Abstract
The study by Naresh et al is, to the best of our knowledge, the first use of parametric mapping to image macrophage infiltration in the heart.
Summary
The elegant study by Naresh and colleagues (1) synthesizes many of the best aspects of molecular magnetic resonance (MR) imaging: Quantitative serial imaging of a well-defined molecular process is performed in vivo, and its results are correlated with sensitive measures of left ventricular function. The technique described adds a valuable tool to the molecular imaging armamentarium. How, then, will myocardial inflammation be imaged with MR imaging? The only clinical experience to date has been with iron oxide nanoparticles (2,3). Their excellent sensitivity, dynamic range, and safety record make them a highly appealing choice. It will be critical, however, for any iron oxide nanoparticle that is used clinically to be well studied and validated in animal models of the disease before it is used in humans. A “group effect” cannot be assumed, even in the case of fairly similar iron oxide nanoparticles. The use of MR imaging–detectable liposomes appears promising, and initial clinical studies with fluorine-containing liposomes are likely to begin shortly. The clinical use of gadolinium-labeled liposomes appears further away, and the approach described by Naresh and colleagues is thus likely to remain confined to preclinical investigation for the foreseeable future. The development of novel anti-inflammatory therapies, however, will require robust imaging tools to shepherd these agents through preclinical studies and into the clinical arena. The approach described by Naresh et al adds a valuable tool to the preclinical molecular imaging armamentarium.
The Setting
Inflammation plays a role in several diseases that affect the heart, including acute coronary syndromes, ventricular remodeling, myocarditis, and transplant rejection. While much attention has been focused on the imaging of inflammation in atherosclerotic plaques, the importance and complexity of inflammation in the myocardium itself is being increasingly realized (4). Recent preclinical investigation suggests that the modulation of inflammatory activity in the myocardium can have substantial effects, both beneficial and deleterious. The development of noninvasive imaging techniques to characterize myocardial inflammation is thus an important endeavor.
T2-weighted MR imaging of the myocardium can be used to detect the edema associated with inflammation. The greatest utility of this technique has been in differentiating acute from chronic myocardial infarction, but it is also of important use in the work-up of myocarditis. T2-weighted MR imaging, however, yields a nonspecific signature of myocardial inflammation, with no cellular or molecular information. Tissue macrophages are highly metabolically active, and positron emission tomographic (PET) imaging of fluorine 18 (18F)-fluorodeoxyglucose (FDG) uptake in the myocardium has thus been used as a measure of myocardial macrophage infiltration. In the preclinical setting, measures can be devised to suppress glucose uptake in the myocardium. In the clinical setting, however, the uptake of 18F-FDG in the myocardium reflects the high metabolic activity of both cardiomyocytes and infiltrating macrophages. Given the nonspecific nature of T2-weighted MR imaging and FDG PET, a clear need exists for specific targeted molecular imaging techniques to be developed to image myocardial inflammation.
The Science
Macrophages are professional phagocytes and will inherently take up imaging agents in the nanoparticulate range. Two platforms have been used to date to exploit this in the myocardium: (a) superparamagnetic iron oxide nanoparticles and (b) MR imaging–detectable liposomes loaded with large amounts of fluorine 19 (19F). The approach described here by Naresh and colleagues (1), who used gadolinium-loaded liposomes to image macrophage infiltration in the myocardium, expands this armamentarium further. The current study also very nicely synthesizes some of the key aspects of molecular MR imaging. How, then, will myocardial inflammation be imaged in both the preclinical and clinical settings? We examine the advantages and disadvantages of the available platforms below.
Iron oxide nanoparticles have been used for almost 2 decades to image monocytes, macrophages, and Kupfer cells in humans in vivo (2,3). Several of these agents have been clinically approved and have been used commercially in thousands of patients. The pharmacokinetics and elimination of these agents is thus well understood and well characterized. It must be stressed that even small changes in size and surface chemistry can drastically change the properties of iron oxide nanoparticles. Many of the commercially available iron oxide nanoparticles are thus not at all suited to in vivo imaging, including preclinical imaging studies. Likewise, iron oxide microparticles have extremely different properties from clinically used nanoparticles and, furthermore, have no record of use in humans. We thus confine our remarks here to what we believe to be the relevant experience with iron oxide particles: The use of long-circulating dextran-coated nanoparticles, which have been used in humans in either identical or similar form.
An initial study (5) with the crossed-linked iron oxide (CLIO) nanoparticles in mice 96 hours after infarction showed that macrophage infiltration into the injured myocardium could be robustly imaged by using a clinically approved dose of the nanoparticle (<3 mg iron per kilogram of body weight). T2*-weighted MR imaging was used to generate signal hypointensity in the vicinity of the nanoparticle in this study. In a subsequent study (6), an off-resonance MR imaging technique provided positive contrast with this platform in the identical model. What are the advantages and disadvantages of this platform? The sensitivity of these agents is excellent, and few, if any, safety concerns related to their use exist. In our experience, the sensitivity of these agents allows reasonably short echo times to be used, and nonspecific susceptibility artifacts are thus rarely a problem. However, the inability to distinguish endogenous iron related to hemorrhage from the injected nanoparticle is a legitimate concern. In the preclinical setting, the attachment of a fluorochrome (which can be imaged noninvasively) to the nanoparticle provides a complete solution to this problem (5). In humans, strategies such as pre- and post imaging or the use of MR/PET-detectable radiolabeled nanoparticles will need to be considered when the suspicion for intramyocardial hemorrhage is high.
