Supplemental Digital Content is available in the text
Keywords: laparoscopic distal gastrectomy, network meta-analysis, reconstruction
Abstract
Background:
There is no consensus regarding which reconstruction methods are superior after laparoscopic distal gastrectomy (LDG). This study compared four reconstruction methods after LDG for gastric cancer.
Methods:
Literature in EMBASE, PubMed, and the Cochrane Library was screened to compare Billroth I (B-I), Billroth II (B-II), Roux-en-Y (RY), and uncut Roux-en-Y (URY) anastomoses after LDG for gastric cancer. A Bayesian network meta-analysis (NMA) was conducted to compare these methods.
Results:
Eighteen studies involving 4347 patients were eligible for our NMA. The operative time in RY anastomosis was longer than that in B-I and B-II anastomoses. Blood loss and risk of gastrointestinal motility dysfunction were greater with RY anastomosis than with URY or B-I anastomosis. Furthermore, URY anastomosis was superior to the other 3 reconstruction methods for preventing food residue. For remnant gastritis, RY anastomosis was significantly superior to B-I and B-II anastomoses, whereas URY anastomosis was significantly superior to B-II anastomosis. In addition, RY and URY anastomoses were better than B-I and B-II anastomoses for preventing bile reflux.
Conclusions:
URY anastomosis tended to be a more favorable reconstruction method after LDG due to its operative simplicity and reduced long-term complications.
1. Introduction
Gastric cancer is the fifth most frequent cancer worldwide and the third most common cause of death from cancer.[1] Gastric cancer accounted for 5.7% of all cancer cases and 8.2% of all cancer-related mortality, with 782,685 deaths, in 2018, thus posing a serious threat to human life and health.[1]
Radical surgery is the only definitive treatment method for gastric cancer.[2] It has been approximately 25 years since Kitano completed the first laparoscopic distal gastrectomy (LDG) in the world in 1994, and its advantage of minimal invasiveness is becoming increasingly accepted.[3] LDG and laparotomy have different surgical approaches, and whether experience gained from laparotomy can be applied to LDG is questionable. Given the wide application of LDG, it is essential to reappraise the reconstruction procedures used in pure laparoscopic surgery.
Several reconstruction procedures (Billroth I (B-I), Billroth II (B-II), Roux-en-Y (RY), and uncut Roux-en-Y (URY) anastomoses) are selectively performed after LDG, according to the choice of individual surgeons. To date, only a few meta-analyses[4–6] have investigated reconstruction procedures after distal gastrectomy. A meta-analysis by Xiong et al[4] in 2013 reported that RY anastomosis had some clinical advantages over B-I anastomosis, and in 2018, an NMA by Cai et al[5] indicated that RY anastomosis was superior to B-I and B-II anastomoses in terms of preventing bile reflux and remnant gastritis. However, a recent meta-analysis by Sun et al[6] in 2018 demonstrated that URY anastomosis is clinically superior to RY anastomosis. Therefore, there is a lack of consensus on which reconstruction method is better. None of the 3 meta-analyses had simultaneously compared 4 anastomosis methods, and they also failed to distinguish laparoscopic surgery from laparotomy.
Thus, we performed a network meta-analysis (NMA), a new analysis method that can both provide comparisons of treatment effects and rank all treatments,[7] comparing four different reconstruction methods to evaluate both the short-term outcomes and the long-term endoscopic findings after LDG.
2. Materials and methods
2.1. Search strategy
Our NMA was conducted based on the PRISMA statement[8] and its extension statement for network meta-analyses[9] to search all relevant studies. Three electronic databases, namely, EMBASE, PubMed and the Cochrane Library, were methodically searched for articles published up to November 2018 with the following combination of keywords and their variants: “laparoscopy”, “stomach neoplasm”, “gastroenterostomy”, “anastomosis, Roux-en-Y”, and “Billroth”. The detailed search strategy for PubMed is shown in Supplementary Fig. 1, and search strategies for EMBASE and the Cochrane Library were similar to it. Additional articles were also checked from the references of the included studies.
2.2. Study selection
The following criteria were used for study selection: Participants: Patients with a pathological diagnosis of primary gastric cancer without metastasis or invasion into adjacent organs and with sufficient cardiopulmonary function to tolerate surgery; interventions and comparisons: only original articles comparing 2 or more different reconstruction procedures (B-I, B-II, RY, and URY anastomoses) after LDG were considered for inclusion, regardless of total laparoscopic surgery or laparoscopic-assist surgery; outcomes: the study needed to report at least one of the following outcomes: operative time, blood loss, anastomotic complication, gastrointestinal motility dysfunction, food residue, remnant gastritis and bile reflux; study design: both randomized controlled trials (RCTs) and nonrandomized controlled trials were included due to the lack of high-quality RCTs.
In the case of the same results reported 2 or more times, only the newest publications were included. Literature selection was agreed upon by 2 independent reviewers, and any the disagreements were resolved by consultation with a third reviewer.
