Abstract
Depression, one of the most common mental health disorders, is among the leading causes of health-related disability worldwide. Although antidepressant treatment has been available for decades, depression remains largely refractory to the prevailing limited treatment approach of monoamine transmission modulation. Fortunately, recent evidence points to a link between depression and inflammatory factors within the innate and the adaptive immune system. The purpose of this review is to evaluate current and potential clinical immunotherapies for depression, as contextually focused by an immunologic lens of the pathophysiologic mechanisms of depression. The utility of pro-inflammatory cytokines (primarily interleukin-1β, interleukin -6 and tumor necrosis factor-α) is considered in their role as screening biomarkers in prediction of treatment response or nonresponse. The evidence base of numerous recent clinical studies is discussed as related to their antidepressant efficacy and favorable safety profile, with consideration of multiple agents that target inflammatory mechanisms linked to depression including nonsteroidal anti-inflammatory pathways (i.e., aspirin, celecoxib), cytokine antagonism (i.e., etanercept, infliximab), N-methyl-D-aspartate receptor (NMDA) receptor antagonism (i.e., ketamine), and modulation of kynurenine pathways (i.e., minocycline). Additionally, new and exciting directions in targeting inflammatory mechanisms in the treatment of depression are underway, and future investigation is also warranted to explore the utility of inflammation in diagnosing depression, guiding clinical treatment decision-making, and monitoring disease burden and relapse risk.
Keywords: Immunotherapy, Mood disorders, Depression, Inflammation
1. Introduction
Depression is among the most common and costly of all psychiatric disorders. Nearly one in four women and one in six men experience depression during their lifetime, and up to 65% of individuals have recurrent episodes of the disorder. Additionally, a recent study of global burden of disease found that mental health disorders were the number one cause of years lived with disability (YLDs) in the world, with depression accounting for nearly half of these years. Moreover, the overall burden of mental health disease has increased by nearly 40% in the past 20 years (Whiteford et al., 2013).
The extensive negative impact that depression can have on one’s emotional, social, and occupational functioning warrants the prioritization of effective prevention and treatment measures. Nearly two thirds of patients do not achieve remission upon initial treatment despite adherence to treatment and are thus considered “treatment resistant” (Andrews et al., 2004; Chisholm et al., 2004; Alexopoulos, 2005; Roose and Schatzberg, 2005). Epidemiological studies have estimated the rates of relapses and recurrences during continued antidepressant treatment to be between approximately 10% and 30% (Fava, 2003, Rush et al., 2006). The narrow focus of antidepressant pharmacological therapies on modulation of monoamine transmission is a limitation, which may contribute to the high rates of refractory treatment outcomes. As such, patients who do not respond to such treatments that rely on monoaminergic mechanisms have few therapeutic options. Furthermore, although the addition of agents such as atypical antipsychotics to traditional antidepressants has yielded some benefit (Nelson and Papakostas, 2009), the majority of patients continue to suffer from debilitating disease.
It is well established that depression is associated with inflammatory factors within the innate and the adaptive immune system as previously reviewed (Irwin and Miller, 2007; Miller et al., 2009; Slavich and Irwin, 2014; Miller and Raison, 2016). This association is supported by evidence that a subset of depressed individuals has an inflammatory component that might be driving their disease process (Pariante, 2016; Wohleb et al., 2016). Moreover, increased levels of pro-inflammatory cytokines including, but not limited to, interleukin (IL)-6, tumor necrosis factor (TNF) α and IL-1β, as well as elevated concentrations of acute phase proteins (Dinan, 2009; Maes, 2011; Leonard and Maes, 2012; Maes et al., 2012) have been identified in depressed individuals. These data correlate with studies showing that, compared to responders, depressed individuals who fail to respond to monoamine reuptake inhibiting agents have abnormal increases in various markers of pro-inflammatory activity. For example, elevated levels of circulating markers of inflammation predict poor response to selective serotonin reuptake inhibitor (SSRI) treatment. In contrast, studies have demonstrated that elevated levels markers of inflammation predict enhanced, rather than attenuated, treatment response to tricyclic antidepressant (TCA) medications, ketamine, and electroconvulsive therapy (ECT) (O’Brien et al., 2007; Yoshimura et al., 2009; Carvalho et al., 2013). In animal models, such conclusions have been corroborated by studies showing that immune activation produces depression-like behaviors in repeatedly stressed animals (Hodes et al., 2014) . Moreover, a rapidly developing and dynamic clinical literature provides evidence that some types of anti-inflammatory treatments can produce antidepressant effects in patients with evidence of inflammation (Köhler et al., 2015; Kohler et al., 2016).
