Abstract
Hydrogen formed by small intestinal bacterial overgrowth in patients with non-constipated irritable bowel syndrome has an inverse relationship with obesity. However, the effect of eradicating small intestinal hydrogen-producing bacterial overgrowth on the body weight of these patients has not yet been reported. The aim of this study was to investigate body weight changes after eradicating small intestinal bacterial overgrowth with rifaximin treatment in patients with non-constipated irritable bowel syndrome.
We reviewed the charts of patients with non-constipated irritable bowel syndrome who showed abdominal symptoms with documented lactulose hydrogen breath test results in order to diagnose small intestinal bacterial overgrowth. A total of 153 patients were enrolled in the study and divided into quartiles according to body mass index (BMI) and body weight.
In the lowest body weight quartile, the BMI and body weight were significantly increased (0.4 kg/m2, P = .038; 0.6 kg, P = .010, respectively) in patients with negative lactulose hydrogen breath tests after rifaximin treatment. However, there was no significant change in body weight in the other quartiles. Despite treatment with rifaximin for 12 weeks, there was no change in BMI or body weight in any group of patients with consistently positive lactulose hydrogen breath tests.
Eradication of hydrogen formed by small intestinal bacterial overgrowth does not cause clinically significant changes in body weight.
Keywords: body weight, hydrogen, irritable bowel syndrome, rifaximin, small intestinal bacterial overgrowth
1. Introduction
Irritable bowel syndrome (IBS) is a functional bowel disorder.[1] According to the Rome III criteria, IBS is characterized by abdominal pain or discomfort during defecation, and altered stool form and passage.[2] Dysregulation of the brain–gut axis, the autonomic nervous system, genetic predisposition, visceral hypersensitivity, and altered levels of gastrointestinal hormones have been suggested as causes of IBS, but recent studies reported that disturbances in the gut microbiota, including small intestinal bacterial overgrowth (SIBO), also play important roles.[3–5] Indeed, the symptoms of IBS are similar to those of SIBO and mounting evidence supports an association between IBS and SIBO.[6,7]
SIBO is characterized by an increase in the number and/or presence of atypical bacteria in the upper gut, which in turn affects intestinal motility and metabolism differently, depending on whether hydrogen gas or methane is produced.[8] Among these gases, we recently showed that hydrogen-forming SIBO was associated with non-constipated IBS and had an inverse relationship with obesity.[8] In other words, the prevalence of hydrogen-forming SIBO in the lower-weight group of patients with non-constipated IBS was higher than in the obese group. However, the effect on body weight following rifaximin treatment to eradicate the SIBO in this lower-weight group has not yet been reported.
Rifaximin is a semi-synthetic, rifamycin-based antibiotic with good antibacterial activity and low gastrointestinal absorption. Its antimicrobial action has been reported to have few side effects, so it is suitable for the long-term treatment of hydrogen-forming SIBO.[9,10] Indeed, in our previous study, clinical resistance to rifaximin did not develop when it was used to treat SIBO for up to 3 months.[11] We have been observing body weight changes in rifaximin-treated patients with hydrogen-forming SIBO since 2015 and we report our findings here.
2. Methods
2.1. Study data
The electronic medical records of consecutive patients who completed a lactulose hydrogen breath test (LHBT) for the diagnosis of SIBO between March 2015 and January 2017 in the Department of Family Medicine and the Health Promotion Center of Ajou University Hospital were reviewed to establish their eligibility for this study. Patients were considered eligible for inclusion if they were over 19 years of age, had IBS symptoms (assessed according to the Rome III diagnostic criteria)[12] at the time of visit, and had undergone esophagogastroduodenoscopy, colonoscopy, or abdominal ultrasonography within the previous 2 years. IBS was defined as recurrent abdominal pain or discomfort (at least 3 days per month in the last 3 months) with an onset at least 6 months before the diagnosis associated with 2 or more of the following:
-
(1)
improvement with defecation,
-
(2)
onset associated with a change in the frequency of stools, and
-
(3)
onset associated with a change in the form of the stools.
The IBS subgroups were classified as constipation-predominant IBS, diarrhea-predominant IBS, and mixed type (both constipation and diarrhea IBS). The exclusion criteria were:
-
(1)
gastrointestinal diseases other than non-constipated IBS, especially constipation-predominant IBS with SIBO that produced methane, inflammatory bowel diseases, or celiac disease;
-
(2)
intake of medications, such as antibiotics, laxatives, non-steroidal anti-inflammatory drugs, and acid-suppressing medications (H2 blocker or proton pump inhibitors) in the previous 2 months;
-
(3)
thyroid disease; and
-
(4)
a history of intestinal surgery (except appendectomy).
