Abstract
Objective:
In recent years, an increasing number of drugs have been proved to be associated with the induction of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV). This article reviews the latest research progress on drug-induced AAV.
Data sources:
We conducted a comprehensive and detailed search of the PubMed database. The search terms mainly included drug-induced, ANCA, and vasculitis.
Study selection:
We summarized the original articles and reviews on drug-induced AAV in recent years. The extracted information included the definition, epidemiology, associated drugs, pathogenesis, clinical features, diagnosis, treatment, and prognosis of drug-induced AAV. We also focused on the differences between drug-induced AAV and primary vasculitis.
Results:
The offending drugs leading to drug-induced AAV are almost from pharmacologic categories and we need to be vigilant when using these drugs. The pathogenesis of drug-induced AAV might be multifactorial. The formation of neutrophil extracellular traps is an important mechanism for the development of drug-induced AAV. The clinical features of drug-induced AAV are similar to those of primary AAV. Understanding the difference between drug-induced AAV and primary AAV is helpful to identify drug-induced AAV. Stopping the offending drug at once after diagnosis may be sufficient for those patients with mild symptoms. Immunosuppressive therapy should only be used in patients with vital organs involvement.
Conclusions:
Patients with drug-induced AAV usually have a good prognosis if they stop using the offending drug immediately. Recent advances in research on AAV are expected to help us better understand the pathogenesis of drug-induced AAV.
Keywords: Anti-neutrophil cytoplasmic antibody, Drug-induced, Vasculitis
Introduction
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a group of autoimmune diseases characterized by the presence of ANCAs and necrotizing inflammation of small and medium vessels, including microscopic polyangiitis (MPA), granulomatosis with polyangiitis and eosinophilic granulomatosis with polyangiitis.[1,2] Diffuse cytoplasmic staining-ANCA (C-ANCA) and perinuclear staining-ANCA (P-ANCA) are two main fluorescence patterns that can be identified by indirect immunofluorescence reactions. The C-ANCA pattern is almost exclusively associated with antibodies against proteinase 3 (PR3). By contrast, the P-ANCA pattern can be caused by many proteins, mainly including myeloperoxidase (MPO), cathepsin G, elastase, β-glucuronidase, and others.[3] Although there is now increasing evidence to support the pathogenic effects of these antibodies in AAV, the mechanism for ANCA production is not fully understood.[4,5]
In recent years, more and more cases of drug-induced vasculitis (DIV) have attracted people's attention. In previous cases, it has been recognized that almost all pharmacological classes of drugs are potentially associated with the development of DIV.[6] As a large proportion of patients with DIV are characterized by ANCA positive, the detection of ANCAs can serve as a warning of the possibility of DIV. So, in some cases, DIV mainly refers to drug-induced AAV.[7,8] Although both drug-induced lupus disease and drug-induced AAV have been classified as autoimmune syndromes,[8–12] it is often difficult to distinguish them because of their similar clinical and experimental features, even some researchers have the opinion that trying to separate them is partly artificial.[8]
This review aims to summarize the definition, epidemiology, associated drugs, pathogenesis, clinical features, diagnosis, treatment, and prognosis of drug-induced AAV. The differences between drug-induced AAV and primary AAV are also the focus of this review.
Definition
Previously, DIV was poorly understood and empirically defined. Ambiguous and undefined terms such as leukocytoclastic vasculitis, allergic vasculitis, hypersensitivity vasculitis, serum sickness, and so on were often used to describe such diseases.[13] Some researchers regarded DIV as a group of vascular inflammatory diseases, in which a specific drug is identified as a suspected cause of the disease while other types of vasculitis are excluded.[6] Similarly, there is no clear definition of drug-induced AAV. Traditionally, drug-induced AAV has only been understood as a class of DIV characterized by ANCA positive. Based on our understanding of the disease, the 2012 International Chapel Hill Consensus Conference classified drug-induced AAV as vasculitis associated with probable etiology.[14] This increased our awareness of this subset of vasculitis.
Epidemiology
Due to the lack of relevant studies, there is no clear epidemiological data to help us calculate the incidence of drug-induced AAV. According to the available prospective and cross-sectional studies, Balavoine et al[15] summarized that the prevalence of propylthiouracil (PTU)-induced AAV ranged from 4% to 64%, with a median prevalence of 30%, while the prevalence of methimazole (MMI)-induced AAV ranged from 0% to 16%, with a median prevalence of 6%.
Associated Drugs
So far, the associated drugs leading to drug-induced AAV are almost from all pharmacologic categories, mainly including anti-thyroid drugs[16–19] and the tumor necrosis factor (TNF) inhibitor.[20–23] Moreover, the following drugs are also showed a possible association with the occurrence of drug-induced AAV: cephotaxime,[24] minocycline,[25] nitrofurantoin,[26] trimethoprim-sulfamethoxazole,[27] vancomycin,[28] isoniazid,[29] rifampicin,[30] D-penicillamine,[31] sulfasalazine,[32] clozapine,[33] thioridazine,[34] allopurinol,[35] atorvastatin,[36] cocaine/levamisol,[37,38] denosumab,[39] hydralazine,[40] isotretinoin,[41] and phenytoin.[42] But most articles are confined to case reports [Table 1].
Table 1.
Medications associated with drug-induced AAV.

