Supplemental Digital Content is available in the text.
Abstract
Summary:
Objective evidence for the role of inhibition of collagen cross-linking in human scar using a nontoxic topical inhibitor, 1,4-diaminobutane (1,4 DAB), in patients with scars at risk for hypertrophic scar formation is presented. The authors used a concentration of 1,4 DAB of 0.8% (weight/volume) in a cream base similar to Glaxal Base. Application was once per day at night. The control was treated with cream base alone. In treatment phase studies at 2 months, tissue biopsies were performed and used to determine a therapeutic effect biochemically in paired scars harvested chosen with typical hypertrophic scars at two major treatment centers. Tissue transglutaminase activity revealed a significant reduction of the ε-(γ-glutamyl)lysine cross-links in the treated scars: 7.96 ± 1.51 pmol/µmol amino acid versus 14.78 ± 3.52 pmol/µmol amino acid. A subset of paired scars (n = 15) was also analyzed for soluble procollagen type III amino propeptide. The effect was a significant increase in procollagen type III amino propeptide in the scars treated with 1,4 DAB compared with sham-treated scars: 47.75 ± 4.6 µg/mg wet weight versus 39.08 ± 6.02 µg/mg wet weight, respectively. Levels of tissue 1,4 DAB was found to be twice as high in the presence of the active cream versus in the tissue of the control group. In subsequent prophylaxis studies, the authors treated 44 breast reduction patients prospectively with active cream to one or the other side in a double-blind randomized fashion. Hardness (in grams) measured using a Rex Durometer at 6 and 12 weeks postoperatively along with photographs were analyzed. The mean value ± SD of 24.98 ± 1.2 g on the active side versus 31.76 ± 1.1 g on the sham side was significantly different (p < 0.05). The patient scale scores of the Patient and Observer Scar Assessment Scale were also requested by survey in a responding 27-patient subgroup at a minimum 1 year postoperatively, and the differences between the two sides were found to be statistically significant, where the mean on the active side was 14.07 ± 1.34 and the mean on the sham side was 21.41 ± 1 (p < 0.05). The results are evidence to support the use of this agent in prevention of hypertrophic scars.
CLINICAL QUESTION/LEVEL OF EVIDENCE:
Therapeutic, II.
Hypertrophic scar formation remains a poorly understood disorder in postsurgical and trauma patients, although studies of its pathogenesis have been reported.1,2 Current treatment modalities rely on steroid injection, silicone gel and pressure garments, in addition to 5-fluorouracil and radiation therapy in keloids, although these scars are different in their pathogenesis. Results to date remain unreliable requiring invasive or cumbersome long-term treatment. We began these studies to attempt to control fibrous proliferation in hypertrophic scars. This led to the discovery of an enzyme-mediated cross-linking of precursor type III collagen in the wound, which forms a nidus for subsequent type III and type I collagen to form fibrils in the ongoing formation of scar.2 This study analyzes the mechanism of action of a biochemical inhibitor of the responsible enzyme, tissue transglutaminase, in reducing the cross-linking of the fibrous tissue matrix and also shows clinical evidence of objective improvement in the scar structurally by scar hardness using durometry and by patient assessment using the validated Patient and Observer Scar Assessment Scale.3
PATIENTS AND METHODS
Analytical Assessments
Thirty patients were prospectively randomized in double-blind fashion to receive 1,4-diaminobutane (1,4 DAB) or control treatment by the hospital research pharmacist in Winnipeg, which supplied both centers with labeled jars of cream that were identical apart from the code on the jars. The study monitors at both centers provided patients with necessary instruction for use of the topical preparations and arranged before and after treatment follow-up. Topical 1,4 DAB was compounded as 0.8% weight/volume in a base that is available commercially (Glaxal Base; Glaxo Canada Ltd., Toronto, Ontario, Canada) and given the early commercial name Fibrostat.4 Arrangements were made for biopsy at 2 months after initiation of the study, as these patients had already had visible scars present for at least 1 month before enrolment. Subsequent work showed that maximum cross-link formation occurs at 3 months after injury (data not shown), and we also showed that maximum hardness in the scars appears at approximately 3 months postoperatively. Inclusion criteria required that two areas on the extremities or torso, as similar as possible, had to be assessable. However, separation of two treatment areas along a single scar simultaneously was allowed. It was desirable for the two scars to be actively hypertrophic as evidenced by their appearance clinically.4 Scars that were previously nonhypertrophic but became raised, red, and symptomatic were treated as well. Patients with dermatoses or malignancy were excluded from all phases, as were female patient who might have become pregnant during the study period or who were lactating. Patient age was not a consideration, unless compliance became an issue. The patients reported all and any adverse events to the study monitors. All were issued consents and numbered jars or cream along with patient instruction for proper application. The study was conducted in accordance with the local ethics committees at the universities of Manitoba and Alberta in keeping with the Declaration of Helsinki.
