Abstract
Extensive, yet disparate, research exists elucidating structural anomalies in individuals with Reading Disability (RD) or ADHD. Despite ADHD and RD being highly comorbid, minimal research has attempted to determine shared patterns of morphometry between these disorders. In addition, there is no published research examining the morphometry of comorbid RD and ADHD (RD/ADHD). Hence, we conducted voxel-based morphometry on the MRI scans of 106 children, ages 8–12 years, with RD, ADHD, or RD/ADHD, and typically developing controls. We found right caudate and superior frontal regions in both RD and ADHD, along with areas specific to RD and to ADHD that are consistent with current theories on these disorders. Perhaps most importantly, we found a potential neurobiological substrate for RD/ADHD. Further, our findings illustrate both shared and specific contributors to RD/ADHD, supporting two current theories on the comorbidity of RD and ADHD, thereby facilitating future work on potential etiologies of RD/ADHD.
Keywords: Reading disability, ADHD, Reading disability/ADHD, Structural neuroimaging, Thalamus, Frontal lobes, Striatum, VBM, Morphometry, Occipital lobes
1. Introduction
Reading Disability (RD) and Attention Deficit Hyperactivity Disorder (ADHD) are two neurodevelopmental disorders that have a comorbidity greater than expected based upon the base rate of either disorder alone, about 25–40% (Boada, Willcutt, & Pennington, 2012; Shaywitz & Shaywitz, 2005). Despite the high comorbidity between these two disorders, the literature is disparate on whether comorbid RD/ADHD is a unique disorder or merely a summation of both RD and ADHD etiologies. As the literature deliberates, any contributions to understanding the neurobiological correlates of comorbid RD/ADHD may have wide-reaching implications in the field. Therefore, the primary purpose of this study was to discover whether there are distinct patterns of gray matter morphometry in children with comorbid RD/ADHD as compared to controls using VBM and if these patterns differ from having either disorder alone. Our secondary purpose was to determine if there are shared neurobiological correlates of RD and ADHD.
1.1. Reading disability
Reading Disability (RD) is often defined as poor word identification and decoding skills (basic reading) despite intact cognitive ability (IQ or other cognitive functions; Pennington, Peterson, & Mcgrath, 2010). There is substantial heterogeneity between theories on the etiology of reading disability. This heterogeneity is likely due to the diversity of symptoms across individuals with the disorder (Tamboer, Scholte, and Vorst, 2015) and to the different operational definitions of reading disability used throughout the literature. In terms of the latter, some researchers used the poor reader definition of reading disability which requires reading ability to be below average despite the child not being intellectually disabled; no IQ-achievement discrepancy is required (Siegel, 1992). Others used the discrepancy definition of reading disability which requires reading ability to be significantly below the child’s measured intellect, following the DSM-IV as well as the USA’s IDEA requirements prior to 2004. The World Health Organization defined development dyslexia as poor word recognition and spelling abilities despite adequate instruction, intelligence and sensory abilities (see Peterson & Pennington, 2012), so dyslexia could be considered a subset of reading disability given the additional spelling requirement. The RD literature utilizes all three definitions. Irrespective of how RD is defined, three theories of reading disability have been utilized more often than the rest in neuroimaging studies: double deficit, dual route, and visual attention.
The double-deficit theory postulates that dyslexia is due to poor phonological awareness, rapid automatized naming, or both (Jednoróg, Gawron, Marchewka, Heim, & Grabowska, 2013; Pugh et al., 2013). The dual route theory suggests that there are two routes to reading: phonological and orthographic, and reading problems can occur due to damage or faulty development in either route (Coltheart, Rastle, Perry, Langdon, & Ziegler, 2001). The visual attention hypothesis states that reading problems are due to poor phonological processing, visual attention, both, or neither (Bosse, Tainturier, & Valdois, 2007). Hence, while there is heterogeneity between theories on the etiology of RD, one commonality across all theories is poor phonological processing, which is the most common deficit found in RD (Ramus et al., 2003). Further support for these theories is found in the morphometry literature.
Previous RD research using VBM analysis has found gray matter abnormalities in the occipital cortex, inferior and lateral temporal cortices, parietal cortex, frontal cortex and the cerebellum. Using the double deficit hypothesis of reading disability as a paradigm, Jednoróg et al. (2013) found children with poor phonological awareness had smaller gray matter volume clusters in the right precentral and left parietal lobe but larger gray matter volume clusters in the left cerebellum and right putamen. Children with poor rapid autonomic naming had the same brain regions implicated but in an opposite volumetric pattern to the poor phonological awareness group. For children with both poor phonological processing and rapid autonomic naming (double deficit), the VBM analysis found decreased gray matter in the right supramarginal gyrus and increased gray matter in the left cerebellum. In a study testing the visual attention theory of reading disability (Stein & Walsh, 1997), the authors found that left posterior STG and middle temporal deviations were associated with poor phonological processing/verbal working memory, and right lateral occipital/superior parietal deviations were related to visual attention deficits based on correlational analyses.
In contrast to these two theories, a considerable amount of research, both structural and functional, has been published related to the dual route model. Two studies proposed a similar model of dyslexia based on fMRI methodology (Pugh et al., 2000; Shaywitz, Lyon, & Shaywitz, 2006). They suggested three circuits are involved with dyslexia: ventral, dorsal and anterior. The ventral circuit includes the left lateral extrastriate and inferior occipital-temporal regions and is involved with rapid recognition of familiar words and letter strings (orthographic route to reading). The dorsal circuit includes left superior temporal and inferior parietal structures and is involved with the decoding of novel words (phonological route). The anterior route is used by individuals with RD to compensate for deficits in posterior functioning and includes the inferior frontal gyrus. Areas homologous to the dorsal and ventral routes in the right hemisphere may be used to compensate as well. All of the areas involved in the dorsal, ventral, and anterior circuits have been implicated in various VBM studies on RD (Black et al., 2012; Hoeft et al., 2007; Im, Raschle, Smith, Ellen Grant, & Gaab, 2015; Linkersdorfer, Lonnemann, Lindberg, Hasselhorn, & Fiebach, 2012; Raschle, Chang, & Gaab, 2012; Richardson and Price, 2009; Richlan, Kronbichler, & Wimmer, 2013; Tamboer et al., 2015; Xia, Hoeft, Zhang, & Shu, 2016). Nonetheless, results are variable regarding whether these clusters are equal to, larger, or smaller than controls across studies (Jednoróg et al., 2013; Pernet, Andersson, Paulesu, & Demonet, 2009). This variability may be related to the heterogeneity of the disorder and/or variations in the operational definitions of RD used, language spoken by the various samples, and ages included in the various samples. Two recent review articles recapitulate this point. Xia, Hancock, and Hoeft (2017) and Ramus, Altarelli, Jednoróg, Zhao, and Scotto di Covella (2017) both cite language, as well as small sample size, as limitations in RD studies that use imaging methodology. Other potential causes of heterogeneity in RD studies include variability in VBM methodology (Ramus, et al., 2017) and not considering RD’s comorbidity with other neurodevelopmental disorders or RD subtypes (Xia et al., 2017). Therefore, future studies (including the current study) should address these methodological shortcomings.
1.2. Attention-Deficit/Hyperactivity Disorder
Attention-Deficit/Hyperactivity Disorder (ADHD) describes children who have heightened levels of inattention and/or hyperactivity/impulsivity for their age (APA, 2013). The most commonly cited theory on the etiology of ADHD is the frontal-striatal theory, which suggests that the prefrontal cortex and basal ganglia are not functioning optimally in ADHD (Barkley, 1997; Castellanos & Proal, 2012; Castellanos et al., 1996). Barkley (1997) found that the worst deficits in ADHD are within the areas of inhibition, working memory, self-regulation, sustained attention, other executive functions, and motor control. Many of these deficits are associated with the prefrontal-striatal circuit (in particular the dorsolateral prefrontal cortex and caudate), especially the cognitive aspects of executive functioning such as working memory, planning, and problem-solving (Castellanos & Proal, 2012; Castellanos et al., 1996; Dang et al., 2014; Monchi, Petrides, Mejia-Constain, and Strafella, 2007). Other structural research has identified additional frontal circuits that may be invovled in executive functions, including the inferior frontal-striatal-cerebellar (Carmona et al., 2005; Makris et al., 2015; Rubia, 2011), prefrontal-posterior parietal (Carmona et al., 2005; Seidman et al., 2006), and orbitofrontal-limbic (Carmona et al., 2005; Makris et al., 2007; Seidman et al., 2011) circuits. These circuits play a role in motor/behavioral inhibition, emotional regulation, selective attention, and visual regulation of attention (Castellanos & Proal, 2012), potentially for both bottom-up and top-down processes, depending upon the region and circuit (Sonuga-Barke, Sergeant, Nigg, & Willcutt, 2008). Moreover, the orbitofrontal-limbic circuit may serve an additional purpose of aiding in delay aversion processing – a behavior often compromised in those with ADHD (Sonuga-Barke et al., 2008). In general, the literature strongly implicates prefrontal (DLPFC, inferior frontal and orbitofrontal), striatal, limbic (cingulate and medial temporal lobe), and cerebellar abnormalities that may give rise to the various ADHD symptoms presented in the literature.
