Supplemental Digital Content is Available in the Text.
A widespread upregulation of cortical GABAA receptors was observed using [18F]flumazenil positron emission tomography in fibromyalgia patients, which correlated with functioning and pain.
Keywords: Fibromyalgia, Flumazenil, PET, GABAA receptor
Abstract
An imbalance between excitatory and inhibitory neurotransmission has been linked to fibromyalgia (FM). Magnetic resonance spectroscopy has shown increased levels of glutamate in the insula and posterior cingulate cortex in FM as well as reduced insular levels of gamma-aminobutyric acid (GABA). Both of these changes have been associated with increased pain sensitivity. However, it is not clear whether excitatory and/or inhibitory neurotransmission is altered across the brain. Therefore, the aim of this study was to quantify GABAA receptor concentration on the whole brain level in FM to investigate a potential dysregulation of the GABAergic system. Fifty-one postmenopausal women (26 FM, 25 matched controls) underwent assessments of pain sensitivity, attention and memory, psychological status and function, as well as positron emission tomography imaging using a tracer for GABAA receptors, [18F]flumazenil. Patients showed increased pain sensitivity, impaired immediate memory, and increased cortical GABAA receptor concentration in the attention and default-mode networks. No decrease of GABAA receptor concentration was observed. Across the 2 groups, GABAA receptor concentration correlated positively with functional scores and current pain in areas overlapping with regions of increased GABAA receptor concentration. This study shows increased GABAA receptor concentration in FM, associated with pain symptoms and impaired function. The changes were widespread and not restricted to pain-processing regions. These findings suggest that the GABAergic system is altered, possibly indicating an imbalance between excitatory and inhibitory neurotransmission. Future studies should try to understand the nature of the dysregulation of the GABAergic system in FM and in other pain syndromes.
1. Introduction
Fibromyalgia (FM) is a chronic pain condition, characterized by widespread pain, sensory hypersensitivities, and cognitive and affective disturbances.13 An increased ratio between excitatory and inhibitory neurotransmission, measured using magnetic resonance spectroscopy (MRS) in the brain, has been suggested to contribute to FM.15,20 This perhaps explains symptom diversity through exaggerated gain setting. In support of an increased excitatory/inhibitory ratio, higher glutamate levels compared with control participants have been observed in patients with FM in the posterior insula, the posterior cingulate cortex, the ventrolateral prefrontal cortex, and the amygdala (systematically reviewed in Ref. 39), some of which changes were related to increased pain sensitivity. Patient studies on inhibitory neurotransmission are less common, but one study reported reduced GABA levels in the insula in FM.15 Animal studies support the notion that an imbalance between (increased) glutamatergic and (decreased) GABAergic neurotransmission in the insular cortex46 or the anterior cingulate cortex (ACC)54 is causally related to pain sensitivity by augmenting central pain processing.
Although useful to measure glutamate and GABA neurotransmitter levels in humans, MRS is associated with difficulties and disadvantages.1 The resonance frequency of both molecules is close to the frequencies of other metabolites, which is why glutamate is often quantified together with glutamine and GABA together with macromolecules. In addition, the in vivo concentration of GABA (1-2 mM) is at the lower end of the detectable range for MRS,42 potentially explaining why so few studies have quantified GABA levels in FM. Finally, voxel sizes in MRS are typically relatively large (on the order of several cubic centimeters), and data acquisition is relatively long, resulting in data on only 1 or perhaps 2 brain regions in most studies.
Positron emission tomography (PET) using the tracer [18F]flumazenil yields quantitative voxel-wise, whole-brain information on cortical GABAA receptors. Flumazenil binds to the benzodiazepine site of GABAA (but not GABAB) receptors, which are densely expressed at inhibitory synapses in the cortex.35 We reasoned that quantifying GABAA receptors using [18F]flumazenil PET allows for investigation of a voxel-wise index of an important part of inhibitory neurotransmission. Cortical GABAA receptor concentration was compared between patients with FM and matched controls. We investigated whether GABAA receptor concentration is related to clinical characteristics of FM, including pain, sensitivity to mechanical stimuli, and functional, affective, and cognitive function. This work is a secondary analysis of a data set we have previously published on Ref. 38.
2. Methods
2.1. Participants
Twenty-six postmenopausal women with FM were included, with the diagnosis confirmed by an experienced rheumatologist (M.-A.F.) according to the 2012 Canadian Guidelines for diagnosis and management of FM.13 Thus, patients with FM had to have chronic widespread pain for at least 3 months that was not explained by any other cause as well as sleep disturbances, fatigue, cognitive complaints, and/or other somatic symptoms and mood disorders to variable degrees. A group of 25 control participants was matched at the group level to the FM group for age, body mass index, education level, income, and physical activity level (based on the short version of the International Physical Activity Questionnaire7). The McGill Institutional Review Board approved the study (certificate A08-M75-11B), and participants gave written informed consent before inclusion. Exclusion criteria for both groups were pain conditions other than FM, uncontrolled medical conditions, any diagnosed psychiatric or neurological disorders, body mass index greater than 30 kg/m2, and alcohol intake more than 15 glasses/week. There was no significant difference in alcohol intake between groups (controls had on average 2.7 ± 4 units/week and FM patients 1.7 ± 3 units/week, P > 0.3). Participants using benzodiazepine medication more than once a week were excluded. Participants using benzodiazepines occasionally (4 patients once a week, 2 patients biweekly) were off medication for at least 48 hours before the PET scan to avoid competitive binding with the radiotracer. Additional details on participants can be found in our previous report.38
2.2. Data acquisition and image processing
Participants took part in three 1.5-hour long sessions: one psychophysical/questionnaire session, one magnetic resonance imaging (MRI) session, and one PET session. Thirty-eight participants out of 51 had the 3 sessions within a 2-week period, 9 within 1 month, and 4 within 3 months.
