Abstract
Objective
Renal fibrosis is the most common manifestation of chronic kidney disease (CKD). Noting that existing treatments of renal fibrosis only slow disease progression but do not cure it, there is an urgent need to identify novel therapies. Hydrogen sulfide (H2S) is a newly discovered endogenous small gas signaling molecule exerting a wide range of biologic actions in our body. This review illustrates recent experimental findings on the mechanisms underlying the therapeutic effects of H2S against renal fibrosis and highlights its potential in future clinical application.
Data sources
Literature was collected from PubMed until February 2019, using the search terms including “Hydrogen sulfide,” “Chronic kidney disease,” “Renal interstitial fibrosis,” “Kidney disease,” “Inflammation factor,” “Oxidative stress,” “Epithelial-to-mesenchymal transition,” “H2S donor,” “Hypertensive kidney dysfunction,” “Myofibroblasts,” “Vascular remodeling,” “transforming growth factor (TGF)-beta/Smads signaling,” and “Sulfate potassium channels.”
Study selection
Literature was mainly derived from English articles or articles that could be obtained with English abstracts. Article type was not limited. References were also identified from the bibliographies of identified articles and the authors’ files.
Results
The experimental data confirmed that H2S is widely involved in various renal pathologies by suppressing inflammation and oxidative stress, inhibiting the activation of fibrosis-related cells and their cytokine expression, ameliorating vascular remodeling and high blood pressure, stimulating tubular cell regeneration, as well as reducing apoptosis, autophagy, and hypertrophy. Therefore, H2S represents an alternative or additional therapeutic approach for renal fibrosis.
Conclusions
We postulate that H2S may delay the occurrence and progress of renal fibrosis, thus protecting renal function. Further experiments are required to explore the precise role of H2S in renal fibrosis and its application in clinical treatment.
Keywords: Hydrogen sulfide, Renal interstitial fibrosis, Chronic kidney disease
Introduction
The burden of chronic kidney disease (CKD) has been recognized as a leading public health problem affecting about 11% of world population.[1,2] Renal fibrosis is the unavoidable consequence of CKD irrespective of the primary underlying insult, which evokes severe clinical problems.[3] It is a complex phenomenon governed by the interplay between different cellular components and intricate networks of signaling pathways, which together lead to loss of renal functionality and replacement of kidney parenchyma with scar tissue.[4] Thus, the effective prevention and management of renal fibrosis are crucial to CKD treatment. However, the pathogenesis of renal fibrosis is not fully elucidated, and existing therapies only slow disease progression but do not cure it. As fibrosis is a disorder associated with multiple pathways and signaling components, there is an urgent need to identify novel therapies to target additional disease mechanisms[5] and using small molecules targeting multiple steps of the fibrotic process may serve as a promising approach to treat the disease.[6]
Description of Hydrogen Sulfide
Over the past decades, researchers have reported the biologic significance and therapeutic potential of endogenous gaseous signaling molecules collectively known as “gasotransmitters.”[5] Hydrogen sulfide (H2S), a member of the gasotransmitter family, has recently been identified and demonstrated to possess important therapeutic characteristics that prevent the development and progression of renal fibrosis in experimental animals. By targeting several important molecular pathways, H2S may represent an alternative or additional therapeutic approach for treating renal fibrosis.
H2S is known for its toxicity at high micromolar concentrations. The mechanism of H2S toxicity has been attributed to its inhibition of cytochrome c oxidase in a similar manner to hydrogen cyanide,[7] breaking down the respiratory and mitochondrial functions in mammals.[8] However, it remains to be investigated whether other unknown mechanisms and processes are related to the toxicity of H2S in vivo.
H2S is highly lipophilic, allowing its rapid transfer through cell membranes without using specific transporters. It exerts a host of biologic effects on various targets as a signaling molecule with physiologic relevance and therapeutic potentials.[5] Emerging evidence suggests that the potential toxicity of H2S can be ameliorated by controlling its concentration in vivo. Nevertheless, in some circumstances, the production of endogenous H2S is insufficient to trigger the desired biologic effects.[9] Thus, efforts have been made to identify suitable exogenous H2S donors. There are both natural and synthetic sources of exogenous H2S donors. Natural donors were noted in some plants, like garlic and onions. Sodium hydrosulfide (NaHS) was one of the first synthetic donors, generating supra-physiologic quantities of H2S spontaneously in solution. However, NaHS has a half-life of only 15 min, limiting its potential as a therapeutic tool.[10] There has been a surge in the research and development of clinically viable H2S donors including allyl disulfide, sodium polysulthionate (SG-1002, ClinicalTrials.gov identifier: NCT01989208), N-acetylcysteine, intravenous sodium sulfide (IK-1001, ClinicalTrials.gov identifier: NCT00879645), Zofenopril and ATB-346 (ClinicalTrials.gov identifier: NCT03291418),[11,12] GYY4137,[13] AP39 (a mitochondrially targeted donor of H2S),[14] and S-propargyl cysteine (also known as ZYZ-802).[15] A study on a variety of H2S donor systems, including the H2S-releasing trigger mechanism, H2S release profiles, byproducts, and potential therapeutic applications, may have the potential of developing H2S-releasing therapeutics.[16]
Metabolism of H2S in Kidney
In the early 1980s, Stipanuk and Beck[17] demonstrated the existence of H2S in rat kidney. The kidney is one of the major organs that regulate endogenous H2S levels.[13] H2S is mainly derived from l-cysteine (l-Cys) in mammals by the enzymes cystathionine beta-synthase (CBS), cystathionine gamma-lyase (CSE), 3-mecaptopyruvate sulfur transferase (3-MST), and cysteine aminotransferase.[18] It is also produced endogenously in the cytoplasm and mitochondria of mammalian cells from d-cysteine (d-Cys) by the enzyme d-amino acid oxidase[19] [Figure 1]. These H2S-producing enzymes are abundantly expressed in the kidney.[20,21] While CBS is the predominant H2S-generating enzyme located in proximal renal tubules,[22,23] CSE appears to be the main H2S-producing enzyme expressed by endothelial cells, mesangial cells, and podocytes in glomeruli, as well as in proximal tubular epithelium, and vascular endothelium of arterioles and peritubular capillaries.[23,24] However, it was also reported that none of these enzymes (CBS, CSE, and 3-MST) are expressed in glomeruli.[25,26] This discrepancy certainly warrants further investigations. Laser-capture microdissection could be used to overcome the limitation of immunohistochemistry, providing more reliable information on the expression of H2S-producing enzymes.[27]
Figure 1.