Corresponding with the different theories proposed, gray matter morphometry studies have found reduced volume in various parts of the prefrontal, parietal, temporal and cingulate cortices, the striatum, the cerebellum, and in total brain volume (Carmona et al., 2005; de Mello et al., 2013; Seidman et al., 2011; Yang et al., 2008). More specifically, multiple experiments have found that children with ADHD have smaller total gray and white matter volume compared to children without it (Carmona et al., 2005; Castellanos et al., 2002; Lim et al., 2013; Seidman et al., 2006; Yang et al., 2008), which persists from childhood into at least adolescence (Castellanos et al., 2002). When examining the frontal-striatal circuit, participants with ADHD have smaller gray matter clusters compared to controls in the dorsolateral prefrontal cortex, inferior frontal cortex, caudate, putamen, and anterior cingulate (Carmona et al., 2005; de Mello et al., 2013; Makris et al., 2015; Seidman et al., 2011; Tremols et al., 2008; see Krain and Castellanos, 2006 or Seidman, Valera, & Makris, 2005 for a review). In addition, reduced gray matter has been found in individuals with ADHD in the cerebellum and temporal-parietal regions (Carmona et al., 2005; Depue, Burgess, Bidwell, Willcutt, and Banich, 2011a; Lim et al., 2013; Pironti et al., 2014; van ‘t Ent et al., 2007; Villemonteix et al., 2015) and in orbitofrontal and limbic structures (Carmona et al., 2005; Frodl & Skokauskas, 2012; Krain & Castellanos, 2006; Seidman et al., 2006; van ‘t Ent et al., 2007). Nonetheless, not all studies find reduced gray matter volume in these structures. For example, some researchers have found that people with ADHD have larger clusters in the dorsolateral prefrontal cortex, orbitofrontal cortex, caudate, putamen, inferior parietal cortex and/or temporal cortex (Makris et al., 2015; Moreno-Alcázar et al., 2016; Seidman et al., 2011, 2005), while others have found the basal ganglia, amygdala, and hippocampus to be commensurate in size to controls (Pironti et al., 2014). This variability may be related to the heterogeneity of symptomology and behavioral deficits found in ADHD (e.g., about 20% of individuals with ADHD do not present with an executive function deficit, and some have other deficits as well; Nigg, Willcutt, Doyle, & Sonuga-Barke, 2005) and medication status (i.e., medication naïve versus chronically treated; Villemonteix et al, 2015). Despite the heterogeneity found in the literature, there is sufficient evidence from gray matter morphometry studies to support a frontal-striatal and/or orbitofrontal-limbic deficit in many individuals with ADHD.
1.3. Comorbid ADHD and reading disability
Etiological research on why RD and ADHD are frequently comorbid is disparate. Some suggest ADHD and RD are two separate disorders that share select genetic, neurobiological and/or cognitive contributors which lead to their comorbidity (McGrath et al., 2012; Willcutt et al., 2001). Others suggest comorbid RD/ADHD is a unique subtype from RD and ADHD alone (Rucklidge & Tannock, 2002). Alternatively, RD, ADHD and RD/ADHD could be different manifestations of the same neurodevelopmental process (Gilger & Kaplan, 2001).
The first position, that of separate disorders with shared neurobiological and cognitive contributors, currently has the most support. Children with RD often have poor phonological processing (Lyon, Fletcher, & Barnes, 2003), whereas those with ADHD often have poor inhibitory control (Barkley, 1997). Children with comorbid RD/ADHD tend to display both sets of problems (Klorman et al., 1999; Korkman & Pesonen, 1994; Rucklidge & Tannock, 2002; Willcutt et al., 2001; Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005), suggesting they possess shared deficits between the two disorders instead of a unique set of deficits specific to the comorbidity. Current research supports two potential sources of shared etiology: slow processing speed (McGrath et al., 2012; Shanahan et al., 2006) and/or poor focused auditory attention/rote verbal short-term memory (Kibby & Cohen, 2008). For example, Shanahan et al. (2006) found that measures of processing speed helped account for the relationship between RD and ADHD. Nonetheless, the authors stated slow processing speed was not sufficient to explain the extent of the shared variance between RD and ADHD, although neither disorder is fully explained without accounting for the impact of processing speed. Kibby and Cohen (2008) found reduced digit span forward performance in children with RD and in those with ADHD, along with short-term memory deficits specific to each disorder. The comorbid RD/ADHD group exhibited all the deficits found in the RD and ADHD groups but no additional deficits. Research on genetic origins also has found shared candidate genes between RD and ADHD (Willcutt, Betjemann, Mcgrath, & Pennington, 2010).
In contrast, Rucklidge and Tannock (2002) suggested that comorbid RD/ADHD could be its own unique subtype separate from RD and ADHD. More specifically, they found only the RD/ADHD group had poor rapid automatized naming of numbers and colors, as well as slower and less accurate responses, when compared to RD, ADHD, and controls. While coming to a different conclusion, McGrath et al. (2012) did find that processing speed predicted group membership in the comorbid group over predicting ADHD or RD alone, being related to both the reading and inattention symptom dimensions. Finally, given that both are neurodevelopmental disorders and there is a high comorbidity between the two, some suggest RD and ADHD are different manifestations of the same neurodevelopmental process (Gilger & Kaplan, 2001; Pettersson et al., 2013; Visser, 2003).
Currently, there is limited research on the neurobiological basis of comorbid RD/ADHD, and none was found using morphometry. An early structural imaging study performed by Hynd, Semrud-Clikeman, Lorys, Novey, & Eliopulos (1990) found that both ADHD and RD were associated with reduced right frontal volume. In a later study using tracing, Kibby, Kroese, Krebbs, Hill, & Hynd (2009) found that the right pars triangularis was smaller in those with ADHD, regardless of RD status; thus, the comorbid group shared similar reductions in pars triangularis size as ADHD. Furthermore, right pars triangularis size was associated with both rapid naming (a common problem in both RD and ADHD) and attention problems. Kibby, Fancher, Markanen, and Hynd (2008) showed RD diagnosis was associated with reduced cerebellar asymmetry regardless of ADHD status, suggesting RD and comorbid RD/ADHD had similar atypicalities in the cerebellum. ADHD diagnosis was not associated with differences in cerebellum structure; nonetheless, anterior vermis volume correlated with both phonological awareness and ADHD symptoms, indicating another potential source of shared etiology. Therefore, based upon the limited MRI literature available, comorbid RD/ADHD may stem from additive neurobiological and cognitive effects of the separate disorders (Hynd, et al., 1990; Kibby et al., 2008, 2009) or could be a manifestation of the same neurodevelopmental disorder.
1.4. Specific aims
This study had two objectives. One aim was to determine which brain areas are disparate in the comorbid group as compared to controls and whether these overlap with the RD group, ADHD group, or are unique to RD/ADHD, as there is a dearth of morphology research on this group. Given the limited amount of structural neuroimaging research comparing RD to ADHD in the same study, the second aim was to determine whether there are shared brain areas affected in both disorders, as well as areas that are specific to each disorder alone. Based on the VBM literature reviewed, we hypothesized that temporal-parietal, occipital-inferior temporal, and inferior frontal gyri along with the cerebellum would be smaller in RD compared to controls, and that the prefrontal, basal ganglia, anterior cingulate, temporal-parietal, and cerebellar regions would be smaller in ADHD compared to controls. Based upon the additive notion of comorbid RD and ADHD, individuals with comorbid RD/ADHD were hypothesized to exhibit decreased volume in brain regions that may be shared by both disorders (such as the temporal-parietal, inferior frontal and cerebellar regions), as well as in regions that may be disorder specific (e.g., occipital-inferior temporal gyrus, basal ganglia, anterior cingulate). The comorbid group was not expected to exhibit a unique gray matter pattern compared to ADHD or RD. Nevertheless, due to the limited quantitative MRI research on comorbid RD/ADHD and small sample size, the hypotheses were exploratory, and both corrected and uncorrected whole brain analyses are reported to guide future research.
2. Methods
2.1. Participants
Participants included 106 children, 8–12 years of age, 87% Caucasian, and 51% male. They were recruited through larger, NIH-funded projects that examined neuropsychological characteristics of children with Attention-Deficit/Hyperactivity Disorder, reading disability, comorbid RD/ADHD, and typically developing controls (TDC). Only participants who completed MRI scans without substantial motion artifacts were included in this study (ADHD n =41; RD n =17; RD/ADHD n =16; controls n =32). All children had an IQ > 79. Groups did not differ on gender, maternal education/SES, or age (ps > .10). Groups did differ in handedness [F (3,102) =4.34, p =.006], such that more children with ADHD were left-handed than in the other three groups. Please refer to Table 1 for further participant demographic details. Children were recruited from local schools, through referrals from physicians and psychologists, and through flyers and media advertisements in southern Illinois, eastern Missouri, and western Kentucky, representing a community sample from a rural area. As compensation for participating in the larger study, families received a free neuropsychological evaluation on their child, and the children received a T-shirt.
Table 1.
Participant Demographic Data.
| Groups | Total N | Number of Females | ||
|---|---|---|---|---|
| Sample | Controls | 32 | 14 | |
| RD | 17 | 11 | ||
| ADHD | 41 | 23 | ||
| RD/ADHD | 16 | 6 | ||
| Total | 106 | 54 | ||
| Groups | Mean | Standard Deviation | Confidence intervals [95%] | |
| Age | Controls | 9.66 | 1.38 | 9.16–10.15 |
| RD | 9.24 | 1.35 | 8.54–9.93 | |
| ADHD | 9.61 | 1.39 | 9.17–10.05 | |
| RD/ADHD | 9.13 | 1.54 | 8.50–9.95 | |
| Total | 9.49 | 1.4 | 9.22–9.76 | |
| Handednessb* | Controls | 92.19 | 7.06 | 89.64–94.73 |
| RD | 80.59 | 26.09 | 67.17–94.00 | |
| ADHD | 77.32 | 29.84 | 67.90–86.73 | |
| RD/ADHD | 95.93 | 2.29 | 92.27–98.98 | |
| Total | 85.09 | 22.86 | 80.69–89.50 | |
| Maternal SESa | Controls | 37.17 | 17.7 | 30.44–43.91 |
| RD | 38.31 | 16.6 | 29.46–47.16 | |
| ADHD | 37.11 | 15.08 | 32.01–42.21 | |
| RD/ADHD | 35.64 | 19.48 | 24.39–46.89 | |
| Total | 37.12 | 16.58 | 33.74–40.49 |
Notes:
Maternal SES was measured using the Hollingshead Four-Factor Index of Socioeconomic Status (1975).
Handedness was measured using the Edinburgh Handedness Inventory (1971).
p-value less than 0.05.
