Abstract
Background:
Discordant results about the causal relationship between hormone replacement therapy use (HRT) and lung cancer risk in women had been reported. We therefore conducted a meta-analysis of cohort studies to evaluate this association.
Methods:
The PubMed and Embase databases were searched. Fixed- or random-effects model was used to pool the study-specific relative risks (RRs) with corresponding 95% confidence intervals (CIs). Sensitivity analysis, publication bias, and subgroup analysis were performed.
Results:
A total of 13 cohort studies met the inclusion criteria. Combined results indicated that compared with nonusers, women with HRT use were at a decreased risk (RR: 0.95, 95% CI: 0.91-0.99, I2 = 30.8%, P for heterogeneity = .137). In subgroup analysis by geographic area, smoking statue, type of hormones, and histology type of lung cancer, no significant association between HRT use and lung cancer was observed in most subgroups except in those studies which reported risk estimates adjusted for age, body mass index, smoking, and other confounders (RR: 0.95, 95 CI: 0.91-0.99, I2 = 33.0%, P for heterogeneity = .214). Both Begg funnel plot and Egger test (P = .243) suggested no evidence for publication bias.
Conclusion:
Our meta-analysis suggests ever use of HRT is associated with a decreased risk of lung cancer in women.
Keywords: lung cancer, risk factors, hormone replacement therapy, meta-analysis
1. Introduction
Lung cancer is the most common diagnosed cancer and first leading cause of cancer death.[1] In 2019, an estimated 228,150 men and women will be diagnosed with lung cancer and 142,670 will die from this disease in the United States.[1] The etiology of lung cancer is largely unclear. Tobacco smoking is believed to be the established risk factor for lung cancer.[2,3] Women appear more likely than men to develop adenocarcinoma, especially among never smokers and have better survival than men globally.[2,3] Moreover, estrogen and progesterone receptor expression in normal and lung tumor cells were confirmed, estrogens may promote lung tumorigenesis, and progesterone inhibited growth of progesterone receptor-positive non-small-cell lung cancers cells.[4,5] Based on the experimental evidences and discrepancies on lung cancer pathology, risk factors, and prognosis between men and women, the role of female hormonal factors in lung cancer development had been the subject of speculation for many years. Hormone replacement therapy (HRT) is the most effective treatment for menopausal symptoms in postmenopausal women, as well as younger patients who are into early menopause due to surgery, chemotherapy, or radiotherapy. In recent years, a great number of epidemiological studies have assessed the relationship between HRT use and lung cancer risk, however, mixed results were reported.[6–22] Some studies found a significant inverse or nonsignificant inverse relation[6–8,14,15]; some found an increased risk, although the association was not statistically significant.[17,20,22] In order to delineate the role of HRT use in lung cancer risk, a systematic search of the literature and a meta-analysis of cohort studies were performed.
2. Methods
We reported this meta-analysis in accordance with PRISMA Statement.[23] Ethical approval is not required, as this study is a meta-analysis of published studies.
2.1. Literature search
Two databases PubMed and Embase databases) were searched (updated to May 9, 2019). Our search terms involved hormone, reproductive factors, menstrual factors, lung cancer, and risk. The process of search was shown step by step in Supplemental Table 1. Additional studies were reviewing the reference lists of eligible studies. No language limitation was set.
2.2. Selection criteria and exclusion criteria
We included cohort studies that assessed the association between HRT use and lung cancer risk in women. Adjusted risk estimates with 95% CIs were reported necessarily. If multiple studies pertained to the same subjects, the one with the largest sample sizes or the longest follow-up years was included. Cohort studies with crude risk estimates, case-control studies, conference abstracts, reviews, and letters without original data provided were excluded.
2.3. Data extraction
Two reviewers independently extracted the following information: author, year of publication, country, the period of cohort enrolled, the number of lung cancer cases and participants, adjusted risk estimates and their 95% CIs in maximally multivariable-adjusted models, and confounder for control. Any disagreements were solved by discussion to reach a consensus.
