Abstract
Objective:
Systemic sclerosis (SSc) is a remarkably systemic heterogeneous connective tissue disease with many organs involved. The heart is one of the major organs involved, carrying the threat of sudden cardiac death, especially in diffuse cutaneous SSc. This review summarizes the pathophysiology, types, new diagnostic approaches, and imaging and novel therapies of primary cardiac complications while underlining the effects of recently developed non-contrast cardiovascular magnetic resonance (CMR) in early diagnosis.
Data sources:
Medline and Embase were searched for articles published up to July 2019. A combination of Medical Subject Headings (MeSH) terms and keywords pertaining to SSc (“Scleroderma, Systemic” OR “Systemic sclerosis” OR" SSc”), AND cardiology (“cardiology” OR “heart” OR “cardiac”) were applied to the search strategies.
Study selection:
Literature was mainly printed in English and Chinese about cardiac complications in systemic sclerosis. After selected simply on the title and abstract, the articles were included for the full text. Article type was not limited.
Results:
Relevant cardiac manifestations are complex, including arrhythmias, pericardial effusion, myocardial dysfunction, and valvular diseases. Even though the symptoms of cardiac complications are well known, unfortunately, they appear to be poor prognostic factors. As systemic sclerosis with cardiac complications has a high mortality rate and patients might have a poor quality of life, it is essential to promote early diagnosis and treatment. With the advent of non-invasive imaging techniques, such as CMR, early diagnosis of cardiac complications in SSc is becoming more effective.
Conclusions:
Cardiac complications play an essential role in SSc and carry the threat of sudden cardiac death. More basic and clinical studies are warranted to develop better management of cardiac involvement in patients with SSc.
Keywords: Complications, Diagnosis, Heart, Scleroderma, Systemic, Treatment
Introduction
Systemic sclerosis (SSc), also called scleroderma, is a markedly systemic heterogeneous connective tissue disease, characterized by dysregulation of innate and adaptive immunity, microvascular damage and generalized fibrosis in multiple organs, and represents a major clinical challenge for physicians and patients.[1,2] In addition, the involvement of most organs occurs early in the disease duration, such as the Raynaud phenomenon, lung fibrosis, scleroderma renal crisis, and cardiac complications [Figure 1].[3] Heart involvement in SSc was first identified in 1926 by Heine,[4] who found from an autopsy that a patient with SSc had pathological changes in coronary arteries, pericardium, and myocardium. The heart is one of the organs involved early in SSc with diverse presentations of cardiac symptoms.[5] Cardiac manifestations include cardiac fibrosis, myositis, conduction system abnormalities, coronary artery disease, pericardial disease, and heart failure.[3] Cardiac involvement appears to be the key cause of mortality, with an estimated clinical prevalence of 15% to 35%, and 27.2% of deaths in SSc patients are due to cardiac disease.[6,7] The urgent emerging need regarding cardiac disease in this population is acknowledged amongst different medical specialties namely rheumatologists and cardiologists.[8] This disease requires further discussion as cardiologists do not appear to be aware of the distinct characteristics of this population. For example, the appearance of arrhythmia such as atrial fibrillation should be evaluated differently in SSc patients compared to controls, and novel imaging modalities could help in this regard.[9] In addition, “general cardiology practice guidelines” do not seem to apply to SSc patients as this specific group of patients must be treated earlier compared to non-SSc individuals with arrhythmias.[10] Therefore, early diagnosis and timely management are imperative.
Figure 1.
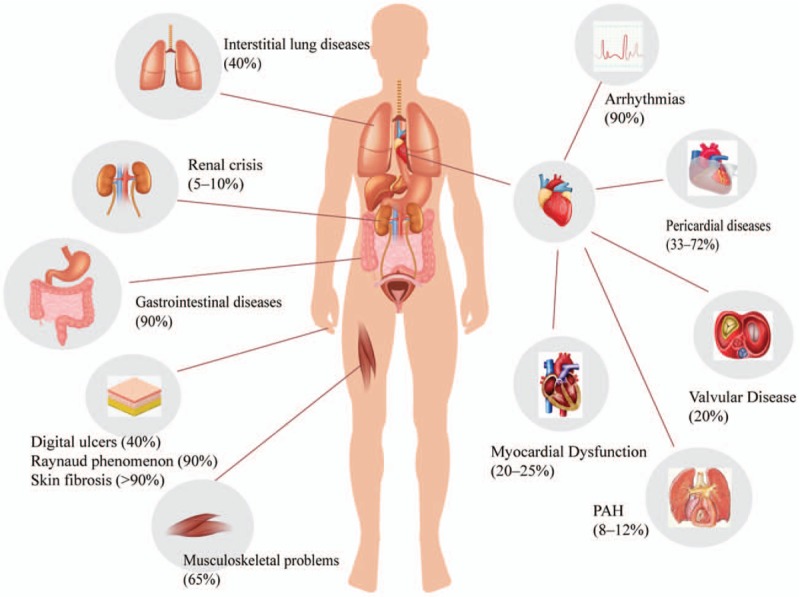
Organ complications associated with systemic sclerosis and classification of cardial complication.
