced was the cost of the procedure itself and the additional costs associated with travelling to and staying in another country. Participants said they used savings or borrowed money to help with the costs:
It's really frustrating and just kind of stressful … We had a 6-month-old at that time … and we all chose the best option would be to go as a family. Obviously, financially it was a huge burden. It was really expensive to take us all.
In paying for this [the osseointegrated prosthetic implant], I understood that I might be reimbursed for none of it and so far, that is the case. I was in a financial position to make this decision—not many others are.
The cost factor was also a challenge, which had to be financed in my own case. It was approximately $13,000 to $15,000 for the 31 days my husband and I stayed there. This amount did not include personal transportation—car rental, Opal card for trains, scooter rental—or food, etc. This cost was an additional $2,000 to $3,000. Manitoba Health paid for both of our flights … [We] took a line of credit [to] pay for it [the implant].
Some participants said it was a challenge to receive government funding for an osseointegrated prosthetic implant. They were denied coverage because the procedure was deemed experimental or prosthetic because they needed a referral from a local prosthetist or surgeon. However, many local professionals would not take them on due to the risks associated with osseointegrated prosthetic implants:
Having a hard time to find a surgeon locally to sign off things … I have the forms ready, but I don't have a surgeon willing to fill it out.
Talking to people who have been denied … it impacted my decision in filling the form out … At the time I was looking into it, few previous applications had been denied … [But the] outlook of being approved is low … It just didn't seem exciting to go down that route.
I was denied on the grounds of treatment's experimental … They would like to have had more information from specialists.
Access to Treatment
For a few participants who opted to receive an osseointegrated prosthetic implant, traveling outside the country for the surgery and rehabilitation had an impact on their work, and the long journey carried particular challenges for someone using a socket prosthesis:
The need to go to another country to have the surgery done necessitated that I take the full 28 days off work that were required by this program. Had I had the surgery done at home, I would only have been off for the time needed to recover from the acute phase post surgery—probably not more than 10 days.
One of the challenges I faced when going [out of country] for osseointegration surgery was the actual 22-plus hour flight and layover, while wearing a socket prosthetic. It was very difficult to even try to get to the washroom, remove your pants, to remove the socket prosthetic and then try to hop back to your seat using two crutches, or having to use the onboard wheelchair to get back to your seat. It was not doable. I personally chose to wear my prosthetic, so I could at least get off my seat and stand in the aisles for a few minutes at a time.
Some caregivers also felt the impact of the extended, out-of-country travel:
My role in Canada after the accident, my life pretty well went on hold just looking after our household … But that's very different from picking up your whole life and leaving for [another country].
Limitations of the Osseointegrated Prosthetic Implant
People also reported limitations to receiving an osseointegrated prosthetic implant. These included infections around the implant and issues with maintaining the implant.
Infection
While overseas for their surgery, patients had received instructions on how to keep the area around the implant clean. However, most experienced some sort of infection after returning home. For some people, receiving treatment was straightforward, but others had doubts about whether physicians in Canada would be able to treat an infection around an implant that they may not be familiar with:
I got two infections, around the stoma [the opening in the skin where the implant enters the limb] … [The treatment] was pretty straightforward, did blood work, put me on IV [intravenous] antibiotic for five days … very easy.
I developed a slight infection a week ago. They [emergency physician in Ontario] didn't know it was an infection… I received [in Australia] a big packet of instructions about the leg … if I were concerned about infection, I should take a picture … and also in the instruction material they give you a set of questions that they want you to answer when you send the picture … The surgeon is very concerned that people here are not familiar with it [the implant] … I needed someone that was open-minded about things that are not mainstream.
I was very left on my own, I felt, when I got the infection. I called them [the out-of-country surgeon] to get their advice … [Later] some of the doctors [emergency physicians in Ontario] told me, “This is your fault, because you went and got this done. This is a brand-new procedure, and no one knows anything about it. This is your fault that you got your infection.” … [It was a] total of 12 months that I was on antibiotics … I was able to do everything [day-to-day activities] except for the time I would need to go to the hospital for the IV antibiotic.
Maintaining the Implant
Amputees and their caregivers had to learn step by step how to maintain the implant. If they encountered any maintenance problems, the overseas implant team had advised them to send a video and they would be further instructed. This was done as a precaution, in case patients could not find a local professional to help them with their maintenance issues:
A [medical] resident took my iPad and videoed [the maintenance process] for me, and I watched it very carefully and talked it through with [the overseas implant team] … so now I am capable of doing it myself.
Some participants had concerns about whether parts would be available and whether their prosthetist would know how to maintain the implant. Some patients' prosthetists took the initiative to learn more about the implant from the overseas implant team, but others did not, which created challenges in maintaining the implant. An additional stress for participants was the need to pay for parts out of pocket:
About 2 months ago, my grub screw seized into my connector which holds the prosthetic leg onto the implant. I was able to use the safety bolt in the interim, but nevertheless it caused me a considerable amount of distress and worry. The Canadian distributor … was useless, carried no parts, didn't get back to my prosthetist till 5 days after his initial call to them asking for a warrantied part replacement. I had to contact the [out-of-country medical] team myself, [and they] immediately sent the replacement parts by overnight FedEx. Parts are extremely expensive. I am using my mechanical knee as a shower leg and needed a second connector to be able to don/doff the shower leg. This cost me an additional $2,000 U.S. [$2,600 CAD].
Discussion
We interviewed three types of patients with lower-limb amputation (along with three family members and caregivers): one person who had recently undergone amputation surgery and was not yet using a prosthetic limb, three amputees who had only ever used a socket prosthesis, and nine people who were able to compare the socket prosthesis with the osseointegrated prosthetic implant, which they had now received.
Amputees and caregivers shared their personal experiences about the burden and struggle of living with lower-limb amputation. This condition had an impact on their daily lives, well-being, relationships with friends and family, work, and finances. Both types of prosthesis carry out-of-pocket costs for most people.
The main limitation of the socket prosthesis, the current treatment available in Ontario, is the difficulty in keeping a good, comfortable fit. Participants described how an uncomfortable socket led to skin irritation and pain, making even the simplest tasks a challenge. This in turn led to social isolation, the need to carefully plan activities, and an overall poorer quality of life, for the amputee and caregiver alike.
Switching to an osseointegrated prosthetic implant involves a lengthy, complex process, requiring the patient to travel out of Canada for approximately a month. Most participants described the expensive investment for this treatment, a cost that most had to pay out of pocket because they were denied out-of-country coverage. However, one patient was able to receive partial coverage. The treatment—including the surgery, recovery, rehabilitation, and accommodation—cost people approximately $100,000 each. In addition, they felt the impact on their day-to-day lives and ability to work due to their extended stay overseas.
Despite these barriers, participants with an osseointegrated prosthetic implant reported that it had greatly improved their quality of life. They valued the ability to walk better for longer periods, without blisters or pain, and the ability to generally live with less stress and more ease. Skin infection around the implant was the main limitation reported. These were treated with antibiotics, but one person found it hard to get care from local physicians to manage an implant infection. Overall, despite the surgery and associated risks, people who had received an osseointegrated prosthetic implant had no regrets about going through the procedure.
Conclusions
Amputees and caregivers viewed osseointegrated prosthetic implants as a positive alternative to socket prosthetics in treating lower-limb amputation. People who had received an osseointegrated prosthetic implant said they now had better mobility and quality of life than before receiving the osseointegrated prosthetic implant and that their ability to perform day-today activities at home and at work had improved. The participants also described limitations, however, particularly the ongoing risk of infection and potential for problems with implant maintenance. Cost and access to the osseointegration procedure (owing to a lack of public funding and the need for extended overseas travel) were important barriers to this treatment. People still using a socket prosthesis said cost was the only factor preventing them from getting an osseointegrated prosthetic implant. It is important to note that the people we spoke with may not be fully representative of all people who have or are candidates for osseointegrated prosthetic implants; of note, all people with osseointegrated prosthetic implants with whom we spoke were satisfied overall with their implants and had not experienced any serious adverse events.
CONCLUSIONS OF THE HEALTH TECHNOLOGY ASSESSMENT
In studies of osseointegrated prosthetic implants for lower-limb amputation, the mean duration of follow-up was between 1 and 5 years. These studies showed that patients' functional outcomes improved with osseointegrated prosthetic implants (GRADE: Low), but their emotional health did not improve (GRADE: Low). Osseointegrated prosthetic implants can lead to serious adverse events such as bone infection or fracture (GRADE: High), which may require additional surgeries and may negatively impact emotional health. However, chronic poor socket fit and limited function can also impact mental health and quality of life for amputees using socket prostheses.
We identified two economic evaluations that modelled osseointegrated prosthetic implants compared with conventional socket prostheses for people with lower-limb amputation. The reported ICERs varied from $16,632 AUD per QALY gained (2016/17 AUD) when accounting for only prosthetic services, to €83,374 per QALY gained (2009 EUR) when the full care pathway was included, from implantation to rehabilitation and complications. Given their differing perspectives, cost inputs, and methodological approaches, both studies were considered partially applicability to the Ontario context, and we therefore conducted our own economic analysis.
Our economic analysis found that, in patients with a lower-limb amputation eligible for osseointegrated prosthetic implants, osseointegration had higher costs and greater QALYs gained compared with those living with an uncomfortable socket prosthesis. Results were sensitive to parameter uncertainties and model assumptions. The ICER for the osseointegrated prosthesis compared to an uncomfortable socket prosthesis was $94,987 per QALY gained in the reference case. There was a high degree of uncertainty in the reference case, in which the probability of osseointegration being cost-effective was 54.2% at a willingness-to-pay value of $100,000 per QALY gained.
Our budget impact analysis indicated that publicly funding osseointegrated prosthetic implants for a small population of people with above-the-knee amputation who cannot tolerate a socket prosthesis and are eligible for osseointegration surgery may result in extra spending ranging from $1.5 million in year 1 to $0.6 million in year 5, for a 5-year total of $5.3 million.
We interviewed 16 amputees and caregivers. They viewed osseointegrated prosthetic implants as a positive alternative to socket prostheses in treating lower-limb amputation. Patients who had received an osseointegrated prosthetic implant said they had better mobility, quality of life, and ability to perform day-to-day activities at home and at work. They also described limitations, particularly the ongoing risk of infection and potential problems in maintaining the implant. Cost and access to the implant procedure (lack of public funding, the need for extended overseas travel) were seen as important barriers to this treatment. People still using a socket prosthesis said money was the only factor preventing them from getting the implant.
Acknowledgments
This report was developed by a multidisciplinary team from Health Quality Ontario, which is now the Quality business unit at Ontario Health. The clinical epidemiologist was Shayan Sehatzadeh, the health economist was Sean Tiggelaar, the patient and public partnership analyst was Ammara Shafique, and the medical librarian was Corinne Holubowich.
The medical editors were Amy Zierler and Jeanne McKane. Others involved in the development and production of this report were Merissa Mohamed, Claude Soulodre, Kara Cowan, Kathryn Schwarz, Sarah McDowell, Vivian Ng, Andrée Mitchell, Amy Lang, Nancy Sikich, and Irfan Dhalla.