2.4. Statistical analysis
RR was used as a common measure of the association between HRT and lung cancer risk. To compute the summary RR for ever HRT users vs non-users, when one study reported more than one category fell into the range considered for ever HRT use, we first combined the corresponding estimates with fixed-effects model before combined with other studies. The method was widely used in previous meta-analyses.[24–26] Subgroup analyses were performed according to geographic area, smoking statue, confounder, type of hormones, and histologic subtype. Publication bias was estimated by the Begg funnel plot and Egger test at the P < .10 level of significance.[27,28] We conducted a sensitivity analysis by excluding one study at a time to examine whether its effect on the overall results. The Higgin I2 statistic and Q test were used to assess the heterogeneity.[29] The value of I2 ranges from 0% to 100%.[30] When P for Q test < .1, the heterogeneity was considered to be significant. In the presence of significant heterogeneity, the combined risk estimates were computed using random-effects models, otherwise, fixed-effects models were adopted.[31] All analyses were performed in Stata version 12.0 (StataCorp. College Station, TX).
3. Result
The flowchart of study selection was presented in Figure 1. A total of 432 articles were identified from PubMed and Embase databases. Five cohort studies as potentially available studies were identified through additional search. After excluding the duplicates from two databases and reviewing the titles and abstracts, 67 studies with full-text were assessed. Fifty four studies were further excluded as these studies were reported as case-control studies, conference abstracts, meta-analyses, reviews, notes, comments, or study protocols. Therefore, 13 cohort studies were identified in this meta-analysis.[6–18]
Figure 1.
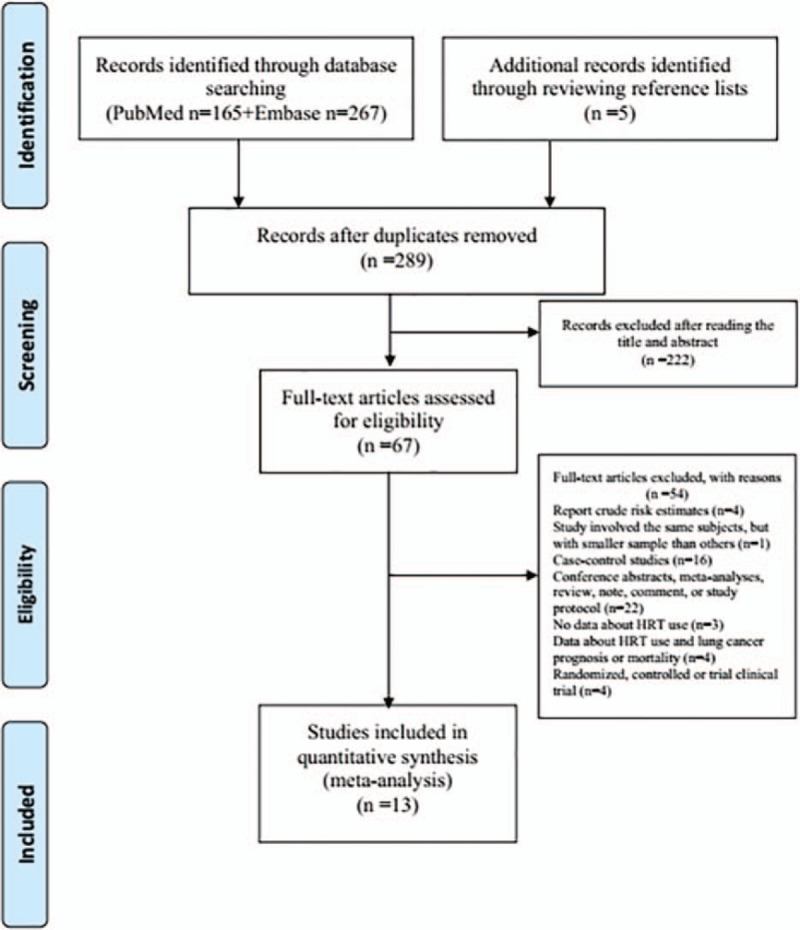
Flow chart for the process of selecting eligible publications.
Table 1 shows the main characteristic of included studies. A total of 11,391 lung cases were diagnosed in 968,440 participants. These studies were performed in USA, China, Singapore, Canada, Japan, and Italy, and published between 2005 and 2015. The follow-up year ranges from five to thirty. Various confounders, such as age, smoking history, body mass index (BMI), education, ethnicity, dietary factors, oral contraceptive use, personal disease history, reproductive factor, and menstrual factors, were taken into consideration in original studies.