2.3. Data extraction and quality assessment
The required information, including population characteristics (country, design, study year, mean age, sex, and tumor characteristics) and outcome factors (blood loss, operative time, anastomotic leakage, anastomotic bleeding, anastomotic stenosis, gastrointestinal motility dysfunction, food residue, remnant gastritis, and bile reflux), was independently extracted by 2 reviewers using the same form. Any conflicts between the 2 reviewers were resolved thorough discussions with another reviewer.
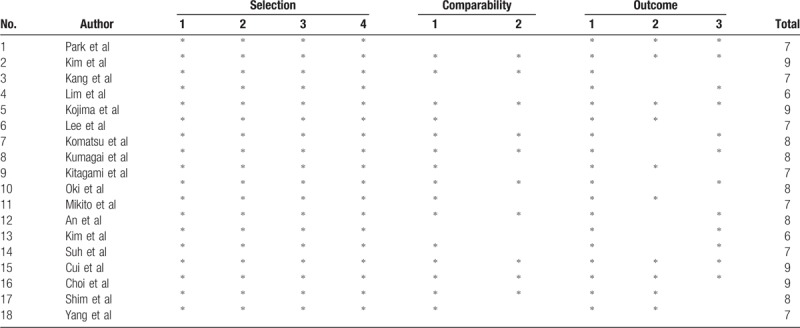
The risk of bias for the included studies was assessed by 2 independent authors using the Newcastle-Ottawa scoring system.[10]
2.4. Data analysis
For categorical data, treatment effects were expressed as odds ratios (ORs) and 95% confidence intervals (95%CIs). For continuous data, treatment effects were expressed as standardized mean differences (SMDs) and 95%CIs. Surface under the cumulative ranking curve (SUCRA) was used to calculate the hierarchy of treatments for each intervention. The SUCRA value ranges from 0 to 1, and a higher SUCRA value indicates a better efficacy.[11] Comparison-adjusted funnel plots were used to identify publication bias and small-study effects. Inconsistency was evaluated using an inconsistency check (‘ifplot’ procedure of Stata version 13.0). Discrepancies between direct comparison and network comparison were also utilized to identify inconsistency. WinBUGS version 1.4.3 software and statistical model described by Chaimani et al[12] with a random-effects model were used to perform our NMA. Graphs of the statistical results obtained from WinBUGS version 1.4.3 software were generated by Stata version 13.0 and GraphPad Prism version 7.0a.
All of the information we considered was extracted from published papers, so it was not necessary to obtain ethical approval.
3. Results
3.1. Study characteristics
After a comprehensive inspection, 17 retrospective studies[13–29] and 1 RCT[30] were included in our NMA (Fig. 1). One study simultaneously compared B-I, B-II, RY, and URY anastomoses; 3 studies compared B-I and B-II anastomoses; 8 studies compared B-I and RY anastomoses; 2 studies compared B-I and URY anastomoses; 3 studies compared B-II and RY anastomoses; and 1 study compared B-II and URY anastomoses. Table 1 shows the characteristics of these studies. In total, 4347 patients were included in our analysis: 2362 were treated with B-I anastomosis; 1132 were treated with B-II anastomosis; 714 were treated with RY anastomosis; and 139 were treated with URY anastomosis.
Figure 1.
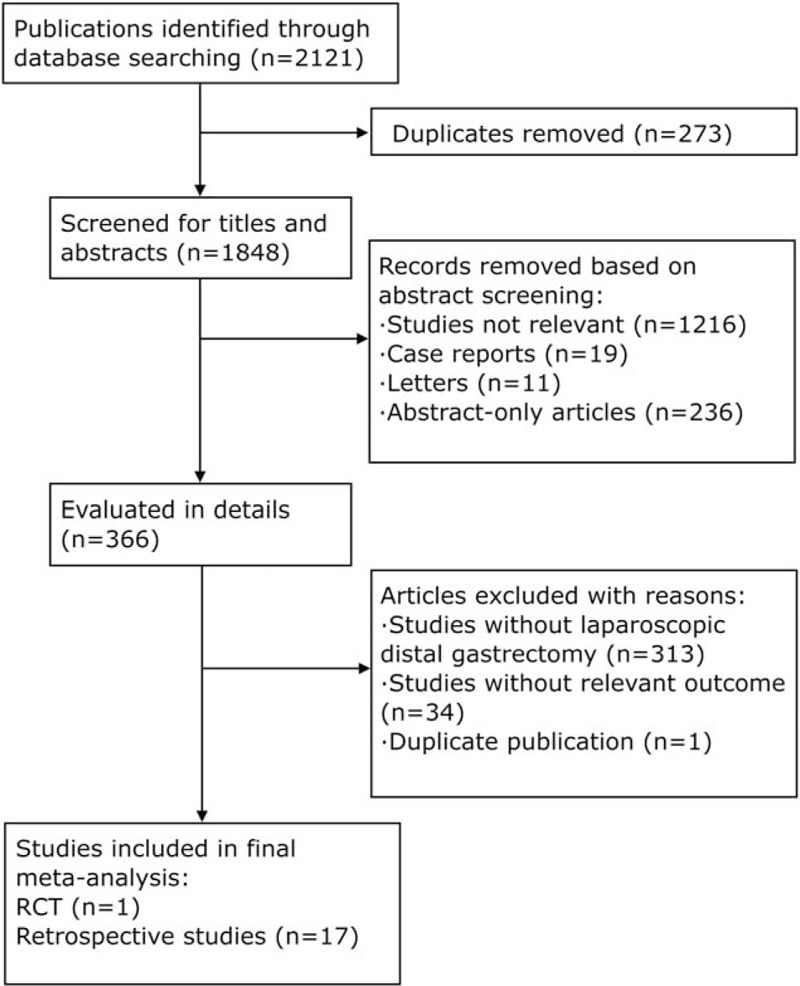
Flow diagram for the identification of eligible studies. RCT = randomized controlled trial.