Cardiovascular magnetic resonance (CMR), as a newly developed non-invasive and non-radiative technique can assess cardiac function and perform tissue characterization.[11] CMR has the capability to detect presentations such as edema, infiltration, ischemia, and fibrosis of the cardiac muscles for the early diagnosis of cardiac involvement in SSc. CMR is attractive as reduced scan time and affordable for identifying SSc patients at high risk for cardiac involvement.[9]
The aim of this review is to introduce the high prevalence and high mortality in SSc presenting with cardiac symptoms, and the relevant diagnosis and management of SSc. In addition, we highlighted the diagnostic potential of CMR in the evaluation of SSc patients with primary cardiac involvement.
Physiopathology of Cardiac Complications
Cardiac involvement in SSc patients is often clinically occult. Demonstrated by echocardiography, electrocardiography (ECG), computed tomographic (CT), and magnetic resonance imaging, the existence of reversible functional and vasospastic abnormalities of the heart has been observed in SSc patients at an early stage.[5] At this point in time, patients usually have no clinical symptoms; however, as the disease progresses, permanent structural abnormalities of the small coronary arteries and arterioles might result in reduced coronary flow reserve which leads to myocardial microcirculation disturbances.[12] Myocardial fibrosis occurs later in SSc, leading to diastolic and systolic dysfunction.[13] Both ventricles can be affected by myocardial fibrosis, causing impaired relaxation of myocardial tissue, increased ventricular mass, and decreased movement of the ventricular walls during diastole.[5,14] Notably, atherosclerosis and macrovascular heart disease are regarded as major roles in the development of cardiac involvement in SSc in some studies.[15]
Patterns of Cardiac Complications
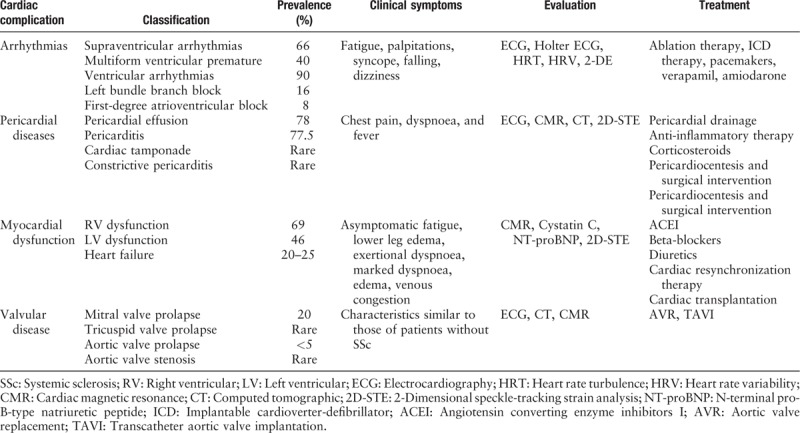
Cardiac involvement in SSc can affect all structures of the heart, contributing to arrhythmias, pericardial diseases, myocardial dysfunction, heart failure, and valvular diseases. The main characteristics of patients with SSc-related heart disease are summarized in Table 1.
Table 1.
