Supplemental Digital Content is available in the text
Keywords: Metataxonomics, Internal transcribed spacer amplicons, Cerebrospinal fluid, Diagnosis, Genotype, Cryptococcal meningitis
Abstract
Background:
Cryptococcal meningitis is a severe infectious disease associated with high morbidity and mortality. Rapidity and accuracy of diagnosis contribute to better prognosis, but readily available tools, such as microscopy, culture, and antigens do not perform well all the time. Our study attempted to diagnose and genotype cryptococcus in the cerebrospinal fluid (CSF) samples from patients with cryptococcal meningitis using the approach of metataxonomics of Internal Transcribed Spacer (ITS) amplicons.
Methods:
The CSF samples were collected from 11 clinically suspected cryptococcal meningitis patients and four non-infectious controls. Samples were recruited from the First Affiliated Hospital of Fujian Medical University Hospital, Fuzhou Fourth Hospital and the 476th Hospital of Chinese People's Liberation Army from December 2017 to December 2018. ITS1 ribosomal deoxyribonucleic acid (rDNA) genes of 15 whole samples were amplified by universal forward primer ITS1 (CTTGGTCATTTAGAGGAAGTAA) and reverse primer ITS2 (GCTGCGTTCTTCATCGATGC), sequenced by Illumina MiSeq Benchtop Sequencer. The results were confirmed by sanger sequencing of ITS1 region and partial CAP59 gene of microbial isolates from 11 meningitic samples. Pair-wise comparison between infectious group and control group was conducted through permutational multivariate analysis (PERMANOVA) in R software.
Results:
The 30,000 to 340,000 high-quality clean reads were obtained from each of the positively stained or cultured CSF samples and 8 to 60 reads from each control. The samples from 11 infected patients yielded detectable cryptococcal-specific ITS1 DNA with top abundance (from 95.90% to 99.97%), followed by many other fungal groups (each <1.41%). ITS genotype was defined in 11 CSF samples, corresponding to ITS type 1, and confirmed by Sanger sequencing. A statistically significant difference (r2 = 0.65869, P = 0.0014) between infectious group and control group was observed.
Conclusions:
The metataxonomics of ITS amplicons facilitates the diagnosis and genotype of cryptococcus in CSF samples, which may provide a better diagnostic approach of cryptococcal infection.
Introduction
Cryptococcal meningitis is a life-threatening disease among both immunosuppressed individuals and healthy hosts, accounting for approximately half a million deaths per year particularly in patients with acquired immune deficiency syndrome (AIDS).[1] Rapidity and accuracy of diagnosis contribute to better prognosis, however, with the readily available tools, such as microscopy, culture, and antigens do not perform well all the time. Fungal culture remains to be gold standard for diagnosis, but it is time-consuming and insensitive to low pathogen burdens during the early disease phase. India Ink is also a common method for identifying the encapsulated cryptococcus, yet its sensitivity is highly unstable and operator-dependent.[2,3] Application of latex agglutination test and lateral flow assay are helpful for improving the sensitivity and detection speed, but it involves subjectivity in the interpretation of false-negative result.[4] DNA probes and polymerase chain reaction (PCR) techniques provide information regarding the diagnosis as well as classification. However, they limited their target a group of candidate pathogens when cryptococcal infection with atypical manifestations is unnoticed by physicians.
Dramatic advances in high-throughput sequencing, namely deep sequencing, and bioinformatics have allowed for a quick and accurate metataxonomic/metagenomic diagnosis, and profiling of microbial communities in many types of clinical samples including cerebrospinal fluid (CSF).[5–7] While shotgun metagenomics enables all DNA from a sample being sequenced, and it can be cost-ineffective for identification in human samples where microbes are a minor component. For bacteria or fungi, rDNA genes and their associated Internal Transcribed Spacer (ITS) have been adopted as common marker genes due to their superior ability to recognize the inter and intraspecific variation.[8] When these combined with bioinformatic analysis produce a relatively unbiased and sensitive approach that could distinguish major species or subspecies at high abundance within short turn-around time,[6] indicating possible pathogens in the clinical samples. Unfortunately, there is no report on deep sequencing of ribosomaldeoxyribonucleic acid (rDNA) amplicons directly from the cerebrospinal fluid for diagnosing cryptococcal meningitis.
Hence, in this study, the unique reads of cryptococcal genome were detected in 15 CSF samples, aiming to establish an appropriate metataxonomic diagnostic and sub-typing workflow for diagnosis of cryptococcal meningitis.
