Abstract
Objective:
To review the diagnosis of chronic wound biofilms and discuss current treatment approaches.
Data sources:
Articles included in this review were obtained from the following databases: Wanfang, China National Knowledge Infrastructure, PubMed, and the Web of Science. We focused on research published before August 2019 with keywords including chronic wound, biofilm, bacterial biofilms, and chronic wound infection.
Study selection:
Relevant articles were selected by carefully reading the titles and abstracts. Further, different diagnosis and clinical treatment methods for chronic wound biofilm were compared and summarized from the selected published articles.
Results:
Recent guidelines on medical biofilms stated that approaches such as the use of scanning electron microscopy and confocal laser scanning microscopy are the most reliable types of diagnostic techniques. Further, therapeutic strategies include debridement, negative pressure wound therapy, ultrasound, antibiotic, silver-containing dressing, hyperbaric oxygen therapy, and others.
Conclusion:
This review provides the identification and management of biofilms, and it can be used as a tool by clinicians for a better understanding of biofilms and translating research to develop best clinical practices.
Keywords: Biofilm, Chronic wound, Diagnosis, Therapy
Introduction
A wound is termed chronic when it cannot achieve anatomical and functional integrities through normal, orderly, and timely repair processes under the influence of various internal or external factors.[1] Wounds are injuries that have not healed and have no tendency to heal after more than one month of treatment.[2] A bacterial biofilm (BBF) in a chronic wound is a membranous tissue formed by bacteria attached to the wound bed and fused with extracellular matrix (ECM) secreted by the film.[3] It is composed of bacteria and their products, ECM, necrotic tissue, and so on.[2] Clinic is more common in pressure ulcer, diabetic foot ulcer, lower extremity arteriovenous ulcer, and other chronic wounds.[4,5] For example, the annual incidence of foot ulcers in diabetic patients is 1% to 4% in the United States, with a lifetime risk of occurrence between 15% and 25%.[3] In 2006, the cost of the treatment, amputation, rehabilitation, and long-term care of diabetic foot ulcers in the United States totaled $10.9 billion[6]; approximately 85% of amputations are preceded by this types of ulcers. These figures will increase as the number of diabetes diagnoses is expected to rise. Pressure/decubitus ulcers are a common problem in nursing homes, rehabilitation clinics, and home care patients. Further, venous leg ulcers affect 1% of the worldwide population.[3] Surgical site infections occur in 5% of procedures and are an increasingly common type of post-operative complication; an average of 0.5% of the total hospital budget in the United States is allocated to manage these infections in affected patients.[7] The science of how a wound heals is fascinating, and new discoveries clarifying the mechanisms of physiologic wound repair are constantly being reported. In recent years, the role of BBFs in the formation of chronic wounds has attracted increased attention. The BBF may be an important factor that impairs the healing of chronic wounds.[8] In this review, the basic concepts of BBF will be discussed, with a focus on current practices in the treatment of chronic wounds and future directions in wound care.
Definition and Structural Characteristics of BBF
In 1978, the Canadian scholar Costerton first proposed the concept of “biofilm”. Thereafter, scientists used this concept to describe microbial colonies embedded in an extracellular polymeric matrix secreted by themselves. A BBF has a growth pattern corresponding to plankton cells formed on the surface of inert or active materials, and they adapt to the living environment during the growth of the bacteria. Its structure includes bacteria and extracellular polymeric substance (EPS) secreted by themselves.[9] This phenotype is the best condition for bacteria to inhabit, and is different from free bacteria that have been widely studied in the laboratory. The main components of a BBF include proteins, polysaccharides, extracellular DNA (eDNA), water, and so on. The formation of a biofilm is a dynamic process; it has been found that bacteria can form a mature biofilm on a wound within 24 h.[10] The formation of a BBF includes four stages.[5,11–13] (1) Adhesion: the wound bed contains organic or inorganic nutrients on which bacteria get attached, and most are implanted biomaterials and their own tissue lesions. (2) Reproduction: when bacteria are adhered to the wound surface, they initiate gene expression, secrete a large number of EPS, attract each other to form a microbial colony, and thereafter form a mushroom structure. (3) Maturation: bacteria are buried deep in the matrix and become mature biofilms. (4) Shedding: when the biofilm matures, a small cluster of bacterial cells separate from the biofilm, spread to other environments, and cause infections; thus, chronic wound infection occurs repeatedly in clinics. Further, recent studies have found biofilms resistant to anti-microbial agents; biofilms may be 1000 times more resistant to anti-microbial agents than ordinary-free organisms.[14] The eDNA in biofilm plays an important role in drug resistance. Chiang et al found that the protective effect of eDNA makes Pseudomonas aeruginosa resistant to aminoglycoside drugs.[15] Evidence showed that eDNA had anti-microbial activity; chelating cations could make the cells split, stabilize the lipopolysaccharide and outer membrane of bacteria.[16] By adding DNA lyase to the biofilm formation process, it was found that DNA lyase could not act on the mature biofilm or mucinous biofilm of P. aeruginosa.[17] eDNA is an important component of biofilm matrix in both Gram-negative or Gram-positive bacteria.[18] The production of eDNA is related to quorum sensing (QS) in the wild-type biofilm of P. aeruginosa, and the regulation of QS system can lead to cell cleavage and provide eDNA for the biofilm.[15]
Clinical Diagnosis of BBF in Chronic Wound
It is unlikely that bacterial aggregates in biofilms in the wound can be visualized with the naked eye because they are often less than 100 μm in size and lack macroscopically distinguishable features.[19] Clinical workers usually need to use a bacterial culture to detect bacteria in the wound; however, the diagnosis of chronic infection caused by BBF lacks accuracy. Currently, there is no specific clinical manifestation for the diagnosis of biofilm.[20] Previous studies have shown that the clinical symptoms of BBF that colonize wounds are similar to those of chronic infection wounds, such as pale wound bed, yellow exudate, necrotic tissue, and clear tissue fluid.[21] Some scholars have used granulation tissue morphology and color as the criteria for identifying BBF.[22] Bacterial species and distribution in the biofilm of the wound were summarized and used as one of the diagnostic criteria by extracting the specimens of different kinds of chronic wounds.[23] In 2003, Parsek et al[24] put forward the diagnostic criteria of Parsek-Singh experiment: (1) the relationship between the bacterial infection and wound surface tissue; (2) the pathological examination of the wound tissue, which showed that bacteria were gathered and encapsulated by a matrix; (3) infection in local tissues, with or without systemic infection; and (4) the resistance of bacteria to conventional antibiotics. In 2012, a World Biofilm Seminar summarized the clinical diagnostic criteria of a biofilm infection: (1) pale and edema wound bed; (2) a fragile granulation tissue; (3) large amount of yellow exudate; (4) necrotic and rotting tissue; (5) wound pain; and (6) pungent smell.[25] This criteria was updated in 2017, which included: (1) recalcitrant to treatment with antibiotics or antiseptics; (2) treatment failure despite using appropriate antibiotics or antiseptics; (3) delayed healing; (4) cycles of recurrent infection/exacerbation; (5) excessive moisture and wound exudate; (6) low-level chronic inflammation; and (7) low-level erythema.[26] Recent guidelines on medical biofilms by the European Society of Clinical Microbiology and Infectious Diseases study group for biofilms stated that approaches such as the use of scanning electron microscopy (SEM) and confocal laser scanning microscopy are the most reliable types of diagnostic techniques. The SEM technique can identify biofilms in wounds that do not show any evidence of acute infection.[27] However, these imaging techniques are highly specialized and not practical in a typical clinical setting.[28] To improve the accuracy and scientific nature of the clinical diagnosis of BBF, some new methods have been developed, such as polymerase chain reactions, fluorescence in situ hybridization, and denaturing gradient gel electrophoresis.
