Abstract
Ovarian cancer (OC) is one of the most common malignant tumors in female reproductive system and most OC cases are diagnosed at an advanced stage with the overall 5-year survival rate below 40%. The function of CD247 enhances T-cell antigen receptor (TCR) signaling cascade and it is necessary for assembling of the TCR/CD3 complex on the surface of T lymphocytes. It is well established that defective CD247 function leads to impaired activation of T cells upon engagement of the TCR.
Flow cytometry was used to examine the difference of CD247+ T lymphocyte between the OC and ovarian cyst, immunohistochemistry analysis was used to investigate the correlation between CD247 expression and clinicopathologic features of epithelial OC patients.
Our study showed that the expression of CD247 in peripheral blood lymphocytes from patients with OC is decreased compared with ovarian cyst patients and the expression of CD247 in tumor infiltrating lymphocytes with cancer tissue is decreased compared with adjacent tissues. We showed that abnormal expression of CD247 was related with differentiation and classification in OC.
Our findings suggested that CD247-targeted treatment could be used as a potential therapeutic strategy for OC.
Keywords: ovarian cancer, CD247, flow cytometry, immunohistochemistry
1. Introduction
Ovarian cancer (OC) is one of the three major malignancies of the female reproductive system and the leading lethal gynecological cancer worldwide. It accounts for approximately 200,000 new cases per year globally.[1] Due to the fact that the disease often progresses before symptoms appear, most OC cases are diagnosed at an advanced stage with the overall 5-year survival rate below 40%.[2,3] While initial remission is often achieved, most patients relapse and die of the complications. To solve this issue, the options of new diagnosis and treatment, including T-cell-based immunotherapies, have been explored in OC. Tumor microenvironment (TME) is a pathologic environment composed of tumor cells, stromal cells, cytokines, and immune cells.[4] OC is a kind of immunogenic tumor which can evade immune surveillance through a variety of immunosuppressive methods and spread through peritoneal implants mainly. Understanding the role of cytokines, immune and inflammatory responses in the TME may be a key to understand the progression of OC.
The CD247, as known as CD3ζ-chain or T-cell antigen receptor (TCR)-Z, is a 16-kDa molecule which constitutes part of the TCR complex. It is essential for recognition (activation) of the antigenic peptide that is presented by the human leukocyte antigen on antigen presenting cells or tumor cells.[5,6] Downregulation of CD247 has frequently been found to be accompanied by decreased expression of other T-cell-associated signal-transducing molecules, such as ZAP-70 and p56lck,[7,8] decreased calcium flux,[9] and T-cell apoptosis.[10] Studies had demonstrated that the presence of tumor-infiltrating lymphocytes (TILs) is associated with improved clinical outcome in OC patients.[11–13] Numerous studies showed that the TME is immunosuppressive that impaired the function of the innate (NK cells) and adaptive (T cells) immune systems. With CD247 downregulation, which is an indispensable molecule in the structure, expression, and function of the TCR and the NK-cell-activating receptors, T-cell responsiveness, and proliferative capacity will be changed.[14–16] Reduced levels of CD247 in both TILs and peripheral blood lymphocytes was associated with many cancers including the gastric carcinoma, head and neck cancer.[17,18]
However, only very few studies have showed a correlation between the expression of CD247 in OC patients and the clinical and pathologic characteristics of patients. In this study, we aimed to study the relationship between them and to provide new ideas for the diagnosis and treatment of OC.
2. Materials and methods
2.1. Clinical samples
All clinical tissue samples for immunohistochemistry were obtained from the Third Affiliated Hospital of Soochow University (Changzhou, China) between January 2008 and December 2010. These specimens, including 102 cases of primary epithelial OC (8 well-differentiated cases, 19 moderately differentiated cases, 75 poorly differentiated cases), 84 cases of serous cystadenocarcinoma, 11 cases of mucinous cystadenocarcinoma, 3 cases of endometrioid carcinoma, and 4 cases of clear cell carcinoma were collected from patients who underwent surgery without chemotherapy and radiotherapy. Detailed clinicopathologic variables of the patients are summarized in Table 1. The study design was approved by the ethics committee of Soochow University. Written informed consent was obtained from every patient.