Since 1946, anti-thyroid drugs (ATD) have been gradually used,[43,44] especially in Graves’ patients.[45] ATD are simple molecules in the group of thioamides. According to the different molecular structures of ATD, they can be divided into two major categories: derivatives of thiouracil (PTU and benzylthiouracil [BTU]) and methyl-mercapto-imidazole (MMI and carbimazole [CMZ]).[45,46] However, many side effects began to be seen soon after people started taking ATD. Besides granulocytosis and acute liver injury,[47] several cases of ATD-induced AAV have also been described subsequently and most of them were PTU-induced AAV.[48,49] So far, there were more than 200 cases of ATD-induced AAV have been reported. Approximately 90% of the ATD-induced AAV were related to PTU, while cases induced by MMI, CMZ, and BTU were relatively rare.[17–19] Studies have shown that ANCA production is related to the duration of ATD,[50,51] those patients taking PTU for more than 18 months should pay more attention to serum ANCA and it is not recommended to take PTU for more than 3 years. However, when PTU-induced AAV occurs, conversion from PTU to MMI is still not recommended, as it has been reported that this will lead to the recurrence of drug-induced AAV.[52]
In the past few years, biologics agents are increasingly used in rheumatic diseases. Puzzlingly, more and more biologics-induced autoimmune diseases have been gradually reported, including a wide range of organ-specific autoimmune diseases and systemic diseases.[53] Anti-TNF-α drugs are a class of biologics agents that widely used in rheumatoid arthritis, ankylosing spondylitis, and other autoimmune diseases. Studies have shown that repeated use of these drugs causes about 10% of patients to develop autoantibodies, such as anti-nuclear antibodies (ANA), anti-cardiolipin antibodies and anti-dsDNA.[54] Although uncommon, some patients were also found to develop AAV after receiving anti-TNF-α drugs.[20–23]
Cocaine is one of the most common and widely used drugs. It stimulates people by increasing dopamine levels and inhibiting its reuptake. Levamisole is an immunomodulator and anthelmintic drug widely used as an adulterant of cocaine.[37,38] Levamisole adulterates cocaine mainly because they have similar properties and levamisole enhances the effects of cocaine.[55,56] According to previous data, at least 66% of cocaine samples contained levamisole.[57] Levamisole was banned from the US market in 1999 because of serious side effects. Severe rash and neutropenia are the most common complications of levamisole. In the last decade, levamisole has gained renewed attention as an adulterant in cocaine. A series of adverse side effects of levamisole-adulterated cocaine is known as cocaine/levamisole-associated autoimmune syndrome (CLAAS).[55] Neutropenia and vasculitis are also the most common clinical manifestations of CLAAS.[55,56] The positive serum ANCA in some patients with CLAAS makes people realize that the formation of ANCAs may be the cause of the development of CLAAS.[37,38] Due to the high prevalence of levamisole in cocaine, it is difficult to distinguish whether the development of CLAAS is because of the synergistic effect of levamisole and cocaine or the result of levamisole alone.
Pathogenesis
Etiology of AAV mainly includes genetic factors, epigenetic factors, and environmental factors.[5] Drug as an environmental factor can trigger the development of AAV. Primary AAV and drug-induced AAV share a partial pathway in pathogenesis. To date, the pathogenesis of drug-induced AAV is still poorly understood. Here we summarize the role of genetic and epigenetic factors in AAV and the correlation between drug-induced AAV and neutrophil extracellular traps (NETs) [Figure 1].
Figure 1.

The role of NET formation in the development of drug-induced AAV. In some patients with drug-induced AAV DNase I activity is reduced and NET degradation is weakened. The persistence of NETs results in the generation of ANCAs. Pro-inflammatory cytokines such as IL-1β and TNF prime neutrophils then ANCAs binds to the primed neutrophils. This binding leads to the excessive activation of these neutrophils and eventually to the formation of NETs. Histones and MMPs in NETs can damage vascular endothelial cells. AAV: ANCA-associated vasculitis; ANCA: Anti-neutrophil cytoplasmic antibody; IL: Interleukin; MMPs: Matrix metalloproteinases; MPO: Myeloperoxidase; NET: Neutrophil extracellular trap; PR3: Proteinase 3; TNF: Tumor necrosis factor.
Genome-wide association studies identified several genes associated with AAV susceptibility. Major histocompatibility complex class II genes have the strongest association with AAV.[58,59] Genetic factors were associated with ANCA specificity rather than with the clinical manifestation. PR3-ANCA was associated with human leukocyte antigen (HLA)-DP and MPO-ANCA was associated with HLA-DQ.[58] The pathogenesis of these genes in AAV remains to be further studied.
Epigenetic modifications that induce gene silencing mainly includes histone H3 lysine 27 trimethylation (H3K27me3) and DNA methylation.[60,61] The decrease of H3K27me3 is associated with the abnormal expression of MPO and PR3 in patients with AAV.[60] DNA methylation is associated with MPO and PRTN3 the gene that encodes PR3 expression. Hypomethylation of MPO and PRTN3 was seen in patients with active AAV and DNA methylation generally increased in remission.[61] Some drugs, such as hydrazine, inhibit DNA methylation and induce self-reactivity in T cells.[62,63] Activated T cells further induce B cells and plasma cells produce autoantibodies. These studies indicated that the abnormal epigenetic modification is associated with the inappropriate expression of PR3 and MPO in patients with AAV.
