Abstract
Background:
Endostatin, a biologically active fragment of collagen XVIII, has been observed in patients with ischemic heart disease. The aim of the present study was to investigate whether endostatin overexpression could attenuate cardiac hypertrophy by inhibiting the cyclic adenosine monophosphate-protein kinase A (cAMP-PKA) signaling pathway.
Methods:
This study was examined in vivo in rats and in vitro in primary neonatal rat cardiomyocytes treated with angiotensin (Ang) II to model cardiac hypertrophy. Twenty-four male Sprague-Dawley rats were randomized into adenovirus (Ad)-green fluorescent protein, Ang II, Ad-endostatin, and Ang II + Ad-endostatin groups (n = 6 in each group). Four weeks later, all the rats were weighed and sacrificed after transthoracic echocardiography. Cardiac function was evaluated by transthoracic echocardiography, cardiomyocyte size was evaluated by hematoxylin-eosin staining. Levels of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) were evaluated by quantitative reverse-transcription polymerase chain reaction or Western blotting, PKA level was evaluated by Western blotting, and cAMP level was evaluated by enzyme-linked immunosorbent assay. Statistical significance among multiple groups was evaluated by one-way analysis of variance.
Results:
Endostatin overexpression reduced the increases in left ventricle (LV) mass (P = 0.0063), LV mass/body weight (BW) (P = 0.0013), interventricular septal thickness (IVS) in diastole (P = 0.0013), IVS in systole (P = 0.0056), left ventricular posterior wall thickness (LVPW) in diastole (P = 0.0291), LVPW in systole (P = 0.0080), heart weight (HW) (P = 0.0138), HW/BW (P = 0.0001), and HW/tibial length (P = 0.0372) in Ang II-treated rats. In addition, endostatin overexpression reduced cardiomyocyte cross-sectional area expansion, and reduced the levels of ANP and BNP in Ang II-treated rats (P = 0.0251 and 0.0477 for messenger RNA [mRNA]), and primary neonatal rat cardiomyocytes (P = 0.0188 and P = 0.0024 for mRNA; P = 0.0023 and 0.0013 for protein, respectively). Additionally, endostatin overexpression reduced the increase of cAMP (P = 0.0054) and PKA (P = 0.0328) levels in cardiomyocytes treated with Ang II. Treatment with cAMP reversed the effects of endostatin overexpression on ANP (P = 0.0263) and BNP (P = 0.0322) levels in cardiomyocytes induced by Ang II.
Conclusion:
Endostatin overexpression could alleviate cardiac hypertrophy by inhibiting the cAMP-PKA signaling pathway.
Keywords: Endostatin, Cardiac hypertrophy, Cardiomyocytes, Cyclic adenosine monophosphate, Protein kinase A
Introduction
Cardiovascular diseases remain a major cause of death worldwide.[1] Cardiac remodeling has garnered considerable attention as an important pathophysiologic process during cardiovascular disease. Myocardial fibrosis and hypertrophy are two of the most important changes that are observed during cardiac remodeling.[2] Cardiac hypertrophy is an important adaptive response to pathological stimuli, including myocardial infarction, hypertension, pressure overload, and the activation of the renin-angiotensin system.[3] Cardiac hypertrophy is characterized by increased heart weight (HW) and cell size, and increase in gene expression, such as atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP).[4]
Endostatin, a 20,000 C-terminal fragment derived from Collagen XVIII, is a potent inhibitor of angiogenesis as demonstrated in both in vitro and in vivo studies.[5,6] In addition, endostatin is involved in multiple physiological and pathological processes including sepsis, acute kidney injury, and fibrosis.[7–9] Whether endostatin is involved in cardiac hypertrophy is yet to be deciphered.
Cyclic adenosine monophosphate-protein kinase A (cAMP-PKA) is a threonine/serine kinase consisting of two regulatory and two catalytic sub-units, each of those is capable of binding two cAMP molecules. After binding to cAMP, specific conformation change in the regulatory sub-unit occurs, resulting in the activation and release of the catalytic sub-units. PKA levels in the heart are significantly increased during cardiac hypertrophy as demonstrated in rat models.[10] However, the downstream signaling pathway of endostatin on attenuating cardiac hypertrophy is unknown. Therefore, we hypothesized that endostatin attenuates cardiac hypertrophy by inhibiting PKA signaling pathway. The present study was performed to investigate the effects of endostatin on cardiac hypertrophy induced by angiotensin (Ang) II and the downstream signaling pathways.
Methods
Ethical approval
Male Sprague-Dawley (SD) rats (Vital River Biological Co., Ltd, Beijing, China) weighing 160 to 180 g were used in this study. Rats were housed in a temperature-controlled room with 12 to 12 h light-dark cycle with free access to standard chow and tap water. All procedures were approved by the Experimental Animal Care and Use Committee of Nanjing Medical University and performed in accordance with the Guidelines for the Care and Use of Laboratory Animals (NIH publication No. 85-23, revised 1996). Every effort was made to minimize the number of animals and reduce their suffering.