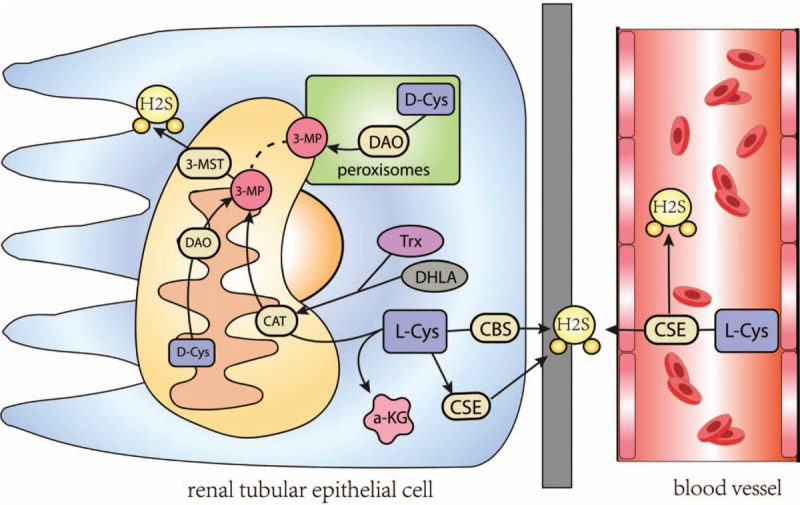
Schematic representation of the production of H2S from d- and l-cys. d-cysis metabolized by DAO to an achiral 3MP, which is also produced by CBS, CSE, CAT from l-cys in the presence of a-ketoglutarate (aKG). 3MP is metabolized by 3MP sulfurtransferase to H2S. CBS: Cystathionine beta-synthase; CSE: Cystathionine gamma-lyase; CAT: Cysteine aminotransferase; DAO: d-amino acid oxidase; 3MP: 3-mercaptopyruvate.
Role of H2S in Renal Homeostasis
H2S plays an important role in renal homeostasis. It causes vasodilation and increases renal blood flow (RBF) and the glomerular filtration rate, reduces blood pressure, regulates vascular tone in synergy with (nitric oxide) NO, increases the excretion of Na+, K+ in the urine, and acts as an O2 sensor in the kidney, especially under hypoxic circumstance.[27] Besides, emerging evidence support the idea that H2S has epigenetic effects by modulating DNA methylation,[28] histone deacetylase activity,[29] and microRNA expression.[30] As neither NaHS administration nor inhibition of endogenous H2S influenced renin activity, H2S may only modulate renal activity when RAS(rennin angiotensin system) is overactivated.[31] However, the biologic mechanisms of H2S signaling are not well understood.[32]
In CKD rat models, H2S is depleted by the down-regulation of the H2S-producing enzymes CBS, CSE, and 3-MST in the kidney.[33] In diabetic nephropathy, reactive oxygen species (ROS)-mediated matrix metalloproteinase-9 (MMP-9) activity regulates the expression of CBS and CSE.[34] Hyperhomocysteinemia increases DNA methylation of the CSE promoter, leading to the repression of CSE transcription and reduced H2S production.[35] Moreover, the mechanism of H2S production involved an increase in cGMP, and augmentation of inducible nitric oxide synthase (iNOS) expression, which is also called nitric oxide-H2S signaling in high glucose-stimulated podocytes.[36] Therefore, H2S maintains a balance in the kidneys through a variety of mechanisms.
H2S and Mechanisms of Renal Fibrosis and Regression
Fibrosis occurs in many tissues and organs with a similar constellation of pathogenic processes. Major cellular events in tubulointerstitial fibrosis include inflammation, oxidative stress, fibroblast activation and expression of fibrotic-related cytokines, vascular remodeling and high blood pressure, tubular apoptosis, as well as autophagy. Each of these pathologic features could contribute to the progression of fibrosis in its own unique way, but together they constitute a core set of fibrogenic events that result in the ultimate destruction of renal parenchyma and loss of kidney function.[37]
Targeting these mechanisms of tubulointerstitial fibrosis might provide a useful way to delay renal fibrosis. As H2S is widely produced in the kidneys, and has diverse and widespread biologic functions [Figure 2], it may serve as a useful therapeutic agent against renal fibrosis. And several recent studies have demonstrated that, at low micromolar concentrations, H2S exhibits important therapeutic characteristics that target multiple molecular pathways, thereby preventing the development and progression of several pathologies of renal fibrosis.
Figure 2.
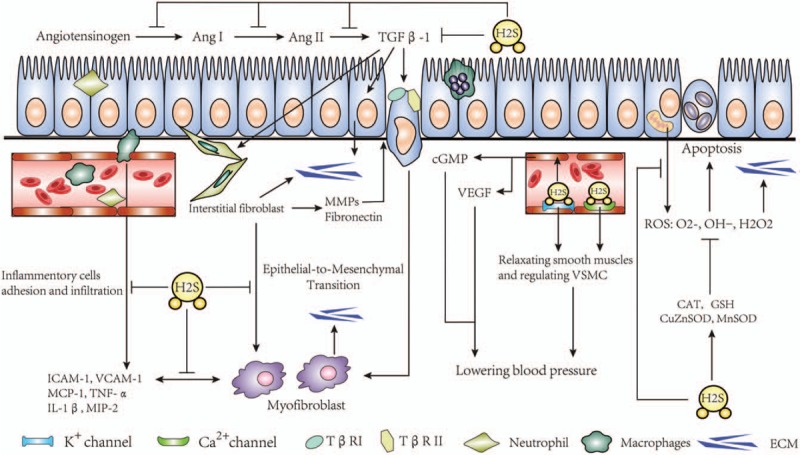
The mechanism of anti-fibrotic role of H2S in renal fibrosis.
H2S Exhibits Anti-Renal Fibrotic Effect by Inhibiting Inflammation
Inflammation plays a key role in the initial stage of renal fibrosis.[38] Tissue fibrosis typically occurs in uncontrolled inflammation.[39] Renal fibrosis is always accompanied by T lymphocytes, monocytes, macrophages, mast cells, and dendritic cell infiltration when kidney damage begins. The classic view is that inflammation and fibrosis interact in a paracrine manner, whereby inflammatory cells secrete profibrotic cytokines such as TGF-β1, monocyte chemotactic protein 1 (MCP-1), and tissue inhibitor of metalloproteinase via the nuclear factor-κB pathway or other pathways, which act on resident fibroblasts and tubular cells to promote fibrogenesis.[37] H2S plays an anti-inflammatory effect by inhibiting the activation of inflammatory cells. In the unilateral ureteral occlusion (UUO) model, small doses of H2S could suppress the renal interstitial infiltration of CD68+ macrophages cells[23] and drive macrophages toward the anti-inflammatory M2 phenotype.[40,41] However, Lin et al reported that H2S only reduced neutrophil infiltration but did not suppress macrophage infiltration.[41] The authors speculated that the increase of CD68+ cells may reflect a surge of anti-inflammatory M2 cells which contribute to kidney tissue remodeling by enhancing tubular cell proliferation and repair as well as inducing maladaptive repair of fibrosis.[42] Hence, the role of H2S on macrophage polarization in renal fibrosis requires further investigation. Furthermore, H2S inhibits the activation of inflammatory molecules such as intercellular adhesion molecule-1, vascular cell adhesion molecule-1, MCP-1, tumor necrosis factor-α, interleukin-1β, and macrophage inflammatory protein-2.[23,43] Leukocyte adhesion to vascular endothelium can be suppressed by H2S by inhibiting chemotaxis and infiltration of neutrophils and lymphocytes. H2S was also able to mitigate renal injury in high fat diet-induced obese mice through the reduction of kidney inflammation by down-regulating the expression of nuclear factor-kappa B[44] and in a streptozotocin (STZ)-induced diabetic rat model.[45] In addition, in an angiotensin II (ANG II)-induced kidney model, exogenous H2S (released by GYY4137) improved inflammation by reversing the expression of miR-129 through an epigenetic mechanism.[46] These studies indicate that the anti-fibrotic effects of H2S is closely linked to the suppression of inflammation. Nonetheless, how H2S attenuates inflammation mechanistically remains to be elucidated.