As an important part of innate immunity, NETs are extracellular structures composed of granule proteins and chromatin that kill bacteria.[64,65] The formation of NETs is strictly regulated and disordered regulation of NETs is an important cause of ANCA production.[66,67] Infectious factors stimulated neutrophils to form NETs, which are mainly degraded by serum endonuclease DNase I.[68] In patients with MPA, DNase I activity is reduced and NET degradation is weakened. The persistence of NETs can destroy the tolerance to MPO and generate MPO-ANCA.[66] Pro-inflammatory cytokines such as interleukin-1β and TNF prime neutrophils, then primed neutrophils express ANCA specific antigens. ANCAs binds to these antigens and the Fc region of ANCAs binds to the Fcγ receptor on neutrophils. This binding leads to the excessive activation of these neutrophils and eventually to the formation of NETs.[69–71] Histones and matrix metalloproteinases in NETs can damage vascular endothelial cells.[72,73] In conclusion, the formation of NETs and ANCAs forms a vicious circle in the pathogenesis of AAV.
In healthy conditions, Semaphorin 4D (SEMA4D) receptors on neutrophils interact with plexin B2 ligands on endothelial cells to negatively regulate neutrophils activation. While in patients with AAV, SEMA4D is proteolytically cleaved from the surface of neutrophils. Alterations in SEMA4D-plexin B2 interactions can lead to the formation of NETs.[74,75] These studies further illustrate the important role of NET formation in the pathogenesis of AAV.
PTU induces abnormal NETs that are difficult to digest by DNase I. It is speculated that the metabolites of PTU may mask the DNase I recognition sites.[76] Both cocaine and levamisole induce NET formation and also augment the release of B-cell activating factor.[77] In addition, hydralazine can also significantly induce the formation of NETs.[63] As mentioned above, NETs induced by these drugs lead to the formation of ANCAs.
Some drugs such as minocycline and clozapine did not significantly induce NET formation or impair NET degradation.[63] This may suggest that some unknown mechanisms are also involved in the pathogenesis of drug-induced AAV.
Difference Between Drug-induced AAV and Primary AAV
The clinical manifestations of drug-induced AAV are similar to those of primary AAV. It is difficult to distinguish drug-induced AAV from primary AAV based on clinical manifestations. In addition, there are no unique clinicopathological or laboratory markers that can distinguish drug-induced AAV from primary AAV.[6] The clinical manifestations and severity of AAV induced by different drugs may vary greatly, and it is very difficult to generalize about all types of drug-induced AAV.
ATD-induced AAV is the most common drug-induced AAV, so we mainly summarize the difference between ATD-induced AAV and primary AAV, which may provide useful information for us to identify drug-induced AAV and primary AAV. According to some previous retrospective studies comparing ATD-induced AAV with primary AAV, we can summarize the following contents [Table 2].
Table 2.
Difference between ATD-induced AAV and primary AAV.
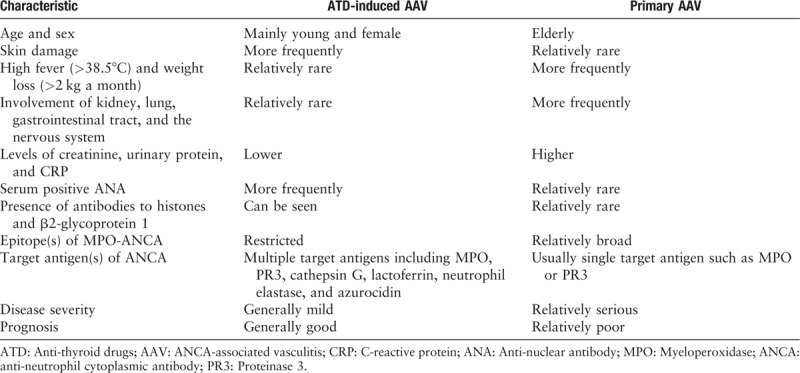
In clinical manifestations, ATD-induced AAV mainly occurs in young women, while primary AAV usually occurs in the elderly, with a similar probability between men and women. This difference is related to thyroid disease mainly involving young women.[10,78–80] ATD-induced AAV more frequently has skin damage, and primary AAV is more characterized by high fever (>38.5°C), weight loss (>2 kg a month), and the involvement of kidney, lung, gastrointestinal tract, and the nervous system. There is no significant difference in the involvement of eye, ear, nose, throat, cardiovascular, and joint pain.[10,79,80] As long as the offending drug is stopped in time, the severity of ATD-induced AAV is usually milder than that of primary AAV. The prognosis of ATD-induced AAV is also generally better than that of primary AAV.[10,78–80]
In laboratory tests, ATD-induced AAV is also slightly different from primary AAV. First, compared with primary AAV, ATD-induced AAV showed lower levels of creatinine, urinary protein, and C-reactive protein. This is consistent with the observed milder clinical manifestations of ATD-induced AAV.[10,78–80] Second, the ANA positive rate of ATD-induced AAV is significantly higher than that of primary AAV.[10,79,80] Third, ATD-induced AAV can also be detected in the presence of antibodies to β2-glycoprotein 1 and histones, which are relatively rare in primary AAV.[8,10] In addition, the epitopes of anti-MPO antibodies in ATD-induced AAV can be more limited than in primary AAV.[81] This is consistent with our observation that almost all patients with ATD-induced AAV presented positive MPO-ANCA instead of PR3-ANCA.[10,78–80] Lastly, ANCAs typically recognize many target antigens in ATD-induced AAV including lactoferrin, cathepsin G, azurocidin, and neutrophil elastase. In contrast, ANCAs generally recognize only one target antigen, MPO or PR3, in primary AAV.[48,82,83]
Diagnosis
Early diagnosis of drug-induced AAV and cessation of the offending drug immediately are crucial to the prognosis of drug-induced AAV. Currently, there is no clear definition of the diagnostic criteria of drug-induced AAV, which is still an exclusive diagnosis. The difference between drug-induced AAV and primary AAV mentioned above can help us to identify drug-induced AAV. Furthermore, we suggest that the diagnosis of drug-induced AAV should be considered when the following conditions are met: (1) Patients should first meet the 2012 Chapel Hill Consensus Conference definition for AAV. (2) The clinical symptoms are related to the use of the offending drug and relieved with discontinuation. (3) Serum ANCA is positive. (4) Excluding diseases with similar characteristics, in particular infections, malignancies, and other types of vasculitis.[6]
If the diagnosis remains difficult, tissue biopsy is strongly encouraged to confirm the definitive diagnosis.[6]
Treatment
To date, there is no standard treatment strategy for drug-induced AAV. Corticosteroid and immunosuppressants used to treat primary AAV may not be appropriate for most patients with drug-induced AAV. The treatment strategy for drug-induced AAV should vary from patient to patient depending on the severity of the disease [Figure 2].[6] For those with mild symptoms including arthralgia, fever, weight loss, and without organ involvement, stopping the offending drug at once after diagnosis may be sufficient to induce disease remission. Active management is reserved for patients with more severe conditions. Prednisone at 1 mg/kg for 1 to 2 months with gradually reduced dose is required for those with severe and active organ involvement. For patients with important organs involved, immunosuppressive agents (especially cyclophosphamide) may be necessary. Furthermore, patients with massive pulmonary alveolar hemorrhage or rapidly progressive glomerulonephritis should be treated with intravenous injections of 7 to 15 mg/kg of methylprednisolone per day for 3 consecutive days or even plasmapheresis.[84–86]
Figure 2.

