Abstract
Objective:
Adult-onset Still's disease (AOSD) is a rare but clinically well-known polygenic systemic autoinflammatory disease. In this review, we aim to present frontiers in the pathogenesis, clinical features, diagnosis, biomarkers, disease course, prognosis, and treatment in AOSD.
Data sources:
We retrieved information from the PubMed database up to July 2019, using various search terms and relevant words, including AOSD and Still's disease.
Study selection:
We included data from peer-reviewed journals. Both basic and clinical studies were selected.
Results:
Pathogenesis of AOSD involves genetic background, infectious triggers, and immunopathogenesis, mainly the activation of macrophages and neutrophils followed by a cytokine storm. Diagnosis and prognosis evaluation of AOSD is still challenging; therefore, there is an urgent need to identify better biomarkers. Biologic agents, including interleukin (IL)-1β, IL-6, and tumor necrosis factor-α antagonists in the treatment of AOSD, have good prospect.
Conclusion:
This review highlights the advances in pathogenesis, potential biomarkers, disease course, and treatment in AOSD.
Keywords: Adult-onset Still's disease, Biomarkers, Disease course, Pathogenesis, Treatment
Introduction
Adult-onset Still's disease (AOSD) is a rare but clinically well-known multi-systemic autoinflammatory disorder. It is typically characterized by a high spiking fever, an evanescent skin rash, polyarthralgia, sore throat, leukocytosis, and hyperferritinemia.[1–3] AOSD was first defined by Bywaters[4] in 1971 after description of fourteen adult patients whose clinical manifestations closely resembled the systemic juvenile idiopathic arthritis (previously named Still's disease). The incidence of AOSD has been reported at 0.16 (per 100,000 persons) in France,[5] 0.22 in Japan,[6] and 0.4 in northern Norway.[7] AOSD usually affects young adults, and the median age at diagnosis is 36 years old.[7] Females seem to be more affected in some studies, accounting for approximately 70% of the patients with AOSD,[8] while in a recent study AOSD is considered to have a similar incidence in men and women. Asian patients are reported to have a significantly higher in-hospital mortality rate.[9]
Progresses have been achieved in the complex pathogenesis of AOSD in the last few decades. In this review, we focus on the frontiers in the pathogenesis arising from recent studies, and aim to update information about disease course and prognosis in AOSD.
Pathogenesis
The etiology of AOSD is still unclear, while there is evidence that various mechanisms contribute to the pathogenesis of AOSD, mainly including genetic susceptibility, infectious triggers, activation of inflammation, and deficient resolution of inflammation [Figure 1].
Figure 1.

Genetic background and environmental triggers like PAMPs and DAMPs are the beginning points of inflammation in AOSD. They drive to stimulate macrophages and activate NLPR3 inflammasomes. Then NLRP3 inflammasomes facilitate caspase-1 activation, leading to the proteolytic cleavage of pro-IL-1β and pro-IL-18 to its bioactive and mature forms, which further generate a burst of a cytokine storm with IL-6, IL-8, and TNF-α involvement. Neutrophils are also extensively activated in AOSD and release more NETs, which can further stimulate NLRP3 activation. Activated neutrophils also generate more S100 proteins, responsible for the amplified inflammatory response. Besides these two important innate immune cells, adaptive immune cells like NK cells and T cells are also involved in the pathogenesis of AOSD. The amount and function of NK cells are deficient in AOSD, but Th1 and Th17 cells are elevated, which contribute to the activation of macrophages or neutrophils in AOSD by producing more IFN-γ and IL-17. Besides, deficiency in the resolution of inflammation, including decreased TGF-β and Treg cells, also plays a role in the cytokine storm in AOSD. Notably, macrophage activation leads to release of ferritin, which may exacerbate inflammation in AOSD by unclear mechanisms. AGEs: Advanced glycation end products; AOSD: Adult-onset Still's disease; DAMP: Damage associated molecular pattern; ER: Endoplasmic reticulum; HMGB1: High mobility group box-1; IL: Interleukin; MIF: Macrophage inhibitory factor; NET: Neutrophil extracellular trap; NETosis: NET formation; NLRP3: NACHT, LRR, and PYD domains-containing protein 3; PAMP: Pathogen associated molecular pattern; ROS: Reactive oxygen species; SAA1: Serum amyloid A1; TNF: Tumor necrosis factor.
Genetic background
AOSD is categorized as a multigenic disorder.[10] Familial trend has not been reported for AOSD yet, but some studies have found that genetic susceptibility and polymorphisms were associated with AOSD. Associations of AOSD patients and human leucocyte antigen (HLA) antigens, including HLA-Bw35 (first described), -B17, -B18, -B35, -DR2, -DR4, -DR5, -DQ1, -DRw6, -DRB1, and -DQB1 have been described in different ethnic groups.[11–15] Polymorphisms in genes of interleukin-18 (IL-18), serum amyloid A1, and macrophage inhibitory factor (MIF) may affect the susceptibility of patients with AOSD.[16–19] But there are no significant associations of FcγR or Mediterranean fever gene polymorphisms with AOSD.[20–22]
Infectious triggers
It has long been suspected that infections, especially viral infections, are potential triggers of AOSD due to the similar symptoms between them. AOSD patients often present similar manifestations with viral infections, including abrupt high fever, sore throat, and rash before the onset or relapse of disease.[10,23] Over the past decades, many cases have reported infection with pathogens in AOSD patients, including rubella virus, measles morbillivirus, mumps virus, Epstein-Barr virus, hepatitis A virus, hepatitis B virus, hepatitis C virus, human immunodeficiency virus, cytomegalovirus (CMV), parvovirus B19, adenovirus, echovirus, human herpesvirus 6, influenza virus, parainfluenza viruses, coxsackievirus, Yersinia enterocolitica, Campylobacter jejuni, Chlamydia trachomatis, Chlamydia pneumoniae, Mycoplasma pneumoniae, and Borrelia burgdorferi.[24–29] However, data based on a cohort study is lacking. Recently, a study consisting of 100 AOSD patients has investigated the presence of antibodies against virus, virus DNA load, and nucleic acid sensors (IFI16 and AIM2).[30] It shows that CMV infection is strongly related to the initiation or amplification of inflammatory responses in AOSD. It is suggested that viral infection may trigger the initiation or relapse of AOSD.[30]
Activation of inflammation
The activation and amplification of inflammation, characterized by a cytokine storm, is the hallmark of AOSD.[3,10,31] It has been recognized that the initiation and facilitation of inflammation are mainly driven by innate immune cells while adaptive immune cells also participate in it. Among them, macrophage and neutrophil activation play a major role in the pathogenesis of AOSD.[10] Natural killer (NK) cells and T cells are also reported to be involved in the amplified inflammatory response.
