Abstract
Background:
Blunted facial affect is a common negative symptom of schizophrenia. Additionally, assessing the trustworthiness of faces is a social cognitive ability that is impaired in schizophrenia. Currently available pharmacologic agents are ineffective at improving either of these symptoms, despite their clinical significance. The hypothalamic neuropeptide oxytocin has multiple prosocial effects when administered intranasally to healthy individuals and shows promise in decreasing negative symptoms and enhancing social cognition in schizophrenia. Although two small studies have investigated oxytocin’s effects on ratings of facial trustworthiness in schizophrenia, its effects on facial expressivity have not been investigated in any population.
Methods:
We investigated the effects of oxytocin on facial emotional expressivity while participants performed a facial trustworthiness rating task in 33 individuals with schizophrenia and 35 age-matched healthy controls using a double-blind, placebo-controlled, cross-over design. Participants rated the trustworthiness of presented faces interspersed with emotionally evocative photographs while being video-recorded. Participants’ facial expressivity in these videos was quantified by blind raters using a well-validated manualized approach (i.e., the Facial Expression Coding System (FACES)).
Results:
While oxytocin administration did not affect ratings of facial trustworthiness, it significantly increased facial expressivity in individuals with schizophrenia (Z=−2.33, p=0.02) and at trend level in healthy controls (Z=−1.87, p=0.06).
Conclusions:
These results demonstrate that oxytocin administration can increase facial expressivity in response to emotional stimuli and suggest that oxytocin may have the potential to serve as a treatment for blunted facial affect in schizophrenia.
Keywords: Oxytocin, schizophrenia, facial expressivity, trustworthiness, social cognition, blunted affect
INTRODUCTION:
The negative symptoms of schizophrenia include reduced emotional expression in the face, voice, and body, poverty of speech and thought, anhedonia, and reduced motivation and social interest. The most prominent form of blunted emotional expressivity is blunted facial affect (Blanchard and Cohen, 2006, Tremeau et al., 2005). It is commonly present early in the course of the disorder, even before the development of frank psychotic symptoms, and typically continues into the chronic phase of the illness after psychotic symptoms are successfully treated with antipsychotic medications (Gur et al., 2006). Indeed, analysis of childhood home movies of people who later developed schizophrenia and their unaffected siblings reveal subtle abnormalities in facial expressivity many years before the onset of overt illness (Walker et al., 1993). People with schizophrenia have reduced facial expressivity during social interactions and when viewing emotional films (Berenbaum and Oltmanns, 1992), and have difficulty consciously amplifying their facial expressions to emotional stimuli (Kring and Moran, 2008). Furthermore, blunted facial affect has been linked with worse quality of life and functional outcomes in individuals with schizophrenia (Gur et al., 2006). Despite, its clinical relevance, current pharmacological treatments are ineffective at remediating blunted affect in schizophrenia (Green, 2016).
Deficits in social cognition are also prominent in schizophrenia (Foussias et al., 2015) and are strong predictors of functional outcomes (Green, 2016). One example of a disrupted social cognitive function that is important for proper social functioning in schizophrenia is the assessment of trustworthiness in faces (Winston et al., 2002). Studies investigating trustworthiness ratings in schizophrenia have produced mixed results with patients rating faces as more (Baas et al., 2008b), less (Pinkham et al., 2008), or identically trustworthy to control subjects (Couture et al., 2008). These conflicting findings may be partially explained by the influence that affective information has on trustworthiness judgments. A recent study showed that individuals with schizophrenia rate faces as less trustworthy than healthy controls only after negative affective primes (i.e., negatively valenced evocative photos shown immediately before the face), but not after neutral or positive primes, and that lower trust ratings were associated with greater severity of feelings of suspiciousness and persecution (Hooker et al., 2011). Despite the clinical relevance of disruption of social cognition in general, and ratings of facial trustworthiness in particular, there are no currently available pharmacotherapies for these deficits in schizophrenia or in any disorder.
There is growing interest in using the neuropeptide oxytocin to treat both the negative symptoms and social cognitive deficits of schizophrenia. Interestingly, blunted affect has rarely been studied as the focus of treatment in schizophrenia--perhaps due to an underlying assumption that it may not be malleable (Pinkham et al., 2008). This is in contrast to negative symptoms and various social cognitive deficits, which have been the focus of a number of studies in schizophrenia, particularly those using oxytocin. For example, several longitudinal clinical trials have found that oxytocin administration can decrease negative symptoms measured by a semi-structured interview like the Positive and Negative Syndrome Scale (PANSS, for review see (Feifel et al., 2016), but see (Horta de Macedo et al., 2014, Dagani et al., 2016, Weiser et al., 2015)). However, due to the limitations of these measures (e.g., only one of seven questions in the PANSS negative subscale concerns blunted affect and is not limited to facial affect) and the small sample sizes involved, no study has reported on effects of oxytocin specifically on blunted affect. On the other hand, intranasal oxytocin administration has been shown to improve multiple aspects of social cognition, such as theory of mind, in schizophrenia (Woolley et al., 2014, Pedersen et al., 2011) (however, see (Cacciotti-Saija et al., 2015)) and one small study found a non-significant trend for a single dose of oxytocin to decrease trustworthiness ratings of untrustworthy faces in individuals with schizophrenia (Pedersen et al., 2011) (though a second small study found no effect (Gibson et al., 2014)). In sum, despite the interest in oxytocin as a treatment for schizophrenia, significant uncertainty remains regarding which symptoms and deficits, if any, are affected by oxytocin administration.
Blunted facial affect and social cognitive deficits such as disrupted assessment of facial trustworthiness are thought to be related to different underlying neural circuit dysfunction. In particular, blunted affect has been linked to amygdala hyperactivity and weaker prefrontal cortex (PFC)-amygdala coupling, while deficits in ratings of facial trustworthiness have been linked to hypo-activity of multiple regions including the amygdala, ventrolateral PFC, superior temporal sulcus and fusiform face area (Pinkham et al., 2008, Baas et al., 2008a). Despite the lack of studies focusing on oxytocin effects on blunted affect, emerging evidence suggests that oxytocin may specifically normalize the neural circuitry dysfunction that is believed to underlie blunted facial affect. For example, oxytocin administration decreases amygdala activity during presentation of fearful faces and strengthens amygdala-PFC coupling at rest in healthy individuals and those with schizophrenia (Kirsch et al., 2005, Sripada et al., 2013, Shin et al., 2015). Thus, in the current study, we simultaneously investigated the effects of a single-dose of oxytocin on blunted affect and ratings of facial trustworthiness, two functionally distinct impaired domains in schizophrenia that are due to dysfunction in distinct neural circuits.