The psychophysical/questionnaire session served to assess different diagnostic domains of FM, specifically pain sensitivity, affective disturbances through questionnaires, and cognitive function through 2 validated cognitive tasks. Participants were asked to mark their current pain level on an 11-point numeric scale ranging from 0 (no pain) to 10 (worst bearable pain) and to complete questionnaires on depression (Beck Depression Inventory2), anxiety (Hospital Anxiety and Depression Scale53), and functional status (Fibromyalgia Impact Questionnaire [FIQ],3 patients with FM were instructed to refer to their clinical pain, and controls were given no specific instructions). Cognitive function of participants was assessed using the Attention Network Task10 and the Auditory Consonant Trigram test45 because it has been shown that memory and attention are the most affected cognitive domains in FM.50 Finally, pressure pain threshold and pressure pain tolerance were assessed using a calibrated hand-held pressure algometer with a 1-cm diameter round tip applied at constant rate on the thumbnail of the nondominant hand. Participants verbally indicated when the first sensation of pain occurred (pain threshold) and when the pain became intolerable (pain tolerance level). The average pressure of 3 assessments was used as pain threshold and tolerance threshold, respectively.
In the MRI session, a structural scan was acquired for coregistration of PET images. T1-weighted images were acquired using a 3-Tesla Tim Trio Siemens MRI scanner (Siemens, Erlangen, Germany) with a 12-channel head coil and a 3D magnetization-prepared rapid acquisition by gradient echo sequence (repetition time 2300 ms, echo time 2.98 ms, flip angle 9°, field of view 256 mm, 192 slices in the sagittal plane, resolution 1 × 1 × 1 mm, acquisition time: 10 minutes). Images from other MRI sequences were acquired, but the results are not presented here (see Ref. 38 for more details).
In the PET session, data were acquired using an ECAT High-Resolution Research Tomograph (Siemens Medical Solution, Knoxville, TN), which has a spatial resolution of 2.3 to 3.4 mm at full width at half maximum. The radiopharmaceutical [18F]flumazenil was synthesized as published previously.29 After a transmission scan for attenuation correction (137Cs-source), approximately 370 MBq of [18F]flumazenil was injected intravenously as a slow bolus over 60 seconds. List-mode data were acquired for 60 minutes after injection and were subsequently binned into fully 3D sinograms for a total of 17 time-frames (40, 20, 2 × 30, 3 × 60, 4 × 150, 3 × 300, and 3 × 600 seconds). Raw PET images were reconstructed by fully 3D-filtered back projection by a 3D-reprojection method and corrected for participants' head motion. The nondisplaceable binding potential (BPND) maps were computed because they represent the signal arising from the fraction of radiotracer that is specifically bound to the benzodiazepine site of GABAA receptors, using the idSURF method with the eroded white-matter segments as reference region.17 Higher specific binding of [18F]flumazenil can be due to higher receptor concentration or greater ligand affinity.23 Because there is no evidence for GABAA receptors to be structurally different in FM, we refer to BPND values as GABAA receptor concentration in the remainder of the article. Please note that subcortical regions are not well represented in the gray-matter mask because the pipeline was specifically designed for cortical gray matter. Resulting BPND maps in the Montreal Neurological Institute standard space (ICBM15230) was spatially blurred with a 7 × 7 × 7-mm full width at half maximum Gaussian smoothing kernel.
2.3. Statistical analyses
Pressure pain thresholds and tolerance, questionnaire scores, and performance in the attention network task were analyzed using independent-sample two-sided t-tests to compare patients with FM and control participants. A two-way repeated-measures analysis of variance was used to assess the interaction between performance for the different recall delays (within-subject factor Recall delay, 4 levels) and Group (2 levels: patients and controls) in the Auditory Consonant Trigram task, followed by pair-wise post hoc tests.
Whole-brain, voxel-wise statistical analysis was performed in SPM8 (revision 4667, Wellcome Trust Centre for Neuroimaging); a general linear model was applied to the BPND maps to (1) compare FM patients with control participants, including age as a covariate of no interest and (2) investigate voxel-wise regressions with clinical scores and cognitive performance, including age as a covariate of no interest. For statistical inference, a voxel-based threshold of P < 0.001 uncorrected and a cluster extent threshold of k > 53, calculated in SPM8 based on the smoothness of the image and random-field theory,49 were used. Voxel-wise regression analyses were considered exploratory in nature, and therefore, we did not correct for the number of regressions tested.
3. Results
3.1. Participants
Patients and control participants were well matched: no significant group differences were observed for age, body mass index, education level, income, and physical activity level (Table 1).
Table 1.
Participants' demographic and clinical data.