Liposomes have a long history and established safety record in humans. In the diagnostic arena they have been used to enhance the accuracy of ultrasonography for many years. Two strategies have been used to make liposomal nanoparticles visible at MR imaging: 19F and gadolinium. Loading the liposomes with 19F has allowed their uptake by macrophages and their subsequent infiltration into the myocardium to be imaged with 19F-MR imaging (7). The advantage of this approach lies in the complete absence of background signal. Its principal disadvantage lies in its lower sensitivity.
The liposomes used by Naresh and colleagues (1) had a size distribution of 500 nm ± 250. The circulation half-life of the liposomes was 90 minutes, which is adequate for uptake by circulating monocytes. The longitudinal relaxivity (r1) of the liposomes was 3.8 mmol−1 ⋅ sec−1, which is two orders of magnitude lower than the transverse relaxivity (r2*) of iron oxide nanoparticles. This difference in relaxivities, however, is mitigated somewhat because the inherent R1 (longitudinal relaxation rate) of the myocardium is significantly less than its R2* (transverse relaxation rate). Indeed, the kinetic curves in the study suggest that the sensitivity of the liposome was good and that it is able to depict early macrophage infiltration after myocardial infarction more robustly than its fluorine-labeled counterpart (1,7). By dual-labeling the liposomes with gadolinium and a fluorochrome, the authors were nicely able to confirm with microscopy that the liposomes were indeed taken up by macrophages infiltrating the infarct (1).
The Practice
The presence of macrophages in infarcted myocardium is well established, and molecular imaging approaches add little value if they do not permit both serial and quantitative imaging to be performed. Serial imaging of mice with infarcts that were injected with 19F-labeled liposomes has been performed, but robust quantification was complicated by the pharmacokinetics and lower sensitivity of the agent (7). Several indirect approaches have been used to image the time course of macrophage infiltration in healing infarcts. These include imaging the time course of cathepsin and myeloperoxidase activity in the myocardium after infarction (8,9). These enzymes are secreted by infiltrating macrophages, and the time course of their activity presumably closely mirrors that of the macrophage infiltrate. Indeed, imaging these enzymes produced a time course very similar to that produced by the liposomes injected by Naresh and colleagues (1). Macrophage activity in the infarcted myocardium is detectable by 24 hours, peaks between 72 and 96 hours, and dissipates 10–14 days after infarction. The strength of the current study is that serial imaging was performed in the same animals. This provides a cleaner, yet similar, result to the studies involving myeloperoxidase and cathepsin imaging (8,9), in which cohorts of animals were imaged and euthanized at separate time points.
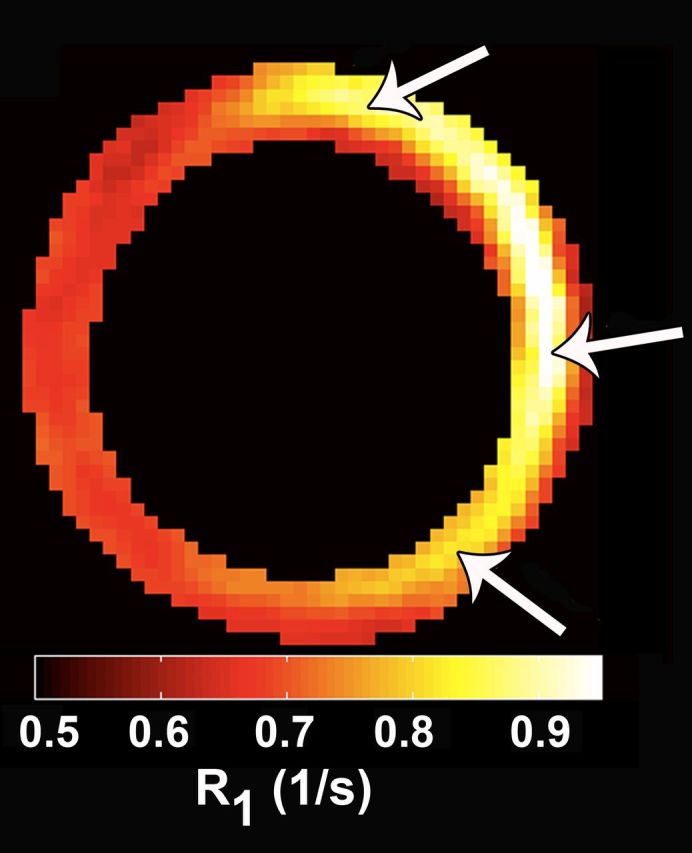
Accurate quantification of the signal produced by a molecular imaging agent is of major importance. In the case of molecular MR imaging, this involves the generation of maps of either the transverse (R2*) or the longitudinal (R1) relaxation rate. (T1 and T2* maps are simply the reciprocals of the relaxation rate maps.) These maps are immune to factors such as coil loading and sensitivity, which can change substantially from study to study. Both R2* and R1 maps have been used to quantify the uptake of molecular imaging agents in the context of cell death imaging (10,11). The article by Naresh et al is, however, to the best of our knowledge, the first use of parametric mapping to image macrophage infiltration in the heart. The authors have spent considerable time, through their study of arterial spin labeling (12), in optimizing their R1 mapping technique, and the maps produced in this study are thus of the highest quality. The ability to visualize a gradient in macrophage infiltration from the center of the infarct to the border zones on the basis of accurate R1 mapping is indeed praiseworthy.