We used a well-validated paradigm of facial trustworthiness and objectively quantified ratings of facial expressivity in response to the standardized emotionally evocative stimuli presented in the task. We included matched healthy individuals in our study to determine the specificity of any oxytocin effects to individuals with schizophrenia. Using a double-blind, placebo-controlled, cross-over study design, we video-recorded participants while they rated the trustworthiness of faces that were immediately preceded by emotionally evocative primes. We then quantified the frequency of positive and negative valence facial expressions displayed in these videos in response to the affective primes using an objective manualized coding system validated for use in individuals with schizophrenia (Kring and Sloan, 2007). By having participants rate facial trustworthiness while being video-recorded, we could simultaneously examine the effects of schizophrenia and oxytocin on facial trustworthiness ratings and facial expressivity while also maximizing consistency between subjects and study sessions. Indeed, the use of standardized stimuli and objective behavioral outcome measures may overcome many of the substantial limitations of more classic semi-structured interview-based symptom scales (Cohen et al., 2008). We hypothesized that oxytocin administration would increase facial expressivity in response to emotionally evocative photographs irrespective of valence in individuals with schizophrenia and in healthy controls. Additionally, we hypothesized that oxytocin would normalize the accuracy of ratings of facial trustworthiness in individuals with schizophrenia, particularly improving trust ratings after negative affective primes, but not after positive or neutral primes.
METHODS:
Participants
Thirty-three individuals with a schizophrenia spectrum disorder (SZ): (26 schizophrenia; 6 schizoaffective; 1 schizophreniform) were recruited from across the San Francisco Bay Area and thirty-five age-matched healthy controls (HC) were recruited through online advertisements. Healthy participants had no Axis I DSM-IV disorder within the last year or a lifetime history of a psychotic disorder. All participants had no neurological disorders or substance dependence within the last six months and had a negative urine toxicology test at each visit. All diagnoses were verified by the Structured Clinical Interview for DSM-IV (M.B. First, 2002). Patients were on a stable dose of psychiatric medications for at least one month and throughout the study. Written informed consent was obtained from each participant in accordance with the University of California, San Francisco Committee on Human Research.
Procedures
Testing was performed in a randomized, double-blind, cross-over design, with the two testing days separated by at least one week. On each test day, 40 IU of oxytocin (Novartis, Switzerland) or matched placebo was self-administered via nasal spray by alternating insufflations every 15 seconds between each nostril over a five-minute timeframe (Feifel et al., 2010). On each testing day, participants completed behavioral testing and were simultaneously video-recorded. Previous work has shown that intranasal oxytocin administration begins to have physiological effects within 30 minutes and lasts for at least 90 minutes (Norman et al., 2011). Therefore, in the current study, behavioral testing began 60 minutes post-administration and continued for no longer than 120 minutes. For further justification of dosage and timing, see Supplementary Materials.
Social Judgment Task (SJT)
In the SJT, participants rate the trustworthiness of faces after the presentation of an affective ‘prime’ photograph just prior to presentation of the face photograph (for detailed methods see Figure 1, Supplementary Materials and (Adolphs et al., 1998, Hooker et al., 2011)). Participants viewed black and white photographs of 49 unfamiliar male and female faces in natural poses, each presented three times paired with three different priming images (negative, neutral, and positive), taken from the International Affective Picture System (IAPS), for a total of 147 trials. Primes were randomly assigned to faces and the face-prime pairs were presented in a fixed, pseudo-random order, such that none of the faces appeared twice in a row. Primes were presented for 1 second, followed by a face for 7 seconds (or until the subject responded), followed by an inter-trial interval of 1.5 seconds. Participants rated each face on a 7-point scale according to how trustworthy it appeared: −3 (very untrustworthy) to +3 (very trustworthy). Task instructions emphasized that the prime photographs and faces were not related, and that the participant was to rate only the trustworthiness of the faces. Participants were asked to imagine trusting the person in a very serious situation (e.g., with all their money or with their life). Two versions of the SJT with different faces and priming images were used for each testing day and order was randomized between subjects. Previous work demonstrated that the faces used on each test day did not differ in ratings of trustworthiness (Hooker et al., 2011). Prior to performing the task, participants were given a set of seven practice trials providing an opportunity to familiarize themselves with the task and raise any questions they might have.
Figure 1. Experimental Setup of Social Judgment Task.
Oxytocin was delivered intranasally 60 minutes before testing. In a randomized order, either a positive, neutral, or negative affective prime image was presented for 1 second before a photo of a face was displayed for 7 seconds or until the participant made a rating of the trustworthiness of the face. After the rating, a “+” screen was shown for 1.5 seconds before the next set of photos was presented for a total of 147 trials. The task was presented on a laptop computer using E-prime professional version 2.0. Photos of the actual task were in color.
Facial Expressivity Coding
Facial expressivity was quantified using videos of participants while they completed the SJT (video length depended on how fast each participant completed the task (Range: 8–24 minutes, M=13.11, SD=3.13)). Video-recording during this paradigm minimized experimental heterogeneity as all participants had similar experiences (e.g., seeing the same photos) without any variability due to an interviewer being present during the task. Furthermore, having a behavioral task that required active input from participants encouraged participants to maintain focus on the computer screen throughout the task and minimized any self-consciousness about being video recorded. While the SJT has never been used to study facial expressivity before, the IAPS pictures that are part of the SJT have frequently been used to elicit facial expressivity in multiple populations including schizophrenia (e.g., (Wolf et al., 2005, Peterman et al., 2015)). Thus, the SJT is an efficient method to simultaneously study facial expressivity in response to emotionally evocative stimuli and judgments about facial trustworthiness.
Videos were coded independently by four raters using the Facial Expression Coding System (FACES) (Kring and Sloan, 2007), a behavioral coding system validated for use in schizophrenia that is based on a two-dimensional model of emotion, where each emotion varies on both valence (positive/negative) and intensity (weak/intense). FACES ratings have been correlated with facial muscle activity, individual-difference measures of expressiveness and personality, skin conductance, heart rate, and reports of experienced emotion (Kring and Sloan, 2007). FACES has also been used with and validated on participants with schizophrenia (Kring and Sloan, 2007, Aghevli et al., 2003). Coders were blind to diagnostic status, drug condition, and to results of the behavioral task. Inter-rater reliability for FACES is very high (r = 0.70–0.99) when coded by trained experimenters, and FACES ratings converge with ratings using Ekman’s rating system for facial expressions (i.e., EMFACS) (Ekman and Friesen, 1976). The intra-class correlation between our two sets of two raters was calculated across participants. There were no significant differences between the ratings between raters, and therefore composite scores are taken from total frequency of expressions (Kring et al., 1994). Because the variance due to coders is not ignored, the coefficient can be interpreted as an index of agreement rather than consistency (Shrout and Fleiss, 1979). Our inter-rater reliability for FACES was excellent, with an average intraclass correlation coefficient of 0.92 for the schizophrenia group and 0.90 for the controls.