3.2. Increased pain sensitivity in patients with FM
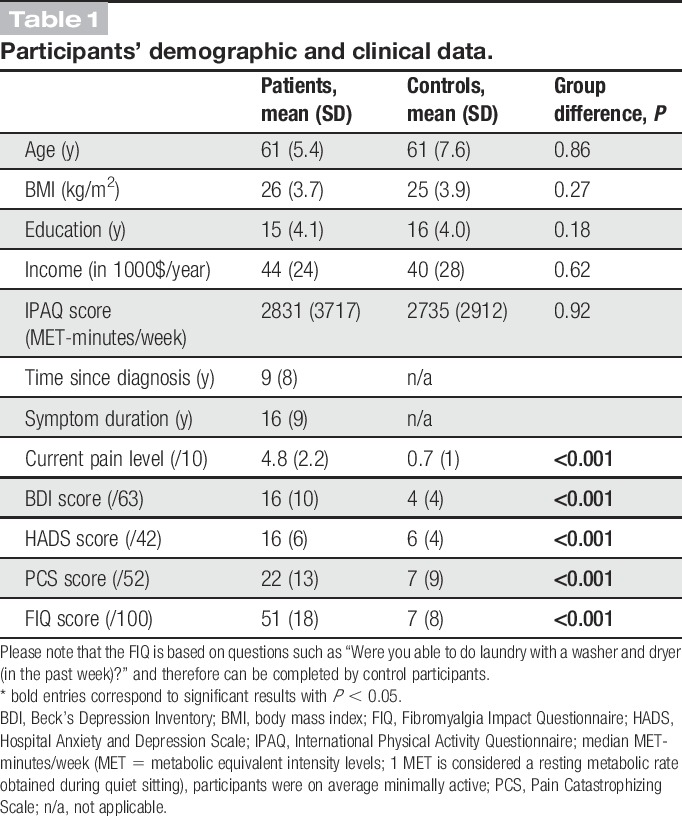
Compared with control participants, patients with FM had higher current pain levels (mean ± SD, controls: 0.7 ± 0.9, FM: 4.7 ± 2.2, T = 8.8, P < 0.0001) and were more sensitive to pressure stimuli (pain threshold, controls: 5.3 ± 1.8 kg, FM: 3.8 ± 1.5 kg, T = −3.4, P = 0.0016; pain tolerance, controls: 8.4 ± 2.0 kg, FM: 6.9 ± 2.0 kg, T = −2.6, P = 0.0126). Furthermore, patients with FM had higher scores on the anxiety and depression scales (HAD_total, controls: 6.2 ± 4.1, FM: 15.1 ± 6.2, T = 5.52 P < 0.0001; HAD_A, controls: 4.7 ± 3.3, FM: 8.8 ± 3.1, T = 3.9, P < 0.0001; HAD_D, controls: 1.5 ± 1.2, FM: 6.3 ± 4.3, T = 4.7, P < 0.0001; BDI, controls: 3.6 ± 3.6, FM: 16.2 ± 10.0, T = 31.5, P = 0.0001), on the pain catastrophizing scale (controls: 6.8 ± 8.6, FM: 22.3 ± 13.0, T = 43.6, P < 0.0001) and the FIQ (controls: 7.0 ± 8.1, FM: 50.5 ± 17.9, T = 11.2, P < 0.0001) compared with control participants (Fig. 1). It is important to note that because the FIQ was administered without specific instructions to the elderly control participants, their scores likely reflect the impact of some sort of pain on their daily function. Indeed, some of the controls indicated some pain from, eg, osteoarthritis in the initial interview.
Figure 1.
Mean pressure pain sensitivity and mean questionnaire scores for each group. Patients with fibromyalgia have lower pressure pain threshold and tolerance levels compared with control participants. Patients with fibromyalgia have higher pain, depression and anxiety scores, catastrophizing scores, and fibromyalgia impact scores compared with control participants. BDI, Beck depression inventory; CPL, current pain level; FM, patients with fibromyalgia; FIQ, Fibromyalgia Impact Questionnaire; HAD, hospital anxiety and depressions scale; PCS, pain catastrophizing scale; ***P < 0.001, **P < 0.005, *P < 0.05.
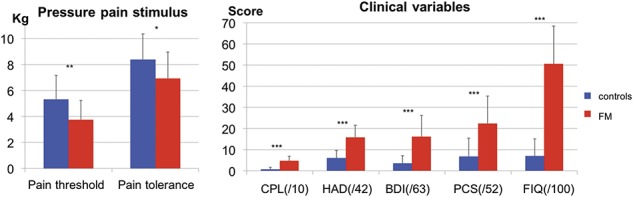
3.3. Delayed recall is affected but not attention in the FM sample
The Auditory Consonant Trigram test was used to assess immediate memory, and results showed that, in accordance with previous studies,45 when a delay was introduced, patients with FM recalled a significantly smaller number of consonant letters compared with control participants. This is shown by the statistically significant interaction between Group and Recall Delay (F(3, 147) = 107.8, P = 0.034, Fig. 2 left panel). Post hoc pair-wise comparisons showed that the number of consonant letters recalled was significantly smaller in patients with FM at the 9-second delay (controls: 12.6 ± 2.8, FM: 10.7 ± 3.3, T = 2.2, P = 0.032), 18-second delay (controls: 10.6 ± 3.0, FM: 8.8 ± 2.9, T = 2.3, P = 0.028), and 36-second delay (controls: 11.4 ± 2.4, FM: 9.5 ± 2.9, T = 2.6, P = 0.013), but no difference was observed at immediate recall (controls: 15.0 ± 0.0, FM: 14.9 ± 0.3, T = 2.6, P = 0.13).
Figure 2.
Impaired immediate memory in patients with fibromyalgia. Left panel, patients with fibromyalgia recalled fewer consonant letters compared with control participants as soon as a recall delay was introduced. Patients and control participants were both able to recall trigrams with no delay, but the longer the delay between test and recall (higher difficulty), the lesser the patients could recall the trigrams, which show a significant impairment of immediate memory in patients with fibromyalgia. Right panel, no difference was observed in the performance on the attention network task between patients with fibromyalgia and control participants. FM, patients with fibromyalgia; NS, nonsignificant, *P < 0.05.
In the Attention Network Task, there was no significant difference between groups in reaction time for the different networks: conflict (controls: 90.4 ± 30.26, FM: 108.77 ± 48.95, T = −1.6, P = 0.115, Fig. 2, right panel), orienting (controls: 22.00 ± 25.26, FM: 24.23 ± 28.13, T = −0.3, P = 0.767), or alerting (controls: 4.76 ± 21.78, FM: 3.96 ± 21.16, T = 0.1, P = 0.895). The number of errors neither differed significantly between groups (controls: 1.8 ± 2.5, FM: 2.2 ± 2.4, T = −0.57, P = 0.572).
