Abstract
Background:
Artificial intelligence-assisted image recognition technology is currently able to detect the target area of an image and fetch information to make classifications according to target features. This study aimed to use deep neural networks for computed tomography (CT) diagnosis of perigastric metastatic lymph nodes (PGMLNs) to simulate the recognition of lymph nodes by radiologists, and to acquire more accurate identification results.
Methods:
A total of 1371 images of suspected lymph node metastasis from enhanced abdominal CT scans were identified and labeled by radiologists and were used with 18,780 original images for faster region-based convolutional neural networks (FR-CNN) deep learning. The identification results of 6000 random CT images from 100 gastric cancer patients by the FR-CNN were compared with results obtained from radiologists in terms of their identification accuracy. Similarly, 1004 CT images with metastatic lymph nodes that had been post-operatively confirmed by pathological examination and 11,340 original images were used in the identification and learning processes described above. The same 6000 gastric cancer CT images were used for the verification, according to which the diagnosis results were analyzed.
Results:
In the initial group, precision-recall curves were generated based on the precision rates, the recall rates of nodule classes of the training set and the validation set; the mean average precision (mAP) value was 0.5019. To verify the results of the initial learning group, the receiver operating characteristic curves was generated, and the corresponding area under the curve (AUC) value was calculated as 0.8995. After the second phase of precise learning, all the indicators were improved, and the mAP and AUC values were 0.7801 and 0.9541, respectively.
Conclusion:
Through deep learning, FR-CNN achieved high judgment effectiveness and recognition accuracy for CT diagnosis of PGMLNs.
Trial Registration:
Chinese Clinical Trial Registry, No. ChiCTR1800016787; http://www.chictr.org.cn/showproj.aspx?proj=28515.
Keywords: Faster region-based convolutional neural networks, Perigastric metastatic lymph nodes, Deep learning, Gastric cancer
Introduction
The incidence of gastric cancer globally ranks fifth among malignancies and third among tumor-related deaths.[1,2] In China, gastric cancer has a high incidence, high mortality and poor prognosis; by the time of diagnosis, cancer cells have mostly infiltrated and metastasized, and in this respect, lymph node metastasis is the main form of gastric cancer metastasis.[3] According to the latest National Comprehensive Cancer Network (NCCN) guidelines for gastric cancer, pre-operative neoadjuvant chemotherapy can be considered as long as the pre-operative assessment indicates that the metastatic lymph node is positive, which has been regarded as Type 1 evidence.[4] However, surgery is still the most effective method of gastric cancer treatment. Complete dissection of metastatic lymph nodes is key for the success of radical surgery for gastric cancer and has been jointly recommended by the NCCN guidelines and the Japanese Gastric Cancer Guidelines.[5] Therefore, effective and standardized dissection of metastatic lymph nodes can significantly improve the prognosis and the 5-year survival rate of patients.[6]
Thus, it is extremely important to conduct a complete and accurate clinical pre-operative evaluation of gastric lymph node metastasis, which requires a simple and effective evaluation method. Currently, the most commonly used and reliable assessment method is the evaluation of gastric lymph nodes metastasis and tumor staging through enhanced computed tomography (CT).[7] However, the complex grouping of the gastric lymph nodes leads to technical difficulty in CT evaluation, and false-negative and false-positive (FP) results on perigastric metastatic lymph nodes (PGMLNs) are inevitable.[8] In the traditional method, the radiologist interprets enhanced abdominal CT images and thereby determines whether lymphatic metastasis has occurred based on the size, shape, and density of the lymph nodes. However, it often remains challenging for the radiologist to make correct and timely judgments under time constraints by combining the above-described factors, especially for large numbers of cases for which the accuracy of diagnosis will inevitably decrease and errors are likely to occur. Therefore, it is necessary to develop more convenient, faster, and more accurate software or systems that can assist radiologists in quickly identifying PGMLNs.
In recent years, steady progress has been made in deep learning technology. Some clinical studies have already been conducted on pathological diagnosis and the identification of lymphoma, lung nodules, prostate cancer, and lymph nodes[9–13] have already been conducted. Artificial intelligence-assisted image recognition technology is currently able to detect the target area of an image and make classifications according to the detected target features, which is similar to the diagnosis process of the radiologist. This approach also represents a new solution for the above problems. Based on deep learning of medical imaging knowledge and network construction, the medical image artificial intelligence-assisted automatic recognition system, which is called faster region-based convolutional neural networks (FR-CNN) established in our hospital, can identify specific lesions through the identification and labeling of lesions, with automatic volume delineation and three-dimensional reconstruction of target areas. In this study, we aimed to identify PGMLNs in enhanced CT images using deep learning networks, and we continuously improved the accuracy of the network through routine deep learning and precision deep learning using CT images with pathologically confirmed metastatic lymph nodes.