The objective of this review to build upon prior research that has systematically examined the mechanisms linking immune dysfunction to depression, and to evaluate current and potential clinical immunotherapies for depression, as contextually focused by an immunologic lens of the pathophysiologic mechanisms of depression. The utility of this review is for clinical researchers and practitioners given its focus on evaluating the evidence base of numerous recent clinical studies which have tested the antidepressant efficacy and favorable safety profile of multiple agents that target inflammatory mechanisms linked to depression including nonsteroidal anti-inflammatory pathways (i.e., aspirin, celecoxib) cytokine antagonism (i.e., etanercept, infliximab), N-methyl-D-aspartate receptor (NMDA) receptor antagonism (i.e., ketamine), and modulation of kynurenine pathways (i.e., minocycline). Additionally, this review is aligned with the interests of basic and clinical neuroscientists who seek to gain an updated review on the current and potential immunotherapies for depression for consideration in development of new strategies to monitor depression risk, predict outcome, and treat depression.
2. Inflammatory pathophysiology of depression
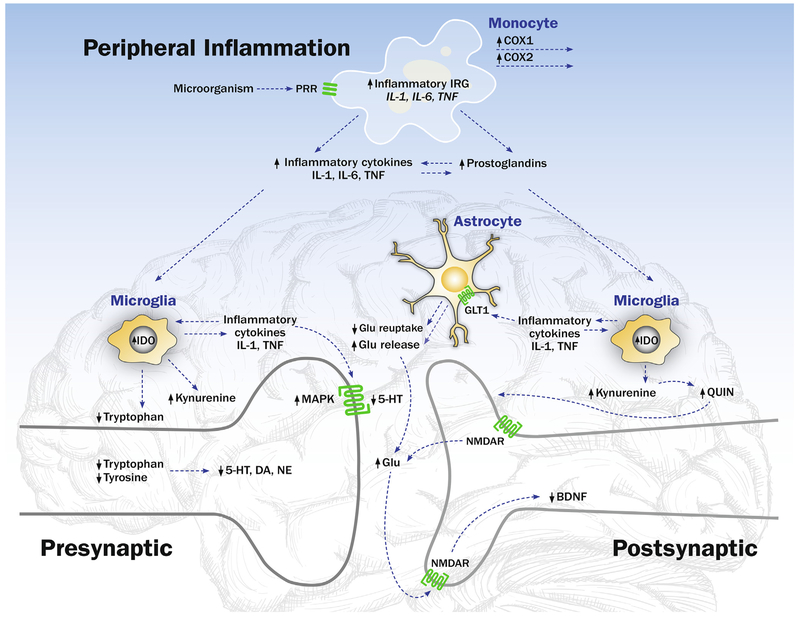
Aside from being classified as either acute or chronic, inflammatory processes are also divided into central and peripheral components. During the classic peripheral inflammatory process, innate immune cells sense pathologic changes within all tissues of the body, including the central nervous system (CNS). Innate immune cells, such as monocytes or macrophages and dendritic cells, circulate in the body and use invariant receptors to sense alterations in tissues through damage-associated molecular patterns (DAMPs) (Heppner et al., 2015). When pattern recognition receptors (PRRs) of innate immune cells recognize highly conserved features of microbes or pathogen-associated molecular patterns (PAMPs) (i.e., pattern recognition), an increase in inflammatory activity or an acute phase response occurs both locally, at the site of injury or infection, and systemically (Medzhitov, 2008). (Fig. 1) One of the best characterized families of these PRRs is Toll-like receptors (TLRs). Innate immune cells use TLR and other pattern recognition strategies to identify conserved components of microbes to activate inflammatory and antimicrobial innate immune responses. For example, specific ligands, such as lipopolysaccharide (LPS; i.e., a membrane component of Gram negative bacteria) bind to TLR4 and activate intracellular transcription factors nuclear factor-κB (NF-κB) and activator protein 1 (AP-1). In turn, these transcription factors drive the expression of pro-inflammatory immune response genes such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1 (IL-1), that lead to translation and production of respective pro-inflammatory cytokines, the main effectors of the inflammatory response. Inflammatory cytokines are further subdivided into anti-inflammatory cytokines, such as interleukin (IL)-10, and pro-inflammatory cytokines, including TNF-α and IL-6 among others. These molecular messengers also allow for communication with members of the adaptive immune system, including B- and T-cells (Jiang et al., 2014; Sokol and Luster, 2015). Inflammatory cytokines, especially TNF-α and IL-6, are the primary molecular targets for cytokine antagonist therapies for depression, as discussed below.
Fig. 1.