Macrophage Activation
Many biomarkers reflecting macrophage activation are increased in patients with AOSD and correlated with disease activity, including macrophage-colony stimulating factor and interferon-γ (IFN-γ).[32,33] Their activation is triggered by danger signals such as pathogen-associated molecular patterns or damage-associated molecular patterns (DAMPs).[31,34] The role of several DAMPs in AOSD pathogenesis have been well illustrated, including high mobility group box-1, advanced glycation end products, S100 proteins, soluble CD163, MIF, and neutrophil extracellular traps (NETs).[34] These danger signals initiate macrophages activation via specific Toll-like receptors, and then facilitate activation of NACHT, LRR, and PYD domains-containing protein 3 (NLRP3) inflammasomes. Upon activation, NLRP3 inflammsomes upregulate caspase-1 activity, leading to the proteolytic cleavage of pro-IL-1β and IL-18 to its bioactive and mature forms from their ex-forms.[31,35,36] Then IL-1β and IL-18 further promote immune cells to produce a large amount of pro-inflammatory cytokines, including IL-6, IL-8, IL-17, and tumor necrosis factor (TNF)-α, as well as IL-1β and IL-18 themselves.[31,35–37] These cytokines activate downstream pathways and thus contribute to an amplified inflammatory response, which is called a cytokine storm. Macrophage activation leads to increased release of ferritin. It is well recognized that AOSD is characterized by high levels of ferritin in serum, called “hyperferritinemic syndrome.” Ferritin is produced by macrophages, liver cells, and Kupfer cells,[38] and is now discovered to play a role as a pro-inflammatory cytokine.[39] It can activate an iron-independent signaling cascade, resulting in phosphatidylinositol 3-kinase - nuclear factor kappa-B (PI3K-NFκB) activation and dramatically enhanced expression of pro-inflammatory mediators like IL-1β, inducible nitric oxide synthase, regulated upon activation normal T cell expressed and secreted factor, inhibitor of NF-κB, and intra-cellular adhesion molecule 1 in hepatic stellate cells.[39] Furthermore, ferritin synthesis is also regulated by the bursting pro-inflammatory cytokine in AOSD, such as IL-1β.[40,41] Ferritin sub-units are divided into heavy (H) sub-units and light (L) sub-units, and ferritin enriched with L-sub-units (L-ferritin) is found in liver and spleen in the normal condition.[42,43] In some AOSD patients with macrophage activation syndrome (MAS), increased levels of H-ferritin and its imbalance with L-ferritin are found in bone marrow and liver, and H-ferritin levels are correlated with disease severity.[44] Similarly, enhanced tissue H-ferritin expression and a strong infiltrate of CD68+/H-ferritin+ cells are found in the lymph nodes and skin of AOSD patients. Moreover, a positive correlation between H-ferritin levels as well as CD68+/H-ferritin+ cells number and disease severity is reported.[45,46] Therefore, it is hypothesized that ferritin could be involved in amplifying inflammation of AOSD as an oxygen radical donor or by an unknown mechanism that is yet to be determined.[47] More studies need to be carried out.
Neutrophil Activation
Neutrophil activation is also a hallmark of AOSD. CD64 (FcγR1), a neutrophil activation marker, is upregulated in active AOSD.[48] CXC-chemokine ligand-8 (ex-IL-8), a primary chemokine known to be involved in neutrophils recruitment and activation, is also elevated in AOSD patients.[49] Recently, evidence of increased NET formation in AOSD has been first proved.[37,50] NETs are web-like structures released by neutrophils in both infectious and non-infectious inflammatory conditions. NETs are composed of chromatin filaments coated with histones, DNA, and granular proteases.[51,52] The spontaneous enhancement of NETs in AOSD is dependent on the high level of reactive oxygen species.[37] Moreover, the function of NETs in AOSD is to activate NLRP3 and macrophages in AOSD.[37] Thus, a novel link between neutrophils and macrophages is established by NET formation in AOSD.
NK Cells Deficiency
Lower percentages, decreased absolute numbers and defective cytotoxic function of NK cells have been reported in AOSD, which may contribute to persistent macrophage and lymphocyte activation.[53,54] In acute AOSD, NK cells also have a stronger ability to secret IFN-γ; moreover, their expression of IL-12 and IL-15 receptors are also upregulated, which promotes their IFN-γ production.[54] NK T cell deficiency is also present in active AOSD and is found to be correlated with NK cell dysfunction.[55]
T Cells Imbalance
Significant higher IFN-γ-producing Th1 cells and Th1/Th2 cells ratio have been found in peripheral blood in AOSD patients. Notably, a positive correlation between Th1 cell level as well as Th1/Th2 ratio and serum IL-18 levels is found.[56] Increased concentrations of α-soluble receptor of IL-2 (CD25) in AOSD may suggest the pathogenetic role of T cell activation in AOSD.[57] Th17 cells are also elevated in active untreated AOSD patients, and correlated with the disease activity score and pro-inflammatory cytokine levels.[58] Th17 cells may contribute to the pathogenesis of AOSD by producing the pro-inflammatory cytokine IL-17 to stimulate neutrophil recruitment. Decreased proportions of CD4+, CD4+CCR7+, CD4+CD62L−, and CD8+CD62L− cells and increased proportions of CD8+ naïve T cells are found in AOSD patients. Moreover, proportions of CD4+ effector memory T cells, CD8+ naïve T cells, and CD8+ effector memory T cells are significantly associated with the systemic score.[59]
Deficient resolution of inflammation
Deficiency in resolution of inflammation has been hypothesized to play a role in the pathogenesis of AOSD.[3,31] However, the hypothesis lacks sufficient supporting data. A study published in 2010 discovered a diminished level of circulating CD4+CD25high Treg cells and the transforming growth factor-β in AOSD patients, and the level was inversely correlated with disease activity of AOSD and on the rise after clinical remission.[60] No other data of deficiency in anti-inflammatory cytokines in patients with AOSD has ever been reported. Conversely and surprisingly, levels of immune-suppressive cytokines are mostly found to be increased in AOSD and may act as a potential biomarker. IL-10, a classical anti-inflammatory cytokine, shows elevated serum levels in AOSD, and the levels of IL-10 are associated with disease activity.[61] Similarly, a significantly higher level of IL-37 and its positive correlation with disease activity are detected in serum in AOSD patients.[62] IL-37 can attenuate the production of IL-1β, IL-18, IL-6, and TNF-α in peripheral blood mononuclear cells from patients with AOSD.[62] According to the above results, it is suggested that there exists a feedback loop from pro-inflammatory cytokines to the upregulation of anti-inflammatory cytokines.[62]
Clinical Features and Diagnosis
Four symptoms of AOSD are cardinal: fever, arthritis or arthralgia, skin rash, and leukocytes >10,000/mm3 with neutrophils >80%.[31,63] Other manifestations are sore throat, odynophagia, myalgia, myositis, lymphadenopathy, splenomegaly, pericarditis, myocarditis, pleuritis, lung disease, and hepatitis. Life-threatening complications may also be present, including pulmonary arterial hypertension, fulminant hepatitis, MAS, disseminated intravascular coagulopathy, myocarditis, acute respiratory syndrome, and thrombotic microangiopathy.[3,31,64] Main laboratory findings include elevated erythrocyte sedimentation rate and C-reactive protein levels.[10,31] Mild to moderate liver abnormalities are common.[10,31] Anti-nuclear antibodies and rheumatoid factor are mostly negative.[8,10,31]
The current recognized diagnosis of AOSD is based on clinical manifestations and still lacks specific diagnostic tests. Exclusion of mimickers is extremely necessary, mainly including infectious, malignant, autoimmune, and some other autoinflammatory diseases.[2,10,31] Several diagnostic criteria have been proposed, and two of them are validated in clinical practice and research: the Yamaguchi and Fautrel criteria.[65,66] Yamaguchi criteria is most widely used.[67,68] A combination of Yamaguchi criteria with glycosylated ferritin (GF) ≤20% can reach a diagnosis sensitivity of 98.2% and specificity of 98.6%.[68]