Analytical Methods
Topical 1,4 DAB was shown to be active for up to 12 months (data on file), although hospital stock was rotated every 3 months. Control treatment differed from the active cream only in that 1,4 DAB was omitted. Patients and caregivers could not distinguish between the active and control preparations.
Biochemical analysis of the content of 1,4 DAB was carried out on 10 representative samples of active cream and 50-mM standard samples on high-performance liquid chromatography by an independent laboratory of an industrial partner. Each batch subsequently used in the breast reduction group was tested by the formulating laboratory (data on file; Dermal Therapy Research, Inc., London, Ontario, Canada). A similar method was used to measure levels of 1,4 DAB in eight punch biopsy tissue samples from scars revised at 3 months in patients who had given consent and who requested this and is described in the Appendix. (See Appendix, Supplemental Digital Content 1, http://links.lww.com/PRS/D848.)
Procollagen Type III Amino Propeptide Radioimmunoassay Technique
Radioimmunoassay was chosen for its specificity in measuring subtypes of collagen in the tissue. Antibodies raised in sheep to human procollagen type III amino propeptide and adsorbed onto particles that can be radioactively iodinated were purchased. The radioactivity from the adsorbed particles onto procollagen type III amino propeptide which is attached to the testing well can then be quantitated in a gamma counter.
The homogenization supernatant and digestion supernatant were prepared for procollagen type III amino propeptide radioimmunoassay from 15 patients by dilution with phosphate-buffered saline, pH 7.4: 1:50 to 1:100 dilutions were used for preparation of the homogenization supernatant samples and 1:10 to 1:25 dilutions were used for preparation of the digestion supernatant samples. Assays were carried out according to the manufacturer’s instructions on as many samples possible with each kit (Farmos Diagnostica, Finland). Gamma counting was performed on an LKB Wallac 1260 Mutigamma (LKB Instruments, Mt. Waverly, Victoria, Australia).
Clinically Based Assessments
Patient Durometer Testing
Forty-eight breast reduction patients were enrolled and cream treatment randomized blindly to one or the other breast, once per day at night, starting at 1 week postoperatively for a 5-day run in on an intent-to-treat basis. If agreeable and tolerating the treatment at the second-week follow-up, patients were enrolled and photographed with a durometer measured as the average of three readings along the vertical breast scar using a Rex durometer (Rex Gauge Company, Inc., Buffalo Grove, Ill.) at 6 and 12 weeks postoperatively. Duredness is a measurement of hardness recorded in grams by a pressure strain gauge, that produces a set deformational force when applied to the surface of the scar and then is compared to the opposite sham-treated side. Digital readout is compared for three separate areas on the scar and averaged. The model used was the 00, as this is best for skin measurement. The vertical scar was chosen for consistency in measuring hardness, as many of the patients underwent vertical reduction mammaplasty.
Patient and Observer Scar Assessment Scale
Of the 44 patients evaluated for scar duredness, 27 were assessable by returned completed survey forms at least 1 year postoperatively. The patients reported on seven parameters, rating each from 1 to 10.3 They were able to compare both scars with each other and the surrounding normal skin for subjective and objective criteria such as pain and itch, color, stiffness, regularity, and subjective hardness.