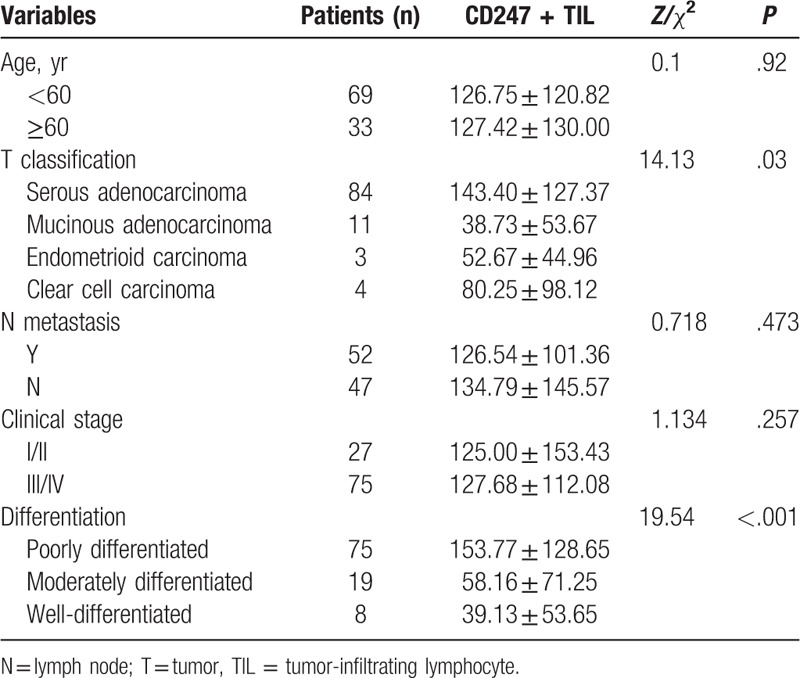
Table 1.
Correlation between clinicopathologic features and CD247 expression.
2.2. Flow cytometry
2.2.1. Flow cytometric analysis of CD45, CD3, CD4, CD8, and CD247 expression of PBMC
Whole blood were collected from consented benign ovarian cyst (as control group) and OC (as cancer group), for peripheral blood mononuclear cell (PBMC) isolations using Ficoll gradient centrifugation as described similar to Kulkarni et al.[19] For surface staining, membrane-labeled antibodies of CD45, CD3, CD4, CD8 were added into the tube respectively and then incubated with peripheral blood of patients at 4°C for 30 minutes in the dark. Then hemolysin was put into the test tube and incubated for 8 minutes, centrifuging it for 1200 rpm for 5 minutes and discarding the supernatant. Each tube was added with diluted fixed solution (the fixed solution and diluent were prepared according to 1:3) and was incubated for 8 minutes in 4°C, then centrifuging it 1200 rpm for 5 minutes and discarding the supernatant. Permeabilization buffer (the permeabilization buffer and double distilled water were prepared according to 1:9) was added to each tube for intracellular CD247 staining. Cells were resuspended, incubated at 4°C for 30 minutes in the dark and centrifuged for 1200 rpm for 5 minutes. After discarding the supernatant, 1% FBS Hanks was added into the test tube to resuspend the cells, then the cells were immediately analyzed on a FACSCanto II cytometer (BD).
2.2.2. Flow cytometric analysis of CD45, CD3, CD4, CD8, and CD247 expression of TIL
The TILs were isolated from tumor tissue as described similar to Thomas et al.[20] The fresh tissue of OC and adjacent tissue were rinsed by 1% FBS Hanks solution and was cut up with scissors. Then enzymes were added in the tissue for digesting for 30 minutes at 37°C incubator. The tissue was grinded thoroughly, filtered and transfered to the 15 mL centrifuging tube. The supernatant was discarded after centrifuging 1500 rpm for 5 minutes. Phorbol 12-myristate 13-acetate (PMA) and ionomycin was used for resuspension and then transfer to 96-well plat for incubating for 1 hour at 37°C. Then the cells were transfered to the 96-well plate and centrifuging at 1500 rpm for 5 minutes and add antibodies (CD45, CD3, CD4, CD8) with 1% FBS Hanks for surface staining. Incubated the cells for 30minutes at 4°C and centrifuged at 1500 rpm for 5 minutes, then discarded the supernatant carefully. Intracellular antibody for CD247 was stained after permeabilization solution was used. The cells were centrifuged at 1500 rpm for 5 minutes and resuspended with 1% FBS Hanks and transfer to the flow tube for analyzing.
