Abstract
Background:
This study aims to assess the clinical efficacy and safety of omadacycline for the treatment of acute bacterial infections in adult patients through meta-analysis.
Methods:
PubMed, Embase, ClinicalTrials.gov, and Cochrane databases were searched up to May 2019. Only randomized controlled trials (RCTs) that evaluated omadacycline and other comparators for treating acute bacterial infections in adult patients were included. The primary outcome was the clinical response rate at the posttreatment evaluation, whereas the secondary outcomes were risk of an adverse event (AE) and mortality.
Results:
Four RCTs were included. Overall, omadacycline had a clinical response rate noninferior to comparators in the treatment of acute bacterial infection in the modified intent-to-treat population (odds ratio [OR], 1.31; 95% confidence interval [CI], 1.04–1.65; I2 = 0%) and in the clinically evaluable population (OR, 1.53; 95% CI, 1.11–2.11; I2 = 0%). Furthermore, no significant differences were found between omadacycline and comparators for the risk of treatment-emergent AEs (OR, 1.13; 95% CI, 0.60–2.14; I2 = 93%), treatment-related AEs (OR, 0.70; 95% CI, 0.46–1.04; I2 = 56%), serious AEs (OR, 1.01; 95% CI, 0.64–1.58; I2 = 0%), and discontinuation of study drug due to an AE (OR, 0.78; 95% CI, 0.47–1.29; I2 = 0%). However, in the clinical trial, NCT02877927, in which omadacycline was used in only oral form, the reported incidence of nausea and vomiting were 30.2% (111/368) and 16.9% (62/368), respectively. Finally, the mortality rate was similar between omadacycline and comparator in the treatment of acute bacterial infection (OR, 1.32; 95% CI, 0.47–3.67; I2 = 0%).
Conclusion:
The clinical efficacy of omadacycline is not inferior to that of comparators in the treatment of acute bacterial infections in adult patients, and this antibiotic is also well tolerated.
Keywords: omadacycline, acute bacterial infection, complicated skin and skin structure infection, community-acquired bacterial pneumonia
1. Introduction
Omadacycline is a new aminomethylcycline, a semisynthetic compound derived from tetracycline class.[1] Like older tetracyclines, omadacycline exhibits activity against a broad spectrum of bacteria, including gram-positive, gram-negative, anaerobic, and atypical pathogens.[2–8] More, omadacycline has the additional advantage than older tetracyclines that it remains active against antibiotic-resistant bacteria carrying the major efflux and target protection resistance determinants.[9,10] In the global surveillance investigations,[2–5,8,10,11] omadacycline exhibits potent in vitro activity against methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci, penicillin-resistant S pneumoniae, and extended-spectrum β-lactamase-producing Enterobacteriaceae. Recently, several randomized trials have assessed the clinical efficacy and safety of omadacycline for treating acute bacterial skin and skin structure infection (ABSSSI) and community-acquired bacterial pneumonia (CABP) in adult patients.[12–15] However, an updated meta-analysis comparing the efficacy and safety of omadacycline and other comparators for the treatment of acute bacterial infection in adult patients is lacking. Therefore, we conducted this meta-analysis to provide a real-time evidence on the efficacy and safety of omadacycline in adult patients with acute bacterial infection.
2. Methods
2.1. Study search and selection
All clinical studies were identified through a systematic review of the literature in PubMed, Embase, ClinicalTrials.gov, and Cochrane databases until May 2019 using the following search terms: “omadacycline,” “Nuzyra,” and “PTK-0796.” Only randomized controlled studies that compared the clinical efficacy and adverse effects of omadacycline and other comparators in the treatment of adult patients with acute bacterial infections were included. All languages of publication could be included. However, we excluded articles if they were in vitro studies or pharmacokinetic–pharmacodynamic assessment. Two reviewers (SHL and SPC) searched and examined publications independently to avoid bias. Any disagreement was resolved and decided by a 3rd reviewer (Lai). The following data were extracted from all the included studies: authorship, year of publication, study design, countries, antibiotic regimens of omadacycline and comparators, outcomes, and adverse events (AEs). The modified intent-to-treat (MITT) population consisted of all patients in the ITT population who had a confirmed diagnosis in accordance with the study protocol criteria. The clinically evaluable (CE) population included patients from the MITT population who had a qualifying infection as per the criteria for trial entry, received a trial drug, did not receive any antibacterial agent not assigned within the trial that could confound interpretation of the results, and had an assessment of outcome during the protocol-defined window. The microbiologically evaluable population included patients in the CE population from whom at least 1 bacterial pathogen was isolated from blood or infected tissue at baseline. For evaluating safety, the ITT population that included all patients who received any amount of intravenous study drug was used. The ethical approval was not necessary for meta-analysis in our institute.