Statistical Analysis
Statistical methods were by Mann-Whitney U testing and Kolmogorov-Smirnov normal distribution testing on the chart data, as the sample sizes are relatively small. The paired t test was used on some sample testing because the cream and punch biopsy specimens were all taken from the patients used as their own controls, resulting in the power of the study being doubled by doing so. The 95 percent confidence level was chosen, and all the results were considered significant at the p < 0.05 level.
RESULTS
Seventy-eight patients in total were enrolled from two major plastic surgery units. However, 30 were assessable for the biochemical analyses. Specimens from one patient were lost because of laboratory error during the procollagen type III amino propeptide analysis. In addition, laboratory error accounted for two more samples being lost during the 1,4 DAB analysis in elective 3-month scar revision samples. Of the 48 breast reductions enrolled for durometry studies, 44 were assessable at 12 weeks, and 27 returned Patient and Observer Scar Assessment Scale surveys. That only 44 patients returned for 12-week assessment was attributable to four withdrawing after the 5-day run for inability to meet the follow-up schedule. The average age of the patients was 29 years (range, 5 to 53 years). Ten male subjects and 50 female subjects were studied (Fig. 1). The biochemical analyses are illustrated in Figures 2 through 4. The results in Figure 2 reveal ε-(γ-glutamyl)lysine cross-link levels of 7.96 ± 1.51 pmol/µmol amino acid versus 14.78 ± 3.52 pmol/µmol amino acid for 1,4 DAB–treated versus control-treated scars, respectively. 1,4 DAB–treated scars also had a significant (p < 0.05) elevation of soluble procollagen type III amino propeptide, being 47.75 ± 4.6 µg/mg wet weight versus 39.08 ± 6.02 µg/mg wet weight of tissue in the control-treated scars, and insoluble levels of procollagen type III amino propeptide were not significantly affected (Fig. 3).
Fig. 1.
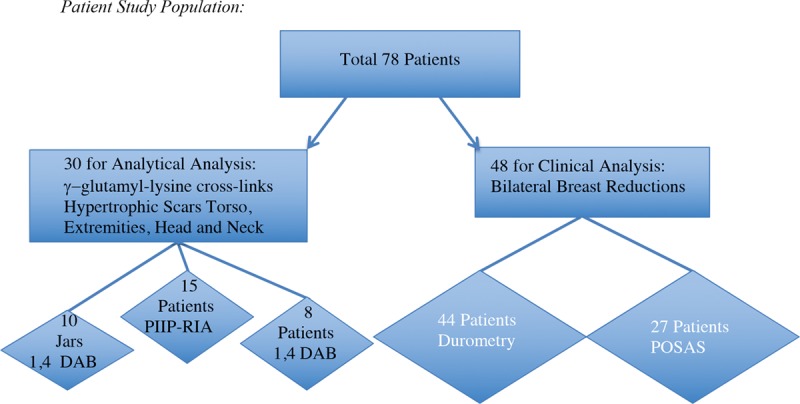
Early enrolment for biochemical analysis were from two centers. All patients accepted with recent scars were able to be studied in pairs (n = 30). Later, 48 breast reduction patients from one center were enrolled and 44 studied by durometry. Four of the patients enrolled after breast reduction dropped out because of personal reasons. Twenty-seven patients returned Patient and Observer Scar Assessment Scale questionnaires at 1-year follow-up. The total enrollment was 78 patients for both types of analysis. 1,4 DAB, 1,4-diaminobutane; PIIP-RIA, procollagen type III amino propeptide radioimmunoassay; POSAS, Patient and Observer Scar Assessment Scale.
Fig. 2.