Animal grouping
Twenty-four rats were randomized into four groups: adenovirus-green fluorescent protein (Ad-GFP), Ang II, Ad-endostatin, and Ang II + Ad-endostatin groups (n = 6 in each group). Rats in the Ad-GFP group were subjected to a 4-week infusion of saline (Nanjing Biochannel Biotechnology Co., Ltd, Nanjing, China) using mini-osmotic pumps (ALZET Osmotic Pumps, Cupertino, CA, USA), those were surgically implanted below the neck, with adenovirus expressing GFP (108 TU/mL, 100 μL; Genechem, Shanghai, China) administered via tail vein injection; rats in the Ang II group were subjected to a 4-week infusion of Ang II (500 ng·kg–1·d–1; Sigma, St. Louis, MO, USA) using mini-osmotic pumps, with 100-μL saline administered via tail vein injection; rats in the Ad-endostatin group were subjected to a 4-week infusion of saline using mini-osmotic pumps, with adenovirus expressing both GFP and endostatin (108 TU/mL, 100 μL) administered via tail vein injection; and rats in the Ang II + Ad-endostatin group were subjected to a 4-week infusion of Ang II (500 ng·kg–1·d–1) using mini-osmotic pumps, with adenovirus expressing both GFP and endostatin (108 TU/mL, 100 μL) administered via tail vein injection.
After transthoracic echocardiography, rats were weighed and then sacrificed with an overdose of pentobarbital (100 mg/kg, i.v.). The heart was weighed and the tibial length (TL) was measured. A part of the left ventricle (LV) was sectioned for hematoxylin-eosin (HE) staining and the remaining tissue was used for quantitative reverse-transcription polymerase chain reaction (qRT-PCR).
Echocardiography
After 4-weeks of endostatin overexpression, transthoracic echocardiography was performed under isoflurane anesthesia using an ultrasound (Vevo 2100; VisualSonics, Toronto, Canada) with a 21-MHz probe. The LV mass, interventricular septal thickness in diastole (IVSd), interventricular septal thickness in systole (IVSs), left ventricular posterior wall thickness in diastole (LVPWd), and left ventricular posterior wall thickness in systole (LVPWs) were measured. The LV mass to body weight (BW) ratio was calculated. Measurements over three consecutive cardiac cycles were then averaged.
HE staining
Heart sections (5 μm) were examined after HE staining (Nanjing Biochannel Biotechnology Co., Ltd.) to measure the cross-sectional area of cardiomyocytes. Three to five random fields were selected from each of the three sections from each animal and observed under a light microscope (Olympus Corporation, Tokyo, Japan). Images were then acquired and analyzed using the Image-Pro Plus software (Media Cybernetics, Inc., Rockville, MD, USA).
Masson staining
Cardiac sections (5 μm) were examined by Masson staining (Nanjing Biochannel Biotechnology Co., Ltd.) according to the manufacture instruments to measure the fibrosis of cardiomyocytes. Three to five random fields (around 30–50 cells per field) were selected from each of three sections from each animal for observation under a light microscope (Carl Zeiss GmbH, Oberkochen, Germany). Images were analyzed using Image-Pro Plus software (Media Cybernetics, Inc.).
Culture of cardiomyocytes isolated from neonatal rats
Primary cardiomyocytes were isolated from 1- to 2-day-old newborn SD rats (Vital River Biological Co.).[11] Hearts were excised and digested with collagenase type II (Worthington Biochemical Corp., Lakewood, NJ, USA) and pancreatin (Sigma) in phosphate buffer saline (PBS). The atria and great vessels were discarded. The ventricles were cut into small pieces and further digested with collagenase type II and pancreatin. Cells harvested after digestion, and then collected and cultured in Complete Dulbecco modified Eagle medium (Nanjing Biochannel Biotechnology Co., Ltd.) supplemented with 10% fetal bovine serum (Nanjing Biochannel Biotechnology Co., Ltd.), 1% penicillin, and 1% streptomycin for 2 to 4 h to reduce fibroblasts. The cardiomyocytes were then cultured at 37°C with 5% CO2. Ang II (10−6 mol/L) was used to induce cardiomyocytes hypertrophy.
Western blotting analysis
Cultured cells were sonicated in radio immunoprecipitation assay (RIPA) lysis buffer and homogenized. Cell debris was removed by centrifugation at 4°C for 10 min at 12,000 × g and the supernatants were collected. Approximately 30 to 50 μg of protein was separated by gel electrophoresis, transferred to a polyvinylidene-fluoride membrane, and probed with primary antibodies against ANP and BNP (Abcam, San Francisco, MA, USA), and PKA (Cell Signaling Technology, Danvers, MA, USA); and glyceraldehyde-3-phosphate dehydrogenase (Abcam) as an internal control. Images were analyzed using Image-Pro Plus software.
qRT-PCR
RNA was isolated from heart tissues or cultured cells using Trizol (Invitrogen Inc., Waltham, CA, USA). Total RNA (0.5 μg) was reverse transcribed to complementary DNA. qRT-PCR was performed using an ABI Prism 7000 sequencer (Applied Biosystems, Waltham, CA, USA). Primer sequences are shown in Table 1. The relative level of target messenger RNA (mRNA) expression was expressed as 2−ΔΔCt.