A child was diagnosed with reading disability (RD) in one of two ways, following the guidelines of Pennington, Peterson, and Mcgrath (2008). Children were classified as a ‘poor reader’ if the child’s performance was one standard deviation or more below the mean on two out of three measures of basic reading ability: the Woodcock Johnson Tests of Achievement III (WJ-III; Woodcock, McGrew, & Mather, 2001), the Gray Oral Reading Test-4th edition (Gort-4; Wiederholt & Bryant, 2001) or the Boder Test of Reading-Spelling Patterns (Boder & Jarrico, 1982). The second way children were diagnosed was by a discrepancy definition; that is, if a child’s reading performance on two of the basic reading measures previously mentioned was significantly lower than expected based on their IQ, using the regression formula from the State of Washington which controlled for the correlation between the IQ and achievement measures. Both diagnostic criteria were utilized following the suggestion of Pennington, Peterson, and Mcgrath (2008), as children from both groups have similarly reduced phonological processing, which is the core deficit in RD (Brady and Shankweiler, 1991; Stanovich, 1988; Swank, 1994). It also aids generalization across studies, as some prior work used a poor reader definition (e.g., Siegel, 1992) and others used a discrepancy definition (e.g., Eckert et al., 2005). A child only had to meet one definition to be included in the RD group.
Attention-deficit/hyperactivity disorder was diagnosed by a child clinical neuropsychologist using DSM-IV criteria, as this was the most recent DSM edition available at the time of data collection. As part of the diagnostic process, interview data from the parent to determine symptoms, the age of onset and impairment were used, as well as questionnaires. Questionnaires included parent and teacher ratings of attention problems and hyperactivity/impulsivity from the Behavior Assessment System for Children, Second Edition (BASC-2; Reynolds & Kamphaus, 2004) to verify the severity of symptoms was above average and appeared across the school and home settings.
Children who met criteria for both RD and ADHD were placed into the comorbid RD/ADHD group. Specifically, the child clinical neuropsychologist ensured that reading problems occurred on at least two measures and that ADHD symptoms occurred across settings (academic and non-academic). Because of the small size of the RD/ADHD group, ADHD was not broken down further into subtypes for VBM analysis. Nonetheless, ADHD and RD/ADHD did not differ in the proportion of ADHD subtypes (X2 =.03, p = .86), with ADHD having 22 children with ADHD-PI and 19 with ADHD-C, and RD/ADHD having 9 children with ADHD-PI and 7 with ADHD-C.
Participants were classified as a TDC if they did not meet inclusion criteria for ADHD or RD. Participants in all groups met the exclusionary criteria. Children were excluded from the study if they had a history of medical or neurological disorders (e.g., traumatic brain injury, prolonged high fever), any significant perinatal complications (e.g., prematurity under 36 weeks,), or severe environmental problems (e.g., suspected abuse).
2.2. Procedures
2.2.1. MRI data collection
Children were scanned for 8 min on a Philips Intera 1.5 Tesla scanner. A 3-D, fast spin, gradient reversal acquisition protocol was used to acquire T1-weighted images with a TR of 30 ms, a TE of 4.6 ms, and a flip angle of 35. All images included 200 axial slices, spaced 0.8 mm apart, with a slice thickness of 1.6 mm, a FOV of 256 mm by 256 mm, and a voxel size of .89 mm × .89 mm × 1.6 mm. The child’s head was stabilized with padding to reduce motion artifacts, and noise-reducing headphones were provided to facilitate comfort in the scanner.
2.2.2. MRI data preprocessing and analysis
Children were excluded if there was too much motion in the structural scan that interfered with preprocessing, as noted above, resulting in the 106 participants included in this study. Preprocessing and analyses were conducted in SPM 8, using Ashburner’s (2010) protocol for preprocessing with VBM. The images were manually reoriented to the anterior and posterior commissures. Manual reorientation was followed by segmentation using New Segment and DARTEL (create Templates and Normalized to MNI Space batch scripts) for realignment and normalization (including modulation) to the MNI template. This study did not use a custom template for normalization, as research conducted in middle childhood that compared the MNI template typically used for normalization with a custom template for normalization did not find sufficient differences to warrant the added ambiguity in interpreting results (Hoeft et al., 2007). Finally, the images were smoothed with an 8 mm Gaussian Kernel.
A whole brain approach was utilized as our goal was to compare the three clinical groups to determine where they had shared versus dissimilar brain morphology. While an a priori approach has many benefits, it was not used in this study for two reasons: (1) If the groups were contrasted on the ROIs implicated in both RD and ADHD, over ten regions would need to be compared based on the literature reviewed, which is extensive given our cell size; (2) There is a lack of research on the comorbid group, making a whole brain approach beneficial to guide future ROI research on this group. Hence, a one-way analysis of covariance was conducted with the four groups (ADHD, RD, RD/ADHD, and controls), regressing out effects of gender, age, handedness and total intracranial volume (TIV). TIV was the sum of white matter, gray matter, and cerebral spinal fluid segmented files provided from the segment pre-processing step in SPM. An implicit mask with an absolute threshold of 0.2 intensity value was used.
The first contrast utilized an FDR correction (p < .05) to minimize family-wise error and a cluster extent threshold of 20 voxels; it also was corrected for non-isotropic smoothness using the VBM8 toolbox (developed by Christian Gasser). This contrast subtracted the combined clinical group’s (RD/ADHD, RD, and ADHD) gray matter maps from the control group’s maps. The purpose of this overarching contrast was to identify the brain regions that are affected in our clinical groups in total, considering the potential shared etiologies of RD and ADHD, and to enhance power given the small sample size of the various clinical groups. Follow-up contrasts were used to determine which specific clinical group(s) was driving the clusters from the overarching contrast. Initially, these follow-up comparisons were performed using FDR, but none yielded significant clusters with this technique, similar to many studies on both RD and ADHD using whole-brain VBM (e.g., Depue, Burgess, Bidwell, Willcutt, and Banich, 2011b; Makris et al., 2015; Tamboer et al., 2015). Thus, following these studies’ methods, the subsequent contrasts were performed uncorrected, but they were masked with the map from the overarching contrast to limit clusters to regions found to be significant with FDR correction previously. While this may remove unique clusters for the individual groups, the raw spmT maps are available for review on NeuroVault (https://neurovault.org/collections/3593/) All of the follow-up contrasts found in the maps were significant at p < .001. Brain regions were identified and visualized using the toolbox, xjview (http://www.alivelearn.net/xjview).
3. Results
The overarching analysis subtracted gray matter volume maps of the combined disorder group from the control group, as described above. The results from this contrast are presented in Table 2 and Fig. 1. The contrast identified bilateral clusters in superior frontal gyri, middle frontal gyri, orbitofrontal gyri, ventral medial frontal gyri, insulae, striatum, and thalami. There were right hemisphere clusters in the inferior frontal gyrus (pars orbitalis), middle occipital lobe, posterior cingulate/anterior lingual gyrus, superior temporal gyrus, and calcarine/cuneus. There were left hemisphere clusters in the middle temporal gyrus/superior temporal sulcus, inferior occipital lobe, precentral gyrus (BA 6), occipital lobe, and supramarginal gyrus. A second contrast subtracted the control group from the combined disorder group, which displayed no significant results and was not analyzed further.
Table 2.
Significant Clusters at Peak-Level from Omnibus Contrast Using FDR (Control > Disorder).
| Peak MNI Coordinate | Hemisphere | Peak Voxel Location | Number of Voxels | t-value |
|---|---|---|---|---|
| −45, −55.5, 9 | Left | Posterior Middle Temporal Gyrus and STS | 122 | 6.64 |
| 7.5, 19.5, 3 | Right | Anterior Caudate and Putamen | 746 | 5.72 |
| −12, 19.5, −6 | Left | Anterior Caudate and Putamen | 752 | 5.45 |
| 28.5, 31.5, 54 | Right | Superior Frontal Gyrus* | 1811 | 5.26 |
| 42, −1.5, −6 | Right | Insula | 413 | 5.24 |
| −7.5, −16.5, 6 | Bilateral | Thalamus | 804 | 4.84 |
| 13.5, 51, −1.5 | Right | Medial Orbital Frontal | 133 | 4.53 |
| −36, 58.5, 13.5 | Left | Middle Frontal Gyrus | 468 | 4.4 |
| −39, −90, −10.5 | Left | Inferior Occipital Gyrus | 52 | 4.4 |
| −42, 1.5, 12 | Left | Insula | 188 | 4.24 |
| − 4.5, 64.5, −22.5 | Left | Medial Superior Frontal Gyrus | 40 | 4.22 |
| − 55.5, −3, 24 | Left | Precentral Gyrus (Brodmann 6) | 94 | 4.13 |
| − 24, −78, 25.5 | Left | Occipital Lobe | 43 | 4.1 |
| 42, −79.5, 3 | Right | Middle Occipital Gyrus | 37 | 3.91 |
| − 22.5, 25.5, 60 | Left | Superior Frontal Gyrus | 25 | 3.81 |
| 4.5, −58.5, 10.5 | Right | Posterior Cingulate and Anterior Lingual | 69 | 3.66 |
| 13.5, −63, 19.5 | Right | Calcarine and Cuneus | 41 | 3.65 |
| 66, −21, 3 | Right | Superior Temporal Gyrus | 24 | 3.61 |
| −51, −43.5, 24 | Left | Supramarginal Gyrus | 27 | 3.56 |
Cluster contains the following brain regions: bilateral middle, orbitofrontal and ventral medial frontal gyri, right superior frontal gyrus, and right inferior frontal gyrus (pars orbitalis). AAL atlas and Damasio (1995) were used to help identify regions.
Fig. 1.
Overarching contrast. This figure displays the reduced gray matter found in the overarching contrast.