3.4. Upregulation of cortical GABAA receptor concentration in FM
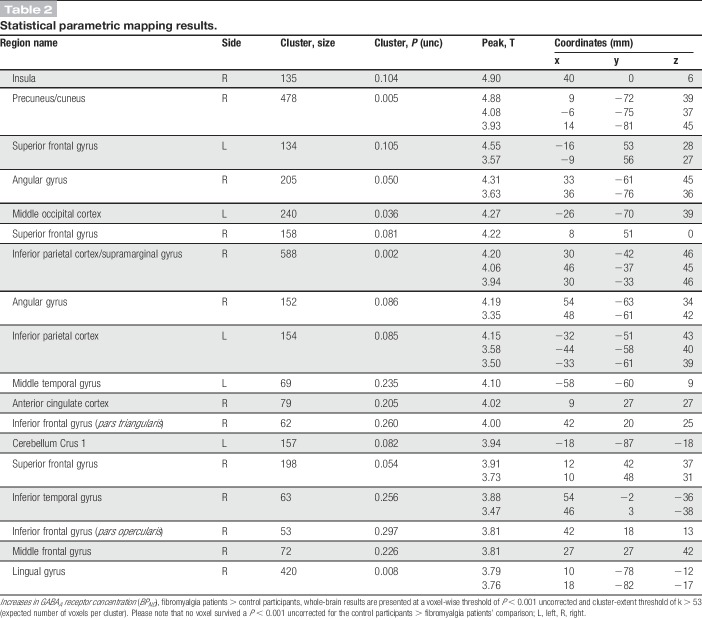
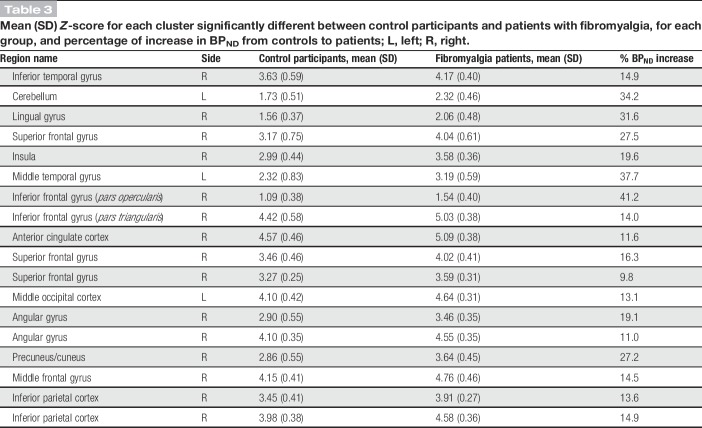
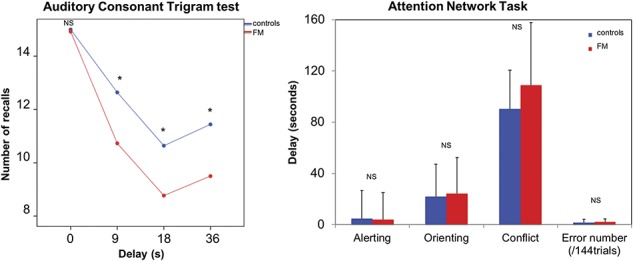
In controls, the distribution of GABAA receptors was similar to what has been previously reported in healthy subjects with the highest concentrations in the visual cortex (Fig. 3).28,29,47 Six clusters showed higher GABAA receptor concentration in patients with FM compared with controls, including the right precuneus/cuneus, superior frontal gyrus, right angular gyrus, middle occipital cortex, inferior parietal/supramarginal gyrus, and lingual gyrus (Fig. 4 and Table 2). Mean cluster BPND was 11% to 31% higher in patients with FM compared with control participants (Table 3). No region showed significantly lower GABAA receptor concentration in patients compared with controls.
Figure 3.
Mean BPND map per group. Whole-brain flumazenil BPND map representing GABAA receptor concentration in control participants (left panel) and in patients with fibromyalgia (right panel). Axial images are displayed in neurological convention, ie, the left hemisphere is on the left.
Figure 4.
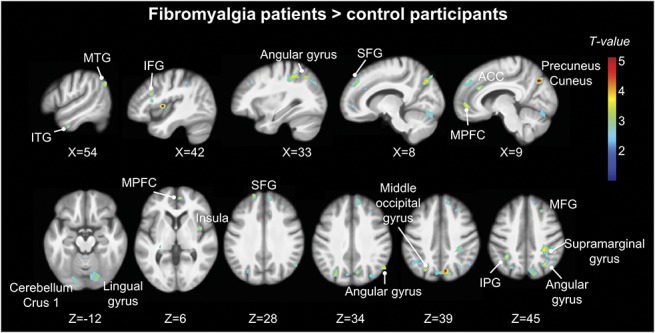
Higher GABAA receptor concentration in patients with fibromyalgia. Whole-brain map of higher GABAA receptor concentration (flumazenil BPND) in patients compared with control participants. T-values are presented at a voxel-wise threshold of P < 0.001 and cluster-extent threshold of k > 53, overlaid on the mean anatomical image of the whole sample (N = 51). ACC, anterior cingulate cortex; IPG, inferior parietal gyrus; ITG, inferior temporal gyrus; MFG, medial frontal gyrus; MPFC, medial prefrontal cortex; MTG, middle temporal gyrus; SFG, superior frontal gyrus. Axial images are displayed in neurological convention, ie, the left hemisphere is on the left.
Table 2.
Statistical parametric mapping results.
Table 3.
Mean (SD) Z-score for each cluster significantly different between control participants and patients with fibromyalgia, for each group, and percentage of increase in BPND from controls to patients; L, left; R, right.