Therapeutic Strategies
Wound debridement is the first key step in the removal of BBF. Sharp debridement is commonly used in clinical practice to remove inactivated tissue, slough and necrotic tissue, foreign bodies, and poor healing tissues, which provide an attachment point for bacterial colonization and biofilm formation; thus, it is important to remove necrotic tissue and foreign bodies in time.[29] Late debridement and residues of necrotic tissues and foreign bodies can lead to bacterial colonization of Staphylococcus aureus and P. aeruginosa, which can cause secondary infections. To improve patient tolerance to debridement, painless debridement has gained considerable attention.[30] Hydrosurgical debridement is a painless debridement technique developed recently, and its basic principle is the application of precisely controlled ultrasonic fine water flow to remove carrion, tissue fragments, colonies, and so on from the wound bed based on liquid jet technology, while keeping the wound bed clean and moist. Caputo et al[31] used this technique to debride wounds in 22 patients with chronic leg ulcer; 19 patients with similar ulcers were treated with surgical debridement as the control group. Results showed that ultrasonic atomization of water flow debridement was quicker than that of surgical debridement. In addition, the use of gauze, physiological saline, and other materials was reduced, in addition to the decrease in the pain. Therefore, ultrasonic atomization technology can improve the effectiveness and safety of debridement. In future applications, it is necessary to explore its characteristics, operating methods, and cost-effectiveness, to popularize this technique.
Negative pressure wound therapy (NPWT) has been a widely used method for wound treatment in the last 20 years.[32,33] This therapy can improve local blood flow, reduce tissue edema, promote the growth of granulation tissue, and effectively reduce the number of bacteria.[34] In 2012, Ngo et al[35] first reported that the number of bacteria in BBF significantly decreased after treatment using negative pressure combined with silver foam for 2 weeks, by establishing an in vitro model of P. aeruginosa biofilm. It is speculated that the micromorphology of the wound tissue caused by negative pressure may destroy the original thickness structure of BBF, control the spread of bacteria in the membrane, and thus effectively reduce the wound infection. Further, Phillips et al used an infected pig-skin biofilm as a research object.[36] It was found that NPWT combined with different flushing solutions can effectively remove bacteria in the biofilm in the wound. In 2016, Wang et al[37] used concanavalin A staining in vitro and found that negative pressure environment could reduce biofilm formation compared with normal pressure environment based on observations through a fluorescence microscope. In the model of a rabbit ear biofilm infection, the early treatment of S. aureus infection with NPWT could effectively inhibit the formation of the biofilm; however, it could not clear the mature biofilm.[13] In addition, in this in vitro experiment, the authors found that a negative pressure environment can reduce the total amount of eDNA in the S. aureus biofilm. Further, as eDNA plays an important role in bacterial drug resistance, it is suggested that negative pressure may play a role in reducing bacterial drug resistance. However, there is a lack of research and direct evidence in this area. Negative pressure wound therapy instillation is an improvement on NPWT and one of the treatment methods for biofilms.[13] Phillips et al[36] found that flushing NPWT-binding active anti-bacterial substances could enhance the bacterial clearance of the wound by NPWT and destroy the BBF effectively.
Ultrasound can destroy and remove biofilms via electron-hole pairs and foaming. Nursing staff certified to perform wound treatments can administer ultrasonic treatment independently after training. The effect of ultrasound combined with antibiotics on micro-organisms was studied by Teresa et al[38] It was found that ultrasound could significantly enhance the bactericidal efficacy of gentamicin against P. aeruginosa and Escherichia coli. Zhu et al[39] found that when the parameters of high intensity focused ultrasound were set to a focal length of 150 mm and an output frequency of 40 W, linear scanning radiation, scanning speed of 3 mm/s, scanning length of 10 mm, and scanning interval of 5 mm, it can kill P. aeruginosa and destroy its biofilm structure to a certain extent. Ultrasound, as a physical cleaning method, has been well developed in clinical wound nursing in recent years.