A positive LHBT was defined by one of the following criteria: a baseline hydrogen value >20 ppm[13,14] and/or an increase in hydrogen above a baseline value >20 ppm between 15 and 90 minutes after lactulose ingestion.[15,16] LHBT was achieved under standard conditions. The patients were asked to refrain from antibiotic use for 2 weeks. They were also asked to discontinue probiotics for a week. To minimize basal hydrogen excretion, carbohydrate dietary restriction and avoidance of smoking for at least 24 hours prior to the test and during the test were recommended. Fasting for 12 hours before the procedure was required. Before the examination, the patients used 20 mL of antiseptic mouthwash (0.05% chlorhexidine) to eliminate fermentation by oral bacteria. End-expiratory breath samples were collected just before the ingestion of 10 g of lactulose in a 20-mL water solution. Samples were taken every 20 minutes for 90 minutes using 2 collapsible bags with a mouthpiece and a T-valve. The first bag was used to collect the dead space air and the second bag was used to collect end-expiratory air. A breath sample was aspirated from the second bag into a 20-mL plastic syringe. The hydrogen concentration in the sample was measured immediately using a BreathTracker SC Quintron gas chromatograph (Quintron Instrument Company, Milwaukee, WI).
The patients were treated as described previously.[11] Briefly, those who had positive LHBTs were given rifaximin at 1200 mg a day. The LHBT was re-administered every 4 weeks. To assess normalization, a follow-up LHBT was done for patients who reported an improvement in abdominal symptoms. After 12 weeks of treatment, rifaximin treatment was discontinued since most patients reported improvement in their abdominal symptoms, although some patients still had abnormal LHBT results. The maximal treatment duration was 12 weeks.
A total of 475 patients were eligible for the study. Of these, 206 were positive for LHBT at the initial visit and 269 were negative. Of the 206 positive LHBT patients, 53 were not followed-up. Therefore, a total of 153 patients with positive LHBT results were analyzed for this study. Of the patients with documented positive LHBT results at the initial visit, 83 patients had negative results during follow-up LHBTs after treatment with rifaximin for 4, 8, or 12 weeks (Fig. 1). Ethics committee approval was received for this study from The Institutional Review Board of Ajou University Hospital (Decision Date: 31.07.2017/Decision No: AJIRB-MED-MDB-17-207).
Figure 1.
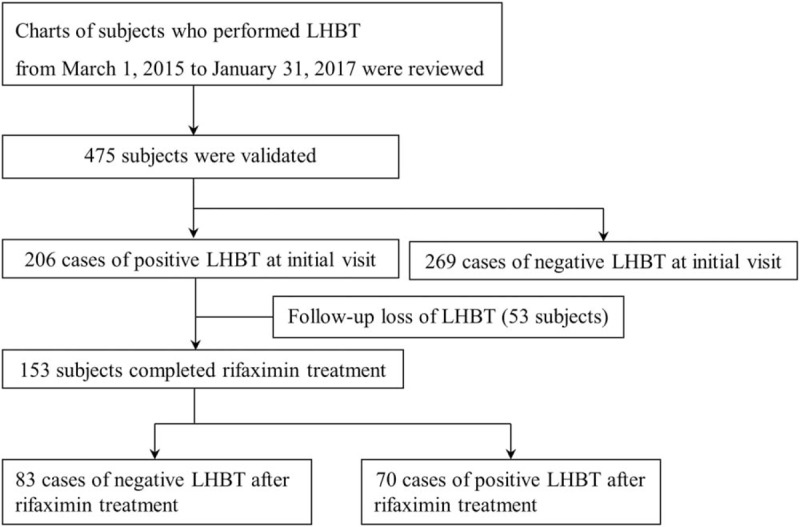
Flow chart of study patients. LHBT = lactulose hydrogen breath test.
2.2. Measurements
Hypertension was defined as systolic blood pressure of 140 mm Hg or diastolic blood pressure of 90 mm Hg or the use of antihypertensive medication. Diabetes was defined as fasting blood glucose >126 mg/dL or the use of oral hypoglycemic agents or insulin. Dyslipidemia was defined as previously diagnosed hyperlipidemia and currently taking cholesterol medication. Data on alcohol consumption were collected by means of a self-reported questionnaire. Alcohol consumption in patients was calculated and then converted to weekly alcohol consumption (grams of ethanol per week) by the graduated frequency method.[17] At the time of the hospital visit, the patients were asked about the amount of their moderate physical activity as follows: “During the last 7 days, on how many days did you do moderate physical activities for at least 30 minutes (e.g., bicycling at a regular pace, swimming at a regular pace?)”. The frequency of weekly, moderate physical activity was then recorded. Patients who had regularly smoked cigarettes during the past year were considered to be current smokers.
2.3. Statistical analyses
All continuous variables are presented as mean ± standard deviation. Categorical variables are expressed as numbers and percentages. Continuous variables and categorical variables were analyzed using an independent t-test and the chi-squared test, respectively. The patients were divided into quartiles to observe how rifaximin treatment affected body mass index (BMI) or body weight. We performed the Kolmogorov–Smirnov test to determine whether BMI or body weight was normally distributed according to quartiles and the P-value was >.05. Therefore, a paired t-test was performed to evaluate BMI and mean body weight change after rifaximin treatment. In addition, we performed a power analysis on the paired t-test. A sample size of 22 achieved 60% power to detect a mean of the paired differences of 0.2, with a known standard deviation of the differences of 0.4 and a significance level (alpha) of 0.05000 using a 2-sided paired z-test. A statistically significant difference was considered to be P < .05. All statistical analyses were performed using SPSS 20.0 (SPSS, Chicago, IL).