Classification, prevalence, symptomatology, valuation and treatment of cardiac complications in SSc.[19,20,25,26,29,34,35,40]
Arrhythmias
Arrhythmias are the most frequent cardiac complications according to the large European League Against Rheumatism Scleroderma Trials and Research database, which indicated that among the 128 SSc-related deaths, 26% of deaths were attributed to cardiac origin, while arrhythmias accounted for 6% of deaths in total. Arrhythmias are not only associated with high mortality, but are also related to a poor prognosis.[16] A combination of the direct effects of microvascular injury, the subsequent development of fibrosis, and an autonomic dysfunction results in arrhythmia.[5] In addition, myocardial edema has been proposed as an important causative factor of ventricular tachycardia and ventricular fibrillation.[9] Moreover, drugs might also induce arrhythmias, such as methotrexate, which is used as a treatment for scleroderma, and despite a small probability known, drugs may induce right bundle block and ventricular arrhythmias.[17,18]
The patterns of arrhythmias are various. In a study of 53 consecutive patients with SSc, 42% presented with one or more abnormal features in ECG. In 24-h Holter ECG monitoring, 66% of patients with abnormal features were found to have supraventricular arrhythmias, 40% were found to have multiform ventricular premature beats, and 90% were found to have ventricular arrhythmias.[19] In a prospective study, 16% and 8% of SSc patients had been observed with left bundle branch block and a first-degree atrioventricular block, respectively.[20] With disease progression, fatigue, palpitations, syncope, and dizziness are the common clinical manifestations of patients with arrhythmias. Although arrhythmias are quite common in SSc and can lead to high mortality, they may also be present in the general population, making an early and accurate diagnosis difficult but important to improve patient prognosis.[17]
To screen early lesions, the most frequently used instruments are ECG and 24-h Holter ECG, which can detect problems quickly and economically. Although 24-h Holter ECG can credibly record supraventricular and ventricular tachycardia, it does not perform tissue characterization.[9] One of the latest progress for evaluating subclinical myocardial dysfunction is to use a two-dimensional speckle-tracking strain analysis (STE), which is more sensitive and accurate than other technologies.[21] CMR, as by measuring the extent of viable tissue and fibrosis after contrast enhancement can detect structural and functional impairments and tissue characterization.[8] As an important causative factor, myocardial edema detected by T2-weighted CMR imaging alters myocardial T2-relaxation. Late gadolinium-enhancement (LGE) is considered to be the best way to detect myocardial fibrosis, and the superior imaging time for detection is 10 to 20 min after the intravenous gadolinium injection. During this time, maximal differences could be found between normal myocardium, fibrotic tissue, and normal myocardium.[22] A study found that approximately 75% of SSc patients had an arrhythmia in an LGE pattern, even though 25% of patients who had a negative LGE pattern had an arrhythmia.[23] Moreover, CMR with LGE has been certified as a predictive tool for ventricular tachyarrhythmia in ischaemic and non-ischaemic cardiomyopathy with ventricular dysfunction.[24]
The early diagnosis of cardiac complications in SSc is prepared for early management treatments of arrhythmias in SSc patients usually according to the same principles applied to the general population. It was emphasized that SSc individuals should have earlier intervention compared to non-SSc individuals. According to general cardiology practice guidelines, ablation therapy, implantable cardioverter-defibrillator (ICD) therapy, and pacemakers can be considered, among these therapies pacemaker implantation is the first choice for complete heart block and other serious bradyarrhythmia.[20] Additionally, CMR can be an effective predictor for ICD therapy.[24] Anti-arrhythmic drugs must be used individualized for SSc patients because concomitant drugs for the treatment of other organ involvement in SSc patients should also be taken into account. For example, beta-blockers should be chosen carefully because they might aggravate Raynaud phenomenon even though they are widely used in the treatment of arrhythmias to improve vasoconstriction.[14] Compared with the side effects of other drugs, calcium channel blockers such as verapamil might be recommended to treat atrial or intra-nodal tachycardia. In terms of conduction disorders, hydroxychloroquine is safer than chloroquine.[17] Domperidone, as a prokinetic drug for gastrointestinal complications in SSc, can increase the risk of serious ventricular arrhythmia and sudden cardiac death (SCD).[25] Currently, although amiodarone might worsen pulmonary fibrosis in immune-allergic pneumonia, it is the most effective anti-arrhythmic drug.[20]
Pericardial diseases
Pericardial involvement in SSc is usually mild and asymptomatic, and it was reported that symptomatic pericardial disease ranges from only 7% to 20% of SSc patients.[24] However, pericardial involvement is frequently found in 33% to 72% patients in autopsy studies, and in 41% of patients in echocardiography, and these results imply that we underestimate this situation.[26,27] Fibrinous pericarditis, pericardial adhesions or effusions without pericarditis have been found in the autopsy of SSc patients.[28] The involvement of the pericardium consists of acute pericarditis, constrictive pericarditis, and pericardial effusion, among which pericardial effusion is more common and is always accidentally diagnosed by echocardiography[29], and pericardial effusion accounts for 15% to 43% of patients in echocardiographic findings, and 78% in autopsy studies.[30]
In one retrospective study, the characteristics and causes of pericardial effusion in SSc were described as inflammation, especially during the early course of the disease. The major pericardial pathology was consistent with pericardial fibrosis, perivascular infiltration of inflammatory cells, and non-specific fibrotic pericardial thickening with adhesion.[27] Pericardial disease in SSc is usually silent, while the manifestations always occur after other clinical features of scleroderma appear.[28] With disease progression, patients might present with the common symptoms of pericardial effusion, such as chest pain, chest tightness, dyspnoea, and fever.