Methods
Description of samples
A total of 15 CSF samples were included in our research, and all were processed through India Ink staining or fungal cultivation according to the accepted diagnostic criteria for cryptococcal meningitis. Eight CSF samples were collected from the First Affiliated Hospital of Fujian Medical University Hospital, with six additional specimens from Fuzhou Fourth Hospital and one from the 476th Hospital of Chinese People's Liberation Army from December 2017 to December 2018. Each CSF sample was split into two parts: India Ink microscopy or culture and ITS metataxonomic analysis. Samples for metataxonomic study were flash-frozen instantly after lumber puncture was completed and stored at −80°C until analysis. Identification of cryptococcus was conducted in a double-blind manner.
DNA extraction from CSF and from cultural strains
To better fit our local laboratory conditions and increase the concentration of DNA samples, we have refined the traditional workflow of DNA extraction.[9] CSF specimens were centrifuged for 15 min at 14,000 r/min, followed by phosphate buffer solution (PBS) washing twice. The 200 mL of ultra-pure water was added to resuspend the packed cells. After 15 min, the samples were inactivated at 100°C and were bead-beat on vortex mixer (Qilingbeier, China) for two cycles of 3 minutes each. Next, the suspension was treated by QIAamp DNA Mini Kit (Qiagen, Germany) according to the manufacturer's instructions for extracting total CSF deoxyribonucleic acid.
ITS rDNA gene identification and etiologic typing
ITS1 rDNA gene was amplified by universal forward primer ITS1 (CTTGGTCATTTAGAGGAAGTAA) and reverse primer ITS2 (GCTGCGTTCTTCATCGATGC).[10] Barcoded with unique index sequence, amplicons were submitted to Illumina MiSeq Benchtop Sequencer with 2 × 250 bp paired-end method at Genesky Biotechnologies, Inc. (Shanghai, China). A series of pipelines, including Trim Galore, FLASH2, Mothur, Usearch, and UPARSE were used to merge the paired reads, removed the unqualified sequences, such as reads over the error rate of 2 or quality score of <20, fragments shorter than 100 base pairs in length, chimeras and singletons.[11]
Optimized sequencing data was clustered with the similarity of 97% into distinct operational taxonomic units (OTUs) and interpreted based on the UNITE (https://unite.ut.ee/), reducing redundant efforts on filtering human or non-fungal records. Being the most complete database for fungal ITS sequences, UNITE consists of 741,222 unique ITS reads, representing 73,929 fungal species. Cryptococcus detection in the samples was considered as dominant etiology and should be aligned to the typical standard sequences for strains of ITS Type 1–7 as previously described.[12]
Statistical analysis
Based on the results of gold standard tests, specimens were assigned to control group or infectious group. Pair-wise comparison between them was conducted through permutational multivariate analysis (PERMANOVA) in R software.
DNA confirmation of etiology
If the culture-based assay was positive, then the microbial isolates were processed similarly as the CSF DNA extraction, followed by polymerase chain reaction targeted at ITS1 region and partial CAP59 gene. The candidate regions were amplified by PCR and sequenced by ABI PRISM 3730XI DNA analyzer (Applied Biosystems, USA).
Results
Clinical data and cultural identification of 15 subjects
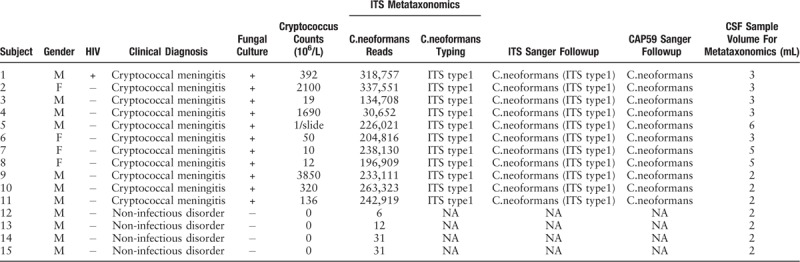
The study including 11 male and 4 female adults. Eleven of them were suspected with cryptococcal meningitis, in consideration of their prominent clinical manifestations of headache and fever with sub-acute or chronic onset, and the increased opening pressure of CSF. Among these suspected cases, one patient was complicated with AIDS. Other four subjects with no indications of microbial infection were also submitted to following microscopic or cultural examination. All suspected cases were confirmed with cryptococcal meningitis on the basis of the positively-stained mycetes and colonies grown on blood plates (Table 1).
Table 1.
Clinical data of 15 subjects, with determination of ITS metagenomics, conventional fungal tests and underlying confirmation follow-up.