Biomarkers for AOSD
Serum ferritin
As discussed, serum ferritin is a biomarker of AOSD, while its specificity is poor. Four systemic diseases can be included in the “hyperferritinemic syndrome:” AOSD, MAS, catastrophic anti-phospholipid syndrome, and septic shock.[69] High serum ferritin levels are closely correlated with disease activity and associated with systemic pattern, recurrent flares, occurrence of MAS, and poor prognoses.[70–72] Aside from total ferritin levels, the GF level has also been investigated.[73,74] The normal GF level is >50% total ferritin level, but it decreases to 20% to 50% in inflammatory condition owing to the ferritin production.[73,75] In AOSD, GF level can even drop to under 20%.[75] A combination of ferritin >5 × upper limit of normal and GF ≤20% can reach a diagnostic specificity of 92.9%.[75]
Cytokine levels
Serum pro-inflammatory cytokine levels like IL-1β, IL-18, IL-6, TNF-α, and IFN-γ are elevated in AOSD and are associated with disease activity.[31,76,77] Among them, the role of IL-18 as a biomarker of AOSD is mostly studied. Evidences have suggested that IL-18 is a potential biomarker to diagnose AOSD and assess disease activity.[78–82] High levels of IL-18 are associated with RHL, hepatitis, steroid dependence, and systemic pattern.[79–82] Moreover, increased IL-1β is also associated with systemic pattern, whereas high levels of IL-6 are associated with arthritis pattern.[57,77] Serum anti-inflammatory cytokines, such as IL-10 and IL-37, are also elevated in AOSD and can be used as biomarkers to assess disease activity of AOSD.[61,62]
Other potential biomarkers
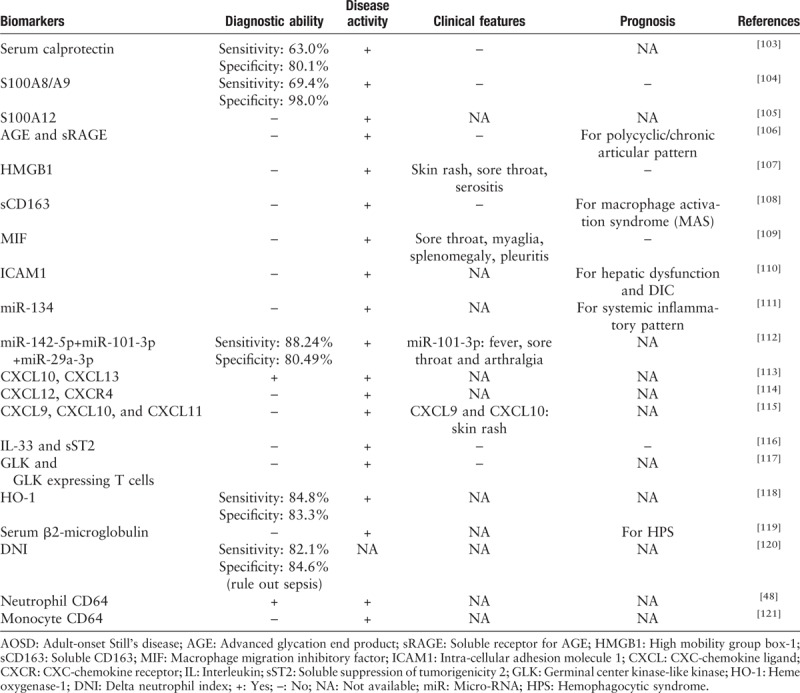
Other biomarkers, including DAMPs, microRNAs, and chemokines, have been proposed in different studies [Table 1]. These biomarkers may shed new insights into pathogenesis and treatment of AOSD.
Table 1.
Potential biomarkers of AOSD.
Disease Course and Prognosis
Several AOSD disease courses have been described.[83] A phenotypic trichotomy illustrates three different clinical courses of AOSD: (i) monocyclic/self-limited course, defined as a single episode (2 months to 1 year) followed by sustained remission throughout the whole follow-up period; (ii) polycyclic/intermittent course, with recurrent systemic flares between remissions; (iii) chronic course, at least one episode of persistent symptoms lasting longer than 1 year.[3,83] The chronic course is the most frequent one.[70,84,85] We notice that this definition of AOSD disease course is strongly based on the follow-up time, and it does not clearly illustrate the condition of treatment during remission. Yet another description takes the condition of drug withdrawal into account: the monocyclic course is either self-limited or includes drug-free remission, in which treatments after remission can be progressively tapered and finally stopped without relapse after a few months.[31] The recurrent or polycyclic course is characterized by AOSD relapses after a few months or years under immunomodulatory treatment or after its discontinuation.[31]
Recent data have suggested that these three patterns may be grouped into only two: a systemic form and a chronic articular form.[10,86] This phenotypic dichotomy may be more useful in applying different treatments for patients with AOSD.[87]
As introduced above, severe life-threatening complications or even death can occur in AOSD, and some patients have poor response to corticosteroid treatment. However, few studies have focused on the prognostic factors of these situations in AOSD. In 2009, a study analyzed clinical features and prognosis of 61 cases of AOSD in China. It defined the cyclic course as a favorable outcome of AOSD and the chronic course or death as an unfavorable outcome. It has shown that pleuritis, interstitial pneumonia, elevated ferritin levels, and failure of fever to subside after 3 days of prednisolone at 1 mg kg−1·d−1 are unfavorable prognostic factors for patients with AOSD.[72]
So far there is no exact report of the long-term survival rate for AOSD based on large samples. In a long-term follow-up study of Still's disease (eight AOSD patients followed up for 14 ± 5 years), two AOSD patients died.[88] In the retrospective study of 61 AOSD cases mentioned above, patients with disease duration over 2 years present a mortality of 12%.[72]
Treatment
Before the biologic era, treatment options were limited to nonsteroidal anti-inflammatory drug (NSAIDs), corticosteroids, and conventional disease-modifying antirheumatic drugs (cDMARDs). The efficacy and safety of NSAIDs are not satisfying, but it can be used as a temporary supportive treatment.[10,89,90] Corticosteroid therapy is used as the first-line treatment for AOSD, with an initial dosage at 0.5 to 1.0 mg/kg per day.[1,91] To a large extent, the duration of treatment is based on the patients’ response to the drugs and disease course. The response to corticosteroids is rapid within few days.[3,29] Usually, the tapering of corticosteroids starts after 4 to 6 weeks of therapy,[3] when the symptoms and the inflammatory laboratory parameters are normalized. Polycyclic and chronic AOSD need a long-term continuous treatment. Patients with severe visceral involvement or MAS should achieve intravenous, high-dosages corticosteroids.[1] Methotrexate (MTX) is still frequently used in AOSD for its steroid-sparing effect. If MTX fails to control the disease, other cDMARDs may be taken into consideration.[3] In a few retrospective studies, cyclosporine A has been proved to be as effective as MTX in the treatment of AOSD patients with both systemic and chronic articular involvement.[89,92] Azathioprine can be effective in controlling cutaneous eruptions or acute pericarditis in systemic AOSD.[89] Tacrolimus is also suggested to be useful in refractory AOSD in a report of six cases.[93] Other DMARDs like hydroxychloroquin, leflunomide, sulfasalazine (SSZ), cyclophosphamide, and intravenous immunoglobulin have also been applied with various response rates, but most of them seem to be ineffective and have more frequent adverse events.[89] Notably, the use of SSZ may trigger the onset of MAS in AOSD.[94]
In patients lacking clinical response with corticosteroids or cDMARDs (refractory AOSD), biologic agents should be applied.[95] Cytokine inhibitors targeting IL-1β, IL-6, TNF-α, and potentially IL-18 can suppress the inflammatory response in AOSD.[96,97] TNF-α blockers are the first biologic agents used for AOSD, but they turn out to be of limited efficacy and must be reserved for patients with chronic articular AOSD.[98] IL-1β inhibitors are now the primary choice for treating autoinflammatory diseases, and anakinra is the first one to show convincing and sustained efficacy in treating both systemic and articular AOSD.[99,100] It is reported that tocilizumab is the only IL-6 antagonists used in refractory AOSD patients, and the clinical response is strong, rapid and sustainable, with a corticosteroid-sparing effect.[101,102] Recently a retrospective study enrolling 27 AOSD patients has shown that biologic treatment may depend on the phenotypic dichotomy: a substantial response for tocilizumab in chronic articular type and anakinra in systemic type.[87]
Conclusions
In the past decades, progress has been made in the possible etiology of AOSD and identification of its diagnostic and prognostic biomarkers. Yet no reliable prediction of the treatment response and outcome of AOSD is available. Given the recent insights in AOSD pathogenesis and a better understanding of its disease course, new therapeutic targets, and clinical management may be approved to improve the outcome of AOSD in the future.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81671589, 81871272).