Treatment strategy for patients with drug-induced AAV. AAV: ANCA-associated vasculitis; ANCA: Anti-neutrophil cytoplasmic antibody.
Individual disease courses are unpredictable and each patient requires careful monitoring. The duration of immunosuppressive therapy in patients with drug-induced AAV remains uncertain. It is generally believed that the duration of immunosuppressive treatment of drug-induced AAV should be shorter than that of primary AAV.[87] Compared with primary AAV, maintenance therapy of drug-induced AAV may not be necessary as long as the offending drug stop being used. Patients with drug-induced AAV usually do not relapse once the offending drug is withdrawn and the disease is in remission.[87]
Prognosis
According to previous studies, the prognosis of drug-induced AAV is generally significantly better than that of primary AAV.[7,84,86,87] Most patients with drug-induced AAV can achieve complete remission after stop using the offending drug without further treatment. A small number of patients with organ involvement can also achieve a good prognosis after immunosuppressive therapy. Very few patients develop end-stage renal disease because of not discontinuation of the offending drug timely.[16,87] In long-term follow-up studies, the incidence of end-stage renal disease and the mortality rate were both lower after discontinuation of the offending drug and no recurrence was observed.[16,87]
Conclusions
The offending drugs leading to drug-induced AAV are almost from every pharmacologic class. Genetic factors, epigenetic factors and the formation of NETs are important mechanisms for the development of drug-induced AAV. Patients treated with drugs that may induce AAV must be closely monitored. ANCA is an effective tool for early diagnosis of drug-induced AAV. Understanding the difference between drug-induced AAV and primary AAV may be helpful in identifying drug-induced AAV. Once diagnosed, the offending drug must be stopped immediately. Most patients can be relieved when they stop using the offending drug. Immunosuppressive therapy should only be used in patients with vital organs involvement to prevent further disease progression. The duration of immunosuppressive therapy in patients with drug-induced AAV should be much shorter than that in primary AAV, and long-term maintenance therapy is generally not required. The prognosis of drug-induced AAV is generally better than that of primary AAV.
Conflicts of interest
None.
Footnotes
How to cite this article: Weng CH, Liu ZC. Drug-induced anti-neutrophil cytoplasmic antibody-associated vasculitis. Chin Med J 2019;132:2848–2855. doi: 10.1097/CM9.0000000000000539
References
- 1.Cornec D, Cornec-Le Gall E, Fervenza FC, Specks U. ANCA-associated vasculitis - clinical utility of using ANCA specificity to classify patients. Nat rev Rheumatol 2016; 12:570–579. doi: 10.1038/nrrheum.2016.123. [DOI] [PubMed] [Google Scholar]
- 2.Khan I, Watts RA. Classification of ANCA-associated vasculitis. Curr Rheumatol Rep 2013; 15:383.doi: 10.1007/s11926-013-0383-6. [DOI] [PubMed] [Google Scholar]
- 3.Radice A, Sinico RA. Antineutrophil cytoplasmic antibodies (ANCA). Autoimmunity 2005; 38:93–103. doi: 10.1080/08916930400022673. [DOI] [PubMed] [Google Scholar]
- 4.Jarrot PA, Kaplanski G. Pathogenesis of ANCA-associated vasculitis: an update. Autoimmun Rev 2016; 15:704–713. doi: 10.1016/j.autrev.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Nakazawa D, Masuda S, Tomaru U, Ishizu A. Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat Rev Rheumatol 2019; 15:91–101. doi: 10.1038/s41584-018-0145-y. [DOI] [PubMed] [Google Scholar]
- 6.Merkel PA. Drug-induced vasculitis. Rheum Dis Clin North Am 2001; 27:849–862. doi: 10.1016/S0889-857X(05)70239-8. [DOI] [PubMed] [Google Scholar]
- 7.Radic M, Martinovic Kaliterna D, Radic J. Drug-induced vasculitis: a clinical and pathological review. Neth J Med 2012; 70:12–17. [PubMed] [Google Scholar]
- 8.Wiik A. Drug-induced vasculitis. Curr Opin Rheumatol 2008; 20:35–39. doi: 10.1097/BOR.0b013e3282f1331f. [DOI] [PubMed] [Google Scholar]