H2S Attenuats Oxidative Stress in Renal Fibrosis
Oxidative stress is a serious imbalance between the production of ROS (such as O2-, OH., H2O2), reactive nitrogen species, and loss of the anti-oxidative enzyme system.[47] It has an important pathogenic role in the development of many diseases, including renal fibrosis.[48] The imbalance of pro-oxidants or free radicals can oxidize macromolecules such as proteins, lipids, and nucleic acids, and alter redox-sensitive pathways resulting in subsequent cell and tissue injuries. Dysregulation of anti-oxidant mechanisms not only promotes a fibrotic milieu but also leads to mitochondrial dysfunction and further exacerbates kidney injury.[49] NAD(P)H oxidase (NOX) is a major source for renal ROS,[50] which are important mediators and modulators of specific intracellular signal transduction pathways by activating redox-sensitive kinases. H2S ameliorates oxidative stress by inhibiting mitochondrial ROS generation, acting as an oxygen sensor that restores oxygen balance, and increasing medullary flow in renal medulla.[51–53] H2S can also inhibit high glucose-induced NOX4, the ROS sources, by activating AMP-activated protein kinase (AMPK), and decrease matrix protein accumulation by recruiting iNOS to generate NO in renal epithelial cells.[54] In addition to acting as a direct ROS scavenger, H2S increased the expression/activity of anti-oxidative enzymes including copper-zinc superoxide dismutase and manganese superoxide dismutase,[55] up-regulated antioxidant haemoxygenase-1, SIRT1,[9] and glutathione levels,[55,56] and promoted the transcription of anti-oxidant genes via the activation of Nrf2 anti-oxidant pathway.[45,57] These observations suggest that the anti-oxidative role of H2S is important for preventing renal fibrosis.
H2S Inhibits the Activation of Fibrosis-Related Cells and Their Expression of Fibrotic Cytokines
Phenotypic transition to myofibroblasts are one of major cellular events of renal fibrosis.[37] Most studies have implicated epithelial cells, fibroblasts, pericytes, inflammatory cells, and bone-marrow-derived “fibrocytes” as probable myofibroblast precursors.[37,58–61] Fibroblast activation and epithelial-to-mesenchymal transition (EMT) are important steps in myofibroblast formation. Fibroblasts and tubular epithelial cells can be activated by growth factors such as TGF-β1, which are released from infiltrating mononuclear cells and interstitial fibroblasts. Activated TGF-β1 initiates its cellular actions across multiple cell types by binding with the TGF-β type II receptor, leading to gene expression, cytoskeleton reorganization, and cellular transformation into myofibroblasts in a Smad2/3-dependent manner.[62,63]Other non-Smad pathways, such as various branches of MAP kinase pathways, also contribute to myofibroblasts formation.[64]
Current anti-fibrotic strategies in renal fibrosis employ pharmacologic therapies targeting the myofibroblasts. For instance, inhibition of GLI1/GLI2, the transcriptional effectors of the hedgehog (Hh) pathway which are important for myofibroblast proliferation, could suppress renal fibrosis.[65] Fluorofenidone [1-(3-fluorophenyl)-5-methyl-2-(1H)-pyridone, AKF-PD] showed potent anti-fibrotic properties by inhibiting myofibroblasts proliferation in renal disease.[66–68] Moreover, calcitriol could effectively block myofibroblast activation from interstitial fibroblasts, suggesting its potential in the treatment of renal fibrosis.[69]
Because TGF-β1 is the most vital cytokine regulating myofibroblasts, many studies are focused on the effects of H2S on renal myofibroblast activation induced by TGF-β1. H2S counteracted Ang II- and TGF-β1-induced EMT through mechanisms involving direct inactivation of TGF-β1.[70] Exogenous H2S also inhibited the activation of myofibroblasts and extracellular matrix production partially by attenuating the phosphorylation of Smad3 and inducing Smad7, which blocks TGF-β1-Smad signaling through hindrance of TβRII.[10,23] Furthermore, Guo et al[71] demonstrated that NaHS inhibits EMT by reducing the expression of TGF-β receptor type I (TβR I) and TβR II, attenuating TGF-β1-induced increase of β-catenin expression and MAPK/ERK phosphorylation, and inhibiting the TGF-β1-induced nuclear translocation of β-catenin.[10,23]
Another effect of H2S on renal fibrosis is its inhibition of the renin-angiotensin-aldosterone system (RAAS). The kidney contains all components of the RAAS.[6] Angiotensin II is a potent profibrotic factor that stimulates collagen synthesis through the TGF-β1-dependent[72] and -independent[73] signaling pathways. In renovascular hypertension animal models, H2S could also lower the serum levels of angiotensin II by down-regulating cellular cAMP production.[74] In human endothelial cells, H2S inhibited the activity of angiotensin-converting enzyme.[75] Moreover, endogenous H2S suppressed the release of renin in As4.1 and renin-rich renal cells.[76] MMPs represent another important group of fibrosis-related cytokines. Although some MMPs suppressed fibrosis through the degradation of ECM components, MMP-2 and -9 were both associated with the progression of renal fibrosis.[77] Noticeably, NaHS treatment down-regulated the renal expression of MMP-2 and MMP-9 in diabetic kidney disease rats.[78,79] While the effects of H2S on other major signaling targets in renal fibrosis, such as BMP7 (bone morphogenetic protein 7) and connective tissue growth factor,[80] await further studies, the abilities of H2S in blocking the activation of fibrosis-related cells and the biologic effects of fibrotic cytokines highlight its anti-fibrosis potential.
H2S Ameliorates Vascular Remodeling and High Blood Pressure
Renal vascular remodeling could result in the pathologic changes of peritubular capillaries, which may play a critical role in providing oxygen and nutrition to tubules and maintaining glomerular filtration rate. It was noted that kidney failure was characterized by a progressive loss of interstitial microvasculature, which correlated directly with the development of renal fibrosis.[81] By activating ATP-sensitive potassium channels, H2S produced by vascular smooth muscle cells leads to the hyperpolarization of cytomembrane and relaxation of smooth muscle to ensure the blood flow volume of kidney. It also serves as a sensor monitoring the oxygen contents of the renal medulla and regulating the blood flow in the renal cortex.[82,83] Furthermore, H2S is a potent inhibitor of phosphate-induced calcification and osteoblastic differentiation of vascular smooth muscle cells (VSMC).[84] In addition, H2S down-regulated the ERK/MAPK signal pathway to inhibit the expression of proliferating cell nuclear antigen,[85] which promotes cell proliferation and vascular remodeling.[86] Therefore, to certain extent, the supplementation of exogenous H2S can counter low kidney irrigation and hyperplasia of smooth muscle cells.