Conflicts of interest
None.
Footnotes
How to cite this article: Wang MY, Jia JC, Yang CD, Hu QY. Pathogenesis, disease course, and prognosis of adult-onset Still disease: an update and review. Chin Med J 2019;132:2856–2864. doi: 10.1097/CM9.0000000000000538
References
- 1.Govoni M, Bortoluzzi A, Rossi D, Modena V. How I treat patients with adult onset Still's disease in clinical practice. Autoimmun Rev 2017; 16:1016–1023. doi: 10.1016/j.autrev.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Mahroum N, Mahagna H, Amital H. Diagnosis and classification of adult Still's disease. J Autoimmun 2014; 48-49:34–37. doi: 10.1016/j.jaut.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Giacomelli R, Ruscitti P, Shoenfeld Y. A comprehensive review on adult onset Still's disease. J Autoimmun 2018; 93:24–36. doi: 10.1016/j.jaut.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Bywaters EG. Still's disease in the adult. Ann Rheum Dis 1971; 30:121–133. doi: 10.1136/ard.30.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magadur-Joly G, Billaud E, Barrier JH, Pennec YL, Masson C, Renou P, et al. Epidemiology of adult Still's disease: estimate of the incidence by a retrospective study in west France. Ann Rheum Dis 1995; 54:587–590. doi: 10.1136/ard.54.7.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wakai K, Ohta A, Tamakoshi A, Ohno Y, Kawamura T, Aoki R, et al. Estimated prevalence and incidence of adult Still's disease: findings by a nationwide epidemiological survey in Japan. J Epidemiol 1997; 7:221–225. doi: 10.2188/jea.7.221. [DOI] [PubMed] [Google Scholar]
- 7.Evensen KJ, Nossent HC. Epidemiology and outcome of adult-onset Still's disease in Northern Norway. Scand J Rheumatol 2006; 35:48–51. doi: 10.1080/03009740510026616. [DOI] [PubMed] [Google Scholar]
- 8.Cagatay Y, Gul A, Cagatay A, Kamali S, Karadeniz A, Inanc M, et al. Adult-onset Still's disease. Int J Clin Pract 2009; 63:1050–1055. doi: 10.1111/j.1742-1241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 9.Mehta BY, Ibrahim S, Briggs W, Efthimiou P. Racial/ethnic variations in morbidity and mortality in adult onset Still's disease: an analysis of national dataset. Semin Arthritis Rheum 2019; doi: 10.1016/j.semarthrit.2019.04.004 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10.Gerfaud-Valentin M, Jamilloux Y, Iwaz J, Seve P. Adult-onset Still's disease. Autoimmun Rev 2014; 13:708–722. doi: 10.1016/j.autrev.2014.01.058. [DOI] [PubMed] [Google Scholar]
- 11.Terkeltaub R, Esdaile JM, Decary F, Harth M, Lister J, Lapointe N. HLA-Bw35 and prognosis in adult Still's disease. Arthritis Rheum 1981; 24:1469–1472. doi: 10.1002/art.1780241203. [DOI] [PubMed] [Google Scholar]
- 12.Pouchot J, Sampalis JS, Beaudet F, Carette S, Decary F, Salusinsky-Sternbach M, et al. Adult Still's disease: manifestations, disease course, and outcome in 62 patients. Medicine (Baltimore) 1991; 70:118–136. doi: 10.1097/00005792-199103000-00004. [PubMed] [Google Scholar]
- 13.Wouters JM, Reekers P, van de Putte LB. Adult-onset Still's disease. Disease course and HLA associations. Arthritis Rheum 1986; 29:415–418. doi: 10.1002/art.1780290316. [DOI] [PubMed] [Google Scholar]
- 14.Fujii T, Nojima T, Yasuoka H, Satoh S, Nakamura K, Kuwana M, et al. Cytokine and immunogenetic profiles in Japanese patients with adult Still's disease. Association with chronic articular disease. Rheumatology (Oxford) 2001; 40:1398–1404. doi: 10.1093/rheumatology/40.12.1398. [DOI] [PubMed] [Google Scholar]
- 15.Joung CI, Lee HS, Lee SW, Kim CG, Song YH, Jun JB, et al. Association between HLA-DR B1 and clinical features of adult onset Still's disease in Korea. Clin Exp Rheumatol 2003; 21:489–492. [PubMed] [Google Scholar]
- 16.Chen DY, Chen YM, Chen HH, Hsieh CW, Lin CC, Lan JL. Functional association of interleukin 18 gene -607 (C/A) promoter polymorphisms with disease course in Chinese patients with adult-onset Still's disease. J Rheumatol 2009; 36:2284–2289. doi: 10.3899/jrheum.090316. [DOI] [PubMed] [Google Scholar]
- 17.Sugiura T, Kawaguchi Y, Harigai M, Terajima-Ichida H, Kitamura Y, Furuya T, et al. Association between adult-onset Still's disease and interleukin-18 gene polymorphisms. Genes Immun 2002; 3:394–399. doi: 10.1038/sj.gene.6363922. [DOI] [PubMed] [Google Scholar]
- 18.Yashiro M, Furukawa H, Asano T, Sato S, Kobayashi H, Watanabe H, et al. Serum amyloid A1 (SAA1) gene polymorphisms in Japanese patients with adult-onset Still's disease. Medicine (Baltimore) 2018; 97:e13394.doi: 10.1097/md.0000000000013394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang FF, Huang XF, Shen N, Leng L, Bucala R, Chen SL, et al. A genetic role for macrophage migration inhibitory factor (MIF) in adult-onset Still's disease. Arthritis Res Ther 2013; 15:R65.doi: 10.1186/ar4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JJ, Kim JK, Shim SC, Choe JY, Kim TH, Jun JB, et al. MEFV gene mutations and their clinical significance in Korean patients with adult-onset Still's disease. Clin Exp Rheumatol 2013; 31: 3 Suppl 77: 60–63. [PubMed] [Google Scholar]
- 21.Nonaka F, Migita K, Jiuchi Y, Shimizu T, Umeda M, Iwamoto N, et al. Increased prevalence of MEFV exon 10 variants in Japanese patients with adult-onset Still's disease. Clin Exp Immunol 2015; 179:392–397. doi: 10.1111/cei.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woo JH, Sung YK, Lee JS, Chung WT, Choe JY, Song GG, et al. Association of Fcgamma receptor polymorphisms with adult onset Still's disease in Korea. J Rheumatol 2009; 36:347–350. doi: 10.3899/jrheum.071254. [DOI] [PubMed] [Google Scholar]
- 23.Jamilloux Y, Gerfaud-Valentin M, Martinon F, Belot A, Henry T, Seve P. Pathogenesis of adult-onset Still's disease: new insights from the juvenile counterpart. Immunol Res 2015; 61 (1-2):53–62. doi: 10.1007/s12026-014-8561-9. [DOI] [PubMed] [Google Scholar]
- 24.Ohta A, Yamaguchi M, Tsunematsu T, Kasukawa R, Mizushima H, Kashiwagi H, et al. Adult Still's disease: a multicenter survey of Japanese patients. J Rheumatol 1990; 17:1058–1063. [PubMed] [Google Scholar]