- 9.Aloush V, Litinsky I, Caspi D, Elkayam O. Propylthiouracil-induced autoimmune syndromes: two distinct clinical presentations with different course and management. Semin Arthritis Rheum 2006; 36:4–9. doi: 10.1016/j.semarthrit.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Bonaci-Nikolic B, Nikolic MM, Andrejevic S, Zoric S, Bukilica M. Antineutrophil cytoplasmic antibody (ANCA)-associated autoimmune diseases induced by antithyroid drugs: comparison with idiopathic ANCA vasculitides. Arthritis ResTher 2005; 7:R1072–R1081. doi: 10.1186/ar1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pillinger MH, Staud R. Propylthiouracil and antineutrophil cytoplasmic antibody associated vasculitis: the detective finds a clue. Semin Arthritis Rheum 2006; 36:1–3. doi: 10.1016/j.semarthrit.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Wiik A. Clinical and laboratory characteristics of drug-induced vasculitic syndromes. Arthritis Res Ther 2005; 7:191–192. doi: 10.1186/ar1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calabrese LH, Duna GF. Drug-induced vasculitis. Current Opin Rheumatol 1996; 8:34–40. doi: 10.1097/00002281-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013; 65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 15.Balavoine AS, Glinoer D, Dubucquoi S, Wemeau JL. Antineutrophil cytoplasmic antibody-positive small-vessel vasculitis associated with antithyroid drug therapy: how significant is the clinical problem? Thyroid 2015; 25:1273–1281. doi: 10.1089/thy.2014.0603. [DOI] [PubMed] [Google Scholar]
- 16.Yang J, Yao LP, Dong MJ, Xu Q, Zhang J, Weng WW, et al. Clinical Characteristics and outcomes of propylthiouracil-induced antineutrophil cytoplasmic antibody-associated vasculitis in patients with Graves’ disease: a median 38-month retrospective cohort study from a single institution in China. Thyroid 2017; 27:1469–1474. doi: 10.1089/thy.2017.0468. [DOI] [PubMed] [Google Scholar]
- 17.Gumà M, Salinas I, Reverter JL, Roca J, Valls-Roc M, Juan M, et al. Frequency of antineutrophil cytoplasmic antibody in Graves’ disease patients treated with methimazole. J Clin Endocrinol Metab 2003; 88:2141–2146. doi: 10.1210/jc.2002-021383. [DOI] [PubMed] [Google Scholar]
- 18.Mavrakanas TA, Bouatou Y, Samer C, de Seigneux S, Meyer P. Carbimazole-induced, ANCA-associated, crescentic glomerulonephritis: case report and literature review. Ren Fail 2013; 35:414–417. doi: 10.3109/0886022x.2012.760356. [DOI] [PubMed] [Google Scholar]
- 19.Frigui M, Kechaou M, Haddouk S, Masmoudi A, Kaddour N, Masmoudi H, et al. Benzylthiouracil induced ANCA-positive vasculitis: study of three cases and review of the literature. Ann Endocrinol (Paris) 2008; 69:517–522. doi: 10.1016/j.ando.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Ginsberg S, Rosner I, Slobodin G, Boulman N, Rozenbaum M, Kaly L, et al. Etanercept treatment-related c-ANCA-associated large vessel vasculitis. Clin Rheumatol 2016; 35:271–273. doi: 10.1007/s10067-015-3134-4. [DOI] [PubMed] [Google Scholar]
- 21.Ashok D, Dubey S, Tomlinson I. C-ANCA positive systemic vasculitis in a patient with rheumatoid arthritis treated with infliximab. Clin Rheumatol 2008; 27:261–264. doi: 10.1007/s10067-007-0712-0. [DOI] [PubMed] [Google Scholar]
- 22.Simms R, Kipgen D, Dahill S, Marshall D, Rodger RS. ANCA-associated renal vasculitis following anti-tumor necrosis factor alpha therapy. Am J Kidney Dis 2008; 51:e11–e14. doi: 10.1053/j.ajkd.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 23.Parekh K, Ching D, Rahman MU, Stamp LK. Onset of Wegener's granulomatosis during therapy with golimumab for rheumatoid arthritis: a rare adverse event? Rheumatology (Oxford) 2010; 49:1785–1787. doi: 10.1093/rheumatology/keq101. [DOI] [PubMed] [Google Scholar]
- 24.Feriozzi S, Muda AO, Gomes V, Montanaro M, Faraggiana T, Ancarani E. Cephotaxime-associated allergic interstitial nephritis and MPO-ANCA positive vasculitis. Ren Fail 2000; 22:245–251. doi: 10.1081/jdi-100100869. [DOI] [PubMed] [Google Scholar]
- 25.Lenert P, Icardi M, Dahmoush L. ANA (+) ANCA (+) systemic vasculitis associated with the use of minocycline: case-based review. Clin Rheumatol 2013; 32:1099–1106. doi: 10.1007/s10067-013-2245-z. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal A, Agrawal A, Nathan K, Roy S. Rare adverse effect of a common drug: nitrofurantoin-induced ANCA-associated vasculitis. BMJ Case Rep 2015; 2015:bcr2014209253.doi: 10.1136/bcr-2014-209253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodring T, Abraham R, Frisch S. A case of probable trimethoprim-sulfamethoxazole induced circulating antineutrophil cytoplasmic antibody-positive small vessel vasculitis. Dermatology Online J 2017; 23: 13030/qt3j9537pg. [PubMed] [Google Scholar]
- 28.Pingili CS, Okon EE. Vancomycin-induced leukocytoclastic vasculitis and acute renal failure due to tubulointerstitial nephritis. Am J Case Rep 2017; 18:1024–1027. doi: 10.12659/ajcr.905214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan CD, Smith A, Rodriguez ER. Systemic necrotizing vasculitis induced by isoniazid. Cardiovasc Pathol 2014; 23:181–182. doi: 10.1016/j.carpath.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Ji G, Zeng X, Sandford AJ, He JQ. Rifampicin-induced antineutrophil cytoplasmic antibody-positive vasculitis: a case report and review of the literature. Int J Clin Pharmacol Ther 2016; 54:804–807. doi: 10.5414/CP202576. [DOI] [PubMed] [Google Scholar]