Antibiotic treatment of wounds has always been controversial; generally, only when the wound is accompanied by inflammatory reactions such as redness, swelling, heat, pain, or symptoms of bacteremia, and whole-body anti-bacterial treatment is considered.[26] For chronic wounds with BBF but no symptoms of infection, the efficacy of systemic anti-bacterial therapy was reduced by 25% to 30%. The drug resistance of bacteria after biofilm formation can increase to 1000 to 1500 times of that in the free state,[40] and improper use of antibiotics can promote membrane formation.[41] Antibiotic resistance of micro-organisms within a biofilm can have a significant influence on wound healing in mammalian medicine. When wound isolates are grown in the biofilm phenotypic state, they exhibit enhanced tolerance to antibiotics. This tolerance of a biofilm occurs through phenotypic rather than genotypic changes. Many studies have reported the evidence of antibiotic-resistant isolates in biofilms, in particular methicillin-resistant S. aureus, vancomycin-resistant Enterococcus, and multi-drug resistant Acinetobacter baumannii,[22,42] and; therefore, it is suggested that antibiotics should be used in combination with other antibiotics. Meanwhile, antibiotics should be used according to the structural characteristics of BBF.[43] For example, fluoroquinolones have the strongest scavenging effect on biofilms, but imipenem and ceftazidime have a weaker effect. Macrolides have the strongest penetrating effect on the bacterial extracellular polysaccharide matrix, while fluoroquinolones and β-lactams are the second, with aminoglycosides being the weakest.[44] At present, the combination of traditional antibiotics and specific anti-BBF agents against BBF is the research focus, and it includes using the combination of linazolamine and acetylcysteine. Acetylcysteine can degrade extracellular polysaccharides and destroy bacterial adhesion; therefore, it can inhibit the formation of BBF. Further, the combination of the two can play a synergistic effect, effectively reduce the formation of BBF in Staphylococcus epidermidis.[45] Although new anti-bacterial agents are being developed globally, there are many research studies on single active ingredients, lacking clinical trials and comprehensive pharmacokinetic analysis, and this is expected to become another research hotspot to conquer BBF in the future.
Nanoparticles are versatile and bioactive, and they are becoming increasingly popular for use as a biofilm-targeting approach. Nanoparticles with intrinsic anti-microbial activity, primarily inorganic materials such as silver, can act as biofilm-targeting agents or as nanocoatings. Owing to their flexible chemical structures, they can also function as drug delivery vehicles (nanocarriers) with organic nanoparticles, accounting for over two-thirds of the systems approved for use in humans. Further, both inorganic and organic nanoparticles can be combined or modified by adding molecules (hybrid nanoparticles) to enhance their biological properties or provide multi-functionality. Excellent in-depth reviews on the principles and current applications of nanoparticles, particularly silver, are available.[9] Silver-containing dressing is recognized as a broad-spectrum anti-bacterial dressing. Silver ion dressing is the first choice in the treatment of BBF wounds. When the concentration of silver ion is as high as 5 to 10 g /mL, 90% of the bacteria in the wound BBF could be cleared within 24 h and 100% within 48 h.[46] The silver ions can prevent various micro-organisms including bacteria and fungi from competing with the host cell for oxygen and nutrients, inhibit the production of the metabolic toxin, reduce the expression of the growth factor and the local anti-inflammatory effect, and effectively control the growth of the micro-organisms in the wound environment[47] thereby significantly improving the healing of the wound.
Honey has a very high osmotic pressure and low pH value, and it contains hydrogen peroxide and acetone aldehyde and other bactericidal components; it can reduce bacterial adhesion, inhibit biofilm formation, interfere with QS, hinder the formation of early biofilm structure, and remove or destroy established biofilms.[48] According to the guidelines of the European Wound Management Association, clinical wound nurses can choose different types and concentrations of honey according to the type of bacteria and the stage in which they are located, to perform effective clinical nursing care of the wound.[49] Maddocks et al[50] found that honey inhibited the specific adhesion of S. aureus, P. aeruginosa, and Streptococcus pyogenes to fibronectin, fibrinogen, and collagen, respectively, and prevented it from attaching to human keratinocytes. Meluca honey at 8% concentration inhibited 95% of the biofilm formation of S. aureus, which could reach 97% if the concentration reached 10%, while only 50% of the biofilm formation was inhibited by artificial honey. The minimum inhibitory concentrations of Meluca honey on S. aureus, P. aeruginosa, and S. pyogenes biofilms were 16%, 50%, and 30%, respectively.
Traditional Chinese medicine (TCM) has a long history in the treatment of chronic wounds and has unique advantages in the prevention and treatment of BBF infection. Wound nurses should actively learn TCM anti-bacterial therapy in clinics, understand the advantages of TCM anti-bacterial therapy, and actively organize multi-disciplinary joint diagnosis and treatment in clinical nursing, for example, carry out joint wound treatment with the TCM department.[39] The advantages of TCM should be integrated into the clinical nursing of wound. Study by Gong et al[51] showed that the oral decoction of peony bark (125 mg/L) and ginger (250 mg/L) could inhibit Candida albicans biofilms to a certain extent. It was found that a wet compress of gallnut ethanol extract had a scavenging effect on P. aeruginosa biofilms. The minimum inhibitory concentration (MIC) was 19.5 μg/mL, and two times ethanol extract of gallnut had a complete scavenging effect on the P. aeruginosa biofilm.[52] Chen et al[53] found that andrographolide with the concentration of 30 μg/mL could interfere with the bacterial aggregation of P. aeruginosa wild strain, reduce the adhesion force of P. aeruginosa and destroy the biofilm structure by wet dressing or lavage within 72 h.
Maggot debridement therapy refers to the use of sterile medical maggots to nibble away necrotic tissue and bacteria that hinder wound healing, reduce inflammation, and promote tissue regeneration.[54] The advantage of this therapy is that the debridement using maggots does not affect the healthy tissue around the wound. Maggots can enter deep wounds and pathogens that are difficult to reach by surgery, such as lurking pathogens and sinuses, which can easily form BBF because of anaerobic bacteria. The excrement or secretion of maggots after ingesting rotten meat contains unique collagenase, trypsin, chymotrypsin, and anti-bacterial phthalein, which decomposes necrotic tissue into semi-liquid foam, and then it is digested to degrade the bacteria.[55] An aseptic maggot is used to remove Gram-positive bacteria biofilms, but bacteria-pre-treated maggots fed with many kinds of bacteria can inhibit Gram-negative bacteria biofilms more effectively than the aseptic maggot, such as P. aeruginosa and others.[56] The debridement of maggots in the future may be a promising method for the removal of biofilms; however, the mechanism needs to be further studied to better grasp the application methods and timing in clinical practice.