2.2. Definitions and outcomes
The primary outcome was investigator-assessed clinical response at the posttreatment evaluation (7–14 days after the last dose of a trial drug) in the MITT and the CE population, and clinical response was defined as the resolution of clinical signs and symptoms of acute bacterial infection, or improvement to the extent that no further antimicrobial therapy was necessary. Secondary outcomes included early clinical responses, the risk of AEs, including treatment-emergent AEs (TEAEs), treatment-related AEs, serious AEs, and discontinuation because of AEs and mortality.
2.3. Data analysis
This study used the Cochrane Risk of Bias Assessment tool to assess the quality of enrolled randomized controlled trials (RCTs) and the risk of bias.[16] Statistical analyses were conducted using the software Review Manager, version 5.3. The degree of heterogeneity was evaluated with the Q statistic generated from the Chi-squared test. The proportion of statistical heterogeneity was assessed using the I2 measure. Heterogeneity was considered significant when the P was <.10 or I2 was >50%. The random-effect model was used when data were significantly heterogeneous, and the fixed-effect model was used when data were homogenous. Pooled odds ratio (OR) and 95% confidence intervals (CIs) were calculated for outcome analyses.
3. Results
3.1. Study selection and characteristics
The search program yielded 172 references. After excluding 69 duplications, the remaining 103 abstracts were screened. Among them, we retrieved ten articles for full-text review. Finally, 4 studies[12–15] fulfilling the inclusion criteria were included in this meta-analysis (Fig. 1). All studies[12–15] were randomized, multicenter studies designed to compare the clinical efficacy and safety of omadacycline with other comparators for adult patients with acute bacterial infection (Table 1). All studies[12–15] were multicenter, and 2 studies[13,14] were multinational. Three studies[12,13,15] focused on ABSSSIs, in which linezolid was the comparator and 1 study[14] focused on CABP in which moxifloxacin was the comparator. Except 1 study[15] comparing oral omadacycline and linezolid, 3 studies[12–14] focused on the initially intravenous, injection of omadacycline, and comparators. However, the antibiotics used in these RCTs are not 1st-line antibiotics commonly used to treat SSTIs. Almost all the domains in each study were classified as having a low risk of bias (Fig. 2).
Figure 1.

Study selection process flow.
Table 1.
Characteristics of included studies.
Figure 2.