Other organs such as lung and kidney have wide pathological changes in this disease. Currently, many studies have also focused on the correlation among renal crisis, pulmonary hypertension (PAH) and pericardial effusion in SSc. Pericardial effusion in PAH of scleroderma generally represents right heart failure and hemodynamic instability. Pericardial effusion, as one of the predictors for mortality in PAH, has been well examined with echocardiography.[31] A study suggested that cardiac tamponade used in diuretic or heart failure, the diminished renal cortical perfusion would cause renal ischemia in SSc.[30] While reporting a case of a female with scleroderma related renal crisis, a Japanese study indicated that moderate asymptomatic pericardial effusion may predict the occurrence of early or future renal crisis in scleroderma.[32] Interestingly, a descriptive retrospective study performed in SSc patients with symptomatic pericardial effusion in Thailand described that renal disease and PAH rarely occurred in patients with large pericardial effusion or cardiac tamponade, since not a single patient with pericardial effusion demonstrated renal disease and PAH.[27] There are many mechanisms of renal crisis, PAH and pericardial effusion in scleroderma postulated based on available evidence, but the real mechanism is still unknown and warrants further investigation.
It is essential to screen the silent pericardial involvement in SSc because of the poor prognosis. Echocardiography has been suggested as the initial inspective instrument in the diagnosis of pericardial effusion. CT imaging and ECG can be used in the diagnosis of pericardial disease. A bright signal area would also be presented in CMR imaging for pericardial effusion and when cardiac tamponade occurs, through measurement of the extent of viable tissue and fibrosis after contrast enhancement, the imaging would appear with right ventricular (RV) compression.[33] Additionally, the method of monitoring pericardial LGE provides an opportunity for quantitative assessment of active pericarditis and treatment response.[15] STE is useful in visualization of pericardial effusion and pericardial thickening.[34]
There is no specific treatment for pericardial diseases in SSc, but non-steroidal anti-inflammatory therapy with careful monitoring of renal function can be used in acute pericarditis. In cases of cardiac tamponade or pericardial constriction, pericardiocentesis and surgical intervention must be considered. Corticosteroids are useful in the setting of SSc associated myocarditis.[17] Since studies certified that pericardial effusion is associated with PAH or renal crisis, once pericardial effusion is diagnosed, echocardiography is vital to evaluate whether the effusion is progressive, to assess the hemodynamic effects, and to monitor the response to therapy. With large significant pericardial effusions and PAH, the first step is to use vasoactive therapy to stabilize pulmonary artery pressure and right heart function. Moreover, cautious pericardial drainage should be considered.[28,31]
Myocardial dysfunction
Even though myocardial dysfunction is not as frequent as arrhythmias and pericardial diseases in SSc, it is important as a predictor of mortality and might be the most life-threatening condition in patients with SSc.[13] Myocardial dysfunction includes left ventricular (LV) dysfunction, RV dysfunction, and heart failure. The prevalence of reduced left ventricular ejection fraction (LVEF) is greatly underestimated.[35] LV or RV dysfunction includes systolic and diastolic abnormalities. LV systolic dysfunction accounted for 1.4% of the 570 patients in a multicentre study in France.[36] Compared to LV systolic dysfunction, diastolic dysfunction is more frequently associated with an increased risk of mortality.[36] However, in a study of 54 patients, up to 46% of patients had LV dysfunction during exercise, although a low prevalence occurred at rest; the most common abnormality by echocardiography was elevated right ventricular systolic pressure in 69% patients.[37] The prevalence of congestive heart failure is 20% to 25%, with symptoms of marked dyspnoea, edema, and venous congestion. According to some studies, myocardial fibrosis which is the hallmark of cardiac involvement of SSc, may lead to myocardial dysfunction.[38] Patchy fibrosis, secondary to both repeated ischemia and/or immuno-inflammatory damage, also leads to diastolic dysfunction and mechanical remodeling, which could further induce conduction disturbances and arrhythmias. Saito et al[39] indicated that LV dysfunction could be affected by RV dysfunction and pulmonary hemodynamic, reflecting ventricular interdependence in SSc. Moreover, a study on LV diastolic dysfunction in SSc indicated that acute myocarditis, microvascular coronary artery disease, advanced age, and duration of SSc were associated with LV diastolic dysfunction. However, RV dysfunction has usually been reported to be caused by lung vascular disease, interstitial lung disease, and PAH.[29,33,39] The mechanism of heart failure remains controversial regarding myocardial fibrosis. Current hypotheses include vessel obliteration and arteriolar endothelial injury resulting in fibrosis.[30]
As a predictor of LV diastolic dysfunction, reduced tissue Doppler E’ velocity is independently associated with death.[40] STE, providing measurements of LV regional and global strain in three orthogonal directions (longitudinal, circumferential, and radial) can measure both strain and LVEF to assess LV function. Importantly, three orthogonal directions measured in LV were independent predictors of impaired functional capacity.[21] CMR is an important tool in evaluating RV function and morphology and in assessing heart failure etiology (ischaemic or non-ischaemic). In addition, in a clinical study, CMR, particularly in T1 mapping and extracellular volume quantification, can act as a potential tool for when LV dysfunction or irreversible myocardial damage occurs.[41] Akkus et al[42] found that cystatin C and N-terminal pro-B-type natriuretic peptide levels may be considered screening biomarkers for the early detection of right heart failure in SSc patients.