3.5. GABAA receptor concentration is positively associated with clinical scores
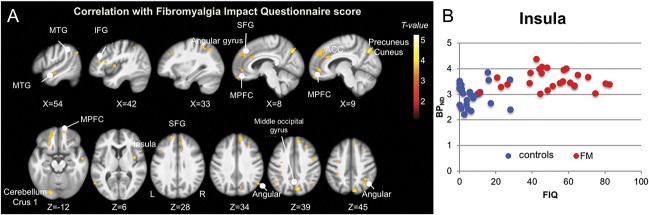
GABAA receptor concentration was associated with functional impairment across the 2 groups, as shown by the whole-brain regression analysis of flumazenil BPND onto FIQ scores. The pattern for this association overlaps with regions showing increased GABAA receptor concentration in patients (Fig. 5).
Figure 5.
(A) Regression between GABAA receptor concentration and Fibromyalgia Impact Questionnaire (FIQ) scores. Whole-brain map of the positive association between GABAA receptor concentration (flumazenil BPND) and FIQ scores across all subjects. T-values are presented at a voxel-wise threshold of P < 0.001 and cluster-extent threshold of k > 53, overlaid on the mean anatomical image of the whole sample (N = 51). Axial images are displayed in neurological convention, ie, the left hemisphere is on the left. (B) Scatter plot of GABAA receptor concentration extracted from the insular cluster and FIQ score. The insular cluster was chosen because of the highest T-value in the group comparison; the scatter plot only serves to illustrate the positive association of flumazenil BPND and FIQ score for the whole sample. FM, patients with fibromyalgia.
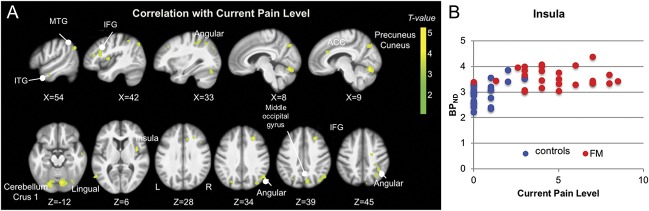
GABAA receptor concentration was also associated with participants' pain, as shown by the whole-brain regression analysis between GABAA receptor concentration and current pain level. The pattern for this association overlaps with regions showing increased GABAA receptor concentration in patients (Fig. 6).
Figure 6.
(A) Regression between GABAA receptor concentration and current pain level (CPL). Whole-brain map of the positive association between GABAA receptor concentration (flumazenil BPND) and current pain level across all subjects. T-values are presented at a voxel-wise threshold of P < 0.001 and cluster-extent threshold of k > 53, overlaid on the mean anatomical image of the whole sample (N = 51). Axial images are displayed in neurological convention, ie, the left hemisphere is on the left. (B) Scatter plot of GABAA receptor concentration extracted from the insular cluster and current pain level. The insular cluster was chosen because of the highest T-value in the group comparison; the scatter plot only serves to illustrate the positive association between flumazenil BPND and current pain level for the whole sample. FM, patients with fibromyalgia.
GABAA receptor concentration in the hippocampus (Montreal Neurological Institute coordinates: −28, −19, −9, pclus < 0.001, k > 53) was negatively associated with participant's performance in the Auditory Consonant Trigram memory task (recall delay of 18 seconds).
There was no significant whole-brain correlation with attentional measures, pressure pain thresholds, or tolerance levels.
4. Discussion
In this study, we demonstrated for the first time widespread upregulation of GABAA receptors in patients with FM using [18F]flumazenil PET to quantify GABAA receptor concentration. We showed that the binding potential of flumazenil was more than 10% higher in FM compared with control participants, reflecting a significant increase in GABAA receptor concentration. There was no significant GABAA receptor concentration downregulation in patients with FM. The observed change is almost certainly driven by neuronal GABAA receptors because the concentration of GABAA receptor mRNA in neurons is nearly 2 magnitudes greater than in astrocytes.16 However, it is unlikely to be driven by increased concentration of neurons in the gray matter because we also observed GABAA receptor upregulation in regions without any significant gray-matter change.38 Because GABA is the most abundant neurotransmitter in the brain and is involved in a myriad of brain functions, an upregulation of GABAA receptors across multiple brain regions could have important consequences. More specifically, GABAA (and not GABAB) receptors contribute to cortical levels of excitability as demonstrated with short-interval intracortical inhibition.31,44,52 In rodent chronic pain models, increased neuronal excitability and decreased inhibition have been demonstrated in the spinal cord6 and in the brain, mainly in the ACC,51 that were positively associated with pain sensitivity.22 The present results are therefore in line with previous reports suggesting an imbalance between neuronal excitation and inhibition in FM and other chronic pain conditions.48
There are several mechanisms that might underlie the observed increase in GABAA receptor concentration. Common to all explanations is a decrease in GABA neurotransmitter concentration: a decrease in GABA neurotransmitter would lead to a compensatory increase in receptor concentration18 that we observed in FM. However, the increase in receptor concentration would still be insufficient because symptoms persist in FM. The notion of decreased GABA levels has been supported in the insula in diabetic neuropathy36 and has been associated with higher pain levels in the ACC in knee osteoarthritis.40 This is also supported by another PET study showing that flumazenil BPND correlated inversely with plasma levels of GABA.25 GABA levels could be decreased, for example, by a primary defect in (one of) the enzyme(s) that converts glutamate into GABA, ie, glutamic acid decarboxylase,14 which would increase glutamate concentration and result in lower GABA concentration. As outlined in the introduction, increased glutamate levels have indeed been observed in FM and other chronic pain conditions.11,15,20 Alternatively, GABAA receptors might become excitatory instead of being inhibitory21,41 because of a change in the neuron's depolarization pattern and as has been described for the spinal cord in rodent models of chronic pain.4 In this scenario, GABA neurotransmitter production would be reduced to compensate for the switch from inhibition to excitation.