We are grateful to the following experts for lending their expertise to the development of this report: Alison Davis (The Ottawa Hospital Rehabilitation Centre), Nancy Dudek (The Ottawa Hospital Rehabilitation Centre), Wade Gofton (The Ottawa Hospital), Richard Jenkinson (Sunnybrook Health Sciences Centre), Simon Kelley (The Hospital for Sick Children), Amanda Mayo (St. John's Rehab and Sunnybrook Health Sciences Centre), Dan Mead (Orthotics Prosthetics Canada), John Murnaghan (Sunnybrook Health Sciences Centre), James Waddell (St. Michael's Hospital), and William Wai Lun Wong (University of Waterloo).
We also thank our lived experience participants who generously gave their time to share their stories with us for this report.
The statements, conclusions, and views expressed in this report do not necessarily represent the views of those we consulted.
ABBREVIATIONS
- 6MWT
6-Minute Walk Test
- ADP
Assistive Devices Program
- AMP
Amputee Mobility Predictor
- AMPPRO
Amputee Mobility Predictor Prosthesis
- CI
Confidence interval
- EQ-5D
EuroQol 5-dimension quality of life questionnaire
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- ICER
Incremental cost-effectiveness ratio
- IPL
Integral Leg Prosthesis; also known as ESKA Endo-Exo Femur-Prosthesis
- IV
intravenous
- NICE
National Institute for Health and Care Excellence
- OGAP-OPL
Osseointegration Group of Australia–Osseointegration Prosthetic Limb
- OHIP
Ontario Health Insurance Plan
- OPRA
Osseointegrated Prostheses for the Rehabilitation of Amputees Implant System
- Q-TFA
Questionnaire for Persons with a Transfemoral Amputation
- QALY
Quality-adjusted life-year
- ROBINS-I
Risk of Bias in Non-randomized Studies of Interventions
- SD
Standard deviation
- SF-6D
6-Dimension Short Form Health Survey
- SF-36
36-Item Short Form Health Survey
- TUG
Timed Up and Go
GLOSSARY
- Adverse event
An adverse event is any unexpected problem that happens during or as a result of treatment, regardless of the cause or severity.
- Budget impact analysis
A budget impact analysis estimates the financial impact of adopting a new health care intervention on the current budget (i.e., its affordability). It is based on predictions of how changes in the intervention mix impact the level of health care spending for a specific population. Budget impact analyses are typically conducted for a short-term period (e.g., 5 years). The budget impact, sometimes referred to as the net budget impact, is the estimated cost difference between the current scenario (i.e., the anticipated amount of spending for a specific population without using the new intervention) and the new scenario (i.e., the anticipated amount of spending for a specific population following the introduction of the new intervention).
- Cost-effective
A health care intervention is considered cost-effective when it provides additional benefits, compared with relevant alternatives, at an additional cost that is acceptable to a decision-maker based on the maximum willingness-to-pay value.
- Cost-effectiveness acceptability curve
In economic evaluations, a cost-effectiveness acceptability curve is a graphical representation of the results of a probabilistic sensitivity analysis. It illustrates the probability of health care interventions being cost-effective over a range of different willingness-to-pay values. Willingness-to-pay values are plotted on the horizontal axis of the graph, and the probability of the intervention of interest and its comparator(s) being cost-effective at corresponding willingness-to-pay values are plotted on the vertical axis.
- Cost-effectiveness analysis
Used broadly, “cost-effectiveness analysis” may refer to an economic evaluation used to compare the benefits of two or more health care interventions with their costs. It may encompass several types of analysis (e.g., cost-effectiveness analysis, cost–utility analysis). Used more specifically, “cost-effectiveness analysis” may refer to a specific type of economic evaluation in which the main outcome measure is the incremental cost per natural unit of health (e.g., life-year, symptom-free day) gained.
- Cost–utility analysis
A cost–utility analysis is a type of economic evaluation used to compare the benefits of two or more health care interventions with their costs. The benefits are measured using quality-adjusted life-years (QALYs), which capture both the quality and quantity of life. In a cost–utility analysis, the main outcome measure is the incremental cost per quality-adjusted life-year gained.
- Deterministic sensitivity analysis
Deterministic sensitivity analysis is an approach used to explore uncertainty in the results of an economic evaluation by varying parameter values to observe the potential impact on the cost-effectiveness of the health care intervention of interest. One-way sensitivity analysis accounts for uncertainty in parameter values one at a time, whereas multiway sensitivity analysis accounts for uncertainty in a combination of parameter values simultaneously.
- Disutility
A disutility is a decrease in utility (i.e., a decrease in preference for a particular health outcome) typically resulting from a particular health condition (e.g., a symptom or complication).
- Fatigue failure
“Fatigue failure” is a term used in materials science; it refers to a breakage or collapse resulting from repeated cycles of loading and unloading. With regard to lower-limb osseointegration, the term refers to damage to the osseointegrated prosthetic implant owing to repeated use.
- Fixation
In lower-limb osseointegration, fixation is the procedure whereby the femoral bone is connected to the metal implant.
- Health-related quality of life
Health-related quality of life is a measure of the impact of a person's health status on their quality of life. Health-related quality-of-life tools allow the effects of chronic illness, treatment, and disability on a person's quality of life to be measured.
- Incremental cost
An incremental cost is the additional cost, typically per person, of a health care intervention versus a comparator.
- Incremental cost-effectiveness ratio (ICER)
The incremental cost-effectiveness ratio (ICER) is a summary measure that indicates, for a given health care intervention, how much more a consumer must pay to get an additional unit of benefit relative to an alternative intervention. It is obtained by dividing the incremental cost by the incremental effectiveness. Incremental cost-effectiveness ratios are typically presented as the cost per life-year gained or the cost per quality-adjusted life-year gained.
- K-level
The K-level is a rating system used by the U.S. Medicare health insurance program to indicate the extent of a person's disability and their potential for rehabilitation. Ratings range from 0 (no potential to walk independently, even with a prosthesis) to 4 (exceeds basic ambulation skills).
- Markov model
A Markov model is a type of decision-analytic model used in economic evaluations to estimate the costs and health outcomes (e.g., quality-adjusted life-years gained) associated with using a particular health care intervention. Markov models are useful for clinical problems that involve events of interest that may recur over time (e.g., stroke). A Markov model consists of mutually exclusive, exhaustive health states. Patients remain in a given health state for a certain period of time before moving to another health state based on transition probabilities. The health states and events modelled may be associated with specific costs and health outcomes.
- Monte Carlo simulation
Monte Carlo simulation is an economic modelling method that derives parameter values from distributions rather than fixed values. The model is run several times, and in each iteration, parameter values are drawn from specified distributions. This method is used in microsimulation models and probabilistic sensitivity analysis.
- Probabilistic sensitivity analysis (PSA)
A probabilistic sensitivity analysis (PSA) is used in economic models to explore uncertainty in several parameters simultaneously. It is done using Monte Carlo simulation. Model inputs are defined as a distribution of possible values. In each iteration, model inputs are obtained by randomly sampling from each distribution, and a single estimate of cost and effectiveness is generated. This process is repeated many times (e.g., 10,000 times) to estimate the number of times (i.e., the probability) that the health care intervention of interest is cost-effective.
- Quality-adjusted life-year (QALY)
The quality-adjusted life-year (QALY) is a generic health outcome measure commonly used in cost–utility analyses to reflect the quantity and quality of life-years lived. The life-years lived are adjusted for quality of life using individual or societal preferences (i.e., utility values) for being in a particular health state. One year of perfect health is represented by one quality-adjusted life-year.
- Reference case
The reference case is a preferred set of methods and principles that provide the guidelines for economic evaluations. Its purpose is to standardize the approach of conducting and reporting economic evaluations so that results can be compared across studies.
- Revision
In lower-limb osseointegration, a revision is a surgery performed to replace or compensate for a failed implant or to correct undesirable outcomes of the previous surgery.
- Scenario analysis
A scenario analysis is used to explore uncertainty in the results of an economic evaluation. It is done by observing the potential impact of different scenarios on the cost-effectiveness of a health care intervention. Scenario analyses include varying structural assumptions from the reference case.
- Sensitivity analysis
Every economic evaluation contains some degree of uncertainty, and results can vary depending on the values taken by key parameters and the assumptions made. Sensitivity analysis allows these factors to be varied and shows the impact of these variations on the results of the evaluation. There are various types of sensitivity analysis, including deterministic, probabilistic, and scenario.
- Soft tissue refashioning
Soft tissue refashioning is a surgical procedure to remove excess soft tissue (muscle) from the end of the stump to allow for an improved prosthetic fit.
- Tornado diagram
In economic evaluations, a tornado diagram is used to determine which model parameters have the greatest influence on results. Tornado diagrams present the results of multiple one-way sensitivity analyses in a single graph.
- Utility
Utilities are values that represent people's preferences for various health states. Typically, utility values are anchored at 0 (death) and 1 (perfect health). In some scoring systems, a negative utility value indicates a state of health valued as being worse than death. Utility values can be aggregated over time to derive quality-adjusted life-years, a common outcome measure in economic evaluations.
- Willingness-to-pay value
A willingness-to-pay value is the monetary value a health care consumer is willing to pay for added health benefits. When conducting a cost–utility analysis, the willingness-to-pay value represents the cost a consumer is willing to pay for an additional quality-adjusted life-year. If the incremental cost-effectiveness ratio is less than the willingness-to-pay value, the health care intervention of interest is considered cost-effective. If the incremental cost-effectiveness ratio is more than the willingness-to-pay value, the intervention is considered not to be cost-effective.
