Supplemental Digital Content is available in the text
Keywords: Acute surgical pain, Risk assessment, Stratified counseling, Multimodal analgesia
Abstract
Background:
Post-operative pain is unpleasant for patients and may worsen surgical recovery. Peri-operative multimodal analgesia has been used for many years; however, its efficacy still needs improvement. In the present study, a thorough peri-operative pain counseling and stratified management program based on risk assessment was implemented, with the goal of improving post-operative analgesia and patient satisfaction.
Methods:
This prospective, controlled, pilot study included 361 patients who underwent elective surgery. Of these 361 patients, 187 received peri-operative pain risk assessment and stratified analgesia and counseling (stratified analgesia group), while 174 received conventional multimodal analgesia (conventional group). The two groups were compared regarding the post-operative pain intensity, rescue analgesia administration, post-operative quality of recovery as assessed via the quality of recovery 40 questionnaire, total dosage of peri-operative opioids, analgesic satisfaction, and analgesic costs.
Results:
Compared with the conventional group, the stratified analgesia group reported decreased pain intensity during motion at 24 h post-operatively and required lower dosages of rescue analgesia (P = 0.03). The total quality of recovery 40 questionnaire score and the scores for physical wellbeing and pain were significantly better in the stratified analgesia group than the conventional group (P = 0.04); the stratified analgesia group also reported better scores for analgesic satisfaction (P = 0.03) and received lower dosages of opioids (P = 0.03). Analgesic costs were lower in the stratified analgesia group than the conventional group; the cost-effective ratio was 109 in the conventional group and 62 in the stratified analgesia group.
Conclusions:
The analgesic efficacy was improved by the implementation of stratified analgesia based on surgical pain risk assessment and counseling. This stratified analgesia protocol increased the patients’ analgesic satisfaction and improved the quality of recovery without increasing healthcare costs. The present findings may help improve the efficacy of peri-operative multimodal analgesia in clinical practice.
Clinical Trial Registry:
NCT02728973; https://clinicaltrials.gov/ct2/show/NCT02728973?term=NCT02728973&draw=2&rank=1.
Introduction
Acute post-operative pain is not only an unpleasant experience that decreases the psychological and somatic wellbeing of the patients, but is also linked to adverse outcomes such as the development of chronic pain and discomfort and a prolonged recovery.[1] However, 20 years after the introduction of multimodal analgesia, this pain management strategy still achieves only moderate efficacy and results in increased opioid consumption. Acute moderate-to-severe post-operative pain is experienced by more than 50% of patients in the USA[2] and in China.[3] Uncontrolled post-operative pain adversely affects physical functioning, recovery, and quality of life, especially mental status.[4] Thus, there is an urgent need to improve the efficacy of multimodal analgesia.
The individualization of pain management is an ideal that has yet to be met. To bridge the analgesic needs of the patients and the clinicians’ perceptions of the patients’ needs, the pre-operative characteristics of patients that render them susceptible to severe and prolonged pain need to be recognized because patients at high risk of severe post-operative pain often need more progressive measures to control acute surgical pain.[5] In addition, patients’ misconceptions about the possible adverse effects of analgesic medications must be addressed; for example, because patients with chronic pain pre-operatively are more likely to develop addiction to analgesic medication post-operatively,[6] some older adult patients in China request lower than recommended dosages of analgesics because they believe that surgical pain is inevitable and that medication abuse must be avoided.[3] There is a substantial gap between the patients’ needs and the peri-operative pain management in clinical practice.[7]
Although opioids remain the standard therapy for post-operative pain, the misuse and tolerance of such analgesic medications is a prominent issue that is associated with increased mortality, morbidity, and readmission after surgery.[6–8] However, several meta-analyses have reported that patient-controlled analgesia (PCA) achieves favorable outcomes regarding acute post-operative pain, post-operative complications (such as pulmonary embolism and PCA-related adverse events), and duration of post-operative hospitalization.[9–11]
According to a previous logistic regression analysis (unpublished data), the risk factors for acute severe post-operative pain are the type of surgery, American Society of Anesthesiologists (ASA) grade, use of PCA, and presence of chronic pain before surgery. These results were used to formulate the peri-operative pain risk scale (PPRS)-CYMZ 2.0 and create a stratified analgesic model for groups of patients with varying risks of post-operative pain in which patients at higher risk of pain are administered more potent analgesic techniques and medications.[12] In the present study, patients were assessed to determine the stratified level of analgesia they required, and groups of patients with different risks of pain (low, intermediate, and high) received the appropriate recommended combinations of analgesic techniques and medications. The patients’ post-operative pain intensity, recovery quality, analgesic satisfaction, and analgesic costs were assessed. The goal of the present study was to optimize the efficacy of peri-operative multimodal analgesia.