Deep sequencing of ITS rDNA gene from CSF samples
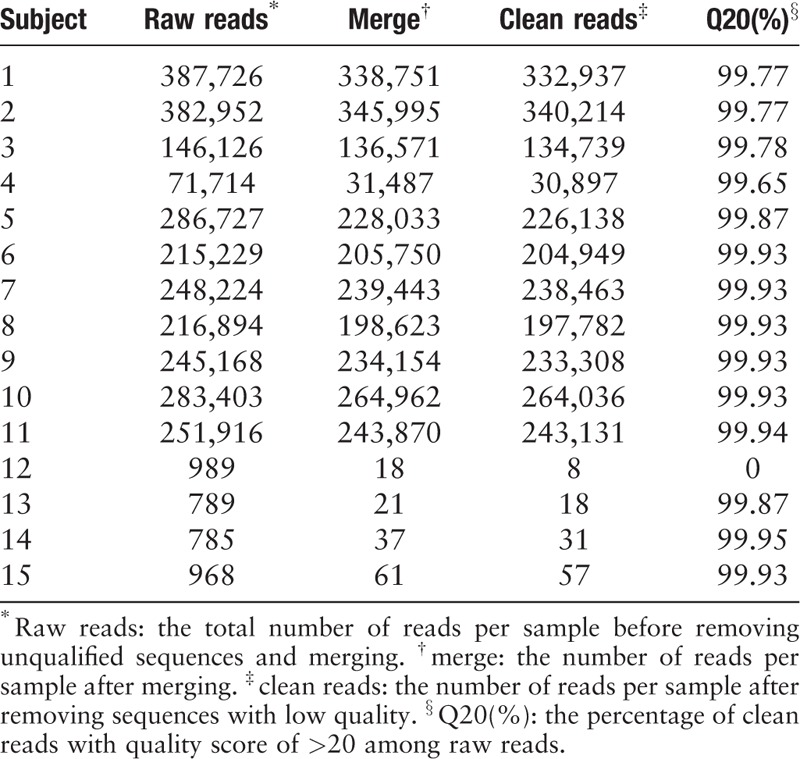
As shown in Tables 1–3, the 30,000 to 340,000 high-quality clean reads were obtained from each of the 11 positively cultured CSF samples and 8 to 60 reads from each control were obtained after trimming the reads with low quality or those that do not resemble for alignment. To minimize the interference for subsequent evaluation, we simultaneously filtered the primer pairs and the base-mismatching fragments.
Table 3.
Results of permutational multivariate analysis.
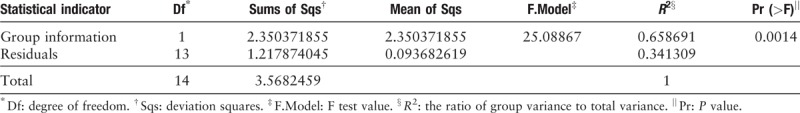
Table 2.
Sampling depth found by ABI PRISM 3730XI DNA analyzer from 15 cerebrospinal fluid samples.
Bioinformatic analysis
PCR amplicons from each specimen were assigned to diverse organisms, and the OTUs were ranked according to the number of hit reads (Detailed in Supplementary Table 1). All samples from 11 infected patients yielded detectable cryptococcal-specific ITS1 DNA with top abundance (from 95.90%–99.97%), and further aligned as the previous research described,[12] corresponding to ITS type 1. This was followed by many other fungal groups, accounting for only a small proportion (each <1.41%) [Detailed in Figure 1]. To eliminate the interference of the contaminants introduced from sample processing, we included specimens from 4 non-infectious cases as control group. OTU data related to background contamination demonstrated different distribution patterns from those associated with true infection. A statistically significant difference (r2 = 0.65869, P = 0.0014) between the two groups was observed with permutational multivariate analysis (PERMANOVA) [Table 3 and Figure 2]. As suggested by Mongkolrattanothai et al,[13] reads per million (RPM) ratio was utilized to avoid misidentification of pathogen when the value is more than 10, and RPM ratio is defined as RPMsample/RPMNTC for any given species. Likewise, microorganisms that have an enormous data gap of several orders of magnitude should also be filtered out as contaminants. Therefore, those with Cryptococcus as dominant fungal taxonomic unit reasonably shed light on the initial diagnosis of cryptococcal central nervous system (CNS) infection.
Figure 1.