APPENDICES
Appendix 1: Literature Search Strategies
Clinical Evidence Search
Search date: June 5, 2018
Databases searched: Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, CRD Health Technology Assessment Database, and NHS Economic Evaluation Database
Database: EBM Reviews – Cochrane Central Register of Controlled Trials <April 2018>, EBM Reviews – Cochrane Database of Systematic Reviews <2005 to May 31, 2018>, EBM Reviews -Health Technology Assessment <4th Quarter 2016>, EBM Reviews – NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2018 Week 23>, Ovid MEDLINE(R) ALL <1946 to June 04, 2018>
Search Strategy:
-
1
exp Amputation/ (61508)
-
2
Amputation, Traumatic/ (5935)
-
3
Amputation Stumps/ (5461)
-
4
Amputees/ (3674)
-
5
(amputat∗ or amputee∗).ti,ab,kf. (87411)
-
6
or/1–5 (109545)
-
7
Artificial Limbs/ (9604)
-
8
Prosthesis Implantation/ (14366)
-
9
“Prostheses and Implants”/ (58520)
-
10
Prosthesis Design/ (52033)
-
11
(artificial adj2 (implant∗ or prosthes?s or prosthetic∗)).ti,ab,kf. (4590)
-
12
or/7–11 (127521)
-
13
Extremities/ (56328)
-
14
exp lower extremity/ (465976)
-
15
exp Leg Bones/ (185832)
-
16
Leg Injuries/ (13710)
-
17
(leg or legs or low∗ extremity or low∗ extremities or low∗ limb or low∗ limbs or knee or knees or transfemoral or trans femoral or transtibial or trans tibial).ti,ab,kf. (704634)
-
18
or/13–17 (1135896)
-
19
18 and (6 or 12) (61384)
-
20
Osseointegration/ (10158)
-
21
(osseointegrat∗ or osseo integrat∗ or OIP).ti,ab,kf. (17715)
-
22
Bone-Implant Interface/ (744)
-
23
(bone implant∗ interfac∗ or bone prosthes?s interfac∗ or ((boneanchor∗ or bone anchor∗) adj2 (prosthes?s or prosthet∗))).ti,ab,kf. (2979)
-
24
(percutaneous or Intraosseous or intra osseous).ti,ab,kf. (334378)
-
25
(direct skeletal or endo exo∗ or endoexo∗).ti,ab,kf. (1317)
-
26
(integral leg adj2 (prosthes?s or prosthetic∗)).ti,ab,kf. (4)
-
27
or/20–26 (360829)
-
28
19 and 27 (2776)
-
29
exp Animals/ not Humans/ (15599682)
-
30
28 not 29 (1800)
-
31
Case Reports/ or Comment.pt. or Editorial.pt. or Congresses.pt. (3536483)
-
32
30 not 31 (1654)
-
33
limit 32 to english language [Limit not valid in CDSR; records were retained] (1460)
-
34
33 use medall,cctr,coch,clhta,cleed (1047)
-
35
exp amputation/ (61508)
-
36
traumatic amputation/ (6465)
-
37
amputation stump/ (5461)
-
38
amputee/ (3674)
-
39
(amputat∗ or amputee∗).tw,kw. (88544)
-
40
or/35–39 (110186)
-
41
exp limb prosthesis/ (13941)
-
42
prosthesis implantation/ (14366)
-
43
prosthesis/ (73305)
-
44
prosthesis design/ (52033)
-
45
(artificial adj2 (implant∗ or prosthes?s or prosthetic∗)).tw,kw,dv. (4745)
-
46
or/41–45 (146027)
-
47
limb/ (59338)
-
48
exp lower limb/ (465976)
-
49
exp leg injury/ (212469)
-
50
exp leg bone/ (185832)
-
51
(leg or legs or low∗ extremity or low∗ extremities or low∗ limb or low∗ limbs or knee or knees or transfemoral or trans femoral or transtibial or trans tibial).tw,kw. (707325)
-
52
or/47–51 (1254486)
-
53
52 and (40 or 46) (69151)
-
54
osseointegration/ (10158)
-
55
(osseointegrat∗ or osseo integrat∗ or OIP).tw,kw,dv. (18338)
-
56
bone implant interface/ (744)
-
57
(bone implant∗ interfac∗ or bone prosthes?s interfac∗ or ((boneanchor∗ or bone anchor∗) adj2 (prosthes?s or prosthet∗))).tw,kw,dv. (3030)
-
58
(percutaneous or Intraosseous or intra osseous).tw,kw,dv. (339348)
-
59
(direct skeletal or endo exo∗ or endoexo∗).tw,kw,dv. (1336)
-
60
(integral leg adj2 (prosthes?s or prosthetic∗)).tw,kw,dv. (4)
-
61
or/54–60 (366375)
-
62
53 and 61 (3082)
-
63
(exp animal/ or nonhuman/) not exp human/ (10438865)
-
64
62 not 63 (2560)
-
65
Case Report/ or Comment/ or Editorial/ or conference abstract.pt. (8673313)
-
66
64 not 65 (1923)
-
67
limit 66 to english language [Limit not valid in CDSR; records were retained] (1671)
-
68
67 use emez (564)
-
69
34 or 68 (1611)
-
70
69 use medall (985)
-
71
69 use emez (564)
-
72
69 use coch (2)
-
73
69 use cctr (60)
-
74
69 use clhta (0)
-
75
69 use cleed (0)
-
76
remove duplicates from 69 (1122)
Economic Evidence Search
Search date: June 5, 2018
Databases searched: Ovid MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Centre for Reviews and Dissemination (CRD) Health Technology Assessment Database, and National Health Service (NHS) Economic Evaluation Database
Database: EBM Reviews – Cochrane Central Register of Controlled Trials <April 2018>, EBM Reviews – Cochrane Database of Systematic Reviews <2005 to May 31, 2018>, EBM Reviews -Health Technology Assessment <4th Quarter 2016>, EBM Reviews – NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2018 Week 23>, Ovid MEDLINE(R) ALL <1946 to June 04, 2018>
Search Strategy:
-
1
exp Amputation/ (61508)
-
2
Amputation, Traumatic/ (5935)
-
3
Amputation Stumps/ (5461)
-
4
Amputees/ (3674)
-
5
(amputat∗ or amputee∗).ti,ab,kf. (87411)
-
6
or/1–5 (109545)
-
7
Artificial Limbs/ (9604)
-
8
Prosthesis Implantation/ (14366)
-
9
“Prostheses and Implants”/ (58520)
-
10
Prosthesis Design/ (52033)
-
11
(artificial adj2 (implant∗ or prosthes?s or prosthetic∗)).ti,ab,kf. (4590)
-
12
or/7–11 (127521)
-
13
Extremities/ (56328)
-
14
exp lower extremity/ (465976)
-
15
exp Leg Bones/ (185832)
-
16
Leg Injuries/ (13710)
-
17
(leg or legs or low∗ extremity or low∗ extremities or low∗ limb or low∗ limbs or knee or knees or transfemoral or trans femoral or transtibial or trans tibial).ti,ab,kf. (704634)
-
18
or/13–17 (1135896)
-
19
18 and (6 or 12) (61384)
-
20
Osseointegration/ (10158)
-
21
(osseointegrat∗ or osseo integrat∗ or OIP).ti,ab,kf. (17715)
-
22
Bone-Implant Interface/ (744)
-
23
(bone implant∗ interfac∗ or bone prosthes?s interfac∗ or ((boneanchor∗ or bone anchor∗) adj2 (prosthes?s or prosthet∗))).ti,ab,kf. (2979)
-
24
(percutaneous or Intraosseous or intra osseous).ti,ab,kf. (334378)
-
25
(direct skeletal or endo exo∗ or endoexo∗).ti,ab,kf. (1317)
-
26
(integral leg adj2 (prosthes?s or prosthetic∗)).ti,ab,kf. (4)
-
27
or/20–26 (360829)
-
28
19 and 27 (2776)
-
29
economics/ (257267)
-
30
economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (810769)
-
31
economics.fs. (405976)
-
32
(econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗).ti,ab,kf. (805711)
-
33
exp “costs and cost analysis”/ (558350)
-
34
(cost or costs or costing or costly).ti. (245773)
-
35
cost effective∗.ti,ab,kf. (289830)
-
36
(cost∗ adj2 (util∗ or efficacy∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)).ab,kf. (190613)
-
37
models, economic/ (11453)
-
38
markov chains/ or monte carlo method/ (73524)
-
39
(decision adj1 (tree∗ or analy∗ or model∗)).ti,ab,kf. (37423)
-
40
(markov or markow or monte carlo).ti,ab,kf. (117194)
-
41
quality-adjusted life years/ (35659)
-
42
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).ti,ab,kf. (62788)
-
43
((adjusted adj1 (quality or life)) or (willing∗ adj2 pay) or sensitivity analys∗s).ti,ab,kf. (102420)
-
44
or/29–43 (2388831)
-
45
28 and 44 (84)
-
46
45 use medall,coch,cctr,clhta (34)
-
47
28 use cleed (0)
-
48
or/46–47 (34)
-
49
limit 48 to english language [Limit not valid in CDSR; records were retained] (31)
-
50
exp amputation/ (61508)
-
51
traumatic amputation/ (6465)
-
52
amputation stump/ (5461)
-
53
amputee/ (3674)
-
54
(amputat∗ or amputee∗).tw,kw. (88544)
-
55
or/50–54 (110186)
-
56
exp limb prosthesis/ (13941)
-
57
prosthesis implantation/ (14366)
-
58
prosthesis/ (73305)
-
59
prosthesis design/ (52033)
-
60
(artificial adj2 (implant∗ or prosthes?s or prosthetic∗)).tw,kw,dv. (4745)
-
61
or/56–60 (146027)
-
62
limb/ (59338)
-
63
exp lower limb/ (465976)
-
64
exp leg injury/ (212469)
-
65
exp leg bone/ (185832)
-
66
(leg or legs or low∗ extremity or low∗ extremities or low∗ limb or low∗ limbs or knee or knees or transfemoral or trans femoral or transtibial or trans tibial).tw,kw. (707325)
-
67
or/62–66 (1254486)
-
68
67 and (55 or 61) (69151)
-
69
osseointegration/ (10158)
-
70
(osseointegrat∗ or osseo integrat∗ or OIP).tw,kw,dv. (18338)
-
71
bone implant interface/ (744)
-
72
(bone implant∗ interfac∗ or bone prosthes?s interfac∗ or ((boneanchor∗ or bone anchor∗) adj2 (prosthes?s or prosthet∗))).tw,kw,dv. (3030)
-
73
(percutaneous or Intraosseous or intra osseous).tw,kw,dv. (339348)
-
74
(direct skeletal or endo exo∗ or endoexo∗).tw,kw,dv. (1336)
-
75
(integral leg adj2 (prosthes?s or prosthetic∗)).tw,kw,dv. (4)
-
76
or/69–75 (366375)
-
77
68 and 76 (3082)
-
78
Economics/ (257267)
-
79
Health Economics/ or Pharmacoeconomics/ or Drug Cost/ or Drug Formulary/ (131754)
-
80
Economic Aspect/ or exp Economic Evaluation/ (431875)
-
81
(econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗).tw,kw. (830390)
-
82
exp “Cost”/ (558350)
-
83
(cost or costs or costing or costly).ti. (245773)
-
84
cost effective∗.tw,kw. (300869)
-
85
(cost∗ adj2 (util∗ or efficac∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)).ab,kw. (198266)
-
86
Monte Carlo Method/ (58972)
-
87
(decision adj1 (tree∗ or analy∗ or model∗)).tw,kw. (41180)
-
88
(markov or markow or monte carlo).tw,kw. (122160)
-
89
Quality-Adjusted Life Years/ (35659)
-
90
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw,kw. (66580)
-
91
((adjusted adj1 (quality or life)) or (willing∗ adj2 pay) or sensitivity analys∗s).tw,kw. (121888)
-
92
or/78–91 (2027599)
-
93
77 and 92 (125)
-
94
93 use emez (52)
-
95
limit 94 to english language [Limit not valid in CDSR; records were retained] (48)
-
96
49 or 95 (79)
-
97
96 use medall (29)
-
98
96 use emez (48)
-
99
96 use coch (0)
-
100
96 use cctr (2)
-
101
96 use clhta (0)
-
102
96 use cleed (0)
-
103
remove duplicates from 96 (62)
Grey Literature Search
Performed: June 5–6, 2018
Websites searched:
HTA Database Canadian Repository, Alberta Health Technologies Decision Process reviews, Canadian Agency for Drugs and Technologies in Health (CADTH), Institut national d'excellence en santé et en services sociaux (INESSS), Institute of Health Economics (IHE), McGill University Health Centre Health Technology Assessment Unit, National Institute for Health and Care Excellence (NICE), Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers, Australian Government Medical Services Advisory Committee, Centers for Medicare & Medicaid Services Technology Assessments, Institute for Clinical and Economic Review, Ireland Health Information and Quality Authority Health Technology Assessments, Washington State Health Care Authority Health Technology Reviews, ClinicalTrials.gov, PROSPERO, EUnetHTA, Tuft's Cost-Effectiveness Analysis Registry
Keywords used:
osseointegration, intraosseous, bone anchor, direct skeletal, prosthesis, prostheses, prosthetic, amputation
Results (included in PRISMA): 1
Ongoing clinical trials (ClinicalTrials.gov): 1
Ongoing HTAs (PROSPERO/EUnetHTA): 2
Health State Utilities Search
Search date: June 11, 2018
Database searched: Ovid MEDLINE
Database: Ovid MEDLINE(R) ALL <1946 to June 08, 2018>
Search Strategy:
-
1
exp Amputation/ (19741)
-
2
Amputation, Traumatic/ (4521)
-
3
Amputation Stumps/ (2992)
-
4
Amputees/ (3061)
-
5
(amputat∗ or amputee∗).ti,ab,kf. (40398)
-
6
or/1–5 (49332)
-
7
Artificial Limbs/ (6218)
-
8
Prosthesis Implantation/ (12334)
-
9
“Prostheses and Implants”/ (43481)
-
10
Prosthesis Design/ (47200)
-
11
(artificial adj2 (implant∗ or prosthes?s or prosthetic∗)).ti,ab,kf. (2035)
-
12
or/7–11 (100614)
-
13
Extremities/ (23202)
-
14
exp lower extremity/ (154546)
-
15
exp Leg Bones/ (88561)
-
16
Leg Injuries/ (8940)
-
17
(leg or legs or low∗ extremity or low∗ extremities or low∗ limb or low∗ limbs or knee or knees or transfemoral or trans femoral or transtibial or trans tibial).ti,ab,kf. (297782)
-
18
or/13–17 (473332)
-
19
18 and (6 or 12) (34075)
-
20
Osseointegration/ (9024)
-
21
(osseointegrat∗ or osseo integrat∗ or OIP).ti,ab,kf. (8692)
-
22
Bone-Implant Interface/ (313)
-
23
(bone implant∗ interfac∗ or bone prosthes?s interfac∗ or ((boneanchor∗ or bone anchor∗) adj2 (prosthes?s or prosthet∗))).ti,ab,kf. (1433)
-
24
(percutaneous or Intraosseous or intra osseous).ti,ab,kf. (133225)
-
25
(direct skeletal or endo exo∗ or endoexo∗).ti,ab,kf. (591)
-
26
(integral leg adj2 (prosthes?s or prosthetic∗)).ti,ab,kf. (1)
-
27
or/20–26 (148358)
-
28
19 and 27 (1708)
-
29
Quality-Adjusted Life Years/ (10215)
-
30
(quality adjusted or adjusted life year∗).tw. (13366)
-
31
(qaly∗ or qald∗ or qale∗ or qtime∗).tw. (8598)
-
32
(illness state$1 or health state$1).tw. (5534)
-
33
(hui or hui1 or hui2 or hui3).tw. (1268)
-
34
(multiattribute∗ or multi attribute∗).tw. (749)
-
35
(utility adj3 (score$1 or valu∗ or health∗ or cost∗ or measure∗ or disease∗ or mean or gain or gains or index∗)).tw. (11926)
-
36
utilities.tw. (5991)
-
37
(eq-5d or eq5d or eq-5 or eq5 or euro qual or euroqual or euro qual5d or euroqual5d or euro qol or euroqol or euro qol5d or euroqol5d or euro quol or euroquol or euro quol5d or euroquol5d or eur qol or eurqol or eur qol5d or eurqol5d or euro?qul or eur?qul5d or euro∗ quality of life or European qol).tw. (8678)
-
38
(euro∗ adj3 (5 d or 5d or 5 dimension∗ or 5dimension∗ or 5 domain∗ or 5domain∗)).tw. (2999)
-
39
(sf36∗ or sf 36∗ or sf thirtysix or sf thirty six).tw. (19302)
-
40
(time trade off$1 or time tradeoff$1 or tto or timetradeoff$1).tw. (1652)
-
41
((qol or hrqol or quality of life).ti. or ∗quality of life/) and ((qol or hrqol∗ or quality of life) adj2 (increas∗ or decreas∗ or improve∗ or declin∗ or reduc∗ or high∗ or low∗ or effect or effects of worse or score or scores or change$1 or impact$1 or impacted or deteriorate$)).ab. (26217)
-
42
Cost-Benefit Analysis/ and (cost effectiveness ratio∗ and (perspective∗ or life expectanc∗)).tw. (2796)
-
43
∗quality of life/ and (quality of life or qol).ti. (46416)
-
44
quality of life/ and ((quality of life or qol) adj3 (improve∗ or chang∗)).tw. (20443)
-
45
quality of life/ and ((quality of life or qol) adj (score$1 or measure$1)).tw. (10016)
-
46
quality of life/ and health-related quality of life.tw. (25916)
-
47
quality of life/ and ec.fs. (8953)
-
48
quality of life/ and (health adj3 status).tw. (7668)
-
49
(quality of life or qol).tw. and cost-benefit analysis/ (10473)
-
50
models, economic/ (8785)
-
51
or/29–50 (135166)
-
52
28 and 51 (28)
-
53
limit 52 to english language (25)
Health State Utility Values Search (Leg Prosthetics and Filter)
Search date: June 27, 2018
Database searched: Ovid MEDLINE
Database: Ovid MEDLINE(R) ALL <1946 to June 26, 2018>
Search Strategy:
-
1
Artificial Limbs/ (6191)
-
2
Prosthesis Implantation/ (12333)
-
3
“Prostheses and Implants”/ (43430)
-
4
Prosthesis Design/ (47245)
-
5
(artificial adj2 (implant∗ or prosthes?s or prosthetic∗)).ti,ab,kf. (2039)
-
6
or/1–5 (100607)
-
7
Extremities/ (23206)
-
8
exp lower extremity/ (154642)
-
9
exp Leg Bones/ (88637)
-
10
Leg Injuries/ (8949)
-
11
(leg or legs or low∗ extremity or low∗ extremities or low∗ limb or low∗ limbs or knee or knees or transfemoral or trans femoral or transtibial or trans tibial).ti,ab,kf. (298200)
-
12
or/7–11 (473844)
-
13
6 and 12 (14948)
-
14
Quality-Adjusted Life Years/ (10183)
-
15
(quality adjusted or adjusted life year∗).tw. (13338)
-
16
(qaly∗ or qald∗ or qale∗ or qtime∗).tw. (8582)
-
17
(illness state$1 or health state$1).tw. (5503)
-
18
(hui or hui1 or hui2 or hui3).tw. (1268)
-
19
(multiattribute∗ or multi attribute∗).tw. (751)
-
20
(utility adj3 (score$1 or valu∗ or health∗ or cost∗ or measure∗ or disease∗ or mean or gain or gains or index∗)).tw. (11909)
-
21
utilities.tw. (5983)
-
22
(eq-5d or eq5d or eq-5 or eq5 or euro qual or euroqual or euro qual5d or euroqual5d or euro qol or euroqol or euro qol5d or euroqol5d or euro quol or euroquol or euro quol5d or euroquol5d or eur qol or eurqol or eur qol5d or eurqol5d or euro?qul or eur?qul5d or euro∗ quality of life or European qol).tw. (8635)
-
23
(euro∗ adj3 (5 d or 5d or 5 dimension∗ or 5dimension∗ or 5 domain∗ or 5domain∗)).tw. (2987)
-
24
(sf36∗ or sf 36∗ or sf thirtysix or sf thirty six).tw. (19272)
-
25
(time trade off$1 or time tradeoff$1 or tto or timetradeoff$1).tw. (1644)
-
26
((qol or hrqol or quality of life).ti. or ∗quality of life/) and ((qol or hrqol∗ or quality of life) adj2 (increas∗ or decreas∗ or improve∗ or declin∗ or reduc∗ or high∗ or low∗ or effect or effects of worse or score or scores or change$1 or impact$1 or impacted or deteriorate$)).ab. (26165)
-
27
Cost-Benefit Analysis/ and (cost effectiveness ratio∗ and (perspective∗ or life expectanc∗)).tw. (2781)
-
28
∗quality of life/ and (quality of life or qol).ti. (46300)
-
29
quality of life/ and ((quality of life or qol) adj3 (improve∗ or chang∗)).tw. (20423)
-
30
quality of life/ and ((quality of life or qol) adj (score$1 or measure$1)).tw. (10001)
-
31
quality of life/ and health-related quality of life.tw. (25835)
-
32
quality of life/ and ec.fs. (8939)
-
33
quality of life/ and (health adj3 status).tw. (7655)
-
34
(quality of life or qol).tw. and cost-benefit analysis/ (10449)
-
35
models, economic/ (8775)
-
36
or/14–35 (134921)
-
37
13 and 36 (208)
-
38
limit 37 to english language (197)
Appendix 2: Selected Excluded Studies—Clinical Evidence
| Citation | Primary Reason for Exclusion |
|---|---|
| Sullivan J, Uden M, Robinson KP, Sooriakumaran S. Rehabilitation of the transfemoral amputee with an osseointegrated prosthesis: the United Kingdom experience. Prosthet Orthot Int. 2003;27(2):114–20 | Different device that was not specific to intervention of interest |
| Hagberg K, Branemark R, Gunterberg B, Rydevik B. Osseointegrated transfemoral amputation prostheses: prospective results of general and condition-specific quality of life in 18 patients at 2-year follow-up. Prosthet Orthot Int. 2008;32(1):29–41 | Same patients were included in the study by Branemark et al, 201410 |
| Hagberg K, Branemark R. One hundred patients treated with osseointegrated transfemoral amputation prostheses–rehabilitation perspective. J Rehabil Res Dev. 2009;46(3):331–44 | Includes patients before OPRA protocol; patients with OPRA protocol were reported by Branemark et al, 201410 |
| Aschoff HH, Kennon RE, Keggi JM, Rubin LE. Transcutaneous, distal femoral, intramedullary attachment for above-the-knee prostheses: an endo-exo device. J Bone Joint Surg Am. 2010;92 Suppl 2:180–6 | The study was the interim report of the study by Juhnke et al, 20156 |
| Hagberg K, Hansson E, Branemark R. Outcome of percutaneous osseointegrated prostheses for patients with unilateral transfemoral amputation at two-year follow-up. Arch Phys Med Rehabil. 2014;95(11):2120–7 | Most patients were included in the study by Branemark et al, 201410 |
| Guirao L, Samitier CB, Costea M, Camos JM, Majo M, Pleguezuelos E. Improvement in walking abilities in transfemoral amputees with a distal weight bearing implant. Prosthet Orthot Int. 2017;41(1):26–32 | Different device that was not specific to intervention of interest |
| Al Muderis M, Lu W, Tetsworth K, Bosley B, Li JJ. Single-stage osseointegrated reconstruction and rehabilitation of lower limb amputees: the Osseointegration Group of Australia Accelerated Protocol-2 (OGAAP-2) for a prospective cohort study. BMJ Open. 2017;7(3):e013508 | Protocol for a study |
| Al Muderis M, Lu W, Glatt V, Tetsworth K. Two-stage osseointegrated reconstruction of post-traumatic unilateral transfemoral amputees. Mil Med. 2018;183(suppl 1):496–502 | Same patients were included in the study by Al Muderis et al, 201634 |
Abbreviation: OPRA, Osseointegrated Prostheses for the Rehabilitation of Amputees Implant System.