Some metal ions have a certain bactericidal ability. In addition to the extensive use of silver ions in the management of infected wounds, transition metal gallium has recently been found to inhibit and kill P. aeruginosa in zooplankton and BBF.[57] In the model of pulmonary infection in rats, early gallium therapy can reduce the number of bacteria by about 1000 times, which indicates that gallium has good potential in treating both acute and chronic infections.[39,58,59]
Phage therapy
Phages are found in abundance and can be isolated from a wide range of environments. They are usually specific to narrow host ranges, and due to their self-replication, a low dosage is sufficient. Their high mutation rate helps them to adapt as the host bacteria undergoes genetic alterations to survive in a given environment. Phages have been effective in eradicating biofilms of single or mixed bacterial species and can lyse a biofilm grown on a chronic wound.[60]
Lactoferrin
It is an important non-heme iron-binding glycoprotein in milk, and its anti-bacterial activity is the most remarkable. Lactoferrin can inhibit and kill many micro-organisms, including Gram-positive, Gram-negative aerobes, anaerobes, and some fungi.[59]In vitro experiments showed that through adhesion and decomposing the extracellular polysaccharide of BBF, lactoferrin accelerates infiltration into the membrane to kill bacteria.[61]
Extracellular polymeric substance
Treatment for the composition and structure of EPS has been a popular therapy in recent years. Extracellular polysaccharide degrading enzymes are typical examples, such as the glucose hydrolase (glucanase and insoluble glucanase), dispersin B, which can destroy the matrix of pathogenic biofilms in the oral cavity. Glycoside hydrolases are used to degrade biofilms on wounds infected with mixed bacteria (S. aureus and P. aeruginosa).[62–64] Lysozyme (bacteriophage-encoded peptidoglycan hydrolases) can destroy bacteria in biofilms by degrading peptidoglycan in the bacterial cell wall.[65] The engineered peptidoglycan hydrolases can bind to different anti-bacterial substances, and then cleave bacteria by binding to different binding sites of peptidoglycan. This method has been applied specifically to S. aureus.[66] It has been proved to be effective in killing bacteria and removing biofilms.
Cationic anti-microbial peptides
These peptides are a new family of anti-bacterial peptides found in recent years. It is a low molecular cationic peptide rich in arginine, which is widely distributed in animals, plants, and insects.[67] It has high efficiency and broad spectrum anti-bacterial activity, and does not cause drug resistance and adverse reactions like other antibiotics; thus, it has good application prospects. It has been reported that RNA III inhibitory peptide is very effective in the treatment of severe microbial infections, including highly resistant bacteria such as methicillin-resistant S. aureus.[68]
QS system
Because a quorum-sensing signal system plays a central role in regulating bacterial pathogenic factors, researchers assume that it may be a new target for the control of infectious diseases.[59] It is hoped that it can inhibit the expression of pathogenic factors to achieve a therapeutic purpose.[69] Therefore, signal molecular inhibitors in the QS system have been paid increasing attention in biomembrane therapy. For example, an in vitro study found that QS system autoinducer inhibitor can effectively inhibit bacterial adhesion and dissemination.[70] Quorum-sensing inhibitor type I autoinducing peptide can dissolve methicillin-resistant S. aureus, which is clustered on the surface of titanium, making it more sensitive to rifampicin and levofloxacin.
Phytochemicals
Plant-based chemicals called phytochemicals are being increasingly explored as possible anti-therapeutic agents as they can kill micro-organisms with diverse mechanisms of action with a minimal chance for bacteria to develop resistance. Phytochemicals such as 7-hydroxycoumarin (7-HC), indole-3-carbinol (I3C), salicylic acid, and saponin have shown inhibitory activity against the planktonic culture of E. coli and S. aureus, and they were also able to restrict the growth of the biofilm partially. The phytochemicals I3C and 7-HC had a more pronounced effect on QS inhibition and bacterial motility for both E. coli and S. aureus.[71]
Hyperbaric oxygen therapy (HBOT) uses 100% oxygen at pressures greater than atmospheric pressure. HBOT has been successfully used as adjunctive therapy for wound healing.[72] It can increase tissue metabolism, which can not only reduce the exudation and edema of damaged tissue, improve the local blood circulation, but also promote the formation of neovascularization, accelerate the establishment of collateral circulation, and accelerate the repair of epithelial tissue.[73] Zhang et al[74] studied the efficacy of hyperbaric oxygen in the treatment of submandibular cellulitis in children, and found that hyperbaric oxygen could promote the absorption of inflammation, accelerate wound healing, reduce the infection rate of post-operative incision, and shorten hospitalization time. Cimsit et al[75] also suggested that hyperbaric oxygen can effectively control wound infection and accelerate wound healing. However, if hyperbaric oxygen is overused or treated incorrectly, adverse reactions such as barotrauma, oxygen poisoning, and decompression sickness will occur. Therefore, for the application of hyperbaric oxygen in biofilm chronic wounds, it is necessary to follow normal operation, strictly control the oxygen pressure and speed, and so on, to avoid unnecessary injury.
Other methods include scavenging enzyme and anti-oxidant enzymes, including alginase lyase, deoxyribonuclease I, poly-phosphate kinase, and others.[75] Natural products such as proanthocyanidins in North American cranberry juice, ursolic acid in black sandalwood, and green tea polyphenols all have a good inhibitory effect on biofilms.[76] The type II DNA-binding proteins can destroy the integrity of the eDNA structure.[77] Integration host factor with high affinity can specifically bind to nucleic acid protein in biomembranes, and it has been widely used in animal models.[78,79] There are also genetic engineering drugs and stem cell therapy.[80] Current therapeutic approaches being devised and used in the clinic are shown in Figure 1.
Figure 1.
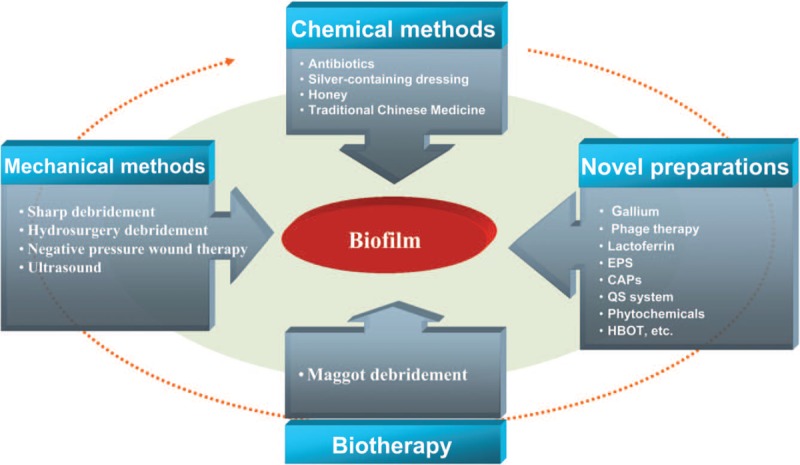
Current therapeutic approaches. CAPs: Cationic antimicrobial peptides; EPS: Extracellular polymeric substance; HBOT: Hyperbaric oxygen therapy; QS: Quorum-sensing.