Table 1.
Characteristics of the included studies of the relationship between lung cancer risk and hormone replacement therapy.
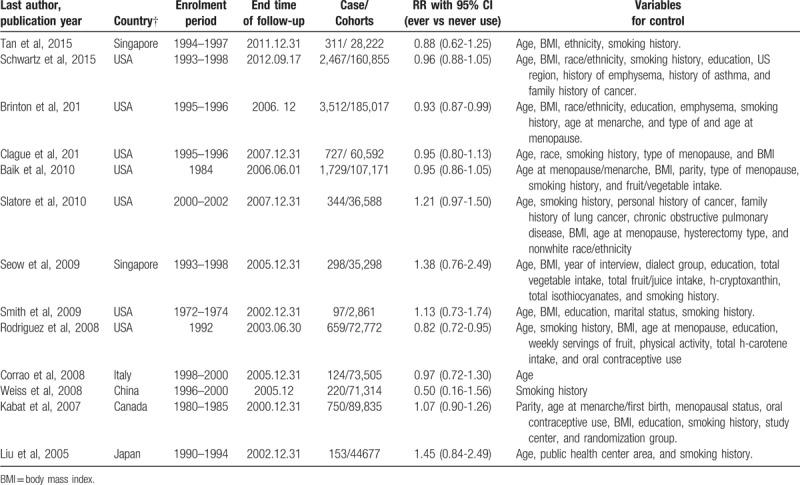
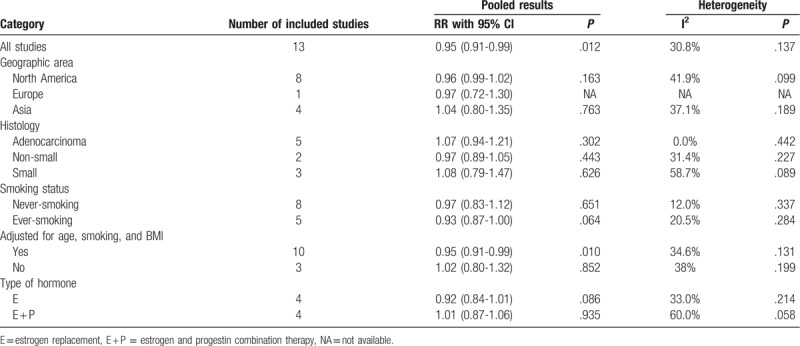
Figure 2 shows the RRs with corresponding 95% CIs for each study and the pooled result. No significant heterogeneity was observed (I2 = 30.8%, P or heterogeneity = .137). The pooled RR and 95% CI were 0.95 (0.91-0.99). Furthermore, subgroup analyses were performed according to geographic region, smoking statue, confounders controlled, type of hormones, and histologic subtype (Table 2). Significant heterogeneity was found in subgroup of small lung cancer (I2 = 58.7%, P for heterogeneity = .089) and estrogen and progestin combination therapy (I2 = 60.0%,P for heterogeneity = .058). Subgroup analyses showed that the significant association was only observed in those studies with age, BMI, smoking, and other confounders considered (RR: 0.95, 95% CI: 0.91-0.99, I2 = 34.6% p for heterogeneity = 0.131). The pooled RRs and 95% CIs were 0.96 (0.99-1.02), 0.97 (0.72-1.30), 1.04 (0.80-1.35), 1.07 (0.94-1.21), 0.97 (0.89-1.05), 1.08 (0.79-1.47), 0.97 (0.83-1.12), 0.95 (0.91-0.99), 0.92 (0.84-1.01), and 1.01 (0.87-1.06) for North America, Europe, Asia, adenocarcinoma, non-small lung cancer, small lung cancer, never-smoking, ever-smoking, estrogen, and estrogen and progestin combination therapy, respectively.
Figure 2.