Methods
Ethical approval
This prospective, controlled, pilot study was conducted in a single university hospital. The study protocol was approved by the institutional review board of this hospital and was registered in the Clinical Trial Registry (NCT02728973). All clinical practices and observations were conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from each patient before the study was conducted.
Study design and patients
This study recruited patients who underwent elective surgery from January 2017 to September 2018. The reporting of this trial followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.[13]
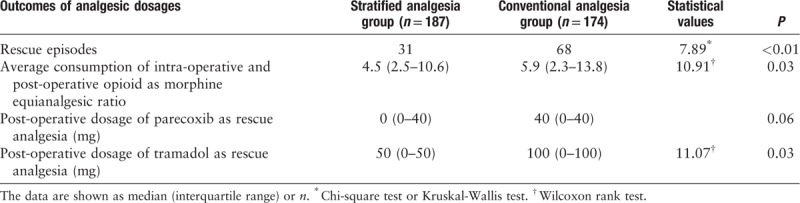
Patients were recruited from the departments of gynecology, general surgery, orthopedic surgery, plastic surgery, thoracic surgery, breast surgery, and urological surgery. The inclusion criteria were: patients who underwent elective surgery and provided written informed consent for follow-up, age range from 16 to 85 years, body mass index between 18 and 30 kg/m2, and ASA grade between I and III. The exclusion criteria were: ASA grade of greater than III; refusal of study participation; intra-operative cardiac arrest; peri-operative mental diseases; pregnancy; patients who received monitored anesthesia care outside of the operating room; age younger than 16 years or older than 80 years; surgery of the head, neck, or heart; participation in other clinical trials; and patients who could not be extubated in the post-anesthesia care unit and needed further treatment in the intensive care unit. The following pre-operative data were collected from the patients 1 week before surgery: age, sex, body mass index, diagnosis, malignancy status, history of smoking (smoking for at least 1 year before surgery), ASA grade, pain intensity assessed using a numerical rating scale, and analgesic use.
Patient characteristics and anesthesia
All patients underwent general anesthesia with endotracheal intubation. Anesthesia was induced with 0.10 to 0.15 mg/kg of midazolam (or 0.15–0.20 mg/kg of etomidate for patients older than 65 years), 2.0 to 2.5 mg/kg of propofol, 0.3 to 1.0 μg/kg of sufentanil citrate, and 0.08 to 0.12 mg/kg of vecuronium. Anesthesia was maintained via the inhalation of 1% to 3% sevoflurane and continuous intravenous infusion of remifentanil at 7 to 8 μg·kg−1·h−1 and propofol at 25 to 75 μg·kg−1·min−1 via a microperfusion pump. Anesthetic depth was maintained at a bispectral index of between 40 and 60 (Covidien, Boulder, CO, USA). All patients were extubated in the post-anesthesia care unit after receiving full reversal with flumazenil and neostigmine and meeting the criteria for extubation. Patients who reported a visual analog scale score for pain of more than 4 or reported interrupted sleep due to pain were administered rescue analgesia (40 mg of intravenous parecoxib or 50 mg of intravenous flurbiprofen) before being transferred to the surgical ward; each administration of rescue analgesia was counted as one episode. All patients had a Steward score of ≥4 before being discharged from the post-anesthesia care unit.
Conventional peri-operative multimodal analgesia
Conventional multimodal analgesia was selected based on the consensus judgement of the anesthesiologists and surgeons, and comprised either a single analgesic injection, PCA (intravenous, epidural, or peripheral nerve block), or oral analgesic tablets to achieve a visual analogue scale score for pain of <4.