2.3. Monoclonal antibodies and reagents
The monoclonal antibodies used were as follows: peridininchlorophyl protein-conjugated anti-CD45 (cat number: 652803, Lot number 8103002; BD), AC7-conjugated anti-CD4 (cat number: 555349, Lot number: 7354542; BD), PC7-conjugated anti-CD8 (cat number: 557746, Lot number 4341615; BD Biosciences), fluorescein isothiocyanate-conjugatedand anti-CD3 (Lot number: 8115819; BD Biosciences), phycoerythrin-conjugated anti-CD247 (cat number: 558448, Lot number: 6007597; BD), PMA, and ionomycin were purchased from Sigma Aldrich.
2.4. Immunohistochemistry
The primary antibodies used for immunohistochemistry were commercially available, including CD247 antibody (ab42824; Abcams, CO). Formalin-fixed, paraffin-embedded consecutive sections (4-μm thick) were heated at 85°C for 2 hours and then cooled at room temperature for 20 minutes. The slides were immersed in dimethylbenzene 3 times for deparaffinage 15 minutes each time and then consecutively hydrated in 100%, 95%, and 75% ethanol for 5 minutes. For antigen retrieval, slides were heated at 125°C for 5 minutes in 2% EDTA-citrate antigen retrieval solution (MVS-0099; Fuzhou Maixin Biotech Co, Ltd, Fuzhou, China) in a pressure cooker. Subsequently, slides were rinsed with phosphate-buffered saline (PBS-0061; Fuzhou Maixin Biotech Co, Ltd) for 3 times and then immersed in hydrogen peroxide at room temperature for 30 minutes to block endogenous peroxidase, followed by incubation with 3% BSA at 37°C for 30 minutes to block nonspecific binding. Next, the slides were incubated with CD247 antibody (1:200 diluted using antibody diluent) at 4°C for 14 hours. A MaxVisionTM rapid immunohistochemistry kit (KIT-5020; Fuzhou Maixin Biotech Co, Ltd) was used in the present study, and the binding process of secondary antibody was carried out according to the manufacturer's instructions. A DAB substrate kit (DAB-0031; Fuzhou Maixin Biotech Co, Ltd) was employed and its staining process was conducted according to the manufacturer's instructions. After staining, the sections were counterstained using hematoxylin, followed by dehydration through ethanol and xylene.
2.5. Evaluation of immunohistochemical staining and statistical analysis
All slides were independently examined by 2 senior pathologists who were blinded to the clinical parameters of patients. The immunostaining density of CD247 was assessed according to the H-score method: H-score = (% unstained TILs × 0) + (% weakly TILs × 1) + (% moderately TILs × 2) + (% strongly TILs × 3). The H-scores ranged from 0 (100% negative TILs) to 300 (100% strong staining TILs). Results from the 2 pathologists were averaged and used in the statistical analysis. All data were expressed as the mean ± standard deviation. Statistical analysis was performed using SPSS version 22.0 (SPSS Inc, Chicago, IL). The expression of CD247+ T cells in PBMCs and TIL of ovarian carcinoma was compared by Student t test. The difference among all the groups was compared by Kruskal–Wallis test and Mann–Whitney U test. P < .05 was considered statistically significant.
3. Results
3.1. CD247+ T cells were downregulated in PBMCs and TIL of ovarian carcinoma
The PBMCs were obtained from OC patients (10 cases, as tumor group) and ovarian cyst (10 cases, as normal group). Flow cytometry analyses showed that the ratio of CD4+CD247+ T cells and CD8+CD247+ T cells of PBMCs were significantly downregulated in OC patients than in ovarian cyst (P < .05) (Fig. 1).
Figure 1.