Methods
Patients and ethical approval
This retrospective study was conducted in the Department of Gastrointestinal Surgery of the Affiliated Hospital of Qingdao University from January 2011 to May 2018. It was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University and waived informed consent due to the retrospective nature of this study. A total of 750 patients with gastric cancer who had been gastroscopically diagnosed were included, and they were pre-operatively subjected to CT scans (PHILIPS Brilliance iCT, Eindhoven, Netherlands). Of these, 250 patients underwent surgical treatment within 2 weeks of CT examination, and the presence of PGMLNs was confirmed by post-operative pathology. This study was registered in the Chinese Clinical Trial Registry (No. ChiCTR1800016787).
Grouping and sessions
In this study, two deep learning sessions were performed. The first deep learning session was performed on the initial group. After exclusion, 18,780 enhanced CT images and 1371 labeled CT images from 313 patients in the initial group were used in the identification. The second deep learning session was performed in the precision group. After exclusion, 11,340 enhanced CT images and 1004 labeled CT images from 189 patients in the precision group were used in the identification. The specific grouping and flow chart are shown in Figure 1.
Figure 1.
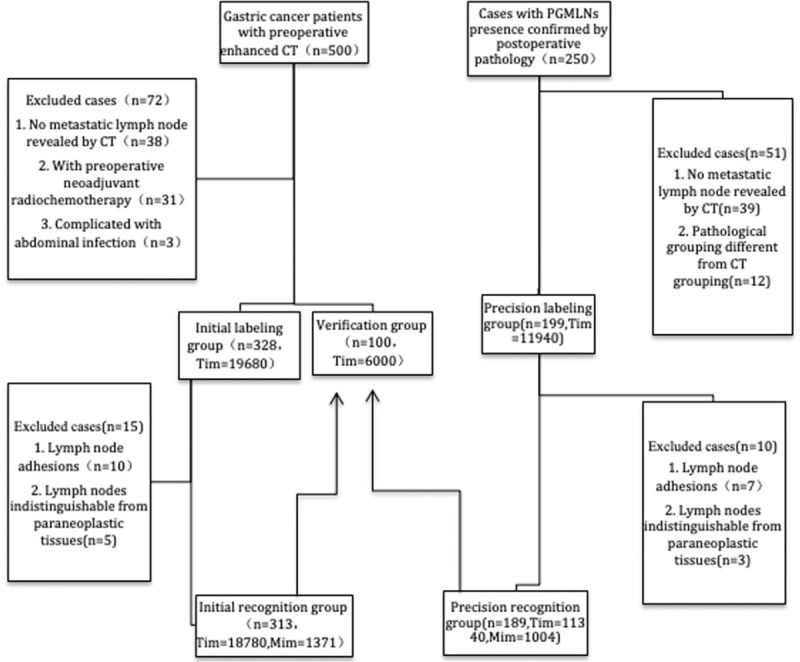
Patient grouping, exclusion and testing sessions of this study. CT: Computed tomography; Mim: Marked images; PGMLNs: Perigastric metastatic lymph nodes; Tim: Total images.
Study method
CT scan
Before CT examination, all of the patients were asked to fast for 8 to 12 h and not take any heavy-metal drugs for 1 week. They were intra-muscularly injected with 10 mg of anisodamine hydrochloride (654-2) and asked to drink 800 to 1200 mL of lukewarm water 10 to 15 min before the CT scan to ensure that more than 3/4 of the total stomach volume was filled and the lesions were completely covered by water. The scanning range was from the mediastinum top to the umbilical plane, and the scan was completed within a single breath-hold after inhaling. Triphasic contrast enhancement scanning was performed with 80 to 100 mL of non-ionic contrast agent (Ultravist, 300 mgI/mL; Bayer Schering Pharma AG, Germany) (the total amount was 1.5 mg/kg body weight, which was injected into the patient's median vein of the elbow with a high-pressure syringe, at 3.0 mL/s), and the arterial phase was scanned with the contrast agent tracking and automatic triggering. The portal venous-phase scan was performed 20 to 40 s after the arterial scan, while the equilibration phase scan was performed 1 min after the arterial scan. The scanning parameters were as follows: helical: 64 × 0.625; gantry rotation time: 0.7 s; transverse image reconstruction slice thickness: 1 mm; slice spacing: 0.7 mm; crown and sagittal pitch: 1.0; slice thickness: 1.0 mm; tabletop movement speed: 12 mm/s; tube voltage: 120 kV; tube current: 260 to 320 mAs; image reconstruction slice thickness: 5 mm.