Table 1.
List of utilized primers for qRT-PCR.

Measurement of cAMP levels
Primary cardiomyocytes were homogenized in lysis buffer. Total protein in the homogenate was extracted and measured using the protein bicinchoninic acid assay kit (Thermo Fisher Scientific, Waltham, MA, USA). The cAMP levels in cardiomyocytes were determined using an enzyme immunoassay kit (R&D Systems Inc., Minneapolis, MN, USA) following the manufacturer's instructions.
Immunofluorescence
Cardiomyocytes were fixed with 4% paraformaldehyde for 15 min at room temperature and incubated with a blocking solution consisting of 1% bovine serum albumin for 1 h. The cells were incubated with α-actinin (Abcam) at 4°C overnight and the following day washed three times in PBS and incubated in secondary antibodies (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) for 2 h at room temperature. Then, 4′,6-diamidino-2-phenylindole (Life Technology, Waltham, CA, USA) was counterstained of the nucleus. Fluorescent cell imaging was achieved using a microscope (ZEISS, Germany).
Statistical analysis
Data were presented as mean ± standard error. Statistical significance among multiple groups was evaluated by one-way analysis of variance with Bonferroni post-hoc test using GraphPad Prism 7.0 (GraphPad Software Inc., San Diego, CA, USA). Two-tailed P-value <0.05 was considered statistically significant.
Results
Endostatin overexpression attenuates Ang II-induced cardiac hypertrophy in rats
Echocardiography showed that Ang II administration in rats increased LV mass (P = 0.0019), LV/BW (P = 0.0012), IVSd (P = 0.0001), IVSs (P = 0.0008), LVPWd (P = 0.0002), and LVPWs (P = 0.0007). Endostatin overexpression attenuated the increase in LV mass (P = 0.0063), LV/BW (P = 0.0013), IVSd (P = 0.0013), IVSs (P = 0.0056), LVPWd (P = 0.0291), and LVPWs (P = 0.0080) in rat hearts induced by Ang II administration [Figure 1].
Figure 1.
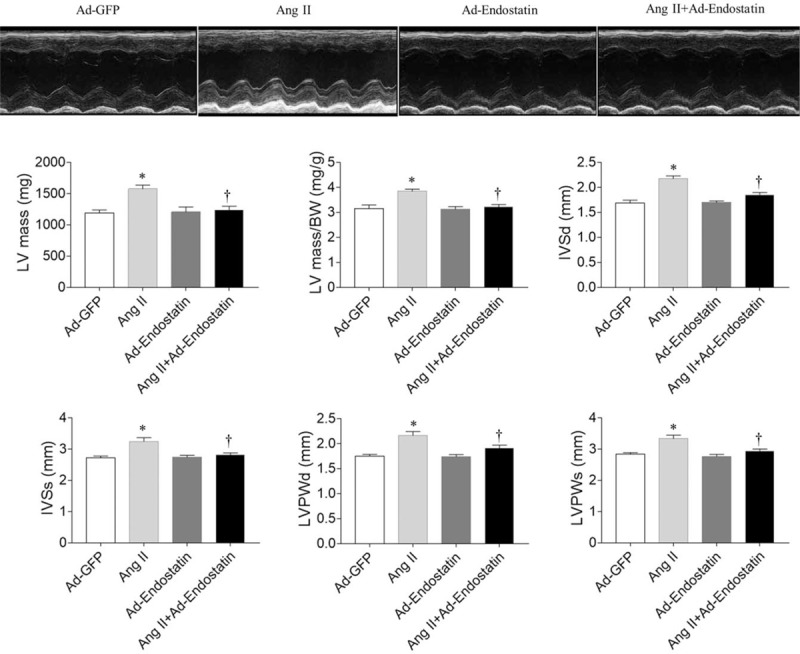
Effects of endostatin overexpression on cardiac hypertrophy in Ang II-treated rats under transthoracic echocardiography. Endostatin overexpression attenuated the increases in LV mass, LV mass/BW, IVSd, IVSs, LVPWd, LVPWs in Ang II-treated rats. Results are expressed as mean ± SE, n = 6. ∗P < 0.05 vs. the Ad-GFP group; †P < 0.05 vs. the Ad-endostatin group. Ad-GFP group: Rats treated with adenovirus expressing green fluorescent protein; Ang II group: Rats treated with Ang II; Ad-endostatin group: Rats treated with adenovirus expressing endostatin; Ang II + Ad-endostatin group: Rats treated with Ang II and adenovirus expressing endostatin. Ad-GFP: Adenovirus-green fluorescent protein; Ang II: Angiotensin II; BW: Body weight; IVSd: Interventricular septal thickness in diastole; IVSs: Interventricular septal thickness in systole; LV: Left ventricle; LVPWd: Left ventricular posterior wall thickness in diastole; LVPWs: Left ventricular posterior wall thickness in systole; SE: Standard error.