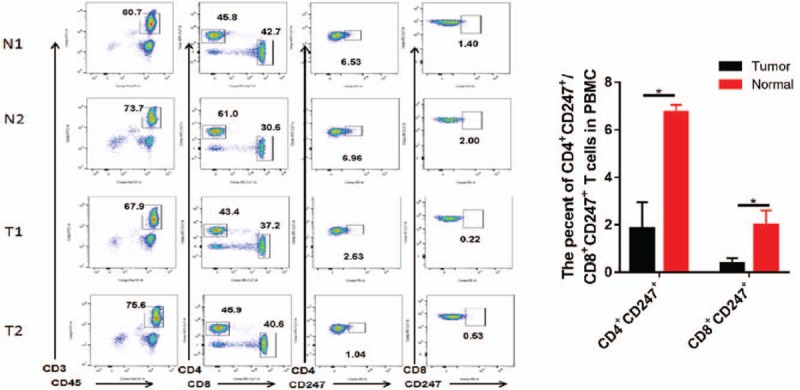
CD3+CD45+, CD4+CD247+, and CD8+CD247+T-cell detection with flow cytometry is shown. T = peripheral blood mononuclear cell (PBMC) from the ovarian cancer patients, N = PBMC from the ovarian cyst patients. Results are presented as mean ± standard deviation. ∗P < .05 (Student t test).
Ovarian TILs were obtained from OC patients (10 cases). We found that the frequencies of CD4+CD247+ T cells were significantly downregulated in OC cancer tissues than in adjacent tissues, the expression of CD4+CD247+ and CD8+CD247+ T cells in TILs also expressed significantly lower percentage in OC cancer tissues than in adjacent tissues (Fig. 2). Meanwhile, we found that the percentage of CD4+CD247+ T cells and CD8+CD247+ T cells were significantly downregulated in OC tissues than in PBMCs (P < .05) (Fig. 2).
Figure 2.
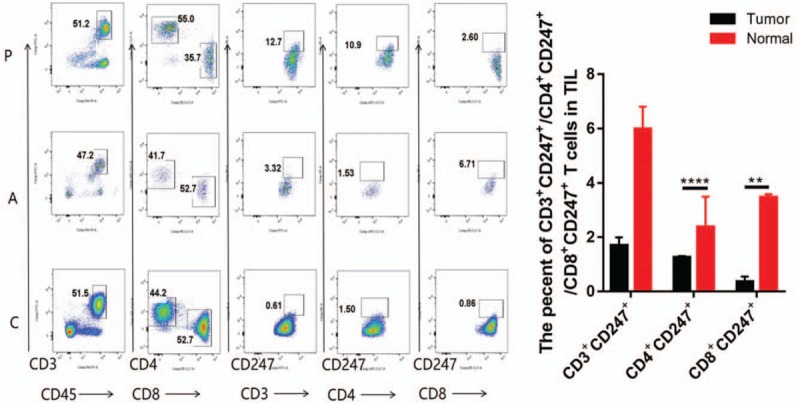
CD3+CD45+, CD3+CD247+, CD4+CD247+, and CD8+CD247+T-cell detection with flow cytometry is shown. C = Tumor-infiltrating lymphocyte (TIL) from ovarian cancer tissues, A = TIL from adjacent tissues, P = T cells from peripheral blood mononuclear cell with ovarian cancer. Results are presented as mean ± standard deviation. ∗P < .05, ∗∗P < .01 (Student t test).
3.2. Immunohistochemistry
CD247 staining was observed in various proportions of tumor cells and TILs. It was localized in the cytoplasm of T-lymphocyte cells (brown granular). In all 102 cases of primary epithelial OC, 84 cases (82.35%) were serous cystadenocarcinoma with a staining score of 143.40 ± 127.37, 11 cases (9.89%) were mucinous cystadenocarcinoma with a staining score of 38.73 ± 53.67. Three cases (2.94%) were endometrioid carcinoma and 4 cases (3.92%) were clear cell carcinoma, with a staining score of 52.67 + 44.96 and 80.25 + 98.12, respectively, which revealed significant differences between the 4 subgroups (P < .05; Table 1, Fig. 3).
Figure 3.