As described above, decreased GABA levels in chronic pain have been observed in certain brain regions using MRS, specifically the insula and the ACC. Presumably, these areas were investigated because they are important pain-processing regions.33,37,43 Our study replicates the findings of increased GABAA receptor concentration in the ACC and the insula. However, because it afforded a whole-brain analysis approach, impossible with MRS, increases in GABAA receptor concentration were observed in additional brain regions. The regions with increased GABAA receptor concentration seem to belong to 2 main brain networks: the attention network (superior frontal gyrus and supramarginal gyrus) and the default-mode network (precuneus and medial prefrontal cortex). Interestingly, enhanced connectivity between the medial prefrontal cortex, a key node of the default-mode network, and other regions of the default-mode network has been linked to patients' degree of rumination about their pain.27 Furthermore, intrinsic connectivity within the default-mode network has been observed to be inversely related to GABA levels in the default-mode network.24 Therefore, our findings of increased GABAA receptor concentration in the default-mode network and perhaps the attention network might reflect the hypervigilance and impaired disengagement from pain commonly observed in FM and other chronic pain conditions.13 However, it should be noted that we did not observe a (linear) relationship between GABAA receptor concentration and catastrophizing scores or performance in the attention network task, which should at least partly be related to hypervigilance and impaired disengagement. Nevertheless, increased GABAA receptor concentration was positively correlated with functional status (FIQ score) and current pain levels, and negatively correlated, in the hippocampus, with performance in the memory task, indicating that there likely is a clinical relevance of altered receptor levels. This notion is supported by the observation that intracortical inhibition, as measured with short-interval intracortical inhibition, is reduced in FM and correlates with fatigue.32
It is important to point out that because the correlations observed between GABAA levels and clinical measure are present only across groups, it does not appear that an imbalance between increased excitation and decreased inhibition is specific to FM but might be a common feature across different types of chronic pain. Indeed, increased insular glutamate levels have been reported in painful diabetic neuropathy36 and migraine.5 Decreased GABA levels have been reported in the insula in painful diabetic neuropathy,36 and GABA levels have been associated with higher pain levels in the ACC in knee osteoarthritis.40 Also, in this study, the scatter plots of the correlations of GABAA receptor concentration and FIQ scores, respectively, current pain levels, clearly show that some degree of functional impairment/current pain in the controls (eg, back pain, osteoarthritis, and ankle pain) contributed to the significant correlation. Thus, it is unlikely that dysregulation of excitatory and inhibitory neurotransmitter systems constitutes the etiology of FM or of any other pain condition for which increased glutamate/decreased GABA has been reported. Rather, it might be a factor contributing to the maintenance of various symptoms across different chronic pain conditions. Importantly, it might be a factor that can be therapeutically targeted. For example, pregabalin, which blocks the voltage-dependent calcium channel and involved in glutamate release through an increase in Ca2+ influx,8,12 has been shown to reduce glutamate levels in the posterior insula of patients with FM,19 and memantine, an NMDA receptor antagonist, improved symptoms in FM compared with placebo.34
5. Limitations
This study has some limitations that should be noted. Although using [18F]flumazenil PET to measure GABAA receptors has the advantage that it allows whole-brain (cortical) quantification, it does not directly measure GABA levels. Nevertheless, the observed GABAA receptor alterations indicate some sort of dysregulation of the GABAergic system, perhaps resulting from reduced GABA levels. To truly comment on an altered imbalance between increased excitatory and decreased inhibitory neurotransmission, concentrations of inhibitory and excitatory neurotransmitters ought to be measured. Furthermore, GABAA receptor concentration was not measured in subcortical areas or in the spinal cord, and GABAB receptor levels cannot be assessed with flumazenil. This limits our interpretation of the present results as different neurotransmitter systems in different regions could interact to result in the observed increase in cortical GABAA receptor levels. In addition, measuring GABAA receptors in vivo in humans is a coarse measurement and does not reveal subcellular or molecular processes. Indeed, different diseases have been related to decreased GABA levels in the brain (reviewed in Ref. 42) that might be related to different pathophysiological mechanisms. However, macro-level findings often seem to reflect finer metrics of GABA function and provide a link between up-stream biochemistry and macro-level behavioral function.9,42 In addition, there could be instances in which it is sufficient or even advantageous to target more down-stream processes, for example, because of redundant or compensatory neurobiological pathways. In support of this notion, (spinal) administration of GABAergic compounds shows considerable promise to reverse pain phenotypes in animal models.26
6. Conclusion and perspectives
We show that cortical GABAA receptor concentration is upregulated in FM and associated with pain levels and function. This upregulation occurs in a widespread network of brain regions and is not restricted to typical pain-processing regions. This finding adds evidence to the imbalance between excitatory and inhibitory neurotransmission hypothesis, which seems to apply to different chronic pain conditions. Future studies should test for glutamate and GABA neurotransmitter levels as well as the expression of receptors in the same patients, ideally in longitudinal designs, to further investigate the imbalance hypothesis.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplemental digital content
A video abstract accompanying this article can be found at http://links.lww.com/PAIN/A879.
Supplementary Material
Acknowledgments
This work was supported by a competitive Pfizer Neuropathic Pain Award.