The Effects of the Peripheral and CNS Inflammatory Signaling on Monoamine and Glutaminergic Neurotransmission. Innate immune cells such as monocytes use invariant, pattern recognition receptors (PRR), to sense highly conserved features of microbes or pathogen-associated molecular patterns. Activation of this PRR such as Toll-like receptor-4 TLR4 induces increases of intracellular transcription factors such as nuclear factor-κB (NF-κB) and activator protein 1 (AP-1), which drive the expression of pro-inflammatory immune response genes such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1 (IL-1), and translation and production of respective pro-inflammatory cytokines such as IL-1, IL-6, and TNF. The release of inflammatory cytokines induces increases in the activity of cyclooxygenases (COX-1 and COX-2), that generates increases in prostaglandins and the promotion of further increases in inflammation. In the brain, microglia, analogous to the macrophages of the peripheral immune system, are primary cellular recipients of peripheral inflammatory signals. Activation of these microglia lead to a shift in their morphology to an amoeboid shape with release of pro-inflammatory cytokines (IL-6, TNF-α, IL-1β) within the CNS. Microglial release of pro-inflammatory cytokines regulate monoamine metabolism by activating mitogen-activated protein kinase (MAPK), which increases the number and activity of presynaptic reuptake pumps, and acts to reduce the availability of monoamines — serotonin (5-HT), dopamine (DA) and noradrenaline (NE) in the neuronal synapse. Microglial release of proinflammatory cytokines also activates the enzyme indoleamine 2,3 dioxygenase (IDO), which metabolizes tryptophan into kynurenine and decreases the availability of tryptophan for serotonin (5HT) synthesis. Addditionally, activated microglia convert kynurenine into quinolinic acid, which binds to the N-methyl-d-aspartate receptor (NMDAR), a glutamate receptor, leading to increased release of glutamate (Glu), an excitatory amino acid neurotransmitter. Inflammatory cytokines also act on astrocytes and induce a reduction of glutamate reuptake and an increase in the release of glutamate. Excessive amounts of glutamate bind to extrasynaptic NMDARs, which can lead to a reduction in synthesis of brain-derived neurotrophic factor (BDNF) with effects on neuronal integrity, including neurogenesis.
In addition, to the effects of inflammatory cytokines on the regulation of the innate- and adaptive immune system, the release inflammatory cytokines also induces increases in the activity of cyclooxygenases (COX-1 and COX-2), (Fig. 1) which generate increases in prostaglandins (Sokol and Luster, 2015) and in turn promote further increases in inflammation. Inhibition of COX-1 and COX-2 enzymatic activity is the target of non-steroidal anti-inflammatory drugs (NSAID), which have also been proposed as novel neuroimmune treatments of depression (see below).
A further component of the body’s inflammatory response lies within the central nervous system (CNS), and immune reactions within the CNS are labelled collectively as neuroinflammation (Bentivoglio et al., 2011), which is also a target of neuroimmune therapies for depression. Although the CNS contains a variety of inflammatory cells, microglia and astrocytes are the most pivotal members. Microglia are analogous to the macrophages of the peripheral immune system, whereas astrocytes possess numerous functions including maintenance of ion concentrations within and outside neurons along with support of the blood brain barrier (Bentivoglio et al., 2011; Jiang et al., 2014).
At the organismic level, the CNS integrates information regarding general physiological conditions, as well as the extra-organismic perceived environment, to steer the inflammatory response in the periphery and to amplify signals within the CNS to influence neurotransmitters and neurocircuits, which coordinate behavioral changes such as alterations in sleep, motivation and reward (i.e., anhedonia), and depressive symptoms (i.e., depressed mood, anxiety). Indeed, microglia serve as the primary cellular recipients of these inflammatory signals. When “activated”, the cellular morphology of microglia becomes amoeboid with release of pro-inflammatory cytokines (IL-6, TNF-α, IL-1β) within the CNS. (Fig. 1)
Relevant to depression, activation of microglia can alter monoamine metabolic pathways, which are well known to be the principal neurotransmitters that regulate arousal- and reward mechanisms. (Fig. 1) The molecular pathways by which microglial release of pro-inflammatory cytokines (i.e., IL-1β, TNFα) regulate monoamine metabolism are several fold. First, these central inflammatory signals activate mitogen-activated protein kinase (MAPK), which increases the number and activity of presynaptic reuptake pumps, and acts to reduce the availability of monoamines — serotonin (5-HT), dopamine (DA) and noradrenaline (NE) in the neuronal synapse. Secondly, these proinflammatory cytokines also activate the enzyme indoleamine 2,3 dioxygenase (IDO). This enzyme metabolizes tryptophan into kynurenine, and in so doing, decreases the availability of this primary amino acid precursor for serotonin. Thirdly, activated microglia also convert kynurenine into quinolinic acid. When quinolinic binds to the N-methyl-d-aspartate receptor (NMDAR), a glutamate receptor, there is increased release of glutamate, which is an excitatory amino acid neurotransmitter. This molecular process is thought to be the target of ketamine, a non-competitive NMDA receptor antagonist, in its antidepressant action, as described below. Finally, inflammatory cytokine can act on astrocytes and induce both a reduction in glutamate reuptake and an increase in the release of glutamate. When excessive amounts of glutamate bind to extrasynaptic NMDARs, there is a reduction in synthesis of brain-derived neurotrophic factor (BDNF) with effects on neuronal integrity, including neurogenesis. Interestingly in its antidepressant effect, ketamine appears to potentiate BDNF.