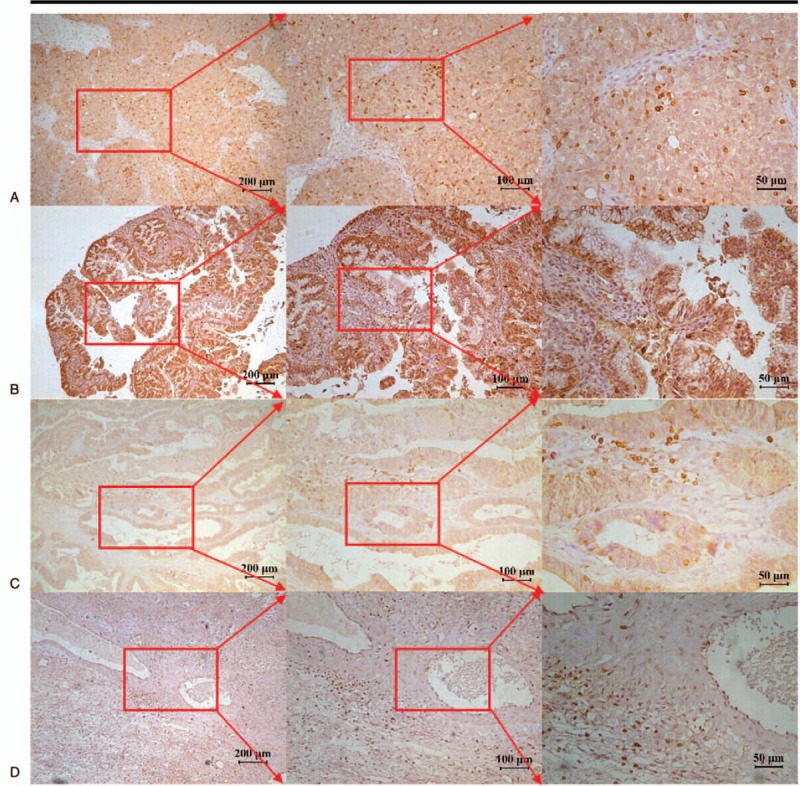
CD247 immunohistochemical staining in different types of ovarian cancer. (A) Serous adenocarcinoma, (B) mucinous adenocarcinoma, (C) endometrioid adenocarcinoma, and (D) clear cell carcinoma.
3.3. Relationship with clinicopathologic variables with IHE
In the 102 OC patients, 75 cases (75.53%) were poorly differentiated, 19 cases (18.63%) were moderately differentiated, and 8 patients (7.84%) were well differentiated, with a staining score of 153.77 ± 128.65, 58.16 ± 71.25, and 39.13 ± 53.65, respectively (U = 19.54, P < .001; Table 1, Fig. 4).
Figure 4.
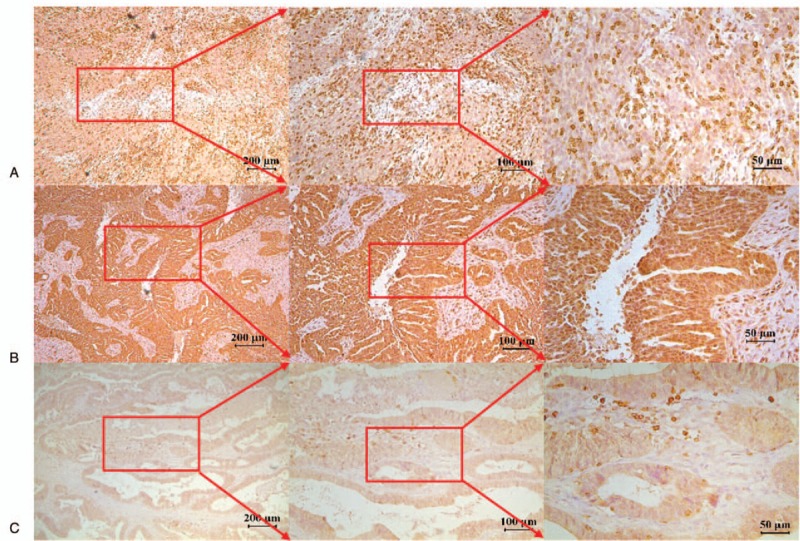
CD247 immunohistochemical staining in 3 types differentiation of ovarian cancer. (A) Poorly differentiated case. (B) Moderately differentiated case. (C) Well-differentiated case.