Endostatin overexpression attenuated the increase in HW (P = 0.0138), HW/BW (P = 0.0001), and HW/TL (P = 0.0372) in rat hearts induced by Ang II administration [Figure 2A]. Enlarged heart and thickened ventricular wall were obviously observed in Ang II-induced cardiac hypertrophy model, which was reversed by endostatin overexpression [Figure 2B and 2C]. HE staining demonstrated that Ang II increased the size of cardiomyocytes in rat hearts (P = 0.0001), and endostatin overexpression reduced the increase in cardiomyocyte size of rat hearts induced by Ang II administration (P = 0.0070) [Figure 2C]. ANP and BNP levels in rat hearts were increased after Ang II administration (P = 0.0012 and P = 0.0099, respectively), but were reduced by endostatin overexpression (P = 0.0251 and P = 0.0477, respectively) [Figure 2D].
Figure 2.
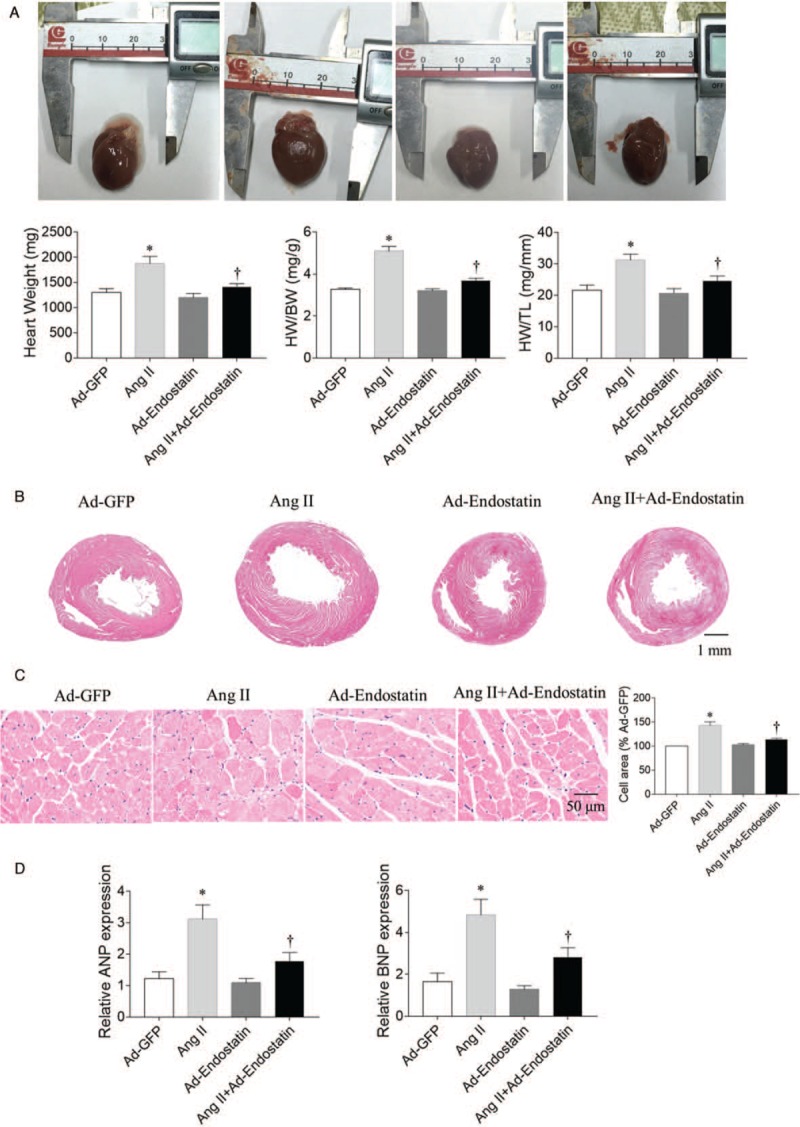
Effects of endostatin overexpression on cardiac hypertrophy in Ang II-treated rats. (A) Endostatin overexpression attenuated the increases in HW, HW/BW and HW/TL in Ang II-treated rats. (B) Representative photographs with heart tissue sections in four groups (hematoxylin-eosin staining, bar: 1 mm). (C) Endostatin overexpression attenuated the increase in cardiomyocyte cross-sectional area in Ang II-treated rats (hematoxylin-eosin staining, bar: 50 μm). (D) Endostatin overexpression reduced the increase in ANP and BNP mRNA levels in Ang II-treated rats. Results are expressed as mean ± SE, n = 6. ∗P < 0.05 vs. the Ad-GFP group; †P < 0.05 vs. the Ad-endostatin group. Ad-GFP group: Rats treated with adenovirus expressing green fluorescent protein; Ang II group: Rats treated with Ang II; Ad-endostatin group: Rats treated with adenovirus expressing endostatin; Ang II + Ad-endostatin group: Rats treated with Ang II and adenovirus expressing endostatin. Ad-GFP: Adenovirus-green fluorescent protein; Ang II: Angiotensin II; ANP: Atrial natriuretic peptide; BNP: Brain natriuretic peptide; BW: Body weight; HW: Heart weight; mRNA: Messenger RNA; TL: Tibial length; SE: Standard error.