Angiotensin converting enzyme inhibitors I (ACEI) and beta-blockers can be used in systolic dysfunction. Even though the treatment of diastolic heart failure lacks evidence, ACEI and beta-blockers may also be considered. Diuretics should be added when necessary. Cardiac resynchronization therapy and transplantation should be considered for SSc patients with left bundle branch block-associated diastolic dysfunction and end-stage systolic dysfunction, respectively, usually advised when intra-cardiac transmission delay (electrocardiographic QRS wave duration >0.15 s).[43] The data reported indicates that ivabradine can serve as an alternative drug for myocardial dysfunction, which is a selective inhibitor of the sinus node; in addition, vasodilators such as bosentan, sildenafil, and iloprost can improve LV function.[14]
Valvular diseases
Because of the low prevalence of valvular involvement in SSc patients, it is not considered a typical manifestation.[44] It has been reported that the mitral valve lesion is the most frequent in valvular involvement, of which mitral valve prolapse accounts for 60%, although most do not have hemodynamic consequences and are asymptomatic.[45] Additionally, degenerative aortic valve stenosis is a common manifestation in SSc patients. In addition, according to recent studies, premature onset of severe aortic valve stenosis (AS) may be another cardiac complication.[3,45] The clinical symptoms of valvular diseases have characteristics similar to those of patients without SSc.[46]
The mechanism of valvular diseases in SSc remains unknown. It has been reported that the underlying inflammatory burden, immune system activation, widespread microvascular and macrovascular damage, endothelial dysfunction, and fibroblast activity might play important roles in accelerating the progression in SSc.[43] To date, echocardiography allows measurement of valvular dimensions and diagnosis of valvular involvement in SSc, while cardiac CT and CMR can also be considered.[15] Interestingly, it is common for LGE to detect many valvular diseases, especially in aortic stenosis.[22] Ferrari et al[47] reported a patient with aortic stenosis and SSc who required aortic valve replacement. Another study indicated that transcatheter aortic valve implantation (TAVI) was safer and more effective than surgical aortic valve replacement (SAVR). The avoidance of general anesthesia, sternotomy/autotomy of the chest anatomy may be the potential advantages of TAVI over SAVR, making TAVI a viable choice for patients with specific comorbidities.[43]
Conclusions
Cardiac complications play an essential role in SSc and carry the threat of SCD, especially in diffuse cutaneous SSc. In addition, the danger of SCD is tremendous and has a high prevalence, as reported in 21% to 54% of SSc patients, while it is highly likely caused by malignant ventricular tachycardia.[48] Early diagnosis and management of cardiac complication are helpful for slowing the development of myocardial fibrosis, myocardial ischemia, and immuno-inflammatory damage, thus improving the quality of life of patients with SSc. This review mainly described the new early diagnosis and management of various types of cardiac involvement in SSc. Even though modern imaging and biomarkers allow for earlier detection of complications, effective therapy of cardiac complications is still under development. Currently, facing the cardiac implications in SSc, what we can do is symptomatic treatment and intervention in the early stage of disease. However, more studies are needed in the future to determine how to prevent cardiac pathological changes, how to decrease this incidence, and how to control cardiovascular risk factors. In addition, more basic and clinical studies are warranted to investigate and develop better management of cardiac involvement in patients with SSc.
Acknowledgements
The authors thank Kun Zhao from the School of Medicine, Zhejiang University who provided comments that greatly improved the manuscript.
Funding
This work was supported by grants from Zhejiang Medical and Health Science and Technology Program (No. 2018KY422), and General Research Program of Zhejiang Provincial Department of Education (No. Y201941733).
Conflicts of interest
None.