In the 102 epithelial OC patients, 27 cases (26.47%) were in early stage (stages I and II), and 75 cases were in late stage (73.53%), including stages III and IV. The staining score of CD247 in patients of early and late stages was 125.00 ± 153.43 and 127.68 ± 112.08, respectively (U = 1.134, P = .257; Table 1). However, there was no statistical significance among these subgroups.
In the 102 epithelial OC patients, 99 cases had the history of lymphadenectomy in which 47 patients (47.47%) had no lymphatic metastasis and 52 patients (52.53%) exhibited lymphatic metastasis, with a staining score of 134.79 ± 145.57 and 126.54 ± 101.36, respectively (U = 0.718, P = .473; Table 1).
4. Discussion
In several types of tumors, including OC, the presence of TILs is associated with a good prognosis.[21–24] The presence of TILs within the TME is considered to be an indication of the host immune response to tumor antigens. It was assumed CD247 could be the basis of T-lymphocyte cell functional deficit. Phosphorylation of the CD247 is an essential step in the intracellular signaling pathway leading to T-lymphocyte activation. The function of CD247 enhances the TCR signaling cascade and it is necessary for assembling of the TCR/CD3 complex on the surface of T lymphocytes.[25,26] It is well established that defective CD247 function leads to impaired activation of T cells upon engagement of the TCR.[27,28] Downregulation of CD247 has been described in many malignancies such as melanoma, cervical, pancreatic, ovarian, breast and head and neck cancer.[17,18,29,30] In this study, we observed that the expression of CD247 in peripheral blood lymphocytes from patients with ovarian carcinomas is decreased compared with peripheral blood lymphocytes from ovarian cyst patients and the expression of CD247 in TILs with cancer tissue is decreased compared with adjacent tissues. This may help explain, at least in part, why patients with OC have impaired T-cell response. Meanwhile, we found that the percentage of CD247+TILs were significantly lower in OC tissues than in PBMCs, that means the suppressive influences of the TME was obviously in OC. Another research showed that CD247 expression was negatively correlated with clinical stage, tumor progression and lymph node metastasis of patients and higher expression was in favor of better 5-year survival including stomach cancer, oral cancer, melanoma and esophageal cancer.[31,32] But in our study, CD247+TIL of OC patients with lymph node metastasis was less than the patient without metastasis, but is no statistical significance. The mechanisms of CD247 downregulation are still not fully understood. Secretion of arginase-1 by activated macrophages, granulocytes, and MDSC has been shown to induce the loss of CD247 and is an important mechanism of T-cell suppression in tumor-bearing mice and humans.[33,34]
Despite satisfactory chemotherapy regimens for OC, its 5-year survival rate has not been significantly improved in the past 50 years, partly due to the drug resistance of the classical chemotherapy regimens of platinum and paclitaxel.[35,36] At present, there is no obvious effective molecular biomarkers for the early screening of OC and most of methods for OC are aimed to finding new treatment rather than preventive measures.[37] With the development of immunology, it is recognized that immunologic factors are closely related to the development and outcome of tumor. There are many pathologic categories in OC. Our study found than CD247+TIL in serous adenocarcinoma were more than in mucinous adenocarcinoma and CD247+TIL in poorly differentiated cancer were more than in well-differentiated cancer, which was consistent with CD8+TIL in OC, so we could use the characteristic of OC for the immunotherapy. There were no statistical significance in CD247+TIL between early stage OC and late stage OC, which is different from another cancers including stomach cancer in which higher expression was in favor of good survival, this suggests that even in its early stages, the patients of OC has poor immunity and poor outcome. Pathological examination also found that expression of CD247+TIL in cancer nest was significantly less than that in cancer stromal, which was consistent with the distribution of CD4+TIL and CD8+TIL in OC.
Collectively, we showed that CD247 was downregulated in OC, and such abnormal expression of CD247 is related with differentiation and classification in OC. Study have demonstrated Transgenic expression of a CD3-zeta signaling chimeric antigen receptor recovered hyporesponsive T cells to full effector functions.[38] However, how to increase the CD247+TIL and improve the survival on the clinical outcomes of OC needed to be further investigated, including both in vitro experiment and animal model.
Author contributions
Conceptualization: Xiaohui Rui, Jingting Jiang.
Data curation: Ming Xu, Lijuan Shi.
Formal analysis: Yi Zhou.