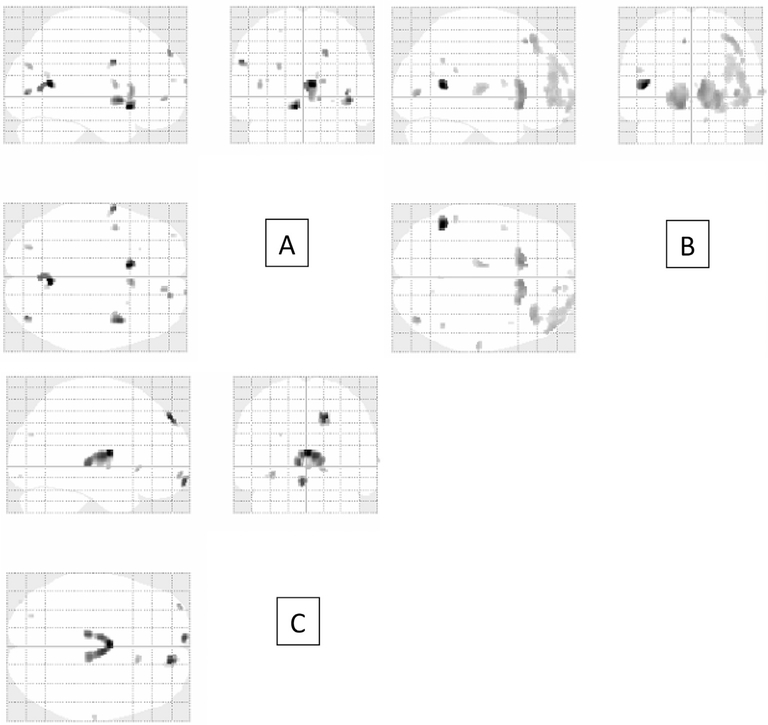
The first follow-up contrast subtracted the RD group’s gray matter maps from the control group’s maps. The regions that contributed to the overarching analysis include clusters in bilateral calcarine/lingual gyri, bilateral insulae, left caudate/subcallosal gyrus, left precentral (BA 6), right superior frontal gyrus, and right anterior caudate, along with some additional occipital clusters that were small. Cluster size and location are found in Table 3 and Fig. 2, respectively.
Table 3.
Peak Brain Regions found in Follow-up Analyses.
| Contrasts | Peak MNI Coordinate | Hemisphere | Peak Voxel Location | Number of Voxels | T-value |
|---|---|---|---|---|---|
| Control-RD | −9, 16.5, −12 | Left | Subcallosal Gyrus/Caudate | 91 | 3.86 |
| 6, −58.5, 9 | Bilateral | Calcarine/Lingual | 89 | 3.85 | |
| 42, 6, −4.5 | Right | Insula | 133 | 3.67 | |
| −58.5, 1.5, 33 | Left | Precentral (Brodmann 6) | 25 | 3.61 | |
| 19.5, 54, 39 | Right | Superior Frontal Gyrus | 20 | 3.49 | |
| 39, −81, 1.5 | Right | Middle Occipital Gyrus | 14 | 3.56 | |
| 6, 18, 3 | Right | Caudate | 68 | 3.48 | |
| 13.5, 48, −4.5 | Right | Medial Orbital | 11 | 3.4 | |
| −42, 3, 9 | Left | Insula | 18 | 3.37 | |
| −24, −79.5, 27 | Left | Superior Occiptal Lobe | 14 | 3.37 | |
| 16, 69, 12 | Right | Superior Medial Frontal | 5 | 3.37 | |
| 10.5, −61.5, 16.5 | Right | Calcarine | 7 | 3.26 | |
| Control-ADHD | −45, −55.5, 9 | Left | Middle Temporal | 121 | 5.89 |
| −12, 18, −10.5 | Left | Caudate and Putamen | 379 | 4.6 | |
| 7.5, 19.5, 3 | Right | Caudate and Putamen | 462 | 4.48 | |
| 40.5, −79.5, 1.5 | Right | Middle Occipital Gyrus | 31 | 4.19 | |
| 37.5, 31.5, 49.5 | Right | Middle Frontal Gyrus | 73 | 4.12 | |
| −9, 24, 49.5 | Left | SMA/Superior Frontal | 11 | 4.12 | |
| 48, 49.5, −4.5 | Right | Inferior Frontal, pars orbitalis | 305 | 3.93 | |
| −9, −18, 6 | Left | Thalamus | 47 | 3.86 | |
| 64.5, −21, 3 | Right | Superior Temporal | 18 | 3.75 | |
| 25.5, 64.5, −12 | Right | Superior and Middle Frontal Gyrus (orbital part) | 106 | 3.72 | |
| 13.5, 52.5, 3 | Right | Superior Medial Frontal | 26 | 3.72 | |
| 46.5, −54, 33 | Right | Angular Gyurs | 8 | 3.67 | |
| −52.5, −43.5, 22.5 | Left | Inferior Parietal (Supramarginal) | 11 | 3.61 | |
| 12, 61.5, −22.5 | Right | Superior Frontal Gyrus | 6 | 3.46 | |
| −42, 3, 12 | Left | Insula | 8 | 3.37 | |
| Control-ADHD/RD | 0, −6, 10.5 | Right and Left | Thalamus | 479 | 3.65 |
| 16.5, 51, 43.5 | Right | Superior Frontal Gyrus | 42 | 3.57 | |
| −6. 66, −15 | Left | Medial Frontal Gyrus | 33 | 3.51 | |
| −33, 61.5, −9 | Left | Middle Frontal Gyrus (orbital part) | 11 | 3.33 | |
| 15, 21, −4.5 | Right | Caudate | 12 | 3.3 |
Note: These contrasts have been masked with the omnibus contrast.
Fig. 2.
Follow-up analyses. A shows reduced gray matter in the RD group compared to controls; B shows reduced gray matter in the ADHD group compared to controls; C shows reduced gray matter in the RD/ADHD group compared to controls.
The second follow-up contrast subtracted the ADHD group’s gray matter maps from the control group’s gray matter maps (Table 3 and Fig. 2). The regions that contributed to the overarching analysis included clusters in bilateral anterior caudate and putamen, left middle temporal gyrus and STS, right middle occipital, right middle frontal, right pars orbitalis, right superior and middle frontal (orbital part), and left thalamus. Small clusters also were found in the right superior temporal and bilateral inferior parietal gyri.
The final contrast subtracted the comorbid RD/ADHD group’s gray matter maps from the control group’s maps (Table 3 and Fig. 2). The regions contributing to the overarching analysis include bilateral thalami, right superior frontal, and left medial frontal gyri. Small clusters were found in the left middle frontal gyrus (orbital part) and right anterior caudate as well.
Most of the regions found were consistent with prior literature. However, the large calcarine/lingual cluster in the RD group and the bilateral thalamic clusters in the RD/ADHD were not expected. See Fig. 3 for a plot of the contrast score distribution for the bilateral lingual cluster and Fig. 4 for a plot of the contrast score distribution for the bilateral thalamus cluster. Thus, follow-up analyses were performed to help understand our findings. As portions of the lingual area may be involved with letter-level processing (Fisher, Cortes, Griego, & Tagamets, 2012; Mechelli, Humphreys, Mayall, Olson, & Price, 2000), performance on the Colorado Perceptual Speed test (DeFries, Plomin, Vandenberg, & Kuse, 1981) was analyzed in relation to the Lingual/Calcarine cluster from the control minus RD contrast. The Colorado Perceptual Speed Test measures quick and accurate letter/number selection from similar foils. The contrast score from the bilateral Lingual/Calcarine cluster was correlated with performance on the Colorado Perceptual Speed test, r = −0.212 (p =.034). The RD/ADHD group was the only clinical group with a bilateral thalamic cluster. The thalamus has been implicated in processing speed. Hence, we examined this cluster in relation to simple reaction time to determine whether volume of this cluster was related to basic response rate. Reaction time was measured with a lab-based computer program that calculated response time for pressing the space bar to an auditory tone within a 500–2500 ms time frame. There was a significant correlation between the thalamic contrast score from the control minus RD/ADHD contrast and simple reaction time, r = −0.27 (p = .005).
Fig. 3.
Bilateral Lingual Cluster in the Control Minus RD contrast. The glass brain on the left shows the location of the bilateral lingual cluster found in the RD contrast. The graph on the right shows the distribution of contrast scores broken down by group.
Fig. 4.
Bilateral Thalamus Cluster in the Control Minus Comorbid RD/ADHD. The glass brain on the left shows the location of the bilateral thalami cluster from the RD/ADHD contrast. The graph on the right shows the distribution of contrast scores broken down by group.
4. Discussion
This study is among the first to assess the neurobiological correlates of comorbid RD/ADHD using structural imaging. Findings related to the first objective are partially consistent with two theories on the etiology of comorbid RD/ADHD—one suggesting that comorbid RD/ADHD is a unique subtype, the other suggesting that comorbidity arises from a shared etiology between the separate disorders. In terms of the second objective, findings support some prior volume-based work on the unique neurobiological contributors to RD and to ADHD, along with providing potential shared neurobiological correlates for RD and ADHD.
4.1. Reading disability
According to the dual-route model of reading, RD may arise from abnormal orthographic processing, which has been linked to the ventral circuit for reading (including the lateral extrastriate and left fusiform), and/or poor phonological processing, which has been linked to the dorsal circuit (including left temporal-inferior parietal regions; Pugh et al., 2000; Shaywitz et al., 2006). While areas consistent with the traditional dorsal route were not found, small lateral extrastriate clusters were found. Occipital regions have been found in other VBM studies on RD in middle childhood that used a basic reading definition (Eckert et al., 2005; Jednoróg et al., 2013). Further, the RD group had a significant reduction in volume compared to controls in the lingual gyrus, supporting an early ventral stream deficit. Consistent with this supposition, this cluster’s size was correlated with rapid letter processing. Areas within the bilateral occipital striate (e.g., posterior lingual) may be part of the network that is analyzing letters (Richlan et al., 2013) and feeding the ventral and dorsal routes of reading. However, since this relationship is correlational, more careful experimental design is needed to determine whether the lingual differences lead to orthographic processing weaknesses or whether environmental factors cause the orthographic weakness and the corresponding lingual reductions.