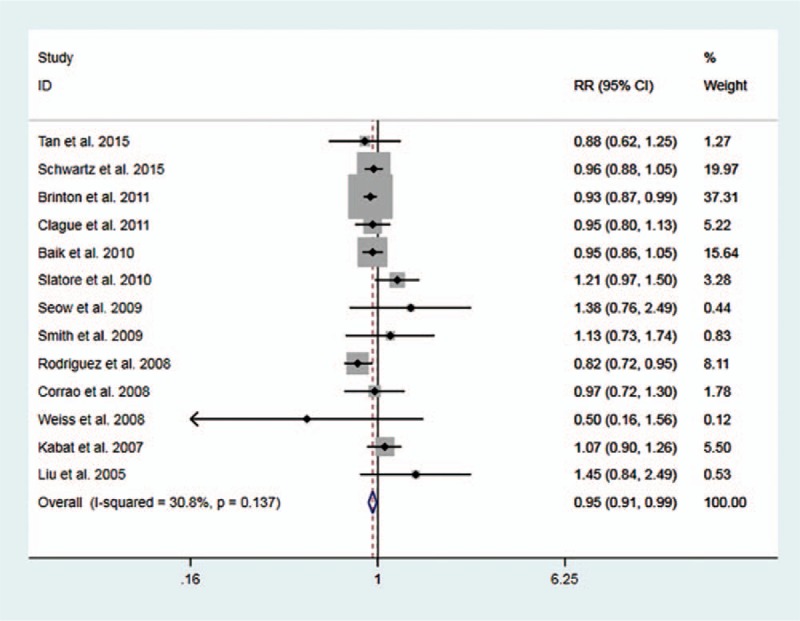
Forest plot for study-specific and pooled RRs and 95% CIs of HRT use and lung cancer risk.
Table 2.
Results of subgroup analysis.
Sensitivity analyses were performed to assess the influence of a single study on the overall risk estimate by excluding one study in each turn. Sensitivity analyses indicated that our results were not robustness (Table 3). The Begg funnel plot does not show any substantial asymmetry (Fig. 3). The Egger test suggested no evidence of publication bias (P = .243).
Table 3.
Results of sensitivity analyses.
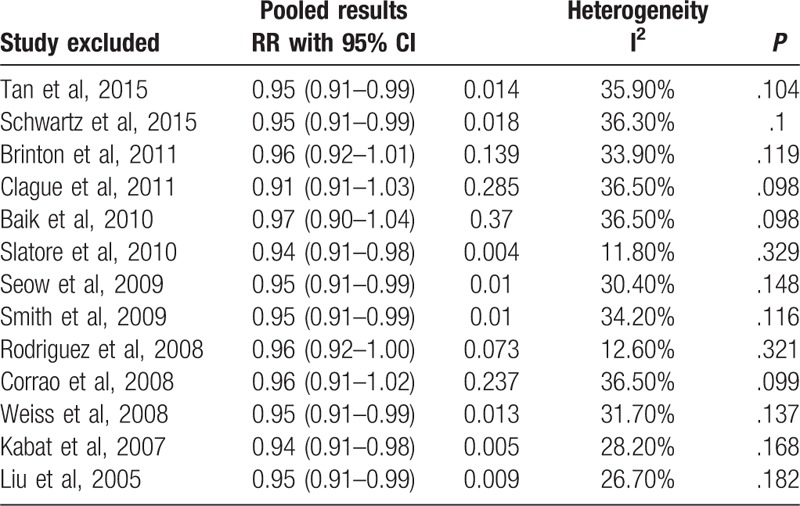
Figure 3.
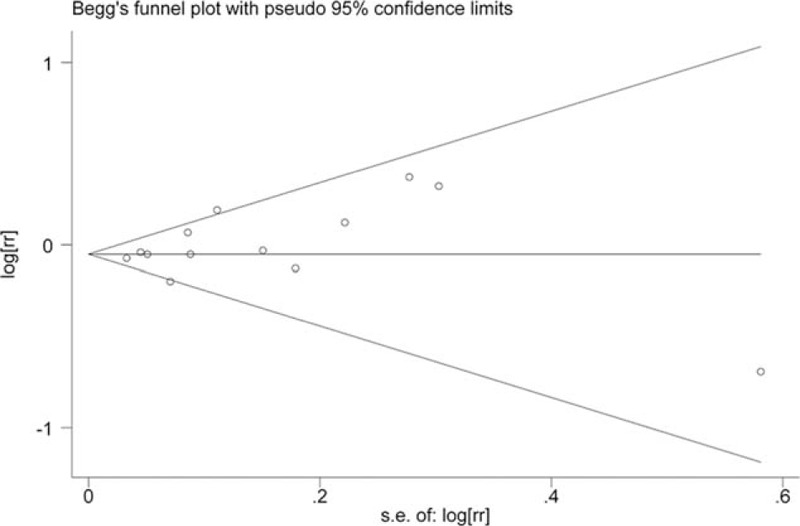
Begg funnel plot.