Renal injury may be counteracted specifically by etiologic treatments (such as blood pressure and blood glucose control), which ameliorate architectural disruption and fibrosis. Globally, CKD due to hypertension contributed to 23% of the overall increase in CKD disability-adjusted-life-years.[87] Controlling blood pressure could be useful for anti-fibrotic treatment, of which H2S is a newly discovered regulator. De et al showed that endogenous H2S is involved in the maintenance of basal blood pressure and the progression of hypertension.[88] In renal tissues of Dahl rats, H2S donor inhibited salt-sensitive hypertension, reversed aortic structural remodeling, and inhibited RAS activation.[89] Recent studies have further identified H2S as the endothelium-derived hyperpolarizing factor that directly induces vasorelaxation.[90,91] Blood pressure lowering mechanisms of H2S involve the sulfhydration of K-ATP channels on VSMCs,[92] up-regulation of cyclic guanosine monophosphate (cGMP) by inhibition of cGMP phosphodiesterases,[93,94] and activation of free vascular endothelial growth factor[95] as well as calcium signaling.[96] H2S could cause an increase of RBF, GFR, and urinary excretion of Na+ and K+,[27] leading to reduced blood pressure. In addition, H2S prevents the activation of the BMP4/COX-2 pathway in hypertension, which may be involved in its ameliorative effects on endothelial impairment,[97] providing new target for prevention and therapy of hypertension. Collectively, H2S protects renal blood vessels by inhibiting vascular remodeling and lowering blood pressure.
H2S Stimulates Tubular Cell Regeneration and Inhibits Apoptosis, Autophagy, and Hypertrophy
Proximal tubule, a specialized epithelial segment vulnerable to injury, plays a central role in the progression of renal fibrosis.[98] Tubular cell apoptosis is a major pathway of kidney fibrosis and mitochondrial damage.[99] Apoptosis of tubule epithelial cells gives rise to a reduction of the tubular compartment, the formation of atubular glomeruli,[100] and a scarring-like, fibrotic healing process of the interstitial compartment.[101] In a renal ischemia/reperfusion injury model, H2S has been shown to stimulate tubular regeneration.[102,103] In UUO and re-implantation models, H2S was found to induce tissue regeneration and possess anti-apoptotic properties.[10] However, this anti-apoptotic effects of H2S were not observed in another study using the UUO models.[41] It is noteworthy that an acute increase in renal tubular apoptosis following UUO with a trending decline shortly thereafter, suggesting the initiation of a more fibrotic phenotype.[104]
The role of H2S in proximal tubular autophagy and renal injury is complex. Autophagy is a cellular process of degradation of cytoplasmic contents, including protein aggregates and dysfunctional organelles.[105] H2S is likely protective early in injury though it may promote apoptosis or cell degeneration if the injury is too severe.[98] The profibrotic function of autophagy is related to the regulation of tubular cell death, interstitial inflammation, and the production of profibrotic factors.[106] In STZ-induced diabetes mellitus kidney disease model and in the 5/6 nephrectomy animal model,[55] H2S improved renal tissue fibrosis by inhibiting autophagy.[107] In rats, exogenous H2S was shown to reduce renal ischemic renal injury injury by up-regulating endoplasmic reticulum stress-induced autophagy.[108] Furthermore, amelioration of high glucose-induced kidney injury by NaHS involves AMPK stimulation and mTORC1 inhibition leading to reduced kidney epithelial cell hypertrophy and increased matrix protein expression.[109] The distinct roles of H2S in apoptosis vs. autophagy may account for the different observations in various models and stages of the lesion. Despite some discrepancies, most studies suggested a protective role of H2S in renal tubular cells for renal fibrosis.
Limitations
There are some limitations in previous research. First, H2S measurement in some of these studies may be unreliable and overestimated partly be due to lack of sensitive measuring technique and its volatile nature.[5] Moreover, in most studies, only CBS and/or CSE levels were measured, and little is known about the role of 3-MST in fibrosis-producing renal cells. And GYY4137, as a donor of H2S, was considered exacerbate cisplatin-induced nephrotoxicity in mice possibly through promoting inflammation, oxidative stress, and apoptotic response, which also need further discussion for application.[110] There is also lacking of mechanistic studies of the regulatory role of H2S in renal structure. Thus, additional studies will be needed to decipher not only the mechanistic actions of H2S in renal fibrosis, but also the therapeutic application of H2S.
Future Perspectives and Clinical Application
Although many studies have focused on the role of H2S in renal fibrosis, the role of H2S in the development of renal fibrosis needs to be further studied with the in-depth research on the pathogenesis of renal fibrosis. Take mitochondrial biosynthesis as an example. Mitochondrial biosynthesis plays an important role in the occurrence and development of CKD.[111] Recent studies have shown that the transcription factor activation protein PPARγ-activated protein family (PGC) has an important role in mitochondrial biosynthesis.[112,113] Thus, more studies are needed to know whether H2S improve mitochondrial function and inhibit renal interstitial fibrosis by regulating the expression and biologic activity of the PGC protein family.
Drugs that can be used to cure renal fibrosis are currently unavailable.[114,115] Due to the many promising actions of H2S against renal fibrosis, there has been a surge in the research and development of clinically viable H2S donors. Sulfide salts, such as NaHS, deliver H2S in supra-physiologic amounts with potential off-target effects, making it not a useful therapeutic tool.[10] In contrast, GYY4137 is a water-soluble donor molecule that allows for slow release of H2S, leading to a sustained elevation of plasma H2S levels.[83] However, the effects of GYY4137 is not robust due to limited target specificity. It is found that AP39, which is a mitochondria-targeted H2S donor, can overcome this specificity issue.[14] Though still at the early stages of development, other H2S-releasing drugs including sodium polysulthionate (SG-1002), intravenous sodium sulfide (IK-1001), Zofenopril, and ATB-346, also show considerable promise. Currently, there are clinical trials registered at clinicaltrials.gov on the effects of intravenous sodium sulfide (IK-1001) and N-acetylcysteine in impaired renal function. However, the study of intravenous sodium sulfide (IK-1001) was terminated due to the inability of developing a rapid and reliable assay to detect sulfide concentrations. Furthermore, N-acetylcysteine was found to have no short-term effect on creatinine levels and did not decrease urine protein excretion within 48 h of treatment. Further studies will be needed to develop better H2S donors for H2S therapeutics. Attention should be paid to the chemistry of H2S donors, particularly the identity and release of reactive byproducts, and the physiologic actions of H2S. Another area is to focus on the potential to intervene fibrosis by targeting the pathway of endogenous H2S-producing enzymes.[116]
Conclusions
As a newly discovered endogenous gas molecule beside carbon monoxide and nitrogen oxide, H2S has been proved to have many physiologic functions. In renal fibrosis related research, we postulate that H2S may delay the occurrence and progress of renal fibrosis, thus protecting renal function. However, further experiments are required to explore the precise role of H2S in renal fibrosis, and its therapeutic potential in clinical treatment.
Acknowledgements
The authors thank Prof. Chi-Chung Hui (Department of Molecular Genetics, University of Toronto) for helping us revise the article.
Funding
This project was supported by the National Natural Science Foundation of China (No. 81300610, No. 81600582, and No. 81400749) and College Students’ Innovative Undertaking Plan of Central South University (No. CX2015529 and No. CX2015530)
Conflicts of interest
None.
Footnotes
How to cite this article: Wang Y, Xing QQ, Tu JK, Tang WB, Yuan XN, Xie YY, Wang W, Peng ZZ, Huang L, Xu H, Qin J, Xiao XC, Tao LJ, Yuan QJ. Involvement of hydrogen sulfide in the progression of renal fibrosis. Chin Med J 2019;132:2872–2880. doi: 10.1097/CM9.0000000000000537
Yu Wang and Qi-Qi Xing contributed equally to the study.