Labeling process
Three experienced radiologists carefully examined the full-sequence triphasic contrast-enhanced CT images of the upper abdomen, and they observed the relevant locations, such as the cancer periphery, the hepatogastric gap, and the periphery of the important blood vessels. The radiologists also labeled 1371 upper abdominal enhanced CT images on metastatic lymph nodes by using the standardized window-adjusting technique coupled with local scaling and measuring functions, and they combined the information in the corresponding imaging report according to the latest diagnostic criteria for upper abdominal contrast-enhanced CT for metastatic lymph nodes of gastric cancer. Then, a radiologist from our own hospital further labeled the metastatic lymph nodes according to their sizes, shapes, and enhancement density values according to the following criteria. Size: lymph nodes greater than 10 mm were considered to be likely metastatic. Shape: lymph nodes with irregular or spiculated edges, round or near-round shape, a short diameter to long diameter ratio of ≥0.7, a high-density periphery but a low-density center, a lower peak value of arteriovenous enhancement than vessels in the same slice in the enhanced CT image and a cystic, nibbling and significantly enhanced appearance after enhancement were generally considered metastatic. Enhancement density difference: an arterial phase CT value of ≥70 Hu or a venous-phase CT value of ≥80 Hu was used as the diagnostic criterion for a positive lymph node. The locations of the metastatic lymph nodes in the spiral CT three-phase enhanced scan images were labeled. At the same time, the labeling results were evaluated by three experienced radiologists and one gastrointestinal surgeon; in case of a dispute among the four doctors, the opinion of an additional radiologist was sought, and a consensus was reached after discussion. A total of 1371 labeled images (approximately three images per patient) were chosen as images with diagnostic value. These images were exported to a deep neural network for the deep neural network platform to learn and were used in combination with the included upper abdominal enhanced CT images of each patient to establish the image databases for artificial intelligence learning and training.
After consulting several experienced gastrointestinal surgeons, radiologists, and pathologists of our hospital, we decided to infer the imaging result from the pathological result, for which the pathologists provided the post-operative pathology reports of most recent 250 patients with gastric cancer. Three senior radiologists used the metastatic lymph nodes diagnosed in the pathology report to relabel 1004 upper abdominal enhanced CT images with metastatic lymph nodes based on the patient's hospitalization number, the image number, and the hospitalization number for continued deep learning of the enhanced CT automatic recognition system on metastatic lymph nodes of gastric cancer. This approach was intended to further improve the artificial intelligence system such that its accuracy in identifying the actual metastatic lymph nodes of lesions would be improved.
Training and identification processes
The training process included two phases. In the first phase, initial training was conducted with 1371 labeled abdominal CT images of lymph node metastasis and the 18,780 original CT images as training data. ImageNet was pre-trained as visual geometry group 16 with 13 convolution layers and three fully connected layers, which were used to initialize the feature extraction network, and all of the new layers were randomly initialized by the weights that were extracted from the zero-mean Gaussian distribution with a standard deviation of 0.01. The training process was also performed in two phases, which included 80,000 iterations of training on the region proposal network candidate regions (the learning rate of the first 60,000 iterations was 0.001 and that of the next 20,000 iterations was 0.0001) and 40,000 iterations of classification and regression based on the eigenvectors of candidate regions (the learning rate of the first 30,000 iterations was 0.001 and that of the next 10,000 iterations was 0.0001), with a momentum of 0.9 and a weight decay of 0.0005. The scales of the anchors of the region generation network were set to 1282, 2562, and 5122, respectively. The aspect ratios of the anchors were set to 0.5, 1, and 2, respectively. During the training process, the error between the predicted value and the actual value was calculated, based on which the deep learning network parameters, such as the weight, were adjusted using end-to-end back-propagation and stochastic gradient descent. Network convergence was achieved by minimizing the loss function being reduced through sequential iterations.
Because the training results and the test results obtained after the above-described training processes were not satisfactory, we added the second phase of model parameter fine-tuning and testing. In this second phase, after multiple sessions of discussion and consultation among senior gastrointestinal surgeons, imaging specialists, and pathologists in our hospital, we decided to infer the imaging result from the pathological result. The pathologists provided the post-operative pathology reports of the most recent 250 patients with gastric cancer, and three senior radiologists used the metastatic lymph nodes diagnosed in the pathology reports to relabel 1004 upper abdominal enhanced CT images with metastatic lymph nodes. These images, when coupled with the 11,340 original CT images were used for the continued deep learning of the enhanced CT automatic recognition system of metastatic lymph nodes of gastric cancer. By implementing this approach, the artificial intelligence system was improved such that its accuracy in identifying actual metastatic lymph nodes of lesions was increased. The second phase of the training process used the final model obtained from the final training of the first phase for the initialization of the feature extraction network, and the above-described two-phase training process was also adopted.
Statistical analysis
Statistical analysis of the data was performed using the SPSS 20.0 analysis software (SPSS Inc., Chicago, IL, USA). Comparisons among count data were performed using the Chi-square test. P < 0.05 was considered statistically significant. The sensitivity, specificity (FP rate) and accuracy of the artificial intelligence diagnosis system for metastatic lymph nodes were also analyzed, and the receiver operating characteristic (ROC) and precision-recall (PR) curves were generated. The ROC curve was mainly used as the basis of the analysis, and the area under the ROC curve (AUC) of the FR-CNN-based artificial intelligence-assisted diagnosis was calculated using the trapezoidal method.