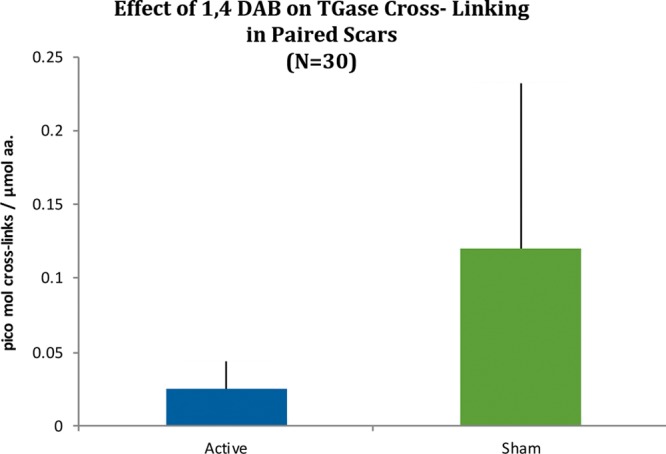
High-pressure liquid chromatography: box-and-whisker plot results for 30 paired scar specimens expressed in picomoles of ε-(γ-glutamyl)lysine isopeptide bonds in digests. Kolmogorov-Smirnov test was not normally distributed but applied Mann-Whitney U test was significant (p < 0.05). 1,4 DAB, 1,4-diaminobutane; TGase, tissue transglutaminase.
Fig. 4.
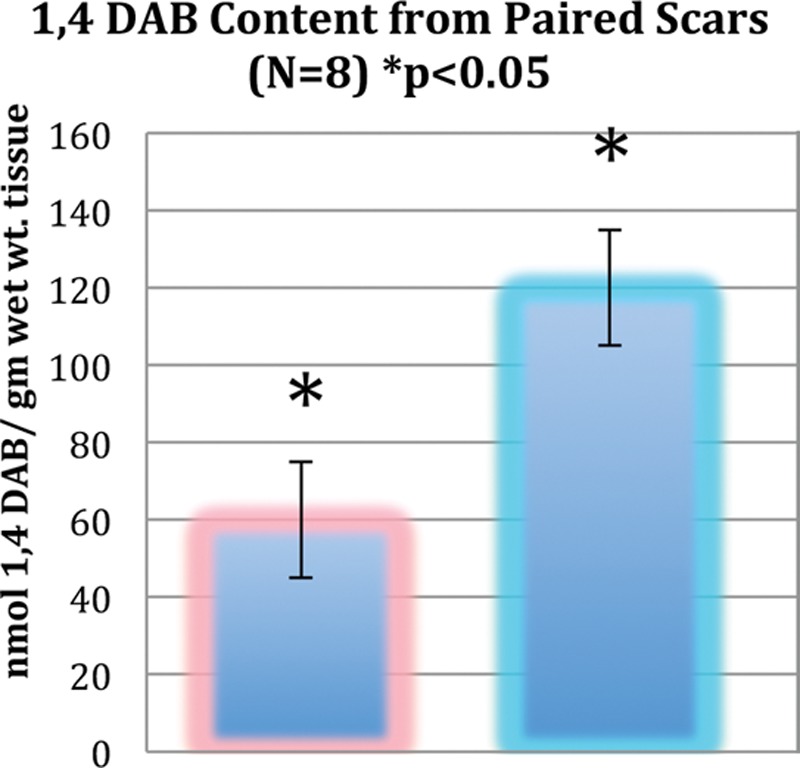
1,4 DAB content (in nanomoles per milligram wet weight of tissue) after thawing from eight random skin samples harvested in triplicate at the time of elective scar revision at 3 months. Paired scars treated with 1,4 DAB are shown in blue and control-treated scars are shown in pink; t test (p < 0.05) demonstrates twice physiologic levels in active test scars (outside independent laboratory testing performed by industrial partner). 1,4 DAB, 1,4-diaminobutane.
Fig. 3.