- 31.Kang S, Cho MH, Hyun H, Kim JH, Ko JS, Kang HG, et al. A pediatric case of a D-penicillamine induced ANCA-associated vasculitis manifesting a pulmonary-renal syndrome. J Korean Med Sci 2019; 34:e173.doi: 10.3346/jkms.2019.34.e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denissen NH, Peters JG, Masereeuw R, Barrera P. Can sulfasalazine therapy induce or exacerbate Wegener's granulomatosis? Scand J Rheumatol 2008; 37:72–74. doi: 10.1080/03009740701607117. [DOI] [PubMed] [Google Scholar]
- 33.Jaunkalns R, Shear NH, Sokoluk B, Gardner D, Claas F, Uetrecht JP. Antimyeloperoxidase antibodies and adverse reactions to clozapine. Lancet 1992; 339:1611–1612. doi: 10.1016/0140-6736(92)91877-b. [DOI] [PubMed] [Google Scholar]
- 34.Greenfield JR, McGrath M, Kossard S, Charlesworth JA, Campbell LV. ANCA-positive vasculitis induced by thioridazine: confirmed by rechallenge. Br J dermatol 2002; 147:1265–1267. doi: 10.1046/j.1365-2133.2002.05000_2.x. [DOI] [PubMed] [Google Scholar]
- 35.Choi HK, Merkel PA, Niles JL. ANCA-positive vasculitis associated with allopurinol therapy. Clin Exp rheumatol 1998; 16:743–744. [PubMed] [Google Scholar]
- 36.Haroon M, Devlin J. A case of ANCA-associated systemic vasculitis induced by atorvastatin. Clin Rheumatol 2008; 27: Suppl 2: S75–S77. doi: 10.1007/s10067-008-1020-z. [DOI] [PubMed] [Google Scholar]
- 37.Lotscher F, Krusche M, Ruffer N, Kubacki T, Person F, Kotter I. Cocaine-induced ANCA-associated renal disease: a case-based review. Rheumatol Int 2019; 39:2005–2014. doi: 10.1007/s00296-019-04410-9. [DOI] [PubMed] [Google Scholar]
- 38.Marquez J, Aguirre L, Munoz C, Echeverri A, Restrepo M, Pinto LF. Cocaine-levamisole-induced vasculitis/vasculopathy syndrome. Curr Rheumatol Rep 2017; 19:36.doi: 10.1007/s11926-017-0653-9. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez A, Lozier M, Adkinson BC, Ilaiwy A. c-ANCA vasculitis after initiation of denosumab. BMJ Case Rep 2019; 12:e228336.doi: 10.1136/bcr-2018-228336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar B, Strouse J, Swee M, Lenert P, Suneja M. Hydralazine-associated vasculitis: overlapping features of drug-induced lupus and vasculitis. Semin Arthritis Rheum 2018; 48:283–287. doi: 10.1016/j.semarthrit.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Mangodt TC, Joos R, Siozopoulou V, Cortoos PJ, Baeten H, Docx M, et al. Perinuclear antineutrophil cytoplasmic antibody-positive vasculitis, oligoarthritis, tendinitis, and myositis associated with isotretinoin in a 15-year-old boy: case report and review of literature. Pediatr Dermatol 2018; 35:e173–e177. doi: 10.1111/pde.13445. [DOI] [PubMed] [Google Scholar]
- 42.Parry RG, Gordon P, Mason JC, Marley NJ. Phenytoin-associated vasculitis and ANCA positivity: a case report. Nephrol Dial Transplant 1996; 11:357–359. doi: 10.1093/oxfordjournals.ndt.a027268. [DOI] [PubMed] [Google Scholar]
- 43.Astwood EB, Vanderlaan WP. Treatment of hyperthyroidism with propylthiouracil. Ann Intern Med 1946; 25:813–821. [DOI] [PubMed] [Google Scholar]
- 44.Poate H. Thyrotoxicosis treated with 2-carbethoxythio-1-methylglyoxaline (neo mercazole). Lancet 1953; 1:879–881. doi: 10.1016/s0140-6736(53)92005-4. [DOI] [PubMed] [Google Scholar]
- 45.Cooper DS. Antithyroid drugs. New Engl J Med 2005; 352:905–917. doi: 10.1056/NEJMra042972. [DOI] [PubMed] [Google Scholar]
- 46.Borson-Chazot F, Caron P, Glinoer D, Wemeau JL. Antithyroid drugs: what kind of prescription? Presse Med 2014; 43:105–108. doi: 10.1016/j.lpm.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Glinoer D, Cooper DS. The propylthiouracil dilemma. Curr Opin Endocrinol Diabetes Obes 2012; 19:402–407. doi: 10.1097/MED.0b013e3283565b49. [DOI] [PubMed] [Google Scholar]
- 48.Dolman KM, Gans RO, Vervaat TJ, Zevenbergen G, Maingay D, Nikkels RE, et al. Vasculitis and antineutrophil cytoplasmic autoantibodies associated with propylthiouracil therapy. Lancet 1993; 342:651–652. doi: 10.1016/0140-6736(93)91761-a. [DOI] [PubMed] [Google Scholar]
- 49.Vogt BA, Kim Y, Jennette JC, Falk RJ, Burke BA, Sinaiko A. Antineutrophil cytoplasmic autoantibody-positive crescentic glomerulonephritis as a complication of treatment with propylthiouracil in children. J Pediatr 1994; 124:986–988. doi: 10.1016/s0022-3476(05)83199-3. [DOI] [PubMed] [Google Scholar]