Appendix 3: Critical Appraisal of Clinical Evidence
Table A1:
Risk of Bias Among Nonrandomized Studies (ROBINS-I)
| Author, Year | Confounding | Study Participation Selection | Classification of Interventions | Deviations From Intended Intervention | Missing Data | Measurement of Outcomes | Selection of Reported Results |
|---|---|---|---|---|---|---|---|
| Tilander et al, 201031 | No | Yesa | No | No | No | No | No |
| Branemark et al, 201410 | No | No | No | No | No | No | No |
| Tilander et al, 201730 | No | No | No | No | No | No | No |
| Hagberg, 201829 | No | No | No | No | No | No | No |
| van de Meent et al, 201333 | No | No | No | No | No | No | Yesb |
| Juhnke et al, 20156 | No | No | No | No | No | No | No |
| Al Muderis et al, 201632 | No | No | No | No | No | No | No |
| Al Muderis et al, 201634 | No | No | No | No | No | No | Yesc |
| Al Muderis et al, 201735 | No | Unknown | No | No | No | No | Yesc |
Abbreviation: Q-TFA, Questionnaire for Persons with a Transfemoral Amputation; ROBINS-I, Risk of Bias in Non-randomized Studies—of Interventions; SF-36, 36-Item Short Form Health Survey.
Patients were those who attended the osseointegrated clinic.
Did not report all subscales of Q-TFA.
Did not report all subscales of Q-TFA and SF-36.
Table A2:
GRADE Evidence Profile for Comparison of Osseointegrated Prosthetic Implants and Conventional Socket Prostheses for People With Lower-Limb Amputation
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Outcomes measured | |||||||
| Q-TFA | |||||||
| 3 (observational) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Not detected | None | ⊕⊕ Low |
| 6MWT | |||||||
| 2 (observational) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Not detected | None | ⊕⊕ Low |
| TUG | |||||||
| 2 (observational) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Not detected | None | ⊕⊕ Low |
| SF-36 physical component summary | |||||||
| 2 (observational) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Not detected | None | ⊕⊕ Low |
| SF-36 mental component summary | |||||||
| 1 (observational) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Not detected | None | ⊕⊕ Low |
| Infection | |||||||
| 8 (observational) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Not detected | Other considerations (+2)a | ⊕⊕⊕⊕ High |
| Bone fracture | |||||||
| 6 (observational) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Not detected | Other considerations (+2)a | ⊕⊕⊕⊕ High |
| Explant | |||||||
| 7 (observational) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Not detected | Other considerations (+2)a | ⊕⊕⊕⊕ High |
| Device mechanical complications | |||||||
| 6 (observational) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Not detected | Other considerations (+2)a | ⊕⊕⊕⊕ High |
| Noninfectious soft tissue and bone complications | |||||||
| 2 (observational) | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Not detected | Other considerations (+2)a | ⊕⊕⊕⊕ High |
Abbreviations: 6MWT, 6-Minute Walk Test; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; SF-36, 36-Item Short Form Health Survey; TUG, Timed Up and Go test; Q-TFA, Questionnaire for Persons with a Transfemoral Amputation.
Observational studies start at a low GRADE level because of inherent limitations in study design. However, we upgraded the quality of the evidence because these studies can provide high-quality evidence of adverse effects associated with an intervention, thereby allowing us to infer a strong association from even a limited number of events. The GRADE level for adverse events was high because we were certain that these events had occurred.
Appendix 4: Selected Excluded Studies—Economic Evidence
| Citation | Primary Reason for Exclusion |
|---|---|
| Kaulback K, Jones, A. Osseointegrated prosthetic implants for lower limb amputation: a review of clinical effectiveness, cost-effectiveness and guidelines. CADTH rapid response report: summary with critical appraisal. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health. 2017. | Review |
| Al Muderis MM, Lu WY, Li JJ. Kaufman K, Orendurff M, Highsmith MJ, Lunseth PA, Kahle JT. Clinically relevant outcome measures following limb osseointegration; systematic review of the literature. J Orthop Trauma. 2018;32(2):e64-e75. | Review |
| Haggstrom EE, Hansson E, Hagberg K. Comparison of prosthetic costs and service between osseointegrated and conventional suspended transfemoral prostheses. Prosthet Orthot Int. 2013;37(2):152–160. | Cost comparison |
Appendix 5: Results of Applicability Checklists for Studies Included in the Economic Literature Review
Table A3:
Assessment of the Applicability of Studies Evaluating the Cost-Effectiveness of Osseointegrated Prosthetic Implants
| Author, Year, Country of Publication | Is the study population similar to the question? | Are the interventions similar to the question? | Is the health care system studied sufficiently similar to Ontario? | Were the perspectives clearly stated? If yes, what were they? | Are estimates of relative treatment effect from the best available source? | Are all future costs and outcomes discounted? If yes, at what rate? | Is the value of health effects expressed in terms of quality-adjusted life-years? | Are costs and outcomes from other sectors fully and appropriately measured and valued? | Overall Judgmenta |
|---|---|---|---|---|---|---|---|---|---|
| Frossard et al, 201772 | Yes, but does not specify if individuals could not achieve a comfortable fit and/or walk comfortably | Yes | No (Australia) | Yes, the study took an Australian state prosthetic care provider perspective | Partially, costs taken from real-world data (which were limited), while a literature search informed utility values | No | Yes | No (the study did not use a societal perspective) | Partially applicable |
| Hansson et al, 201873 | Yes, but does not specify if individuals could not achieve a comfortable fit and/or walk comfortably | Yes | No (Sweden) | Yes, the study took a Swedish health care perspective | Partially, no mention of literature search; estimates primarily from single study (missing Hagberg, 201829) | No | Yes | No (the study did not use a societal perspective) | Partially applicable |
Note: Response options for all items were “yes,” “partially,” “no,” “unclear,” and “NA” (not applicable).
Overall judgment may be “directly applicable,” “partially applicable,” or “not applicable.”
Appendix 6: Additional Tables—Primary Economic Evaluation
Table A4:
Clinical Requirements for People Receiving Osseointegrated Prosthetic Implants
| Patient Selection Criteria | Contraindications |
|---|---|
|
|
Table A5:
Population Demographics of Simulated Cohort
| Reference | Sample Size | Sex Ratio (M:F) | Mean Age at Implant, Years |
|---|---|---|---|
| Sullivan et al, 200374 | 11 | NRa | NR |
| Hagberg et al, 200813 | 18 | 0.80 | 45 |
| Hagberg and Branemark, 200925 | 100 | 1.56 | NR |
| Tillander et al, 201031 | 39 | 1.16 | 49 |
| Branemark et al, 201410 | 51 | 1.21 | 44 |
| Hagberg et al, 201426 | 39 | 0.77 | 44 |
| Aschoff et al, 20109 | 37 | 4.28 | 44 |
| van de Meent et al, 201333 | 22 | 4.50 | 46 |
| Juhnke et al, 20156 | 39 | 4.30 | 45 |
| Al Muderis et al, 201632 | 86 | 3.09 | 48 |
| Khemka et al, 201575 | 4 | 3.00 | 55 |
| Khemka et al, 201676 | 3 | 0.50 | 49 |
| Al Muderis et al, 20167 | 50 | 2.12 | 48 |
| Al Muderis et al, 201735 | 22 | 3.40 | 46 |
| Weighted average | 2.53 | 46 |
Abbreviations: F, female; M, male; NR, not reported.
Studies that did not report characteristics were excluded from the calculation of the weighted average.
Table A6:
Inputs for Deterministic Sensitivity Analyses
| Variable | Mean | Source | Lower Bound Estimate | Upper Bound Estimate | Calculation Method |
|---|---|---|---|---|---|
| Yearly probability of clinical event | |||||
| Implant extraction | 0.018 | Al Muderis et al, 201632; Al Muderis et al, 201634; Tilander et al, 201031; Tilander et al, 201730; Branemark et al, 201410; Hagberg, 201829 | 0.0057 | 0.058 | Based on 95% CI |
| Permanent implant extractiona | 0.68 | Al Muderis et al, 201632; Al Muderis et al, 201634; Tilander et al, 201031; Tilander et al, 201730; Branemark et al, 201410; Hagberg, 201829; Juhnke et al, 20156 | 0.00 | 1.00 | Range of estimates in literature |
| Superficial infection | 0.22 | Al Muderis et al, 201632; Al Muderis et al, 201634; Tilander et al, 201031; Tilander et al, 201730; Branemark et al, 201410; Hagberg, 201829 | 0.16 | 0.30 | Based on 95% CI |
| Deep infection | 0.033 | Al Muderis et al, 201632; Al Muderis et al, 201634; Tilander et al, 201031; Tilander et al, 201730; Branemark et al, 201410; Hagberg, 201829 | 0.013 | 0.077 | Based on 95% CI |
| Soft tissue refashioning | 0.18 | Al Muderis et al, 201632; Al Muderis et al, 201634 | 0.12 | 0.26 | Based on 95% CI |
| Fractures | 0.027 | Al Muderis et al, 201632; Al Muderis et al, 201634; Branemark et al, 201410; Hagberg, 201829 | 0.0093 | 0.080 | Based on 95% CI |
| Stump revisions | 0.026 | Hansson et al, 201845 | 0.013 | 0.18 | Expert opinion |
| Utilities | |||||
| Implant surgery and recovery | 0.682 | Hagberg et al, 201426 | 0.653 | 0.711 | Based on 95% CI |
| Osseointegrated prothesis | 0.692 | Hagberg et al, 201426 | 0.658 | 0.726 | Based on 95% CI |
| Uncomfortable socket | 0.653 | Hagberg et al, 201426 | 0.623 | 0.683 | Based on 95% CI |
| Implant extraction | 0.653 | Hagberg et al, 201426 | 0.623 | 0.683 | Based on 95% CI |
| Reimplant | 0.682 | Hagberg et al, 201426 | 0.653 | 0.711 | Based on 95% CI |
| Costs, $ | |||||
| Uncomfortable socket prosthesis | 358 | SoB | 216 | 784 | Expert opinion |
| Implant device | 36,500 | Montreal surgeryb | 18,250 | 40,150 | Assumption |
| Two-stage Osseo surgery, inpatient stay | 32,477 | OCCI | 26,398 | 38,555 | Based on 95% CI |
| Deep infection, inpatient stay | 14,535 | OCCI | 12,045 | 17,025 | Based on 95% CI |
| Soft tissue refashioning, inpatient stay | 8,313 | OCCI | 6,285 | 10,341 | Based on 95% CI |
| Fracture, inpatient stay | 12,141 | OCCI | 11,175 | 13,107 | Based on 95% CI |
| Stump revision, inpatient stay | 8,313 | OCCI | 6,285 | 10,341 | Based on 95% CI |
| Implant extraction, impatient stay | 14,979 | OCCI | 14,170 | 15,787 | Based on 95% CI |
| Reimplant, inpatient stay | 24,357 | OCCI | 19,798 | 28,916 | Based on 95% CI |
| Miscellaneous | |||||
| Average age at implant, years | 46 | 30 | 50 | Expert opinion | |
| Model time horizon | Lifetime | 5 years | Lifetime | Assumption | |
Abbreviations: CI, confidence interval; SoB, Ontario Physician Schedule of Benefits; OCCI, Ontario Case Costing Initiative.