Results
General patient information
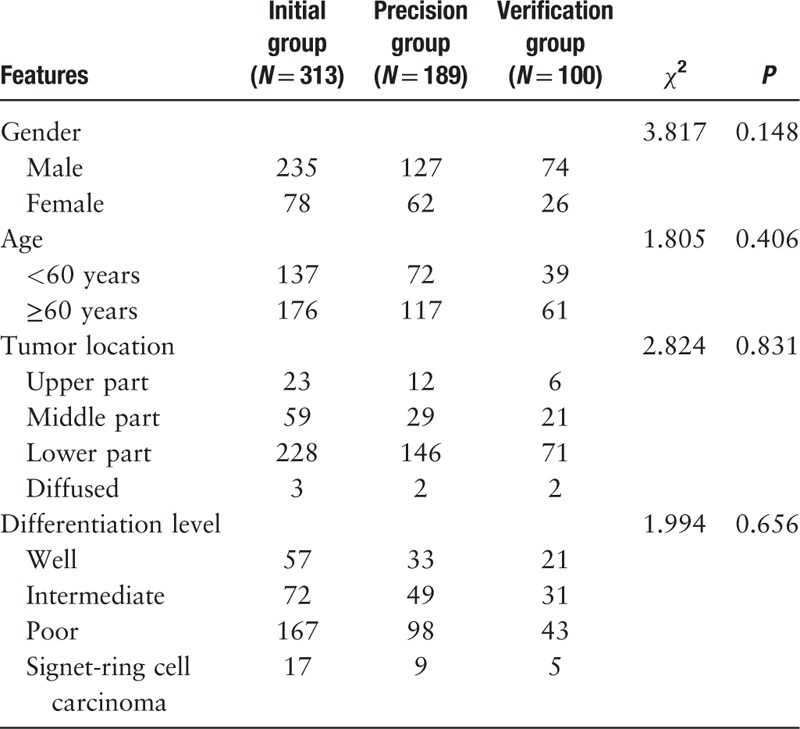
A total of 602 patients, 436 males (72.4%) and 166 females, with an average age of 61.1 years (range, 20–91 years), were included in this study. Sex, age, tumor location, and differentiation level were not significantly different among the initial group, the precision group and the verification group (P > 0.05). For the tumor location, the incidence of lower gastric cancer was the highest and diffused gastric cancer was the lowest in trend. For the differentiation level, the incidence of poorly differentiated adenocarcinoma was the highest and signet-ring cell carcinoma was the lowest in trend [Table 1].
Table 1.
Basic information of 602 patients with gastric cancer (n).
Clinical verification of the FR-CNN-assisted CT imaging diagnosis of the gastric lymph nodes
Verification of the initial group after training
First, to fully grasp the training effectiveness in the training process, we examined the precision rates and the recall rates of the nodule classes of the training set and the validation set, based on which PR curves were generated, as shown in Figure 2A.
Figure 2.
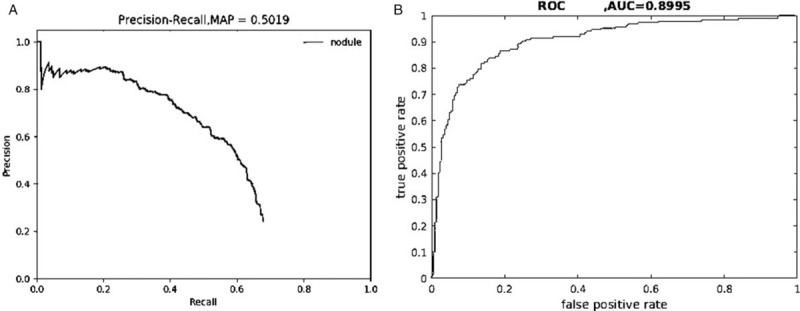
Precision-recall curve (A) and ROC curve (B) of the initial group after training. AUC: Area under the curve; MAP: Mean average precision; ROC: Receiver operating characteristic.
An AUC of 0.5019, that is, AP = 0.5019, was calculated from the curve. Because only one class (nodule) was included in this study, mAP = AP = 0.5019. Next, to reflect the regression and classification results of the testing process more visually and completely, we counted the numbers of true positives (TPs)/FPs of all of the labeled metastatic lymph node regions of the test set. The TP and FP rates under different probability thresholds were calculated and plotted as ROC curves, as shown in Figure 2B. The AUC was calculated as 0.8995, that is, AUC value = 0.8995, which accurately and comprehensively reflects the effectiveness of the trained model on the test data set.