- 50.Gunton JE, Stiel J, Clifton-Bligh P, Wilmshurst E, McElduff A. Prevalence of positive anti-neutrophil cytoplasmic antibody (ANCA) in patients receiving anti-thyroid medication. Eur J Endocrinol 2000; 142:587.doi: 10.1530/eje.0.1420587. [DOI] [PubMed] [Google Scholar]
- 51.Vanek C, Samuels MH. Central nervous system vasculitis caused by propylthiouracil therapy: a case report and literature review. Thyroid 2005; 15:80–84. doi: 10.1089/thy.2005.15.80. [DOI] [PubMed] [Google Scholar]
- 52.Ahmed K, Rao S, Simha V. Antineutrophil cytoplasmic antibody-positive vasculitis in a patient with graves disease: cross-reaction between propylthiouracil and methimazole. Endocr Pract 2010; 16:449–451. doi: 10.4158/ep09304.Cr. [DOI] [PubMed] [Google Scholar]
- 53.Gutierrez-Gonzalez LA. Biological therapy-induced systemic vasculitis. Current Rheumatol Rep 2016; 18:39.doi: 10.1007/s11926-016-0588-6. [DOI] [PubMed] [Google Scholar]
- 54.Ziolkowska M, Maslinski W. Laboratory changes on anti-tumor necrosis factor treatment in rheumatoid arthritis. Curr Opin Rheumatol 2003; 15:267–273. doi: 10.1097/00002281-200305000-00014. [DOI] [PubMed] [Google Scholar]
- 55.Cascio MJ, Jen KY. Cocaine/levamisole-associated autoimmune syndrome: a disease of neutrophil-mediated autoimmunity. Curr Opin Hematol 2018; 25:29–36. doi: 10.1097/moh.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 56.Brunt TM, van den Berg J, Pennings E, Venhuis B. Adverse effects of levamisole in cocaine users: a review and risk assessment. Arch Toxicol 2017; 91:2303–2313. doi: 10.1007/s00204-017-1947-4. [DOI] [PubMed] [Google Scholar]
- 57.Casale JF, Colley VL, Legatt DF. Determination of phenyltetrahydroimidazothiazole enantiomers (levamisole/dexamisole) in illicit cocaine seizures and in the urine of cocaine abusers via chiral capillary gas chromatography-flame-ionization detection: clinical and forensic perspectives. J Anal Toxicol 2012; 36:130–135. doi: 10.1093/jat/bkr025. [DOI] [PubMed] [Google Scholar]
- 58.Lyons PA, Rayner TF, Trivedi S, Holle JU, Watts RA, Jayne DR, et al. Genetically distinct subsets within ANCA-associated vasculitis. New Engl J Med 2012; 367:214–223. doi: 10.1056/NEJMoa1108735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rahmattulla C, Mooyaart AL, van Hooven D, Schoones JW, Bruijn JA, Dekkers OM, et al. Genetic variants in ANCA-associated vasculitis: a meta-analysis. Ann Rheum Dis 2016; 75:1687–1692. doi: 10.1136/annrheumdis-2015-207601. [DOI] [PubMed] [Google Scholar]
- 60.Ciavatta DJ, Yang J, Preston GA, Badhwar AK, Xiao H, Hewins P, et al. Epigenetic basis for aberrant upregulation of autoantigen genes in humans with ANCA vasculitis. J Clin Invest 2010; 120:3209–3219. doi: 10.1172/JCI40034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones BE, Yang J, Muthigi A, Hogan SL, Hu Y, Starmer J, et al. Gene-specific DNA methylation changes predict remission in patients with ANCA-associated vasculitis. J Am Soc Nephrol 2017; 28:1175–1187. doi: 10.1681/asn.2016050548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cornacchia E, Golbus J, Maybaum J, Strahler J, Hanash S, Richardson B. Hydralazine and procainamide inhibit T cell DNA methylation and induce autoreactivity. J Immunol 1988; 140:2197–2200. [PubMed] [Google Scholar]
- 63.Irizarry-Caro JA, Carmona-Rivera C, Schwartz DM, Khaznadar SS, Kaplan MJ, Grayson PC. Brief report: drugs implicated in systemic autoimmunity modulate neutrophil extracellular trap formation. Arthritis Rheumatol 2018; 70:468–474. doi: 10.1002/art.40372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science 2004; 303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 65.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 2007; 176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakazawa D, Shida H, Tomaru U, Yoshida M, Nishio S, Atsumi T, et al. Enhanced formation and disordered regulation of NETs in myeloperoxidase-ANCA-associated microscopic polyangiitis. J Am Soc Nephrol 2014; 25:990–997. doi: 10.1681/asn.2013060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schreiber A, Rousselle A, Becker JU, von Massenhausen A, Linkermann A, Kettritz R. Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc Natl Acad Sci U S A 2017; 114:E9618–E9625. doi: 10.1073/pnas.1708247114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A 2010; 107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heeringa P, Rutgers A, Kallenberg CGM. The net effect of ANCA on neutrophil extracellular trap formation. Kidney Int 2018; 94:14–16. doi: 10.1016/j.kint.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 70.Jennette JC, Falk RJ. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol 2014; 10:463–473. doi: 10.1038/nrrheum.2014.103. [DOI] [PubMed] [Google Scholar]