Symptom Severity
To assess the positive and negative symptoms of schizophrenia, a subset (n=23) of patients were rated by trained raters using the PANSS (Kay et al., 1987). A limited number of patients were administered the PANSS because this measure was not implemented until later in the study.
Medications
In order to better describe our patient population, we calculated benztropine and chlorpromazine (CPZ) equivalents for patients using a standardized conversion table(Andreasen et al., 2010).
Statistical Analyses
We plotted variable distributions to examine skewness and kurtosis and tested normality using the Shapiro-Wilk test, and identified potential outliers using stem-and-leaf and box plots. We found no outliers for the normally distributed data. We then examined group differences in demographic variables using independent samples t tests and chi-square tests.
Our primary outcome of interest was frequency of participants’ facial expressions (total, negative, and positive) during the SJT. Because video lengths varied with speed of completing the task, we calculated the number of expressions (defined by the face changing from neutral to non-neutral expression and then back to neutral) per five-minute interval (i.e., number of expressions divided by number of five-minute intervals, e.g., 6 expressions over 15 minutes would yield 2 expressions per five-minute interval). Our inter-rater reliability for our primary outcome of interest (frequency of facial expressions) was excellent, with an average intra-class correlation coefficient ≥0.90 (see Supplementary Materials for details). We conducted non-parametric tests to model non-normally distributed data (i.e., lack of any facial expression, particularly for the SZ group, leading to a preponderance of zeroes). Differences in frequency of facial expressions were examined using the Wilcoxon Signed-Rank Test and the Mann-Whitney U Test. We calculated oxytocin-induced changes in total facial expressivity (i.e., frequency of facial expressions on the oxytocin day – placebo day), then examined differential effects of oxytocin on facial expressivity by group. We also examined within-subject changes in facial expressivity by drug and emotional valence (positive and negative), within and between groups. We adjusted for multiple comparisons in our analyses using Benjamini and Yekutieli (B-Y) corrections for family-wise error (Narum, 2006) (see Supplementary Materials).
In addition to our primary outcome, we also examined average duration per expression and average intensity per expression. For these analyses, we excluded participants who had zero expressions on at least one of the testing days. We used non-parametric Wilcoxon Signed-Rank Test to examine within-subject differences and Mann-Whitney U Test for between-subject differences.
We also examined effects of oxytocin and affect priming on ratings of trustworthiness during the SJT. We performed a 2x3x2 mixed factorial ANOVA with Drug (oxytocin, placebo) and Priming (negative, neutral, positive) as within-subjects factors and Group (SZ, HC) as the between-subjects factor. We followed up significant interactions with simple effects ANOVAs to examine the Group effect and tests of within-subject factors to examine the Priming effect. Because of previous findings that oxytocin had a specific effect on ratings of untrustworthy faces (Pedersen et al., 2011), we also examined the effect of oxytocin on ratings of trustworthiness specifically for facial stimuli, irrespective of the affect priming photographs, that were rated as low, average, or high trustworthiness by a separate healthy sample (Adolphs et al., 1998). We performed a 2x3x2 mixed factorial ANOVA with Drug (oxytocin, placebo) and normative Trustworthiness ratings (low, average, high) as within-subjects factors and Group (SZ, HC) as the between-subjects factor.
RESULTS:
Demographic Data
Demographic and descriptive variables are presented in Table 1. Groups were well matched on age. HCs had significantly more years of education than individuals with SZ. There was a higher proportion of non-Hispanic Caucasians in the HC group.
Table 1.
Demographic and clinical information
| Schizophrenia Patients (N = 33) | Healthy Controls (N = 35) | ||||
|---|---|---|---|---|---|
| Mean/N | SD/% | Mean/N | SD/% | p-value | |
| Demographics | |||||
| Age (years) | 42.9 | 10.1 | 42.0 | 13.2 | 0.75 |
| Range | 23–61 | - | 20–64 | - | |
| Education Level | 13.3 | 2.1 | 15.4 | 1.9 | 0.001** |
| Gender | |||||
| Male | 30 | 90.9% | 33 | 94.3% | |
| Female | 3 | 9.1% | 2 | 5.7% | |
| Diagnosisa | |||||
| Schizophrenia | 26 | 78.8% | - | - | |
| Schizoaffective | 6 | 18.2% | - | - | |
| Schizophreniform | 1 | 3.0% | - | - | |
| On a Mood Stabilizerb | 8 | 24.2% | - | - | |
| Race | 0.13 | ||||
| Caucasian | 9 | 27.3% | 19 | 54.3% | |
| African American | 8 | 24.2% | 6 | 17.1% | |
| Latino/Hispanic | 3 | 9.1% | 5 | 14.3% | |
| Asian American | 11 | 33.3% | 5 | 14.3% | |
| Native American | 1 | 3.0% | 0 | 0.0% | |
| Other | 1 | 3.0% | 0 | 0.0% | |
| Clinical Symptoms (N = 23)a | |||||
| Positive | 15.4 | 3.6 | - | - | |
| Negative | 14.7 | 5 | - | - | |
| General | 30 | 7.2 | - | - | |
| Quality of Life | 24.4 | 7.2 | - | - | |
| Medications Equivalents | |||||
| Cogentin | 0.8 | 1.6 | - | - | |
| Range | 0–7.5 | - | |||
| Chlorpromazine | 389 | 345 | - | - | |
| Range | 0–1655 | - | |||
p≤0.01
p≤0.05
Based on the Positive and Negative Symptom Scale for DSM-IV (PANSS) administered by trained clinical interviewers.
Mood stabilizers include Depakote, Lithium, and Lamictal.
Group Differences in Facial Expressivity During Placebo
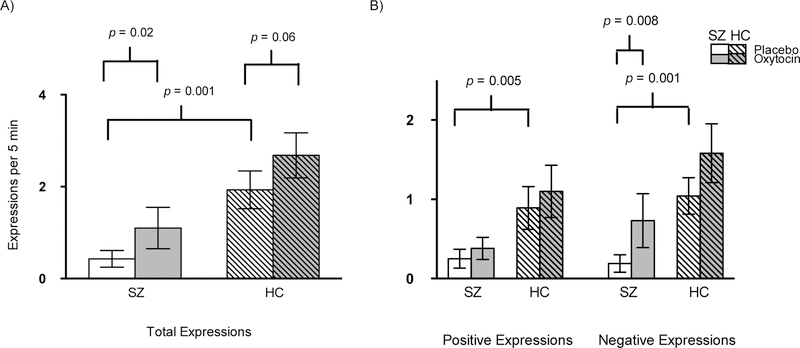
There was no difference in placebo-day expressivity between non-Hispanic Caucasians and the other ethnic groups (p=0.27) in the HC group, therefore we did not include ethnicity in any subsequent analyses. Consistent with extensive literature on blunted affect in SZ (Kring and Elis, 2013), SZ participants displayed fewer total (both negative and positive valence) facial expressions while viewing emotionally evocative pictures compared to HCs on the placebo day (Figure 2; p’s<0.01).