3. Results
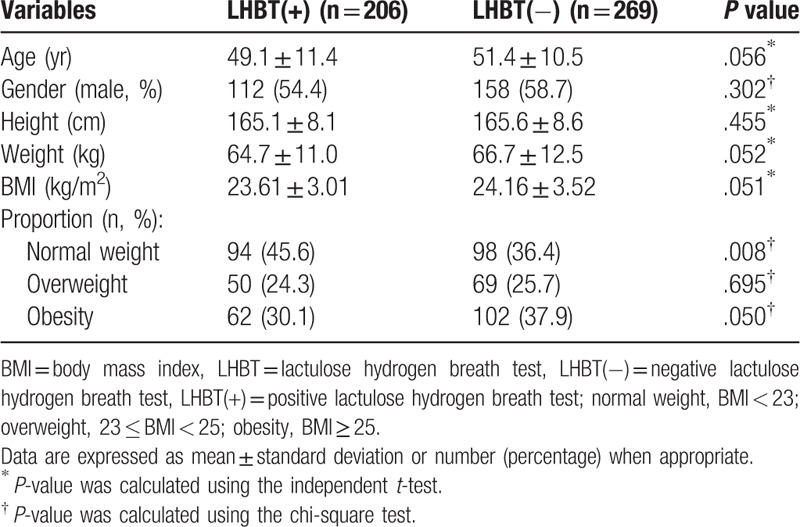
The general characteristics of the study patients according to the LHBT results at the initial visit are summarized in Table 1. Of the total of 475 patients who were eligible for this study, 206 patients had a positive initial LHBT and 269 had a negative initial LHBT. Their mean ages were 49.1 ± 11.4 and 51.4 ± 10.5, respectively. Among the positive initial LHBT patients, 112 (54.4%) were males. In the negative LHBT patients, 158 (58.7%) were males. There was no statistically significant difference in mean age, gender, height, weight, or BMI between the positive LHBT and negative LHBT patients. However, the prevalence of obesity was higher in the negative LHBT group than in the positive LHBT group.
Table 1.
General characteristics of the study subjects according to LHBT results in initial visit (n = 475).
Among the positive initial LHBT patients, 83 patients had negative LHBTs in follow-up testing conducted after rifaximin treatment, including 34 patients who had negative LHBTs after 4 weeks of treatment with rifaximin, 26 patients who had negative LHBTs after 8 weeks of treatment, and 23 patients with negative LHBTs after 12 weeks of treatment. Seventy patients had sustained positive LHBTs in follow-ups conducted after 12 weeks of rifaximin treatment. The initially positive LHBT patients were divided into 2 groups according to their follow-up LHBT results. Metabolic and social parameters were analyzed to determine whether any factors before rifaximin treatment might have affected weight change according to improvement in the LHBT after rifaximin treatment (Table 2). Except for current smoking status, there was no significant difference in any factor between the groups.
Table 2.
Comparison of pre-treatment metabolic and social parameters between the group which converted to negative LHBT after rifaximin treatment and the other group which remained positive LHBT after rifaximin treatment (n = 153).
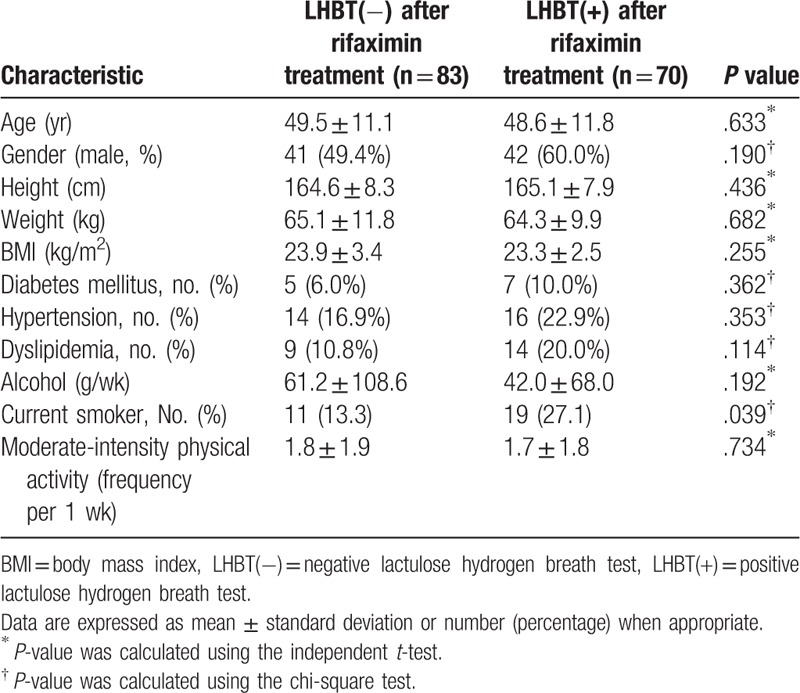
Figure 2 shows the effect of rifaximin treatment on BMI and body weight according to BMI and body weight quartiles. There was a statistically significant increase in BMI and body weight only in the lowest quartile group when the LHBT results were converted to negative after treatment with rifaximin (BMI increase of 0.4 kg/m2, P = .038; body weight increase of 0.6 kg, P = .010) (Fig. 2 a and b). There was no change in BMI or body weight in the second and higher quartiles, even though the LHBTs were converted to negative. In addition, despite the use of rifaximin for up to 3 months, no statistically significant change in BMI or body weight was observed in any of the groups whose LHBT remained positive (Fig. 2 c and d).