References
- 1.Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet 2017; 389:1238–1252. doi: 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012; 379:815–822. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 3.Meguid El Nahas A, Bello AK. Chronic kidney disease: the global challenge. Lancet 2005; 365:331–340. doi: 10.1016/S0140-6736(05)17789-7. [DOI] [PubMed] [Google Scholar]
- 4.Lovisa S, Zeisberg M, Kalluri R. Partial epithelial-to-mesenchymal transition and other new mechanisms of kidney fibrosis. Trends Endocrinol Metab 2016; 27:681–695. doi: 10.1016/j.tem.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Dugbartey GJ. Diabetic nephropathy: a potential savior with ‘rotten-egg’ smell. Pharmacol Rep 2017; 69:331–339. doi: 10.1016/j.pharep.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Song K, Li Q, Yin XY, Lu Y, Liu CF, Hu LF. Hydrogen sulfide: a therapeutic candidate for fibrotic disease? Oxid Med Cell Longev 2015; 2015:458720.doi: 10.1155/2015/458720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Truong DH, Eghbal MA, Hindmarsh W, Roth SH, O’Brien PJ. Molecular mechanisms of hydrogen sulfide toxicity. Drug Metab Rev 2006; 38:733–744. doi: 10.1080/03602530600959607. [DOI] [PubMed] [Google Scholar]
- 8.Dorman DC, Moulin FJ, McManus BE, Mahle KC, James RA, Struve MF. Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: correlation with tissue sulfide concentrations in the rat brain, liver, lung, and nasal epithelium. Toxicol Sci 2002; 65:18–25. doi: 10.1093/toxsci/65.1.18. [DOI] [PubMed] [Google Scholar]
- 9.Hou CL, Wang MJ, Sun C, Huang Y, Jin S, Mu XP, et al. Protective effects of hydrogen sulfide in the ageing kidney. Oxid Med Cell Longev 2016; 2016:7570489.doi: 10.1155/2016/7570489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin S, Lian D, Liu W, Haig A, Lobb I, Hijazi A, et al. Daily therapy with a slow-releasing H2S donor GYY4137 enables early functional recovery and ameliorates renal injury associated with urinary obstruction. Nitric Oxide 2018; 76:16–28. doi: 10.1016/j.niox.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Wallace JL, Vaughan D, Dicay M, MacNaughton WK, de Nucci G. Hydrogen sulfide-releasing therapeutics: translation to the clinic. Antioxid Redox Signal 2018; 28:1533–1540. doi: 10.1089/ars.2017.7068. [DOI] [PubMed] [Google Scholar]
- 12.Szabo C, Papapetropoulos A. International Union of Basic and Clinical Pharmacology. CII: pharmacological modulation of H2S levels: H2S donors and H2S biosynthesis inhibitors. Pharmacol Rev 2017; 69:497–564. doi: 10.1124/pr.117.014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo R, Hu S, Liu Q, Han M, Wang F, Qiu M, et al. Hydrogen sulfide upregulates renal AQP-2 protein expression and promotes urine concentration. FASEB J 2019; 33:469–483. doi: 10.1096/fj.201800436R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad A, Olah G, Szczesny B, Wood ME, Whiteman M, Szabo C. AP39, a mitochondrially targeted hydrogen sulfide donor, exerts protective effects in renal epithelial cells subjected to oxidative stress in vitro and in acute renal injury in vivo. Shock 2016; 45:88–97. doi: 10.1097/SHK.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian X, Li X, Ma F, Luo S, Ge R, Zhu Y. Novel hydrogen sulfide-releasing compound, S-propargyl-cysteine, prevents STZ-induced diabetic nephropathy. Biochem Biophys Res Commun 2016; 473:931–938. doi: 10.1016/j.bbrc.2016.03.154. [DOI] [PubMed] [Google Scholar]
- 16.Powell CR, Dillon KM, Matson JB. A review of hydrogen sulfide (H2S) donors: chemistry and potential therapeutic applications. Biochem Pharmacol 2018; 149:110–123. doi: 10.1016/j.bcp.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J 1982; 206:267–277. doi: 10.1016/j.jrras.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimura H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide 2014; 41:4–10. doi: 10.1016/j.niox.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Shibuya N, Koike S, Tanaka M, Ishigami-Yuasa M, Kimura Y, Ogasawara Y, et al. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat Commun 2013; 4:1366.doi: 10.1038/ncomms2371. [DOI] [PubMed] [Google Scholar]
- 20.Dugbartey GJ, Talaei F, Houwertjes MC, Goris M, Epema AH, Bouma HR, et al. Dopamine treatment attenuates acute kidney injury in a rat model of deep hypothermia and rewarming - The role of renal H2S-producing enzymes. Eur J Pharmacol 2015; 769:225–233. doi: 10.1016/j.ejphar.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 21.Dugbartey GJ, Bouma HR, Strijkstra AM, Boerema AS, Henning RH. Induction of a Torpor-like state by 5’-AMP does not depend on H2S production. PLoS One 2015; 10:e0136113.doi: 10.1371/journal.pone.0136113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan X, Zhang J, Xie F, Tan W, Wang S, Huang L, et al. Loss of the protein cystathionine beta-synthase during kidney injury promotes renal tubulointerstitial fibrosis. Kidney Blood Press Res 2017; 42:428–443. doi: 10.1159/000479295. [DOI] [PubMed] [Google Scholar]
- 23.Song K, Wang F, Li Q, Shi YB, Zheng HF, Peng H, et al. Hydrogen sulfide inhibits the renal fibrosis of obstructive nephropathy. Kidney Int 2014; 85:1318–1329. doi: 10.1038/ki.2013.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bos EM, Wang R, Snijder PM, Boersema M, Damman J, Fu M, et al. Cystathionine gamma-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J Am Soc Nephrol 2013; 24:759–770. doi: 10.1681/ASN.2012030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagahara N, Ito T, Kitamura H, Nishino T. Tissue and subcellular distribution of mercaptopyruvatesulfurtransferase in the rat: confocal laser fluorescence and immunoelectron microscopic studies combined with biochemical analysis. Histochem Cell Biol 1998; 110:243–250. doi: 10.1007/s004180050286. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto J, Sato W, Kosugi T, Yamamoto T, Kimura T, Taniguchi S, et al. Distribution of hydrogen sulfide (H(2)S)-producing enzymes and the roles of the H(2)S donor sodium hydrosulfide in diabetic nephropathy. Clin Exp Nephrol 2013; 17:32–40. doi: 10.1007/s10157-012-0670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koning AM, Frenay AR, Leuvenink HG, van Goor H. Hydrogen sulfide in renal physiology, disease and transplantation--the smell of renal protection. Nitric Oxide 2015; 46:37–49. doi: 10.1016/j.niox.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Guo Q, Feng X, Xue H, Teng X, Jin S, Duan X, et al. Maternal renovascular hypertensive rats treatment with hydrogen sulfide increased the methylation of AT1b gene in offspring. Am J Hypertens 2017; 30:1220–1227. doi: 10.1093/ajh/hpx124. [DOI] [PubMed] [Google Scholar]