The authors thank Lina Naso for interviewing and testing the participants, Alysha Ahmed for her help with participant recruitment, and the personnel at the McConnell Brain Imaging Centre for expert support of MR and PET imaging. The authors thank the participants for taking part in the study.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Alger JR. Quantitative proton magnetic resonance spectroscopy and spectroscopic imaging of the brain. Top Magn Reson Imaging 2010;21:115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–71. [DOI] [PubMed] [Google Scholar]
- [3].Bennett RR. The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol 2005;23:S154–62. [PubMed] [Google Scholar]
- [4].Bravo-Hernández M, Corleto JA, Barragán-Iglesias P, González-Ramírez R, Pineda-Farias JB, Felix R, Calcutt NA, Delgado-Lezama R, Marsala M, Granados-Soto V. The α5 subunit containing GABAA receptors contribute to chronic pain. PAIN 2016;157:613–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bridge H, Stagg CJ, Near J, Lau CI, Zisner A, Cader MZ. Altered neurochemical coupling in the occipital cortex in migraine with visual aura. Cephalalgia 2015;35:1025–30. [DOI] [PubMed] [Google Scholar]
- [6].Cervero F. Spinal cord hyperexcitability and its role in pain and hyperalgesia. Exp Brain Res 2009;196:129–37. [DOI] [PubMed] [Google Scholar]
- [7].Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95. [DOI] [PubMed] [Google Scholar]
- [8].Cunningham MO, Woodhall GL, Thompson SE, Dooley DJ, Jones RSG. Dual effects of gabapentin and pregabalin on glutamate release at rat entorhinal synapses in vitro. Eur J Neurosci 2004;20:1566–76. [DOI] [PubMed] [Google Scholar]
- [9].Duncan NW, Wiebking C, Northoff G. Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans—a review of multimodal imaging studies. Neurosci Biobehav Rev 2014;47:36–52. [DOI] [PubMed] [Google Scholar]
- [10].Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci 2002;14:340–7. [DOI] [PubMed] [Google Scholar]
- [11].Fayed N, Andres E, Rojas G, Moreno S, Serrano-Blanco A, Roca M, Garcia-Campayo J. Brain dysfunction in fibromyalgia and somatization disorder using proton magnetic resonance spectroscopy: a controlled study. Acta Psychiatr Scand 2012;126:115–25. [DOI] [PubMed] [Google Scholar]
- [12].Fink K, Dooley DJ, Meder WP, Suman-Chauhan N, Duffy S, Clusmann H, Göthert M. Inhibition of neuronal Ca2+ influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology 2002;42:229–36. [DOI] [PubMed] [Google Scholar]
- [13].Fitzcharles MA, Ste-Marie PA, Goldenberg DL, Pereira JX, Abbey S, Choinière M, Ko G, Moulin DE, Panopalis P, Proulx J, Shir Y; National Fibromyalgia Guideline Advisory Panel. 2012 Canadian Guidelines for the diagnosis and management of fibromyalgia syndrome: executive summary. Pain Res Manag 2013;18:119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fitzgerald CT, Carter LP. Possible role for glutamic acid decarboxylase in fibromyalgia symptoms: a conceptual model for chronic pain. Med Hypotheses 2011;77:409–15. [DOI] [PubMed] [Google Scholar]
- [15].Foerster BR, Petrou M, Edden RAE, Sundgren PC, Schmidt-Wilcke T, Lowe SE, Harte SE, Clauw DJ, Harris RE. Reduced insular γ-aminobutyric acid in fibromyalgia. Arthritis Rheum 2012;64:579–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fraser DD, Duffy S, Angelides KJ, Perez-Velazquez JL, Kettenmann H, MacVicar BA. GABAA/benzodiazepine receptors in acutely isolated hippocampal astrocytes. J Neurosci 1995;15:2720–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Funck T, Paquette C, Evans A, Thiel A. Surface-based partial-volume correction for high-resolution PET. NeuroImage 2014;102:674–87. [DOI] [PubMed] [Google Scholar]
- [18].Harris JB. Denervation supersensitivity—some pharmacological curiosities. Trends Neurosci 1980;3:224–5. [Google Scholar]
- [19].Harris RE, Napadow V, Huggins JP, Pauer L, Kim J, Hampson J, Sundgren PC, Foerster B, Petrou M, Schmidt-Wilcke T, Clauw DJ. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology 2013;119:1453–64. [DOI] [PubMed] [Google Scholar]
- [20].Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, Clauw DJ. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum 2009;60:3146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Huang YJ, Grau JW. Ionic plasticity and pain: the loss of descending serotonergic fibers after spinal cord injury transforms how GABA affects pain. Exp Neurol 2018;306:105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hubbard CS, Khan SA, Xu S, Cha M, Masri R, Seminowicz DA. Behavioral, metabolic and functional brain changes in a rat model of chronic neuropathic pain: a longitudinal MRI study. NeuroImage 2015;107:333–44. [DOI] [PubMed] [Google Scholar]
- [23].Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang S-C, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007;27:1533–9. [DOI] [PubMed] [Google Scholar]
- [24].Kapogiannis D, Reiter DA, Willette AA, Mattson MP. Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. NeuroImage 2013;64:112–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Klumpers UMH, Veltman DJ, Drent ML, Boellaard R, Comans EFI, Meynen G, Lammertsma AA, Hoogendijk WJG. Reduced parahippocampal and lateral temporal GABAA-[11C]flumazenil binding in major depression: preliminary results. Eur J Nucl Med Mol Imaging 2010;37:565–74. [DOI] [PubMed] [Google Scholar]
- [26].Knabl J, Zeilhofer UB, Crestani F, Rudolph U, Zeilhofer HU. Genuine antihyperalgesia by systemic diazepam revealed by experiments in GABAA receptor point-mutated mice. PAIN 2009;141:233–8. [DOI] [PubMed] [Google Scholar]
- [27].Kucyi A, Moayedi M, Weissman-Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J Neurosci 2014;34:3969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].la Fougère C, Grant S, Kostikov A, Schirrmacher R, Gravel P, Schipper HM, Reader A, Evans A, Thiel A. Where in-vivo imaging meets cytoarchitectonics: the relationship between cortical thickness and neuronal density measured with high-resolution [18F]flumazenil-PET. NeuroImage 2011;56:951–60. [DOI] [PubMed] [Google Scholar]