- 25.Wouters JM, van der Veen J, van de Putte LB, de Rooij DJ. Adult onset Still's disease and viral infections. Ann Rheum Dis 1988; 47:764–767. doi: 10.1136/ard.47.9.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Escudero FJ, Len O, Falco V, de Sevilla TF, Sellas A. Rubella infection in adult onset Still's disease. Ann Rheum Dis 2000; 59:493.doi: 10.1136/ard.59.6.490c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van de Putte LB, Wouters JM. Adult-onset Still's disease. Bailliere's Clin Rheumatol 1991; 5:263–275. [DOI] [PubMed] [Google Scholar]
- 28.Perez C, Artola V. Adult Still's disease associated with Mycoplasma pneumoniae infection. Clin Infect Dis 2001; 32:E105–E106. doi: 10.1086/319342. [DOI] [PubMed] [Google Scholar]
- 29.Kadar J, Petrovicz E. Adult-onset Still's disease. Best Pract Res Clin Rheumatol 2004; 18:663–676. doi: 10.1016/j.berh.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Jia J, Shi H, Liu M, Liu T, Gu J, Wan L, et al. Cytomegalovirus infection may trigger adult-onset Still's disease onset or relapses. Front Immunol 2019; 10:898.doi: 10.3389/fimmu.2019.00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feist E, Mitrovic S, Fautrel B. Mechanisms, biomarkers and targets for adult-onset Still's disease. Nat Rev Rheumatol 2018; 14:603–618. doi: 10.1038/s41584-018-0081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsui K, Tsuchida T, Hiroishi K, Tominaga K, Hayashi N, Hada T, et al. High serum level of macrophage-colony stimulating factor (M-CSF) in adult-onset Still's disease. Rheumatology (Oxford) 1999; 38:477–478. doi: 10.1093/rheumatology/38.5.477. [DOI] [PubMed] [Google Scholar]
- 33.Hoshino T, Ohta A, Yang D, Kawamoto M, Kikuchi M, Inoue Y, et al. Elevated serum interleukin 6, interferon-gamma, and tumor necrosis factor-alpha levels in patients with adult Still's disease. J Rheumatol 1998; 25:396–398. [PubMed] [Google Scholar]
- 34.Jung JY, Suh CH, Kim HA. The role of damage-associated molecular pattern for pathogenesis and biomarkers in adult-onset Still's disease. Expert Rev Mol Diagn 2019; 19:459–468. doi: 10.1080/14737159.2019.1615449. [DOI] [PubMed] [Google Scholar]
- 35.Hsieh CW, Chen YM, Lin CC, Tang KT, Chen HH, Hung WT, et al. Elevated expression of the NLRP3 inflammasome and its correlation with disease activity in adult-onset Still disease. J Rheumatol 2017; 44:1142–1150. doi: 10.3899/jrheum.161354. [DOI] [PubMed] [Google Scholar]
- 36.Church LD, Cook GP, McDermott MF. Primer: inflammasomes and interleukin 1beta in inflammatory disorders. Nat Clin Pract Rheumatol 2008; 4:34–42. doi: 10.1038/ncprheum0681. [DOI] [PubMed] [Google Scholar]
- 37.Hu Q, Shi H, Zeng T, Liu H, Su Y, Cheng X, et al. Increased neutrophil extracellular traps activate NLRP3 and inflammatory macrophages in adult-onset Still's disease. Arthritis Res Ther 2019; 21:9.doi: 10.1186/s13075-018-1800-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zandman-Goddard G, Shoenfeld Y. Ferritin in autoimmune diseases. Autoimmun Rev 2007; 6:457–463. doi: 10.1016/j.autrev.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 39.Ruddell RG, Hoang-Le D, Barwood JM, Rutherford PS, Piva TJ, Watters DJ, et al. Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase C zeta/nuclear factor kappaB-regulated signaling in rat hepatic stellate cells. Hepatology 2009; 49:887–900. doi: 10.1002/hep.22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei Y, Miller SC, Tsuji Y, Torti SV, Torti FM. Interleukin 1 induces ferritin heavy chain in human muscle cells. Biochem Biophys Res Commun 1990; 169:289–296. doi: 10.1016/0006-291x(90)91466-6. [DOI] [PubMed] [Google Scholar]
- 41.Rogers JT, Bridges KR, Durmowicz GP, Glass J, Auron PE, Munro HN. Translational control during the acute phase response. Ferritin synthesis in response to interleukin-1. J Biol Chem 1990; 265:14572–14578. [PubMed] [Google Scholar]
- 42.Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood 2002; 99:3505–3516. doi: 10.1182/blood.v99.10.3505. [DOI] [PubMed] [Google Scholar]
- 43.Knovich MA, Storey JA, Coffman LG, Torti SV, Torti FM. Ferritin for the clinician. Blood Rev 2009; 23:95–104. doi: 10.1016/j.blre.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruscitti P, Cipriani P, Di Benedetto P, Ciccia F, Liakouli V, Carubbi F, et al. Increased level of H-ferritin and its imbalance with L-ferritin, in bone marrow and liver of patients with adult onset Still's disease, developing macrophage activation syndrome, correlate with the severity of the disease. Autoimmun Rev 2015; 14:429–437. doi: 10.1016/j.autrev.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Ruscitti P, Ciccia F, Cipriani P, Guggino G, Di Benedetto P, Rizzo A, et al. The CD68(+)/H-ferritin(+) cells colonize the lymph nodes of the patients with adult onset Still's disease and are associated with increased extracellular level of H-ferritin in the same tissue: correlation with disease severity and implication for pathogenesis. Clin Exp Immunol 2016; 183:397–404. doi: 10.1111/cei.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruscitti P, Cipriani P, Ciccia F, Di Benedetto P, Liakouli V, Berardicurti O, et al. H-ferritin and CD68(+) /H-ferritin(+) monocytes/macrophages are increased in the skin of adult-onset Still's disease patients and correlate with the multi-visceral involvement of the disease. Clin Exp Immunol 2016; 186:30–38. doi: 10.1111/cei.12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehta B, Efthimiou P. Ferritin in adult-onset still's disease: just a useful innocent bystander? Int J Inflam 2012; 2012:298405.doi: 10.1155/2012/298405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Komiya A, Matsui T, Nogi S, Iwata K, Futami H, Takaoka H, et al. Neutrophil CD64 is upregulated in patients with active adult-onset Still's disease. Scand J Rheumatol 2012; 41:156–158. doi: 10.3109/03009742.2011.644325. [DOI] [PubMed] [Google Scholar]
- 49.Kasama T, Furuya H, Yanai R, Ohtsuka K, Takahashi R, Yajima N, et al. Correlation of serum CX3CL1 level with disease activity in adult-onset Still's disease and significant involvement in hemophagocytic syndrome. Clin Rheumatol 2012; 31:853–860. doi: 10.1007/s10067-012-1952-1. [DOI] [PubMed] [Google Scholar]
- 50.Ahn MH, Han JH, Chwae YJ, Jung JY, Suh CH, Kwon JE, et al. Neutrophil extracellular traps may contribute to the pathogenesis in adult-onset Still's disease. J Rheumatol 2019; doi: 10.3899/jrheum.181058 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 51.Cooper PR, Palmer LJ, Chapple IL. Neutrophil extracellular traps as a new paradigm in innate immunity: friend or foe? Periodontology 2000 2013; 63:165–197. doi: 10.1111/prd.12025. [DOI] [PubMed] [Google Scholar]