Stratified analgesia counseling and implementation
One week before surgery, the patients, which were selected to undergo stratified analgesia counseling and implementation (stratified analgesia group), were interviewed to determine their risk of pain and issues with pain management. The risk of pain was evaluated with the pre-validated PPRS-CYMZ 2.0,[12] which comprises a checklist of seven items that produce a total score of 0 to 14. The seven items of the PPRS-CYMZ 2.0 are: type of surgery, whether the surgical procedure is minimally invasive or not, estimated duration of surgery, pre-operative chronic pain at the surgical sites, ASA grade, risk factors for chronic post-operative pain (comprising the presence of pre-operative anxiety, presence of pre-operative neuropathy, and pre-operative use of analgesia for more than 1 month), and the malignancy status of the disease requiring surgery. Each item was assigned a score from a maximum of 5 to a minimum of 0. The details of the PPRS-CYMZ 2.0 are described in Supplementary 1.
Patients with a total stratified PPRS-CYMZ 2.0 score of 1 to 3 were rated as having a low risk of developing moderate-to-severe pain after surgery, those with a total score of 4 to 7 were rated as intermediate risk, and those with a total score of 8 to 14 were rated as high risk. Each patient was informed about their risk of pain and the implementation of peri-operative stratified analgesia; one anesthesiologist who was specialized in the pre-operative evaluation of surgical patients explained the principles of pain risk evaluation and the administration of stratified analgesia. The formatted words used for patient counseling were as follows:
-
A.
You are going to receive the procedure xxx to treat your disease of xxx. Based on your previous medical history, previous pain intensity, and the scheduled procedure, your risk of developing acute moderate-to-severe pain post-operatively is low/moderate/high. We will develop a continuous peri-operative pain management protocol for you, which we hope will help reduce your pain before and after surgery.
-
B.
Because you have a low/moderate/high risk of surgical pain, you require oral preventive analgesia before surgery. We will also use local wound infiltration or a peripheral nerve block that blocks the pain transmission from your incision to your brain. A self-controlled device will administer local anesthetics, opioid analgesics, or non-opioid analgesics based on your individual situation.
-
C.
During your hospitalization, our staff will closely monitor your pain intensity, especially if pain interrupts your sleep, affects your extremities or bowel movements, or is experienced during activities such as coughing and walking. Please do not hesitate to tell your nurses or doctors if you are experiencing pain; we will deal with these problems and help you to achieve better pain control. We will also conduct a short interview with you about your recovery during post-operative hospitalization.
-
D.
All pain medications will be given under the supervision of anesthesiologists until your discharge from the hospital. If you feel any discomfort such as shortness of breath, nausea, or vomiting during hospitalization, please inform your physician, who will adjust your therapy or treat you accordingly.
The implementation of stratified analgesia involved a multidisciplinary team that comprised anesthesiologists, attending surgeons, and nursing staff. The stratified analgesia algorithm was a peri-operative continuous analgesia protocol that included pre-operative preventive analgesia, and intra- and post-operative analgesia based on pain risk stratification (Supplementary 2). Rescue analgesia was administered to patients who experienced sudden burst pain (visual analog scale score for pain of greater than 4), exacerbation of pain during motion, or interrupted sleep because of pain. Bridge analgesia was administered during the period immediately before hospital discharge. The patients with the highest risk of surgical pain were administered the most potent analgesic techniques or medications. Preventive analgesia was administered 24 h pre-operatively for all surgical patients. The moderate or high-risk groups received PCA and local wound infiltration combined with nerve blocks. All daily dosages of recommended analgesic medications in clinical practice are outlined in Supplementary 2. Patients were given advice regarding analgesia after hospital discharge, including the recommended analgesia after discharge and the need for referral to a specialist if any complication developed.