Verification using the precision group after training
To more accurately and comprehensively verify that the second phase enhanced the effectiveness of the whole system, we examined the precision rates and the recall rates of the nodule classes of the training set and the validation set, based on which the PR curves were plotted, as shown in Figure 3A.
Figure 3.
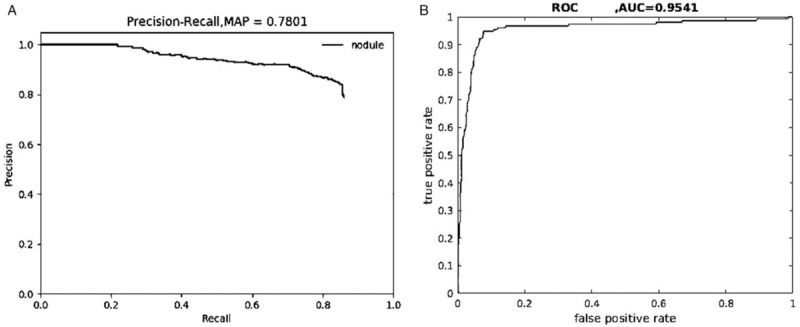
Precision-recall curve (A) and ROC curve (B) of the precision group after training. AUC: Area under the curve; MAP: Mean average precision; ROC: Receiver operating characteristic.
An AUC of 0.7801, that is, AP = 0.7801, was calculated from the curve. In this study, only one class (nodule) was included, mAP = AP = 0.7801, which indicates that the training effectiveness of the FR-CNN was significantly improved. Next, to more visually and completely reflect the regression and classification results of the testing process, we counted the numbers of TPs/FPs of all of the labeled metastatic lymph node regions of the test set. Based on those results, the TP and FP rates under different probability thresholds were calculated and plotted as ROC curves, as shown in Figure 3B. The AUC was calculated to 0.9541, that is, AUC value = 0.9541, which was significantly improved compared with the AUC value of the first phase (0.8995). Figure 4 shows the identification result of pathologically confirmed metastatic lymph nodes by the FR-CNN.
Figure 4.
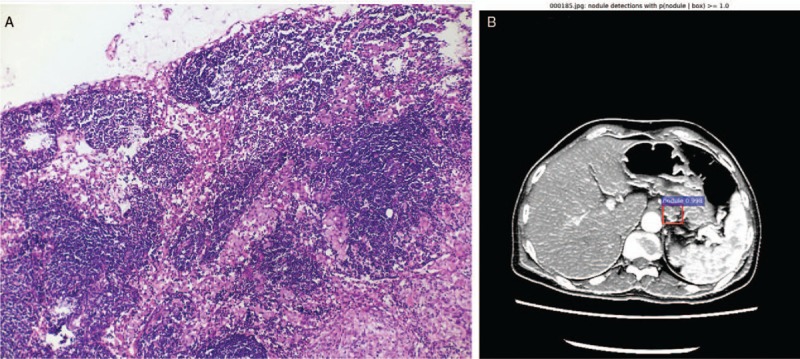
The pathology result (A, hematoxylin and eosin staining, original magnification ×100) and identification result by the FR-CNN of metastatic lymph nodes (B). FR-CNN: Faster region-based convolutional neural networks.
Discussion
Due to the presence of rich lymphatic vessels and lymph nodes, the most common metastatic route of gastric cancer is lymphatic metastasis.[14] Currently, the most comprehensive classification of gastric lymph nodes is the method defined by the Japanese Classification of Gastric Cancer, which has also been recognized by the American Joint Committee on Cancer and the International Union Against Cancer's.[15] Surgery and adjuvant chemotherapy currently remains the most common gastric cancer treatment. In particular, surgery is typically the first choice of treatment for gastric cancer, and lymph node dissection the most critical step in radical gastrectomy, consistent with the increased focus on lymph node dissection among scholars.[16] Most studies have shown that the number of dissected gastric lymph nodes is an independent predictive factor in gastric cancer prognosis, and the intra-operative dissection of more than 15 lymph nodes is the minimum standard recognized by various guidelines and studies.[17–19] However, accurate pre-operative evaluation of gastric lymph nodes remains a challenge for surgeons. Although the development of CT, magnetic resonance imaging, and ultrasound endoscopy has provided a new platform for lymph node identification in recent years, these methods still heavily rely on the subjectivity and experience of the doctors, and; therefore, the accuracy of recognition is not ideal.[20–22] The emergence of artificial intelligence has brought new opportunities and challenges to the development of medicine. Artificial intelligence-based deep neural networks have made remarkable achievements in the fields of breast cancer, lung cancer, lymphoma, and colorectal cancer, but have barely touched the field of gastric cancer. In this study, we developed a deep neural network to identify PGMLNs, and we continuously improved the recognition accuracy of PGMLNs through the initial learning phase and the precision learning phase, thus aiming to partially replace the manual recognition of PGMLNs and guide the diagnosis and treatment of gastric cancer.