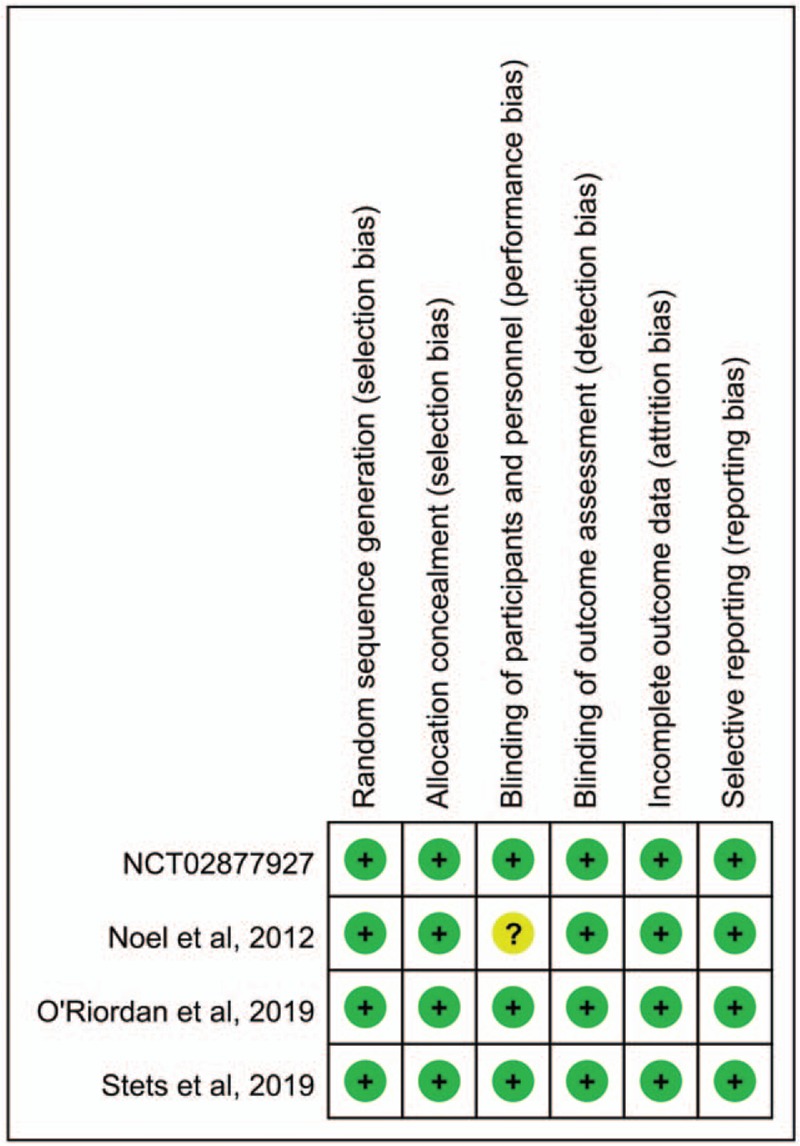
Risk of bias per study and domain.
3.2. Clinical efficacy
Overall, omadacycline had a clinical response rate in MITT population not inferior to comparators in the treatment of acute bacterial infection (OR, 1.31; 95% CI, 1.04–1.65; I2 = 0%; Fig. 3) in the pooled analysis of 4 studies.[12–15] In the CE population, omadacycline remained noninferior to comparators in terms of clinical response rate in the pooled analysis of five studies (OR, 1.53; 95% CI, 1.11–2.11; I2 = 0%). The noninferiority of omadacycline remained the same in sensitivity test after randomly excluding individual study. In the subgroup analysis of patients with ABSSSIs, omadacycline exhibited noninferior clinical response rate to linezolid among both MITT and CE populations (MITT populations, OR, 1.35; 95% CI, 1.02–1.77; I2 = 28%. CE population, OR, 1.60; 95% CI, 1.08–2.38; I2 = 0%). According to different type of ABSSSIs, no significant difference regarding clinical response rate was observed between omadacycline and linezolid in terms of major abscess (OR, 1.44; 95% CI, 060–3.49; I2 = 52%), wound infection (OR, 2.02; 95% CI, 0.26–15.96; I2 = 53%) and cellulitis (OR, 1.83; 95% CI, 0.83–4.07). Among overall ME population, no significant difference was observed between omadacycline and comparator in the pooled analysis of 3 studies[12–14] (OR, 1.71; 95% CI, 0.97–3.03; I2 = 3%). The similar trend between omadacycline and comparator was noted for treating infection with S aureus (OR, 1.06; 95% CI, 0.62–1.83; I2 = 0%), and MRSA (OR, 0.95; 95% CI, 0.38–2.34; I2 = 0%).
Figure 3.
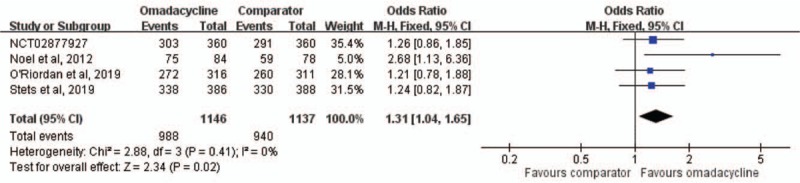
Overall clinical response rates for omadacycline and comparators in the treatment of acute bacterial infections. CI = confidence interval.