Table 1.
The characteristics of the included researches.
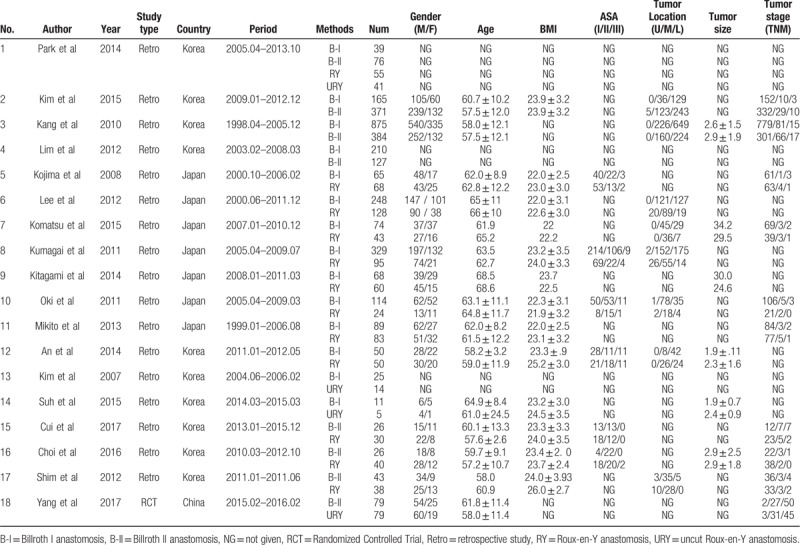
A network graph of the included studies is shown in Figure 2. Four nodes were compared in the network graph, in which the size of the nodes was associated with the number of patients undergoing a certain type of anastomosis, and the thickness of the lines was related to the number of direct comparisons between 2 reconstruction methods. The Newcastle-Ottawa scoring system (Table 2) showed high quality without obvious bias among the studies. Comparison-adjusted funnel plots (Supplementary Fig. 2) followed a symmetrical distribution, and no obvious publication bias was identified.
Figure 2.
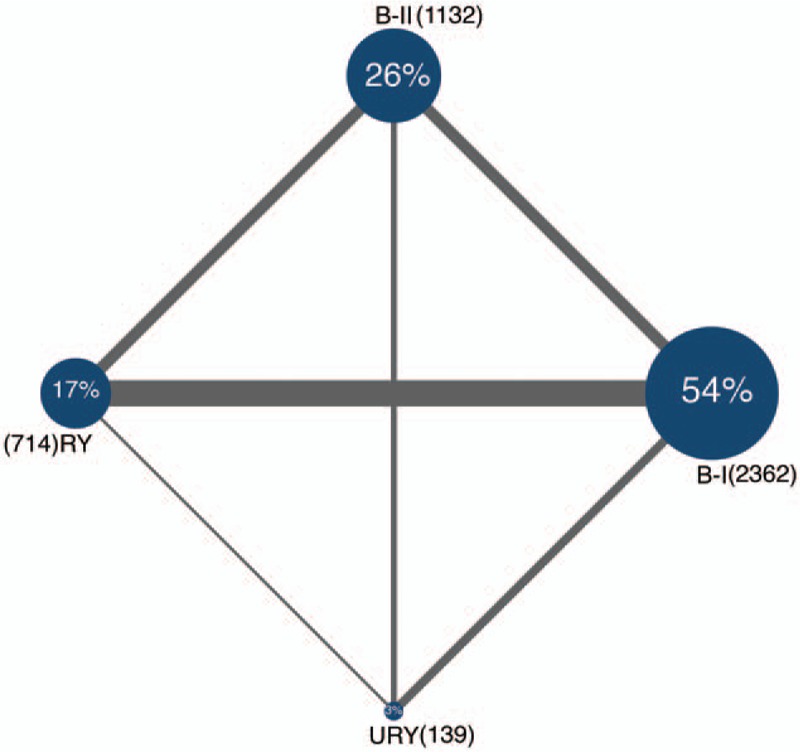
Network graph of the included studies. B-I = Billroth I anastomosis, B-II = Billroth II anastomosis, RY = Roux-en-Y anastomosis, URY = uncut Roux-en-Y anastomosis.