Endostatin overexpression attenuates Ang II-induced cardiac fibrosis in rats
Masson staining demonstrated that cardiac fibrosis was significantly increased in Ang II treated rats (P = 0.0001). Endostatin overexpression reduced the increase in cardiac fibrosis induced by Ang II (P = 0.0003) [Figure 3].
Figure 3.
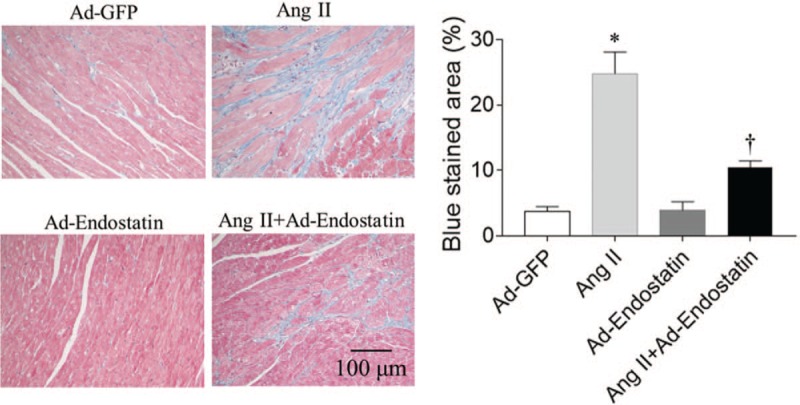
Effects of endostatin overexpression on cardiac fibrosis in Ang II-treated rats (Masson staining, bar: 100 μm). Endostatin overexpression attenuated the increase in cardiac fibrosis levels in Ang II-treated rats. Results are expressed as mean ± SE, n = 6. ∗P < 0.05 vs. the Ad-GFP group; †P < 0.05 vs. the Ad-endostatin group. Ad-GFP group: Rats treated with adenovirus expressing green fluorescent protein; Ang II group: Rats treated with Ang II; Ad-endostatin group: Rats treated with adenovirus expressing endostatin; Ang II + Ad-endostatin group: Rats treated with Ang II and adenovirus expressing endostatin. Ad-GFP: Adenovirus-green fluorescent protein; Ang II: Angiotensin II; SE: Standard error.
Endostatin overexpression attenuates Ang II-induced hypertrophy in primary cardiomyocytes
Ang II administration increased the size of primary cardiomyocytes (P = 0.0002), while endostatin overexpression reduced the increase induced by Ang II treatment (P = 0.0190) [Figure 4A]. The levels of ANP and BNP mRNA (P = 0.0020 and P = 0.0001, respectively) and protein (P = 0.0022 and P = 0.0002, respectively) in primary cardiomyocytes were increased after Ang II treatment, but the increases in mRNA (P = 0.0188 and P = 0.0024, respectively) and protein (P = 0.0023 and P = 0.0013, respectively) of ANP and BNP were reduced by endostatin overexpression [Figure 4B].
Figure 4.
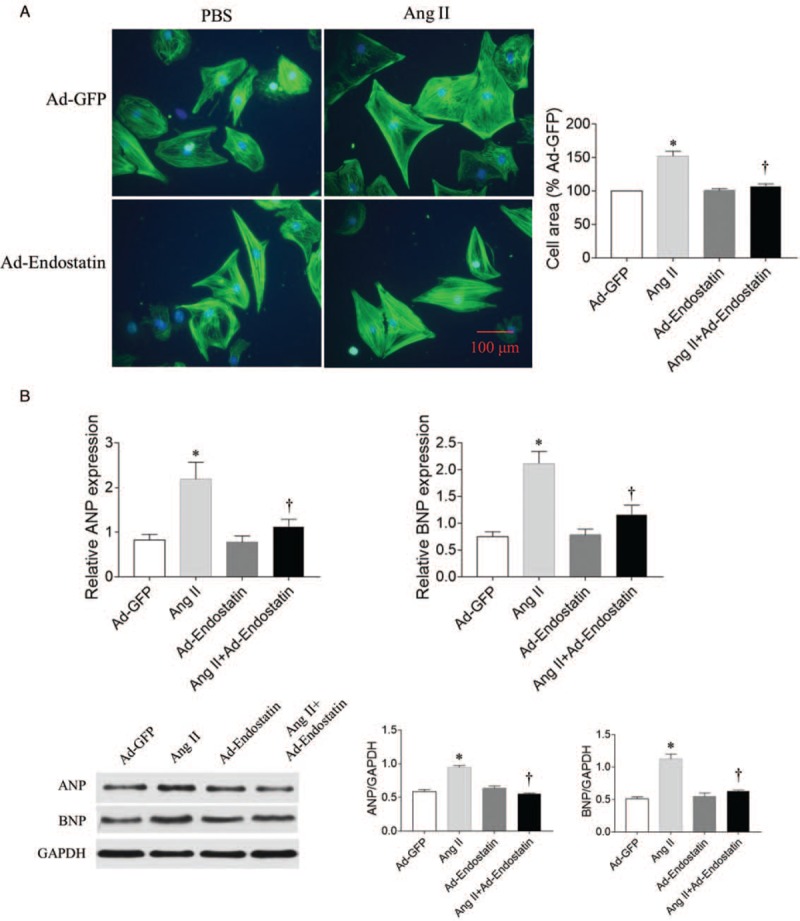
Effects of endostatin overexpression on hypertrophy in Ang II-treated primary cardiomyocytes. (A) Endostatin overexpression attenuated the increase in cardiomyocyte size in Ang II-treated cardiomyocytes (Immunofluorescence, Bar: 100 μm). (B) Endostatin overexpression reduced the increase in ANP and BNP mRNA and protein levels in Ang II-treated cardiomyocytes. Results are expressed as mean ± SE, n = 6. ∗P < 0.05 vs. the Ad-GFP group; †P < 0.05 vs. the Ad-endostatin group. Ad-GFP group: Cardiomyocytes treated with adenovirus expressing green fluorescent protein; Ang II group: Cardiomyocytes treated with Ang II; Ad-endostatin group: Cardiomyocytes treated with adenovirus expressing endostatin; Ang II + Ad-endostatin group: Cardiomyocytes treated with Ang II and adenovirus expressing endostatin. Ad-GFP: Adenovirus-green fluorescent protein; Ang II: Angiotensin II; ANP: Atrial natriuretic peptide; BNP: Brain natriuretic peptide; mRNA: Messenger RNA; SE: Standard error.