3.3. Adverse events
No significant differences were found between omadacycline and comparators for the risk of TEAEs (OR, 1.13; 95% CI, 0.60–2.14; I2 = 93%), treatment-related AEs (OR, 0.70; 95% CI, 0.46–1.04; I2 = 56%), serious AEs (OR, 1.01; 95% CI, 0.64–1.58; I2 = 0%), and discontinuation of study drug due to an AE (OR, 0.78; 95% CI, 0.47–1.29; I2 = 0%) (Fig. 4). Regarding common AEs, no significant difference was observed between omadacycline and comparators in terms of nausea (OR, 1.51; 95% CI, 0.52–4.40; I2 = 92%), vomiting (OR, 2.04; 95% CI, 0.75–5.59; I2 = 81%), diarrhea (OR, 0.51; 95% CI, 0.16–1.60; I2 = 79%), constipation (OR, 1.38; 95% CI, 0.66–2.88; I2 = 0%), and headache (OR, 1.08; 95% CI, 0.67–1.74; I2 = 0%). Finally, the mortality rate was similar between omadacycline and comparator in the treatment of acute bacterial infection (OR, 1.32; 95% CI, 0.47–3.67; I2 = 0%).
Figure 4.
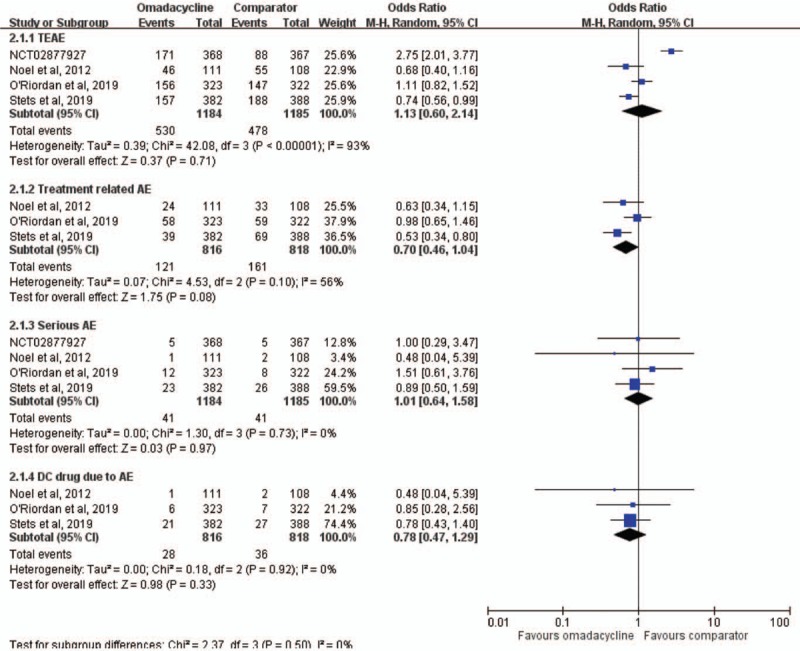
Adverse event risks with omadacycline and comparators in the treatment of acute bacterial infections. AE = adverse event, CI = confidence interval, TEAEs = treatment-emergent AEs.
4. Discussion
This 1st meta-analysis based on 4 RCTs found that the clinical efficacy of omadacycline was not inferior to that of other comparators in the treatment of patients with acute bacterial infection. First, the overall pooled clinical response rate of omadacycline in treating acute bacterial infections including ABSSSI and CABP was 86.2% in MITT population and 93.0% in CE population, and it was not inferior than that of comparator (82.7% in MITT population and 89.6% in CE population). Second, pooled clinical response rate of omadacycline for treating ABSSSI in this meta-analysis was also not inferior to that of linezolid in both MITT and CE population. Third, in the subgroup analysis of different type of ABSSSI and pathogens, omadacycline exhibited the clinical response rate similar to linezolid. Finally, the mortality of patients with acute bacterial infection treated with omadacycline was only 0.84%, which was not significantly different from that seen in comparators (0.65%). In summary, all these findings indicated that omadacycline can be an effective therapeutic option in the treatment of acute bacterial infection, particularly ABSSSI.