Investigation: Bin Xu, Dawei Zhu, Xiaohui Rui, Dachuan Zhang.
Methodology: Wenfeng Ye, Yi Zhou, Ming Xu, Jingting Jiang.
Project administration: Yi Zhou, Xiaohui Rui, Lijuan Shi.
Resources: Dachuan Zhang.
Visualization: Bin Xu.
Writing – original draft: Wenfeng Ye.
Writing – review & editing: Jingting Jiang.
Footnotes
Abbreviations: OC = ovarian cancer, PBMC = peripheral blood mononuclear cell, TCR = T-cell antigen receptor, TILs = tumor-infiltrating lymphocytes, TME = tumor microenvironment.
How to cite this article: Ye W, Zhou Y, Xu B, Zhu D, Rui X, Xu M, Shi L, Zhang D, Jiang J. CD247 expression is associated with differentiation and classification in ovarian cancer. Medicine. 2019;98:51(e18407).
WY, YZ, and BX are contributed equally to the work.
All persons gave their informed consent prior to their inclusion in the study
This study was supported by the National Key R&D Program (No. 2018YFC1313400), the National Science and Technology supporting Program (No. 2015BAI12B12), the Joint Research Fund for Overseas Chinese, Hong Kong and Macao Scholars (No. 31729001), the National Natural Science Foundation of China (No. 31570877, No. 31570908), and the Key R&D Project of Science and Technology Department of Jiangsu Province (BE2018645).
The authors have no conflicts of interest to disclose.
References
- [1].Narod S. Can advanced-stage ovarian cancer be cured? Nat Rev Clin Oncol 2016;13:255–61. [DOI] [PubMed] [Google Scholar]
- [2].Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- [3].Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol 2008;9:730–56. [DOI] [PubMed] [Google Scholar]
- [4].Hede K. Environmental protection: studies highlight importance of tumor microenvironment. J Natl Cancer Inst 2004;96:1120–1. [DOI] [PubMed] [Google Scholar]
- [5].Germain RN, Stefanova I. The dynamics of T cell receptor signaling: complex orchestration and the key roles of tempo and cooperation. Annu Rev Immunol 1999;17:467–522. [DOI] [PubMed] [Google Scholar]
- [6].Leo A, Wienands J, Baier G, et al. Adapters in lymphocyte signaling. J Clin Invest 2002;109:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kurt RA, Urba WJ, Smith JW, et al. Peripheral T lymphocytes from women with breast cancer exhibit abnormal protein expression of several signaling molecules. Int J Cancer 1998;78:16–20. [DOI] [PubMed] [Google Scholar]
- [8].Rabinowich H, Banks M, Reichert TE, et al. Expression and activity of signaling molecules in T lymphocytes obtained from patients with metastatic melanoma before and after interleukin 2 therapy. Clin Cancer Res 1996;2:1263–74. [PubMed] [Google Scholar]
- [9].Whiteside TL. Immune cells in the tumor microenvironment. Mechanisms responsible for functional and signaling defects. Adv Exp Med Biol 1998;451:167–71. [PubMed] [Google Scholar]
- [10].Rabinowich H, Reichert TE, Kashii Y, et al. Lymphocyte apoptosis induced by Fas ligand- expressing ovarian carcinoma cells. Implications for altered expression of T cell receptor in tumor-associated lymphocytes. J Clin Invest 1998;101:2579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005;102:18538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hwang WT, Adams SF, Tahirovic E, et al. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol 2012;124:192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003;348:203–13. [DOI] [PubMed] [Google Scholar]
- [14].Baniyash M, Sade-Feldman M, Kanterman J. Chronic inflammation and cancer: suppressing the suppressors. Cancer Immunol Immunother 2014;63:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Baniyash M. TCR zeta-chain downregulation: curtailing an excessive inflammatory immune response. Nat Rev Immunol 2004;4:675–87. [DOI] [PubMed] [Google Scholar]
- [16].Zea AH, Rodriguez PC, Culotta KS, et al. L-Arginine modulates CD3zeta expression and T cell function in activated human T lymphocytes. Cell Immunol 2004;232:21–31. [DOI] [PubMed] [Google Scholar]
- [17].Ishigami S, Natsugoe S, Tokuda K, et al. CD3-zetachain expression of intratumoral lymphocytes is closely related to survival in gastric carcinoma patients. Cancer 2002;94:1437–42. [DOI] [PubMed] [Google Scholar]