Substantial research has implicated two monoamine neurotransmitters, glutamate and dopamine, in the induction of depressive- and anhedonic symptoms and behaviors following inflammatory exposure. For example, it is known that (LPS) mediates inflammation induced depressive-like behavior by activating the aforementioned IDO pathway, and it appears that this effect is due in part to increased glutaminergic transmission through NMDA receptor activation. In mice, a recent study by Walker et al. demonstrated that administering low dose ketamine, therefore blocking NMDA mediated glutamate transmission, ameliorates the depressive like behavior following exposure to LPS (Walker et al., 2013). Similarly, in humans, a cross sectional study of 50 untreated depressed individuals found that elevated plasma and cerebrospinal fluid (CSF) levels of C-reactive protein (CRP) were associated with increased basal ganglia glutamate levels. Such increased glutamate, in turn, was associated with increased anhedonia and psychomotor retardation in this patient sample (Haroon et al., 2016) . Additionally, alterations in dopamine synthesis, release, or reuptake appear to contribute to inflammation induced behavioral deficits consistent with anhedonia and amotivation. In patients receiving chronic (i.e., 4 to 6 weeks) of interferon-α treatment, activity of the basal ganglia is reduced in association with behavioral symptoms of anhedonia, depression, and fatigue, which correlate with alterations in presynaptic striatal dopamine function (Capuron et al., 2012). Importantly, animal studies have revealed that motivational deficits commonly found in depressed patients may be due to selective loss of dopamine function, because blockade of dopamine uptake, but not serotonin or norepinephrine, increases selection of high effort activity (i.e., increased motivation) (Yohn et al., 2016). Together, these findings provide further evidence that inflammatory stimuli, including inflammatory cytokines, target basal ganglia and dopamine function to induce behavioral changes especially anhedonia and motivational symptoms. Chronic, low grade immune activation seen in major depressive disorder appears to contribute to modulation of glutaminergic and/or dopaminergic mechanisms that underlie motivational deficits in depression, which has implications for understanding why antidepressant therapies that target only serotonin often lack efficacy.
Neuro-glial interactions also appear to be altered in depression which may be due to activation or priming of microglial cells (Ben Achour and Pascual, 2010). In a post mortem analysis by Steiner et al., brain tissue of acutely depressed patients who had committed suicide was obtained and compared to that of controls. In depressed patients, both the subgenual anterior cingulate and anterior midcingulate cortices, two brain regions reliably implicated in the pathophysiology of depression among other mood disorders, showed evidence of increased density of microglia in the intermediate “activated” form (Steiner et al., 2011). Similarly, another post-mortem study of depressed patients who had committed suicide investigated the morphology and distribution of cells immunostained for the macrophage-specific marker ionized calcium binding adaptor molecule 1 (IBA1) within the white matter of the dorsal anterior cingulate cortex (dACC) white matter. As compared to controls, the dACC of depressed patients showed a higher proportion of immune reactive, “primed” microglia as compared to this brain region in controls (Torres-Platas et al., 2014). Increasingly it is thought that microglial activation may be associated with neuroprogression of major depressive disorder, as characterized by increasing persistence of depressive episodes in persons with a history of depression. To investigate this possibility, Seitawan and colleagues cross-sectionally evaluated depressed patients as compared to non-depressed controls using translocator protein (TSPO) total distribution volume (VT), a marker of microglial activation, and characterized whether this marker of neuroinflammation was associated with duration of untreated major depressive disorder, and with total illness duration and antidepressant exposure (Setiawan et al., 2018). Indeed, the degree of microglial activation (i.e., TSPO VT) was highly related to the persistence of major depression, in which duration of untreated major depression, total illness duration, and duration of antidepressant exposure, combined, contributed to over 50% of the variance in microglial activation in prefrontal cortex, ACC, and insula. Although these data are cross-sectional, the causal association between the accumulated illness burden and microglial activation is implicated by evidence that antidepressant treatment mitigates yearly increases in TSPO VT seen with untreated depression duration (Setiawan et al., 2018).
Another function of microglia is to prune synpases and alter synaptic function, in response to alteration from neuroinflammatory states (Chung and Barres, 2012; Aguzzi et al., 2013; Zhan et al., 2014). As such, in the context of mood disorders, it is unclear whether the “activated” microglia are responding in a reparative manner or, by virtue of their activation, are inducing further pathological disturbances of brain physiology (Yirmiya et al., 2015). Whatever the exact mechanism may be, both preclinical and ongoing clinical studies suggest that glial factors released during the neuroinflammatory cascade influence neurocircuitry, synaptic plasticity, and neuron formation in ways that eventually manifest as symptoms of mood disturbance (Bhattacharya and Drevets, 2016).