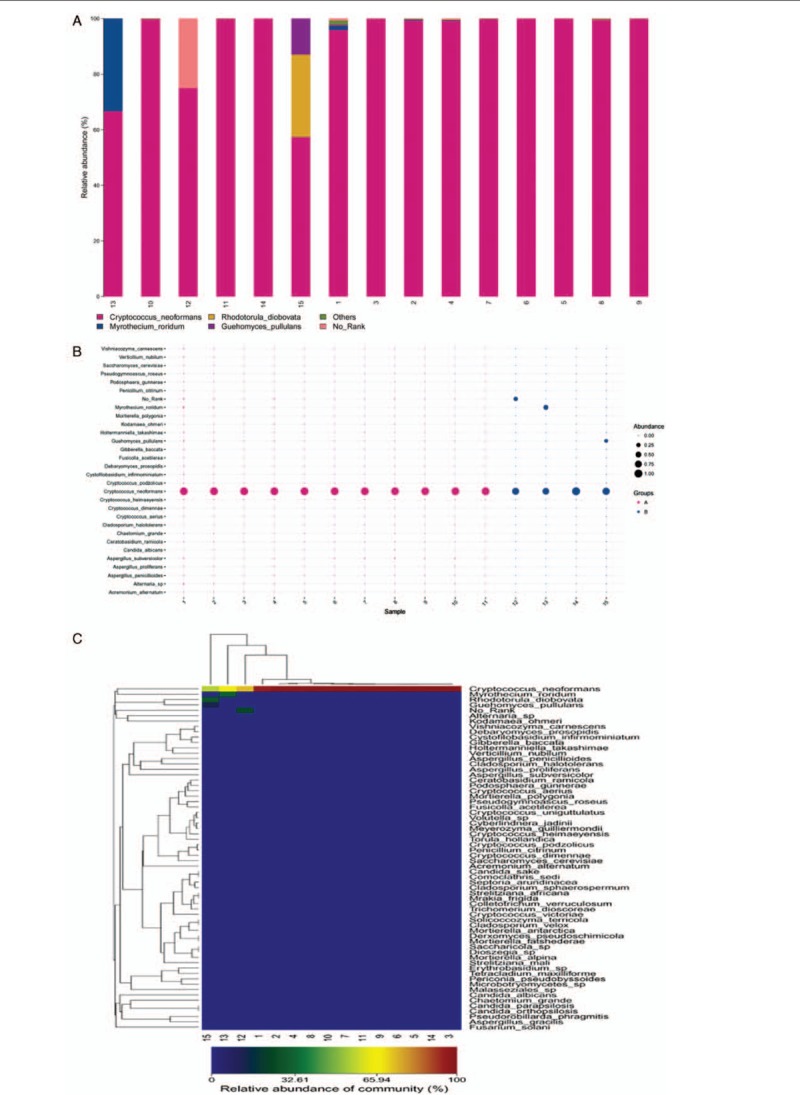
Species distribution of fungal OTU in cerebrospinal fluid sample. All samples from 11 infected patients yielded detectable cryptococcal-specific ITS1 DNA with top abundance. (A) Column diagram of fungal OTU distribution in CSF. Abscissa values represent sample number, coordinate values represent species abundance, and the corresponding colors represent different species as shown in the figure. (B) Bubble map of fungal OTU distribution in CSF. Figure in the abscissa represents sample number, ordinate represents different species. Different colors illustrate different groups, and the size of the bubbles represents the species abundance. (C) Heat map of fungal OTU distribution in CSF. Each column represents a different sample, each row represents a different species, and the corresponding color represents the abundance of species. Colors from blue to red indicate species abundance from small to large. OTU: Operational taxonomic unit.
Figure 2.
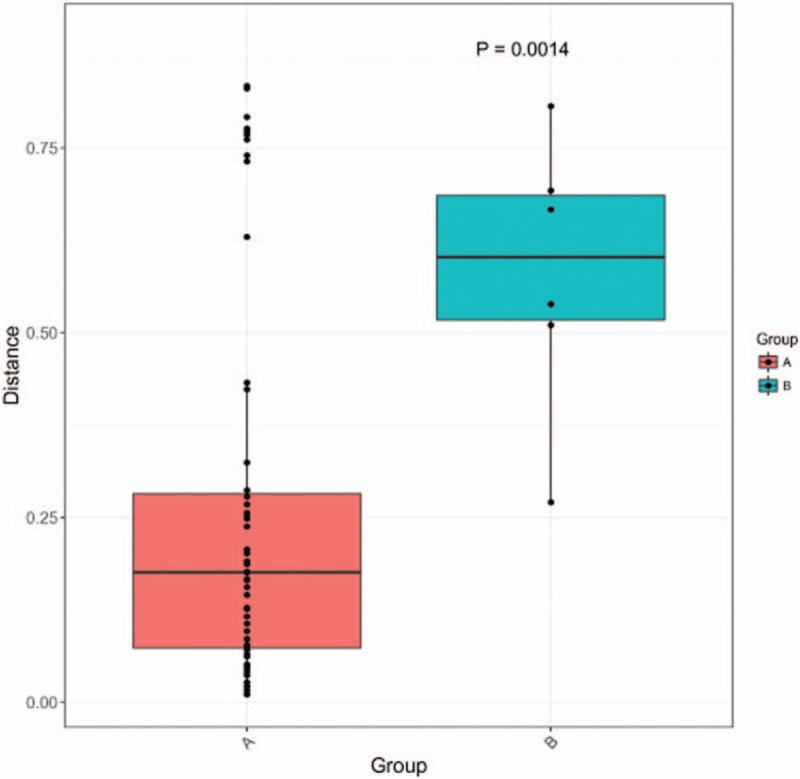
Results of permutational multivariate analysis. Group A represents samples from 11 clinically suspected cryptococcal meningitis patients, while Group B stands for samples from non-infectious patients. A statistically significant difference (r2 = 0.65869, P = 0.0014) between the two groups was observed with permutational multivariate analysis (PERMANOVA).