Advantages of the FR-CNN in PGMLN recognition
With the continuous development of artificial intelligence in medicine, an increasing number of artificial intelligence-related studies have been conducted, and the identification of metastatic lymph nodes of tumors is an application that has received significant interest. Ehteshami Bejnordi et al[23] investigated deep learning through artificial intelligence on the features of metastatic lymph nodes in female patients with breast cancer and compared its recognition results with those of 11 pathologists, and they found that the recognition accuracy of the artificial intelligence algorithm was significantly higher than that of the expert group. Ichimasa et al[11] studied learning through artificial intelligence on T1 stage colorectal cancer to facilitate radical surgery via endoscopic dissection. They analyzed lymph node metastasis and compared the data with various guidelines such as those of the NCCN, the European Society for Medical Oncology, and the Japanese Society for Cancer of the Colon and Rectum. They found that artificial intelligence can prevent unnecessary secondary surgery on lymph node-negative patients with colorectal cancer who have undergone endoscopic dissection. The FR-CNN established in this study can reconstruct the distribution of lymph nodes in the body from multiple angles, which is conducive for more accurately understanding lymph node morphology and, thereby, improving the detection rate while ensuring the detection specificity. At the same time, in the experience of the radiologist, when measuring lymph node diameter on axial CT images, some lymph nodes show a shorter diameter in the axial position, and thus, the measured diameter can be underestimated due to the limitations displaying of different parts of the lymph nodes on the section. In contrast, the FR-CNN enables comprehensive lymph node observation from multiplanar reconstruction images to avoid false-negative results caused by the above-described factors. Some structures, such as vascular cross-section or perigastric adipose tissue, cannot be distinguished from lymph nodes even in thin-layer scanning, but by using the FR-CNN three-dimensional reconstruction and restoration technique, lymph nodes and surrounding nodular tissue can be accurately identified by examining continuous CT images, such that the FP rate of the detected lymph nodes can be reduced. Therefore, FR-CNN enables us to observe the location, number, size, shape, and density of the lymph nodes from different angles. The algorithm can provide comprehensive judgment, thereby improving the accuracy of lymph node staging in gastric cancer.
Significance of the FR-CNN for pre-operative neoadjuvant chemotherapy
The latest NCCN guidelines have regarded pre-operative neoadjuvant chemotherapy for gastric cancer as Type 1 evidence, and a series of studies have been conducted to demonstrate the importance of pre-operative neoadjuvant chemotherapy for gastric cancer. Eom et al[24] conducted a comparative study on pre-operative neoadjuvant chemotherapy and direct surgery in patients with Stage III/IV (M0) gastric cancer, and they found that the 5-year survival of the neoadjuvant treatment group was significantly higher than that of the surgery group, without increasing post-operative complication incidence. Sakuramoto et al[25] also conducted a comparative study on patients with advanced gastric cancer who orally received S-1 pre-operatively and those undergoing direct surgery, and they found that the application of S-1 significantly improved both the prognosis and the 5-year survival of patients. In the above studies, pre-operative gastric lymph node metastasis-positive patients were used in the main inclusion group for neoadjuvant treatment, and thus, the determination of pre-operative gastric lymph node metastasis will directly affect the choice of neoadjuvant therapy. However, due to the enormous workload of interpreting CT scans, the clinical description of lymph node metastasis in the CT report is not detailed, and the information on grouping and number of PGMLNs in the report is often ambiguous. These issues make it difficult for clinicians to make pre-operative choices regarding neoadjuvant therapy. In this study, we found that the recognition accuracy of the FR-CNN for PGMLNs was 95.4%, allowing a fast and accurate pre-operative determination of the number, locations, and diameters of metastatic lymph nodes while providing important bases for tumor staging of patients. As a result, the optimal pre-operative neoadjuvant treatment can be better determined for patients with advanced gastric cancer, and we can develop more reasonable treatment plans.
Significance of the FR-CNN in endoscopic submucosal dissection and lymph node dissection
Lymph node dissection is the most important step in radical gastrectomy. The number of lymph nodes to be dissected determines the prognosis of the patient. A series of in-depth studies have been conducted with regard to the recommendation that at least 15 lymph nodes be dissected in various guidelines, and it was found that the dissection of more than 29 lymph nodes can better improve the prognosis of patients.[26] In addition to the number of lymph nodes to be dissected, some investigations have confirmed that the anatomical location of PGMLNs is also an important factor that affects patient prognosis.[27] Moreover, for patients with early gastric cancer who have undergone non-curing endoscopic resection, many studies have been performed to elucidate the predictors of lymph node metastasis, which revealed that the number of lymphangioma invasions, the emergence of papillary adenocarcinoma, the invasion to lymphatic vessels, and the positive vertical margin are all important predictors.[28,29] Therefore, an accurate pre-operative understanding of the main distribution area and the number of PGMLNs is very significant for lymph node dissection in radical gastrectomy and also provides important references for the endoscopic dissection of early gastric cancer. The largest obstacle faced in this regard is the misdiagnosis and missed diagnosis of PGMLNs via CT, mainly caused by inexperience of the radiologist or the overwhelming image interpretation workload. The development of the FR-CNN fundamentally solves the above problems, eliminating human error caused by inexperience of the radiologist and work fatigue. It also optimizes the choice of the surgical plan and the intra-operative lymph node dissection strategy under the premise of ensuring the accuracy of recognition.