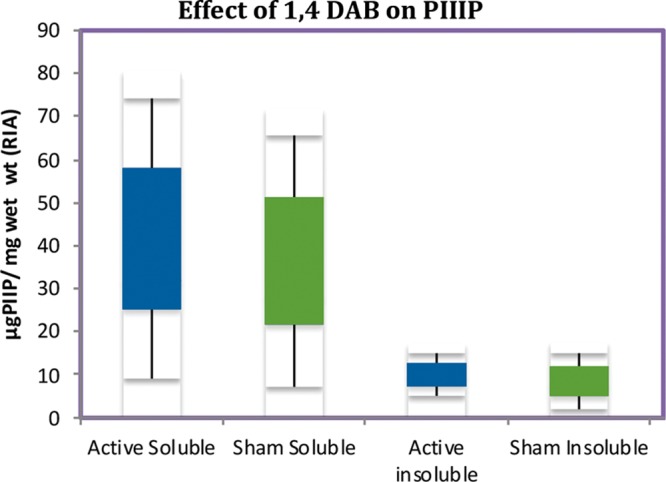
Radioimmunoassay results of 15 paired scar specimens expressed in micrograms of procollagen type III amino propeptide per milligram tissue wet weight after thawing. The insoluble material after exhaustive extraction is compared to the soluble fraction. 1,4 DAB results are also shown as box-and-whisker graphs and show significance (p < 0.05) compared with control-treated scars with respect to soluble procollagen type III amino propeptide only, by Mann-Whitney U test. The Kolmogorov-Smirnov normality test was shown to be valid. PIIIP, procollagen type III amino propeptide; RIA, radioimmunoassay; 1,4 DAB, 1,4-diaminobutane.
The levels of 1,4 DAB in scar tissue at the time of surgical scar revision was measured from a subset of 10 patients, of which eight were assessable. These patients had three punch biopsies performed just before the scar excision after the third month of the study. Twice physiologic levels of 1,4 DAB were recorded in the treated scars compared with the control-treated tissue, with the difference being significant (p < 0.05) (Fig. 4).
A mean duredness (which is hardness measured by a Rex strain gauge durometer in grams) in 44 breast reduction patients was significantly different when comparing the 1,4 DAB–treated breast scars with control scars as seen by the box plot of the 12-week duredness average values in grams (Fig. 5) (active, 24.98 ± 1.2 g; sham, 31.76 ± 1.1 g; p < 0.0002), which is interpreted clinically as a softer, more pliable and elastic scar, in the presence of 1,4 DAB. The 6-week data did not reach statistical significance. Patient and Observer Scar Assessment Scale scores from 27 assessable patients answering the breast scar survey a minimum of 1 year postoperatively revealed average scores with means of 14.07 ± 1.34 on the active side versus 21.41 ± 1.46 on the sham side (p < 0.0005), where the lower the score, the more normal the appearance of the scar compared with controls (Figs. 6 and 7).
Fig. 5.
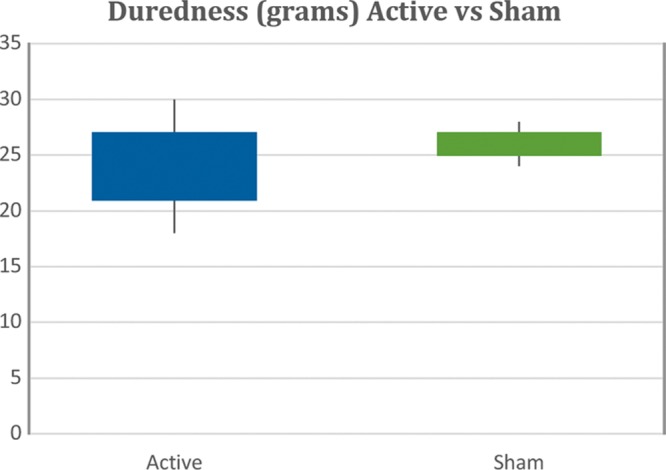
Scar hardness in grams using a Rex durometer from 44 paired breast reduction scars. Box plot shows highly significant difference at 12 weeks after run for active side versus sham-treated scars by Mann-Whitney U test (p < 0.00003). The Kolmogorov-Smirnov test is valid.
