To the Editor
A mutation of the FLT3 receptor tyrosine kinase caused by an internal tandem duplication, FLT3-ITD, is the most frequent molecular anomaly found in 28-34% of cytogenetically normal acute myeloid leukemia (AML) patients, and confers a poor prognosis [1]. The drug midostaurin inhibits FLT3 cell signaling, thereby inducing apoptosis of leukemic cells. The agent shows great promise as a multi-targeted therapy in the treatment of a disease with historically dismal outcomes [2, 3]. Schlenk et al (2019) recently reported that although midostaurin as a continued single-agent maintenance therapy results in improved event-free survival (EFS) rates, its associated toxicities, particularly cardiac and pulmonary, need to be further elucidated [4]. One case of midostaurin-induced pneumonitis has previously been reported in an AML patient with FLT3 mutation prior to bone marrow transplant [5]. Three incidents of fatal pulmonary toxicity of unknown etiology were also reported in early studies of the drug [6]. The role of FLT3 inhibitors as post-transplant maintenance therapy is emerging. Here, we describe one case of a FLT3+ AML patient developing interstitial lung disease while on midostaurin therapy post-allogeneic stem cell transplant. To our knowledge, this is the first report of pulmonary toxicity post-allogeneic transplant. Here, we aim to further characterize midostaurin-induced interstitial lung disease and provide insight into its prevention and early detection.
A 66-year-old woman with no history of lung disease was diagnosed with AML. Bone marrow biopsy showed myelomonocytic leukemia. Molecular studies confirmed FLT3+ ITD mutation. No tyrosine kinase domain mutation was identified. She completed an induction therapy of 7 days of cytarabine infusion followed by 3 days of daunorubicin infusion (7 + 3) and entered complete remission. She then received two doses of high dose cytarabine, but soon relapsed. Bone marrow biopsy and molecular studies at the time showed FLT3+ ITD AML again. She was then started on salvage induction with cladribine, cytarabine, filgrastim and mitoxantrone (CLAG-M).
The safety of midostaurin combined with anthracycline plus cytarabine-based chemotherapy has been investigated thoroughly as a front-line therapy [2]. In a refractory setting, however, midostaurin was shown to have minimal efficacy in a Phase II trial as single agent [7]. Many patients in the trial showed significant blast count reductions, but none achieved complete remission. Our patient received their induction treatment before Food and Drug Administration (FDA) approval of midostaurin as a front-line therapy, not to mention the limited access to midostaurin of their initial treating physician. Thus, upon institutional approval, midostaurin was approved to be used off-label combined with CLAG-M. It was thought that adding an FLT3+ targeted therapy agent would be beneficial. Per the FDA label of the approved front-line therapy, midostaurin was dosed at 50 mg twice daily from day 8 to day 21. A subsequent repeat bone marrow biopsy showed complete molecular remission.
Eleven months after initial diagnosis, our patient received a 5/10 haploidentical allogeneic transplant. Post-transplant cyclophosphamide (PTCy) was administered per Hopkins’ haploidentical protocol as prophylaxis for graft-versus-host disease (GVHD). She tolerated the transplant process and continued midostaurin therapy at 25 mg PO daily.
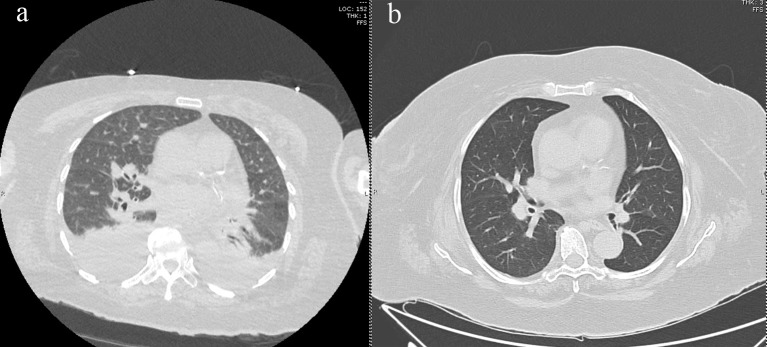
Seven months post-transplant, the patient was hospitalized for worsening shortness of breath and worsening hypoxemia without fevers or leukocytosis. Blood cultures were negative and induced sputum grew normal respiratory flora. Infectious workup was also negative for Pneumocystis jirovecii, aspergillus, Epstein-Barr virus, cytomegalovirus, and a respiratory viral panel. Pulmonary function test (PFT) showed no evidence of pulmonary obstruction. At the time of admission, the patient was taking rivaroxaban for pulmonary embolism, but entilation/perfusion (V/Q) scan showed no evidence of new embolus. High-resolution computed tomography (HRCT) of the chest showed bilateral smooth interlobular septal thickening with scattered ground glass opacities and pulmonary interstitial edema (Fig. 1a). Brain natriuretic peptide (BNP) was 140 pg/mL. Transthoracic echocardiogram (TTE) showed an ejection fraction of 61% and right ventricle systolic pressure elevated to 55 mm Hg. A bubble study did not show evidence of shunting.