- 29.Leucker TM, Nomura Y, Kim JH, Bhatta A, Wang V, Wecker A, et al. Cystathionine gamma-lyase protects vascular endothelium: a role for inhibition of histone deacetylase 6. Am J Physiol Heart Circ Physiol 2017; 312:H711–H720. doi: 10.1152/ajpheart.00724.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber GJ, Pushpakumar SB, Sen U. Hydrogen sulfide alleviates hypertensive kidney dysfunction through an epigenetic mechanism. Am J Physiol Heart Circ Physiol 2017; 312:H874–H885. doi: 10.1152/ajpheart.00637.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao X, Bian JS. The role of hydrogen sulfide in renal system. Front Pharmacol 2016; 7:385.doi: 10.3389/fphar.2016.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu CN, Tain YL. Hydrogen sulfide in hypertension and kidney disease of developmental origins. Int J Mol Sci 2018; 19:E1438.doi: 10.3390/ijms19051438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Modis K, Coletta C, Erdelyi K, Papapetropoulos A, Szabo C. Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J 2013; 27:601–611. doi: 10.1096/fj.12-216507. [DOI] [PubMed] [Google Scholar]
- 34.Kundu S, Pushpakumar SB, Tyagi A, Coley D, Sen U. Hydrogen sulfide deficiency and diabetic renal remodeling: role of matrix metalloproteinase-9. Am J Physiol Endocrinol Metab 2013; 304:E1365–E1378. doi: 10.1152/ajpendo.00604.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li JJ, Li Q, Du HP, Wang YL, You SJ, Wang F, et al. Homocysteine triggers inflammatory responses in macrophages through inhibiting CSE-H2S signaling via DNA hypermethylation of CSE promoter. Int J Mol Sci 2015; 16:12560–12577. doi: 10.3390/ijms160612560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee HJ, Feliers D, Mariappan MM, Sataranatarajan K, Choudhury GG, Gorin Y, et al. Tadalafil integrates nitric oxide-hydrogen sulfide signaling to inhibit high glucose-induced matrix protein synthesis in podocytes. J Biol Chem 2015; 290:12014–12026. doi: 10.1074/jbc.M114.615377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 2011; 7:684–696. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Zheng L, Yuan X, Liu C, Yuan Q, Xie F, et al. Mefunidone ameliorates renal inflammation and tubulointerstitial fibrosis via suppression of IKKbeta phosphorylation. Int J Biochem Cell Biol 2016; 80:109–118. doi: 10.1016/j.biocel.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Meng XM, Tang PM, Li J, Lan HY. Macrophage phenotype in kidney injury and repair. Kidney Dis 2015; 1:138–146. doi: 10.1159/000431214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kluth DC, Erwig LP, Rees AJ. Multiple facets of macrophages in renal injury. Kidney Int 2004; 66:542–557. doi: 10.1111/j.1523-1755.2004.00773.x. [DOI] [PubMed] [Google Scholar]
- 41.Lin S, Visram F, Liu W, Haig A, Jiang J, x Mok A, et al. GYY4137, a slow-releasing hydrogen sulfide donor, ameliorates renal damage associated with chronic obstructive uropathy. J Urol 2016; 196:1778–1787. doi: 10.1016/j.juro.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 42.Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int 2011; 80:915–925. doi: 10.1038/ki.2011.217. [DOI] [PubMed] [Google Scholar]
- 43.Sen U, Givvimani S, Abe OA, Lederer ED, Tyagi SC. Cystathionine beta-synthase and cystathionine gamma-lyase double gene transfer ameliorate homocysteine-mediated mesangial inflammation through hydrogen sulfide generation. Am J Physiol Cell Physiol 2011; 300:C155–C163. doi: 10.1152/ajpcell.00143.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu D, Gao B, Li M, Yao L, Wang S, Chen M, et al. Hydrogen sulfide mitigates kidney injury in high fat diet-induced obese mice. Oxid Med Cell Longev 2016; 2016:2715718.doi: 10.1155/2016/2715718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou X, Feng Y, Zhan Z, Chen J. Hydrogen sulfide alleviates diabetic nephropathy in a streptozotocin-induced diabetic rat model. J Biol Chem 2014; 289:28827–28834. doi: 10.1074/jbc.M114.596593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber GJ, Pushpakumar S, Tyagi SC, Sen U. Homocysteine and hydrogen sulfide in epigenetic, metabolic and microbiota related renovascular hypertension. Pharmacol Res 2016; 113:300–312. doi: 10.1016/j.phrs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Butterfield DA, Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci 2019; 20:148–160. doi: 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang H, Chen X, Su Y, Paueksakon P, Hu W, Zhang MZ, et al. p47(phox) contributes to albuminuria and kidney fibrosis in mice. Kidney Int 2015; 87:948–962. doi: 10.1038/ki.2014.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okamura DM, Pennathur S. The balance of powers: redox regulation of fibrogenic pathways in kidney injury. Redox Biol 2015; 6:495–504. doi: 10.1016/j.redox.2015.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care 2008; 31: Suppl 2: S170–S180. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- 51.Dugbartey GJ. The smell of renal protection against chronic kidney disease: hydrogen sulfide offers a potential stinky remedy. Pharmacol Rep 2018; 70:196–205. doi: 10.1016/j.pharep.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 52.Jiang D, Zhang Y, Yang M, Wang S, Jiang Z, Li Z. Exogenous hydrogen sulfide prevents kidney damage following unilateral ureteral obstruction. Neurourol Urodyn 2014; 33:538–543. doi: 10.1002/nau.22450. [DOI] [PubMed] [Google Scholar]
- 53.Sen U, Basu P, Abe OA, Givvimani S, Tyagi N, Metreveli N, et al. Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. Am J Physiol Renal Physiol 2009; 297:F410–F419. doi: 10.1152/ajprenal.00145.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee HJ, Lee DY, Mariappan MM, Feliers D, Ghosh-Choudhury G, Abboud HE, et al. Hydrogen sulfide inhibits high glucose-induced NADPH oxidase 4 expression and matrix increase by recruiting inducible nitric oxide synthase in kidney proximal tubular epithelial cells. J Biol Chem 2017; 292:5665–5675. doi: 10.1074/jbc.M116.766758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung KJ, Jang HS, Kim JI, Han SJ, Park JW, Park KM. Involvement of hydrogen sulfide and homocysteine transsulfuration pathway in the progression of kidney fibrosis after ureteral obstruction. BiochimBiophys Acta 2013; 1832:1989–1997. doi: 10.1016/j.bbadis.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 56.Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J 2004; 18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 57.Huang P, Shen Z, Liu J, Huang Y, Chen S, Yu W, et al. Hydrogen sulfide inhibits high-salt diet-induced renal oxidative stress and kidney injury in Dahl rats. Oxid Med Cell Longev 2016; 2016:2807490.doi: 10.1155/2016/2807490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao Y, Liu J, Peng Y, Xiong X, Huang L, Yang H, et al. GSTA3 attenuates renal interstitial fibrosis by inhibiting TGF-beta-induced tubular epithelial-mesenchymal transition and fibronectin expression. PLoS One 2016; 11:e0160855.doi: 10.1371/journal.pone.0160855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tennakoon AH, Izawa T, Kuwamura M, Yamate J. Pathogenesis of type 2 epithelial to mesenchymal transition (EMT) in renal and hepatic fibrosis. J Clin Med 2015; 5:E4.doi: 10.3390/jcm5010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, et al. Origin and function of myofibroblasts in kidney fibrosis. Nat Med 2013; 19:1047–1053. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol 2010; 6:643–656. doi: 10.1038/nrneph.2010.120. [DOI] [PubMed] [Google Scholar]