cAMP-PKA signaling pathway
cAMP levels were increased in Ang II treated cardiomyocytes (P = 0.0002), while endostatin overexpression reduced the increase of cAMP levels induced by Ang II in primary cardiomyocytes (P = 0.0054). PKA expression was increased in Ang II treated cardiomyocytes (P = 0.0207), while endostatin overexpression reduced the increase of PKA levels induced by Ang II in primary cardiomyocytes (P = 0.0328) [Figure 5A]. The effect of endostatin overexpression on reducing the increase in ANP and BNP levels induced by Ang II were reversed by cAMP administration (P = 0.0263 and P = 0.0322, respectively) [Figure 5B].
Figure 5.
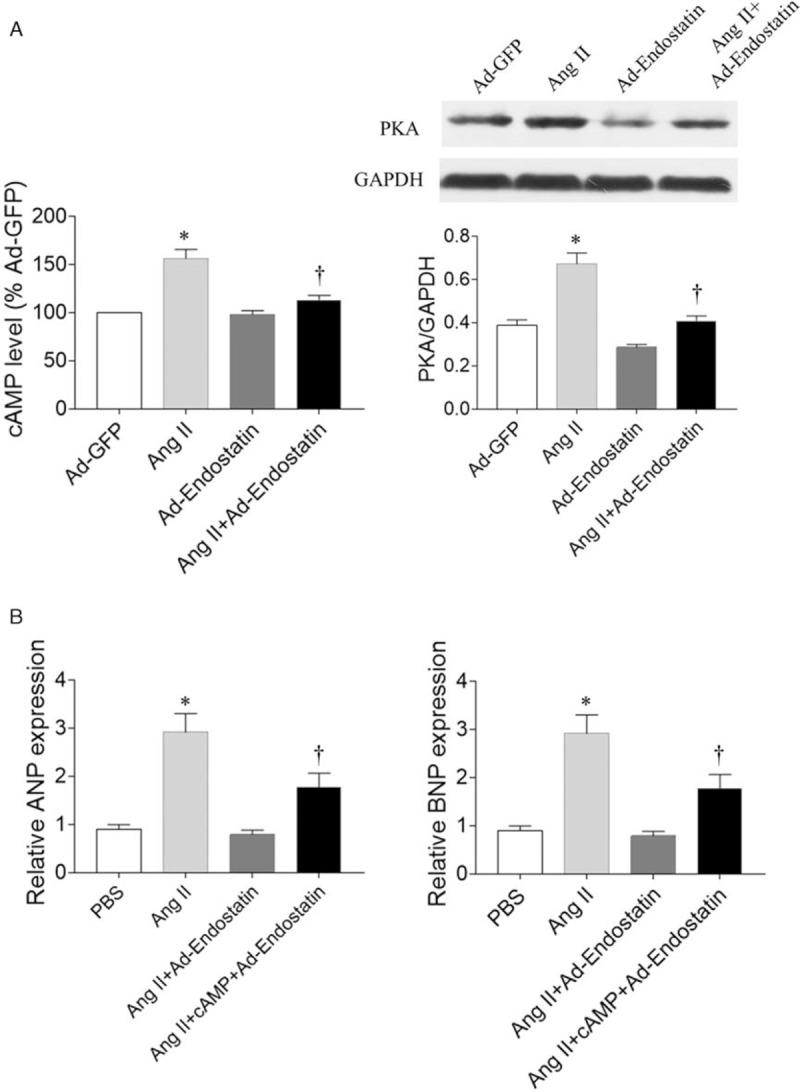
Signaling pathway in primary cardiomyocytes. (A) Endostatin overexpression reduced the increase in cAMP and PKA levels in Ang II-treated cardiomyocytes. (B) Treatment with cAMP reversed the effects of endostatin overexpression by reducing the increase in ANP and BNP levels in cardiomyocytes induced by Ang II. Results are expressed as mean ± SE, n = 6. ∗P < 0.05 vs. the Ad-GFP group (A) or PBS group (B); †P < 0.05 vs. the Ang II group (A) or Ang II + Ad-endostatin group (B). Ad-endostatin group: Cardiomyocytes treated with adenovirus expressing endostatin; Ad-GFP group: Cardiomyocytes treated with adenovirus expressing green fluorescent protein; Ang II group: Cardiomyocytes treated with Ang II; Ang II + Ad-endostatin group: Cardiomyocytes treated with Ang II and adenovirus expressing endostatin; Ang II + cAMP + Ad-endostatin group: Cardiomyocytes treated with Ang II, cAMP, and adenovirus expressing endostatin. Ad-GFP: Adenovirus-green fluorescent protein; Ang II: Angiotensin II; ANP: Atrial natriuretic peptide; BNP: Brain natriuretic peptide; cAMP: Cyclic adenosine monophosphate; PBS: Phosphate buffer saline; PKA: Protein kinase A; SE: Standard error.
Discussion
Cardiac hypertrophy is characterized by increased HW and cell size, and increased gene expression.[12–14] In the present study, cardiac hypertrophy induced by Ang II in rats was attenuated by endostatin overexpression. Endostatin overexpression attenuated primary cardiomyocytes hypertrophy induced by Ang II treatment by inhibiting the cAMP-PKA signaling pathway.