Outcome assessments
Resident anesthesiologists performed the follow-up of surgical recovery and recorded the following outcomes. Patients were assessed for acute post-operative pain at rest or during motion at 12, 24, 48, and 72 h post-operatively. Sleep scores were assessed using a 0 to 10 point scale on the mornings of post-operative days 2, 3, and 4; a sleep score of 0 point represented no sleep at all, while a score of 10 points represented excellent sleep. The intra- and post-operative opioid consumptions were calculated as an equianalgesic ratio to morphine.[14]
Quality of recovery was assessed using the Quality of Recovery 40 questionnaire (QoR-40) at two time points: the day before surgery and 48 h after surgery. The 40-item QoR-40 provides a total score and sub-scores in five dimensions: patient support, comfort, emotions, life ability, physical wellbeing and pain.[15] Satisfaction scores were assessed at the time of hospital discharge, and were based on patient satisfaction with analgesia, satisfaction with the surgical procedure, and satisfaction with hospitalization; satisfaction was scored using a 0 to 10 point scale, with 0 point representing complete dissatisfaction and 10 points representing complete satisfaction.
The assessed analgesic costs included the direct analgesic costs (analgesic medication and catheters), indirect analgesic costs (costs of the prophylactic or therapeutic use of medications or techniques to treat analgesia-related adverse events such as nausea and vomiting), and total costs (sum of the direct and indirect analgesic costs). All costs were calculated based on a national drug price list produced by municipal health administration; considering a discount rate of 3% as a reference for the calculation of costs, the cost-effective ratio (CER) was calculated by dividing the total analgesic costs of all patients by the total analgesic satisfaction scores.
Statistical analysis
The statistical analyses were performed using SPSS 20.0 software (International Business Machines Corporation, Amund City, NY, USA). It was assumed that the implementation of stratified analgesia and counseling would decrease the visual analog scale score for pain during motion by at least 1 point at 24 h post-operatively compared with conventional analgesia. With a statistical power of 80% at the 0.05 significance level with a standard deviation of 0.8 and an assumed dropout rate of 15%, 160 subjects were required in each group. The level of statistical significance was set at 0.05. Data were expressed as mean ± standard deviation (SD) or median (interquartile range) for continuous variables, and as total number (percent frequency) for categorical variables. A group t-test, Wilcoxon rank test or Kruska-Wallis test were used to compare the results for continuous variables. The Chi-squared test was used to compare the results for categorical variables, while Fisher exact test was used for categorical variables when the number of events was less than five. P values of <0.05 were considered to indicate statistical significance. Baseline characteristics were compared between the conventional analgesia group and the stratified analgesia group. Other outcomes were analyzed in accordance with the data distribution.
Results
Four hundred and fifty-one patients who underwent surgery in the period from January 2017 to September 2018 were screened for eligibility. Three hundred and sixty-one patients were enrolled and finished the follow-up during hospitalization. One hundred and eighty-seven patients received stratified analgesia evaluation and counseling (stratified analgesia group), while 174 received conventional analgesia (conventional analgesia group). Figure 1 shows the study flow diagram in accordance with the STROBE statement. The two groups were comparable regarding pre-operative and demographic characteristics including age, sex, ASA grade, pre-operative pain or use of analgesics, and duration of anesthesia. The per-protocol percentage of patients in the stratified analgesia group was 98.4%, and three patients reported pain before sleep post-operatively but refused rescue analgesia; all patients were included in the statistical analysis. All patients received general anesthesia with endotracheal intubation [Table 1].
Figure 1.
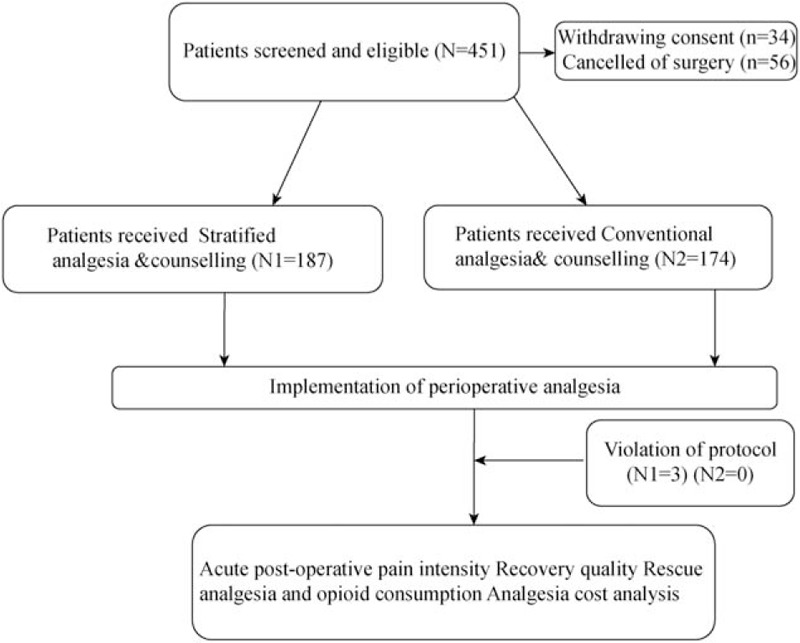
Study flow diagram in accordance with the STROBE statement.