Though the CNS is generally thought to be a protected and immunologically isolated organ, there are a variety of pathways of communication between the peripheral immune system and the and central nervous system (CNS) (Anthony et al., 2012). For example, peripheral pro-inflammatory cytokines communicate with the CNS through neural mechanisms, humoral mechanisms, blood brain barrier transport mechanisms, and cellular processes. First, regarding neural mechanisms, inflammatory cytokines such as IL-1β, or pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide, can act on the vagus nerve (as reviewed (Dantzer et al., 2008)), which directly projects to multiple brain regions. Second, inflammatory cytokines can also enter the brain by volume diffusion as the circumventricular organs and the choroid plexus of the CNS host macrophage-like cells expressing Toll-like receptors (TLRs), which when activated produce inflammatory cytokines (Vitkovic et al., 2000). Additionally, circulating levels of IL-1β can activate IL-1 receptors on endothelial cells, which coordinate the local production of prostaglandin E2, thereby triggering immune activation in the brain. Third, as previously reviewed (Banks, 2015), multiple immunomodulatory molecules are transported in and out of the CNS by the blood brain barrier. Rather than being passive diffusion or unregulated leakage related to dysfunction of the blood brain barrier, the CNS coordinates this active process. Fourth, monocytes can traffic to the vasculature of the brain and its parenchyma, when these immune cells become activated. Again, the CNS partly coordinates this process in that microglial cells, and possibly astrocytes, can be stimulated by peripheral inflammatory signals to produce CC-chemokine ligand 2 (CCL2; also known as MCP1) which leads to attraction of monocytes to the brain. (D’Mello et al., 2009) Finally, mounting clinical evidence demonstrates that a bidirectional relationship between the gut microbiota and the CNS, termed microbiota-gut-brain (MGB) axis, which impacts the pathogenesis of a number of disorders linked to inflammation and their association with increased risk of mood disorders (Petra et al., 2015).
3. Link between inflammation and depression
3.1. Elevated levels of inflammatory cytokines in depression: human studies
As reported in several meta-analyses, patients with depressive disorders show elevated levels of pro- and anti-inflammatory cytokines, as compared to matched controls, with reported increases in levels of IL-2, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, interferon (IFN)-γ, CRP, and TNF-α (Dowlati et al., 2010; Dahl et al., 2014; Haapakoski et al., 2015). Yet, among this wide-range of inflammatory cytokines, robust and consistent elevations are primarily reported for CRP, IL-6, IL-1 (including IL-1β), and TNF-α. A recent systematic review and meta-analysis evaluated 11 longitudinal cohort studies (8 CRP studies and 3 IL-6 studies) conducted in the US, UK, and Australia in order to determine if elevated levels of these cytokines were prospectively associated with increased depressive symptoms. Using a mixed-effects model, elevated levels of both CRP (adjusted weighted-mean effect size r′ = 0.046, p < 0.0005) and IL-6 (adjusted weighted-mean effect size r′ = 0.097, p-value = 0.06) were significantly associated with subsequent increases in depressive symptoms (Valkanova et al., 2013). There was moderate heterogeneity between CRP studies (Q = 11.21, p = 0.08, 12 = 46.5) (Valkanova et al., 2013). Another recent meta-analysis evaluated twenty-nine studies in order to compare levels of soluble interleukin-2 receptor (sIL-2R) and cytokines in the blood between patients with major depressive disorder and controls, and found that sIL-2R, TNF-α and IL-6 serum levels in depressed patients were significantly higher than those of healthy controls (SMD = 0.555, p < 0.001, SMD = 0.567, p = 0.010; SMD = 0.680, p < 0.001 respectively). Meta-regression analysis identified mean age of all subjects as a significant factor impacting high heterogeneity levels of IL-6 (Liu et al., 2012).
Despite these reported increases in inflammatory cytokines in certain depressed patient populations, it is not possible to infer that inflammation is a uniform consequence of depression. There is striking variability of cytokine findings between different studies, in that not all subjects with depression show increases in these inflammatory cytokines, and not all persons with elevated inflammatory cytokines have depression. It is more likely that inflammation is associated with depression in a subtype of depressed patients who may have certain vulnerabilities or biological predispositions for developing inflammatory cytokine-associated depression (Lotrich, 2015).
It is important to note that the above studies only investigated peripheral inflammatory markers, which could be influenced by a variety of other confounding factors such as body mass index, waist circumference, and blood pressure, and the confounding influence of these factors on the association between depression and inflammation has not be adequately considered in the vast majority of studies. Indeed, in studies that adjusted for confounding factors such as waist circumference and blood pressure, depression effects on inflammation were absent (Krogh et al., 2014). More recent studies have attempted have begun to examine the relationship between central levels of inflammation and depressive symptoms. For example, Felger et al. demonstrated that peripheral plasma levels of CRP in untreated depressed individuals correlated with CSF levels of CRP, TNFα, and IL-6 levels, which in turn were associated with anhedonia and increased depressive symptoms (Felger et al., 2018). This increase of CSF levels of TNFα and Il-6 in depressed individuals was further corroborated by a systematic review and meta-analysis of 69 studies, which also demonstrated an increase of these two inflammatory markers in the brain parenchyma in depression (Enache et al., 2019).