Footnotes
How to cite this article: Nie LY, Wang XD, Zhang T, Xue J. Cardiac complications in systemic sclerosis: early diagnosis and treatment. Chin Med J 2019;132:2865–2871. doi: 10.1097/CM9.0000000000000535
References
- 1.Denton CP, Khanna D. Systemic sclerosis. Lancet 2017; 390:1685–1699. doi: 10.1016/s0140-6736(17)30933-9. [DOI] [PubMed] [Google Scholar]
- 2.Coghlan JG, Wolf M, Distler O, Denton CP, Doelberg M, Harutyunova S, et al. Incidence of pulmonary hypertension and determining factors in patients with systemic sclerosis. Eur Respir J 2018; 51:1701197.doi: 10.1183/13993003.01197-2017. [DOI] [PubMed] [Google Scholar]
- 3.Champion HC. The heart in scleroderma. Rheum Dis Clin North Am 2008; 34:181–190. doi: 10.1016/j.rdc.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heine J. Über ein eigenartiges Krankheitsbild von diffuser Skelerosis der Haut und innerer Organe. Virchows Arch Pathol Anat Physiol Klin Med 1926; 262:351–382. doi: 10.1007/BF01892189. [Google Scholar]
- 5.Kahan A, Coghlan G, McLaughlin V. Cardiac complications of systemic sclerosis. Rheumatology (Oxford) 2009; 48: Suppl 3: 45–48. doi: 10.1093/rheumatology/kep110. [DOI] [PubMed] [Google Scholar]
- 6.Allanore Y, Meune C. Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol 2010; 28:S48–S53. [PubMed] [Google Scholar]
- 7.Wangkaew S, Prasertwitayakij N, Phrommintikul A, Puntana S, Euathrongchit J. Causes of death, survival and risk factors of mortality in Thai patients with early systemic sclerosis: inception cohort study. Rheumatol Int 2017; 37:2087–2094. doi: 10.1007/s00296-017-3846-7. [DOI] [PubMed] [Google Scholar]
- 8.Caforio ALP, Adler Y, Agostini C, Allanore Y, Anastasakis A, Arad M, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J 2017; 38:2649–2662. doi: 10.1093/eurheartj/ehx321. [DOI] [PubMed] [Google Scholar]
- 9.Mavrogeni SI, Sfikakis PP, Dimitroulas T, Koutsogeorgopoulou L, Markousis-Mavrogenis G, Poulos G, et al. Prospects of using cardiovascular magnetic resonance in the identification of arrhythmogenic substrate in autoimmune rheumatic diseases. Rheumatol Int 2018; 38:1615–1621. doi: 10.1007/s00296-018-4110-5. [DOI] [PubMed] [Google Scholar]
- 10.Gargani L, Voilliot D, D’Alto M, Agoston G, Moreo A, Serra W, et al. Pulmonary circulation on the crossroads between the left and right heart in systemic sclerosis: a clinical challenge for cardiologists and rheumatologists. Heart Fail Clin 2018; 14:271–281. doi: 10.1016/j.hfc.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Bull S, White SK, Piechnik SK, Flett AS, Ferreira VM, Loudon M, et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart 2013; 99:932–937. doi: 10.1136/heartjnl-2012-303052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahan A, Nitenberg A, Foult JM, Amor B, Menkes CJ, Devaux JY, et al. Decreased coronary reserve in primary scleroderma myocardial disease. Semin Arthritis Rheum 1985; 28:637–646. doi: 10.1002/art.1780280607. [DOI] [PubMed] [Google Scholar]
- 13.Kahan A, Allanore Y. Primary myocardial involvement in systemic sclerosis. Rheumatology (Oxford) 2006; 45: Suppl 4: i14–i17. doi: 10.1093/rheumatology/kel312. [DOI] [PubMed] [Google Scholar]
- 14.Dimitroulas T, Giannakoulas G, Karvounis H, Garyfallos A, Settas L, Kitas GD. Micro- and macrovascular treatment targets in scleroderma heart disease. Curr Pharm Des 2014; 20:536–544. [DOI] [PubMed] [Google Scholar]
- 15.Lambova S. Cardiac manifestations in systemic sclerosis. World J Cardiol 2014; 6:993–1005. doi: 10.4330/wjc.v6.i9.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tyndall AJ, Bannert B, Vonk M, Airò P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010; 69:1809–1815. doi: 10.1136/ard.2009.114264. [DOI] [PubMed] [Google Scholar]
- 17.Vacca A, Meune C, Gordon J, Chung L, Proudman S, Assassi S, et al. Cardiac arrhythmias and conduction defects in systemic sclerosis. Rheumatology (Oxford) 2014; 53:1172–1177. doi: 10.1093/rheumatology/ket377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng JN, Yang QR, Zhu GQ, Pan L, Xia JX, Wang Q. Comparative efficacy and safety of immunosuppressive therapies for systemic sclerosis related interstitial lung disease: a Bayesian network analysis. Mod Rheumatol 2019; Epub ahead of print. doi: 10.1080/14397595.2019.1640343. [DOI] [PubMed] [Google Scholar]