4. Discussion
In the current meta-analysis of 13 cohort studies, we found that HRT use was associated with a decrease risk of lung cancer in women. Before interpreting our results further, important issues need to be considered.
4.1. Dose-response
Eight of 13 studies evaluated the lung cancer risk with duration of HRT use.[5–10,15,16] Most of these studies indicated that there was no trend with duration of use. Kabat et al found that the associated trend over levels of duration of HRT use was of borderline statistical significance (P = .07). Specifically, women with ten years or more of HRT use were at elevated risk (RR:1.51, 95% CI: 1.14-1.99). Assessment of a dose-risk relationship in a meta-analysis of epidemiology studies provides evidence for a suspected causal relationship between exposure and disease. However, we cannot perform a dose-response analysis of duration of HRT use according to the method proposed by Greenland et al[32] and Orsini et al,[33] as insufficient data (i.e., at least 3 quantitative categories and the number of cases and person-years in each category) was reported in original studies. Therefore, the shape of dose-response relationship is still unclear.
4.2. The role in the carcinogenesis of lung cancer subtypes
Our major analysis indicated that women with HRT use were associated with a decreased lung cancer risk. In subgroup analysis, a non-significantly increased risk was observed in adenocarcinoma (RR: 1.07, 95% CI: 0.94-1.21) and small cell lung cancer (RR: 1.08, 95% CI: 0.79-1.47), but not in non- small cell lung cancer (RR: 0.97, 95% CI: 0.89-1.05). Lung adenocarcinoma is the predominant histological subtype of lung cancer in women. The discrepancy in lung cancer subtypes associated with HRT is open to discussion.
4.3. The gap between observational studies and randomized controlled trials
Subgroup analysis of four randomized controlled trials in a meta-analysis showed a positive association with borderline significance between HRT use and lung cancer (RR = 1.18, 95% CI 0.99-1.42).[32] However, result from our meta-analysis of cohort studies indicated a decreased risk of lung cancer in HRT users. The significance of the discrepancy is unclear. Compared with cohort studies, randomized controlled trials have several merits, such as blindness, randomness, and standardization. After careful reading those four randomized controlled trials, several unique characteristics should be proposed. First, all randomized controlled trials were not originally designed to assess the relationship between HRT use and lung cancer incidence. The subjects were carefully selected, and thus it is not representative of the general population. Second, the simple size was relatively small. Third, the follow-up time was relatively short. Therefore, observational studies may better reflect a relatively true real-world study.
4.4. Comparison with other meta-analyses
Several meta-analyses on this issue have been published.[4,34–39] Two meta-analyses reported HRT use was associated with a decreased risk of female lung cancer,[34,38] one found an increased lung adenocarcinoma risk in nonsmoking women with HRT use [35], and three indicated HRT use had no effect on the risk of lung cancer.[4,35,38] Noticeably, findings from previous meta-analyses were mainly based on retrospective case-control studies.[4,34–37] Furthermore, a pooling analysis of observational studies and randomized controlled trials may result in methodological error.[34,35] In the latest meta-analysis by Bae et al,[38] fourteen cohort studies were included. However, 7 of 14 cohort studies provided crude risk estimates. As a meta-analysis of observational studies, the effect of confounding factor and bias (recall and selection bias) are major concerns. To clarify the issue, we performed an updated meta-analysis which only included prospective cohort studies with adjusted risk estimates reported. Thus, a more precise estimation of the relationship between HRT use and lung cancer may be derived.
4.5. Implications for clinical practice
Since HRT use remains the most widely used and effective treatment for postmenopausal symptoms in women, its benefits and harms effect are concern. Previous findings suggested HRT use was associated with an increased risk of breast cancer, endometrial cancer, stroke, and pulmonary embolism, but a decreased risk of colorectal cancer, hip fractures, and diabetes.[39,40] In our meta-analysis of prospective cohort studies, we found that HRT use was associated with a decreased risk of lung cancer. Based on these findings, we concluded those women with HRT use may be reassured by bearing decreased risk of lung cancer and menopausal replacement therapy has a complex pattern of risks and benefits.