Figure 2.
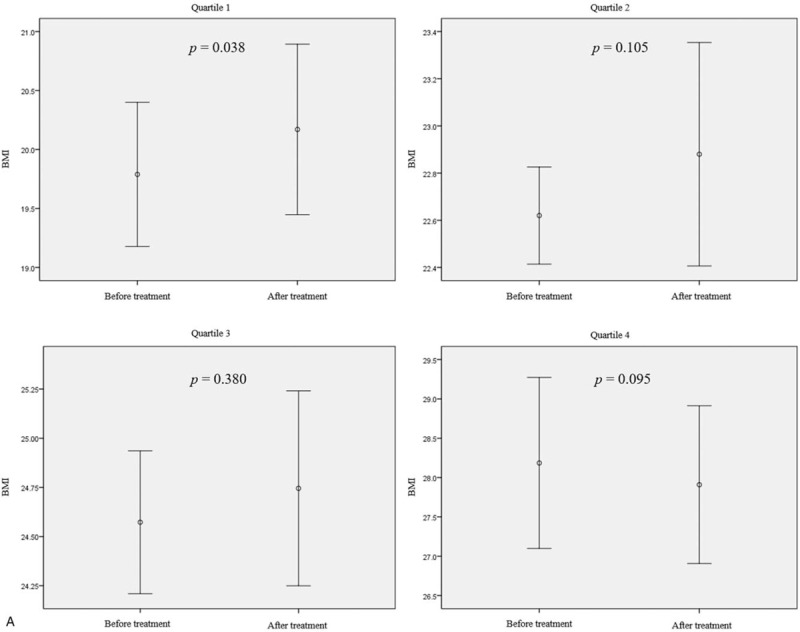
Body mass index (BMI) and body weight changes after treatment with rifaximin in patient groups by pretreatment BMI or body weight quartile. (a and b) The BMI and body weight changes according to quartiles of BMI or body weight in patients with negative LHBTs after rifaximin treatment (n = 83). (c and d) BMI and body weight changes according to the BMI or body weight quartile in patients who had sustained positive LHBT results after 12 wk of rifaximin treatment (n = 70). Mean differences: (a) Q1, +0.40 ± 0.62; Q2, +0.26 ± 0.84; Q3, +0.17 ± 0.81; Q4, −0.28 ± 0.61. (b) Q1, +0.6 ± 1.1; Q2, +0.2 ± 2.7; Q3, +0.6 ± 2.4; Q4, −0.3 ± 1.4. (c) +0.17 ± 0.42; Q2, +0.00 ± 0.57; Q3, +0.21 ± 0.51; Q4, −0.20 ± 0.84. (d) +0.5 ± 0.9; Q2, −0.4 ± 0.6; Q3, −0.6 ± 2.2; Q4, +0.1 ± 1.1. Mean differences are expressed as mean ± standard deviation. P-value was calculated using the paired t-test. BMI = body mass index, LHBT(−) = negative lactulose hydrogen breath test, LHBT(+) = positive lactulose hydrogen breath test, Q1 = 1st quartile, Q2 = 2nd quartile, Q3 = 3rd quartile, Q4 = 4th quartile.
Figure 2 (Continued).