- 19.Ferri C, Bernini L, Bongiorni MG, Levorato D, Viegi G, Bravi P, et al. Noninvasive evaluation of cardiac dysrhythmias, and their relationship with multisystemic symptoms, in progressive systemic sclerosis patients. Arthritis Rheum 1985; 28:1259–1266. doi: 10.1002/art.1780281110. [DOI] [PubMed] [Google Scholar]
- 20.Roberts NK, Cabeen WR, Jr, Moss J, Clements PJ, Furst DE. The prevalence of conduction defects and cardiac arrhythmias in progressive systemic sclerosis. Ann Intern Med 1981; 94:38–40. doi: 10.1097/00000441-198105000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Yiu KH, Schouffoer AA, Marsan NA, Ninaber MK, Stolk J, Vlieland TV, et al. Left ventricular dysfunction assessed by speckle-tracking strain analysis in patients with systemic sclerosis: relationship to functional capacity and ventricular arrhythmias. Arthritis Rheum 2011; 63:3969–3978. doi: 10.1002/art.30614. [DOI] [PubMed] [Google Scholar]
- 22.Mavrogeni SI, Kitas GD, Dimitroulas T, Sfikakis PP, Seo P, Gabriel S, et al. Cardiovascular magnetic resonance in rheumatology: current status and recommendations for use. Int J Cardiol 2016; 217:135–148. doi: 10.1016/j.ijcard.2016.04.158. [DOI] [PubMed] [Google Scholar]
- 23.Tzelepis GE, Kelekis NL, Plastiras SC, Mitseas P, Economopoulos N, Kampolis C, et al. Pattern and distribution of myocardial fibrosis in systemic sclerosis: a delayed enhanced magnetic resonance imaging study. Arthritis Rheum 2007; 56:3827–3836. doi: 10.1002/art.22971. [DOI] [PubMed] [Google Scholar]
- 24.Guaricci AI, De Santis D, Rabbat MG, Pontone G. Cardiac magnetic resonance imaging and primary prevention implantable cardioverter defibrillator therapy: current recommendations and future directions. J Cardiovasc Med (Hagerstown) 2018; 19:223–228. doi: 10.2459/JCM.0000000000000650. [DOI] [PubMed] [Google Scholar]
- 25.van Noord C, Dieleman JP, van Herpen G, Verhamme K, Sturkenboom MC. Domperidone and ventricular arrhythmia or sudden cardiac death: a population-based case-control study in the Netherlands. Drug Saf 2010; 33:1003–1014. doi: 10.2165/11536840-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Byers RJ, Marshall DA, Freemont AJ. Pericardial involvement in systemic sclerosis. Ann Rheum Dis 1997; 56:393–394. doi: 10.1136/ard.56.6.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitchongcharoenying P, Foocharoen C, Mahakkanukrauh A, Suwannaroj S, Nanagara R. Pericardial fluid profiles of pericardial effusion in systemic sclerosis patients. Asian Pac J Allergy Immunol 2013; 31:314–319. doi: 10.12932/ap0305.31.4.2013. [DOI] [PubMed] [Google Scholar]
- 28.Gowda RM, Khan IA, Sacchi TJ, Vasavada BC. Scleroderma pericardial disease presented with a large pericardial effusion--a case report. Angiology 2001; 52:59–62. doi: 10.1177/000331970105200108. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Cambeiro MC, Abu-Assi E, Abumuaileq RR, Raposeiras-Roubin S, Rigueiro-Veloso P, Virgos-Lamela A, et al. Systemic sclerosis: a rare cause of heart failure? Rev Port Cardiol 2015; 34:617.e1–617.e5. doi: 10.1016/j.repc.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez Morales A, Iniesta N, Fernandez-Codina A, Vaz de Cunha J, Perez Romero T, Hurtado Garcia R, et al. Cardiac tamponade and severe pericardial effusion in systemic sclerosis: report of nine patients and review of the literature. Int J Rheum Dis 2017; 20:1582–1592. doi: 10.1111/1756-185x.12952. [DOI] [PubMed] [Google Scholar]
- 31.Dunne JV, Chou JP, Viswanathan M, Wilcox P, Huang SH. Cardiac tamponade and large pericardial effusions in systemic sclerosis: a report of four cases and a review of the literature. Clin Rheumatol 2011; 30:433–438. doi: 10.1007/s10067-010-1667-0. [DOI] [PubMed] [Google Scholar]
- 32.Shu E, Kanoh H, Seishima M. Scleroderma renal crisis following pericardial effusion in a Japanese female. J Dermatol 2014; 41:824–826. doi: 10.1111/1346-8138.12574. [DOI] [PubMed] [Google Scholar]