4.6. Limitations
Several limitations should be acknowledged. First, sensitivity analysis showed our results were not robust. Second, subgroup results should be treated with considerable caution, as the small number of included studies was included. Third, residual confounders may confound the true association, although the multiple -adjusted risk estimates were adopted. Finally, potential publication bias is still a threat to the robustness of our findings.
4.7. Conclusion and future directions
In conclusion, evidence from current prospective cohort studies indicated women with ever HRT use was associated with a decreased risk of lung cancer. Further prospective studies with adequate numbers of lung cancer cases, detailed information on type of hormone use, dose of hormones, duration of HRT use, and histologic type, are needed to confirm and extend these findings.
Author contributions
Conceptualization: Baoping Lang, Chao Jin.
Data curation: Baoping Lang, Chao Jin.
Formal analysis: Baoping Lang, Chao Jin.
Writing – original draft: Baoping Lang, Chao Jin.
Writing – review & editing: Baoping Lang, Chao Jin.
Footnotes
Abbreviations: BMI = body mass index, CIs = confidence intervals, HRT = hormone replacement therapy, RRs = relative risks.
How to cite this article: Jin C, Lang B. Hormone replacement therapy and lung cancer risk in women: a meta-analysis of cohort studies: hormone replacement therapy and lung cancer risk. Medicine. 2019;98:51(e17532).
The authors have no funding and conflicts of interest to disclose.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Jin K, Wu M, Zhou JY, et al. Tobacco smoking modifies the association between hormonal factors and lung cancer occurrence among post-menopausal chinese women. Transl Oncol 2019;12:819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].He F, Xie JX, Liu CL, et al. The relationship of lung cancer with menstrual and reproductive factors may be influenced by passive smoking, cooking oil fumes, and tea intake: A case-control study in Chinese women. Medicine (Baltimore) 2017;96:e8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang Y, Yin Z, Shen L, et al. Menstrual factors, reproductive factors and lung cancer risk: a meta-analysis. Zhongguo Fei Ai Za Zhi 2012;15:701–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ishibashi H, Suzuki T, Suzuki S, et al. Progesterone receptor in non-small cell lung cancer--a potent prognostic factor and possible target for endocrine therapy. Cancer Res 2005;65:6450–8. [DOI] [PubMed] [Google Scholar]
- [6].Tan HS, Tan MH, Chow KY, et al. Reproductive factors and lung cancer risk among women in the Singapore Breast Cancer Screening Project. Lung Cancer 2015;90:499–508. [DOI] [PubMed] [Google Scholar]
- [7].Schwartz AG, Ray RM, Cote ML, et al. Hormone use, reproductive history, and risk of lung cancer: the women's health initiative studies. J Thorac Oncol 2015;10:1004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brinton LA, Gierach GL, Andaya A, et al. Reproductive and hormonal factors and lung cancer risk in the NIH-AARP Diet and Health Study cohort. Cancer Epidemiol Biomarkers Prev 2011;20:900–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Clague J, Reynolds P, Sullivan-Halley J, et al. Menopausal hormone therapy does not influence lung cancer risk: results from the California Teachers Study. Cancer Epidemiol Biomarkers Prev 2011;20:560–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Baik CS, Strauss GM, Speizer FE, et al. Reproductive factors, hormone use, and risk for lung cancer in postmenopausal women, the Nurses’ Health Study. Cancer Epidemiol Biomarkers Prev 2010;19:2525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Slatore CG, Chien JW, Au DH, et al. Lung cancer and hormone replacement therapy: association in the vitamins and lifestyle study. J Clin Oncol 2010;28:1540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Seow A, Koh WP, Wang R, et al. Reproductive variables, soy intake, and lung cancer risk among nonsmoking women in the Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev 2009;18:821–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Smith JR, Barrett-Connor E, Kritz-Silverstein D, et al. Hormone use and lung cancer incidence: the Rancho Bernardo cohort study. Menopause 2009;16:1044–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rodriguez C, Spencer Feigelson H, et al. Postmenopausal hormone therapy and lung cancer risk in the cancer prevention study II nutrition cohort. Cancer Epidemiol Biomarkers Prev 2008;17:655–60. [DOI] [PubMed] [Google Scholar]