Future Prospects
This review provides clarity on the identification and management of biofilms, and it can be used as a tool by clinicians seeking to gain a better understanding of biofilms and a way for translating research to best clinical practice. Diagnostic guidelines are essential for evaluating the treatments of BBF; the efficacy of anti-biofilm treatment must indicate a significant reduction in bacteria as an outcome.[25] BBFs are difficult to diagnose because cultures are not necessarily an accurate indicator of BBF. Thus, to investigate biofilms in vivo, identify an infectious etiology, or evaluate treatments, clear clinical signs, and symptoms of BBF are required.
Currently, researches on biofilms are still in the exploratory stage. With the extensive and in-depth development of related research, people will have a deeper and comprehensive understanding of the chronic recurrent infection caused by biofilms and its drug-resistance mechanism. From as early as 2008, the concept of “biofilm-based wound care,” which aims to successfully remove BBF by inhibiting BBF re-formation, led to the improvement of other therapeutic care schemes (such as skin grafting, skin flap, or negative pressure wound treatment), which could change the wound from a difficult state to a treatable state.[81] An increasing number of wound care experts believe many factors that delay wound healing, such as diabetes mellitus, endocarditis, periodontitis, osteomyelitis, and other systemic diseases; the use of graft and prosthesis like catheter indwelling, artificial heart valve, and joint replacement; patients with low autoimmune function, systemic malnutrition, and cell dysfunction,[25] also increase the risk of BBF formation. Therefore, constantly updating the knowledge of BBF can help identify clinically high-risk patients and treat wounds.
Although successful cases of the clinical removal of BBF have been reported, specific methods and strategies are still in their initial stages. Thus, we predict that future studies will focus on the following: (1) increase in clinical studies on biofilm infections through the combination of clinical and laboratory identification tools to explore the effects of different intervention methods on the removal of biofilm; (2) analysis of the therapeutic target, considering how to exert the synergy and efficiency maximization of combined therapy according to the individual differences of wounds, and summarizing the nursing process of BBF wounds; (3) large-sample randomized controlled trials using active biofilm dressing in clinics and obtaining the best practice evidence; (4) joint diagnosis and treatment of multi-disciplinary medical nursing and emphasizing the importance of wound specialist nursing; and (5) consideration of a drug to penetrate existing biofilms as this feature affects both potential cytotoxicity and anti-bacterial efficacy, and the potential for de novo emergence of anti-microbial resistance.
Funding
This work was supported by grants from the Program on Clinical Research Center for Wound Healing in Hunan province funded under the Science and Technology Department of Hunan Province (No. 2018SK7005), the Guiding Plan of Clinical Medical Technology Innovation in Hunan Province (No. 2018SK50905), the Innovation Platform Program: the Introduction of Foreign Intellectual Special in Hunan Province (No. 2019YZ3035), and National Major Special Research (No. ZDZX2017ZL-04-HN).
Conflicts of interest
None.
Footnotes
How to cite this article: Wei D, Zhu XM, Chen YY, Li XY, Chen YP, Liu HY, Zhang M. Chronic wound biofilms: diagnosis and therapeutic strategies. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000523
References
- 1.Umebayashi M, Megumi H, Lima S, Minarelli A. Development of coverage and its evaluation in the treatment of chronic wounds. Invest Educ Enferm 2017; 35:330–339. doi: 10.17533/udea.iee.v35n3a09. [DOI] [PubMed] [Google Scholar]
- 2.Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle) 2015; 4:560–582. doi: 10.1089/wound.2015.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinton A, Carter T. Chronic wound biofilms: pathogenesis and potential therapies. Lab Med 2015; 46:277–284. doi: 10.1309/LMBNSWKUI4JPN7SO. [DOI] [PubMed] [Google Scholar]
- 4.Barshak MB, Durand ML. The role of infection and antibiotics in chronic rhinosinusitis. Laryngoscope Investig Otolaryngol 2017; 2:36–42. doi: 10.1002/lio2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalan LR, Brennan MB. The role of the microbiome in nonhealing diabetic wounds. Ann N Y Acad Sci 2019; 1435:79–92. doi: 10.1111/nyas.13926. [DOI] [PubMed] [Google Scholar]
- 6.Driver VR, Blume PA. Evaluation of wound care and health-care use costs in patients with diabetic foot ulcers treated with negative pressure wound therapy versus advanced moist wound therapy. J Am Podiatr Med Assoc 2014; 104:147–153. doi: 10.7547/0003-0538-104.2.147. [DOI] [PubMed] [Google Scholar]
- 7.Kathju S, Nistico L, Hall-Stoodley L, Post JC, Ehrlich GD, Stoodley P. Chronic surgical site infection due to suture-associated polymicrobial biofilm. Surg Infect (Larchmt) 2009; 10:457–461. doi: 10.1089/sur.2008.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salisbury AM, Woo K, Sarkar S, Schultz G, Malone M, Mayer DO, et al. Tolerance of biofilms to antimicrobials and significance to antibiotic resistance in wounds. Surg Technol Int 2018; 33:59–66. [PubMed] [Google Scholar]
- 9.Koo H, Allan RN, Howlin RP, Stoodley P, Hall-Stoodley L. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol 2017; 15:740–755. doi: 10.1038/nrmicro.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen PO, Kolpen M, Kragh KN, Kuhl M. Microenvironmental characteristics and physiology of biofilms in chronic infections of CF patients are strongly affected by the host immune response. Apmis 2017; 125:276–288. doi: 10.1111/apm.12668. [DOI] [PubMed] [Google Scholar]