- [29].Massaweh G, Schirrmacher E, la Fougère C, Kovacevic M, Wängler C, Jolly D, Gravel P, Reader AJ, Thiel A, Schirrmacher R. Improved work-up procedure for the production of [18F]flumazenil and first results of its use with a high-resolution research tomograph in human stroke. Nucl Med Biol 2009;36:721–7. [DOI] [PubMed] [Google Scholar]
- [30].Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, Holmes C, Collins L, Thompson P, MacDonald D, Iacoboni M, Schormann T, Amunts K, Palomero-Gallagher N, Geyer S, Parsons L, Narr K, Kabani N, Le Goualher G, Boomsma D, Cannon T, Kawashima R, Mazoyer B. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping ICBM. Philos Trans R Soc Lond B Biol Sci 2001;356:1293–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].McDonnell MN, Orekhov Y, Ziemann U. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res 2006;173:86–93. [DOI] [PubMed] [Google Scholar]
- [32].Mhalla A, Baudic S, Ciampi de Andrade D, Gautron M, Perrot S, Teixeira MJ, Attal N, Bouhassira D. Long-term maintenance of the analgesic effects of transcranial magnetic stimulation in fibromyalgia. PAIN 2011;152:1478–85. [DOI] [PubMed] [Google Scholar]
- [33].Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD. A multisensory investigation of the functional significance of the “pain matrix.” NeuroImage 2011;54:2237–49. [DOI] [PubMed] [Google Scholar]
- [34].Olivan-Blázquez B, Herrera-Mercadal P, Puebla-Guedea M, Pérez-Yus MC, Andres E, Fayed N, López-Del-Hoyo Y, Magallón R, Roca M, Garcia-Campayo J. Efficacy of memantine in the treatment of fibromyalgia: a double-blind, randomised, controlled trial with 6-month follow-up. PAIN 2014;155:2517–25. [DOI] [PubMed] [Google Scholar]
- [35].Olsen RW. GABA and inhibitory synaptic transmission in the brain. Semin Neurosci 1991;3:175–81. [Google Scholar]
- [36].Petrou M, Pop-Busui R, Foerster BR, Edden RA, Callaghan BC, Harte SE, Harris RE, Clauw DJ, Feldman EL. Altered excitation-inhibition balance in the brain of patients with diabetic neuropathy. Acad Radiol 2012;19:607–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain: a review and meta-analysis (2000). Neurophysiol Clin 2000;30:263–88. [DOI] [PubMed] [Google Scholar]
- [38].Pomares FB, Funck T, Feier NA, Roy S, Daigle-Martel A, Ceko M, Narayanan S, Araujo D, Thiel A, Stikov N, Fitzcharles MA, Schweinhardt P. Histological underpinnings of grey matter changes in fibromyalgia investigated using multimodal brain imaging. J Neurosci 2017;37:1090–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pyke TL, Osmotherly PG, Baines S. Measuring glutamate levels in the brains of fibromyalgia patients and a potential role for glutamate in the pathophysiology of fibromyalgia symptoms: a systematic review. Clin J Pain 2017;33:944–54. [DOI] [PubMed] [Google Scholar]
- [40].Reckziegel D, Raschke F, Cottam WJ, Auer DP. Cingulate GABA levels inversely correlate with the intensity of ongoing chronic knee osteoarthritis pain. Mol Pain 2016;12:1744806916650690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Robel S, Sontheimer H. Glia as drivers of abnormal neuronal activity. Nat Neurosci 2016;19:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Schmidt-Wilcke T, Fuchs E, Funke K, Vlachos A, Müller-Dahlhaus F, Puts NAJ, Harris RE, Edden RAE. GABA-from inhibition to cognition: emerging concepts. Neuroscientist 2018;24:501–15. [DOI] [PubMed] [Google Scholar]
- [43].Smallwood RF, Laird AR, Ramage AE, Parkinson AL, Lewis J, Clauw DJ, Williams DA, Schmidt-Wilcke T, Farrell MJ, Eickhoff SB, Robin DA. Structural brain anomalies and chronic pain: a quantitative meta-analysis of gray matter volume. J Pain 2013;14:663–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Stagg CJ, Bachtiar V, Johansen-Berg H. What are we measuring with GABA magnetic resonance spectroscopy? Commun Integr Biol 2014;4:573–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Stuss DT, Stethem LL, Pelchat G. Three tests of attention and rapid information processing: an extension. Clin Neuropsychol 1988;2:246–50. [Google Scholar]
- [46].Watson CJ. Insular balance of glutamatergic and GABAergic signaling modulates pain processing. PAIN 2016;157:2194–207. [DOI] [PubMed] [Google Scholar]
- [47].Wiebking C, Duncan NW, Qin P, Hayes DJ, Lyttelton O, Gravel P, Verhaeghe J, Kostikov AP, Schirrmacher R, Reader AJ, Bajbouj M, Northoff G. External awareness and GABA-A multimodal imaging study combining fMRI and [18F]flumazenil-PET. Hum Brain Mapp 2012;35:173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science 2000;288:1765–9. [DOI] [PubMed] [Google Scholar]
- [49].Worsley KJ, Andermann M, Koulis T, MacDonald D, Evans AC. Detecting changes in nonisotropic images. Hum Brain Mapp 1999;8:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wu YL, Huang CJ, Fang SC, Ko LH, Tsai PS. Cognitive impairment in fibromyalgia: a meta-analysis of case-control studies. Psychosom Med 2018;80:432–8. [DOI] [PubMed] [Google Scholar]
- [51].Yamashita A, Hamada A, Suhara Y, Kawabe R, Yanase M, Kuzumaki N, Narita M, Matsui R, Okano H, Narita M. Astrocytic activation in the anterior cingulate cortex is critical for sleep disorder under neuropathic pain. Synapse 2014;68:235–47. [DOI] [PubMed] [Google Scholar]
- [52].Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol (Lond) 1996;496(pt 3):873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- [54].Zugaib J, Coutinho MR, Ferreira MD, Menescal-de-Oliveira L. Glutamate/GABA balance in ACC modulates the nociceptive responses of vocalization: an expression of affective-motivational component of pain in Guinea pigs. Physiol Behav 2014;126:8–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A video abstract accompanying this article can be found at http://links.lww.com/PAIN/A879.