- [15].Weiss JM, Lacey JV, Jr, Shu XO, et al. Menstrual and reproductive factors in association with lung cancer in female lifetime nonsmokers. Am J Epidemiol 2008;168:1319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Corrao G, Zambon A, Conti V, et al. Menopause hormone replacement therapy and cancer risk: an Italian record linkage investigation. Ann Oncol 2008;19:150–5. [DOI] [PubMed] [Google Scholar]
- [17].Kabat GC, Miller AB, Rohan E. Reproductive and hormonal factors and risk of lung cancer in women: a prospective cohort study. Int J Cancer 2007;120:2214–20. [DOI] [PubMed] [Google Scholar]
- [18].Liu Y, Inoue M, Sobue T, et al. Reproductive factors, hormone use and the risk of lung cancer among middle-aged never-smoking Japanese women: a large-scale population-based cohort study. Int J Cancer 2005;117:662–6. [DOI] [PubMed] [Google Scholar]
- [19].Olsson H, Bladström A, Ingvar C. Are smoking-associated cancers prevented or postponed in women using hormone replacement therapy? Obstet Gynecol 2003;102:565–70. [DOI] [PubMed] [Google Scholar]
- [20].Pukkala E, Tulenheimo-Silfvast A, Leminen A. Incidence of cancer among women using long versus monthly cycle hormonal replacement therapy, Finland 1994–1997. Cancer Causes Control 2001;12:111–5. [DOI] [PubMed] [Google Scholar]
- [21].Persson I, Yuen J, Bergkvist L, et al. Cancer incidence and mortality in women receiving estrogen and estrogen-progestin replacement therapy–long-term follow-up of a Swedish cohort. Int J Cancer 1996;67:327–32. [DOI] [PubMed] [Google Scholar]
- [22].Adami HO, Persson I, Hoover R, et al. Risk of cancer in women receiving hormone replacement therapy. Int J Cancer 1989;44:833–9. [DOI] [PubMed] [Google Scholar]
- [23].Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dong JY, Zhang YH, Qin LQ. Erectile dysfunction and risk of cardiovascular disease: meta-analysis of prospective cohort studies. J Am Coll Cardiol 2011;58:1378–85. [DOI] [PubMed] [Google Scholar]
- [25].Aune D, Lau R, Chan DS, et al. Dairy products and colorectal cancer risk: a systematic review and meta-analysis of cohort studies. Ann Oncol 2012;23:37–45. [DOI] [PubMed] [Google Scholar]
- [26].Gagne JJ, Power MC. Anti-inflammatory drugs and risk of Parkinson disease: a meta-analysis. Neurology 2010;74:995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [28].Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [30].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [32].Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–9. [DOI] [PubMed] [Google Scholar]
- [33].Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata J 2006;6:40–57. [Google Scholar]
- [34].Yao Y, Gu X, Zhu J, et al. Hormone replacement therapy in females can decrease the risk of lung cancer: a meta-analysis. PLoS One 2013;8:e71236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Greiser CM, Greiser EM, Dören M. Menopausal hormone therapy and risk of lung cancer-Systematic review and meta-analysis. Maturitas 2010;65:198–204. [DOI] [PubMed] [Google Scholar]
- [36].Oh SW, Myung SK, Park JY, et al. Hormone therapy and risk of lung cancer: a meta-analysis. J Womens Health (Larchmt) 2010;19:279–88. [DOI] [PubMed] [Google Scholar]
- [37].Chen X, Cai L. Meta-analysis of the effects on hormone replacement therapy and oral contraceptives associated with female lung cancer risk. Wei Sheng Yan Jiu 2009;38:672–6. [PubMed] [Google Scholar]
- [38].Bae JM, Kim EH. Hormonal replacement therapy and the risk of lung cancer in women: an adaptive meta-analysis of cohort studies. J Prev Med Public Health 2015;48:280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA 2013;310:1353–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nelson HD, Humphrey LL, Nygren P, et al. Postmenopausal hormone replacement therapy: scientific review. JAMA 2002;288:872–81. [DOI] [PubMed] [Google Scholar]