In conclusion, the FR-CNN could change the way that pre-operative PGMLNs of gastric cancer are evaluated, allowing transformation from a manual strategy that relies mainly on image interpretation by a radiologist to an artificial intelligence-based strategy that relies on a deep learning network and significantly improves both the recognition accuracy and the recognition speed. In this study, the labeling of metastatic lymph nodes on enhanced CT images by experienced radiologists enabled deep learning of the FR-CNN, and the second phase precision deep learning on PGMLN cases that were post-operatively and pathologically confirmed further improved the recognition accuracy of the FR-CNN and led to satisfactory clinical outcomes. Application of the FR-CNN could facilitate the accurate pre-operative evaluation of PGMLNs in patients with gastric cancer to guide the choice of treatment, such as pre-operative neoadjuvant therapy, endoscopic tumor resection, or intra-operative lymph node dissection in key areas. Moreover, heterogeneity among radiologists may lead to differences in the imaging results, thus affecting the diagnosis and treatment of the disease. However, to some extent, a FR-CNN recognition accuracy equivalent to that of a senior radiologist could help reduce the imbalance in imaging-resource distribution in Chinese medicine, which is conducive to the diagnosis and treatment of gastric cancer.
Conflicts of interest
None.
Footnotes
How to cite this article: Gao Y, Zhang ZD, Li S, Guo YT, Wu QY, Liu SH, Yang SJ, Ding L, Zhao BC, Li S, Lu Y. Deep neural network-assisted computed tomography diagnosis of metastatic lymph nodes from gastric cancer. Chin Med J 2019;132:2804–2811. doi: 10.1097/CM9.0000000000000532
References
- 1.Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res 2018; 10:239–248. doi: 10.2147/CMAR.S149619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Zhou YX, Yang LP, Wang ZX, He MM, Yun JP, Zhang DS, et al. Lymph node staging systems in patients with gastric cancer treated with D2 resection plus adjuvant chemotherapy. J Cancer 2018; 9:660–666. doi: 10.7150/jca.22016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, et al. Gastric Cancer, Version 3. 2016, NCCN Clinical Practice Guidelines in Oncology. J Nati Compr Canc Netw 2016; 14:1286–1312. doi: 10.6004/jnccn.2016.0137. [DOI] [PubMed] [Google Scholar]
- 5.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017; 20:1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanizawa Y, Terashima M. Lymph node dissection in the resection of gastric cancer: review of existing evidence. Gastric Cancer 2010; 13:137–148. doi: 10.1007/s10120-010-0560-5. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Fang M, Wang R, Dong D, Tian J, Liang P, et al. Diagnostic accuracy of dual-energy CT-based nomograms to predict lymph node metastasis in gastric cancer. Eur Radiol 2018; 28:5241–5249. doi: 10.1007/s00330-018-5483-2. [DOI] [PubMed] [Google Scholar]
- 8.Kubota K, Suzuki A, Shiozaki H, Wada T, Kyosaka T, Kishida A. Accuracy of multidetector-row computed tomography in the preoperative diagnosis of lymph node metastasis in patients with gastric cancer. Gastrointest Tumors 2017; 3:163–170. doi: 10.1159/000454923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litjens G, Sanchez CI, Timofeeva N, Hermsen M, Nagtegaal I, Kovacs I, et al. Deep learning as a tool for increased accuracy and efficiency of histopathological diagnosis. Sci Rep 2016; 6:26286.doi: 10.1038/srep26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao S, Dong X, Shen W, Ye Z, Xiang R. Machine learning-based classification of diffuse large B-cell lymphoma patients by eight gene expression profiles. Cancer Med 2016; 5:837–852. doi: 10.1002/cam4.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichimasa K, Kudo SE, Mori Y, Misawa M, Matsudaira S, Kouyama Y, et al. Artificial intelligence may help in predicting the need for additional surgery after endoscopic resection of T1 colorectal cancer. Endoscopy 2018; 50:230–240. doi: 10.1055/s-0043-122385. [DOI] [PubMed] [Google Scholar]
- 12.Song Y, Zhang YD, Yan X, Liu H, Zhou M, Hu B, et al. Computer-aided diagnosis of prostate cancer using a deep convolutional neural network from multiparametric MRI. J Magn Reson Imaging 2018; 48:1570–1577. doi: 10.1002/jmri.26047. [DOI] [PubMed] [Google Scholar]