Indicating the percentage of those who are extracted, that have their implant permanently extracted.
Cost of device in 2018 surgery, conducted in Montreal (Natalie Habra, MD, email communication, June 8, 2018).
Table A7:
Scenario Analyses Inputs—Health State Utilities
| Health State | (Dis)utility | Standard Error | Durationa | Elicitation Method | Reference |
|---|---|---|---|---|---|
| Implant surgery and recovery | |||||
| Hagberg | 0.682 | 0.014 | 1 year | SF-6D | Hagberg et al, 201426 |
| Branemark | 0.732 | Fixed | 1 year | SF-36 to SF-6D | Branemark10; Ara and Brazier, 200951 |
| Osseointegrated prosthesis | |||||
| Hagberg | 0.746 | Fixed | 1 year | SF-36 to SF-6D | Hagberg et al, 200813; Ara and Brazier, 200951 |
| Hagberg | 0.692 | 0.017 | 1 year | SF-6D | Hagberg et al, 201426 |
| Branemark | 0.727 | Fixed | 1 year | SF-36 to SF-6D | Branemark et al, 201410; Ara and Brazier, 200951 |
| Uncomfortable socket prosthesis | |||||
| Hagberg | 0.687 | Fixed | 1 year | SF-36 to SF-6D | Hagberg et al, 200813; Ara and Brazier, 200951 |
| Hagberg | 0.653 | 0.015 | 1 year | SF-6D | Hagberg et al, 201426 |
| Branemark | 0.688 | Fixed | 1 year | SF-36 to SF-6D | Branemark10; Ara and Brazier, 200951 |
| Fracture | |||||
| Honkavaara | –0.120 | 0.009 | 6 months | EQ-5D | Honkavaara et al, 201657 |
| Deep infection | |||||
| Cahill | –0.200 | 0.051 | 6 months | AQoL | Cahill et al, 200877 |
Abbreviations: AQoL, Assessment of Quality of Life; EQ-5D, EuroQol 5-dimension quality of life questionnaire; SF-6D, 6-Dimension Short Form Health Survey; SF-36, 36-Item Short Form Health Survey.
Durations for complications were derived from clinical expert opinion (Richard Jenkinson, MD; Nancy Dudek, MD; email communications, August 2018).
Table A8:
Scenario Analyses Inputs – Other Inputs
| Parameter | Mean | Alpha | Beta | Standard Error | Reference |
|---|---|---|---|---|---|
| Yearly maintenance costs by a prosthetist, $ | |||||
| Osseointegrated prosthesis | 412 | Assumption | |||
| Uncomfortable socket prosthesis | 1,924 | ||||
| Mechanical part replacement | 0.130 | 47 | 322 | ||
| Stump revision rate | 0.186 | 55 | 206 | Assume soft tissue refashioning values | |
| Time-varying rates | |||||
| Superficial infection | Branemark et al, 201410 | ||||
| Year 1 | 0.443 | 29 | 21 | ||
| Year 2+ | 0.230 | 12 | 33 | ||
| Deep infection | Branemark et al, 201410 | ||||
| Year 1 | 0.114 | 6 | 43 | ||
| Year 2+ | 0 | – | – | ||
| Soft tissue refashioning | Branemark et al, 201410 | ||||
| Year 1 | 0 | – | – | ||
| Year 2+ | 0 | – | – | ||
| Fractures | Branemark et al, 201410 | ||||
| Year 1 | 0.0395 | 2 | 47 | ||
| Year 2+ | 0.0216 | 1 | 44 | ||
| Mechanical complications | Branemark et al, 201410 | ||||
| Year 1 | 0.0199 | 1 | 48 | ||
| Year 2+ | 0.160 | 8 | 37 | ||
| Disutilities | |||||
| Fracture | 0.120 | 0.009 | Honkavaara et al, 201657 | ||
| Deep infection | 0.200 | 0.051 | Cahill et al, 200877 | ||
| Utilities | |||||
| Osseointegrated prosthesis (year 1+) | 0.746 | Fixed | Hagberg et al, 200813; Ara and Brazier, 200951 | ||
| Uncomfortable socket prosthesis | 0.687 | Fixed | Hagberg et al, 200813; Ara and Brazier, 200951 | ||
| Implant surgery and recovery | 0.732 | Fixed | Branemark et al, 201410; Ara and Brazier, 200951 | ||
| Osseointegrated prosthesis | 0.727 | Fixed | Branemark et al, 201410; Ara and Brazier, 200951 | ||
| Uncomfortable socket prosthesis | 0.688 | Fixed | Branemark et al, 201410; Ara and Brazier, 200951 | ||
Figure A1: Incremental Cost-Effectiveness Scatterplot—Osseointegrated Prosthetic Implant vs. Uncomfortable Socket Prosthesis.
Abbreviation: QALY, quality-adjusted life-year.
Appendix 7: Additional Tables—Budget Impact Analysis
Table A9:
Annual Costs by Cohort
| Cost, $ | |||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Reference case | |||||
| Osseointegrated prosthetic implant | 77,396 | 2,291 | 2,604 | 2,574 | 2,545 |
| Uncomfortable socket | 595 | 594 | 593 | 591 | 590 |
| Best casea | |||||
| Osseointegrated prosthetic implant | 72,587 | 981 | 1,311 | 1,317 | 1,324 |
| Uncomfortable socket | 2,245 | 2,241 | 2,236 | 2,231 | 2,226 |
| Worst casea | |||||
| Osseointegrated prosthetic implant | 78,106 | 2,988 | 3,292 | 3,252 | 3,214 |
| Uncomfortable socket | 595 | 594 | 593 | 591 | 590 |
From primary economic evaluation.
Table A10:
Target Population for Scenario Analyses
| Number of Amputees Eligible for Osseointegrated Prosthetic Implant, n | |||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Decrease 25% from reference case | 28 | 5 | 5 | 5 | 5 |
| Increase 25% from reference case | 46 | 9 | 9 | 9 | 9 |
| Include all nonvascular above-the-knee amputees | 69 | 70 | 71 | 72 | 73 |
| Include all nonvascular above- or below-the-knee amputees eligible for osseointegration | 66 | 16 | 16 | 16 | 16 |
| Expand number eligible by 5% annually | 37 | 21 | 23 | 24 | 26 |
Appendix 8: Letter of Information
LETTER OF INFORMATION
Health Quality Ontario is conducting a review of Osseointegrated Prosthetic Implant for people with above or below the knee amputation. The purpose is to understand whether this implant should be publicly funded in Ontario.
An important part of this review involves gathering perspectives of patients and caregivers with experience with either an osseointegrated prosthetic implant or above or below the knee amputation. They could have had the osseointegrated prosthetic implant, recently or in the past or could be considering it in the future.
WHAT DO YOU NEED FROM ME
-
✓
Willingness to share your story
-
✓
30 minutes of your time for a phone or videoconference
-
✓
Permission to audio- (not video-) record the Interview
WHAT YOUR PARTICIPATION INVOLVES
If you agree to share your experiences, you will be asked to have an interview with Health Quality Ontario staff. The interview will last 30 minutes. It will be held over the telephone or videoconference. With your permission, the interview will be audio-taped. The interviewer will ask you questions about your or your loved one's condition and your perspectives about treatment options in Ontario.
Participation is voluntary. You may refuse to participate, refuse to answer any questions or withdraw before or at any point during your interview. Withdrawal will in no way affect the care you receive.
CONFIDENTIALITY
All information you share will be kept confidential and your privacy will be protected except as required by law. The results of this review will be published, however no identifying Information will be released or published. Any records containing information from your interview will be stored securely until project completion. After the project completion, the records will be destroyed.
RISKS TO PARTICIPATION
There are no known physical risks to participating. Some participants may experience discomfort or anxiety after speaking about their experience.
IF YOU ARE INTERESTED, PLEASE CONTACT US BEFORE OCTOBER 08, 2018:
Appendix 9: Interview Guide for Participants Who Have Not Received an Osseointegrated Prosthetic Implant
Introduction
Health Quality Ontario is a provincial advisor to the Ministry of Health and Long-Term Care. We do a few things for the ministry, but one of the roles that we have is to conduct health technology assessments that look at technologies and new health services. We review these technologies and new health services for the consideration of public funding.
If any of my questions cause you emotional distress or discomfort, please let me know, and you can feel free to either not answer the question or say as little as you like. Having said that, do you have any questions for me?
History of condition that led to lower-limb amputation
Experience (infection or traumatic event)
Lived Experience
How is your day-to-day routine?
What has been the impact and effect on quality of life?
Did you see any sort of loss of independence?
Did it have an impact on your loved ones/caregivers, work, friends?
Do you feel more comfortable with it now as opposed to before?
Prosthetic/Socket
Decision-making around prosthetic or socket: How did you decide you wanted to go ahead with it … wanted to change it?
Progression of devices for lower-limb amputation
What are the challenge, barriers, and/or benefits of prosthetics or sockets?
Osseointegrated Prosthetic Implant
What can you tell me about the osseointegrated prosthetic implant? How did you hear about it?
What is holding you back from receiving an osseointegrated prosthetic implant?
Barriers/Challenges
Did you face any sort of barrier in terms of distance of travel or cost?
Accessibility of any services?
Appendix 10: Interview Guide for Participants Who Have Received an Osseointegrated Prosthetic Implant
Introduction
Health Quality Ontario is a provincial advisor to the ministry of health and long-term care. We do a few things for the ministry, but one of the roles that we have is to conduct health technology assessments, which look at technologies and new health services. We review these technologies and new health services for the consideration for public funding.
If any of my questions cause you emotional distress or uncomfortable, please let me know, and you can feel free to either not answer the question or say at little as you like. Having said that do you have any questions for me?
History of condition that led to lower-limb amputation
Experience (infection or traumatic event)
Lived Experience After Amputation, After Receiving Prosthetic Socket, and After Receiving Osseointegrated Prosthetic Implant
How is your day-to-day routine? After amputation, after receiving socket, and after receiving implant?
What has been the impact and effect on quality of life? After amputation, after receiving socket, and after receiving implant?
Did you see any sort of loss of independence? After amputation, after receiving socket, and after receiving implant?
Did it have an impact on your loved ones/caregivers, work, friends? After amputation, after receiving socket, and after receiving implant?
Do you feel more comfortable with it now as opposed to before? After amputation, after receiving socket, and after receiving implant?
Prosthetic Socket
Decision-making around prosthetic: How did you decide you wanted to go ahead with it … wanted to change it?
Progression of devices for lower-limb amputation
What are the challenges, barriers, and/or benefits of prosthetics?