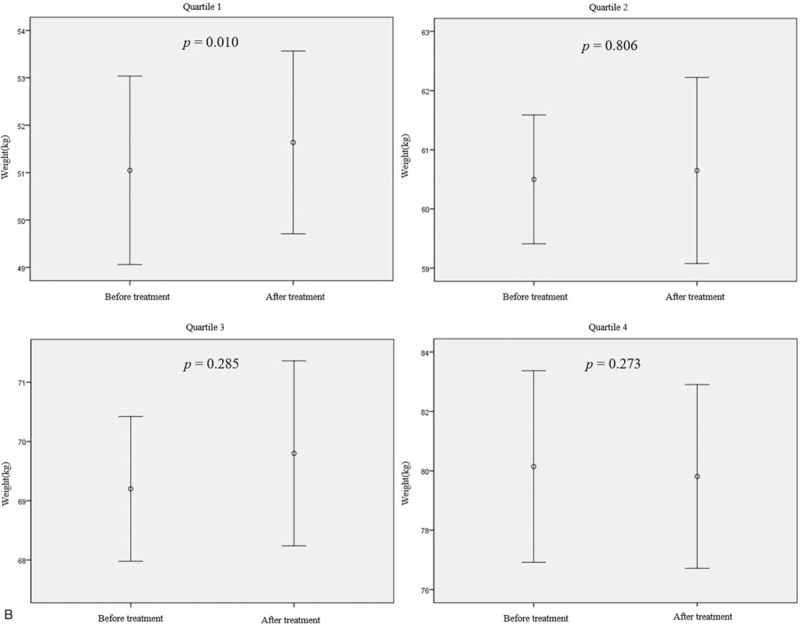
Body mass index (BMI) and body weight changes after treatment with rifaximin in patient groups by pretreatment BMI or body weight quartile. (a and b) The BMI and body weight changes according to quartiles of BMI or body weight in patients with negative LHBTs after rifaximin treatment (n = 83). (c and d) BMI and body weight changes according to the BMI or body weight quartile in patients who had sustained positive LHBT results after 12 wk of rifaximin treatment (n = 70). Mean differences: (a) Q1, +0.40 ± 0.62; Q2, +0.26 ± 0.84; Q3, +0.17 ± 0.81; Q4, −0.28 ± 0.61. (b) Q1, +0.6 ± 1.1; Q2, +0.2 ± 2.7; Q3, +0.6 ± 2.4; Q4, −0.3 ± 1.4. (c) +0.17 ± 0.42; Q2, +0.00 ± 0.57; Q3, +0.21 ± 0.51; Q4, −0.20 ± 0.84. (d) +0.5 ± 0.9; Q2, −0.4 ± 0.6; Q3, −0.6 ± 2.2; Q4, +0.1 ± 1.1. Mean differences are expressed as mean ± standard deviation. P-value was calculated using the paired t-test. BMI = body mass index, LHBT(−) = negative lactulose hydrogen breath test, LHBT(+) = positive lactulose hydrogen breath test, Q1 = 1st quartile, Q2 = 2nd quartile, Q3 = 3rd quartile, Q4 = 4th quartile.
Figure 2 (Continued).
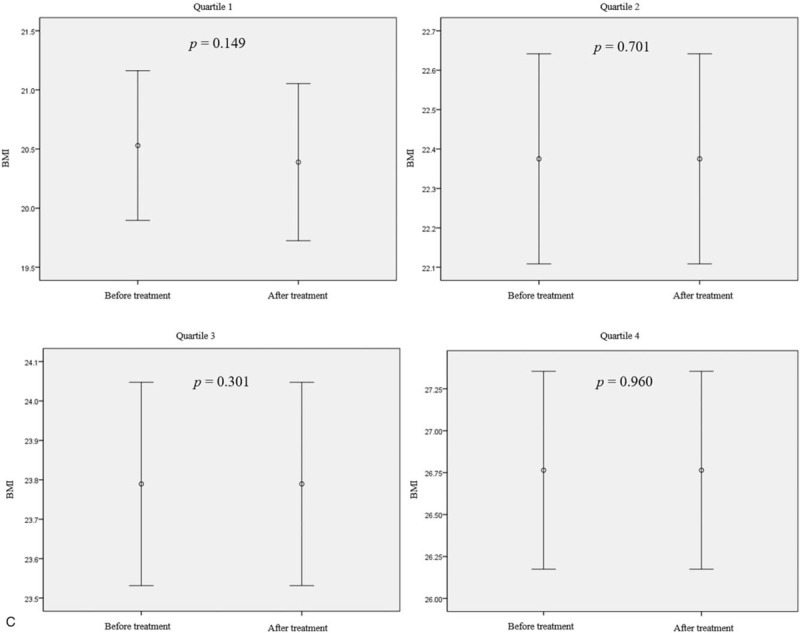
Body mass index (BMI) and body weight changes after treatment with rifaximin in patient groups by pretreatment BMI or body weight quartile. (a and b) The BMI and body weight changes according to quartiles of BMI or body weight in patients with negative LHBTs after rifaximin treatment (n = 83). (c and d) BMI and body weight changes according to the BMI or body weight quartile in patients who had sustained positive LHBT results after 12 wk of rifaximin treatment (n = 70). Mean differences: (a) Q1, +0.40 ± 0.62; Q2, +0.26 ± 0.84; Q3, +0.17 ± 0.81; Q4, −0.28 ± 0.61. (b) Q1, +0.6 ± 1.1; Q2, +0.2 ± 2.7; Q3, +0.6 ± 2.4; Q4, −0.3 ± 1.4. (c) +0.17 ± 0.42; Q2, +0.00 ± 0.57; Q3, +0.21 ± 0.51; Q4, −0.20 ± 0.84. (d) +0.5 ± 0.9; Q2, −0.4 ± 0.6; Q3, −0.6 ± 2.2; Q4, +0.1 ± 1.1. Mean differences are expressed as mean ± standard deviation. P-value was calculated using the paired t-test. BMI = body mass index, LHBT(−) = negative lactulose hydrogen breath test, LHBT(+) = positive lactulose hydrogen breath test, Q1 = 1st quartile, Q2 = 2nd quartile, Q3 = 3rd quartile, Q4 = 4th quartile.
Figure 2 (Continued).