Myocardial hypertrophy and fibrosis are two of the most important changes that are observed during cardiac remodeling.[2,15] Higher serum endostatin levels have been associated with left ventricular dysfunction and an increased risk of heart failure.[16] Endostatin serum levels are significantly elevated in patients with heart failure with reduced ejection fraction compared to controls.[17] In the present study, we demonstrated that endostatin overexpression attenuated the increase in HW, LV mass, IVS, LVPW, and cardiomyocyte cross-sectional area induced by Ang II in rats. The increase in the size of primary cardiomyocytes, and ANP and BNP mRNA and protein levels were reversed by endostatin overexpression. These results demonstrated that endostatin attenuated cardiac hypertrophy induced by Ang II.
Endostatin suppresses extracellular matrix production in fibroblasts in the presence of transforming growth factor-β (TGF-β), prevents TGF-β-induced dermal fibrosis ex vivo in human skin, and ameliorates skin and pulmonary fibrosis induced by bleomycin in vivo.[18] Renal expression of endostatin is increased in aging animals and has been associated with microvascular rarefaction and progressive tubulointerstitial fibrosis.[19] In the present study, we observed that fibrosis in the heart induced by Ang II in rats was inhibited after endostatin overexpression.
PKA inhibitors have been shown to markedly improve hyperglycemia-induced left ventricular hypertrophy.[20] In PKA inhibitor peptide transgenic mouse models, chronic isoproterenol administration failed to induce cardiac hypertrophy, fibrosis, and myocyte apoptosis, and decreased cardiac function.[21] The catalytic β (Cβ) sub-unit of PKA plays an essential role in Ang II-induced cardiac dysfunction. The Cβ null mouse has highlighted the potential of the PKA Cβ sub-unit as a pharmaceutical target for hypertrophic cardiac disease.[22] In the present study, we demonstrated that endostatin overexpression reduced the increase in cAMP and PKA levels induced by Ang II in primary cardiomyocytes. The increase in ANP and BNP mRNA levels induced by Ang II was reduced after endostatin overexpression. These results demonstrated that endostatin attenuates cardiac hypertrophy by inhibiting the cAMP-PKA signaling pathway.
In summary, endostatin overexpression attenuated cardiac hypertrophy and fibrosis, and improved cardiac hypertrophy by inhibiting the cAMP-PKA signaling pathway.
Conflicts of interest
None.