Fig. 6.
The seven parameters of the 10-point Patient and Observer Scar Assessment Scale is shown (copyright © p.p.m. van zuijlen, beverwijk-nl).
Fig. 7.
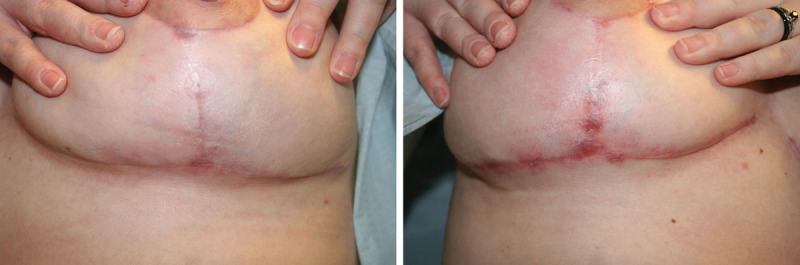
Representative result of one breast reduction patient and the relative improvement in the appearance of scars on the treated (left) side versus the sham (right) side at 12 weeks.
DISCUSSION
The role of tissue transglutaminase in healing wounds is still controversial in the biochemical literature.5,6 Although tissue transglutaminase is considered to be intracellular, an extracellular location has been shown in healing wound tissue and in Dupuytren’s diseased tissue.7,8 Studies in vitro from rhabdomyosarcoma cells reveal that the action of tissue transglutaminase in extracellular fibrillogenesis is inhibited by 1,4 DAB.9 An intracellular location may be involved in apoptosis.10 However, if this were affected by the addition of 1,4 DAB to wound tissue, one would expect to see evidence of this. In studies reported by Linge et al., this was considered an unreliable finding in wound explants studied for apoptosis markers.10
The results in Figure 2 reveal that ε-(γ-glutamyl)lysine cross-link formation by tissue transglutaminase in 1,4 DAB–treated scar is significantly (p < 0.05) below levels seen in control scars from the same patients (7.96 ± 1.51 pmol/µmol amino acid versus 14.78 ± 3.52 pmol/µmol amino acid for 1,4 DAB–treated versus control scars). This occurs with a significant attendant increase in soluble procollagen type III amino propeptide, the putative enzyme substrate in the healing matrix, whereas the insoluble procollagen type III amino propeptide form does not appear to change in content (Fig. 3). This can be explained by an increase in procollagen type III amino propeptide production or by a decrease in the wet weight of the scar. Because the duredness of the scar has decreased to a value of 24.98 ± 1.2 g versus 31.76 ± 1.1 g at 12 weeks in the presence of 1,4 DAB, it is now suggested that it is reducing the insoluble matrix and shifting the procollagen type III amino propeptide pool to a soluble form, and therefore a softer scar results. Once in soluble form, the turnover of procollagen type III amino propeptide would be expected to increase, thus leading to an overall reduction in scar size over time. Procollagen type III amino propeptide amino propeptide is cleaved from the rest of the type III collagen after cross-linking has occurred, forming the nidus on which collagen type I is then laid down by irreducible cross-links. The typical five-band stagger seen in collagen fibers on electron microscopy is a product of the procollagen type III amino propeptide acting as a registration peptide and then being cleaved during fiber formation. The ensuing collagen type I hydrostatic attraction forces are not enzyme-mediated events, therefore supporting a regulatory role for procollagen type III amino propeptide pool size on the degree of collagen matrix formation in the wound.
Tissue transglutaminase has been found to regulate the formation of active transforming growth factor-β, an important cytokine implicated in keloid formation and proliferative fibroblast activity such as hypertrophic burn scars.11–13 It is proposed that control of collagen metabolism at the molecular level may be affected by tissue transglutaminase at a critical point in the pathway to wound matrix formation, and this may be perturbed by the presence of 1,4 DAB.