In addition to the occipital findings, there was reduced volume in bilateral caudate, bilateral insulae, and left Brodmann area 6 in the children with RD, which have been found in the VBM literature (Hoeft et al., 2007; Eckert et al., 2003, 2005; Tamboer et al., 2015), as well as in other structural MRI studies (Brown et al., 2001; Pennington et al., 1999). These areas are implicated in reading: caudate and insula are involved in reading low-frequency words (Fiebach, Friederici, Müller, & Cramon, 2002); the precentral cortex is involved in various aspects of reading and phonological processing (Brown et al., 2001; Jednoróg et al., 2013); and the insula is involved in motor planning of speech (Pennington et al., 1999). In addition, there is some suggestion that individuals with speech sound disorder (SSD) show abnormal caudate and premotor areas related to poor oral praxis (Pennington et al., 2010; Watkins, Dronkers, & Vargha-Khadem, 2002); therefore, it is possible that some speech-related deficit may be impacting the results for our sample, consistent with the motor theory of speech perception (Liberman and Mattingly, 1985). Taken together, these clusters and their purported functions coincide with the phonological route to reading, even though we did not find traditional dorsal circuit areas. Since our results suggest many, distributed brain regions are affected in RD, a detailed analysis that focuses on functional brain networks is warranted to help determine how the caudate, insula, and precentral cortex support the main brain networks for reading. The involvement of the right superior frontal gyrus in the RD group is worthy of additional exploration, suggesting potential prefrontal involvement in some individuals with RD, corresponding with what was found in the ADHD and RD/ADHD groups, as discussed subsequently.
Another point of interest from the RD results was that the left temporoparietal cortices and cerebellum were commensurate to controls. There is evidence that the temporoparietal cortex and cerebellum contribute to reading disability and the dorsal circuit of reading (e.g., Eckert, Berninger, Vaden, Gebregziabher, & Tsu, 2016; Shaywitz et al., 2006), but the following research suggests these brain regions maybe implicated only in select RD populations. Leonard, Eckert, Given, Virginia, & Eden (2006) work in a sample of children with basic reading problems but no comprehension deficits did not show reduced gray matter volume surrounding the left posterior Sylvian fissure (including temporal-inferior parietal regions), but children with both word reading and comprehension deficits did show the classic left temporoparietal reduction that is commonly found in people with reading disability. Furthermore, there is research to suggest that the cerebellum is reduced only in a subset of children with RD (Kibby et al., 2008; Leonard, et al., 2006), which may not be well represented in our sample. Our study used a basic reading problem definition of RD that did not require a reading comprehension deficit, and our results did not include brain regions that may be specific to reading comprehension deficits (Leonard et al., 2006). Instead, our results included regions commonly implicated in orthographic processing (Pugh et al., 2000; Shaywitz et al., 2006), speech and phonological processing (Jednoróg et al., 2013; Pennington et al., 2010; Watkins et al., 2002). Taken together, inter-subject heterogeneity of RD symptoms (see Tamboer’s 2015 meta-analysis), differences in operational definitions of RD (Leonard et al. 2006), languages spoken by the various samples (ours was primarily a monolingual English sample), and ages included in the various samples (many structural studies include individuals older than our sample) could contribute to the variability seen in RD brain morphometry research.
4.2. ADHD
Some of our findings are consistent with the various frontal circuits that are theorized to cause ADHD deficits (Castellanos & Proal, 2012; McAlonan, Cavanaugh, & Wurtz, 2008). We found that children with ADHD displayed reduced gray matter compared to controls in bilateral striate regions (caudate and putamen), as well as right prefrontal regions (some overlapping with the DLPFC). Thus, our findings are commensurate with the frontal-striatal circuit theory of ADHD and prior VBM studies on ADHD (Castellanos and Proal, 2012; Dang et al., 2014). These regions are consistent with the “cool” executive function deficits commonly found in this population, such as planning, working memory, cognitive inhibition, and problem solving (Castellanos and Proal, 2012; Castellanos et al., 1996; Dang et al., 2014; Monchi et al., 2007). Furthermore, the prefrontal clusters extended into right orbitofrontal regions, which is commensurate with previous research that implicates the orbitofrontal-limbic circuit in ADHD (Carmona et al., 2005; Makris et al., 2007; Seidman et al., 2011). The orbitofrontal–limbic circuit is implicated in “hot” EF including behavioral and emotional regulation (Zelazo & Cunningham, 2007) and in delay aversion (Hooper, Luciana, Conklin, & Yarger, 2004; Schulz et al., 2004), problems commonly found in ADHD (Bush, Valera, & Seidman, 2005). Thus, the ADHD group appears to be driving much of the prefrontal clusters found in the overarching analysis.
Furthermore, we found decreased gray matter volume in the left middle temporal gyrus, right superior temporal gyrus, and bilateral inferior parietal lobule in the ADHD group relative to the control group. These findings help support prior research on temporal-parietal dysfunction in ADHD. Carmona et al. (2005) found reduced gray matter in a whole-brain VBM analysis in the parietal and temporal lobes of children with ADHD, as have other studies (Castellanos et al., 2002; Kobel et al., 2010; Krain & Castellanos, 2006). The parietal and temporal lobes may play a role in attention aspects of ADHD (see the meta-analysis by Krall et al., 2015). Reduced temporal, inferior parietal and occipital gray matter may be due to delayed cortical growth in children with ADHD (Shaw et al., 2007). Children with ADHD peak in gray matter development in these regions around 10.6 years, which is later than when controls peak in gray matter development here, around 6.8 years of age. Therefore, the decreased gray matter found in children with ADHD may be from a slowed growth of the posterior cortex.
4.3. Comorbid ADHD and RD
The hypothesis regarding the gray matter morphology of the comorbid group was partially supported, as we found additive effects from both RD and ADHD. The RD/ADHD, ADHD, and RD groups all presented with a right anterior caudate cluster and reductions in the right superior frontal gyrus. Furthermore, reduced left thalamus volume was found in the ADHD group, suggesting reduced left thalamic volume may be a shared characteristic between RD/ADHD and ADHD. Nonetheless, reduced right thalamus and left medial frontal volume were unique to the comorbid group. Evidence suggests that processing speed may be a shared deficit between RD, ADHD, and RD/ADHD (Boada et al., 2012; McGrath et al., 2012), and slower processing speed has been linked to thalamic atrophy and lesions due to aging (Hong et al., 2015; Van Der Werf et al., 2001), multiple sclerosis (Batista et al., 2012) and infarcts (Van Der Werf et al., 2003). Moreover, children with attention problems (Ivanov et al., 2010) and adults with decreased learning ability (Mitchell, 2015) exhibit reduced gray matter volume in the thalamus. Given these findings, it is not unexpected for children experiencing deficits in both attention and reading (a learning problem) to have smaller thalami. The thalamic contrast score from the comorbid group contrast had a significant negative correlation with simple reaction time, supporting processing speed’s relationship to the thalamus in our sample. Processing speed deficits in children with RD/ADHD could arise from under-arousal – leading to slower and variable reaction times (Van der Meere, Stemerdink, & Gunning, 1995) – and/or potentially from working memory deficits (Jacobson et al., 2011). Future research is needed to determine whether thalamic reductions lead to processing speed weaknesses or whether environmental factors cause the processing speed weakness and corresponding thalamic reductions.
Our comorbid group did not show significant differences from our RD group in phonological or orthographic processing. Since the RD/ADHD group did not share the occipital, precentral, or insula clusters found in the RD group under current contrast constraints, perhaps the small sample size and heterogeneity of the RD/ADHD group “washed out” these effects. Sources of heterogeneity included using both the poor reader and discrepancy definitions of RD and including both ADHD-PI and ADHD-C in the sample. Therefore, future research should focus on a larger sample of individuals with comorbid RD/ADHD to address these issues.
When analyzing the theories on comorbid RD/ADHD, our results are partially consistent with two theories: (1) RD/ADHD represents a combination of shared etiologies from RD and ADHD (McGrath et al., 2012; Willcutt et al., 2001), and (2) RD/ADHD is a unique subtype separate from RD and ADHD (Rucklidge & Tannock, 2002). Regarding the first theory, we found all three clinical groups had smaller volumes in right anterior caudate and in right superior frontal regions compared to controls, and the RD/ADHD and ADHD groups showed reduced left thalamus volumes compared to controls. In terms of the unique subtype theory, the RD/ADHD group was the only group to have right thalamus and left medial frontal gyrus clusters being smaller than controls. As the contrast scores in the thalamus were signficantly correlated with simple reaction time, our findings are consistent with the work of McGrath and colleagues and Rucklidge and Tannock (2002) who suggested RD/ADHD may have additional processing speed deficits compared to RD or ADHD. Hence, our findings represent a middle ground indicating comorbid RD/ADHD may have some shared contributors with RD and ADHD, along with unique neurobiological contributors. In contrast, our results do not tend to support Gilger and Kaplan’s (2001) theory that comorbidity is due to RD and ADHD being a manifestation of the same neurodevelopmental process. Although all three clinical groups had right caudate and superior frontal clusters, RD and ADHD differed in more areas than they shared.
4.4. Contributors to RD and ADHD
Another purpose of our study was to determine potential shared neurobiological substrates to RD and ADHD given their high rate of comorbidity. Based upon our sample these include the right anterior caudate and right superior frontal gyrus, which were found in all three clinical groups. Hence, prefrontal-striatal circuit is worthy of further study as a potential source of shared etiology between RD and ADHD. Cognitive executive dysfunction and the dorsolateral prefrontal circuit have been well documented in ADHD (Castellanos and Proal, 2012; Dang et al., 2014; Monchi et al., 2007). Various studies also have demonstrated cognitive executive dysfunction in RD, especially in working memory (Baddeley & Hitch, 1994; Booth, Boyle, & Kelly, 2010; Swanson, 1999). Reduced volume or function of the frontal-striatal circuit is not as well documented in RD, however, despite evidence of executive dysfunction in this group. Thus, this is an area worthy of further research.