Table 1.
Baseline characteristics of all patients in this study.
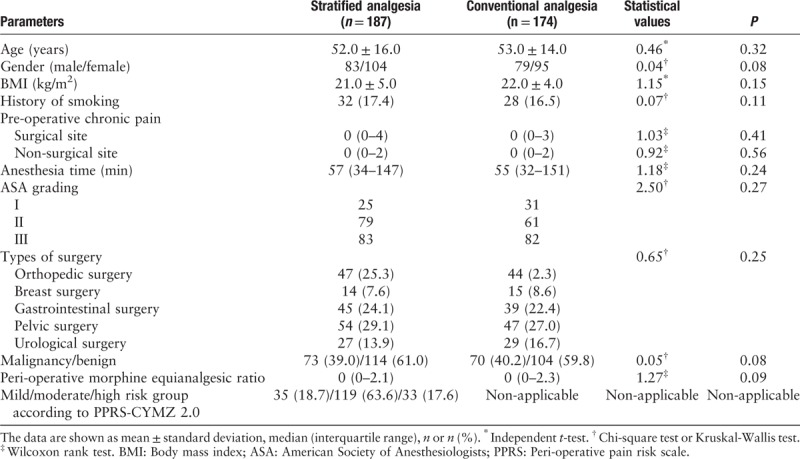
Pain intensity
The visual analog scale scores for pain at rest did not differ between the two groups; however, compared with the stratified analgesia group, the conventional analgesia group reported greater pain intensity during motion at 12 and 24 h post-operatively [Table 2]. The stratified analgesia group reported a better sleep score than the conventional analgesia group for the night at post-operative day 2 (P = 0.02).
Table 2.
Acute post-operative pain intensity and sleep quality.
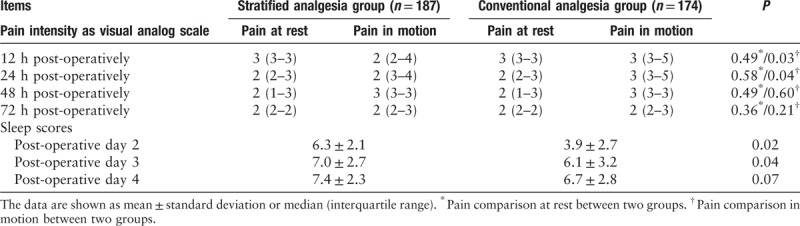
Rescue analgesia
The total cumulative number of rescue analgesia episodes in the conventional analgesia group was significantly greater than that in the stratified analgesia group (68 vs. 31, P < 0.01). Compared with the conventional analgesia group, the stratified analgesia group had a lower cumulative dosage of opioid consumption (P = 0.03), and was administered less doses of tramadol as post-operative rescue analgesia (P = 0.03) [Table 3].
Table 3.
Opioid consumption and rescue analgesia.
Recovery quality
Before the scheduled anesthesia and surgery, the two groups did not significantly differ regarding the QoR-40 scores for pain, emotional status, psychological support, ability to perform activities of daily life, and physical wellbeing. At 48 h post-operatively, the sub-scores for pain and physical wellbeing were significantly better in the stratified analgesia group compared with the conventional analgesia group (P = 0.04) [Figure 2].
Figure 2.