- 11.Smith-Palmer T, Lin S, Oguejiofor I, Leng T, Pustam A, Yang J, et al. In situ confocal raman microscopy of hydrated early stages of bacterial biofilm formation on various surfaces in a flow cell. Appl Spectrosc 2016; 70:289–301. doi: 10.1177/0003702815620539. [DOI] [PubMed] [Google Scholar]
- 12.Lee CK, de Anda J, Baker AE, Bennett RR, Luo Y, Lee EY, et al. Multigenerational memory and adaptive adhesion in early bacterial biofilm communities. Proc Natl Acad Sci U S A 2018; 115:4471–4476. doi: 10.1073/pnas.1720071115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li T, Zhang L, Han LI, Wang G, Yin P, Li Z, et al. Early application of negative pressure wound therapy to acute wounds contaminated with Staphylococcus aureus: an effective approach to preventing biofilm formation. Exp Ther Med 2016; 11:769–776. doi: 10.3892/etm.2016.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seth AK, Geringer MR, Hong SJ, Leung KP, Mustoe TA, Galiano RD. In vivo modeling of biofilm-infected wounds: a review. J Surg Res 2012; 178:330–338. doi: 10.1016/j.jss.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 15.Chiang WC, Nilsson M, Jensen PO, Hoiby N, Nielsen TE, Givskov M, et al. Extracellular DNA shields against aminoglycosides in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 2013; 57:2352–2361. doi: 10.1128/AAC.00001-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulcahy H, Charron-Mazenod L, Lewenza S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog 2008; 4:e1000213.doi: 10.1371/journal.ppat.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L, Hengzhuang W, Wu H, Damkiaer S, Jochumsen N, Song Z, et al. Polysaccharides serve as scaffold of biofilms formed by mucoid Pseudomonas aeruginosa. FEMS Immunol Med Microbiol 2012; 65:366–376. doi: 10.1111/j.1574-695X.2012.00936.x. [DOI] [PubMed] [Google Scholar]
- 18.Cavaliere R, Ball JL, Turnbull L, Whitchurch CB. The biofilm matrix destabilizers, EDTA and DNaseI, enhance the susceptibility of nontypeable Hemophilus influenzae biofilms to treatment with ampicillin and ciprofloxacin. Microbiologyopen 2014; 3:557–567. doi: 10.1002/mbo3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjarnsholt T, Alhede M, Alhede M, Eickhardt-Sørensen SR, Moser C, Kühl M, et al. The in vivo biofilm. Trends Microbiol 2013; 21:466–474. doi: 10.1016/j.tim.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Martin JM, Zenilman JM, Lazarus GS. Molecular microbiology: new dimensions for cutaneous biology and wound healing. J Invest Dermatol 2010; 130:38–48. doi: 10.1038/jid.2009.221. [DOI] [PubMed] [Google Scholar]
- 21.Kwiecinski J, Kahlmeter G, Jin T. Biofilm formation by Staphylococcus aureus isolates from skin and soft tissue infections. Curr Microbiol 2015; 70:698–703. doi: 10.1007/s00284-014-0770-x. [DOI] [PubMed] [Google Scholar]
- 22.Percival SL, Hill KE, Williams DW, Hooper SJ, Thomas DW, Costerton JW. A review of the scientific evidence for biofilms in wounds. Wound Repair Regen 2012; 20:647–657. doi: 10.1111/j.1524-475x.2012.00836.x. [DOI] [PubMed] [Google Scholar]
- 23.Skorobogatykh I, Perunova NB, Kurlaev PP, Bukharin OV. Experimental study of combination of ciprofloxacin and oxytocin on formation of biofilms by opportunistic bacteria (in Russian). Zh Mikrobiol Epidemiol Immunobiol 2010;(6):3–7. [PubMed] [Google Scholar]
- 24.Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol 2003; 57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 25.Hall-Stoodley L, Stoodley P, Kathju S, Hoiby N, Moser C, Costerton JW, et al. Towards diagnostic guidelines for biofilm-associated infections. FEMS Immunol Med Microbiol 2012; 65:127–145. doi: 10.1111/j.1574-695X.2012.00968.x. [DOI] [PubMed] [Google Scholar]
- 26.Schultz G, Bjarnsholt T, James GA, Leaper DJ, McBain AJ, Malone M, et al. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen 2017; 25:744–757. doi: 10.1111/wrr.12590. [DOI] [PubMed] [Google Scholar]
- 27.Hoiby N, Bjarnsholt T, Moser C, Bassi GL, Coenye T, Donelli G, et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect 2015; 21: Suppl 1: S1–S25. doi: 10.1016/j.cmi.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 28.Hurlow J, Blanz E, Gaddy JA. Clinical investigation of biofilm in non-healing wounds by high resolution microscopy techniques. J Wound Care 2016; 25: Suppl 9: S11–S22. doi: 10.12968/jowc.2016.25.Sup9.S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schultz GS, Woo K, Weir D, Yang Q. Effectiveness of a monofilament wound debridement pad at removing biofilm and slough: ex vivo and clinical performance. J Wound Care 2018; 27:80–90. doi: 10.12968/jowc.2018.27.2.80. [DOI] [PubMed] [Google Scholar]
- 30.Kim PJ, Steinberg JS. Wound care: biofilm and its impact on the latest treatment modalities for ulcerations of the diabetic foot. Semin Vasc Surg 2012; 25:70–74. doi: 10.1053/j.semvascsurg.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Caputo WJ, Beggs DJ, DeFede JL, Simm L, Dharma H. A prospective randomised controlled clinical trial comparing hydrosurgery debridement with conventional surgical debridement in lower extremity ulcers. Int Wound J 2008; 5:288–294. doi: 10.1111/j.1742-481X.2007.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matiasek J, Domig KJ, Djedovic G, Babeluk R, Assadian O. The effect of negative pressure wound therapy with antibacterial dressings or antiseptics on an in vitro wound model. J Wound Care 2017; 26:236–242. doi: 10.12968/jowc.2017.26.5.236. [DOI] [PubMed] [Google Scholar]