3.2. Inflammatory processes increase depression risk
Large scale epidemiological studies have correlated inflammatory somatic diseases with an elevated risk for depression and other mood disorders (Slavich and Irwin, 2014). These somatic diseases with inflammatory underpinnings include several autoimmune diseases, such as rheumatoid arthritis, and infectious diseases, such as sepsis and hepatitis (Korczak et al., 2011; Jacob et al., 2017). These findings have been supported by a nationwide, population-based, prospective cohort study using data obtained from Danish longitudinal registers; indeed, 91,637 patients were identified as having hospital contacts for mood disorders (for a total of 78 million person-years of follow-up). Data were adjusted for calendar year, age, and sex and analyzed with survival analysis techniques. This study found a 45% increased risk of depression after hospitalizations secondary to autoimmune disorders (Incidence rate ratios (IRR), 1.45; 95% CI, 1.39–1.52) and 62% increased risk after hospitalizations secondary to infections (IRR, 1.62; 95% CI, 1.60–1.64) (Benros et al., 2013).
Agents that are known to be pro-inflammatory, such as (IFN-α), are often used to treat somatic diseases such as melanoma and hepatitis C, and such pro-inflammatory treatment often induces mild to moderate depressive symptoms in patients (Reichenberg et al., 2005; Eggermont et al., 2008; Friebe et al., 2010). Of note, a contemporary systematic review and meta-analysis of 8 prospective controlled clinical trials investigating patients with malignant melanoma or hepatitis C was performed in order to assess if antidepressant treatment reduced the overall incidence of depression in IFN-treated melanoma and hepatitis C patients. The authors found that SSRI treatment (paroxetine, citalopram, escitalopram) during these trials reduced the overall incidence of major depression during IFN treatment in all patients (n = 589, odds ratio = 0.42; 95% confidence interval, 0.26–0.68; p < 0.001) and was associated with lower mean depression scores after 12 weeks of IFN treatment (g = −0.37; 95% confidence interval −0.59 to −0.18; p < 0.001, n = 375) (Sarkar and Schaefer, 2014).
3.3. Impact of cytokines on kynurenine pathway
Elements of the kynurenine pathway and their interplay with cytokines and CNS neurons are summarized in Fig. 1, as extensively reviewed previously (Dantzer et al., 2008; Miller et al., 2009; Dantzer, 2012). Within CNS microglia, the enzyme indoleamine-pyrrole 2,3-dioxygenase (IDO) plays a role in the metabolism of tryptophan to kynurenine and the subsequent conversion of kynurenine to neurotoxic quinolinic acid. At the same time, cytokine activation shunts metabolic activity from tryptophan to the kynurenine pathway, which further reduces tryptophan hydroxylase driven serotonin (5-HT) synthesis (Muller and Schwarz, 2007; Zoga et al., 2014). These studies are supported by the aforementioned Steiner et al. study which showed that microglia in the anterior cingulate cortex of acutely depressed patients contained greater quinolinic acid (NMDA receptor agonist) expression, implicating pro-inflammatory cytokine induct activation of the kynurenine pathway as a potential link to the immune hypothesis of depression (Steiner et al., 2011). This increase in quinolinic acid and its downstream effects on NMDA receptor modulation has provided molecular rationale for the current studies investigating the use of ketamine as an antidepressant therapy.
3.4. Cytokines and antidepressants
Many in vitro studies have showcased the impact that selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs) have on pro-inflammatory cytokines. An in-vitro analysis of human blood samples demonstrated that antidepressants as a class do not have an uniform impact on cytokine levels. Rather, cytokine levels were variously increased or decreased depending on the particular antidepressant in question (Munzer et al., 2013). Another study utilizing the in-vitro methodology of incubating stimulated blood with various antidepressants including clomipramine, sertraline, and trazodone, found that these medications decreased stimulated production of IFN-γ and the anti-inflammatory cytokine IL-10 (Kopschina Feltes et al., 2017) . Fluoxetine and imipramine treatment led to decreased circulating levels of IL-1ß and TNF-α, in addition to decreased levels of microglial TNF-α and IL-1ß mRNA expression (Obuchowicz et al., 2014). Similarly, both paroxetine and amitriptyline have been found to reduce microglia-mediated neurotoxicity by decreasing TNF-α and IL-1β mRNA levels (Vismari et al., 2012; Liu et al., 2014). Contrary to the trend of decreasing pro-inflammatory markers, sertraline was found to increase peripheral levels of IFN-α and IL-6. Interestingly in this same study, however, sertraline administration resulted in decreased nuclear factor (NF)-κB activity, one of the principal transcription factors involved in regulation of pro-inflammatory cytokine production (Horowitz et al., 2014). Nevertheless, administration of the selective serotonin reuptake inhibitor, citalopram, to mice induces increases infrontal cortical levels of TNFα and IFNγ, and this effect is abolished by treatment with the NSAID, ibuprofen (Horowitz et al., 2014).