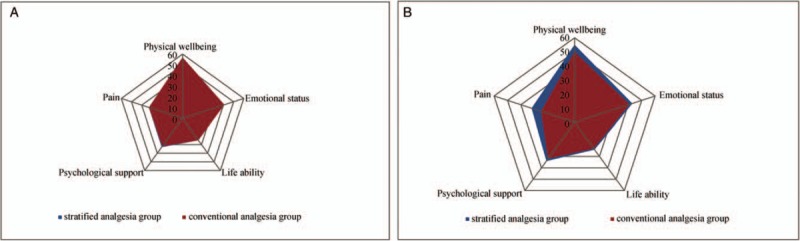
Pre-operative (A) and post-operative (B) QoR-40 scores of two groups. No statistical difference was found in pre-operative scores between two groups, post-operatively, total scores, pain, and physical wellbeing were improved in stratified analgesia group (P < 0.05). QoR-40: Quality of recovery 40 questionnaire.
Analgesic satisfaction and costs
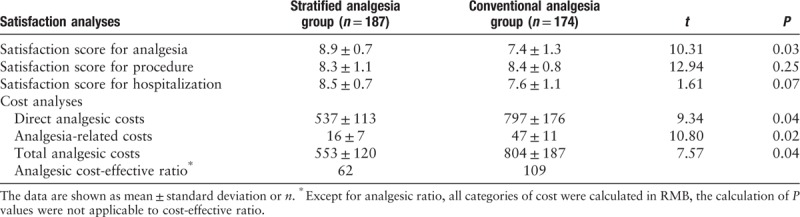
At the time of hospital discharge, the stratified analgesia group was more satisfied with their peri-operative analgesia than the conventional analgesia group; the other scores did not differ between the two groups. The analgesia-related costs and analgesic CER were lower in the stratified analgesia group than the conventional analgesia group (P = 0.04); regarding the CER, each patient saved 47 RMB for every one point increase in analgesic satisfaction [Table 4].
Table 4.
Analgesic costs and satisfaction.
Discussion
This pilot study found that stratified pain management counseling and implementation improved analgesic efficiency, surgical recovery, and patients’ satisfaction. Despite the rapid development of analgesic medications and technique, peri-operative multimodal analgesia is still lagging behind the real needs of surgical patients; according to a national investigation in the USA, over 50% patients still report unsatisfactory pain management after surgery in main tertiary medical centers.[2] The situation is similar in China, where moderate-to-severe surgical pain decreases patient satisfaction.[12] Uncontrolled surgical pain also has a detrimental effect on patients’ sleep, surgical organ rehabilitation, and stress response.[16] Therefore, the present stratified analgesia program was initiated in an attempt to improve the efficacy of peri-operative multimodal analgesia.
The duration and severity of acute and chronic surgical pain are hard to predict, and the risk factors for aggravated surgical pain include at least three dimensions: pre-operative pain, type of surgery, and peri-operative analgesia.[17,18] For example, patients receiving joint replacements are more likely to have long-term pre-operative pain and refractory post-operative pain than patients undergoing other types of surgery.[19] To integrate such risk factors for severe post-operative pain into an optimized peri-operative pain management algorithm, we first performed a statistical analysis to identify the factors that most exacerbated acute post-operative pain, such as type of surgery, presence of malignancy, and ASA grade. We then designed a stratified pain management protocol based on the pre-operative risk assessment for pain, with the goal of promoting the individualization of peri-operative analgesia.