Table 2.
The risk of bias among included researches using the Newcastle-Ottawa Scale.
3.2. NMA results
3.2.1. Short-term outcomes
Short-term outcomes, including operative time, blood loss, time to first flatus, time to first soft diet, length of hospital stay, anastomotic leakage, anastomotic stenosis, anastomotic bleeding, and gastrointestinal motility dysfunction, were measured. If ileus, delayed gastric emptying or Roux stasis syndrome (RSS) were observed, gastrointestinal motility dysfunction could be diagnosed.
Operative time: Operative time was extracted from 14 studies.[13–15,17,18,20,22,24–30] The NMA results (Fig. 3) showed that RY anastomosis had a significantly longer operative time than B-I (SMDs: 0.74, 95%CIs: 0.44, 1.01) and B-II (SMDs: 0.53, 95%CIs: 0.20, 0.86) anastomoses. Nevertheless, no significant differences were found among the other reconstructive methods. SUCRA plots indicated that RY anastomosis had the highest probability of being the worst method for reducing operative time (SUCRA = 0.9%), while B-I anastomosis had the highest probability of being the best method (SUCRA = 92.1%), followed by B-II (SUCRA = 56.2%) and URY (SUCRA = 50.7%) anastomoses (Supplementary Fig. 4). No obvious differences were discovered between network comparison and direct comparison, and no significant differences were detected by the inconsistency check (Supplementary Fig. 3).
Figure 3.
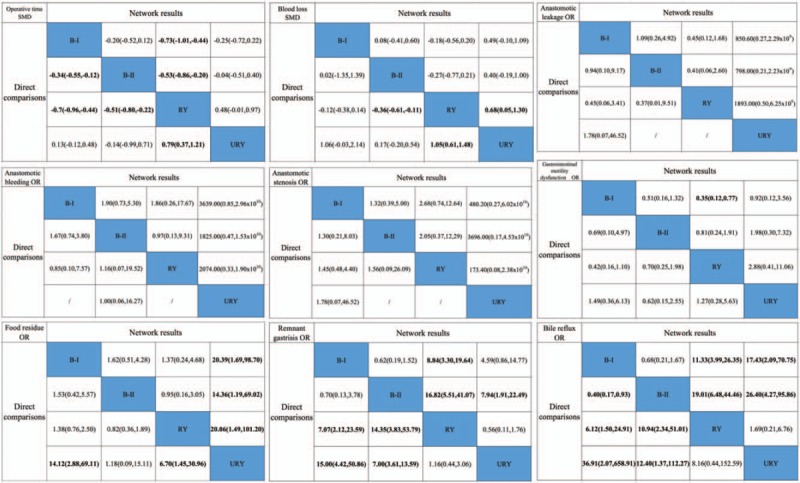
Results of network and direct comparisons between different reconstructions. B-I = Billroth I anastomosis, B-II = Billroth II anastomosis, OR = odds ratio, RY = Roux-en-Y anastomosis, SMD = standardized mean difference, URY = uncut Roux-en-Y anastomosis. The columns represent direct comparisons, and the rows represent all network comparisons.
Blood loss: Blood loss was investigated in 11 studies.[13,14,17,18,20,22,24,25,27,28,30] The NMA results (Fig. 3) demonstrated that URY anastomosis was significantly superior to RY anastomosis in reducing blood loss (SMDs: –0.68, 95%CIs: –1.30, –0.05). However, no significant differences were found among the other reconstruction methods. SUCRA plots (Supplementary Fig. 4) indicated that URY anastomosis had the highest probability of being the best method for reducing blood loss (SUCRA = 95.0%), followed by B-II (SUCRA = 53.2%) and B-I (SUCRA = 41.6%) anastomoses. In contrast, RY anastomosis had the highest probability of being the worst method for reducing blood loss (SUCRA = 10.0%). Network and direct comparison results were mostly comparable, and no significant differences were detected by the inconsistency check (Supplementary Fig. 3).
Anastomotic leakage, anastomotic bleeding, and anastomotic stenosis: The NMA results (Fig. 3) showed no significant differences among B-I, B-II, RY, and URY anastomoses in terms of anastomotic leakage, anastomotic bleeding or anastomotic stenosis. However, the ORs and 95%CIs in the NMA between URY anastomosis and other anastomoses were substantially large, which might be due to small sample effects. Although no significant differences were found by the inconsistency check among B-I, B-II and RY anastomoses, we failed to obtain a comparison loop containing URY anastomosis (Supplementary Fig. 3).