Recent clinical studies have demonstrated that, in addition to their effects on neurotransmitter regulation, antidepressants also possess anti-inflammatory characteristics via impacting pro-inflammatory cytokine production. A meta-analysis of 35 longitudinal studies was recently performed in order to analyze measured inflammatory markers at baseline and after pharmacologic antidepressant treatment. IL-6 levels were found to be decreased with antidepressant treatment regardless of treatment response [effect size: −0.57 (−1.09, −0.04), p = 0.03] (Strawbridge et al., 2015). Similarly, a meta-analysis of twenty-two studies (603 total subjects) evaluated the effect of TCAs, SSRIs, and SNRIs on baseline cytokine levels in depressed patients, and found that overall, IL-6, IL-1β, and TNF-α cytokine levels did not change significantly with the aforementioned treatment. However, subsequent stratification by antidepressant class demonstrated a significant decrease in IL-6 levels in response to SSRI treatment (n = 79 subjects, SMD = 1.45 (95% CI: 2.64, 0.25), Z = 2.4, p = 0.02). Of the six studies that measured IL-1β levels (n = 115 subjects), five used SSRI and one used TCA treatment. There was a significant effect of antidepressant treatment on IL-1β levels (SMD = 0.52 (95% CI: 0.83, 0.20), Z = 3.23, p < 0.001); no stratification by antidepressant class was performed given that five out of the six studies utilized SSRI treatment (Hannestad et al., 2011).
4. Immune biomarkers as predictors of treatment outcomes
The development of biomarkers that can predict treatment response is of clinical importance, with ideal testing being inexpensive and non-labor intensive. These biomarkers would be instrumental in terms of classifying patient subtypes (subtype of depressed patients with elevated immune profiles) and subsequently guiding optimal treatment alternatives if these patients are found to be treatment resistant to conventional antidepressant medication. When considering whether cytokines are associated with treatment outcome, the data in general tend towards a negative correlation between cytokine levels and clinical response (Janssen et al., 2010). In other words, higher levels of proinflammatory cytokines are related to poor response to antidepressant treatment, although the findings on ketamine, as well as ECT, are in the opposite direction as discussed below.
With regards to TNF-α cytokine levels in murine models, TNF-α gene knock-out mice show a lack of response to antidepressant treatment (Duseja et al., 2015), suggesting that the presence of some level of TNF-α is needed for antidepressants to have an effect. Yet, clinical translational studies indicate that high levels TNF-α may interfere with treatment response. In a recent trial of 100 outpatients with major depressive disorder (mean age 32.1 ± 11.9 years, 65% females), the relationship between baseline cytokine levels and prediction of escitalopram treatment response was evaluated. Responders showed lower baseline TNF-α levels in comparison to non-responders (F = 3.52; p = 0.033), indicating that higher baseline levels of TNF-α in depressed patients predict non-response to escitalopram treatment (Eller et al., 2008).
IL-6 may also be an important contribute to treatment response. For example, high baseline levels of serum IL-6 are associated treatment resistance (Lanquillon et al., 2000; Yoshimura et al., 2009). Another contemporary study investigated the differences in baseline IL-6, IL-8, IL-10, TNF-α and sIL-6R levels between three groups: SSRI treatment resistant (6 weeks of treatment) depressed patients (n = 28, 19 female), formerly SSRI treatment resistant patients who became euthymic afterwards (n = 16, 11 female), and healthy controls (n = 24, 14 female). SSRI-resistant depressive patients were found to have higher levels of IL-6 and TNF-α (p = 0.01, p = 0.004 respectively) compared to healthy controls (O’Brien et al., 2007). A more recent investigation by Haroon et al. (2018) further corroborated these findings by measuring concentrations of inflammatory markers in 98 depressed patients who had failed a varying numbers of adequate antidepressant treatment. The authors demonstrated that patients who had three or more failed treatment trials had significantly higher plasma TNFα-, STNF-R2 and IL-6 levels compared to individuals with less failed treatment trials. Thus, it was concluded that measuring inflammatory markers in depressed patients can be useful predictor of treatment resistance (Haroon et al., 2018).