- 33.Tahir S, Malone M, Hu H, Deva A, Vickery K. The effect of negative pressure wound therapy with and without instillation on mature biofilms in vitro. Materials 2018; 11:811.doi: 10.3390/ma11050811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther 2017; 34:599–610. doi: 10.1007/s12325-017-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ngo QD, Vickery K, Deva AK. The effect of topical negative pressure on wound biofilms using an in vitro wound model. Wound Repair Regen 2012; 20:83–90. doi: 10.1111/j.1524-475X.2011.00747.x. [DOI] [PubMed] [Google Scholar]
- 36.Phillips PL, Yang Q, Schultz GS. The effect of negative pressure wound therapy with periodic instillation using antimicrobial solutions on Pseudomonas aeruginosa biofilm on porcine skin explants. Int Wound J 2013; 10: Suppl 1: 48–55. doi: 10.1111/iwj.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang GQ, Li TT, Li ZR, Zhang LC, Zhang LH, Han L, et al. Effect of negative pressure on proliferation, virulence factor secretion, biofilm formation, and virulence-regulated gene expression of Pseudomonas aeruginosa in vitro. Biomed Res Int 2016; 2016:1–7. doi: 10.1155/2016/7986234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teresa CK, Geleana A, Alex S, Tracey V, Devin W, Jeff M, et al. The effects of low-frequency ultrasound (35 kHz) on methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Ostomy Wound Manage 2010; 56:32–43. doi: 10.1016/j.otsr.2010.04.001. [PubMed] [Google Scholar]
- 39.Zhu XJ, Wang Y, Yu JL, Wang Qi, Lu Q, Li FQ. Bactericidal effect of high intensity focused ultrasound on Pseudomonas aeruginosa biofilm in vitro and its spatial structure (in Chinese). Chin J Ultrasound Med 2011; 27:97–101. doi: 10.3969/j.issn.1002-0101.2011.02.001. [Google Scholar]
- 40.Wolcott R, Costerton JW, Raoult D, Cutler SJ. The polymicrobial nature of biofilm infection. Clin Microbiol Infect 2013; 19:107–112. doi: 10.1111/j.1469-0691.2012.04001.x. [DOI] [PubMed] [Google Scholar]
- 41.Li N, Wang L, Yan H, Wang M, Shen D, Yin J, et al. Effects of low-level engineered nanoparticles on the quorum sensing of Pseudomonas aeruginosa PAO1. Environ Sci Pollut Res Int 2018; 25:7049–7058. doi: 10.1007/s11356-017-0947-5. [DOI] [PubMed] [Google Scholar]
- 42.Percival SL, Slone W, Linton S, Okel T, Corum L, Thomas JG. The antimicrobial efficacy of a silver alginate dressing against a broad spectrum of clinically relevant wound isolates. Int Wound J 2011; 8:237–243. doi: 10.1111/j.1742-481x.2011.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varposhti M, Ali AA, Mohammadi P. Synergistic effects of bismuth thiols and various antibiotics against Pseudomonas aeruginosa biofilm. Jundishapur J Microbiol 2014; 7:e9142.doi: 10.5812/jjm.9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghorbani H, Memar MY, Sefidan FY, Yekani M, Ghotaslou R. In vitro synergy of antibiotic combinations against planktonic and biofilm Pseudomonas aeruginosa. GMS Hyg Infect Control 2017; 12:c17.doi: 10.3205/dgkh000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leite B, Gomes F, Teixeira P, Souza C, Pizzolitto E, Oliveira R. Combined effect of linezolid and N-acetylcysteine against Staphylococcus epidermidis biofilms. Enferm Infecc Microbiol Clin 2013; 31:655–659. doi: 10.1016/j.eimc.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 46.Toy LW, Macera L. Evidence-based review of silver dressing use on chronic wounds. J Am Acad Nurse Pract 2011; 23:183–192. doi: 10.1111/j.1745-7599.2011.00600.x. [DOI] [PubMed] [Google Scholar]
- 47.Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res 2015; 52:662–668. doi: 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 48.Yap P, Bakar MFA, Lim H, Carrier D. Antibacterial activity of polyphenol-rich extract of selected wild honey collected in Sabah, Malaysia. J Apicultural Res 2016; 54:1–10. doi: 10.1080/00218839.2016.1151633. [Google Scholar]
- 49.Voidarou C, Alexopoulos A, Plessas S, Karapanou A, Mantzourani I, Stavropoulou E, et al. Antibacterial activity of different honeys against pathogenic bacteria. Anaerobe 2011; 17:375–379. doi: 10.1016/j.anaerobe.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Maddocks SE, Jenkins RE, Rowlands RS, Purdy KJ, Cooper RA. Manuka honey inhibits adhesion and invasion of medically important wound bacteria in vitro. Future Microbiol 2013; 8:1523–1536. doi: 10.2217/fmb.13.126. [DOI] [PubMed] [Google Scholar]
- 51.Gong YJ, Liu H, Feng SY, Chow YH, Sun MJ. Study on the effect of 10 Chinese herbs such as Peony skin on floating bacteria and biofilm of Candida albicans (in Chinese). Chin J Exp Tradit Med Formul 2011; 17:129–132. doi: 10.3969/j.issn.1005-9903.2011.23.039. [Google Scholar]
- 52.Chen Y, Zhao J, Zhang JH, Song H. Effect of galla chinensis aqueous-extracts on pseudomonas aeruginosa biofilm in vitro (in Chinese). J Modern Med Health 2017; 33:3092–3094. doi: 10.3969/j.issn.1009-5519.2017.20.011. [Google Scholar]
- 53.Chen SM, Chen LW, He M, Zeng N. Effect of andrographolide on biofilm formation of Pseudomonas aeruginosa PAO1 and its regulatory mechanism on lasR/rhlR expression (in Chinese). Pharmacol Clin Chin Materia Medica 2014; 30:24–27. [Google Scholar]
- 54.Tian X, Liang XM, Song GM, Zhao Y, Yang XL. Maggot debridement therapy for the treatment of diabetic foot ulcers: a meta-analysis. J Wound Care 2013; 22:462–469. doi: 10.12968/jowc.2013.22.9.462. [DOI] [PubMed] [Google Scholar]
- 55.Davies CE, Woolfrey G, Hogg N, Dyer J, Cooper A, Waldron J, et al. Maggots as a wound debridement agent for chronic venous leg ulcers under graduated compression bandages: a randomised controlled trial. Phlebology 2015; 30:693–699. doi: 10.1177/0268355514555386. [DOI] [PubMed] [Google Scholar]
- 56.Jiang KC, Sun XJ, Wang W, Liu L, Cai Y, Chen YC, et al. Excretions/secretions from bacteria-pretreated maggot are more effective against Pseudomonas aeruginosa biofilms. PLoS One 2012; 7:e49815.doi: 10.1371/journal.pone.0049815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia-Contreras R, Perez-Eretza B, Lira-Silva E, Jasso-Chavez R, Coria-Jimenez R, Rangel-Vega A, et al. Gallium induces the production of virulence factors in Pseudomonas aeruginosa. Pathog Dis 2014; 70:95–98. doi: 10.1111/2049-632X.12105. [DOI] [PubMed] [Google Scholar]