The effectiveness of omadacycline in the treatment of acute bacterial infections including ABSSSI and CABP in adult patients can be supported by in vitro studies. In a global surveillance[3] of 69,246 clinical isolates during 2010 and 2011, 99.9% of S aureus isolates, including MRSA were inhibited by 2 mg/dL of omadacycline, and its potencies were comparable for Streptococcus pneumoniae (MIC50/90 0.06/0.06 mg/dL), and viridans streptococci (MIC50/90 0.06/0.12 mg/dL). In addition, omadacycline remains active against commonly encountered Enterobacteriaceae, including Escherichia coli (MIC50/90 0.5/2 mg/dL), Klebsiella spp (MIC50/90 1/4 mg/dL), and Citrobacter spp (MIC50/90 1/4 mg/dL). Another surveillance[17] of 14,000 clinical isolates from the United States and Europe during 2017 demonstrated similar findings that 96.5% of MRSA, 99.8% of methicillin-susceptible S aureus, 98.6% of S pneumoniae, ≥97.7% of other Streptococcus spp, 99.8% of Hemophilus influenzae, 99.1% of E coli, 87.5% of K pneumoniae can be inhibited by omadacycline. Overall, the potent in vitro activity of omadacycline against clinical isolates, including MRSA, can help explained the great in vivo clinical response in this meta-analysis.
In addition to clinical efficacy, we should consider AE risk while prescribing omadacycline. Nausea, vomiting, and headache were the most common AEs, and the overall incidence of these AEs was comparable with comparators. However, in the clinical trial, NCT02877927,[15] in which omadacycline was used in only oral form, the reported incidence of nausea and vomiting could be up to 30.2% (111/368) and 16.9% (62/368), respectively. In addition to these common AEs, the pooled risks of TEAEs, treatment-related AEs, and serious AEs were similar between omadacycline and comparators. Finally, no significant difference was observed between omadacycline and comparators in terms of discontinuation of the study drug due to an AE. Therefore, the findings of this meta-analysis suggest that omadacycline is as safe as other comparators in the treatment of acute bacterial infection.
This study has several limitations. Only 4 RCTs were considered in this meta-analysis, and only 2 types of acute bacterial infections, ABSSSI and CABP, were included. Fortunately, 2 additional trials[15,18] aim to investigate the efficacy of omadacycline in the clinical setting of acute pyelonephritis and cystitis are ongoing. We can obtain more data to analyze after these trials are completed in the near future. In addition, this study did not assess the cost effect; however, it should be an important issue in the use of novel antibiotics.
In conclusion, omadacycline is as good as comparators in terms of efficacy and tolerance in the treatment of acute bacterial infection in adult patients. Thus, omadacycline is an appropriate option for antibiotic therapy in adult patients with acute bacterial infection.
Author contributions
Conceptualization: Shao-Huan Lan, Shen-Peng Chang, Chih-Cheng Lai, Chien-Ming Chao.
Data curation: Shao-Huan Lan, Shen-Peng Chang, Li-Chin Lu.
Formal analysis: Shao-Huan Lan, Shen-Peng Chang, Li-Chin Lu.
Writing – original draft: Chih-Cheng Lai.
Writing – review & editing: Chien-Ming Chao.
Footnotes
Abbreviations: ABSSSI = acute bacterial skin and skin structure infection, AE = adverse event, CABP = community-acquired bacterial pneumonia, CE = clinically evaluable, MITT = modified intent-to-treat, MRSA = methicillin-resistant Staphylococcus aureus, RCT = randomized controlled trial.
How to cite this article: Lan SH, Chang SP, Lai CC, Lu LC, Chao CM. The efficacy and safety of omadacycline in treatment of acute bacterial infection: a systemic review and meta-analysis of randomized controlled trials. Medicine. 2019;98:51(e18426).
The authors have no funding and conflicts of interest to disclose.