Our peri-operative multimodal analgesia protocol merits further implementation. Compared with conventional multimodal analgesia, the present stratified analgesia protocol reduced the pain score during motion until 24 h post-operatively; the stratified analgesia group also required lower dosages of rescue analgesia, yet reported improved recovery during hospitalization. Our stratified analgesia protocol had several advantages. First, a thorough pre-operative briefing about the potential risk of surgical pain helped balance the patients’ expectations and their compliance with pain management. A successful analgesia program must consider the patients’ characteristics, pertinent analgesic methods, patient compliance with pain management, and timely feedback. The stratified evaluation of surgical pain also encourages patients to report their pain instead of just tolerating it.[20] To increase the efficacy of pain management, apart from counseling, the stratified analgesia protocol based on risk assessment identified those who needed more rigorous analgesic strategies; for example, oral preventive analgesics combined with peripheral nerve block and intra-operative opioids are recommended for patients receiving total knee replacement. The standardization of analgesia also reduces the need for rescue analgesia and associated costs.[21]
Post-operative rechecks and analgesia guidance may help increase patients’ trust in their anesthesiologists and surgeons, and this closer connection between patients and clinicians is beneficial for surgical recovery. The QoR-40 is commonly used to evaluate patients’ surgical recovery in five dimensions.[15] The QoR-40 scores showed that our stratified analgesia program significantly reduced the degree of post-operative pain and discomfort compared with conventional multimodal analgesia. Furthermore, in the moderate-to-high risk group, the general use of wound infiltration and nerve block decreased the use of opioid agents, which is important because peri-operative opioid overuse and misuse are prominent worldwide issues.[22,23] The inappropriate use of opioid agents in surgical patients may be prevented if more adequate analgesia is provided after full consideration of the patients’ risks of developing pain post-operatively. Although numerous studies have developed novel analgesic methods or medications, the present study was not aimed at promoting a certain technique; the stratified analgesia and counseling protocol increased the clinicians’ awareness that surgical pain should be viewed and treated in an individualized way.
This study had several limitations. First, this trial was open-labeled, and the lack of randomization might have introduced selective bias in the analyses of pain intensity and patient recovery. Second, the results may have been affected by confounding factors such as the different way anesthesiologists implemented the stratified analgesia protocol. Third, our risk scale for surgical pain was designed and verified in one tertiary medical center; a multi-centered clinical trial should be conducted to test its application for surgical patients who need more potent peri-operative analgesia. Further studies should explore new methods for opioid-sparing peri-operative multimodal analgesia.
Stratified pain management based on a risk assessment for surgical pain improves the analgesic efficacy and post-operative recovery. Although there is still a gap between the individualization of pain management in surgical patients and the status quo, the present findings might help in the implementation of effective peri-operative multimodal analgesia.
Acknowledgement
The authors thank the surgeons and nurses in the Departments of General Surgery, Urological Surgery, Gynaecology, and Orthopedics.
Funding
This research was funded by grants from the National Clinical Key Discipline Project Sponsored by the Health Bureau of Chongqing Municipality (No. 2016MSXM012) and Chongqing Association for Science and Technology (No. cstc2017shmsA130045).
Conflicts of interest
None.
Supplementary Material
Supplementary Material
Footnotes
How to cite this article: Peng LH, Min S, Jin JY, Wang WJ. Stratified pain management counseling and implementation improving patient satisfaction: a prospective, pilot study. Chin Med J 2019;132:2812–2819. doi: 10.1097/CM9.0000000000000540
References
- 1.Meissner W, Coluzzi F, Fletcher D, Huygen F, Morlion B, Neugebauer E, et al. Improving the management of post-operative acute pain: priorities for change. Curr Med Res Opin 2015; 31:2131–2143. doi: 10.1185/03007995.2015.1092122. [DOI] [PubMed] [Google Scholar]
- 2.Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin 2014; 30:149–160. doi: 10.1185/03007995.2013.860019. [DOI] [PubMed] [Google Scholar]