- 71.Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 2009; 15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumar SV, Kulkarni OP, Mulay SR, Darisipudi MN, Romoli S, Thomasova D, et al. Neutrophil extracellular trap-related extracellular histones cause vascular necrosis in severe GN. J Am Soc Nephrol 2015; 26:2399–2413. doi: 10.1681/asn.2014070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carmona-Rivera C, Zhao W, Yalavarthi S, Kaplan MJ. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis 2015; 74:1417–1424. doi: 10.1136/annrheumdis-2013-204837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nishide M, Nojima S, Ito D, Takamatsu H, Koyama S, Kang S, et al. Semaphorin 4D inhibits neutrophil activation and is involved in the pathogenesis of neutrophil-mediated autoimmune vasculitis. Ann Rheum Dis 2017; 76:1440–1448. doi: 10.1136/annrheumdis-2016-210706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nishide M, Kumanogoh A. The role of semaphorins in immune responses and autoimmune rheumatic diseases. Nat Rev Rheumatol 2018; 14:19–31. doi: 10.1038/nrrheum.2017.201. [DOI] [PubMed] [Google Scholar]
- 76.Nakazawa D, Tomaru U, Suzuki A, Masuda S, Hasegawa R, Kobayashi T, et al. Abnormal conformation and impaired degradation of propylthiouracil-induced neutrophil extracellular traps: implications of disordered neutrophil extracellular traps in a rat model of myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2012; 64:3779–3787. doi: 10.1002/art.34619. [DOI] [PubMed] [Google Scholar]
- 77.Lood C, Hughes GC. Neutrophil extracellular traps as a potential source of autoantigen in cocaine-associated autoimmunity. Rheumatology 2017; 56:638–643. doi: 10.1093/rheumatology/kew256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cao X, Lin W. Clinical study of renal impairment in patients with propylthiouracil-induced small-vessel vasculitis and patients with primary ANCA-associated small-vessel vasculitis. Expe Ther Med 2013; 5:1619–1622. doi: 10.3892/etm.2013.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen Y-X, Zhang WEN, Chen X-N, Yu H-J, Ni L-Y, Xu J, et al. Propylthiouracil-induced antineutrophil cytoplasmic antibody (ANCA)-associated renal vasculitis versus primary ANCA-associated renal vasculitis: a comparative study. J Rheumatol 2012; 39:558–563. doi: 10.3899/jrheum.110931. [DOI] [PubMed] [Google Scholar]
- 80.Hasegawa J, Hoshino J, Sekine A, Hayami N, Suwabe T, Sumida K, et al. Clinical and histological features of antineutrophil cytoplasmic antibody-associated vasculitis related to antithyroid drugs. Clin Nephrol 2018; 89:438–444. doi: 10.5414/cn109364. [DOI] [PubMed] [Google Scholar]
- 81.Ye H, Zhao MH, Gao Y, Guo XH, Wang HY. Anti-myeloperoxidase antibodies in sera from patients with propylthiouracil-induced vasculitis might recognize restricted epitopes on myeloperoxidase molecule. Clin Exp Immunol 2004; 138:179–182. doi: 10.1111/j.1365-2249.2004.02557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gao Y, Zhao MH, Guo XH, Xin G, Gao Y, Wang HY. The prevalence and target antigens of antithyroid drugs induced antineutrophil cytoplasmic antibodies (ANCA) in Chinese patients with hyperthyroidism. Endocr Res 2004; 30:205–213. doi: 10.1081/erc-120037729. [DOI] [PubMed] [Google Scholar]
- 83.Gao Y, Chen M, Ye H, Guo XH, Zhao MH, Wang HY. The target antigens of antineutrophil cytoplasmic antibodies (ANCA) induced by propylthiouracil. Int Immunopharmacol 2007; 7:55–60. doi: 10.1016/j.intimp.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 84.Gao Y, Zhao MH. Review article: drug-induced anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrology 2009; 14:33–41. doi: 10.1111/j.1440-1797.2009.01100.x. [DOI] [PubMed] [Google Scholar]
- 85.Chen M, Gao Y, Guo XH, Zhao MH. Propylthiouracil-induced antineutrophil cytoplasmic antibody-associated vasculitis. Nat Rev Nephrol 2012; 8:476–483. doi: 10.1038/nrneph.2012.108. [DOI] [PubMed] [Google Scholar]
- 86.Grau RG. Drug-induced vasculitis: new insights and a changing lineup of suspects. Curr Rheumatol Rep 2015; 17:71.doi: 10.1007/s11926-015-0545-9. [DOI] [PubMed] [Google Scholar]
- 87.Gao Y, Chen M, Ye H, Yu F, Guo XH, Zhao MH. Long-term outcomes of patients with propylthiouracil-induced anti-neutrophil cytoplasmic auto-antibody-associated vasculitis. Rheumatology 2008; 47:1515–1520. doi: 10.1093/rheumatology/ken321. [DOI] [PubMed] [Google Scholar]