- 52.Delgado-Rizo V, Martinez-Guzman MA, Iniguez-Gutierrez L, Garcia-Orozco A, Alvarado-Navarro A, Fafutis-Morris M. Neutrophil extracellular traps and its implications in inflammation: an overview. Front Immunol 2017; 8:81.doi: 10.3389/fimmu.2017.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park JH, Kim HS, Lee JS, Kim JJ, Jung KH, Park YW, et al. Natural killer cell cytolytic function in Korean patients with adult-onset Still's disease. J Rheumatol 2012; 39:2000–2007. doi: 10.3899/jrheum.111500. [DOI] [PubMed] [Google Scholar]
- 54.Shimojima Y, Kishida D, Ueno KI, Ushiyama S, Ichikawa T, Sekijima Y. Characteristics of circulating natural killer cells and their interferon-gamma production in active adult-onset Still disease. J Rheumatol 2019; doi: 10.3899/jrheum.181192 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 55.Lee SJ, Cho YN, Kim TJ, Park SC, Park DJ, Jin HM, et al. Natural killer T cell deficiency in active adult-onset Still's disease: correlation of deficiency of natural killer T cells with dysfunction of natural killer cells. Arthritis Rheum 2012; 64:2868–2877. doi: 10.1002/art.34514. [DOI] [PubMed] [Google Scholar]
- 56.Chen DY, Lan JL, Lin FJ, Hsieh TY, Wen MC. Predominance of Th1 cytokine in peripheral blood and pathological tissues of patients with active untreated adult onset Still's disease. Ann Rheum Dis 2004; 63:1300–1306. doi: 10.1136/ard.2003.013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi JH, Suh CH, Lee YM, Suh YJ, Lee SK, Kim SS, et al. Serum cytokine profiles in patients with adult onset Still's disease. J Rheumatol 2003; 30:2422–2427. [PubMed] [Google Scholar]
- 58.Chen DY, Chen YM, Lan JL, Lin CC, Chen HH, Hsieh CW. Potential role of Th17 cells in the pathogenesis of adult-onset Still's disease. Rheumatology (Oxford) 2010; 49:2305–2312. doi: 10.1093/rheumatology/keq284. [DOI] [PubMed] [Google Scholar]
- 59.Jung JY, Choi B, Sayeed HM, Suh CH, Kim YW, Kim HA, et al. Characteristic patterns of HLA presentation and T cell differentiation in adult-onset Still's disease. Int J Immunopathol Pharmacol 2018; 32:2058738418791284.doi: 10.1177/2058738418791284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen DY, Chen YM, Chen HH, Hsieh CW, Lin CC, Lan JL. The associations of circulating CD4+CD25high regulatory T cells and TGF-beta with disease activity and clinical course in patients with adult-onset Still's disease. Connect Tissue Res 2010; 51:370–377. doi: 10.3109/03008200903461462. [DOI] [PubMed] [Google Scholar]
- 61.Sun Y, Wang Z, Chi H, Hu Q, Ye J, Liu H, et al. Elevated serum levels of interleukin-10 in adult-onset Still's disease are associated with disease activity. Clin Rheumatol 2019; doi: 10.1007/s10067-019-04642-x [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 62.Chi H, Liu D, Sun Y, Hu Q, Liu H, Cheng X, et al. Interleukin-37 is increased in adult-onset Still's disease and associated with disease activity. Arthritis Res Ther 2018; 20:54.doi: 10.1186/s13075-018-1555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fautrel B. Adult-onset Still disease. Best Pract Res Clin Rheumatol 2008; 22:773–792. doi: 10.1016/j.berh.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Owlia MB, Mehrpoor G. Adult-onset Still's disease: a review. Indian J Med Sci 2009; 63:207–221. doi: 10.4103/0019-5359.53169. [PubMed] [Google Scholar]
- 65.Yamaguchi M, Ohta A, Tsunematsu T, Kasukawa R, Mizushima Y, Kashiwagi H, et al. Preliminary criteria for classification of adult Still's disease. J Rheumatol 1992; 19:424–430. [PubMed] [Google Scholar]
- 66.Fautrel B, Zing E, Golmard JL, Le Moel G, Bissery A, Rioux C, et al. Proposal for a new set of classification criteria for adult-onset still disease. Medicine (Baltimore) 2002; 81:194–200. doi: 10.1097/00005792-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 67.Jiang L, Wang Z, Dai X, Jin X. Evaluation of clinical measures and different criteria for diagnosis of adult-onset Still's disease in a Chinese population. J Rheumatol 2011; 38:741–746. doi: 10.3899/jrheum.100766. [DOI] [PubMed] [Google Scholar]
- 68.Lebrun D, Mestrallet S, Dehoux M, Golmard JL, Granger B, Georgin-Lavialle S, et al. Validation of the Fautrel classification criteria for adult-onset Still's disease. Semin Arthritis Rheum 2018; 47:578–585. doi: 10.1016/j.semarthrit.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 69.Rosario C, Zandman-Goddard G, Meyron-Holtz EG, D’Cruz DP, Shoenfeld Y. The hyperferritinemic syndrome: macrophage activation syndrome, Still's disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med 2013; 11:185.doi: 10.1186/1741-7015-11-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sampalis JS, Esdaile JM, Medsger TA, Jr, Partridge AJ, Yeadon C, Senecal JL, et al. A controlled study of the long-term prognosis of adult Still's disease. Am J Med 1995; 98:384–388. doi: 10.1016/s0002-9343(99)80318-0. [DOI] [PubMed] [Google Scholar]
- 71.Kong XD, Xu D, Zhang W, Zhao Y, Zeng X, Zhang F. Clinical features and prognosis in adult-onset Still's disease: a study of 104 cases. Clin Rheumatol 2010; 29:1015–1019. doi: 10.1007/s10067-010-1516-1. [DOI] [PubMed] [Google Scholar]
- 72.Zeng T, Zou YQ, Wu MF, Yang CD. Clinical features and prognosis of adult-onset still's disease: 61 cases from China. J Rheumatol 2009; 36:1026–1031. doi: 10.3899/jrheum.080365. [DOI] [PubMed] [Google Scholar]
- 73.Van Reeth C, Le Moel G, Lasne Y, Revenant MC, Agneray J, Kahn MF, et al. Serum ferritin and isoferritins are tools for diagnosis of active adult Still's disease. J Rheumatol 1994; 21:890–895. [PubMed] [Google Scholar]
- 74.Vignes S, Le Moel G, Fautrel B, Wechsler B, Godeau P, Piette JC. Percentage of glycosylated serum ferritin remains low throughout the course of adult onset Still's disease. Ann Rheum Dis 2000; 59:347–350. doi: 10.1136/ard.59.5.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fautrel B, Le Moel G, Saint-Marcoux B, Taupin P, Vignes S, Rozenberg S, et al. Diagnostic value of ferritin and glycosylated ferritin in adult onset Still's disease. J Rheumatol 2001; 28:322–329. [PubMed] [Google Scholar]
