Atopic dermatitis (AD) is an inflammatory skin disease characterized by chronic recurrent dermatitis with profound pruritus. Most patients have personal and/or family history of atopic diseases.[1] Allergy to air allergens and food allergens was regarded as an important feature of AD. There have been few studies for contact sensitization in patients with AD. The question remains if patch testing to a greater extent should be used as a screening tool in AD patients, to exclude hidden allergies possibly maintaining or aggravating their skin symptoms. In this study, we compared patch test results between patients with AD and non-AD patients including patients with facial dermatitis, eczema, psoriasis, pruritus and urticaria, and including some healthy population.
A total of 988 patients underwent patch testing. The median age of the study population was 36 years old (range: 7–90 years old), 10.3% of patients were AD. Atopic status (dermatitis, asthma, allergic rhinitis) was assessed in all patients; the diagnosis of AD was established using the Chinese criteria.[1] Patch testing was performed with the Chinese Baseline Series and IQ chamber (Chemotechnique Diagnostics, Malmo ¨, Sweden). The patch test was applied on the upper back for 48 h. And the results were recorded on day 2 and day 7 according to the International Contact Dermatitis Research Group. The incidence of contact sensitization to any allergens in patients with AD (n = 102) and non-AD patients (n = 886) was assessed. Variables not normally distributed were analyzed using t test. The differences in the positivity rate between two groups were analyzed by using the Pearson Chi-square test. A P value <0.05 was considered to indicate statistical significance. Statistical analysis was carried out using SPSS 20.0 (IBM SPSS, Armonk, New York, USA). The Medical Ethics Committee of the Peking University People's Hospital, China approved the study. The study was conducted in accordance with the Declaration of Helsinki and all the study participants provided written informed consent.
Of the 988 patients patch tested, 10.3% (n = 102) had a history of AD. Among the 146 male patients enrolled in the study, the rate of AD was 19.2% (n = 28), and in the 842 female population, the rate of AD was 8.8% (n = 74). There is no significant difference between AD and AD patients.
A total of 78.4% patients with AD were positive to at least one allergen, which was higher than that in non-AD patients (66.8%) [Table 1]. The top three contact allergens in AD patients were Nickel (II) sulfate hexahydrate (33.3%), followed by Cobalt (II) chloride hexahydrate and methylisothiazolinone. And the highest rate of positive allergen in non-AD patients were methylisothiazolinone (15.7%), Nickel (II) sulfate hexahydrate (11.4%) and mercapto mix (10.8%). A total of 33.3% of AD patients had a positive response to nickel sulfate, significantly higher than non-AD patients (P < 0.01); 19.6% of AD patients were positive for cobalt chloride, higher than non-AD patients (9.3%, P < 0.01); 22.5% AD patients and 15.7% non-AD patients had a positive response to methylisothiazolinone (P > 0.05), and 6.9% AD patients and 5.9% non-AD patients were positive to diazolidinylurea (P > 0.05).
Table 1.
Common metal and preservative allergens and their rates of positive patch tests among atopic and non-atopic population, stratified by gender.