Figure 2. Facial expressivity by group and drug condition.
For presentation, means and standard errors are presented. A) Oxytocin effects on frequency of total expressions by group. Means(SD) for oxytocin-induced change for SZ (0.67(2.04)) and HC (0.75(2.20)). B) Oxytocin effects on positive and negative valence expressions by group. Means(SD) for oxytocin-induced change for positive expressions (SZ (0.13(0.79)); HC (0.21(1.68))) and negative expressions (SZ (0.54(1.42)); HC (0.54(1.57))).
Effects of Oxytocin on Facial Expressivity
The distributions were non-normal (see Table 2 for descriptions of the means and standard deviations). Based on the B-Y method, for our primary analysis, we had three comparisons and set the new α to 0.027. Compared to placebo, oxytocin administration significantly increased the frequency of total facial expressions in SZ (Z=−2.33, p=0.02) and non-significantly in HC (Z=−1.87, p=0.06) participants (Figure 2A). There were no differences between groups for oxytocin-induced increases in total facial expressivity (U=568, Z=−0.11, p=0.91). Given that video-length depended on how fast participants completed the task, we examined relationships between video length and our outcome measures. We found that video length did not have an effect on our outcome measure nor did oxytocin have an effect on video length (p’s>0.05).
Table 2.
Distribution of facial expressivity and trustworthiness measures
| Schizophrenia Patients | Healthy Controls | |||
|---|---|---|---|---|
| Tasks | Placebo | Oxytocin | Placebo | Oxytocin |
| Mean(SD) | Mean(SD) | Mean(SD) | Mean(SD) | |
| Facial Expressivity | ||||
| Total | 0.43(1.02) | 1.10(2.56) | 1.93(2.41) | 2.68(2.91) |
| Positive | 0.25(0.70) | 0.38(0.79) | 0.89(1.62) | 1.10(1.96) |
| Negative | 0.19(0.61) | 0.73(1.96) | 1.04(1.35) | 1.58(2.16) |
| Social Judgment Task | ||||
| Negative | 3.72(0.95) | 3.55(0.96) | 3.81(0.93) | 3.77(0.83) |
| Neutral | 4.36(0.61) | 4.37(0.90) | 4.03(0.90) | 4.05(0.81) |
| Positive | 4.45(0.69) | 4.36(0.82) | 4.05(0.98) | 4.01(0.77) |
| Low | 3.61(0.61) | 3.51(0.81) | 3.36(0.89) | 3.25(0.71) |
| Average | 4.36(0.70) | 4.27(0.81) | 4.15(0.98) | 4.22(0.93) |
| High | 4.83(0.88) | 4.77(0.98) | 4.64(1.03) | 4.77(1.03) |
Means and standard deviation are presented for each outcome.
In our secondary analyses, we had seven comparisons and based on the B-Y method set the new α to 0.019. We found that oxytocin significantly increased the frequency of negative valence facial expressions in SZ (Z=−2.67, p<0.008), but not in HC (Z=−1.18, p=0.24), and had no significant effect on the frequency of positive expressions in either group (Figure 2B). Similarly, we found that oxytocin-induced increases in facial expressivity for negative valence expressions were non-significantly greater than for positive expressions in patients with SZ (Z=−2.05, p=0.04). The SZ and HC groups did not differ in oxytocin-induced changes in negative (U=512, Z=−0.83, p=0.41) or positive (U=548, Z=−0.37, p=0.71) valence expressions.
Effects of Oxytocin on Duration and Intensity
Twenty-three of the 33 individuals with SZ (70%) and 12 of the 35 HC (34.3%) had zero expressions on at least one testing day. Only examining the sub-sample of participants that had at least one expression on both testing days, we found that oxytocin did not have an effect on average duration per expression in SZ (mean(SE): oxytocin: 2.04(0.51) vs. placebo: 2.38(0.58); p=0.95) or HC (oxytocin: 1.96(0.18) vs. placebo: 1.95(0.19); p=0.73). Additionally, oxytocin did not have an effect on intensity per expression in SZ (oxytocin: 1.27(0.13) vs. placebo 1.50(0.22); p=0.15) or HC (oxytocin: 1.31(0.05) vs. placebo: 1.35(0.06); p=0.56). SZ did not differ from HC on these measures on the placebo day (p’s>0.4). For this subset of subjects with intensity and duration data for both days, the frequency of negative expressions was still significantly greater while on oxytocin than placebo for SZ (Z=−2.24; p=0.03), but the total and positive expressions were not (p’s>0.07).
Trustworthiness Ratings During SJT
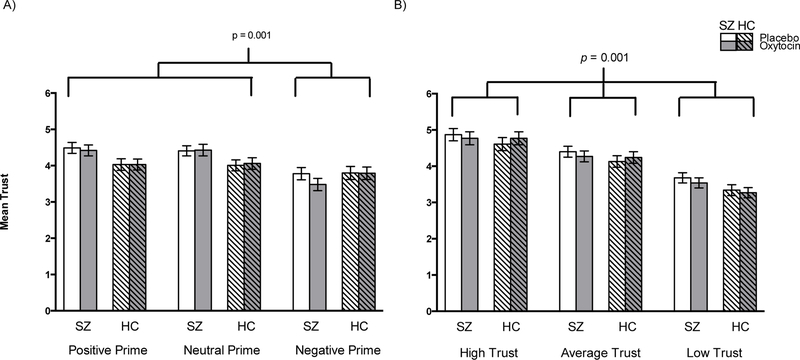
The 2x3x2 ANOVA revealed a main effect of Prime (F(2, 57)=26.49, p=0.001, η2=0.31) and a Prime x Group interaction (F(2, 55)=7.97, p=0.001, η2=0.12, Figure 3). Pairwise comparisons revealed that in both groups, and across Drug conditions, negative primes were associated with significantly lower trust ratings than were neutral or positive primes. Trust ratings between positive and neutral primes, however, did not differ. We then examined the Prime x Group interaction using simple effects ANOVAs, comparing trust ratings between the SZ and HC groups averaged across Drug administration day, followed up by within-subjects contrasts to examine the Prime effect within each group. Looking separately by Group, the main effect of Prime was significant in both SZ and HC (p’s<0.001). There were no significant group differences, however, in any of the Prime conditions (p’s>0.38; Figure 3). Within-subject contrasts revealed that in both groups, negative primes were associated with significantly lower trust ratings than in both the positive and neutral primes (p’s<0.001), while trust ratings during the positive and neutral primes did not differ (p=0.67). There was no significant main effect for Drug (F(2, 58)=0.40, p=0.53), nor were there any significant Drug x Prime (F(2, 57)=1.89, p=0.16), Drug x Group (F(2, 58)=0.68, p=0.42), or Drug x Prime x Group (F(2, 57)=1.04, p=0.36) interactions. Finally, the 2x3x2 ANOVA in which faces were separated by their normative ratings revealed no significant effect of Drug (F(2, 58)=0.13, p=0.72) or Group (F(2, 58)=1.16, p=0.29) nor any significant Drug x Trustworthiness (F(2, 57)=1.72, p=0.18) or Drug x Trustworthiness x Group (F(2, 57)=0.89, p=0.42) interactions. Although we used two different forms, there were no version effects between them for either the Prime or Trustworthiness conditions (p’s>0.3).