- 33.Mavrogeni S, Koutsogeorgopoulou L, Karabela G, Stavropoulos E, Katsifis G, Raftakis J, et al. Silent myocarditis in systemic sclerosis detected by cardiovascular magnetic resonance using Lake Louise criteria. BMC Cardiovasc Disord 2017; 17:187.doi: 10.1186/s12872-017-0619-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rangarajan V, Matiasz R, Freed BH. Cardiac complications of systemic sclerosis and management: recent progress. Curr Opin Rheumatol 2017; 29:574–584. doi: 10.1097/bor.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 35.Porpáczy A, Nógrádi Á, Kehl D, Strenner M, Minier T, Czirják L, et al. Impairment of left atrial mechanics is an early sign of myocardial involvement in systemic sclerosis. J Card Fail 2018; 24:234–242. doi: 10.1016/j.cardfail.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 36.Tennoe AH, Murbraech K, Andreassen JC, Fretheim H, Garen T, Gude E, et al. Left ventricular diastolic dysfunction predicts mortality in patients with systemic sclerosis. J Am Coll Cardiol 2018; 72:1804–1813. doi: 10.1016/j.jacc.2018.07.068. [DOI] [PubMed] [Google Scholar]
- 37.Balaj R, Poanta L, Rednic S. Cardiac involvement in systemic sclerosis. Rom J Intern Med 2012; 50:269–274. [PubMed] [Google Scholar]
- 38.Muresan L, Oancea I, Mada RO, Petcu A, Pamfil C, Muresan C, et al. Relationship between ventricular arrhythmias, conduction disorders, and myocardial fibrosis in patients with systemic sclerosis. J Clin Rheumatol 2018; 24:25–33. doi: 10.1097/RHU.0000000000000623. [DOI] [PubMed] [Google Scholar]
- 39.Saito M, Wright L, Negishi K, Dwyer N, Marwick TH. Mechanics and prognostic value of left and right ventricular dysfunction in patients with systemic sclerosis. Eur Heart J Cardiovasc Imaging 2018; 19:660–667. doi: 10.1093/ehjci/jex147. [DOI] [PubMed] [Google Scholar]
- 40.Hinchcliff M, Desai CS, Varga J, Shah SJ. Prevalence, prognosis, and factors associated with left ventricular diastolic dysfunction in systemic sclerosis. Clin Exp Rheumatol 2012; 30:S30–S37. [PMC free article] [PubMed] [Google Scholar]
- 41.Ntusi NA, Piechnik SK, Francis JM, Ferreira VM, Rai AB, Matthews PM, et al. Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis--a clinical study using myocardial t1-mapping and extracellular volume quantification. J Cardiovasc Magn Reson 2014; 16:21.doi: 10.1186/1532-429X-16-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akkus O, Bozkurt A, Arslantas D, Kaypakli O, Sahin DY, Aktas H, et al. Is cystatin C an evaluative marker for right heart functions in systemic sclerosis? Int J Cardiol 2016; 221:478–483. doi: 10.1016/j.ijcard.2016.07.093. [DOI] [PubMed] [Google Scholar]
- 43.Bissell LA, Anderson M, Burgess M, Chakravarty K, Coghlan G, Dumitru RB, et al. Consensus best practice pathway of the UK Systemic Sclerosis Study group: management of cardiac disease in systemic sclerosis. Rheumatology (Oxford) 2017; 56:912–921. doi: 10.1093/rheumatology/kew488. [DOI] [PubMed] [Google Scholar]
- 44.Bernelli C, Chieffo A, Giustino G, Montorfano M, Latib A, Panoulas VF, et al. Preliminary outcomes after transcatheter aortic valve implantation in patients with systemic sclerosis. EuroIntervention 2015; 10:1464–1467. doi: 10.4244/eijv10i12a255. [DOI] [PubMed] [Google Scholar]
- 45.Sponga S, Basso C, Ruffatti A, Gerosa G. Systemic sclerosis and aortic valve stenosis: therapeutic implications in two cases of aortic valve replacement. J Cardiovasc Med (Hagerstown) 2009; 10:560–562. doi: 10.2459/JCM.0b013e32832c1726. [DOI] [PubMed] [Google Scholar]
- 46.Roldan CA. Valvular and coronary heart disease in systemic inflammatory diseases: systemic disorders in heart disease. Heart 2008; 94:1089–1101. doi: 10.1136/hrt.2007.132787. [DOI] [PubMed] [Google Scholar]
- 47.Ferrari G, Pratali S, Pucci A, Bortolotti U. Aortic valve replacement in systemic sclerosis. J Cardiovasc Med (Hagerstown) 2015; 16: Suppl 1: S60–S61. doi: 10.2459/JCM.0b013e328365aa9d. [DOI] [PubMed] [Google Scholar]
- 48.Bernardo P, Conforti ML, Bellando-Randone S, Pieragnoli P, Blagojevic J, Kaloudi O, et al. Implantable cardioverter defibrillator prevents sudden cardiac death in systemic sclerosis. J Rheumatol 2011; 38:1617–1621. doi: 10.3899/jrheum.100480. [DOI] [PubMed] [Google Scholar]