Gastrointestinal motility dysfunction: Gastrointestinal motility dysfunction was investigated in 15 studies.[13–15,18,19,21–30] The NMA results (Fig. 3) demonstrated a significant superiority of B-I anastomosis to RY anastomosis (ORs: 0.35, 95%CIs: 0.12, 0.77). Nonetheless, no significant differences were found among the other reconstructive methods. Network comparison and direct comparison yielded very similar results, and no significant differences were found by the inconsistency check (Supplementary Fig. 3). SUCRA plots (Supplementary Fig. 4) showed that B-I anastomosis had the highest probability of being the best method for preventing gastrointestinal motility dysfunction (SUCRA = 88.1%), followed by URY (SUCRA = 58.1%) and B-II (SUCRA = 38.3%) anastomoses. In contrast, RY anastomosis had the lowest probability of being the best method for preventing gastrointestinal motility dysfunction (SUCRA = 15.4%).
3.2.2. Long-term endoscopic findings
Long-term endoscopic findings were measured by postoperative endoscopy at 1 year after LDG. These outcomes were categorized according to the “residue, gastritis, bile” (RGB) classification proposed by Kubo et al.[31] Nine studies[13,14,17,21,23,27–30] reported long-term endoscopic findings; 1 study simultaneously compared B-I, B-II, RY, and URY anastomoses; 1 study compared B-I and B-II anastomoses; 3 studies compared B-I and RY anastomoses; 3 studies compared B-II and RY anastomoses; and 1 study compared B-II and URY anastomoses.
Food residue: The NMA results (Fig. 3) indicated a significant superiority of URY anastomosis to B-I (ORs: 0.05, 95%CIs: 0.01, 0.59), B-II (ORs: 0.07, 95%CIs: 0.01, 0.84) and RY (ORs: 0.05, 95%CIs: 0.01, 0.67) anastomoses for preventing food residue. However, no significant differences were found among the other 3 reconstructive methods. Network comparison and direct comparison yielded very similar results, and no significant differences were found by the inconsistency check (Supplementary Fig. 3). SUCRA plots (Supplementary Fig. 4) indicated that URY anastomosis had the highest probability of being the best method for decreasing food residue (SUCRA = 98.9%), followed by B-II (SUCRA = 48.1%), RY (SUCRA = 28.2%) and B-I (SUCRA = 24.6%) anastomoses.
Remnant gastritis: The NMA results (Fig. 3) indicated a significant superiority of RY anastomosis to B-I (ORs: 0.11, 95%CIs: 0.05, 0.30) and B-II (ORs: 0.06, 95%CIs: 0.02, 0.18) anastomoses and a significant superiority of URY anastomosis to B-II anastomosis (ORs: 0.13, 95%CIs: 0.04, 0.52) for preventing remnant gastritis. Nevertheless, no significant differences were found among the other reconstruction methods. Network comparison and direct comparison were yielded very similar results, and no significant differences were found by the inconsistency check (Supplementary Fig. 3). SUCRA plots (Supplementary Fig. 4) indicated that RY anastomosis had the highest probability of being the best method for decreasing remnant gastritis (SUCRA = 95.9%), followed by URY (SUCRA = 69.3%) and B-I (SUCRA = 30.6%) anastomoses. In contrast, B-II anastomosis had the lowest probability of being the best method for preventing remnant gastritis (SUCRA = 4.0%).
Bile reflux: The NMA results (Fig. 3) indicated that both RY and URY anastomoses were significantly superior to B-I (ORs: 0.09, 95%CIs: 0.04, 0.25 and ORs: 0.06, 95%CIs: 0.01, 0.48, respectively) and B-II (ORs: 0.05, 95%CIs: 0.02, 0.15 and ORs: 0.04, 95%CIs: 0.01, 0.23, respectively) anastomoses in preventing bile reflux. However, no significant differences were found between either B-I and B-II anastomoses or between RY and URY anastomoses. Network comparison and direct comparison yielded very similar results, and no significant differences were found by the inconsistency check (Supplementary Fig. 3). SUCRA plots (Supplementary Fig. 4) showed that URY anastomosis had the highest probability of being the best method for preventing bile reflux (SUCRA = 84.7%), followed by RY (SUCRA = 81.8%) and B-I (SUCRA = 28.1%) anastomoses. In contrast, B-II anastomosis had the lowest probability of being the best method for preventing bile reflux (SUCRA = 5.2%).
4. Discussion
In our NMA, we evaluated four reconstruction procedures in 4347 patients who underwent LDG. This is the first NMA to simultaneously compare 4 anastomoses after LDG.
B-I anastomosis is widely used because it conforms to physiological pathways, but due to strong anastomotic tension and a high risk of anastomotic leakage, it cannot be implemented in all patients.[32] B-II anastomosis relieves anastomotic tension, but it results in the most postoperative complications due to changes in the normal physiological pathway.[33] RY anastomosis decreases the rate of common postoperative complications; nevertheless, it causes RSS.[34] In contrast, URY anastomosis can reduce the risk of RSS, but it carries the risk of recanalization of the uncut stapled line.[35]
Roughly, in our NMA, RY anastomosis performed poorly for decreasing operative time and blood loss. Nevertheless, RY and URY anastomoses were better than B-I and B-II anastomoses for preventing adverse long-term endoscopic findings.