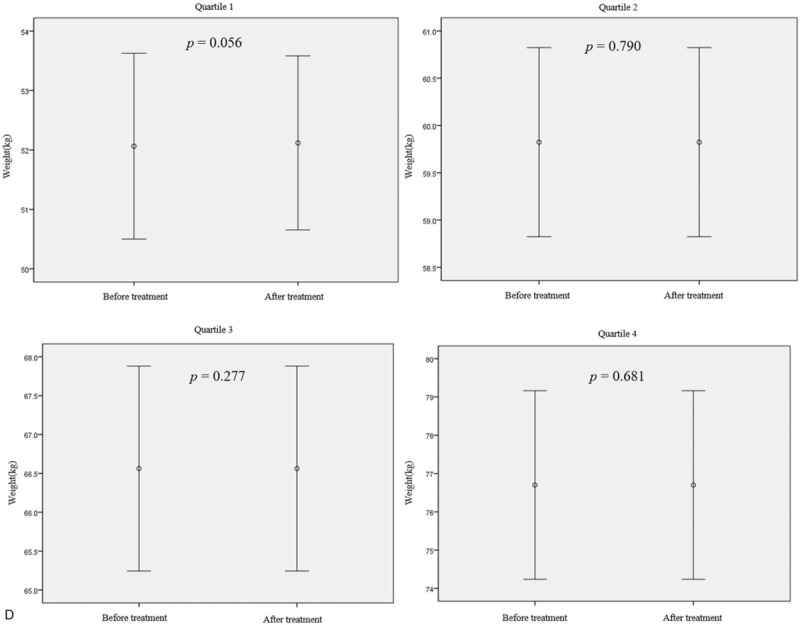
Body mass index (BMI) and body weight changes after treatment with rifaximin in patient groups by pretreatment BMI or body weight quartile. (a and b) The BMI and body weight changes according to quartiles of BMI or body weight in patients with negative LHBTs after rifaximin treatment (n = 83). (c and d) BMI and body weight changes according to the BMI or body weight quartile in patients who had sustained positive LHBT results after 12 wk of rifaximin treatment (n = 70). Mean differences: (a) Q1, +0.40 ± 0.62; Q2, +0.26 ± 0.84; Q3, +0.17 ± 0.81; Q4, −0.28 ± 0.61. (b) Q1, +0.6 ± 1.1; Q2, +0.2 ± 2.7; Q3, +0.6 ± 2.4; Q4, −0.3 ± 1.4. (c) +0.17 ± 0.42; Q2, +0.00 ± 0.57; Q3, +0.21 ± 0.51; Q4, −0.20 ± 0.84. (d) +0.5 ± 0.9; Q2, −0.4 ± 0.6; Q3, −0.6 ± 2.2; Q4, +0.1 ± 1.1. Mean differences are expressed as mean ± standard deviation. P-value was calculated using the paired t-test. BMI = body mass index, LHBT(−) = negative lactulose hydrogen breath test, LHBT(+) = positive lactulose hydrogen breath test, Q1 = 1st quartile, Q2 = 2nd quartile, Q3 = 3rd quartile, Q4 = 4th quartile.
4. Discussion
We found that eradicating hydrogen-forming SIBO by rifaximin treatment increased BMI and body weight in the lowest body weight group. In addition, there was no difference in metabolic or physical activity parameters before rifaximin treatment according to LHBT results after rifaximin treatment. In contrast, there was no significant BMI or weight change in other patient groups, even when the LHBT results converted to negative. Furthermore, there was no considerable BMI or weight change in patients who failed to eradicate the hydrogen-forming SIBO based on the LHBT results.
To the best of our knowledge, there have been no studies on the effect of eradicating hydrogen-producing SIBO on body weight in non-constipated IBS patients, however, some studies have shown a relationship between SIBO and obesity. A recent controlled study reported that SIBO was associated with a high ratio of visceral fat area to subcutaneous fat area in cross-sectional CT images.[18] Another study showed a significant association between obesity and SIBO, although the sample size was small.[19] However, these studies did not examine the correlation between obesity and the type of gas produced by bacteria in the small intestine, nor did they investigate weight changes due to bacterial eradication when SIBO was treated. On the other hand, a recent interesting study prospectively evaluated the prevalence and consequences of SIBO in obese patients before and after bariatric surgery.[20] The study included 378 patients undergoing a hydrogen breath test before and/or after surgery. The results showed that, interestingly, for Roux-en-Y gastric bypass patients, those with hydrogen-forming SIBO had significantly less weight loss and a lower percentage of total weight loss. These results are consistent with our previous findings that hydrogen-producing SIBO was inversely associated with body weight.[8] We conducted the present study to evaluate the effect of rifaximin on body weight when patients with hydrogen-forming SIBO were treated.[11] We hypothesized that the patients would gain weight when hydrogen-forming SIBO was effectively eradicated. Indeed, our data showed that the patients in the lowest body weight quartile group gained weight after treatment with rifaximin. However, the body weight of patients whose LHBTs remained positive was not changed. In addition, we did not observe weight changes after treatment with rifaximin in the second and higher quartile weights, even if the LHBT converted to negative. Furthermore, since clinically significant weight change is considered to be a weight change of more than 5%,[21] the 0.6 kg weight gain in the lowest body weight group of this study is not clinically meaningful. In other words, even though SIBO and obesity are inversely related to each other, this study suggests that elimination of hydrogen-producing intestinal bacteria does not result in clinically significant weight gain, perhaps because the body weight in these groups may result from a variety of factors (e.g., exercise and caloric balance) that may play more important roles than intestinal bacteria. Moreover, in real-life clinical practice, rifaximin treatment for up to 3 months without any caloric restriction or increased calorie intake could be too short to show meaningful weight gain. Furthermore, we performed a power analysis and identified insufficient statistical power, which may be related to the lack of clinical significance in these findings.