- 58.Patriquin GM, Banin E, Gilmour C, Tuchman R, Greenberg EP, Poole K. Influence of quorum sensing and iron on twitching motility and biofilm formation in Pseudomonas aeruginosa. J Bacteriol 2008; 190:662–671. doi: 10.1128/JB.01473-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu YL, Jiang QX, Wang JD. Progress in the treatment of bacterial biofilm in chronic wounds (in Chinese). Chin J Nurs 2014; 49:1382–1386. doi: 10.3761/j.issn.0254-1769.2014.11.023. [Google Scholar]
- 60.Sharma G, Sharma S, Sharma P, Chandola D, Dang S, Gupta S, et al. Escherichia coli biofilm: development and therapeutic strategies. J Appl Microbiol 2016; 121:309–319. doi: 10.1111/jam.13078. [DOI] [PubMed] [Google Scholar]
- 61.Ammons MC, Copie V. Mini-review: lactoferrin: a bioinspired, anti-biofilm therapeutic. Biofouling 2013; 29:443–455. doi: 10.1080/08927014.2013.773317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fleming D, Chahin L, Rumbaugh K. Glycoside hydrolases degrade polymicrobial bacterial biofilms in wounds. Antimicrob Agents Chemother 2017; 61:e01998.doi: 10.1128/AAC.01998-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pleszczynska M, Wiater A, Janczarek M, Szczodrak J. (1→3)-α-D-Glucan hydrolases in dental biofilm prevention and control: a review. Int J Biol Macromol 2015; 79:761–778. doi: 10.1016/j.ijbiomac.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 64.Kaplan JB. Biofilm matrix-degrading enzymes. Methods Mol Biol 2014; 1147:203–213. doi: 10.1007/978-1-4939-0467-9_14. [DOI] [PubMed] [Google Scholar]
- 65.Schmelcher M, Shen Y, Nelson DC, Eugster MR, Eichenseher F, Hanke DC, et al. Evolutionarily distinct bacteriophage endolysins featuring conserved peptidoglycan cleavage sites protect mice from MRSA infection. J Antimicrob Chemother 2015; 70:1453–1465. doi: 10.1093/jac/dku552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Becker SC, Roach DR, Chauhan VS, Shen Y, Foster-Frey J, Powell AM, et al. Triple-acting lytic enzyme treatment of drug-resistant and intracellular Staphylococcus aureus. Sci Rep 2016; 6:25063.doi: 10.1038/srep25063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vila-Farres X, Giralt E, Vila J. Update of peptides with antibacterial activity. Curr Med Chem 2012; 19:6188–6198. doi: 10.2174/092986712804485818. [PubMed] [Google Scholar]
- 68.Schierle CF, Mauricio DLG, Mustoe TA, Galiano RD. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen 2009; 17:354–359. doi: 10.1111/j.1524-475X.2009.00489.x. [DOI] [PubMed] [Google Scholar]
- 69.Solano C, Echeverz M, Lasa I. Biofilm dispersion and quorum sensing. Curr Opin Microbiol 2014; 18:96–104. doi: 10.1016/j.mib.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 70.Anderson JK, Huang JY, Wreden C, Sweeney EG, Goers J, Remington SJ, et al. Chemorepulsion from the quorum signal autoinducer-2 promotes Helicobacter pylori biofilm dispersal. MBio 2015; 6:e379.doi: 10.1128/mBio.00379-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monte J, Abreu AC, Borges A, Simoes LC, Simoes M. Antimicrobial activity of selected phytochemicals against Escherichia coli and Staphylococcus aureus and their biofilms. Pathogens 2014; 3:473–498. doi: 10.3390/pathogens3020473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sarkar S. Beneficial effect of hyperbaric oxygen therapy on an open abdominal laparostomy wound. Hellenic J Surg 2018; 90:90–92. doi: 10.1007/s13126-018-0446-2. [Google Scholar]
- 73.Stabryła P, Kulińska J, Warchoł Ł, Kasielska-Trojan A, Gaszyński W, Antoszewski B. Degloving lower leg injury-the importance of additional treatment: negative pressure and hyperbaric oxygen therapy. Pol Przegl Chir 2018; 90:5–9. doi: 10.5604/01.3001.0011.7453. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y, Gao YX. Effect observation of hyperbaric oxygen in the treatment of children with submaxillary cellulitis (in Chinese). Chin Maternal Child Health Care 2010; 25:4804–4805. doi: 1001-4411(2010)32-4804-02. [Google Scholar]
- 75.Cimsit M, Uzun G, Yildiz S. Hyperbaric oxygen therapy as an anti-infective agent. Expert Rev Anti Infect Ther 2009; 7:1015–1026. doi: 10.1586/eri.09.76. [DOI] [PubMed] [Google Scholar]
- 76.Quave CL, Miriam EC, Compadre CM, Gerren H, Howard H, Beenken KE, et al. Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS One 2012; 7:e28737.doi: 10.1371/journal.pone.0028737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goodman SD, Obergfell KP, Jurcisek JA, Novotny LA, Downey JS, Ayala EA, et al. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal Immunol 2011; 4:625–637. doi: 10.1038/mi.2011.27. [DOI] [PubMed] [Google Scholar]
- 78.Xiong YQ, Estelles A, Li L, Abdelhady W, Gonzales R, Bayer AS, et al. A human biofilm-disrupting monoclonal antibody potentiates antibiotic efficacy in rodent models of both Staphylococcus aureus and Acinetobacter baumannii infections. Antimicrob Agents Chemother 2017; 61:e00904.doi: 10.1128/AAC.00904-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Payne DE, Boles BR. Emerging interactions between matrix components during biofilm development. Curr Genet 2016; 62:137–141. doi: 10.1007/s00294-015-0527-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lopes L, Setia O, Aurshina A, Liu S, Hu H, Isaji T, et al. Stem cell therapy for diabetic foot ulcers: a review of preclinical and clinical research. Stem Cell Res Ther 2018; 9:188.doi: 10.1186/s13287-018-0938-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kucisec-Tepes N. The role of antiseptics and strategy of biofilm removal in chronic wound (in Croatian). Acta Med Croatica 2016; 70:33–42. [PubMed] [Google Scholar]