RY anastomosis involves one more anastomotic site than B-I and B-II anastomoses. In addition, in RY anastomosis, the mesentery needs to be separated, and mesenteric vessels need to be manipulated, unlike in B-I, B-II, or URY anastomosis.[5,6] Therefore, a significantly longer operative time and greater blood loss occur with RY anastomosis. These results are consistent with the results of previous meta-analyses.[4–6]
The OR values for URY anastomosis and the other methods were quite large, which compelled us to consider small sample effects. A possible explanation is that only 2 studies[25,26] with 19 patients analyzed URY anastomosis with regards to anastomotic complications, and one[26] of them was excluded from the network analysis because of the lack of positive results between groups with and without complications. Furthermore, no positive events were observed with URY anastomosis in the remaining study,[25] which decreased comparability between URY anastomosis and the other methods. A recent meta-analysis[6] indicated that URY anastomosis preserved mesenteric continuity and ensured blood supply to the anastomotic site. However, the authors also failed to find a significant difference between RY and URY anastomoses in terms of anastomotic complications. They attributed this lack of difference to the use of cutting-edge technology and a stapling technique.[6]
A previous NMA[5] indicated that B-I anastomosis could be more effective than RY anastomosis for preventing gastrointestinal motility dysfunction because RY anastomosis destroys the physiological continuity of the gastrointestinal tract; this is consistent with our results. RSS, which influences the quality of life of patients, is a gastrointestinal motility dysfunction that is often encountered by surgeons using RY anastomosis.[36–39] A recent meta-analysis[6] demonstrated that for laparotomy, URY anastomosis performed better than RY anastomosis (ORs: 0.14, 95%CIs: 0.04, 0.50) for reducing the risk of RSS by maintaining the completeness and normal electrophysiological conduction of the small intestine. Although the SUCRA value of URY anastomosis was than higher than that of RY anastomosis for preventing gastrointestinal motility dysfunction in our NMA, we failed to find a significant difference. A possible explanation could be that RSS is usually observed in RY anastomosis with limbs longer than 40 cm, as demonstrated by Gustavsson et al.[40] In the studies we included,[19,21,23,24,27–29] the limb length was less than 40 cm.
For preventing food residue, URY anastomosis was more effective than the other methods because URY anastomosis has the advantages of maintaining intestinal integrity, good blood flow at the anastomotic site and normal intestinal peristalsis.[41] For preventing bile reflux, a significant superiority of RY and URY anastomoses to B-I and B-II anastomoses was shown because after URY or RY anastomosis, bile can flow into the excurrent jejunal limb through the second anastomotic site.[5] Remnant gastritis is associated with gastric stump carcinoma.[42] Regarding remnant gastritis, RY and URY anastomoses yielded better results. A possible explanation could be that RY and URY anastomoses have a good anti-reflux ability, which protects the stomach from bile encroachment.[4] Taking all the aforementioned results into consideration, we concluded that URY and RY anastomoses were better than B-I and B-II anastomoses in terms of long-term endoscopic findings.
Nonetheless, there are several limitations of our analysis. First, only 4 studies with 139 patients had investigated URY anastomosis, which may have caused a certain bias due to the small sample size. Third, our results were mostly based on retrospective studies and may have been influenced by inherent selection bias. Furthermore, we did not compare other important outcomes such as body weight change, amount of ingested food and various postgastrectomy symptoms due to a lack of original literature.
5. Conclusion
Both RY and URY anastomoses are superior to B-I and B-II anastomoses in terms of long-term endoscopic findings after LDG. URY anastomosis shows superiority to RY anastomosis in reducing operative blood loss. Furthermore, RY anastomosis is worse than B-I anastomosis in decreasing operative time and preventing gastrointestinal motility dysfunction. Therefore, URY anastomosis seems to be a favorable reconstruction method after LDG due to its superiority because of operative simplicity and fewer long-term complications. However, large prospective multicenter studies and well-designed RCTs, especially for URY anastomosis, are recommended for further validation of this conclusion.
Author contributions
Conceptualization: Wei Fu.
Data curation: Fei Li, Bingyan Wang.
Formal analysis: Yanpeng Ma, Fei Li.
Funding acquisition: Wei Fu.
Methodology: Yanpeng Ma, Xin Zhou, Bingyan Wang, Siyi Lu, Wendong Wang, Shuqing Yu.
Project administration: Xin Zhou, Wei Fu.
Resources: Fei Li.
Software: Yanpeng Ma, Siyi Lu, Shuqing Yu.
Supervision: Wei Fu.
Validation: Wendong Wang.
Visualization: Yanpeng Ma, Wendong Wang.
Writing – original draft: Yanpeng Ma, Shuqing Yu.