- 13.Nishio M, Sugiyama O, Yakami M, Ueno S, Kubo T, Kuroda T, et al. Computer-aided diagnosis of lung nodule classification between benign nodule, primary lung cancer, and metastatic lung cancer at different image size using deep convolutional neural network with transfer learning. Plos One 2018; 13:e0200721.doi: 10.1371/journal.pone.0200721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han TS, Kong SH, Lee HJ, Ahn HS, Hur K, Yu J, et al. Dissemination of free cancer cells from the gastric lumen and from perigastric lymphovascular pedicles during radical gastric cancer surgery. Ann Surg Oncol 2011; 18:2818–2825. doi: 10.1245/s10434-011-1620-8. [DOI] [PubMed] [Google Scholar]
- 15.Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet 2009; 374:477–490. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shang-Guan XC, Chen QY, Li P, Xie JW, Wang JB, Lin JX, et al. Preoperative lymph node size is helpful to predict the prognosis of patients with stage III gastric cancer after radical resection. Surg Oncol 2018; 27:54–60. doi: 10.1016/j.suronc.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Seevaratnam R, Bocicariu A, Cardoso R, Yohanathan L, Dixon M, Law C, et al. How many lymph nodes should be assessed in patients with gastric cancer? A systematic review. Gastric Cancer 2012; 15: Suppl 1: S70–S88. doi: 10.1007/s10120-012-0169-y. [DOI] [PubMed] [Google Scholar]
- 18.Gholami S, Janson L, Worhunsky DJ, Tran TB, Squires MH, 3rd, Jin LX, et al. Number of lymph nodes removed and survival after gastric cancer resection: an analysis from the US Gastric Cancer Collaborative. J Am Coll Surgeons 2015; 221:291–299. doi: 10.1016/j.jamcollsurg.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karpeh MS, Leon L, Klimstra D, Brennan MF. Lymph node staging in gastric cancer: is location more important than number? An analysis of 1,038 patients. Ann Surg 2000; 232:362–371. doi: 10.1097/00000658-200009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi JI, Joo I, Lee JM. State-of-the-art preoperative staging of gastric cancer by MDCT and magnetic resonance imaging. World J Gastroenterol 2014; 20:4546–4557. doi: 10.3748/wjg.v20.i16.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshikawa T, Tanabe K, Nishikawa K, Ito Y, Matsui T, Kimura Y, et al. Accuracy of CT staging of locally advanced gastric cancer after neoadjuvant chemotherapy: cohort evaluation within a randomized phase II study. Ann Surg Oncol 2014; 21: Suppl 3: S385–S389. doi: 10.1245/s10434-014-3615-8. [DOI] [PubMed] [Google Scholar]
- 22.Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer 2009; 12:6–22. doi: 10.1007/s10120-008-0492-5. [DOI] [PubMed] [Google Scholar]
- 23.Ehteshami Bejnordi B, Veta M, Johannes van Diest P, van Ginneken B, Karssemeijer N, Litjens G, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA 2017; 318:2199–2210. doi: 10.1001/jama.2017.14585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eom BW, Kim S, Kim JY, Yoon HM, Kim MJ, Nam BH, et al. Survival benefit of perioperative chemotherapy in patients with locally advanced gastric cancer: a propensity score matched analysis. J Gas Cancer 2018; 18:69–81. doi: 10.5230/jgc.2018.18.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. New Engl J Med 2007; 357:1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 26.Woo Y, Goldner B, Ituarte P, Lee B, Melstrom L, Son T, et al. Lymphadenectomy with optimum of 29 lymph nodes retrieved associated with improved survival in advanced gastric cancer: A 25,000-patient international database study. J Am Coll Surgeons 2017; 224:546–555. doi: 10.1016/j.jamcollsurg.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao B, Zhang J, Zhang J, Chen X, Chen J, Wang Z, et al. Anatomical location of metastatic lymph nodes: an indispensable prognostic factor for gastric cancer patients who underwent curative resection. Scand J Gastroenterol 2018; 53:185–192. doi: 10.1080/00365521.2017.1415371. [DOI] [PubMed] [Google Scholar]
- 28.Park JW, Ahn S, Lee H, Min BH, Lee JH, Rhee PL, et al. Predictive factors for lymph node metastasis in early gastric cancer with lymphatic invasion after endoscopic resection. Surg Endosc 2017; 31:4419–4424. doi: 10.1007/s00464-017-5490-4. [DOI] [PubMed] [Google Scholar]
- 29.Jung DH, Huh CW, Kim JH, Hong JH, Park JC, Lee YC, et al. Risk-stratification model based on lymph node metastasis after noncurative endoscopic resection for early gastric cancer. Ann Surg Oncol 2017; 24:1643–1649. doi: 10.1245/s10434-017-5791-9. [DOI] [PubMed] [Google Scholar]