- 76.Inoue N, Shimizu M, Tsunoda S, Kawano M, Matsumura M, Yachie A. Cytokine profile in adult-onset Still's disease: comparison with systemic juvenile idiopathic arthritis. Clin Immunol 2016; 169:8–13. doi: 10.1016/j.clim.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 77.Chen DY, Lan JL, Lin FJ, Hsieh TY. Proinflammatory cytokine profiles in sera and pathological tissues of patients with active untreated adult onset Still's disease. J Rheumatol 2004; 31:2189–2198. [PubMed] [Google Scholar]
- 78.Colafrancesco S, Priori R, Alessandri C, Perricone C, Pendolino M, Picarelli G, et al. IL-18 serum level in adult onset Still's disease: a marker of disease activity. Int J Inflam 2012; 2012:156890.doi: 10.1155/2012/156890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Priori R, Colafrancesco S, Alessandri C, Minniti A, Perricone C, Iaiani G, et al. Interleukin 18: a biomarker for differential diagnosis between adult-onset Still's disease and sepsis. J Rheumatol 2014; 41:1118–1123. doi: 10.3899/jrheum.130575. [DOI] [PubMed] [Google Scholar]
- 80.Jung KH, Kim JJ, Lee JS, Park W, Kim TH, Jun JB, et al. Interleukin-18 as an efficient marker for remission and follow-up in patients with inactive adult-onset Still's disease. Scand J Rheumatol 2014; 43:162–169. doi: 10.3109/03009742.2013.824023. [DOI] [PubMed] [Google Scholar]
- 81.Girard C, Rech J, Brown M, Allali D, Roux-Lombard P, Spertini F, et al. Elevated serum levels of free interleukin-18 in adult-onset Still's disease. Rheumatology (Oxford) 2016; 55:2237–2247. doi: 10.1093/rheumatology/kew300. [DOI] [PubMed] [Google Scholar]
- 82.Kudela H, Drynda S, Lux A, Horneff G, Kekow J. Comparative study of interleukin-18 (IL-18) serum levels in adult onset Still's disease (AOSD) and systemic onset juvenile idiopathic arthritis (sJIA) and its use as a biomarker for diagnosis and evaluation of disease activity. BMC Rheumatol 2019; 3:4.doi: 10.1186/s41927-019-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cush JJ, Medsger TA, Jr, Christy WC, Herbert DC, Cooperstein LA. Adult-onset Still's disease. Clinical course and outcome. Arthritis Rheum 1987; 30:186–194. doi: 10.1002/art.1780300209. [DOI] [PubMed] [Google Scholar]
- 84.Pay S, Turkcapar N, Kalyoncu M, Simsek I, Beyan E, Ertenli I, et al. A multicenter study of patients with adult-onset Still's disease compared with systemic juvenile idiopathic arthritis. Clin Rheumatol 2006; 25:639–644. doi: 10.1007/s10067-005-0138-5. [DOI] [PubMed] [Google Scholar]
- 85.Colina M, Zucchini W, Ciancio G, Orzincolo C, Trotta F, Govoni M. The evolution of adult-onset Still disease: an observational and comparative study in a cohort of 76 Italian patients. Semin Arthritis Rheum 2011; 41:279–285. doi: 10.1016/j.semarthrit.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 86.Maria AT, Le Quellec A, Jorgensen C, Touitou I, Riviere S, Guilpain P. Adult onset Still's disease (AOSD) in the era of biologic therapies: dichotomous view for cytokine and clinical expressions. Autoimmun Rev 2014; 13:1149–1159. doi: 10.1016/j.autrev.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 87.Vercruysse F, Barnetche T, Lazaro E, Shipley E, Lifermann F, Balageas A, et al. Adult-onset Still's disease biological treatment strategy may depend on the phenotypic dichotomy. Arthritis Res Ther 2019; 21:53.doi: 10.1186/s13075-019-1838-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cabane J, Michon A, Ziza JM, Bourgeois P, Bletry O, Godeau P, et al. Comparison of long term evolution of adult onset and juvenile onset Still's disease, both followed up for more than 10 years. Ann Rheum Dis 1990; 49:283–285. doi: 10.1136/ard.49.5.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Franchini S, Dagna L, Salvo F, Aiello P, Baldissera E, Sabbadini MG. Efficacy of traditional and biologic agents in different clinical phenotypes of adult-onset Still's disease. Arthritis Rheum 2010; 62:2530–2535. doi: 10.1002/art.27532. [DOI] [PubMed] [Google Scholar]
- 90.Wouters JM, van de Putte LB. Adult-onset Still's disease; clinical and laboratory features, treatment and progress of 45 cases. Q J Med 1986; 61:1055–1065. [PubMed] [Google Scholar]
- 91.Siddiqui M, Putman MS, Dua AB. Adult-onset Still's disease: current challenges and future prospects. Open Access Rheumatol 2016; 8:17–22. doi: 10.2147/oarrr.S83948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mitamura M, Tada Y, Koarada S, Inoue H, Suematsu R, Ohta A, et al. Cyclosporin A treatment for Japanese patients with severe adult-onset Still's disease. Mod Rheumatol 2009; 19:57–63. doi: 10.1007/s10165-008-0126-0. [DOI] [PubMed] [Google Scholar]
- 93.Nakamura H, Odani T, Shimizu Y, Takeda T, Kikuchi H. Usefulness of tacrolimus for refractory adult-onset still's disease: report of six cases. Mod Rheumatol 2016; 26:963–967. doi: 10.3109/14397595.2014.933997. [DOI] [PubMed] [Google Scholar]
- 94.Jung JH, Jun JB, Yoo DH, Kim TH, Jung SS, Lee IH, et al. High toxicity of sulfasalazine in adult-onset Still's disease. Clin Exp Rheumatol 2000; 18:245–248. [PubMed] [Google Scholar]
- 95.Cipriani P, Ruscitti P, Carubbi F, Liakouli V, Giacomelli R. Methotrexate: an old new drug in autoimmune disease. Exp Rev Clin Immunol 2014; 10:1519–1530. doi: 10.1586/1744666x.2014.962996. [DOI] [PubMed] [Google Scholar]
- 96.Castaneda S, Blanco R, Gonzalez-Gay MA. Adult-onset Still's disease: advances in the treatment. Best Pract Res Clin Rheumatol 2016; 30:222–238. doi: 10.1016/j.berh.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 97.Ruscitti P, Ursini F, Cipriani P, De Sarro G, Giacomelli R. Biologic drugs in adult onset Still's disease: a systematic review and meta-analysis of observational studies. Exp Rev Clin Immunol 2017; 13:1089–1097. doi: 10.1080/1744666x.2017.1375853. [DOI] [PubMed] [Google Scholar]
- 98.Yoo DH. Treatment of adult-onset still's disease: up to date. Exp Rev Clin Immunol 2017; 13:849–866. doi: 10.1080/1744666x.2017.1332994. [DOI] [PubMed] [Google Scholar]
- 99.Castaneda S, Atienza-Mateo B, Martin-Varillas JL, Serra Lopez-Matencio JM, Gonzalez-Gay MA. Anakinra for the treatment of adult-onset Still's disease. Exp Rev Clin Immunol 2018; 14:979–992. doi: 10.1080/1744666x.2018.1536548. [DOI] [PubMed] [Google Scholar]