Writing – review & editing: Xin Zhou, Wei Fu.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: 95%CIs = 95% confidence intervals, B-I = Billroth I, B-II = Billroth II, LDG = laparoscopic distal gastrectomy, NMA = network meta-analysis, ORs = odds ratios, RCTs = randomized controlled trials, RSS = roux stasis syndrome, RY = Roux-en-Y, SMDs = standardized mean differences, SUCRA = surface under the cumulative ranking curve, URY = uncut Roux-en-Y.
How to cite this article: Ma Y, Li F, Zhou X, Wang B, Lu S, Wang W, Yu S, Fu W. Four reconstruction methods after laparoscopic distal gastrectomy: A systematic review and network meta-analysis. Medicine. 2019;98:51(e18381).
This research was supported by the National Nature Science Foundation of China (No. 81672361).
The authors have no conflicts of interests to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].van de Velde CJ, Peeters KC. The gastric cancer treatment controversy. J Clin Oncol 2003;21:2234–6. [DOI] [PubMed] [Google Scholar]
- [3].Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 1994;4:146–8. [PubMed] [Google Scholar]
- [4].Xiong JJ, Altaf K, Javed MA, et al. Roux-en-Y versus Billroth I reconstruction after distal gastrectomy for gastric cancer: a meta-analysis. World J Gastroenterol 2013;19:1124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cai Z, Zhou Y, Wang C, et al. Optimal reconstruction methods after distal gastrectomy for gastric cancer: a systematic review and network meta-analysis. Medicine 2018;97:e10823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sun MM, Fan YY, Dang SC. Comparison between uncut Roux-en-Y and Roux-en-Y reconstruction after distal gastrectomy for gastric cancer: a meta-analysis. World J Gastroenterol 2018;24:2628–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med 2002;21:2313–24. [DOI] [PubMed] [Google Scholar]
- [8].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- [9].Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–84. [DOI] [PubMed] [Google Scholar]
- [10].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [11].Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71. [DOI] [PubMed] [Google Scholar]
- [12].Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PloS One 2013;8:e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Park JY, Kim YJ. Uncut Roux-en-Y reconstruction after laparoscopic distal gastrectomy can be a favorable method in terms of gastritis, bile reflux, and gastric residue. J Gastric Cancer 2014;14:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kim CH, Song KY, Park CH, et al. A comparison of outcomes of three reconstruction methods after laparoscopic distal gastrectomy. J Gastric Cancer 2015;15:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kang KC, Cho GS, Han SU, et al. Comparison of Billroth I and Billroth II reconstructions after laparoscopy-assisted distal gastrectomy: a retrospective analysis of large-scale multicenter results from Korea. Surg Endosc 2011;25:1953–61. [DOI] [PubMed] [Google Scholar]
- [16].Lim SG, Lee KM, Kim SS, et al. Endoscopic approach for postoperative complications following laparoscopic-assisted gastrectomy in early gastric cancer: literature review. Hepato-gastroenterology 2012;59:1308–12. [DOI] [PubMed] [Google Scholar]
- [17].Kojima K, Yamada H, Inokuchi M, et al. A comparison of Roux-en-Y and Billroth-I reconstruction after laparoscopy-assisted distal gastrectomy. Ann Surg 2008;247:962–7. [DOI] [PubMed] [Google Scholar]
- [18].Lee SW, Tanigawa N, Nomura E, et al. Benefits of intracorporeal gastrointestinal anastomosis following laparoscopic distal gastrectomy. World J Surg Oncol 2012;10:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Komatsu S, Ichikawa D, Kubota T, et al. Clinical outcomes and quality of life according to types of reconstruction following laparoscopy-assisted distal gastrectomy for gastric cancer. Surg Laparosc Endosc Percutan Tech 2015;25:69–73. [DOI] [PubMed] [Google Scholar]
- [20].Kumagai K, Hiki N, Nunobe S, et al. Different features of complications with Billroth-I and Roux-en-Y reconstruction after laparoscopy-assisted distal gastrectomy. J Gastrointest Surg 2011;15:2145–52. [DOI] [PubMed] [Google Scholar]
- [21].Kitagami H, Morimoto M, Nozawa M, et al. Evaluation of the delta-shaped anastomosis in laparoscopic distal gastrectomy: midterm results of a comparison with Roux-en-Y anastomosis. Surg Endosc 2014;28:2137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Oki E, Sakaguchi Y, Ohgaki K, et al. Feasibility of delta-shaped anastomoses in totally laparoscopic distal gastrectomy. Eur Surg Res 2011;47:205–10. [DOI] [PubMed] [Google Scholar]