- 62.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 2002; 110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang P, Luo ML, Song E, Zhou Z, Ma T, Wang J, et al. Long noncoding RNA lnc-TSI inhibits renal fibrogenesis by negatively regulating the TGF-beta/Smad3 pathway. Sci Transl Med 2018; 10:eaat2039.doi: 10.1126/scitranslmed.aat2039. [DOI] [PubMed] [Google Scholar]
- 64.Loeffler I. MKP2 suppresses TGF-beta1-induced epithelial-to-mesenchymal transition through JNK inhibition. Clin Sci 2019; 133:545–550. doi: 10.1042/CS20180881. [DOI] [PubMed] [Google Scholar]
- 65.Kramann R, Fleig SV, Schneider RK, Fabian SL, DiRocco DP, Maarouf O, et al. Pharmacological GLI2 inhibition prevents myofibroblast cell-cycle progression and reduces kidney fibrosis. J Clin Invest 2015; 125:2935–2951. doi: 10.1172/JCI74929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yuan Q, Wang L, Zhang F, Wang R, Fu X, Peng Z, et al. Fluorofenidone suppresses epithelial-mesenchymal transition and the expression of connective tissue growth factor via inhibiting TGF-beta/Smads signaling in human proximal tubular epithelial cells. Pharmazie 2011; 66:961–967. [PubMed] [Google Scholar]
- 67.Yuan Q, Wang R, Peng Y, Fu X, Wang W, Wang L, et al. Fluorofenidone attenuates tubulointerstitial fibrosis by inhibiting TGF-beta(1)-induced fibroblast activation. Am J Nephrol 2011; 34:181–194. doi: 10.1159/000329080. [DOI] [PubMed] [Google Scholar]
- 68.Wang LH, Liu JS, Ning WB, Yuan QJ, Zhang FF, Peng ZZ, et al. Fluorofenidone attenuates diabetic nephropathy and kidney fibrosis in db/db mice. Pharmacology 2011; 88:88–99. doi: 10.1159/000329419. [DOI] [PubMed] [Google Scholar]
- 69.Lucisano S, Buemi M, Passantino A, Aloisi C, Cernaro V, Santoro D. New insights on the role of vitamin D in the progression of renal damage. Kidney Blood Press Res 2013; 37:667–678. doi: 10.1159/000355747. [DOI] [PubMed] [Google Scholar]
- 70.Huang Y, Zhang Z, Huang Y, Mao Z, Yang X, Nakamura Y, et al. Induction of inactive TGF-beta1 monomer formation by hydrogen sulfide contributes to its suppressive effects on Ang II- and TGF-beta1-induced EMT in renal tubular epithelial cells. Biochem Biophys Res Commun 2018; 501:534–540. doi: 10.1016/j.bbrc.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 71.Guo L, Peng W, Tao J, Lan Z, Hei H, Tian L, et al. Hydrogen sulfide inhibits transforming growth factor-beta1-induced EMT via Wnt/catenin pathway. PLoS One 2016; 11:e0147018.doi: 10.1371/journal.pone.0147018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolf G, Zahner G, Schroeder R, Stahl RA. Transforming growth factor beta mediates the angiotensin-II-induced stimulation of collagen type IV synthesis in cultured murine proximal tubular cells. Nephrol Dial Transplant 1996; 11:263–269. [DOI] [PubMed] [Google Scholar]
- 73.Cuevas CA, Gonzalez AA, Inestrosa NC, Vio CP, Prieto MC. Angiotensin II increases fibronectin and collagen I through the beta-catenin-dependent signaling in mouse collecting duct cells. Am J Physiol Renal Physiol 2015; 308:F358–F365. doi: 10.1152/ajprenal.00429.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu M, Liu YH, Goh HS, Wang JJ, Yong QC, Wang R, et al. Hydrogen sulfide inhibits plasma renin activity. J Am Soc Nephrol 2010; 21:993–1002. doi: 10.1681/ASN.2009090949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laggner H, Hermann M, Esterbauer H, Muellner MK, Exner M, Gmeiner BM, et al. The novel gaseous vasorelaxant hydrogen sulfide inhibits angiotensin-converting enzyme activity of endothelial cells. J Hypertens 2007; 25:2100–2104. doi: 10.1097/HJH.0b013e32829b8fd0. [DOI] [PubMed] [Google Scholar]
- 76.Lu M, Liu YH, Ho CY, Tiong CX, Bian JS. Hydrogen sulfide regulates cAMP homeostasis and renin degranulation in As4.1 and rat renin-rich kidney cells. Am J Physiol Cell Physiol 2012; 302:C59–C66. doi: 10.1152/ajpcell.00341.2010. [DOI] [PubMed] [Google Scholar]
- 77.Huen SC, Cantley LG. Macrophages in renal injury and repair. Annu Rev Physiol 2017; 79:449–469. doi: 10.1146/annurev-physiol-022516-034219. [DOI] [PubMed] [Google Scholar]
- 78.Zeng O, Li F, Li Y, Li L, Xiao T, Chu C, et al. Effect of Novel Gasotransmitter hydrogen sulfide on renal fibrosis and connexins expression in diabetic rats. Bioengineered 2016; 7:314–320. doi: 10.1080/21655979.2016.1197743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kundu S, Pushpakumar S, Sen U. MMP-9- and NMDA receptor-mediated mechanism of diabetic renovascular remodeling and kidney dysfunction: hydrogen sulfide is a key modulator. Nitric Oxide 2015; 46:172–185. doi: 10.1016/j.niox.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nastase MV, Zeng-Brouwers J, Wygrecka M, Schaefer L. Targeting renal fibrosis: mechanisms and drug delivery systems. Adv Drug Deliv Rev 2018; 129:295–307. doi: 10.1016/j.addr.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 81.Bohle A, Mackensen-Haen S, Wehrmann M. Significance of postglomerular capillaries in the pathogenesis of chronic renal failure. Kidney Blood Press Res 1996; 19:191–195. doi: 10.1159/000174072. [DOI] [PubMed] [Google Scholar]
- 82.Beltowski J. Hypoxia in the renal medulla: implications for hydrogen sulfide signaling. J Pharmacol Exp Ther 2010; 334:358–363. doi: 10.1124/jpet.110.166637. [DOI] [PubMed] [Google Scholar]
- 83.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, et al. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation 2008; 117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 84.Zavaczki E, Jeney V, Agarwal A, Zarjou A, Oros M, Katko M, et al. Hydrogen sulfide inhibits the calcification and osteoblastic differentiation of vascular smooth muscle cells. Kidney Int 2011; 80:731–739. doi: 10.1038/ki.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang G, Cao K, Wu L, Wang R. Cystathionine gamma-lyase overexpression inhibits cell proliferation via a H2S-dependent modulation of ERK1/2 phosphorylation and p21Cip/WAK-1. J Biol Chem 2004; 279:49199–49205. doi: 10.1074/jbc.M408997200. [DOI] [PubMed] [Google Scholar]