4.5. Limitations and future directions
A strength of our study is that it is the only one to date that examines neurobiological correlates of RD, ADHD, and their comorbidity in a single study using VBM. This is a contribution to the field given the difficulty comparing samples generated from different studies that vary in age, language spoken, ethnocultural factors, and operational definitions used. Perhaps the greatest limitation of this study was the sample size of the RD and RD/ADHD groups. The small samples in the comorbid and reading disordered groups provided lesser power in the analyses when compared to the ADHD group, perhaps masking true group differences from controls, including overlapping areas between RD and RD/ADHD. Small sample sizes in the three groups also may have contributed to why we had null findings when using FDR correction for the separate groups. Nonetheless, our sample sizes are equivalent to many in the published literature, and even large samples can yield insignificance after family-wise error corrections, perhaps due to heterogeneity within the disorders. An additional limitation is that ours is a correlational study. While it is often presumed that brain differences lead to behavioral ones and there is research to support this supposition (Nopoulos et al., 2000), it is also true that experience shapes brain formation, especially in childhood (B. A. Shaywitz et al., 2004). Hence, longitudinal research is needed to determine whether the brain differences are the causes of the disorders or the consequences of faulty environments and behavioral interactions with the environment. Another limitation of the study is that it did not test how most of the reductions in gray matter are related to behavior. Therefore, future studies could use the ROIs found in the present analysis to correlate gray matter volume with behaviors like processing speed, sustained and focused attention, hyperactivity/impulsivity, executive functioning, orthographic and phonological processing, and reading abilities in children. Specifically, a study measuring correlations between various aspects of processing speed and thalamic volume may provide further support for work conducted by McGrath et al. (2012) and Willcutt, Betjemann, Mcgrath, & Pennington (2010), as well as the present findings.
This study briefly discussed the heterogeneous nature of symptoms and etiologies associated with reading disability and ADHD. One contributor to the heterogeneity found in RD is differences in the operational definitions used to define the sample. The field would benefit from assessing gray matter volume using the different definitions of reading disability (RD) that are commonly found in the literature (e.g. discrepancy, poor reader). This is because RD due to a discrepancy may have stronger genetic contributions than RD without such a discrepancy between IQ and achievement (poor reader); further, individuals meeting the poor reader definition may have greater language impairment in areas other than phonological processing than those who meet the discrepancy definition (see Bishop & Snowling, 2004 for a review). Therefore, the findings from such studies would contribute significantly to the field of research on RD definitions and subtypes. For our study, we used a combined definition of basic reading disability that collapsed across the poor reader and discrepancy definitions. Future work should tease apart the role definition plays in the brain areas found. In addition, future studies should address the shortcomings discussed by Xia et al. (2017) and Ramus et al. (2017), such as increasing sample size and including comorbid neurodevelopmental disorders (both partially addressed in this paper) as well as the impact of language impairment or RD subtypes.
Future work also is needed on how ADHD subtypes affect morphometry results. Variability in subtypes used across studies may contribute to the disparity in findings on the neurobiological basis of the disorder. In addition, differences in proportion of subtypes between ADHD and RD/ADHD in our study may have contributed to the limited number of shared areas found, but this is unlikely as groups did not differ in proportions of ADHD-PI and ADHD-C. Another source of variability in all three conditions is that RD and ADHD are polygenetic/multi-factorial. That is, both disorders could result from differing sources of etiology as noted earlier. For example, RD may result from phonological and/or orthographic processing deficits. ADHD may result from executive functioning deficits, but some with ADHD do not have executive impairment and instead have problems in other areas such as temporal-parietal or processing speed dysfunction. Both of these issues would affect the RD/ADHD group. To address this issue, global metaanalyses should be performed in order to attain the large sample sizes needed.
In terms of the RD/ADHD group, while it is believed that this group was reliably diagnosed, it is possible that some children with RD appeared inattentive due to their academic problems and/or some with ADHD appeared to have reading problems due to their inattention, leading them to be placed in the RD/ADHD group, consistent with Pennington, Groisser, and Welsh (1993) phenocopy hypothesis of RD/ADHD. While this is unlikely (see methods) it is possible. Furthermore, more work is needed to understand if the thalamic reduction seen in the RD/ADHD group is a unique pattern or is primarily driven by ADHD symptoms, and if processing speed is the primary behavior deficit linked with thalamic reduction. Finally, it would be interesting to assess thalamic volume in a group of participants with multiple neurodevelopmental disorders, as individuals with varied comorbid neurodevelopmental disorders are commonly seen in psychological and educational settings, and they often have processing speed problems. Studying the thalamus and processing speed in a more diverse group of subjects will help us continue to address the question of whether processing speed deficits are related to thalamus volume, and whether smaller right thalamus size is specifically related to RD/ADHD or is found more broadly across other neurodevelopmental disorders.
Supplementary Material
Acknowledgements
NIH/NICHD funded data collection for this study (R03 HD048752, R15 HD065627). The opinions expressed in this article may not reflect the views of NIH/NICHD.
Footnotes
Conflicts of interest
None.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.bandl.2018.08.004.
References
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- Ashburner J (2010). VBM Tutorial, 1–14. [Google Scholar]
- Baddeley AD, & Hitch GJ (1994). Developments in the concept of working memory. Neuropsychology, 8(4), 485–493. 10.1037/0894-4105.8.4.485. [DOI] [Google Scholar]
- Barkley RA (1997). Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin, 121(1), 65–94. 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Batista S, Zivadinov R, Hoogs M, Bergsland N, Heininen-Brown M, Dwyer MG,… Benedict RHB (2012). Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. Journal of Neurology, 259(1), 139–146. 10.1007/s00415-011-6147-1. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, & Snowling MJ (2004). Developmental dyslexia and specific language impairment: same or different? Psychological Bulletin, 130(6), 858–886. 10.1037/0033-2909.130.6.858. [DOI] [PubMed] [Google Scholar]
- Black JM, Tanaka H, Stanley L, Nagamine M, Zakerani N, Thurston A,… Hoeft F. (2012). Maternal history of reading difficulty is associated with reduced languagerelated grey matter in beginning readers. Neuroimage, 59(3), 3021–3032. 10.1038/ng.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boada R, Willcutt EG, & Pennington BF (2012). Understanding the comorbidity between dyslexia and attention-deficit/hyperactivity disorder. Topics in Language Disorders, 32(3), 264–284. 10.1097/TLD.0b013e31826203ac. [DOI] [Google Scholar]
- Boder, & Jarrico (1982). The Boder Test of Reading-Spelling Patterns. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Booth JN, Boyle JME, & Kelly SW (2010). Do tasks make a difference? Accounting for heterogeneity of performance of children with reading difficulties on tasks of executive function: Findings from a meta-analysis. The British Journal of Developmental Psychology, 28(Pt 1), 133–176. 10.1348/026151009X485432. [DOI] [PubMed] [Google Scholar]
- Bosse ML, Tainturier MJ, & Valdois S (2007). Developmental dyslexia: The visual attention span deficit hypothesis. Cognition, 104(2), 198–230. 10.1016/j.cognition.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Brady SA, & Shankweiler DP (Eds.). (1991). Phonological processes in literacy: A tribute to Isabelle Y. Liberman. Hillsdale, NJ: Lawrence Erlbaurn. [Google Scholar]
- Brown WE, Eliez S, Menon V, Rumsey JM, White CD, & Reiss AL (2001). Preliminary evidence of widespread morphological variations of the brain in dyslexia. Neurology, 56, 781–783. [DOI] [PubMed] [Google Scholar]
- Bush G, Valera EM, & Seidman LJ (2005). Functional neuroimaging of attention-deficit/hyperactivity disorder: A review and suggested future directions. Biological Psychiatry, 57(11), 1273–1284. 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Carmona S, Vilarroya O, Bielsa A, Trèmols V, Soliva JC, Rovira M,… Bulbena A. (2005). Global and regional gray matter reductions in ADHD: A voxel-based morphometric study. Neuroscience Letters, 389(2), 88–93. 10.1016/j.neulet.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP,… Rapoport JL (1996). Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Archives of General Psychiatry, 53, 607–616. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS,… Rapoport JL (2002). Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/ hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 288(14), 1740–1748. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, & Proal E (2012). Large-scale brain systems in ADHD: Beyond the prefrontal- striatal model. Trends in Cognitive Sciences, 16(1), 17–26. 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, & Ziegler J (2001). DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychological Review, 108(1), 204–256. 10.1037/0033-295X.108.1.204. [DOI] [PubMed] [Google Scholar]
- Dang LC, Samanez-Larkin GR, Young JS, Cowan RL, Kessler RM, & Zald DH (2014). Caudate asymmetry is related to attentional impulsivity and an objective measure of ADHD-like attentional problems in healthy adults. Brain Structure and Function, 221(1), 277–286. 10.1007/s00429-014-0906-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mello CB, Suzart A, Rossi U, Gusmão S, Strahler T, De Moura LM,… Jackowski AP (2013). Neuroimaging and Neuropsychological Analyses in a Sample of Children with ADHD - inattentive subtype. Clinical Neuropsychiatry, 10(2), 45–54. [Google Scholar]
- DeFries JC, Plomin R, Vandenberg SG, & Kuse AR (1981). Parent-offspring resemblance for cognitive abilities in the Colorado Adoption Project: Biological, adoptive, and control parents and one-year-old children. Intelligence, 5(3), 245–277. 10.1016/S0160-2896(81)80012-8. [DOI] [Google Scholar]