Figure 3. Mean trustworthiness ratings, placebo vs. oxytocin.
Means and standard errors are presented. A) Oxytocin effects on affective prime trustworthiness by group. Negative prime was associated with significantly lower trustworthiness ratings than positive and neutral primes across drug and group. B) Oxytocin effects on normative trustworthiness of faces by group. Each trust level had significantly different trustworthiness ratings from each other.
Manipulation Checks and Exploratory Analyses
While we found some small significant effects of the order in which oxytocin was administered, these effects did not influence our results for facial expressivity or facial trustworthiness when order was included as a covariate in the model. SZ had significantly greater frequency of positive expressions on the first day (p=0.04) and HC had significantly higher positive, negative and total expressions on the first day (p’s<0.02). For facial trustworthiness, there were no significant order effects. Given the small number of females in our sample and the possibility of sex-specific effects of oxytocin, we re-ran all analyses excluding women. Excluding women did not change any of the results. Our blinding procedure was adequate for SZ such that their guesses when they received oxytocin did not differ from chance (SZ: 60.7%, p=0.26, χ=1.29), but for HC their guesses were significantly worse than chance (HC: 32.3%, p=0.05, χ=3.90). SZ and HC significantly differed in their percentage of accurate guesses (p=0.03, χ=4.80). There were no reported side effects from oxytocin for either SZ or HC.
In exploratory analyses, we conducted spearman correlations between facial expressivity and clinical symptoms and anticholinergic and antipsychotic dosages in the schizophrenia group. We found that oxytocin-induced increases in negative and total expressivity were negatively correlated with positive and general symptoms as well as antipsychotic dosage (for details see Supplementary Materials).
DISCUSSION:
We found that a single intranasal dose of oxytocin significantly increased facial expressivity in response to emotionally evocative photographs in individuals with schizophrenia and non-significantly in healthy controls. Although the number of expressions was relatively small, this is consistent with other studies using FACES to code expressions in schizophrenia and healthy controls (Kring and Neale, 1996). This increase in frequency of facial expression was not associated with a change in the duration or intensity of each expression, although this needs to be interpreted with caution given the small number of participants in this analysis. Similarly, we found a non-significant trend for oxytocin to increase negative valence expressions more than positive valence expressions in the schizophrenia group. This is an intriguing and surprising finding that needs to be interpreted with caution given the trend-level significance. Our results suggest that oxytocin may be an effective pharmacological agent to improve blunted facial affect, a core symptom of schizophrenia, that impairs social interactions (Kring and Moran, 2008) and quality of life (Gur et al., 2006). Given that currently available pharmacotherapies for schizophrenia are generally ineffective for blunted affect and have significant negative side effects (see (Tsapakis et al., 2015) for review), this is an exciting and important discovery.
In the current study, showing emotionally evocative photos immediately before presenting a photo of a face altered ratings of facial trustworthiness, consistent with previous studies, although we did not replicate any group differences in this effect (Hooker et al., 2011). Oxytocin administration did not significantly alter ratings of facial trustworthiness in individuals with schizophrenia or in healthy controls. Previous studies investigating the effects of oxytocin on facial trustworthiness ratings in healthy individuals have been inconsistent, with some reporting oxytocin enhances facial trustworthiness (Kosfeld et al., 2005), but others finding no effect (Lambert et al., 2014). We also failed to replicate the one study that found an effect of oxytocin on facial trustworthiness in schizophrenia (Pedersen et al., 2011) but did replicate the lack of effect found in (Gibson et al., 2014). Given the lack of group differences on trustworthiness ratings in the current study, we could not determine whether oxytocin normalized trustworthiness ratings in schizophrenia. In our study, we used a higher dosage of oxytocin, presented different stimuli, interspersed faces with affective primes, and had an older subject sample than previous studies, all of which may have contributed to any discrepant results.
The mechanisms by which oxytocin increases facial expressivity are unknown. Oxytocin may increase facial expressivity by increasing PFC-amygdala coupling. In schizophrenia, oxytocin may modulate the aberrant overstimulation of the limbic network that is thought to underlie blunted facial affect. For example, severity of blunted facial affect correlates with amygdala hyperactivity during identification of fearful faces (Gur et al., 2007) and during rating of the emotional valence of facial expressions (Lepage et al., 2011). Furthermore, resting-state fMRI reveals that weaker amygdala-pre-frontal cortex (PFC) coupling correlates with higher ratings of flat affect (Anticevic et al., 2012). Evidence from neuroimaging studies on healthy subjects supports this hypothesis: a single administration of oxytocin decreases amygdala activity and strengthens PFC-amygdala coupling at rest and during the presentation of fearful faces (Sripada et al., 2013, Kirsch et al., 2005). Similarly, a single dose of oxytocin has been found to decrease amygdala hyperactivity in individuals with schizophrenia during viewing of emotional faces (Shin et al., 2015). Thus, this potential mechanism could explain how oxytocin can increase expressivity in both individuals with schizophrenia and healthy controls.
Another possibility is that oxytocin may amplify individuals’ subjective experience in response to emotional cues, possibly through increasing empathy (Cohen and Minor, 2010, Barraza and Zak, 2009), thereby modulating facial expressivity. Because individuals with schizophrenia with and without blunted affect typically have normal or even heightened subjective reports of emotional experience (Kring and Elis, 2013, Sweet et al., 1998, Berenbaum and Oltmanns, 1992), and individuals with schizophrenia have been found to have dissociations between emotional expression and emotional experience (Sweet et al., 1998), blunted affect is not likely due to abnormalities in subjective emotional experience. Additionally, few studies have found clear effects of oxytocin on subjective experiences of emotion (Gibson et al., 2014, Abu-Akel et al., 2014) making this hypothesis less likely.
Finally, oxytocin may increase facial expressivity by directly altering parasympathetic regulation of facial musculature, without affecting emotion processing or subjective experience in response to emotional cues. This hypothesis is supported by evidence that oxytocin can affect parasympathetic tone in humans (Gamer and Büchel, 2012) and rodents (Michelini et al., 2003). In order to disambiguate these hypotheses, future studies should investigate the effects of oxytocin administration on simultaneously measured behavioral, subjective, autonomic, and neural responses to emotional stimuli.