Some hypotheses may explain the weight-gain factors in the lowest-weight group. SIBO is associated with increased intestinal permeability by altering tight junction protein expression and promoting pro-inflammatory cytokines.[22,23] Thus, the eradication of SIBO is helpful for the recovery of intestinal epithelial cells.[24] The recovery of intestinal cells after SIBO therapy can help absorb nutrients, thus contributing to weight gain. Therefore, rifaximin therapy may help normalize intestinal motility and improve the absorption capacity of carbohydrates, thus contributing to gain weight.
This study had several limitations. First, even though LHBT is a commonly used method for diagnosing SIBO, it is not a gold-standard diagnostic method. In addition, for participants with rapid intestinal transit time, the accuracy of a breath test using lactulose is relatively lower than tests using glucose.[25,26] However, since a glucose hydrogen breath test cannot diagnose SIBO in the distal intestine, LHBT is more commonly used in clinical practice. Moreover, the North American Consensus recently recommended that not only the glucose breath test but also the LHBT, were valuable for the diagnosis of SIBO.[27] Second, although we strived to include patients who showed good compliance and a physician sufficiently explained the treatment endpoint, some patients were lost before the follow-up LHBT. However, our data had a high follow-up rate (74.3%), meaning that the effect of such cases was inconsequential. Third, this study was a retrospective chart review, which is always in danger of selection and information bias. Fourth, in this study, we did not use Rome IV criteria, which were published in 2016, for the diagnosis of IBS. However, this study built on the results of our previous findings that obesity was inversely correlated with hydrogen-producing intestinal bacteria in patients with non-constipated IBS based on Rome III criteria. Therefore, although Rome IV criteria were present in the diagnosis of non-constipated IBS, patients were recruited based on Rome III for consistency in the study. In addition, since we began collecting data for this study in 2015, the Rome IV criteria were difficult to apply. Fifth, since the endpoint of rifaximin treatment was determined by the monthly LHBT results, it was not possible to identify the time points of BMI or weight change and the duration of rifaximin treatment was different for each patient. In spite of these limitations, our study was the first to investigate the effect of eradicating hydrogen-producing intestinal bacteria on body weight. Furthermore, the study is significant because the results are from actual clinical experience, without any caloric restriction or increased caloric intake by the patients.
In conclusion, a statistically significant weight gain of 0.6 kg was observed after the eradication of hydrogen-producing SIBO in the lowest-weight group of non-constipated IBS patients, but the result was not clinically significant. Therefore, the eradication of hydrogen-producing SIBO did not cause clinically meaningful changes in body weight. Prospective large-scale cohort studies are needed to elucidate the effect of eradicating hydrogen-producing SIBO in the future.
Author contributions
Conceptualization: Seok-Hoon Lee, Kyu-Nam Kim.
Data curation: Seok-Hoon Lee, Nam-Seok Joo, Kyu-Nam Kim.
Formal analysis: Seok-Hoon Lee, Kyu-Nam Kim.
Funding acquisition: Doo-Yeoun Cho, Nam-Seok Joo, Kyu-Nam Kim.
Methodology: Kyu-Nam Kim.
Resources: Seok-Hoon Lee, Doo-Yeoun Cho.
Software: Kyu-Nam Kim.
Supervision: Doo-Yeoun Cho, Nam-Seok Joo, Kyu-Nam Kim.
Validation: Seok-Hoon Lee, Kyu-Nam Kim.
Visualization: Kyu-Nam Kim.
Writing – original draft: Seok-Hoon Lee, Kyu-Nam Kim.
Writing – review & editing: Seok-Hoon Lee, Doo-Yeoun Cho, Nam-Seok Joo, Kyu-Nam Kim.
Kyu-Nam Kim: 0000-0002-1213-5004.
Footnotes
Abbreviations: BMI = body mass index, IBS = irritable bowel syndrome, LHBT = lactulose hydrogen breath test, SIBO = small intestinal bacterial overgrowth.
How to cite this article: Lee SH, Cho DY, Joo NS, Kim KN. Effect of eradicating hydrogen-forming small intestinal bacterial overgrowth with rifaximin on body weight change. Medicine. 2019;98:51(e18396).
Ethics committee approval was received for this study from The Institutional Review Board of Ajou University Hospital (Decision Date: 31.07.2017/Decision No: AJIRB-MED-MDB-17-207).
The requirement for informed consent was waived by the board due to the retrospective nature of the study.
The authors declare that this study did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
The authors have no conflicts of interest to disclose.