Sanger confirmation and classification of Cryptococcus neoformans from cultured-positive strain samples
All the 11 infectious samples demonstrated positive in culture-based isolation, followed by DNA extraction. Fragments of ITS1 gene were amplified via PCR [Figure 3A] and sequenced by Sanger technique, entirely matching the previous metagenetic diagnosis of cryptococcal CNS infection. ITS genotype was defined simultaneously by using BioloMICSNet software (http://www.cbs.knaw.nl/collections/BioloMICSSequences.aspx), suggesting ITS type 1. Additionally, we selected partial region of species-specific housekeeping gene CAP59 as additional evidence for the invasion of cryptococcus neoformans into the CNS, and submitted it to Sanger sequencing. The data demonstrated the presence of cryptoccocus as well [Figure 3B and C]. The ITS metataxonomics, conventional fungal tests and the underlying confirmation of whole 15 subjects were shown [Table 1].
Figure 3.
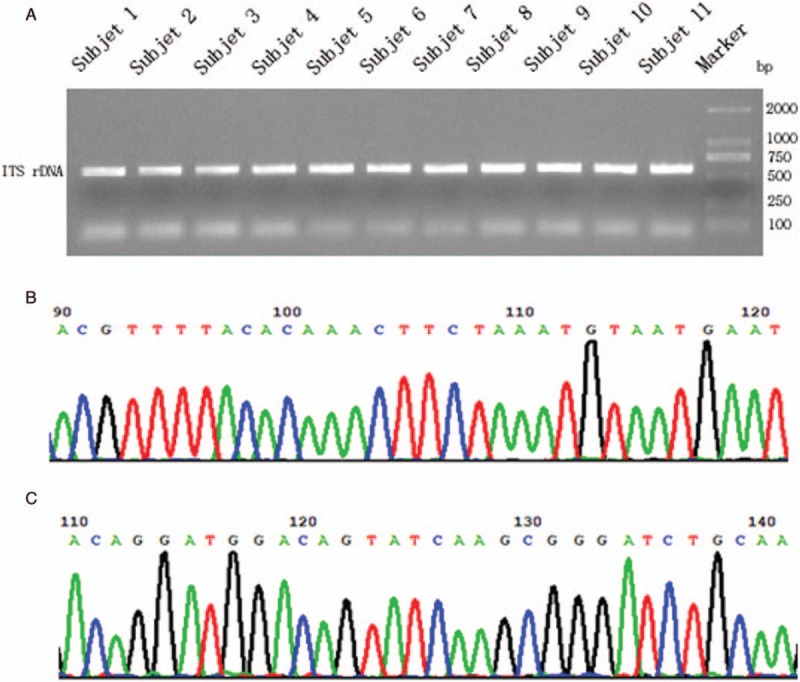
Sanger confirmation and classification of Cryptococcus neoformans from cultured-positive strain samples. (A) Fragments of ITS1 gene were amplified via PCR, separated by agarose gel electrophoresis and visualized by UV light. (B) Sanger sequencing of partial ITS-1 DNA fragment of sample No. 9. (C) Sanger sequencing of partial CAP59 DNA fragment of sample No. 9.
Discussion
Cryptococcus neoformans is the leading cause of fungal meningitis worldwide. Significant mortality and disability bring on considerable burden on health care and medical cost of patients. Timely provision of diagnosis and fungicidal treatment are urgently required, yet diagnosis of cryptococcus infection still remains challenges. Apart from the clinical presentation of cryptococcal disease, other existing diagnostic approaches including the detection of cryptococcal polysaccharide antigen in the body fluids (CSF and serum) by latex agglutination, enzyme-linked immunosorbent assay (ELISA) methods, India ink staining and fungal cultivation. While the latter two approaches are the golden standard of diagnosis. Despite considerably successful diagnosed cases,[14] there are still false-negative results, mainly occurring due to low organism burden or rare capsule-deficient strains.
Molecular-based diagnostics approaches have aided in advancing the diagnostic capabilities.[15] Since the first reported case of neuroleptospirosis in 2014,[6] the application of next-generation sequencing (NGS) technology for comprehensive detection of pathogens from CSF samples has aroused dramatic interest and brought about a revolutionary shift in medical microbiology. However, very few fungal pathogens were reported to be detected via the power of NGS, except for some studies on respiratory tract infection.[16] Related reports were also limited on the invasion of mycetes in CNS until some scientists found their existence in brain parenchyma with Alzheimer's disease.[17] To our knowledge, our research successfully applied NGS as a diagnostic tool for cryptococcal meningitis.