References
- [1].Durães F, Sousa E. Omadacycline: a newly approved antibacterial from the class of tetracyclines. Pharmaceuticals (Basel) 2019;12: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Carvalhaes CG, Huband MD, Reinhart HH, et al. Antimicrobial activity of omadacycline tested against clinical bacterial isolates from hospitals in Mainland China, Hong Kong, and Taiwan: results from the SENTRY Antimicrobial Surveillance Program (2013 to 2016). Antimicrob Agents Chemother 2019;63: pii: e02262-18. doi:10.1128/AAC.02262-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pfaller MA, Huband MD, Rhomberg PR, et al. Surveillance of omadacycline activity against clinical isolates from a global collection (North America, Europe, Latin America, Asia-Western Pacific), 2010-2011. Antimicrob Agents Chemother 2017;61: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pfaller MA, Huband MD, Shortridge D, et al. Surveillance of omadacycline activity tested against clinical isolates from the United States and Europe as Part of the 2016 SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother 2018;62: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pfaller MA, Rhomberg PR, Huband MD, et al. Activities of omadacycline and comparator agents against Staphylococcus aureus isolates from a surveillance program conducted in North America and Europe. Antimicrob Agents Chemother 2017;61: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kohlhoff SA, Huerta N, Hammerschlag MR. In vitro activity of omadacycline against Chlamydia pneumoniae. Antimicrob Agents Chemother 2019;63: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stapert L, Wolfe C, Shinabarger D, et al. In vitro activities of omadacycline and comparators against anaerobic bacteria. Antimicrob Agents Chemother 2018;62: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pfaller MA, Rhomberg PR, Huband MD, et al. Activity of omadacycline tested against Streptococcus pneumoniae from a global surveillance program (2014). Diagn Microbiol Infect Dis 2018;90:143–7. [DOI] [PubMed] [Google Scholar]
- [9].Watkins RR, Deresinski S. Omadacycline: a novel tetracycline derivative with oral and intravenous formulations. Clin Infect Dis 2019;69:890–6. [DOI] [PubMed] [Google Scholar]
- [10].Macone AB, Caruso BK, Leahy RG, et al. In vitro and in vivo antibacterial activities of omadacycline, a novel aminomethylcycline. Antimicrob Agents Chemother 2014;58:1127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pfaller MA, Rhomberg PR, Huband MD, et al. Activity of omadacycline tested against Enterobacteriaceae causing urinary tract infections from a global surveillance program (2014). Diagn Microbiol Infect Dis 2018;91:179–83. [DOI] [PubMed] [Google Scholar]
- [12].Noel GJ, Draper MP, Hait H, et al. A randomized, evaluator-blind, phase 2 study comparing the safety and efficacy of omadacycline to those of linezolid for treatment of complicated skin and skin structure infections. Antimicrob Agents Chemother 2012;56:5650–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].O’Riordan W, Green S, Overcash JS, et al. Omadacycline for acute bacterial skin and skin-structure infections. N Engl J Med 2019;380:528–38. [DOI] [PubMed] [Google Scholar]
- [14].Stets R, Popescu M, Gonong JR, et al. Omadacycline for community-acquired bacterial pneumonia. N Engl J Med 2019;380:517–27. [DOI] [PubMed] [Google Scholar]
- [15].ClinicalTrials.gov. Oral Omadacycline Vs. Oral Nitrofurantoin for the Treatment of Cystitis. Available at: https://www.clinicaltrials.gov/ct2/show/NCT03425396?term=omadacycline&rank=3 Accessed May 1, 2018. [Google Scholar]
- [16].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Huband MD, Pfaller MA, Shortridge D, et al. Surveillance of omadacycline activity tested against clinical isolates from the United States and Europe: results from the SENTRY Antimicrobial Surveillance Programme, 2017. J Glob Antimicrob Resist 2019;19:56–63. [DOI] [PubMed] [Google Scholar]
- [18].ClinicalTrials.gov. Iv or iv/po omadacycline vs. Iv/po levofloxacin for the treatment of acute pyelonephritis. Available at: https://www.clinicaltrials.gov/ct2/show/NCT03757234?term=omadacycline&rank=1 Accessed May 1, 2019. [Google Scholar]