References
- [1].Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016;150:1393–407. e1395. [DOI] [PubMed] [Google Scholar]
- [2].Ford AC, Lacy BE, Talley NJ. Irritable bowel syndrome. N Engl J Med 2017;376:2566–78. [DOI] [PubMed] [Google Scholar]
- [3].Parkes GC, Brostoff J, Whelan K, et al. Gastrointestinal microbiota in irritable bowel syndrome: their role in its pathogenesis and treatment. Am J Gastroenterol 2008;103:1557–67. [DOI] [PubMed] [Google Scholar]
- [4].Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol 2000;95:3503–6. [DOI] [PubMed] [Google Scholar]
- [5].Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA 2015;313:949–58. [DOI] [PubMed] [Google Scholar]
- [6].Holtmann G, Shah A, Morrison M. Pathophysiology of functional gastrointestinal disorders: a holistic overview. Dig Dis 2017;35: Suppl 1: 5–13. [DOI] [PubMed] [Google Scholar]
- [7].Shah ED, Basseri RJ, Chong K, et al. Abnormal breath testing in IBS: a meta-analysis. Dig Dis Sci 2010;55:2441–9. [DOI] [PubMed] [Google Scholar]
- [8].Jung SE, Joo NS, Han KS, et al. Obesity is inversely related to hydrogen-producing small intestinal bacterial overgrowth in non-constipation irritable bowel syndrome. J Korean Med Sci 2017;32:948–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Scarpignato C, Pelosini I. Experimental and clinical pharmacology of rifaximin, a gastrointestinal selective antibiotic. Digestion 2006;73: Suppl 1: 13–27. [DOI] [PubMed] [Google Scholar]
- [10].Scarpellini E, Gabrielli M, Lauritano CE, et al. High dosage rifaximin for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther 2007;25:781–6. [DOI] [PubMed] [Google Scholar]
- [11].Bae S, Lee KJ, Kim YS, et al. Determination of rifaximin treatment period according to lactulose breath test values in nonconstipated irritable bowel syndrome subjects. J Korean Med Sci 2015;30:757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sultan S, Malhotra A. Irritable bowel syndrome. Ann Intern Med 2017;166:ITC81–96. [DOI] [PubMed] [Google Scholar]
- [13].Kerlin P, Wong L. Breath hydrogen testing in bacterial overgrowth of the small intestine. Gastroenterology 1988;95:982–8. [DOI] [PubMed] [Google Scholar]
- [14].Corazza GR, Strocchi A, Gasbarrini G. Fasting breath hydrogen in celiac disease. Gastroenterology 1987;93:53–8. [DOI] [PubMed] [Google Scholar]
- [15].Rhodes JM, Middleton P, Jewell DP. The lactulose hydrogen breath test as a diagnostic test for small-bowel bacterial overgrowth. Scand J Gastroenterol 1979;14:333–6. [DOI] [PubMed] [Google Scholar]
- [16].Posserud I, Stotzer PO, Bjornsson ES, et al. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut 2007;56:802–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Greenfield TK. Ways of measuring drinking patterns and the difference they make: experience with graduated frequencies. J Subst Abuse 2000;12:33–49. [DOI] [PubMed] [Google Scholar]
- [18].Fialho A, Fialho A, Thota P, et al. Higher visceral to subcutaneous fat ratio is associated with small intestinal bacterial overgrowth. Nutr Metab Cardiovasc Dis 2016;26:773–7. [DOI] [PubMed] [Google Scholar]
- [19].Roland BC, Lee D, Miller LS, et al. Obesity increases the risk of small intestinal bacterial overgrowth (SIBO). Neurogastroenterol Motil 2018;30. [DOI] [PubMed] [Google Scholar]
- [20].Sabate JM, Coupaye M, Ledoux S, et al. Consequences of small intestinal bacterial overgrowth in obese patients before and after bariatric surgery. Obes Surg 2017;27:599–605. [DOI] [PubMed] [Google Scholar]
- [21].Stevens J, Truesdale KP, McClain JE, et al. The definition of weight maintenance. Int J Obes (Lond) 2006;30:391–9. [DOI] [PubMed] [Google Scholar]
- [22].Wigg AJ, Roberts-Thomson IC, Dymock RB, et al. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut 2001;48:206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Thompson JR. Is irritable bowel syndrome an infectious disease? World J Gastroenterol 2016;22:1331–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lauritano EC, Valenza V, Sparano L, et al. Small intestinal bacterial overgrowth and intestinal permeability. Scand J Gastroenterol 2010;45:1131–2. [DOI] [PubMed] [Google Scholar]
- [25].Rana SV, Sharma S, Kaur J, et al. Comparison of lactulose and glucose breath test for diagnosis of small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Digestion 2012;85:243–7. [DOI] [PubMed] [Google Scholar]
- [26].Walters B, Vanner SJ. Detection of bacterial overgrowth in IBS using the lactulose H2 breath test: comparison with 14C-D-xylose and healthy controls. Am J Gastroenterol 2005;100:1566–70. [DOI] [PubMed] [Google Scholar]
- [27].Rezaie A, Buresi M, Lembo A, et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: the North American consensus. Am J Gastroenterol 2017;112:775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]