The most promising sequencing-based approaches for microbial detection include metataxonomics and metagenomics.[18] Metataxonomics refers to the deep-sequencing of marker genes, usually in the regions of ribosomal RNA (rRNA) genes that are highly conserved across the taxa, to figure out the microbial distribution or identification of yeast infection. In this research, we followed the proposal of Marchesi and Ravel[19] on terminology, and used the term “metataxonomics” for marker gene sequencing. rRNA gene fractions are the most widely used marker sequences, including the 16S rRNA gene for bacteria, the 18SrRNA gene for eukaryotes, and the ITS regions of the fungal ribosome for fungi.[8] For taxonomy of yeasts, it is the most commonly used technique to sequence ITS regions of rRNA, in particular ITS2 between the 5.8S and 28S genes, after amplification through PCR. ITS2 region is highly variable and has been adopted as the universal fungal barcode sequence for fungi.[8] Our study aimed at exploring the feasibility of metataxonomics of ITS amplicons in CSF for confirming cryptococcal infection. In our study, a large number of unique reads of cryptococcal genome were successfully detected in cryptococcal CSF samples. Consequently, we made efforts to refine the traditional workflow of DNA extraction of cryptococcus with capsules, and the detection limit of cryptococcal genomic DNA by metataxonomics in our laboratory was proved to be at least 0.5pg by testing the standard strain H99 at different concentrations. In other words, in terms of average fungal genome size of 10M, 20 to 30 fungal cells would satisfy the requirement of specimen capacity. With this incomparable advantage of increased analytical sensitivity, metagenomics/metataxomics as a diagnostic tool for CNS fungal infection has boundless prospects in microbiologic methodologies at age of Big Data. Meanwhile, it is time saving. Currently, the turn-around time for mNGS has been reported broadly from approximately 6 h to 7 days (average of 48 h).[20] Unlike traditional method of culture, metagenomics does not rely on growth amplification step, which often takes weeks or requires strict laboratory environment for fastidious fungi to grow.
Another important aspect for cryptococcal infection is genotype. Cryptococcus neoformans was divided into three variants by Kwon et al in 1982,[21] including C. neoformans var. grubii, C. neoformans var. neoformans, and C. neoformans var. gattii. During the late 20th century, it was divided into A, B, C, D, and AD serotypes.[22] At present, nine major molecular types have been recognized: VNI, VNII, VNB, VNIII, and VNIV among C. neoformans isolates, and VGI, VGII, VGIII, and VGIV among C. gattii isolates, presenting numerous differences in the geographical distribution, ecological niches, epidemiology, pathobiology, clinical presentation and molecular characters.[23] Numerous molecular techniques like PCR fingerprinting, AFLP, MLST have been applied to subtype C. neoformans and C. gattii strains. In 2004, Masakazu Katsua et al revealed that each variety of C. neoformans can be clearly differentiated based on its ITS sequence.[12] The ITS types were correlated to polymerase chain reaction fingerprint/random amplification of polymorphic DNA (RAPD) molecular types: ITS type 1 (ATACTAGC) = C. neoformans var. grubii, molecular types VNI+VNII and the serotype A allele of the AD hybrid, VNIIIA; ITS type 2 (ATATAGGC) = the serotype D allele of the AD hybrid, VNIIIB, and C. neoformans var. neoformans, VNIV; and ITS type 3 (GCGCTGGC) and ITS type 7 (ACGCTGGC) = VGI = RAPD type III, ITS type4 (ACACTGAC) = VGII = RAPD type II, ITS type 5: (ACACTGGG) = VGIII = RAPD type I, ITS type 6 (ACACTGGC) = VGIV = RAPD type IV, all corresponding to C. neoformans var. gattii. ITS sequencing is a useful technique for genotyping the three varieties of C. neoformans and for subtyping within C. neoformans var. gattii.
The limitation for all these methods is that they are based on the positive culture of strains. In our study, we attempted to classify directly the subtype of cryptococcus in CSF samples based on ITS amplification sequencing. Eleven samples were perfectly identified to ITS type 1, which were confirmed by PCR with Sanger method. The present study has shown the advantage of ITS gene sequencing approach over the conventional culture-based method, which was evidenced by pathogens that have been identified from the culture-negative CSF samples. Therefore, to the best of our knowledge, this is the first report of genotyping cryptococcus detected in CSF by amplicon sequencing and confirmed by culture and sanger technique. This study revealed further evidence for the delineation of the three varieties within the C. neoformans species complex by providing a fast and informative technique to identify C. neoformans isolates in clinical laboratories to the variety level, without relying on fungal culture.