Osseointegrated Prosthetic Implant
What can you tell me about the osseointegrated prosthetic implant? What was your pathway of care? How did the doctor provide you information, or did you get that information yourself?
How did you decide that you wanted to receive the osseointegrated prosthetic implant? Was it a difficult decision, or, with the information provided, was it easy for you to make the decision?
What was the time that you had to wait for the implant? Did you feel like you needed to wait a long time? Did you feel any sort of anxiety?
Did any other factors have an impact on your decision? Such as cost, the time away from work, etc., or any other commitments you may have?
What was the surgery process?
How long has it been since you had the implant?
Since having the implant, have you experienced any adverse events? Such as infection (soft tissue or deep) or any treatment requiring antibiotics/surgery?
How was the recovery? How long did it take? Were there any complications?
Did you have any bone fractures in any part of the body or any breakage or malfunction of the implant's external parts that required exchange or replacement?
Barrier/Challenges
Did you face any sort of barrier in terms of distance of travel or cost?
Accessibility of any services?
Cost?
Author contributions
This report was developed by a multidisciplinary team from Health Quality Ontario, which is now the Quality business unit at Ontario Health. The clinical epidemiologist was Shayan Sehatzadeh, the health economist was Sean Tiggelaar, the patient and public partnership analyst was Ammara Shafique, and the medical librarian was Corinne Holubowich
KEY MESSAGES
What Is This Health Technology Assessment About?
The loss of a leg is a traumatic event that usually dramatically affects quality of life for amputees and their families. To walk again after an amputation, most people are fitted with a prosthesis (artificial leg). With the conventional type, called a socket prosthesis, friction between the remaining limb and the socket often causes skin problems and pain. These problems can be so severe that people restrict their activity or stop using the prosthesis until their skin has healed.
Lower-limb osseointegrated prosthetic implantation involves inserting a metal rod into the person's leg bone. Over time, the bone grows around the implant (this process is called osseointegration). A metal connection at the end of the implant allows it to be connected to the artificial leg. This technology is not yet widely available in Canada, although a few surgeries have been done in Montreal, with private funding. Some people have travelled overseas for an osseointegrated prosthetic implant, at a cost of about $100,000 per procedure.
This health technology assessment looked at how safe, effective, and cost-effective osseointegrated prosthetic implants are for lower-limb amputees. It also looked at the budget impact of publicly funding them and at the experiences, preferences, and values of people with a lower-limb amputation.
What Did This Health Technology Assessment Find?
Osseointegrated prosthetic implants improve people's ability to walk and function in daily life, but their use can lead to serious adverse events such as bone infection or fractures, which may require additional surgeries.
Compared with conventional socket prostheses for people with chronic socket-related issues, osseointegrated prosthetic implants may be cost-effective, but there is a large degree of uncertainty. We estimate that publicly funding osseointegrated prosthetic implants for people with lower-limb amputations in Ontario would result in additional total spending of about $5.3 million over the next 5 years.
We spoke with nine people with experience of both an osseointegrated prosthetic implant and a conventional socket prosthesis, three people with experience of a conventional socket prosthesis only, and one person who had recently undergone amputation and not yet chosen a prosthesis, as well as three caregivers. Those who had received an osseointegrated prosthetic implant said they had better mobility and quality of life than before receiving their implant but had concerns about the ongoing risk of infection and the potential for problems with implant maintenance. Those with experience of a conventional socket prosthesis only were considering getting an osseointegrated prosthetic implant. These individuals reported that cost was the only factor preventing them from undergoing the procedure.
Contributor Information
Ontario Health (Quality):
Shayan Sehatzadeh, Sean Tiggelaar, Ammara Shafique, and Corinne Holubowich
About Us
This health technology assessment was produced by Health Quality Ontario, which is now the Quality business unit at Ontario Health, the government agency that when fully established will be responsible for ensuring all Ontarians receive high-quality health care where and when they need it.
For more information about Health Quality Ontario, visit hqontario.ca.
For more information about Ontario Health, visit ontariohealth.ca.
REFERENCES
- (1).Imam B, Miller WC, Finlayson HC, Eng JJ, Jarus T. Incidence of lower limb amputation in Canada. Can J Public Health. 2017;108(4):e374–e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Mak AF, Zhang M, Boone DA. State-of-the-art research in lower-limb prosthetic biomechanics-socket interface: a review. J Rehabil Res Dev. 2001;38(2):161–74. [PubMed] [Google Scholar]
- (3).Gailey R, Allen K, Castles J, Kucharik J, Roeder M. Review of secondary physical conditions associated with lower-limb amputation and long-term prosthesis use. J Rehabil Res Dev. 2008;45(1):15–29. [DOI] [PubMed] [Google Scholar]
- (4).Hagberg K, Branemark R. Consequences of non-vascular trans-femoral amputation: a survey of quality of life, prosthetic use and problems. Prosthet Orthot Int. 2001;25(3):186–94. [DOI] [PubMed] [Google Scholar]
- (5).Devan H, Tumilty S, Smith C. Physical activity and lower-back pain in persons with traumatic transfemoral amputation: a national cross-sectional survey. J Rehabil Res Dev. 2012;49(10):1457–66. [DOI] [PubMed] [Google Scholar]
- (6).Juhnke DL, Beck JP, Jeyapalina S, Aschoff HH. Fifteen years of experience with Integral-Leg-Prosthesis: cohort study of artificial limb attachment system. J Rehabil Res Dev. 2015;52(4):407–20. [DOI] [PubMed] [Google Scholar]
- (7).Al Muderis M, Lu W, Tetsworth K, Bosley B, Li JJ. Single-stage osseointegrated reconstruction and rehabilitation of lower limb amputees: the Osseointegration Group of Australia Accelerated Protocol-2 (OGAAP-2) for a prospective cohort study. BMJ Open. 2017;7(3):e013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Li Y, Branemark R. Osseointegrated prostheses for rehabilitation following amputation : the pioneering Swedish model. Unfallchirurg. 2017;120(4):285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Aschoff HH, Kennon RE, Keggi JM, Rubin LE. Transcutaneous, distal femoral, intramedullary attachment for above-the-knee prostheses: an endo-exo device. J Bone Joint Surg Am. 2010;92 Suppl 2:180–6. [DOI] [PubMed] [Google Scholar]
- (10).Branemark R, Berlin O, Hagberg K, Bergh P, Gunterberg B, Rydevik B. A novel osseointegrated percutaneous prosthetic system for the treatment of patients with transfemoral amputation: a prospective study of 51 patients. [Erratum appears in Bone Joint J. 2014 Apr;96-B(4):562]. Bone Joint J. 2014;96-B(1):106–13. [DOI] [PubMed] [Google Scholar]
- (11).Branemark PI, Adell R, Breine U, Hansson BO, Lindstrom J, Ohlsson A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg. 1969;3(2):81–100. [DOI] [PubMed] [Google Scholar]
- (12).Thesleff A, Branemark R, Hakansson B, Ortiz-Catalan M. Biomechanical characterisation of bone-anchored implant systems for amputation limb prostheses: a systematic review. Ann Biomed Eng. 2018;46(3):377–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Hagberg K, Branemark R, Gunterberg B, Rydevik B. Osseointegrated trans-femoral amputation prostheses: prospective results of general and condition-specific quality of life in 18 patients at 2-year follow-up. Prosthet Orthot Int. 2008;32(1):29–41. [DOI] [PubMed] [Google Scholar]
- (14).Staubach KH, Grundei H. [The first osseointegrated percutaneous prosthesis anchor for above-knee amputees]. Biomed Tech (Berl). 2001;46(12):355–61. [DOI] [PubMed] [Google Scholar]
- (15).Food and Drug Administration. OPRA device summary of safety and probable benefit (SSPB) [Internet]. 2015. [cited 2018 Apr]. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf8/H080004B.pdf
- (16).U.S. Department of Veterans Affairs. Osseointegration (OI) for direct skeletal attachment of prosthetic limbs in veterans with amputations [Internet]. Washington (DC): The Department; 2017. [cited 2019 Feb 22]. Available from: http://www.wrnmmc.capmed.mil/Health%20Services/Surgery/Orthopaedics%20and%20Rehabilitation/Osseointegration/SiteAssets/Osseointegration-FactSheet%20VA.pdf [Google Scholar]
- (17).Orthotics Prosthetics Canada. Osseointegrated prosthetic implants in Canada: defining roles and driving a common approach for a world-class standard of care: a white paper report. Ottawa (ON): Orthotics Prosthetics Canada; 2018. [Google Scholar]
- (18).McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- (19).Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook [Internet]. Hamilton (ON): GRADE Working Group; 2013. [cited 2017 Dec]. Available from http://gdt.guidelinedevelopment.org/app/handbook/handbook.html. [Google Scholar]
- (21).Atallah R, Leijendekkers RA, Hoogeboom TJ, Frolke JP. Complications of bone-anchored prostheses for individuals with an extremity amputation: a systematic review. PLoS One. 2018;13(8):e0201821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Leijendekkers RA, van Hinte G, Frolke JP, van de Meent H, Nijhuis-van der Sanden MW, Staal JB. Comparison of bone-anchored prostheses and socket prostheses for patients with a lower extremity amputation: a systematic review. Disabil Rehabil. 2017;39(11):1045–58. [DOI] [PubMed] [Google Scholar]
- (23).van Eck CM RL. Clinical outcome of osseointegrated prostheses for lower extremity amputations: a systematic review of the literature. Curr Orthop Pract. 26(4). [Google Scholar]
- (24).Hebert JS, Rehani M, Stiegelmar R. Osseointegration for lower-limb amputation: a systematic review of clinical outcomes. JBJS Reviews. 2017;5(10):e10. [DOI] [PubMed] [Google Scholar]
- (25).Hagberg K, Branemark R. One hundred patients treated with osseointegrated transfemoral amputation prostheses–rehabilitation perspective. J Rehabil Res Dev. 2009;46(3):331–44. [PubMed] [Google Scholar]
- (26).Hagberg K, Hansson E, Branemark R. Outcome of percutaneous osseointegrated prostheses for patients with unilateral transfemoral amputation at two-year follow-up. Arch Phys Med Rehabil. 2014;95(11):2120–7. [DOI] [PubMed] [Google Scholar]
- (27).Al Muderis MA, Lu W, Glatt V, Tetsworth K. Two-stage osseointegrated reconstruction of post-traumatic unilateral transfemoral amputees. Mil Med. 2018;183(suppl_1):496–502. [DOI] [PubMed] [Google Scholar]