It is known that type III collagen is increased in hypertrophic scars, but not in keloids.14 Furthermore, type III procollagen and the aminopropeptide (procollagen type III amino propeptide), unlike collagen type I, have specific substrate sites for tissue transglutaminase.5 From this, we can hypothesize that inhibition of cross-linking by incorporation of a polyamine such as 1,4 DAB will allow accumulation of soluble procollagen type III amino propeptide in the extracellular tissue (Fig. 3). Weistner et al. have shown that soluble procollagen type III amino propeptide added to fibroblast cultures decreased collagen type I production, suggesting the existence of a negative feedback mechanism, or end-product inhibition.15 It is also speculated that microfibril formation involving type V and XI collagen cross-linking is inhibited by 1,4 DAB and may involve similar effects on intracellular mechanisms. The use of lysyl oxidase inhibitors did not seem to affect this.16
1,4 DAB is well absorbed in its present formulation, with a mean concentration twice that of untreated scars from patients in whom this was studied (Fig. 4). It appears to be safe in vivo at the concentration used, adverse events being rare (phase 2A and 2B studies on file at Bioail Corp., Mississauga, Ontario, Canada). Levels of 1,4 DAB are low compared with normal plasma levels seen in all humans. Further research is needed to study the effects compared to silicone gel and other delivery and dosage regimens.17–19 However, it is recommended that based on the clinical outcomes seen in the current preparation for the prevention of hypertrophic scars in the breast reduction population, the use of 1,4 DAB be considered for future research in the control of hypertrophic scars.
CONCLUSIONS
A role for topical 1,4 DAB in prophylaxis of hypertrophic scar is supported by the data. Its use is proposed to result in a biochemically altered matrix, which is clinically reduced in mechanical properties as measured by a durometer and modified in its structure to appear more acceptable to patients visually and functionally.
ACKNOWLEDGMENTS
This work was supported by a university-industry grant jointly between the Medical Research Council of Canada and Procyon Biopharma, Inc. (London, Ontario, Canada). Technical support was generously provided by the Department of Biochemistry at the University of Manitoba. This study is dedicated to the memory of John Michael Bowness, Ph.D. Gratitude is also expressed to the study nurses, Gail Bloomfield, R.N., and Heather Shankowsky, R.N.; technical assistants Allan Tarr and Faye Kingyens; and Michael Laliberte, M.D., for help in the clinical breast reduction studies.
Supplementary Material
Footnotes
This trial is registered under the name “Role of Topical Putrescine (Fibrostat) for Prevention of Hypertrophic Scars in Mammoplasty Patients,” ClinicalTrials.gov identification number NCT03376620 (https://clinicaltrials.gov/ct2/show/NCT03376620).
Related digital media are available in the full-text version of the article on www.PRSJournal.com.
Disclosure: Dr. Dolynchuk holds the patent rights to 1,4-diaminobutane in treatment of hypertrophic scars and was paid royalties for its commercialization by Vivier Pharma, Montreal, Canada. Dr. Tredget has no financial interests to report.