- 100.Hong D, Yang Z, Han S, Liang X, Ma K, Zhang X. Interleukin 1 inhibition with anakinra in adult-onset Still disease: a meta-analysis of its efficacy and safety. Drug Des Devel Ther 2014; 8:2345–2357. doi: 10.2147/dddt.S73428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma Y, Wu M, Zhang X, Xia Q, Yang J, Xu S, et al. Efficacy and safety of tocilizumab with inhibition of interleukin-6 in adult-onset Still's disease: a meta-analysis. Mod Rheumatol 2018; 28:849–857. doi: 10.1080/14397595.2017.1416924. [DOI] [PubMed] [Google Scholar]
- 102.Li T, Gu L, Wang X, Guo L, Shi H, Yang C, et al. A pilot study on tocilizumab for treating refractory adult-onset Still's disease. Sci Rep 2017; 7:13477.doi: 10.1038/s41598-017-13639-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guo Q, Zha X, Li C, Jia Y, Zhu L, Guo J, et al. Serum calprotectin--a promising diagnostic marker for adult-onset Still's disease. Clin Rheumatol 2016; 35:73–79. doi: 10.1007/s10067-015-3108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim HA, An JM, Nam JY, Jeon JY, Suh CH. Serum S100A8/A9, but not follistatin-like protein 1 and interleukin 18, may be a useful biomarker of disease activity in adult-onset Still's disease. J Rheumatol 2012; 39:1399–1406. doi: 10.3899/jrheum.120079. [DOI] [PubMed] [Google Scholar]
- 105.Bae CB, Suh CH, An JM, Jung JY, Jeon JY, Nam JY, et al. Serum S100A12 may be a useful biomarker of disease activity in adult-onset Still's disease. J Rheumatol 2014; 41:2403–2408. doi: 10.3899/jrheum.140651. [DOI] [PubMed] [Google Scholar]
- 106.Chen DY, Chen YM, Lin CC, Hsieh CW, Wu YC, Hung WT, et al. The potential role of advanced glycation end products (AGEs) and soluble receptors for AGEs (sRAGE) in the pathogenesis of adult-onset still's disease. BMC Musculoskelet Disord 2015; 16:111.doi: 10.1186/s12891-015-0569-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jung JY, Suh CH, Sohn S, Nam JY, Kim HA. Elevated high-mobility group B1 levels in active adult-onset Still's disease associated with systemic score and skin rash. Clin Rheumatol 2016; 35:1937–1942. doi: 10.1007/s10067-016-3314-x. [DOI] [PubMed] [Google Scholar]
- 108.Colafrancesco S, Priori R, Alessandri C, Astorri E, Perricone C, Blank M, et al. sCD163 in AOSD: a biomarker for macrophage activation related to hyperferritinemia. Immunol Res 2014; 60:177–183. doi: 10.1007/s12026-014-8563-7. [DOI] [PubMed] [Google Scholar]
- 109.Zou YQ, Lu LJ, Li SJ, Zeng T, Wang XD, Bao CD, et al. The levels of macrophage migration inhibitory factor as an indicator of disease activity and severity in adult-onset Still's disease. Clin Biochem 2008; 41:519–524. doi: 10.1016/j.clinbiochem.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 110.Chen DY, Lan JL, Lin FJ, Hsieh TY. Association of intercellular adhesion molecule-1 with clinical manifestations and interleukin-18 in patients with active, untreated adult-onset Still's disease. Arthritis Rheum 2005; 53:320–327. doi: 10.1002/art.21164. [DOI] [PubMed] [Google Scholar]
- 111.Liao TL, Chen YM, Hsieh CW, Chen HH, Lee HC, Hung WT, et al. Upregulation of circulating microRNA-134 in adult-onset Still's disease and its use as potential biomarker. Sci Rep 2017; 7:4214.doi: 10.1038/s41598-017-04086-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hu Q, Gong W, Gu J, Geng G, Li T, Tian R, et al. Plasma microRNA profiles as a potential biomarker in differentiating adult-onset Still's disease from sepsis. Front Immunol 2018; 9:3099.doi: 10.3389/fimmu.2018.03099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Han JH, Suh CH, Jung JY, Nam JY, Kwon JE, Yim H, et al. Association of CXCL10 and CXCL13 levels with disease activity and cutaneous manifestation in active adult-onset Still's disease. Arthritis Res Ther 2015; 17:260.doi: 10.1186/s13075-015-0773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Han JH, Ahn MH, Jung JY, Suh CH, Kwon JE, Yim H, et al. The levels of CXCL12 and its receptor, CXCR4, as a biomarker of disease activity and cutaneous manifestation in adult-onset Still's disease. Clin Exp Rheumatol 2019; Epub ahead of print. [PubMed] [Google Scholar]
- 115.Bracaglia C, de Graaf K, Pires Marafon D, Guilhot F, Ferlin W, Prencipe G, et al. Elevated circulating levels of interferon-gamma and interferon-gamma-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann Rheum Dis 2017; 76:166–172. doi: 10.1136/annrheumdis-2015-209020. [DOI] [PubMed] [Google Scholar]
- 116.Han JH, Suh CH, Jung JY, Ahn MH, Kwon JE, Yim H, et al. Serum levels of interleukin 33 and soluble ST2 are associated with the extent of disease activity and cutaneous manifestations in patients with active adult-onset Still's disease. J Rheumatol 2017; 44:740–747. doi: 10.3899/jrheum.170020. [DOI] [PubMed] [Google Scholar]
- 117.Chen DY, Chuang HC, Lan JL, Chen YM, Hung WT, Lai KL, et al. Germinal center kinase-like kinase (GLK/MAP4K3) expression is increased in adult-onset Still's disease and may act as an activity marker. BMC Med 2012; 10:84.doi: 10.1186/1741-7015-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kirino Y, Kawaguchi Y, Tada Y, Tsukamoto H, Ota T, Iwamoto M, et al. Beneficial use of serum ferritin and heme oxygenase-1 as biomarkers in adult-onset Still's disease: a multicenter retrospective study. Mod Rheumatol 2018; 28:858–864. doi: 10.1080/14397595.2017.1422231. [DOI] [PubMed] [Google Scholar]
- 119.Wakabayashi K, Inokuma S, Matsubara E, Onishi K, Asashima H, Nakachi S, et al. Serum beta2-microglobulin level is a useful indicator of disease activity and hemophagocytic syndrome complication in systemic lupus erythematosus and adult-onset Still's disease. Clin Rheumatol 2013; 32:999–1005. doi: 10.1007/s10067-013-2220-8. [DOI] [PubMed] [Google Scholar]
- 120.Park HJ, Ha YJ, Pyo JY, Park YB, Lee SK, Lee SW. Delta neutrophil index as an early marker for differential diagnosis of adult-onset Still's disease and sepsis. Yonsei Med J 2014; 55:753–759. doi: 10.3349/ymj.2014.55.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shimizu T, Kikuchi-Taura A, Tsuji S, Matsushita M, Ohshima S, Saeki Y. Up-regulation of CD64 expression on monocytes in patients with active adult-onset Still disease: a possible biomarker of disease activity. J Clin Rheumatol 2018; Epub ahead of print. doi: 10.1097/rhu.0000000000000931. [DOI] [PubMed] [Google Scholar]