Amplification sequencing and other NGS technologies are currently used mainly for complex environments, such as soil, water, mouth, excrement, or rumen. The microflora structure and abundance were studied and it can effectively reveal the species in these specific environments. CSF is traditionally considered a sterile body site, where any evidences of a microorganism are likely to represent infection. But now we found that CSF included other microorganisms in addition to the pathogenic microorganisms since the application of second NGS technology of CNS, although the microbial content was generally low. NGS was used to identify fungal species present in the CNS of AD patients in 2017.[17] Five fungi were common to all nine AD patients: Alternaria, Botrytis, Candida, Cladosporium, and Malassezia. Interestingly, a great variety of fungal species were also apparent into two control samples.
In our study, in addition to the significantly increased abundance of cryptococcus in the 11 cryptococcal infection cases, a variety of other fungal species were also found. The top three include Myrothecium roridum, Alternaria sp, and Guehomyces pullulans. Some are supposed to contaminate the laboratory but are still worth discussion. Meanwhile, we were unexpectedly found that cryptococcus with microreads was also detected in four groups of non-infectious control samples. Myrothecium roridum is a fungal pathogen of the plants that infects different crops, while there are no report that it can invade human beings and cause illness. So far, it is thought to be associated with laboratory contamination. Alternaria are plant pathogens that produce mycotoxins, and can be found in the nasal and oral cavities as well as in the skin, vagina, and conjunctiva.[24]Guehomyces pullulans grow on lactose cultures at below 5°C. It has been isolated from sea sediment in Antarctica and never reported in any human disease.
The mycobiome of the nasal and oral cavity might presumably constitute infectious reservoir that reaches the CNS through the terminal of the olfactory nerve and small wounds in the gums. Moreover, there is another possibility that fungi pass through the wounds in the skin or traverse the intestinal mucosae. In this regard, Candida, Cladosporium, and Cryptococcus are well-known human pathogens which able to infect the CNS.[20] Regarding this, we provide some bold assumptions. First, there are small amounts of microbial organisms in the CSF, which are traditionally considered sterile. These microbes are too few, also nonviable or uncultivable. Second, other fungi also enter the CNS through the altered blood-brain barrier after cryptococcal infection. Could this be one of the possible reasons which cause poor efficacy of antifungal drugs in some cryptococcal patients? These are all hypotheses that have to be confirmed in further research studies. However, there is limitation in this paper. The sample number is slightly small. In our future work, we will recruit more samples, especially other central nervous system infections.
In conclusion, this study highlights the feasibility of using metataxonomics by ITS of CSF as a diagnostic tool for CNS cryptococcal infections. This is the first study to use amplicon sequencing for studying cryptococcus. Additionally, the first time we performed diagnosis and genotyping of cryptococcus directly from CSF instead of relying on the positive culture of the strains. Meanwhile, we studied the composition of common contaminated fungi and colonized fungi in the CSF and the changes of fungal flora after cryptococcal infection. NGS techniques have impressively expanded our knowledge on the microbial world without cultivation of microorganisms and can overcome the shortage of culture-based approach.
Acknowledgements
The authors sincerely thank the patients that have contributed samples for the purposes of this study.
Funding
This work was supported by a grant from the Startup Fund for Scientific Research, Fujian Medical University (No. 2017XQ1066).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Zhu JT, Lin H, Wu X, Li ZW, Lin AY. Metataxonomics of internal transcribed spacer amplicons in cerebrospinal fluid for diagnosing and genotyping of cryptococcal meningitis. Chin Med J 2019;132:2827–2834. doi: 10.1097/CM9.0000000000000541
Ji-Ting Zhu and Han Lin contributed equally to this work.