Our study has several limitations. First, our study had a relatively small sample size and used a single-dose of oxytocin. We also had a very small number of female participants, which prevented us from determining if there were any sex-specific effects of oxytocin in these paradigms. Second, we did not measure subjective emotional responses in the current study, because such subjective reporting can decrease the intensity of induced emotions (Taylor et al., 2003). Thus, we do not know if oxytocin only affects facial expressivity or if it also has effects on subjective emotional experience. Third, our study design prevented us from addressing whether individuals with schizophrenia are less accurate in their trustworthiness ratings or whether oxytocin can improve the accuracy of their trustworthiness ratings. Fourth, raters may have been able to identify who were the individuals with schizophrenia in the videos, thereby breaking the blind. However, facial expressivity differences between individuals with schizophrenia and controls were not the focus of the current study, and even if raters could tell the patients from the controls, this would not affect our findings of oxytocin effects on facial expressivity. Fifth, despite being worse than chance, healthy controls may have been able to tell which drug they were on, which could have altered the findings. However, previous studies have found that subjects usually cannot tell which drug they are on (Woolley et al., 2014), which suggests that our blinding was adequate and the guessing patterns were spurious. Sixth, the duration of oxytocin’s effect after intranasal administration is unknown and was not addressed in the current study. However, this and other studies suggest that oxytocin has effects for at least a couple of hours. Finally, we did not synchronize the measurement of facial expressivity to the emotional valence of the photographs and there were low frequencies of expression in some individuals. Thus we cannot determine whether any increase in facial expressivity was congruent or incongruent with the valence of the evocative images. Similarly, expressions were scored in terms of frequency and valence, but not with regard to specific emotions. Thus, it remains unclear whether oxytocin increased the ability to facially express specific emotions, or simply increased general expressivity. This distinction is critical because increases in expressivity, in the absence of control or regulation, may not necessarily be adaptive, particularly among individuals that are vulnerable to dysregulated emotion such as those with schizophrenia. Longer treatment trials in larger samples, and with additional autonomic and neural functioning measures, will be necessary to fully elucidate the effects of intranasal oxytocin on emotional expressivity and emotional functioning in people with schizophrenia.
Our approach for eliciting and measuring facial expressivity has several advantages over traditional semi-structured Likert-type symptom rating scales. First, our approach does not require trained clinical interviewers, although it does require trained FACES coders (and coding can be performed at any time after the assessment). Furthermore, with improvements in technology, automated coding of facial expressivity will soon be available (Scherer et al., 2014). Second, our approach minimizes variability that could be introduced during even a structured interview (e.g., differences between assessors in style, appearance, personality etc). Indeed, in our study, all participants saw the same standardized stimuli under the same conditions. Third, from a psychometric perspective, symptom-rating scales are not ideal because they often employ vague rating systems [e.g., “mild”, “moderate” and “severe” categories] (Lukoff et al., 1986) that may be insensitive to subtle changes in the frequency of facial expressivity especially in response to emotional stimuli (Eckert et al., 1996). On the other hand, FACES is an objective rating system that is closely tied to individual behaviors, i.e., coding the presence or absence of each facial expression, as opposed to more gestalt ratings in typical symptom-rating scales. Finally, having participants completing an active behavioral task during presentation of evocative stimuli allows for participant engagement in the task to be verified and measured.
In the current study, we found that oxytocin increases facial expressivity both in individuals with schizophrenia and in healthy controls. This is similar to many commonly used treatments for psychiatric disorders that have similar positive effects when tested in “healthy” individuals. For example, sub-chronic administration of selective serotonin reuptake inhibitor (SSRI) antidepressants to healthy individuals has been found to increase cooperative tendencies, increase confident behaviors, induce a positivity bias in emotion processing, and decrease negative affect related to negative events (Serretti et al., 2010). Similarly, the acetylcholinesterase inhibitor donepezil commonly used in Alzheimer’s Disease, can improve aspects of memory in healthy individuals (Repantis et al., 2010). The lack of specificity of oxytocin’s effect on facial expressivity may suggest that oxytocin is not remediating a specific deficit in schizophrenia. On the other hand, it is also possible that blunted affect exists on a continuum with healthy facial expressivity and that there is not a distinct neural mechanism underlying blunted affect beyond what would be expected from extreme lack of expressivity. Combined with how well-tolerated oxytocin has been in individuals with schizophrenia, even when administered over four months (Busnelli et al., 2016), our results suggest that oxytocin administration may be a way to safely enhance emotional expressivity in anyone, including individuals with blunted affect.
Supplementary Material
Acknowledgements:
The authors thank the current and former members of the Bonding and Attunement in Neuropsychiatric Disorders Lab at the SFVA for their dedicated efforts and contributions to achieving the reported results. The authors also thank the patients for participating in the study.
Funding
Grant support was provided by the Veterans Health Administration Office of Research and Development Career Development Award (CDA) 1IK2CX000758–01A1 and National Institute of Mental Health Diversity Supplement 3R01MH068725–09S1. Dan Mathalon is a consultant to BristolMyersSquibb Inc. Sophia Vinogradov is a consultant to BrainPlasticity Institute.
Footnotes
Disclosures:
The remaining authors (Josh D. Woolley, Brandon Chuang, Chris Fussell, Stefan Scherer, Bruno Biagianti, and Daniel Fulford) declare that they have no financial relationships with competing interests.
Supplementary information is available at the Psychological Medicine website.