- Depue BE, Burgess GC, Bidwell LC, Willcutt EG, & Banich MT (2011a). Behavioral performance predicts grey matter reductions in the right inferior frontal gyrus in young adults with combined type ADHD. Psychiatry Research - Neuroimaging, 182(3), 231–237. 10.1016/j.pscychresns.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue BE, Burgess GC, Bidwell LC, Willcutt EG, & Banich MT (2011b). Behavioral performance predicts grey matter reductions in the right inferior frontal gyrus in young adults with combined type ADHD. Psychiatry Research - Neuroimaging, 18, 231–237. 10.1016/j.pscychresns.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Berninger VW, Vaden KI, Gebregziabher M, & Tsu L (2016). Gray matter features of reading disability: A combined meta-analytic and direct analysis approach. eNeuro, 3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Richards TL, Aylward EH, Thomson J, & Berninger VW (2003). Anatomical correlates of dyslexia: Frontal and cerebellar findings. Brain, 126, 482–494. 10.1093/brain/awg026. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Wilke M, Eckert M, Richards T, Richards A, & Berninger V (2005). Anatomical signatures of dyslexia in children: Unique information from manual and voxel based morphometry brain measures. Cortex, 41, 304–315. 10.1016/S0010-9452(08)70268-5. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD, Müller K, & Cramon DYV (2002). fMRI evidence for dual routes to the mental lexicon in visual word recognition. Journal of cognitive neuroscience, 14, 11–23. 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- Fisher JE, Cortes CR, Griego JA, & Tagamets MA (2012). Repetition of letter strings leads to activation of and connectivity with word-related regions. NeuroImage, 59(3), 2939–12849. 10.1016/j.neuroimage.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, & Skokauskas N (2012). Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatrica Scandinavica, 125(2), 114–126. 10.1111/j.1600-0447.2011.01786.x. [DOI] [PubMed] [Google Scholar]
- Gilger JW, & Kaplan BJ (2001). Atypical brain development: A conceptual framework for understanding developmental learning disabilities. Developmental Neuropsychology, 20(2), 465–481. 10.1207/S15326942DN2002_2. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Ueno T, Reiss AL, Meyler A, Whitfield-Gabrieli S, Glover GH,… Gabrieli JD (2007). Prediction of children’s reading skills using behavioral, functional, and structural neuroimaging measures 54. Behav. Neurosci 121(0735–7044 (Print)), 602–613. [DOI] [PubMed] [Google Scholar]
- Hong Z, Ng KK, Sim SKY, Ngeow MY, Zheng H, Lo JC,… Zhou J (2015). Differential age-dependent associations of gray matter volume and white matter integrity with processing speed in healthy older adults. NeuroImage, 123, 42–50. 10.1016/j.neuroimage.2015.08.034. [DOI] [PubMed] [Google Scholar]
- Hooper CJ, Luciana M, Conklin HM, & Yarger RS (2004). Adolescents’ performance on the iowa gambling task: Implications for the development of decision making and ventromedial prefrontal cortex. Developmental Psychology, 40(6), 1148–1158. 10.1037/0012-1649.40.6.1148. [DOI] [PubMed] [Google Scholar]
- Hynd G, Semrud-Clikeman M, Lorys AR, Novey ES, & Eliopulos D (1990). Brain morphology in developmental dyslexia and attention deficit disorder/hyperactivity. Archives Neurology, 47(8), 919–926. 10.1001/archneur.1990.00530080107018. [DOI] [PubMed] [Google Scholar]
- Im K, Raschle NM, Smith SA, Ellen Grant P, & Gaab N (2015). Atypical sulcal pattern in children with developmental dyslexia and at-risk kindergarteners. Cerebral Cortex, 1–11. 10.1093/cercor/bhu305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I, Bansal R, Hao X, Zhu H, Kellendonk C, Miller L,… Peterson BS (2010). Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. The American Journal of Psychiatry, 167(4), 397–408. 10.1176/appi.ajp.2009.09030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LA, Ryan M, Martin RB, Ewen J, Mostofsky SH, Denckla MB, & Mahone EM (2011). Working memory influences processing speed and reading fluency in ADHD, 17(3), 209–224. 10.1080/09297049.2010.532204.Working. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jednoróg K, Gawron N, Marchewka A, Heim S, & Grabowska A (2013). Cognitive subtypes of dyslexia are characterized by distinct patterns of grey matter volume. Brain Structure and Function, L, 1–11. 10.1007/s00429-013-0595-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibby MY, & Cohen MJ (2008). Memory functioning in children with reading disabilities and/or attention deficit/hyperactivity disorder: A clinical investigation of their working memory and long-term memory functioning. Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence, 14(6), 525–546. 10.1080/09297040701821752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibby MY, Fancher JB, Markanen R, & Hynd GW (2008). A quantitative magnetic resonance imaging analysis of the cerebellar deficit hypothesis of dyslexia. Journal of Child Neurology, 23(4), 368–380. 10.1177/0883073807309235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibby MY, Kroese JM, Krebbs H, Hill CE, & Hynd GW (2009). The pars triangularis in dyslexia and ADHD: A comprehensive approach. Brain and Language, 111(1), 46–54. 10.1016/j.bandl.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klorman R, Hazel-Fernandez LA, Shaywitz SE, Fletcher JM, Marchione KE, Holahan JM,… Shaywitz BA (1999). Executive functioning deficits in attention-deficit/hyperactivity disorder are independent of oppositional defiant or reading disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 38(9), 1148–1155. 10.1097/00004583-199909000-00020. [DOI] [PubMed] [Google Scholar]
- Kobel M, Bechtel N, Specht K, Klarhöfer M, Weber P, Scheffler K,… Penner I-K (2010). Structural and functional imaging approaches in attention deficit/hyperactivity disorder: Does the temporal lobe play a key role? Psychiatry Research, 183(3), 230–236. 10.1016/j.pscychresns.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Korkman M, & Pesonen A (1994). A comparison of neuropsychological test profiles of children with attention deficit – Hyperactivity disorder and/or learning disorder. Journal of Learning Disabilities, 6(27), 383–392. 10.1177/002221949402700605. [DOI] [PubMed] [Google Scholar]
- Krain AL, & Castellanos FX (2006). Brain development and ADHD. Clinical Psychology Review, 26(4), 433–444. 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Krall SC, Rottschy C, Oberwelland E, Bzdok D, Fox PT, Eickhoff SB,… Konrad K (2015). The role of the right temporoparietal junction in attention and social interaction as revealed by ALE meta-analysis. Brain Structure and Function, 220(2), 587–604. 10.1007/s00429-014-0803-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard C, Eckert M, Given B, Virginia B, & Eden G (2006). Individual differences in anatomy predict reading and oral language impairments in children. Brain, 129(12), 3329–3342. 10.1093/brain/awl262. [DOI] [PubMed] [Google Scholar]
- Liberman AM, & Mattingly IG (1985). The motor theory of speech perception revised. Cognition, 21(1), 1–36. [DOI] [PubMed] [Google Scholar]
- Lim L, Marquand A, Cubillo AA, Smith AB, Chantiluke K, Simmons A,… Rubia K (2013). Disorder-specific predictive classification of adolescents with attention deficit hyperactivity disorder (ADHD) relative to autism using structural magnetic resonance imaging. PLoS ONE, 8(5), 1–10. 10.1371/journal.pone.0063660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkersdorfer J, Lonnemann J, Lindberg S, Hasselhorn M, & Fiebach CJ (2012). Grey matter alterations co-localize with functional abnormalities in developmental dyslexia: An ALE meta-analysis. PLoS ONE, 7(8), 10.1371/journal.pone.0043122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon GR, Fletcher JM, & Barnes MC (2003). Learning Disabilites In Mash EJ, & Barkley RA (Eds.). Child Psychopathology (pp. 520–586). (2nd ed.). New York: Guilford. [Google Scholar]
- Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN,… Seidman LJ (2007). Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cerebral Cortex, 17(6), 1364–1375. 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- Makris N, Liang L, Biederman J, Valera EM, Brown AB, Petty C,… Seidman LJ (2015). Toward Defining the Neural Substrates of ADHD: A Controlled Structural MRI Study in Medication-Naïve Adults. Journal of Attention Disorder, 2, 19(11), 944–953. 10.1007/978-1-4939-2914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, & Wurtz RH (2008). Guarding the gateway to cortex with attention in visual thalamus. Nature, 456(7220), 391–394. 10.1038/nature07382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath LM, Pennington BF, Shanahan MA, Barnard HD, Willcutt EG, & Olson RK (2012). A multiple deficit model of reading disability and attention-deficit/hyeractivity disorder. J Child Psychol Psychiatry, 52(5), 547–557. 10.1111/j.1469-7610.2010.02346.x.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Humphreys GW, Mayall K, Olson A, & Price CJ (2000). Differential effects of word length and visual contrast in the fusiform and lingual gyri during. Proceedings of the Royal Society B: Biological Sciences, 267(1455), 1909–1913. 10.1098/rspb.2000.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AS (2015). The mediodorsal thalamus as a higher order thalamic relay nucleus important for learning and decision-making. Neuroscience and Biobehavioral Reviews, 54, 76–88. 10.1016/j.neubiorev.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Mejia-Constain B, & Strafella AP (2007). Cortical activity in Parkinson’s disease during executive processing depends on striatal involvement. Brain, 130(1), 233–244. 10.1093/brain/awl326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Alcázar A, Ramos-Quiroga JA, Radua J, Salavert J, Palomar G, Bosch R,… Pomarol-Clotet E (2016). Brain abnormalities in adults with Attention Deficit Hyperactivity Disorder revealed by voxel-based morphometry. Psychiatry Research: Neuroimaging, 254, 41–47. 10.1016/j.pscychresns.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, & Sonuga-Barke EJS (2005). Causal heterogeneity in attention-deficit/hyperactivity disorder: Do we need neuropsychologically impaired subtypes? Biological Psychiatry, 57(11), 1224–1230. 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Berg S, Castellenos FX, Delgado A, Andreasen NC, & Rapoport JL (2000). Developmental brain anomalies in children with attention-deficit hyperactivity disorder. Retrieved from Journal of Child Neurology, 15(2), 102–108.. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Peterson RL, & Mcgrath LM (2008). Dyslexia In B. F. Pennington’s Diagnosing learning disorders: a neuropsychological framework (2nd ed., pp. 45–82). New York: The Gulford Press; 10.1080/19404151003731125. [DOI] [Google Scholar]
- Pennington BF, Filipek PA, Lefly D, Churchwell J, Kennedy DN, Simon JH,… DeFries JC (1999). Brain morphometry in reading-disabled twins. Neurology, 53 723–723. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Groisser D, & Welsh MC (1993). Contrasting cognitive deficits in attention deficit hyperactivity disorder versus reading disability. Developmental Psychology, 29(3), 511–523. 10.1037/0012-1649.29.3.511. [DOI] [Google Scholar]