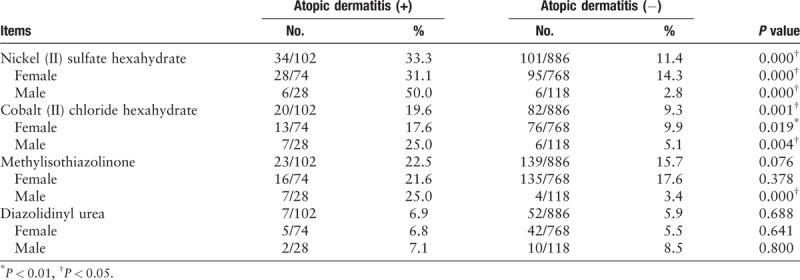
It is known that genetic susceptibility is essential for AD. Null mutations in the filaggrin (FLG) gene were identified as strong genetic risk factors for AD. However, the exact mechanism by which FLG interacts with nickel in the stratum corneum is unknown. It has been speculated that the histidine-rich polypeptide FLG chelates nickel in the epidermis and creates a reservoir that prevents free ions from permeating the skin, where they may induce sensitization or elicitation. This mechanism would explain the findings of in our study that AD predisposes to reactions to other metallic allergens, such as cobalt. Contact sensitization to cobalt chloride was significantly higher in AD patients. Cobalt was named allergen of the year for 2016. Since then, the potential exposure to cobalt and the contact allergy have come into focus. Cobalt allergy was linked to concomitant exposure to nickel. However, it is increasingly recognized as an independent sensitizer.[2] Moreover, in a previous study, the isolated reactions to cobalt chloride were found in AD patients.[3] Sources of exposure to cobalt are often difficult to identify. Recent reports have called attention to new cobalt exposure sources among adult patients such as furniture, laptop computers, and cosmetics, and in children include jewelry, buttons, zippers, snaps, leather shoes, and laptop computers. A previous study found that 50% of patch testing was to metals in pediatric patients with AD.[4] Cobalt chloride is a strong sensitizer and is considered a difficult substance to use for testing, as it may result in an irritant or doubtful reaction.[5] However, exposure to irritants and a history of irritant dermatitis may also be a risk factor for development of allergic contact dermatitis. Irritants can breakdown the skin barrier, allowing allergens to interact with the immune system. Because of this, the association between AD and a positive patch test result reported in this study suggest but do not confirm a relationship between AD and cobalt chloride.
More emollients use in Chinese AD patients, might lead to an increase in contact hypersensitivity to preservatives. Parabens, including methyl, ethyl, propyl, and butyl parabens used alone or in combination are the most common preservatives. As atopic patients usually use creams, lotions, and other products containing biocides, which may increase preservative allergy in these patients.
Funding
This study was supported by a grant from the National Natural Science Foundation of China (No. 81472902).
Conflicts of interest
None.
Footnotes
How to cite this article: Peng F, Schwartz RA, Chen Z, Zhang JZ. High prevalence of contact hypersensitivity to metals and preservatives in Chinese patients with atopic dermatitis. Chin Med J 2019;132:2881–2882. doi: 10.1097/CM9.0000000000000526
References
- 1.Liu P, Zhao Y, Mu ZL, Lu QJ, Zhang L, Yao X, et al. Clinical features of adult/adolescent atopic dermatitis and chinese criteria for atopic dermatitis. Chin Med J 2016; 129:757–762. doi: 10.4103/0366-6999.178960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thyssen JP, Linneberg A, Engkilde K, Menne T, Johansen JD. Contact sensitization to common haptens is associated with atopic dermatitis: new insight. Br J Dermatol 2012; 166:1255–1261. doi: 10.1111/j.1365-2133.2012.10852.x. [DOI] [PubMed] [Google Scholar]
- 3.Thyssen JP, Menné T, Lidén C, Julander A, Jensen P, Jakobsen SS, et al. Cobalt release from implants and consumer items and characteristics of cobalt sensitized patients with dermatitis. Contact Dermatitis 2012; 66:113–122. doi: 10.1111/j.1600-0536.2011.02001.x. [DOI] [PubMed] [Google Scholar]
- 4.Simonsen AB, Duus JJ, Deleuran M, Mortz CG, Skiv L, Sommerlund M, et al. Children with atopic dermatitis may have unacknowledged contact allergies contributing to their skin symptoms. J Eur Acad Dermatol Venereol 2018; 32:428–436. doi: 10.1111/jdv.14737. [DOI] [PubMed] [Google Scholar]
- 5.Rystedt I. Evaluation and relevance of isolated test reactions to cobalt. Contact Dermatitis 1979; 5:233–238. doi: 10.1111/j.1600-0536.1979.tb04857.x. [DOI] [PubMed] [Google Scholar]