Figure 1.
(a) HRCT of thorax demonstrating bilateral pleural effusions, scattered ground glass opacities, and interlobular septal thickening, and (b) HRCT showing resolution of ground glass opacities and pulmonary edema after midostaurin cessation. HRCT: high-resolution computed tomography.
Midostaurin was stopped due to concern of drug-related interstitial lung disease, and subsequently, the patient’s symptoms started improving. Biopsy could not be conducted due to a high risk of bleeding. The patient was diuresed with furosemide and a pulmonary consult recommended starting the patient on prednisone. Repeat HRCT chest 3 months later showed interval resolution of pulmonary edema as well as decrease in lower lobe ground glass opacities (Fig. 1b). Repeat echocardiogram showed improvement in right ventricular systolic pressure to 45 mm Hg. During the course of the treatment, the patient developed pulmonary hypertension with right heart strain that was improved on diuresis with furosemide. She was started on pulmonary hypertension medications sildenafil and macitentan. The respiratory symptoms continued improving, and the steroids were slowly tapered off. The patient’s C-reactive protein (CRP) peaked to 70 mg/dL and normalized at 2.3 mg/dL, and lactate dehydrogenase (LDH) reached a level of 300 U/L and normalized at 199 U/L. The patient continued to be in complete remission, maintaining full chimerism 19 months post-transplant.
Drug-induced lung disease, often a diagnosis of exclusion, is associated with both antineoplastic and non-neoplastic agents. The most common HRCT findings have been described as ground-glass opacities, which are often found in a diffuse pattern in toxicities associated with antineoplastic agents [8, 9]. In our patient, we observed worsening respiratory status with HRCT findings of interlobular septal thickening and scattered ground glass opacities approximately 7 months after allogeneic stem cell transplant and at a midostaurin dose of 25 mg daily. Cessation of midostaurin and treatment with steroids resulted in significant improvement in both clinical symptoms and radiologic findings within 3 months. To our knowledge, although midostaurin has previously been implicated in pulmonary toxicity, this is the first described case of pulmonary toxicity post-allogeneic transplant [5, 6]. Of note, our patient also received PTCy as prophylaxis against GVHD. As cyclophosphamide is also associated with pulmonary toxicity [8], it is possible that its combination with midostaurin only increased the chances of pulmonary complications. Future directions in understanding the risk of interstitial lung injury associated with midostaurin involve identifying the time after transplant that toxicity is likely to occur, whether toxicity is dose dependent, whether the drug can be safely re-introduced after resolution of symptoms and whether its combination with other agents is feasible and safe.
Acknowledgments
None to declare.
Financial Disclosure
George Yaghmour disclose: Pharma bureau speaker for Jazz, Takeda, and Astella Pharmaceutical Company. There is no declaration for others, and no funding was provided.
Conflict of Interest
The authors do not have any conflict of interest.
Informed Consent
Not applicable.
Author Contributions
PV, TK, BY, and GY performed the research and wrote the paper.
References
- 1.Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 2.Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, Thiede C. et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454–464. doi: 10.1056/NEJMoa1614359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Cancer Institute Surveillance Epidemiology and End Results Program. Acute Myeloid Leukemia. 2019.
- 4.Schlenk RF, Weber D, Fiedler W, Salih HR, Wulf G, Salwender H, Schroeder T. et al. Midostaurin added to chemotherapy and continued single-agent maintenance therapy in acute myeloid leukemia with FLT3-ITD. Blood. 2019;133(8):840–851. doi: 10.1182/blood-2018-08-869453. [DOI] [PubMed] [Google Scholar]
- 5.Lee B, Onofrei CD, Sheski F. Drug Induc. Case Reports. 2018. The culprit midostaurin in a case of drug-induced pneumonitis; p. A6647. [Google Scholar]
- 6.Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD, Grandin W. et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105(1):54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 7.Fischer T, Stone RM, Deangelo DJ, Galinsky I, Estey E, Lanza C, Fox E. et al. Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010;28(28):4339–4345. doi: 10.1200/JCO.2010.28.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleverley JR, Screaton NJ, Hiorns MP, Flint JD, Muller NL. Drug-induced lung disease: high-resolution CT and histological findings. Clin Radiol. 2002;57(4):292–299. doi: 10.1053/crad.2001.0792. [DOI] [PubMed] [Google Scholar]
- 9.Akira M, Ishikawa H, Yamamoto S. Drug-induced pneumonitis: thin-section CT findings in 60 patients. Radiology. 2002;224(3):852–860. doi: 10.1148/radiol.2243011236. [DOI] [PubMed] [Google Scholar]