- (28).Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. [DOI] [PubMed] [Google Scholar]
- (29).Hagberg K. Bone-anchored prostheses in patients with traumatic bilateral transfemoral amputations: rehabilitation description and outcome in 12 cases treated with the OPRA implant system. Disabil Rehabil Assist Technol. 2018:1–8. [DOI] [PubMed] [Google Scholar]
- (30).Tillander J, Hagberg K, Berlin O, Hagberg L, Branemark R. Osteomyelitis risk in patients with transfemoral amputations treated with osseointegration prostheses. Clin Orthop Relat Res. 2017;475(12):3100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Tillander J, Hagberg K, Hagberg L, Branemark R. Osseointegrated titanium implants for limb prostheses attachments: infectious complications. Clin Orthop Relat Res. 2010;468(10):2781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Al Muderis M, Khemka A, Lord SJ, van de Meent H, Frolke JP. Safety of osseointegrated implants for transfemoral amputees: a two-center prospective cohort study. J Bone Joint Surg Am. 2016;98(11):900–9. [DOI] [PubMed] [Google Scholar]
- (33).van de Meent H, Hopman MT, Frolke JP. Walking ability and quality of life in subjects with transfemoral amputation: a comparison of osseointegration with socket prostheses. Arch Phys Med Rehabil. 2013;94(11):2174–8. [DOI] [PubMed] [Google Scholar]
- (34).Al Muderis MA, Tetsworth K, Khemka A, Wilmot S, Bosley B, Lord SJ, et al. The Osseointegration Group of Australia Accelerated Protocol (OGAAP-1) for two-stage osseointegrated reconstruction of amputated limbs. Bone Joint J. 2016;98-B(7):952–60. [DOI] [PubMed] [Google Scholar]
- (35).Al Muderis M, Lu W, Li JJ. Osseointegrated Prosthetic Limb for the treatment of lower limb amputations : experience and outcomes. Unfallchirurg. 2017;120(4):306–11. [DOI] [PubMed] [Google Scholar]
- (36).Gailey RS, Roach KE, Applegate EB, Cho B, Cunniffe B, Licht S, et al. The amputee mobility predictor: an instrument to assess determinants of the lower-limb amputee's ability to ambulate. Arch Phys Med Rehabil. 2002;83(5):613–27. [DOI] [PubMed] [Google Scholar]
- (37).Lin SJ, Bose NH. Six-minute walk test in persons with transtibial amputation. Arch Phys Med Rehabil. 2008;89(12):2354–9. [DOI] [PubMed] [Google Scholar]
- (38).Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–8. [DOI] [PubMed] [Google Scholar]
- (39).Hagberg K, Branemark R, Hagg O. Questionnaire for Persons with a Transfemoral Amputation (Q-TFA): initial validity and reliability of a new outcome measure. J Rehabil Res Dev. 2004;41(5):695–706. [PubMed] [Google Scholar]
- (40).Branemark RP, Hagberg K, Kulbacka-Ortiz K, Berlin O, Rydevik B. Osseointegrated percutaneous prosthetic system for the treatment of patients with transfemoral amputation: a prospective five-year follow-up of patient-reported outcomes and complications. J Am Acad Orthop Surg. 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Birtwistle SJ, Wilson K, Porter ML. Long-term survival analysis of total hip replacement. Ann R Coll Surg Engl. 1996;78(3(Pt 1)):180–3. [PMC free article] [PubMed] [Google Scholar]
- (42).Sherk VD, Bemben MG, Bemben DA. BMD and bone geometry in transtibial and transfemoral amputees. J Bone Miner Res. 2008;23(9):1449–57. [DOI] [PubMed] [Google Scholar]
- (43).National Institute for Health and Care Excellence. Process and methods guides. Appendix I: Quality appraisal checklist–economic evaluations [Internet]. London: The Institute; 2012. [cited 2016 Jan]. Available from: https://www.nice.org.uk/process/pmg4/chapter/appendix-i-quality-appraisal-checklist-economic-evaluations [Google Scholar]
- (44).Frossard LA, Merlo G, Burkett B, Quincey T, Berg D. Cost-effectiveness of bone-anchored prostheses using osseointegrated fixation: myth or reality? Prosthet Orthot Int. 2018;42(3):318–27. [DOI] [PubMed] [Google Scholar]
- (45).Hansson E, Hagberg K, Cawson M, Brodtkorb TH. Patients with unilateral transfemoral amputation treated with a percutaneous osseointegrated prosthesis. Bone & Joint Journal. 2018;100-B(4):527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–50. [DOI] [PubMed] [Google Scholar]
- (47).Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada. 4th ed [Internet]. Ottawa (ON): The Agency; 2017. [cited 2018 Jan]. Available from: https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf [Google Scholar]
- (48).TreeAge Pro decision analysis software. TreeAge Software, Inc. Williamstown (MA) Available at: http://www.treeage.com. [Google Scholar]
- (49).Statistics Canada. Life tables, Canada, provinces and territories 1980/1982 to 2014/2016 [Internet]. Ottawa (ON): Statistics Canada; c2018. [updated 2018 June 28; cited 2018 October 17]. Available from: https://www.statcan.gc.ca/eng/reference/refcentre/index?MM=1 [Google Scholar]
- (50).Glanville J, Arber M, Veale T, Garcia S. Sensitivity of a search filter designed to identify studies reporting health state utility values. Paper presented at: Mosaic, 116th Annual Meeting of the Medical Library Association; 2016 May. 15–20; Toronto, ON.
- (51).Ara R, Brazier J. Predicting the short form-6D preference-based index using the eight mean short form-36 health dimension scores: estimating preference-based health-related utilities when patient level data are not available. Value Health. 2009;12(2):346–53. [DOI] [PubMed] [Google Scholar]
- (52).Haggstrom EE, Hansson E, Hagberg K. Comparison of prosthetic costs and service between osseointegrated and conventional suspended transfemoral prostheses. Prosthet Orthot Int. 2013;37(2):152–60. [DOI] [PubMed] [Google Scholar]
- (53).Ministry of Health and Long-Term Care Assistive Devices Program. Limb prostheses (conventional) product manual [Internet]. Toronto (ON): Queen's Printer for Ontario; 2012. [cited 2019 Jan 7]. Available from: http://www.health.gov.on.ca/en/pro/programs/adp/information_technology/docs/limb_prostheses_conventional_manual.pdf [Google Scholar]
- (54).Assistive Devices Program. Limb Prostheses (Conventional) Product Manual. Toronto (ON): Assistive Devices Program; Ministry of Health and Long-Term Care; 2012 Feb. [Google Scholar]
- (55).Frossard L, Berg D, Merlo G. Cost comparison of socket-suspended and bone-anchored trasnfemoral prostheses. J Prosthet Orthot. 2017;29(4):1. [Google Scholar]
- (56).Wolter JM, Cagney RA, McCormack JG. A randomized trial of home vs hospital intravenous antibiotic therapy in adults with infectious diseases. J Infect. 2004;48(3):263–8. [DOI] [PubMed] [Google Scholar]
- (57).Honkavaara N, Al-Ani AN, Campenfeldt P, Ekstrom W, Hedstrom M. Good responsiveness with EuroQol 5-Dimension questionnaire and Short Form (36) Health Survey in 20–69 years old patients with a femoral neck fracture: a 2-year prospective follow-up study in 182 patients. Injury. 2016;47(8):1692–7. [DOI] [PubMed] [Google Scholar]
- (58).Bourke HE, Yelden KC, Robinson KP, Sooriakumaran S, Ward DA. Is revision surgery following lower-limb amputation a worthwhile procedure? A retrospective review of 71 cases. Injury. 2011;42(7):660–6. [DOI] [PubMed] [Google Scholar]
- (59).Ontario Ministry of Finance. Ontario population projections update, 2017–2041 [Internet]. Toronto (ON): The Ministry; c2010. [updated 2018 June 25; cited 2018 July 26]. Available from: https://www.fin.gov.on.ca/en/economy/demographics/projections/table1.html [Google Scholar]
- (60).Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. Patient. 2011;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- (61).Messina J, Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. Patient. 2012;5(3):199–211. [DOI] [PubMed] [Google Scholar]
- (62).Ontario Health Technology Advisory Committee Public Engagement Subcommittee. Public engagement for health technology assessment at Health Quality Ontario—final report from the Ontario Health Technology Advisory Committee Public Engagement Subcommittee [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015. Apr [cited 2018 Apr 30]. Available from: http://www.hqontario.ca/Portals/0/documents/evidence/special-reports/report-subcommittee-20150407-en.pdf [Google Scholar]
- (63).Kvale S. Interviews: an introduction to qualitative research interviewing. Thousand Oaks (CA): Sage; 1996. [Google Scholar]
- (64).Kuzel AJ. Sampling in qualitative inquiry. In: Miller WL, Crabtree BF, editors. Doing qualitative research. Thousand Oaks (CA): Sage; 1999. p. 33–45. [Google Scholar]
- (65).Morse J. Emerging from the data: cognitive processes of analysis in qualitative research. In: Morse J, editor. Critical issues in qualitative research methods. Thousand Oaks (CA): Sage; 1994. p. 23–41. [Google Scholar]
- (66).Patton MQ. Qualitative research and evaluation methods. 3rd ed. Thousand Oaks (CA): Sage; 2002. [Google Scholar]
- (67).Strauss AL, Corbin JM. Basics of qualitative research: techniques and procedures of developing a grounded theory. 2nd ed. Thousand Oaks (CA): Sage; 1998. [Google Scholar]
- (68).Health Technology Assessment International. Introduction to health technology assessment [Internet]. Edmonton (AB): Health Technology Assessment International; 2015. [cited 2018 Apr 30]. Available from: http://www.htai.org/fileadmin/HTAi_Files/ISG/PatientInvolvement/v2_files/Resource/PCI-SG-Resource-Intro_to_HTA__KFacey__Jun13.pdf [Google Scholar]
- (69).Strauss AL, Corbin JM. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;13(1):3–21. [Google Scholar]
- (70).Strauss AL, Corbin JM. Grounded theory methodology: an overview. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. Thousand Oaks (CA): Sage; 1994. p. 273–85. [Google Scholar]
- (71).NVivo qualitative data analysis software. QSR International. Doncaster, Victoria (Australia) Available at: https://www.qsrinternational.com/nvivo/home. [Google Scholar]
- (72).Frossard LA, Merlo G, Burkett B, Quincey T, Berg D. Cost-effectiveness of bone-anchored prostheses using osseointegrated fixation: Myth or reality? Prosthet Orthot Int. 2018;42(3):318–27. [DOI] [PubMed] [Google Scholar]
- (73).Hansson E, Hagberg K, Cawson M, Brodtkorb TH. Patients with unilateral transfemoral amputation treated with a percutaneous osseointegrated prosthesis. Bone Joint J. 2018;100-B(4):527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Sullivan J, Uden M, Robinson KP, Sooriakumaran S. Rehabilitation of the trans-femoral amputee with an osseointegrated prosthesis: the United Kingdom experience. Prosthet Orthot Int. 2003;27(2):114–20. [DOI] [PubMed] [Google Scholar]
- (75).Khemka A, Frossard L, Lord SJ, Bosley B, Al Muderis M. Osseointegrated total knee replacement connected to a lower limb prosthesis: 4 cases. Acta Orthop. 2015;86(6):740–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Khemka A, FarajAllah CI, Lord SJ, Bosley B, Al Muderis M. Osseointegrated total hip replacement connected to a lower limb prosthesis: a proof-of-concept study with three cases. J Orthop Surg Res. 2016;11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Cahill JL, Shadbolt B, Scarvell JM, Smith PN. Quality of life after infection in total joint replacement. J Orthop Surg (Hong Kong). 2008;16(1):58–65. [DOI] [PubMed] [Google Scholar]