References:
- ABU-AKEL A, FISCHER-SHOFTY M, LEVKOVITZ Y, DECETY J & SHAMAY-TSOORY S 2014. The role of oxytocin in empathy to the pain of conflictual out-group members among patients with schizophrenia. Psychological Medicine, 44, 3523–32. [DOI] [PubMed] [Google Scholar]
- ADOLPHS R, TRANEL D & DAMASIO AR 1998. The human amygdala in social judgment. Nature, 393, 470–4. [DOI] [PubMed] [Google Scholar]
- AGHEVLI MA, BLANCHARD JJ & HORAN WP 2003. The expression and experience of emotion in schizophrenia: a study of social interactions. Psychiatry Research, 119, 261–70. [DOI] [PubMed] [Google Scholar]
- ANDREASEN NC, PRESSLER M, NOPOULOS P, MILLER D & HO BC 2010. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biological Psychiatry, 67, 255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANTICEVIC A, REPOVS G & BARCH DM 2012. Emotion effects on attention, amygdala activation, and functional connectivity in schizophrenia. Schizophrenia Bulletin, 38, 967–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAAS D, ALEMAN A, VINK M, RAMSEY NF, DE HAAN EH & KAHN RS 2008a. Evidence of altered cortical and amygdala activation during social decision-making in schizophrenia. Neuroimage, 40, 719–727. [DOI] [PubMed] [Google Scholar]
- BAAS D, VAN’T WOUT M, ALEMAN A & KAHN R 2008b. Social judgement in clinically stable patients with schizophrenia and healthy relatives: behavioural evidence of social brain dysfunction. Psychological Medicine, 38, 747–754. [DOI] [PubMed] [Google Scholar]
- BARRAZA JA & ZAK PJ 2009. Empathy toward strangers triggers oxytocin release and subsequent generosity. Annals of the New York Academy of Sciences, 1167, 182–9. [DOI] [PubMed] [Google Scholar]
- BERENBAUM H & OLTMANNS TF 1992. Emotional experience and expression in schizophrenia and depression. Journal of Abnormal Psychology, 101, 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLANCHARD JJ & COHEN AS 2006. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophrenia Bulletin, 32, 238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSNELLI M, DAGANI J, GIROLAMO G, BALESTRIERI M, PINI S, SAVIOTTI FM, SCOCCO P, SISTI D, ROCCHI M & CHINI B 2016. Unaltered Oxytocin and Vasopressin Plasma Levels in Patients with Schizophrenia After 4 Months of Daily Treatment with Intranasal Oxytocin. Journal of Neuroendocrinology, 28. [DOI] [PubMed] [Google Scholar]
- CACCIOTTI-SAIJA C, LANGDON R, WARD PB, HICKIE IB, SCOTT EM, NAISMITH SL, MOORE L, ALVARES GA, REDOBLADO HODGE MA & GUASTELLA AJ 2015. A double-blind randomized controlled trial of oxytocin nasal spray and social cognition training for young people with early psychosis. Schizophrenia Bulletin, 41, 483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN AS, ALPERT M, NIENOW TM, DINZEO TJ & DOCHERTY NM 2008. Computerized measurement of negative symptoms in schizophrenia. Journal of Psychiatry Research, 42, 827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN AS & MINOR KS 2010. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophrenia Bulletin, 36, 143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUTURE SM, PENN DL, ADDINGTON J, WOODS SW & PERKINS DO 2008. Assessment of social judgments and complex mental states in the early phases of psychosis. Schizophrenia Research, 100, 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGANI J, SISTI D, ABELLI M, DI PAOLO L, PINI S, RAIMONDI S, ROCCHI MB, SAVIOTTI FM, SCOCCO P, TOTARO S, BALESTRIERI M & DE GIROLAMO G 2016. Do we need oxytocin to treat schizophrenia? A randomized clinical trial. Schizophrenia Research, 172, 158–64. [DOI] [PubMed] [Google Scholar]
- ECKERT SL, DIAMOND PM, MILLER AL, VELLIGAN DI, FUNDERBURG LG & TRUE JE 1996. A comparison of instrument sensitivity to negative symptom change. Psychiatry Research, 63, 67–75. [DOI] [PubMed] [Google Scholar]
- EKMAN P & FRIESEN WV 1976. Measuring facial movement. Environmental Psychology and Nonverbal Behavior, 1, 56–75. [Google Scholar]
- FEIFEL D, MACDONALD K, NGUYEN A, COBB P, WARLAN H, GALANGUE B, MINASSIAN A, BECKER O, COOPER J, PERRY W, LEFEBVRE M, GONZALES J & HADLEY A 2010. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biological Psychiatry, 68, 678–80. [DOI] [PubMed] [Google Scholar]
- FEIFEL D, SHILLING PD & MACDONALD K 2016. A Review of Oxytocin’s Effects on the Positive, Negative, and Cognitive Domains of Schizophrenia. Biological Psychiatry, 79, 222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOUSSIAS G, SIDDIQUI I, FERVAHA G, AGID O & REMINGTON G 2015. Dissecting negative symptoms in schizophrenia: opportunities for translation into new treatments. Journal of Psychopharmacology, 29, 116–26. [DOI] [PubMed] [Google Scholar]
- GAMER M & BÜCHEL C 2012. Oxytocin specifically enhances valence-dependent parasympathetic responses. Psychoneuroendocrinology, 37, 87–93. [DOI] [PubMed] [Google Scholar]
- GIBSON CM, PENN DL, SMEDLEY KL, LESERMAN J, ELLIOTT T & PEDERSEN CA 2014. A pilot six-week randomized controlled trial of oxytocin on social cognition and social skills in schizophrenia. Schizophrenia Research, 156, 261–5. [DOI] [PubMed] [Google Scholar]
- GREEN MF 2016. Impact of cognitive and social cognitive impairment on functional outcomes in patients with schizophrenia. Journal of Clinical Psychiatry, 77 Suppl 2, 8–11. [DOI] [PubMed] [Google Scholar]
- GUR RE, KOHLER CG, RAGLAND JD, SIEGEL SJ, LESKO K, BILKER WB & GUR RC 2006. Flat affect in schizophrenia: relation to emotion processing and neurocognitive measures. Schizophrenia Bulletin, 32, 279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUR RE, LOUGHEAD J, KOHLER CG, ELLIOTT MA, LESKO K, RUPAREL K, WOLF DH, BILKER WB & GUR RC 2007. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Archives of General Psychiatry, 64, 1356–66. [DOI] [PubMed] [Google Scholar]
- HOOKER CI, TULLY LM, VEROSKY SC, FISHER M, HOLLAND C & VINOGRADOV S 2011. Can I trust you? Negative affective priming influences social judgments in schizophrenia. Journal of Abnormal Psychology, 120, 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORTA DE MACEDO LR, ZUARDI AW, MACHADO-DE-SOUSA JP, CHAGAS MH & HALLAK JE 2014. Oxytocin does not improve performance of patients with schizophrenia and healthy volunteers in a facial emotion matching task. Psychiatry Research, 220, 125–8. [DOI] [PubMed] [Google Scholar]
- KAY SR, FISZBEIN A & OPLER LA 1987. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin, 13, 261–76. [DOI] [PubMed] [Google Scholar]
- KIRSCH P, ESSLINGER C, CHEN Q, MIER D, LIS S, SIDDHANTI S, GRUPPE H, MATTAY VS, GALLHOFER B & MEYER-LINDENBERG A 2005. Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of Neuroscience, 25, 11489–11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSFELD M, HEINRICHS M, ZAK PJ, FISCHBACHER U & FEHR E 2005. Oxytocin increases trust in humans. Nature, 435, 673–6. [DOI] [PubMed] [Google Scholar]