- Pernet C, Andersson J, Paulesu E, & Demonet JF (2009). When all hypotheses are right: A multifocal account of dyslexia. Human Brain Mapping, 30(7), 2278–2292. 10.1002/hbm.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RL, & Pennington BF (2012). Seminar: Developmental Dyslexia. Lancet, 379(9830), 1997–2007. 10.1016/S0140-6736(12)60198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson E, Anckarsater H, Gillberg C, & Lichtenstein P (2013). Different neurodevelopmental symptoms have a common genetic etiology. The Journal of Child Psychology and Psychiatry, 54(12), 1356–1365. 10.1111/jcpp.12113. [DOI] [PubMed] [Google Scholar]
- Pironti VA, Lai MC, Müller U, Dodds CM, Suckling J, Bullmore ET, & Sahakian BJ (2014). Neuroanatomical abnormalities and cognitive impairments are shared by adults with attention-deficit/hyperactivity disorder and their unaffected first-degree relatives. Biological Psychiatry, 76(8), 639–647. 10.1016/j.biopsych.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Landi N, Preston JL, Mencl WE, Austin AC, Sibley D,… Frost SJ (2013). The relationship between phonological and auditory processing and brain organization in beginning readers. Brain and Language, 125(2), 173–183. 10.1016/j.bandl.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR,… Shaywitz BA (2000). Functional Neuroimaging Studies of Reading and Reading Disability (Developmental Dyslexia). Mental Retardation and Developmental Disabilities, 6(2000), 207–213. . [DOI] [PubMed] [Google Scholar]
- Ramus F, Altarelli I, Jednoróg K, Zhao J, & Scotto di Covella L (2017). Neuroanatomy of developmental dyslexia: Pitfalls and promise. Neuroscience and Biobehavioral Reviews, 84(July 2017), 434–452. 10.1016/j.neubiorev.2017.08.001. [DOI] [PubMed] [Google Scholar]
- Ramus F, Rosen S, Dakin SC, Day BL, Castellote JM, White S, & Frith U (2003). Theories of developmental dyslexia: Insights from a multiple case study of dyslexic adults. Brain, 126(4), 841–865. 10.1093/brain/awg076. [DOI] [PubMed] [Google Scholar]
- Raschle NM, Chang M, & Gaab N (2012). NIH Public Access, 57(3), 742–749. 10.1016/j.neuroimage.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, & Kamphaus RW (2004). Behavior Assessment System for Children, Second Edition (BASC-2). Circle Pines, MN: American Guidance Service. [Google Scholar]
- Richardson FM, & Price CJ (2009). Structural MRI studies of language function in the undamaged brain. Brain Structure and Function, 213(6), 511–523. 10.1007/s00429-009-0211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, & Wimmer H (2013). Structural abnormalities in the dyslexic brain: A meta-analysis of voxel-based morphometry studies. Human Brain Mapping, 34(11), 3055–3065. 10.1002/hbm.22127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K (2011). “Cool” inferior frontostriatal dysfunction in dysfunction in conduct disorder : A review. Bps, 69(12), e69–e87. 10.1016/j.biopsych.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Rucklidge JJ, & Tannock R (2002). Neuropsychological profiles of adolescents with ADHD: Effects of reading difficulties and gender. Journal of Child Psychology and Psychiatry, 43(8), 988–1003. 10.1111/1469-7610.00227. [DOI] [PubMed] [Google Scholar]
- Schulz KP, Ph D, Fan J, Tang CY, Newcorn JH, Buchsbaum MS,… Halperin JM (2004). Response inhibition in adolescents diagnosed with attention deficit hyperactivity disorder during childhood: An event-related fMRI study Kurt (September) The American Journal of Psychiatry, 1650–1657. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Biederman J, Liang L, Valera EM, Monuteaux MC, Brown A,… Makris N (2011). Gray matter alterations in adults with attention-deficit/hyperactivity disorder identified by voxel based morphometry. Biological Psychiatry, 69(9), 857–866. 10.1016/j.biopsych.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, & Makris N (2005). Structural brain imaging of attention-deficit/hyperactivity disorder. Biological Psychiatry, 57(11), 1263–1272. 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K,… Biederman J (2006). Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biological Psychiatry, 60(10), 1071–1080. 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Shanahan MA, Pennington BF, Yerys BE, Scott A, Boada R, Willcutt EG,… DeFries JC (2006). Processing speed deficits in attention deficit/hyperactivity disorder and reading disability. Journal of Abnormal Child Psychology, 34(5), 585–602. 10.1007/s10802-006-9037-8. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D,… Rapoport JL (2007). Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences, 104(49), 19649–19654. 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz BA, Lyon GR, & Shaywitz SE (2006). The role of functional magnetic resonance imaging in understanding reading and dyslexia. Developmental Neuropsychology, 30(1), 613–632. 10.1207/s15326942dn3001_5. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, & Shaywitz BA (2005). Dyslexia (specific reading disability). Biological Psychiatry, 57(11), 1301–1309. 10.1016/j.biopsych.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Blachman B, Pugh KR, Fulbright RK, Skudlarski P,… Gore JC (2004). Development of left occipitotemporal systems for skilled reading in children after a phonologically-based intervention. Biological Psychiatry, 55(7), 685–691. 10.1016/j.biopsych.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Siegel LS (1992). An evaluation of the discrepancy definition of dyslexia. Journal of Learning Disabilities, 25(10), 618–629. 10.1177/002221949202501001. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Sergeant JA, Nigg J, & Willcutt E (2008). Executive dysfunction and delay aversion in attention deficit hyperactivity disorder: Nosologic and diagnostic implications. Child and Adolescent Psychiatric Clinics of North America, 17(2), 367–384. 10.1016/j.chc.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Stanovich KE (1988). The Right and Wrong Places to Look for the Cognitive Locus of Reading Disability. Annals of Dyslexia, 38 10.1007/BF02648254. [DOI] [PubMed] [Google Scholar]
- Stein J, & Walsh V (1997). To see but not to read; the magnocellular theory of dyslexia. Trends in neurosciences, 20, 147–152. [DOI] [PubMed] [Google Scholar]
- Swank LK (1994). Phonological coding abilities: Identification of impairments related to phonologically based reading problems. Topics in Language Disorders, 14, 56–71. 10.1097/00011363-199402000-00007. [DOI] [Google Scholar]
- Swanson HL (1999). Reading comprehension and working memory in learning-disabled readers: Is the phonological loop more important than the executive system? Journal of Experimental Child Psychology, 72(1), 1–31. 10.1006/jecp.1998.2477. [DOI] [PubMed] [Google Scholar]
- Tamboer P, Scholte HS, & Vorst HCM (2015). Dyslexia and voxel-based morphometry: Correlations between five behavioural measures of dyslexia and gray and white matter volumes. Annals of Dyslexia, 65(3), 121–141. 10.1007/s11881-015-0102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremols V, Bielsa A, Soliva J, Raheb C, Carmona S, Tomas J,… Vilarroya O (2008). Differential abnormalities of the head and body of the caudate nucleus in attention deficit-hyperactivity disorder. Psychiatry Research - Neuroimaging, 163, 270–278. 10.1016/j.pscychresns.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Van der Meere J, Stemerdink N, & Gunning B (1995). Effects of presentation rate of stimuli on response inhibition in ADHD children with and without tics. Perceptual and Motor Skills, 81(1), 258–262. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Scheltens P, Lindeboom J, Witter MP, Uylings HBM, & Jolles J (2003). Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia, 41(10), 1330–1344. 10.1016/S0028-3932(03)00059-9. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Tisserand DJ, Visser PJ, Hofman PAM, Vuurman E, Uylings HBM, & Jolles J (2001). Thalamic volume predicts performance on tests of cognitive speed and decreases in healthy aging: A magnetic resonance imagingbased volumetric analysis. Cognitive Brain Research, 11(3), 377–385. 10.1016/S0926-6410(01)00010-6. [DOI] [PubMed] [Google Scholar]
- vant Ent D, Lehn H, Derks EM, Hudziak JJ, Van Strien NM, Veltman DJ, & Boomsma DI (2007). A structural MRI study in monozygotic twins concordant or discordant for attention/hyperactivity problems: Evidence for genetic and environmental heterogeneity in the developing brain. NeuroImage, 35(3), 1004–1020. 10.1007/s11103-011-9767-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemonteix T, De Brito SA, Kavec M, Baleriaux D, Metens T, Slama H,… Massat I (2015). Grey matter volumes in treatment naive vs. chronically treated children with attention deficit/hyperactivity disorder: A combined approach. European Neuropsychopharmacology, 25, 1 10.1016/j.euroneuro.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Visser J (2003). Developmental coordination disorder: A review of research on subtypes and comorbidities. Human Movement Science, 22(4–5), 479–493. 10.1016/j.humov.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Dronkers NF, & Vargha-Khadem F (2002). Behavioural analysis of an inherited speech and language disorder: Comparison with acquired aphasia. Brain, 125, 452–464. 10.1093/brain/awf058. [DOI] [PubMed] [Google Scholar]
- Wiederholt JL, & Bryant BR (2001). Gray Oral Reading Test – (Fourth Edition). Austin, TX: Pro-Ed. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, & Pennington BF (2005). Validity of the executive function theory of attention- deficit/hyperactivity disorder: A meta-analytic review. Psychiatry: Interpersonal and Biological Processes, 57(11), 1336–1346. 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Betjemann RS, Mcgrath LM, & Pennington BF (2010). Etiology and neurophysiology of comorbidity between RD and ADHD: The case for multiple deficit models, 46(10), 1345–1361. 10.1016/j.cortex.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Boada R, Ogline JS, Tunick RA, Chhabildas NA, & Olson RK (2001). A comparison of the cognitive deficits in reading disability and attention-deficit/hyperactivity disorder. Journal of Abnormal Psychology, 110(1), 157–172. 10.1037//0021-843X.110.157. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, & Mather N (2001). Woodcock-Johnson III Tests of Achievement. Itasca, IL: Riverside Publishing. [Google Scholar]
- Xia Z, Hancock R, & Hoeft F (2017). Neurobiological bases of reading disorder Part I: Etiological investigations. Linguistics and Language Compass, 11(4), 10.1111/lnc3.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Hoeft F, Zhang L, & Shu H (2016). Neuroanatomical anomalies of dyslexia: Disambiguating the effects of disorder, performance, and maturation. Neuropsychologia, 81, 68–78. 10.1016/j.neuropsychologia.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Wang P-N, Chuang K-H, Jong Y-J, Chao T-C, & Wu M-T (2008). Absence of gender effect on children with attention-deficit/hyperactivity disorder as assessed by optimized voxel-based morphometry. Psychiatry Research, 164(3), 245–253. 10.1016/j.pscychresns.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, & Cunningham WA (2007). Executive Function: mechanisms underlying emotional regulation. In Gross JJ (Ed.), Handbook of Emotion regulation, Second Edition (pp. 135–158). New York. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.