- 86.Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell 2007; 129 (4):665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 87.Xie Y, Bowe B, Mokdad AH, Xian H, Yan Y, Li T, et al. Analysis of the global burden of disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 2018; 94:567–581. doi: 10.1016/j.kint.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 88.De H, De JB, Tang CS. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun 2004; 313:22–27. doi: 10.1016/j.bbrc.2003.11.081. [DOI] [PubMed] [Google Scholar]
- 89.Huang P, Chen S, Wang Y, Liu J, Yao Q, Huang Y, et al. Down-regulated CBS/H2S pathway is involved in high-salt-induced hypertension in Dahl rats. Nitric Oxide 2015; 46:192–203. doi: 10.1016/j.niox.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 90.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, et al. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 2011; 109:1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang G, Yang G, Jiang B, Ju Y, Wu L, Wang R. H(2)S is an endothelium-derived hyperpolarizing factor. Antioxid Redox Signal 2013; 19:1634–1646. doi: 10.1089/ars.2012.4805. [DOI] [PubMed] [Google Scholar]
- 92.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 2001; 20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang R. Hydrogen sulfide: a new EDRF. Kidney Int 2009; 76:700–704. doi: 10.1038/ki.2009.221. [DOI] [PubMed] [Google Scholar]
- 94.Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Pyriochou A, Roussos C, et al. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol 2010; 30:1998–2004. doi: 10.1161/ATVBAHA.110.209783. [DOI] [PubMed] [Google Scholar]
- 95.Holwerda KM, Burke SD, Faas MM, Zsengeller Z, Stillman IE, Kang PM, et al. Hydrogen sulfide attenuates sFlt1-induced hypertension and renal damage by upregulating vascular endothelial growth factor. J Am Soc Nephrol 2014; 25:717–725. doi: 10.1681/ASN.2013030291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao W, Wang R. H(2)S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol 2002; 283:H474–H480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- 97.Xiao L, Dong JH, Jin S, Xue HM, Guo Q, Teng X, et al. Hydrogen sulfide improves endothelial dysfunction via downregulating BMP4/COX-2 pathway in rats with hypertension. Oxid Med Cell Longev 2016; 2016:8128957.doi: 10.1155/2016/8128957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gewin LS. Renal fibrosis: primacy of the proximal tubule. Matrix Biol 2018; 68–69:248–262. doi: 10.1016/j.matbio.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Han SJ, Noh MR, Jung JM, Ishii I, Yoo J, Kim JI, et al. Hydrogen sulfide-producing cystathionine gamma-lyase is critical in the progression of kidney fibrosis. Free Radic Biol Med 2017; 112:423–432. doi: 10.1016/j.freeradbiomed.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 100.Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int 2009; 75:1145–1152. doi: 10.1038/ki.2009.86. [DOI] [PubMed] [Google Scholar]
- 101.Garcia-Sanchez O, Lopez-Hernandez FJ, Lopez-Novoa JM. An integrative view on the role of TGF-beta in the progressive tubular deletion associated with chronic kidney disease. Kidney Int 2010; 77:950–955. doi: 10.1038/ki.2010.88. [DOI] [PubMed] [Google Scholar]
- 102.Han SJ, Kim JI, Park JW, Park KM. Hydrogen sulfide accelerates the recovery of kidney tubules after renal ischemia/reperfusion injury. Nephrol Dial Transplant 2015; 30:1497–1506. doi: 10.1093/ndt/gfv226. [DOI] [PubMed] [Google Scholar]
- 103.Lobb I, Davison M, Carter D, Liu W, Haig A, Gunaratnam L, et al. Hydrogen sulfide treatment mitigates renal allograft ischemia-reperfusion injury during cold storage and improves early transplant kidney function and survival following allogeneic renal transplantation. J Urol 2015; 194:1806–1815. doi: 10.1016/j.juro.2015.07.096. [DOI] [PubMed] [Google Scholar]
- 104.Truong LD, Petrusevska G, Yang G, Gurpinar T, Shappell S, Lechago J, et al. Cell apoptosis and proliferation in experimental chronic obstructive uropathy. Kidney Int 1996; 50:200–207. doi: 10.1038/ki.1996.303. [DOI] [PubMed] [Google Scholar]
- 105.He L, Wei Q, Liu J, Yi M, Liu Y, Liu H, et al. AKI on CKD: heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int 2017; 92:1071–1083. doi: 10.1016/j.kint.2017.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Livingston MJ, Ding HF, Huang S, Hill JA, Yin XM, Dong Z. Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy 2016; 12:976–998. doi: 10.1080/15548627.2016.1166317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li L, Xiao T, Li F, Li Y, Zeng O, Liu M. Hydrogen sulfide reduced renal tissue fibrosis by regulating autophagy in diabetic rats. Mol Med Rep 2017; 16:1715–1722. doi: 10.3892/mmr.2017.6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ling Q, Yu X, Wang T, Wang SG, Ye ZQ, Liu JH. Roles of the exogenous H2S-mediated SR-A signaling pathway in renal ischemia/reperfusion injury in regulating endoplasmic reticulum stress-induced autophagy in a rat model. Cell Physiol Biochem 2017; 41:2461–2474. doi: 10.1159/000475915. [DOI] [PubMed] [Google Scholar]
- 109.Lee HJ, Mariappan MM, Feliers D, Cavaglieri RC, Sataranatarajan K, Abboud HE, et al. Hydrogen sulfide inhibits high glucose-induced matrix protein synthesis by activating AMP-activated protein kinase in renal epithelial cells. J Biol Chem 2012; 287:4451–4461. doi: 10.1074/jbc.M111.278325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu M, Jia Z, Sun Y, Zhang A, Yang T. A H 2 S donor GYY4137 exacerbates cisplatin-induced nephrotoxicity in mice. Mediators Inflamm 2016; 2016:8145785.doi: 10.1155/2016/8145785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Che R, Yuan Y, Huang S, Zhang A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am J Physiol Renal Physiol 2014; 306:F367–F378. doi: 10.1152/ajprenal.00571.2013. [DOI] [PubMed] [Google Scholar]
- 112.Xu X, Duan S, Yi F, Ocampo A, Liu GH, Izpisua Belmonte JC. Mitochondrial regulation in pluripotent stem cells. Cell Metab 2013; 18:325–332. doi: 10.1016/j.cmet.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 113.Tran MT, Zsengeller ZK, Berg AH, Khankin EV, Bhasin MK, Kim W, et al. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 2016; 531:528–532. doi: 10.1038/nature17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Klinkhammer BM, Goldschmeding R, Floege J, Boor P. Treatment of renal fibrosis-turning challenges into opportunities. Adv Chronic Kidney Dis 2017; 24:117–129. doi: 10.1053/j.ackd.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 115.Breyer MD, Susztak K. The next generation of therapeutics for chronic kidney disease. Nat Rev Drug Discov 2016; 15:568–588. doi: 10.1038/nrd.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Capasso R, Sambri I, Cimmino A, Salemme S, Lombardi C, Acanfora F, et al. Homocysteinylated albumin promotes increased monocyte-endothelial cell adhesion and up-regulation of MCP1, Hsp60 and ADAM17. PLoS One 2012; 7:e31388.doi: 10.1371/journal.pone.0031388. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