References
- 1.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009; 23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Saha DC, Xess I, Jain N. Evaluation of conventional & serological methods for rapid diagnosis of cryptococcosis. Indian J Med Res 2008; 127:483–488. [PubMed] [Google Scholar]
- 3.Thiruchelvan N, Wuu KY, Arseculeratne SN, Ashraful-Haq J. A pseudo-cryptococcal artefact derived from leucocytes in wet India ink mounts of centrifuged cerebrospinal fluid. J Clin Pathol 1998; 51:246–248. doi: 10.1136/jcp.51.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berlin L, Pincus JH. Cryptococcal meningitis. False-negative antigen test results and cultures in nonimmunosuppressed patients. Arch Neurol 1989; 46:1312–1316. doi: 10.1001/archneur.1989.00520480054020. [DOI] [PubMed] [Google Scholar]
- 5.Zhou M, Wang P, Chen S, Du B, Du J, Wang F, et al. Meningitis in a Chinese adult patient caused by Mycoplasma hominis: a rare infection and literature review. BMC infect dis 2016; 16:557.doi: 10.1186/s12879-016-1885-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson MR, Naccache SN, Samayoa E, Biagtan M, Bashir H, Yu G, et al. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. New Engl J Med 2014; 370:2408–2417. doi: 10.1056/NEJMoa1401268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padmanabhan R, Mishra AK, Raoult D, Fournier PE. Genomics and metagenomics in medical microbiology. J Microbiol Methods 2013; 95:415–424. doi: 10.1016/j.mimet.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci U S A 2012; 109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martins Mdos A, Brighente KB, Matos TA, Vidal JE, Hipolito DD, Pereira-Chioccola VL. Molecular diagnosis of cryptococcal meningitis in cerebrospinal fluid: comparison of primer sets for Cryptococcus neoformans and Cryptococcus gattii species complex. Braz J Infect Dis 2015; 19:62–67. doi: 10.1016/j.bjid.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghannoum MA, Jurevic RJ, Mukherjee PK, Cui F, Sikaroodi M, Naqvi A, et al. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog 2010; 6:e1000713.doi: 10.1371/journal.ppat.1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 2013; 10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 12.Katsu M, Kidd S, Ando A, Moretti-Branchini ML, Mikami Y, Nishimura K, et al. The internal transcribed spacers and 5.8S rRNA gene show extensive diversity among isolates of the Cryptococcus neoformans species complex. FEMS Yeast Res 2004; 4:377–388. doi: 10.1016/s1567-1356(03)00176-4. [DOI] [PubMed] [Google Scholar]
- 13.Mongkolrattanothai K, Naccache SN, Bender JM, Samayoa E, Pham E, Yu G, et al. Neurobrucellosis: unexpected answer from metagenomic next-generation sequencing. J Pediatric Infect Dis Soc 2017; 6:393–398. doi: 10.1093/jpids/piw066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen SC, Slavin MA, Heath CH, Playford EG, Byth K, Marriott D, et al. Clinical manifestations of Cryptococcus gattii infection: determinants of neurological sequelae and death. Clin Infect Dis 2012; 55:789–798. doi: 10.1093/cid/cis529. [DOI] [PubMed] [Google Scholar]
- 15.Zhang SX. Enhancing molecular approaches for diagnosis of fungal infections. Future Microbiol 2013; 8:1599–1611. doi: 10.2217/fmb.13.120. [DOI] [PubMed] [Google Scholar]
- 16.Langelier C, Zinter MS, Kalantar K, Yanik GA, Christenson S, O’Donovan B, et al. Metagenomic sequencing detects respiratory pathogens in hematopoietic cellular transplant patients. Am J Respir Crit Care Med 2018; 197:524–528. doi: 10.1164/rccm.201706-1097LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alonso R, Pisa D, Aguado B, Carrasco L. Identification of fungal species in brain tissue from Alzheimer's disease by next-generation sequencing. J Alzheimers Dis 2017; 58:55–67. doi: 10.3233/jad-170058. [DOI] [PubMed] [Google Scholar]
- 18.Breitwieser FP, Lu J, Salzberg SL. A review of methods and databases for metagenomic classification and assembly. Brief Bioinformatics 2017; 20:1–15. doi: 10.1093/bib/bbx120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchesi JR, Ravel J. The vocabulary of microbiome research: a proposal. Microbiome 2015; 3:31.doi: 10.1186/s40168-015-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simner PJ, Miller S, Carroll KC. Understanding the promises and hurdles of metagenomic next-generation sequencing as a diagnostic tool for infectious diseases. Clin Infect Dis 2018; 66:778–788. doi: 10.1093/cid/cix881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon-Chung KJ, Bennett JE, Rhodes JC. Taxonomic studies on Filobasidiella species and their anamorphs. Antonie van Leeuwenhoek 1982; 48:25–38. doi: 10.1007/bf00399484. [DOI] [PubMed] [Google Scholar]
- 22.Lengeler KB, Cox GM, Heitman J. Serotype AD strains of Cryptococcus neoformans are diploid or aneuploid and are heterozygous at the mating-type locus. Infect Immun 2001; 69:115–122. doi: 10.1128/iai.69.1.115-122.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cogliati M. Global molecular epidemiology of cryptococcus neoformans and cryptococcus gattii: an atlas of the molecular types. Scientifica 2013; 2013:675213.doi: 10.1155/2013/675213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solhaug A, Eriksen GS, Holme JA. Mechanisms of action and toxicity of the mycotoxin alternariol: a review. Basic Clin Pharmacol Toxicol 2016; 119:533–539. doi: 10.1111/bcpt.12635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