- 3.Peng LH, Jing JY, Qin PP, Su M. A multicentered cross-sectional study of disease burden of pain of inpatients in southwest China. Chin Med J 2016; 129:936–941. doi: 10.4103/0366-6999.179788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parsons B, Schaefer C, Mann R. Economic and humanistic burden of post-trauma and post-surgical neuropathic pain among adults in the US. J Pain Res 2013; 6:459–469. doi: 10.1016/j.jpain.2013.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kehlet H, Edwards RR, Buvanendran A. Persistent Postoperative Pain: Pathogenic Mechanisms and Preventive Strategies. 14th World Congress of Pain. 2012; Seattle: IASP Press, 133–146. [Google Scholar]
- 6.Gupta A, Nizamuddin J, Elmofty D, Nizamuddin SL, Tung A, Minhaj M, et al. Opioid abuse or dependence increases 30-day readmission rates after major operating room procedures: a national readmissions database study. Anesthesiology 2018; 128:880–890. doi: 10.1097/ALN.0000000000002136. [DOI] [PubMed] [Google Scholar]
- 7.Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res 2017; 10:2287–2298. doi: 10.2147/JPR.S144066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res 2015; 473:2402–2412. doi: 10.1007/s11999-015-4243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudcova J, McNicol E, Quah C, Lau J, Carr DB. Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database Syst Rev 2006; 18:CD003348.doi: 10.1002/14651858.CD003348.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Ballantyne JC, Carr DB, Chalmers TC, Dear KB, Angelillo IF, Mosteller F. Postoperative patient-controlled analgesia: meta-analyses of initial randomized control trials. J Clin Anesth 1993; 5:182–193. doi: 10.1016/0952-8180(93)90013-5. [DOI] [PubMed] [Google Scholar]
- 11.Walder B, Schafer M, Henzi I, Tramèr MR. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. A quantitative systematic review. Acta Anaesthesiol Scand 2001; 45:795–804. doi: 10.1034/j.1399-6576.2001.045007795.x. [DOI] [PubMed] [Google Scholar]
- 12.Peng L, Min S, Ren L, Hao X, Cheng B, Wang P, et al. Prediction and stratified diagnosis and treatment of postoperative pain: a cohort clinical trial (in Chinese). Chin J Anesthesiology 2017; 37:1347–1352. doi: 10.3760/cma.j.issn.0254-1416.2017.11.017. [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61:344–349. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PubMed] [Google Scholar]
- 14.Svendsen K, Borchgrevink P, Fredheim O, Hamunen K, Mellbye A, Dale O. Choosing the unit of measurement counts: the use of oral morphine equivalents in studies of opioid consumption is a useful addition to defined daily doses. Palliat Med 2011; 25:725–732. doi: 10.1177/0269216311398300. [DOI] [PubMed] [Google Scholar]
- 15.Gornall BF, Myles PS, Smith CL, Burke JA, Leslie K, Pereira MJ, et al. Measurement of quality of recovery using the QoR-40: a quantitative systematic review. Br J Anaesth 2013; 111:161–169. doi: 10.1093/bja/aet014. [DOI] [PubMed] [Google Scholar]
- 16.Joshi GP, Ogunnaike BO. Consequences of inadequate postoperative pain relief and chronic persistent postoperative pain. Anesthesiol Clin North United States 2005; 23:21–36. doi: 10.1016/j.atc.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Aubrun F, Valade N, Coriat P, Riou B. Predictive factors of severe postoperative pain in the postanesthesia care unit. Anesth Analg 2008; 106:1535–1541. doi: 10.1213/ane.0b013e318168b2ce. [DOI] [PubMed] [Google Scholar]
- 18.Ip HY, Abrishami A, Peng PW, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology 2009; 111:657–677. doi: 10.1097/ALN.0b013e3181aae87a. [DOI] [PubMed] [Google Scholar]
- 19.Elson DW, Brenkel IJ. Predicting pain after total knee arthroplasty. J Arthroplasty 2006; 7:1047–1053. doi: 10.1016/j.arth.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Pinto PR, Mcintyre T, Ferrero R, Almeida A, Araújo-Soares V. Predictors of acute postsurgical pain and anxiety following primary total hip and knee arthroplasty. J Pain 2013; 14:502–515. doi: 10.1016/j.jpain.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Losina E, Walensky RP, Kessler CL, Emrani PS, Reichmann WM, Wright EA, et al. Cost-effectiveness of total knee arthroplasty in the US: patient risk and hospital volume. Arch Intern Med 2009; 12:1113–1121. doi: 10.1001/archinternmed.2009.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joshi GP, Kehlet H. Postoperative pain management in the era of ERAS: an overview. Best Pract Res Clin Anaesthesiol 2019; doi: 10.1016/j.bpa.2019.07.016 [In Press]. [DOI] [PubMed] [Google Scholar]
- 23.Lavand’homme P, Steyaert A. Opioid-free anesthesia opioid side effects: tolerance and hyperalgesia. Best Pract Res Clin Anaesthesiol 2017; 31:487e98.doi: 10.1016/j.bjane.2014.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