Footnotes
How to cite this article: Dai YJ, Gong JX, Bian R. Effect of endostatin overexpression on angiotensin II-induced cardiac hypertrophy in rats. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000513
You-Jin Dai and Jue-Xiao Gong contributed equally to this work.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 2018; 137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 2.Schiattarella GG, Hill JA. Inhibition of hypertrophy is a good therapeutic strategy in ventricular pressure overload. Circulation 2015; 131:1435–1447. doi: 10.1161/CIRCULATIONAHA.115.013894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol 2003; 65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 4.Miteva K, Van Linthout S, Pappritz K, Muller I, Spillmann F, Haag M, et al. Human endomyocardial biopsy specimen-derived stromal cells modulate angiotensin II-induced cardiac remodeling. Stem Cells Transl Med 2016; 5:1707–1718. doi: 10.5966/sctm.2016-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 1997; 88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 6.Taddei L, Chiarugi P, Brogelli L, Cirri P, Magnelli L, Raugei G, et al. Inhibitory effect of full-length human endostatin on in vitro angiogenesis. Biochem Biophys Res Commun 1999; 263:340–345. doi: 10.1006/bbrc.1999.1342. [DOI] [PubMed] [Google Scholar]
- 7.Martensson J, Jonsson N, Glassford NJ, Bell M, Martling CR, Bellomo R, et al. Plasma endostatin may improve acute kidney injury risk prediction in critically ill patients. Ann Intensive Care 2016; 6:6.doi: 10.1186/s13613-016-0108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martensson J, Vaara ST, Pettila V, Ala-Kokko T, Karlsson S, Inkinen O, et al. Assessment of plasma endostatin to predict acute kidney injury in critically ill patients. Acta Anaesthesiol Scand 2017; 61:1286–1295. doi: 10.1111/aas.12988. [DOI] [PubMed] [Google Scholar]
- 9.Peng Y, Gao M, Jiang Y, Wang K, Zhang H, Xiao Z, et al. Angiogenesis inhibitor endostatin protects mice with sepsis from multiple organ dysfunction syndrome. Shock 2015; 44:357–364. doi: 10.1097/SHK.0000000000000427. [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Yang CX, Chen XR, Liu BX, Li Y, Wang XZ, et al. Alamandine attenuates hypertension and cardiac hypertrophy in hypertensive rats. Amino Acids 2018; 50:1071–1081. doi: 10.1007/s00726-018-2583-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Ngoh GA, Watson LJ, Facundo HT, Jones SP. Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids 2011; 40:895–911. doi: 10.1007/s00726-010-0728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gharaat MA, Kashef M, Jameie B, Rajabi H. Regulation of PI3K and Hand2 gene on physiological hypertrophy of heart following high-intensity interval, and endurance training. J Res Med Sci 2019; 24:32.doi: 10.4103/jrms.JRMS_292_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lino CA, Demasi M, Barreto-Chaves ML. Ubiquitin proteasome system (UPS) activation in the cardiac hypertrophy of hyperthyroidism. Mol Cell Endocrinol 2019; 493:110451.doi: 10.1016/j.mce.2019.110451. [DOI] [PubMed] [Google Scholar]
- 14.Fan C, Li Y, Yang H, Cui Y, Wang H, Zhou H, et al. Tamarixetin protects against cardiac hypertrophy via inhibiting NFAT and AKT pathway. J Mol Histol 2019; 50:343–354. doi: 10.1007/s10735-019-09831-1. [DOI] [PubMed] [Google Scholar]
- 15.Talman V, Ruskoaho H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res 2016; 365:563–581. doi: 10.1007/s00441-016-2431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruge T, Carlsson AC, Ingelsson E, Riserus U, Sundstrom J, Larsson A, et al. Circulating endostatin and the incidence of heart failure. Scand Cardiovasc J 2018; 52:244–249. doi: 10.1080/14017431.2018.1483080. [DOI] [PubMed] [Google Scholar]
- 17.Barroso MC, Boehme P, Kramer F, Mondritzki T, Koehler T, Gulker JE, et al. Endostatin a potential biomarker for heart failure with preserved ejection fraction. Arq Bras Cardiol 2017; 109:448–456. doi: 10.5935/abc.20170144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaguchi Y, Feghali-Bostwick CA. Role of endostatin in fibroproliferative disorders.-as a candidate for anti-fibrosis therapy. Nihon Rinsho Meneki Gakkai kaishi 2013; 36:452–458. doi: 10.2177/jsci.36.452. [DOI] [PubMed] [Google Scholar]
- 19.Lin CH, Chen J, Ziman B, Marshall S, Maizel J, Goligorsky MS. Endostatin and kidney fibrosis in aging: a case for antagonistic pleiotropy? Am J Physiol Heart Circ Physiol 2014; 306:H1692–H1699. doi: 10.1152/ajpheart.00064.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng KC, Chang WT, Kuo FY, Chen ZC, Li Y, Cheng JT. TGR5 activation ameliorates hyperglycemia-induced cardiac hypertrophy in H9c2 cells. Sci Rep 2019; 9:3633.doi: 10.1038/s41598-019-40002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Szeto C, Gao E, Tang M, Jin J, Fu Q, et al. Cardiotoxic and cardioprotective features of chronic beta-adrenergic signaling. Circ Res 2013; 112:498–509. doi: 10.1161/CIRCRESAHA.112.273896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enns LC, Bible KL, Emond MJ, Ladiges WC. Mice lacking the Cbeta subunit of PKA are resistant to angiotensin II-induced cardiac hypertrophy and dysfunction. BMC Res Notes 2010; 3:307.doi: 10.1186/1756-0500-3-307. [DOI] [PMC free article] [PubMed] [Google Scholar]