- [23].Inokuchi M, Kojima K, Yamada H, et al. Long-term outcomes of Roux-en-Y and Billroth-I reconstruction after laparoscopic distal gastrectomy. Gastric Cancer 2013;16:67–73. [DOI] [PubMed] [Google Scholar]
- [24].An JY, Cho I, Choi YY, et al. Totally laparoscopic Roux-en-Y gastrojejunostomy after laparoscopic distal gastrectomy: analysis of initial 50 consecutive cases of single surgeon in comparison with totally laparoscopic Billroth I reconstruction. Yonsei Med J 2014;55:162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kim JJ, Song KY, Chin HM, et al. Totally laparoscopic gastrectomy with various types of intracorporeal anastomosis using laparoscopic linear staplers: preliminary experience. Surg Endosc 2008;22:436–42. [DOI] [PubMed] [Google Scholar]
- [26].Suh YS, Park JH, Kim TH, et al. Unaided stapling technique for pure single-incision distal gastrectomy in early gastric cancer: unaided delta-shaped anastomosis and uncut Roux-en-Y anastomosis. J Gastric Cancer 2015;15:105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cui LH, Son SY, Shin HJ, et al. Billroth II with braun enteroenterostomy is a good alternative reconstruction to Roux-en-Y gastrojejunostomy in laparoscopic distal gastrectomy. Gastroenterology Res Pract 2017;2017:1803851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].In Choi C, Baek DH, Lee SH, et al. Comparison between billroth-II with Braun and Roux-en-Y reconstruction after laparoscopic distal gastrectomy. J Gastrointest Surg 2016;20:1083–90. [DOI] [PubMed] [Google Scholar]
- [29].Shim JH, Oh SI, Yoo HM, et al. Roux-en-Y gastrojejunostomy after totally laparoscopic distal gastrectomy: comparison with Billorth II reconstruction. Surg Laparosc Endosc Percutan Tech 2014;24:448–51. [DOI] [PubMed] [Google Scholar]
- [30].Yang D, He L, Tong WH, et al. Randomized controlled trial of uncut Roux-en-Y vs Billroth II reconstruction after distal gastrectomy for gastric cancer: Which technique is better for avoiding biliary reflux and gastritis? World J Gastroenterol 2017;23:6350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kubo M, Sasako M, Gotoda T, et al. Endoscopic evaluation of the remnant stomach after gastrectomy: proposal for a new classification. Gastric Cancer 2002;5:83–9. [DOI] [PubMed] [Google Scholar]
- [32].Sah BK, Chen MM, Yan M, et al. Gastric cancer surgery: Billroth I or Billroth II for distal gastrectomy? BMC Cancer 2009;9:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tran TB, Worhunsky DJ, Squires MH, et al. To Roux or not to Roux: a comparison between Roux-en-Y and Billroth II reconstruction following partial gastrectomy for gastric cancer. Gastric Cancer 2016;19:994–1001. [DOI] [PubMed] [Google Scholar]
- [34].Hoya Y, Mitsumori N, Yanaga K. The advantages and disadvantages of a Roux-en-Y reconstruction after a distal gastrectomy for gastric cancer. Surg Today 2009;39:647–51. [DOI] [PubMed] [Google Scholar]
- [35].Tu BN, Sarr MG, Kelly KA. Early clinical results with the uncut Roux reconstruction after gastrectomy: limitations of the stapling technique. Am J Surg 1995;170:262–4. [DOI] [PubMed] [Google Scholar]
- [36].Shimoda M, Kubota K, Katoh M, et al. Effect of billroth II or Roux-en-Y reconstruction for the gastrojejunostomy on delayed gastric emptying after pancreaticoduodenectomy: a randomized controlled study. Ann Surg 2013;257:938–42. [DOI] [PubMed] [Google Scholar]
- [37].Masui T, Kubora T, Nakanishi Y, et al. The flow angle beneath the gastrojejunostomy predicts delayed gastric emptying in Roux-en-Y reconstruction after distal gastrectomy. Gastric Cancer 2012;15:281–6. [DOI] [PubMed] [Google Scholar]
- [38].Woodward A, Sillin LF, Wojtowycz AR, et al. Gastric stasis of solids after Roux gastrectomy: is the jejunal transection important? J Surg Res 1993;55:317–22. [DOI] [PubMed] [Google Scholar]
- [39].Mathias JR, Fernandez A, Sninsky CA, et al. Nausea, vomiting, and abdominal pain after Roux-en-Y anastomosis: motility of the jejunal limb. Gastroenterology 1985;88(1 Pt 1):101–7. [DOI] [PubMed] [Google Scholar]
- [40].Gustavsson S, Ilstrup DM, Morrison P, et al. Roux-Y stasis syndrome after gastrectomy. Am J Surg 1988;155:490–4. [DOI] [PubMed] [Google Scholar]
- [41].Zhang YM, Liu XL, Xue DB, et al. Myoelectric activity and motility of the Roux limb after cut or uncut Roux-en-Y gastrojejunostomy. World J Gastroenterol 2006;12:7699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ma Z, Wang Z, Zhang J. Carcinogenicity of duodenogastric reflux juice in patients undergoing gastrectomy. Zhonghua wai ke za zhi [Chinese journal of surgery] 2001;39:764–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