REFERENCES
- 1.Castagnoli C, Peruccio D, Stella M, et al. The HLA-DR beta 16 allogenotype constitutes a risk factor for hypertrophic scarring. Hum Immunol. 1990;29:229–232. [DOI] [PubMed] [Google Scholar]
- 2.Dolynchuk KN. Inhibition of tissue transglutaminase and epsilon (gamma-glutamyl) lysine cross-linking in human hypertrophic scar. Wound Repair Regen. 1996;4:16–20. [DOI] [PubMed] [Google Scholar]
- 3.van de Kar AL, Corion LU, Smeulders MJ, Draaijers LJ, van der Horst CM, van Zuijlen PP. Reliable and feasible evaluation of linear scars by Patient and Observer Scar Assessment Scale. Plast Reconstr Surg. 2005;116:514–522. [DOI] [PubMed] [Google Scholar]
- 4.Dolynchuk KN, Ziesmann M, Serletti JM. Topical putrescine (Fibrostat) in treatment of hypertrophic scars: Phase II study. Plast Reconstr Surg. 1996;97:117–123; discussion 124–125. [DOI] [PubMed] [Google Scholar]
- 5.Bowness JM, Folk JE, Timpl R. Identification of a substrate site for liver transglutaminase on the aminopropeptide of type III collagen. J Biol Chem. 1987;262:1022–1024. [PubMed] [Google Scholar]
- 6.Bensten KD, Lang C, Horslev-Petersen K, Ristelli J. The aminoterminal propeptide of type III procollagen and basement membrane components in serum during wound healing in man. Acta Chir Scand. 1988;154:97–101. [PubMed] [Google Scholar]
- 7.Dolynchuk KN, Pettigrew NM. Transglutaminase levels in Dupuytren’s disease. J Hand Surg Am. 1991;16:787–790. [DOI] [PubMed] [Google Scholar]
- 8.Upchurch HF, Conway E, Patterson MK, Jr, Maxwell MD. Localization of cellular transglutaminase on the extracellular matrix after wounding: Characteristics of the matrix bound enzyme. J Cell Physiol. 1991;149:375–382. [DOI] [PubMed] [Google Scholar]
- 9.Kleman JP, Aeschlimann D, Paulsson M, van der Rest M. Transglutaminase-catalyzed cross-linking of fibrils of collagen V/XI in A204 rhabdomyosarcoma cells. Biochemistry 1995;34:13768–13775. [DOI] [PubMed] [Google Scholar]
- 10.Linge C, Richardson J, Vigor C, Clayton E, Hardas B, Rolfe K. Hypertrophic scar cells fail to undergo a form of apoptosis specific to contractile collagen: The role of tissue transglutaminase. J Invest Dermatol. 2005;125:72–82. [DOI] [PubMed] [Google Scholar]
- 11.Taipale J, Saharinen J, Hedman K, Keski-Oja J. Latent transforming growth factor-beta 1 and its binding protein are components of extracellular matrix microfibrils. J Histochem Cytochem. 1996;44:875–889. [DOI] [PubMed] [Google Scholar]
- 12.Kojima S, Nara K, Rifkin DB. Requirement for transglutaminase in the activation of latent transforming growth factor-beta in bovine endothelial cells. J Cell Biol. 1993;121:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tredget EE, Shankowsky HA, Pannu R, et al. Transforming growth factor-beta in thermally injured patients with hypertrophic scars: Effects of interferon alpha-2b. Plast Reconstr Surg. 1998;102:1317–1328; discussion 1329–1330. [DOI] [PubMed] [Google Scholar]
- 14.Weber L, Meigel WN, Spier W. Collagen polymorphism in pathologic human scars. Arch Dermatol Res. 1978;261:63–71. [DOI] [PubMed] [Google Scholar]
- 15.Weistner M, Krieg T, Hörlein D, Glanville RW, Fietzek P, Müller PK. Inhibiting effect of procollagen peptides on collagen biosynthesis in fibroblast cultures. J Biol Chem. 1979;254:7016–7023. [PubMed] [Google Scholar]
- 16.Fesus I, Thomazy V. Searching for the function of tissue transglutaminase: Its possible involvement in the biochemical pathway to programmed cell death. Adv Exp Med Biol. 1988;231:119–134. [DOI] [PubMed] [Google Scholar]
- 17.O’Brian L, Jones DJ. Silicone gel sheeting for preventing of hypertrophic and keloid scars and for treating existing hypertrophic and keloid scars. Cochrane Database Syst Rev. 2013;9:CD003826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho JW, Cho SY, Lee SR, Lee KS. Onion extract and quercetin induce matrix metalloproteinase-1 in vitro and in vivo. Int J Mol Med. 2010;25:347–352. [PubMed] [Google Scholar]
- 19.Shriharani SN, Magorahis M, Manson PN, et al. The emerging role of antineoplastic agents in the treatment of keloids and hypertrophic scars: A review. Ann Plast Surg. 2010;64:3575–3761. [DOI] [PubMed] [Google Scholar]