Perhaps the most appealing aspect of this study lies in the synthesis of quantitative molecular imaging data with quantitative maps of myocardial strain. The authors used the displacement encoding with stimulated echoes, or DENSE, technique to create maps of circumferential strain and have correlated these with the maps of macrophage infiltration. The high correlation between the two sets of maps is in some ways expected but, more importantly, demonstrates the unique ability of molecular MR imaging readouts to be combined with inherently coregistered readouts of myocardial physiology. No platform other than MR imaging allows molecular and functional data to be integrated in as accurate and powerful a manner.
What limitations, if any, in this study bear mention? Perhaps the most obvious one is the challenge of translating this platform into the clinical arena. While the elimination of small gadolinium-containing chelates is well established, the elimination of gadolinium encapsulated in large liposomes is less understood. The uptake of liposomes by the reticuloendothelial system leads to gadolinium uptake in the liver and spleen, resulting in slower clearance from the body (13). The possibility for gadolinium-associated toxicity would thus need to be thoroughly examined before this technology could be translated to the clinic.
Footnotes
See also the article by Naresh et al.
Disclosures of Potential Conflicts of Interest: D.E.S. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a consultant for Siemens Medical; institution has patent pending with Vital Imaging Agents and receives research support from Siemens Medical. Other relationships: none to disclose. M.N. No potential conflicts of interest to disclose. P.C. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: institution has grants or grants pending with Sanofi; holds stock or stock options in Collagen Medical, Factor 1A, and Catalyst Medical. Other relationships: none to disclose.
References
- 1.Naresh NK, Xu Y, Klibanov AL, et al. Monocyte and/or macrophage infiltration of heart after myocardial infarction: MR imaging by using T1-shortening liposomes. Radiology 2012;264(2):428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med 2003;348(25):2491–2499. [DOI] [PubMed] [Google Scholar]
- 3.Tang TY, Howarth SP, Miller SR, et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study: evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol 2009;53(22):2039–2050. [DOI] [PubMed] [Google Scholar]
- 4.Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 2010;121(22):2437–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sosnovik DE, Nahrendorf M, Deliolanis N, et al. Fluorescence tomography and magnetic resonance imaging of myocardial macrophage infiltration in infarcted myocardium in vivo. Circulation 2007;115(11):1384–1391. [DOI] [PubMed] [Google Scholar]
- 6.Farrar CT, Dai G, Novikov M, et al. Impact of field strength and iron oxide nanoparticle concentration on the linearity and diagnostic accuracy of off-resonance imaging. NMR Biomed 2008;21(5):453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flögel U, Ding Z, Hardung H, et al. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation 2008;118(2):140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nahrendorf M, Sosnovik D, Chen JW, et al. Activatable magnetic resonance imaging agent reports myeloperoxidase activity in healing infarcts and noninvasively detects the antiinflammatory effects of atorvastatin on ischemia-reperfusion injury. Circulation 2008;117(9):1153–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nahrendorf M, Sosnovik DE, Waterman P, et al. Dual channel optical tomographic imaging of leukocyte recruitment and protease activity in the healing myocardial infarct. Circ Res 2007;100(8):1218–1225. [DOI] [PubMed] [Google Scholar]
- 10.Huang S, Chen HH, Yuan H, et al. Molecular MRI of acute necrosis with a novel DNA-binding gadolinium chelate: kinetics of cell death and clearance in infarcted myocardium. Circ Cardiovasc Imaging 2011;4(6):729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sosnovik DE, Schellenberger EA, Nahrendorf M, et al. Magnetic resonance imaging of cardiomyocyte apoptosis with a novel magneto-optical nanoparticle. Magn Reson Med 2005;54(3):718–724. [DOI] [PubMed] [Google Scholar]
- 12.Vandsburger MH, Janiczek RL, Xu Y, et al. Improved arterial spin labeling after myocardial infarction in mice using cardiac and respiratory gated look-locker imaging with fuzzy C-means clustering. Magn Reson Med 2010;63(3):648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unger E, Cardenas D, Zerella A, Fajardo LL, Tilcock C. Biodistribution and clearance of liposomal gadolinium-DTPA. Invest Radiol 1990;25(6):638–644. [DOI] [PubMed] [Google Scholar]