- KRING AM & ELIS O 2013. Emotion deficits in people with schizophrenia. Annual Review of Clinical Psychology, 9, 409–33. [DOI] [PubMed] [Google Scholar]
- KRING AM & MORAN EK 2008. Emotional response deficits in schizophrenia: insights from affective science. Schizophrenia Bulletin, 34, 819–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRING AM & NEALE JM 1996. Do schizophrenic patients show a disjunctive relationship among expressive, experiential, and psychophysiological components of emotion? Journal of Abnormal Psychology, 105, 249. [DOI] [PubMed] [Google Scholar]
- KRING AM & SLOAN DM 2007. The Facial Expression Coding System (FACES): development, validation, and utility. Psychological Assessment, 19, 210–24. [DOI] [PubMed] [Google Scholar]
- KRING AM, SMITH DA & NEALE JM 1994. Individual differences in dispositional expressiveness: development and validation of the Emotional Expressivity Scale. Journal of Personality and Social Psychology, 66, 934. [DOI] [PubMed] [Google Scholar]
- LAMBERT B, DECLERCK CH & BOONE C 2014. Oxytocin does not make a face appear more trustworthy but improves the accuracy of trustworthiness judgments. Psychoneuroendocrinology, 40, 60–8. [DOI] [PubMed] [Google Scholar]
- LEPAGE M, SERGERIE K, BENOIT A, CZECHOWSKA Y, DICKIE E & ARMONY JL 2011. Emotional face processing and flat affect in schizophrenia: functional and structural neural correlates. Psychological Medicine, 41, 1833–44. [DOI] [PubMed] [Google Scholar]
- LUKOFF D, LIBERMAN RP & NUECHTERLEIN KH 1986. Symptom monitoring in the rehabilitation of schizophrenic patients. Schizophrenia Bulletin, 12, 578–602. [DOI] [PubMed] [Google Scholar]
- FIRST MB, GIBBON RLS,M, WILLIAMS JBW 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. Research Version, Patient Edition (SCID-I/P), New York, New York State Psychiatric Institute. [Google Scholar]
- MICHELINI LC, MARCELO MC, AMICO J & MORRIS M 2003. Oxytocinergic regulation of cardiovascular function: studies in oxytocin-deficient mice. American Journal of Physiology-Heart and Circulatory Physiology, 284, H2269–H2276. [DOI] [PubMed] [Google Scholar]
- NARUM SR 2006. Beyond Bonferroni: Less conservative analyses for conservation genetics. Conservation Genetics, 7, 783–787. [Google Scholar]
- NORMAN GJ, CACIOPPO JT, MORRIS JS, KARELINA K, MALARKEY WB, DEVRIES AC & BERNTSON GG 2011. Selective influences of oxytocin on the evaluative processing of social stimuli. Journal of Psychopharmacology, 1313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEDERSEN CA, GIBSON CM, RAU SW, SALIMI K, SMEDLEY KL, CASEY RL, LESERMAN J, JARSKOG LF & PENN DL 2011. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophrenia Research, 132, 50–3. [DOI] [PubMed] [Google Scholar]
- PETERMAN JS, BEKELE E, BIAN D, SARKAR N & PARK S 2015. Complexities of emotional responses to social and non-social affective stimuli in schizophrenia. Frontiers in Psychology, 6, 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINKHAM AE, HOPFINGER JB, PELPHREY KA, PIVEN J & PENN DL 2008. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophrenia Research, 99, 164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REPANTIS D, LAISNEY O & HEUSER I 2010. Acetylcholinesterase inhibitors and memantine for neuroenhancement in healthy individuals: a systematic review. Pharmacological Research, 61, 473–81. [DOI] [PubMed] [Google Scholar]
- SCHERER S, STRATOU G, LUCAS G, MAHMOUD M, BOBERG J, GRATCH J & MORENCY L-P 2014. Automatic audiovisual behavior descriptors for psychological disorder analysis. Image and Vision Computing, 32, 648–658. [Google Scholar]
- SERRETTI A, CALATI R, GORACCI A, DI SIMPLICIO M, CASTROGIOVANNI P & DE RONCHI D 2010. Antidepressants in healthy subjects: what are the psychotropic/psychological effects? European Neuropsychopharmacology, 20, 433–53. [DOI] [PubMed] [Google Scholar]
- SHIN NY, PARK HY, JUNG WH, PARK JW, YUN JY, JANG JH, KIM SN, HAN HJ, KIM SY, KANG DH & KWON JS 2015. Effects of Oxytocin on Neural Response to Facial Expressions in Patients with Schizophrenia. Neuropsychopharmacology, 40, 1919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHROUT PE & FLEISS JL 1979. Intraclass correlations: uses in assessing rater reliability. Psychological Bulletin, 86, 420–8. [DOI] [PubMed] [Google Scholar]
- SRIPADA CS, PHAN KL, LABUSCHAGNE I, WELSH R, NATHAN PJ & WOOD AG 2013. Oxytocin enhances resting-state connectivity between amygdala and medial frontal cortex. International Journal of Neuropsychopharmacology, 16, 255–60. [DOI] [PubMed] [Google Scholar]
- SWEET LH, PRIMEAU M, FICHTNER CG & LUTZ G 1998. Dissociation of affect recognition and mood state from blunting in patients with schizophrenia. Psychiatry Research, 81, 301–8. [DOI] [PubMed] [Google Scholar]
- TAYLOR SF, PHAN KL, DECKER LR & LIBERZON I 2003. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage, 18, 650–659. [DOI] [PubMed] [Google Scholar]
- TREMEAU F, MALASPINA D, DUVAL F, CORREA H, HAGER-BUDNY M, COIN-BARIOU L, MACHER JP & GORMAN JM 2005. Facial expressiveness in patients with schizophrenia compared to depressed patients and nonpatient comparison subjects. American Journal of Psychiatry, 162, 92–101. [DOI] [PubMed] [Google Scholar]
- TSAPAKIS EM, DIMOPOULOU T & TARAZI FI 2015. Clinical management of negative symptoms of schizophrenia: An update. Pharmacology and Therapeutics, 153, 135–47. [DOI] [PubMed] [Google Scholar]
- WALKER EF, GRIMES KE, DAVIS DM & SMITH AJ 1993. Childhood precursors of schizophrenia: facial expressions of emotion. American Journal of Psychiatry, 150, 1654–60. [DOI] [PubMed] [Google Scholar]
- WEISER M, SAPORTA L, LEVI L & FELDMAN R 2015. S. 13.03 RCT administering oxytocin for schizophrenia, a three week add-on study. European Neuropsychopharmacology, S130–S131. [Google Scholar]
- WINSTON JS, STRANGE BA, O’DOHERTY J & DOLAN RJ 2002. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature Neuroscience, 5, 277–83. [DOI] [PubMed] [Google Scholar]
- WOLF K, MASS R, KIEFER F, ECKERT K, STRITZKY AV, HAASEN C, WIEDEMANN K & NABER D 2005. The influence of olanzapine versus risperidone on facial expression of emotions in schizophrenia-preliminary results of a facial electromyogram study. Journal of Clinical Psychopharmacology, 25, 278–281. [DOI] [PubMed] [Google Scholar]
- WOOLLEY JD, CHUANG B, LAM O, LAI W, O’DONOVAN A, RANKIN KP, MATHALON DH & VINOGRADOV S 2014. Oxytocin administration enhances controlled social cognition in patients with schizophrenia. Psychoneuroendocrinology, 47, 116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.