- [18].Upreti D, Zhang ML, Bykova E, et al. Change in CD3zeta-chain expression is an independent predictor of disease status in head and neck cancer patients. Int J Cancer 2016;139:122–9. [DOI] [PubMed] [Google Scholar]
- [19].Kulkarni DP, Wadia PP, Pradhan TN, et al. Mechanisms involved in the down-regulation of TCR zeta chain in tumor versus peripheral blood of oral cancer patients. Int J Cancer 2009;124:1605–13. [DOI] [PubMed] [Google Scholar]
- [20].Thomas ML, Badwe RA, Deshpande RK, et al. Role of adhesion molecules in recruitment of Vdelta1 T cells from the peripheral blood to the tumor tissue of esophageal cancer patients. Cancer Immunol Immunother 2001;50:218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Erdag G, Schaefer JT, Smolkin ME, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res 2012;72:1070–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960–4. [DOI] [PubMed] [Google Scholar]
- [23].Schumacher K, Haensch W, Roefzaad C, et al. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res 2001;61:3932–6. [PubMed] [Google Scholar]
- [24].Nakano O, Sato M, Naito Y, et al. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res 2001;61:5132–6. [PubMed] [Google Scholar]
- [25].D’Oro U, Munitic I, Chacko G, et al. Regulation of constitutive TCR internalization by the zeta-chain. J Immunol 2002;169:6269–78. [DOI] [PubMed] [Google Scholar]
- [26].Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell 1991;64:891–901. [DOI] [PubMed] [Google Scholar]
- [27].Ohno H, Aoe T, Taki S, et al. Developmental and functional impairment of T cells in mice lacking CD3 zeta chains. EMBO J 1993;12:4357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu CP, Ueda R, She J, et al. Abnormal T cell development in CD3-zeta-/- mutant mice and identification of a novel T cell population in the intestine. EMBO J 1993;12:4863–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Upreti D, Pathak A, Kung SK. Development of a standardized flow cytometric method to conduct longitudinal analyses of intracellular CD3zeta expression in patients with head and neck cancer. Oncol Lett 2016;11:2199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pappas J, Wolfson AD, Jung WJ, et al. Differential expression of CD3zeta message and protein in tumor infiltrating lymphocytes from solid tumor specimens and malignant ascites from patients with ovarian carcinoma. Anticancer Res 2009;29:4673–82. [PubMed] [Google Scholar]
- [31].Whiteside TL. Down-regulation of zeta-chain expression in T cells: a biomarker of prognosis in cancer? Cancer Immunol Immunother 2004;53:865–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Matsuda M, Petersson M, Lenkei R, et al. Alterations in the signal-transducing molecules of T cells and NK cells in colorectal tumor-infiltrating, gut mucosal and peripheral lymphocytes: correlation with the stage of the disease. Int J Cancer 1995;61:765–72. [DOI] [PubMed] [Google Scholar]
- [33].Rodriguez PC, Zea AH, DeSalvo J, et al. L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J Immunol 2003;171:1232–9. [DOI] [PubMed] [Google Scholar]
- [34].Ochoa AC, Zea AH, Hernandez C, et al. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res 2007;13:721s–6s. [DOI] [PubMed] [Google Scholar]
- [35].Meyn RE, Stephens LC, Hunter NR, et al. Kinetics of cisplatin-induced apoptosis in murine mammary and ovarian adenocarcinomas. Int J Cancer 1995;60:725–9. [DOI] [PubMed] [Google Scholar]
- [36].Miles GD, Seiler M, Rodriguez L, et al. Identifying microRNA/mRNA dysregulations in ovarian cancer. BMC Res Notes 2012;5:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Santoiemma PP, Powell DJ., Jr Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther 2015;16:807–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rappl G, Riet T, Awerkiew S, et al. The CD3-zeta chimeric antigen receptor overcomes TCR Hypo-responsiveness of human terminal late-stage T cells. PLoS One 2012;7:e30713. [DOI] [PMC free article] [PubMed] [Google Scholar]