On the other hand, the association between inflammation and response to ketamine appears to be in the opposing direction, in which elevated baseline levels of IL-6 predict better treatment responses to ketamine. For example, Yang et al. evaluated the antidepressant efficacy of ketamine, and examined what particular subset of patients who were most likely to benefit from ketamine treatment (Yang et al., 2015). The sample was comprised of 16 depressed inpatients who were treated with intravenous infusion of 0.5 mg/kg ketamine and 24 healthy, matched controls for comparison of baseline cytokines. Patients who responded to treatment had significantly higher baseline IL-6 levels than patients who did not respond. Of note, levels of IL-6 were similar between non-responder patients and control volunteers, although no threshold of IL-6 elevation was identified to predict better response; IL-6 was continuous measured in this study (Yang et al., 2015). Similarly, in a prospective study of 29 depressed patients (14 male, mean age = 42.6) who received ECT (electroconvulsive therapy), depressive symptoms were prospectively evaluated (before ECT treatment, after the second ECT treatment session, and finally at the completion of the index treatment series (Kruse et al., 2018). Results demonstrated that elevated baseline levels of IL-6 were associated with a greater decrease in depressive symptoms scores at the end of ECT index treatment (p = 0.01); moreover, post-hoc analysis revealed that this association was only significant in female patients (p = 0.02). Additionally, fluctuations of IL-6 levels throughout the course of treatment did not correlate with depressive symptoms scores over that time period (Kruse et al., 2018). The findings of this study corroborated the aforementioned study in that higher baseline levels of IL-6 predicted better treatment response. In sum, it appears that circulating levels of IL-6 predict treatment response depending in part on the type of treatment, in which elevated levels of IL-6 are associated with treatment non-response to SSRIs or SNRIs, yet treatment response to ketamine or ECT.
With regards to CRP levels predicting treatment response, a recent study extracted baseline CRP levels in patients from the Genome-Based Therapeutic Drugs for Depression (GENDEP), a multicenter open-label randomized clinical trial. CRP levels were measured with a high-sensitivity method in serum samples from 241 depressed patients randomly allocated to 12-weeks of escitalopram (N = 115) or nortriptyline (N = 126) treatment. Overall, baseline CRP levels differentially predicted treatment outcome with the two antidepressants (CRP-drug interaction: beta = 3.27, 95% CI = 1.65, 4.89). Specifically, a cutoff CRP value of 1 mg/L seemed to account for differential prediction of treatment response. For patients with CRP < 1 mg/L, improvement on the MADRS score was 3 points higher with escitalopram than with nortriptyline treatment. On the other hand, patients with CRP > 1 mg/L, improvement depressive symptoms scores was 3 points higher with nortriptyline than with escitalopram treatment (Uher et al., 2014). Similarly, a recent 2019 study evaluated peripheral CRP levels in 102 treatment-resistant patients with major depressive disorder who were in a depressive episode at the time, 48 treatment-responsive patients with major depressive disorder not experiencing depression at the time of study, 48 patients with depression who were not receiving medication, and 54 healthy non medicated, non-depressed individuals. Compared to the healthy volunteer group, CRP levels were significantly elevated in the treatment resistant depressed group and less elevated in the treatment responsive group. Given that variations in body mass index were adjusted in the analyses, this study suggests that baseline peripheral CRP levels can be useful tool for stratifying patients into a patient group, who may be potentially more responsive to second line antidepressant treatment (Chamberlain et al., 2019).
A pharmacogenetic study investigated the association between interleukin 1β single nucleotide polymorphisms (SNPs) and amygdala and anterior cingulate cortex (ACC) responsiveness to emotional stimuli (emotional responsiveness in these two brain regions has been linked with increased response to antidepressant treatment). Depressed patients (n = 256, 145 women, all Caucasian) were genotyped for variants rs16944, rs1143643, and rs1143634 in the IL1β gene (2q14). Results showed significant associations of GG genotypes of SNPs rs16944 and rs1143643 with nonremission after 6weeks of antidepressant treatment. Additional analyses showed that increased number of G-alleles in both SNPs (rs16944 and rs1143643) was associated with reduced responsiveness of the amygdala and ACC to emotional stimulation (Baune et al., 2010).
As indicated by the above studies, pro-inflammatory cytokines (mainly IL-1β, IL-6 and TNF-α) could be useful biomarkers for investigating the presence or level of inflammation present at baseline during depression screening. Additionally, levels of pre-treatment biomarkers appear to be predictors of treatment response or nonresponse. However, choice of immunological biomarkers for use in the clinical setting is limited by availability of standardized assays for clinical application, with only CRP being routinely available. Unfortunately, circulating levels of IL-1β, IL-6 and TNF-α levels are not yet standardized and are thus more limited to research trials (Dantzer et al., 2011).
5. Anti-inflammatory therapeutic treatment strategies for depression
Here, we review published clinical studies investigating anti-inflammatory agents and their impact on depressive symptoms. All study specific characteristics and outcomes are summarized in Table 1. Current ongoing trials involving anti-inflammatory agents and their antidepressant effects will also be discussed. Studies with inflammatory markers as primary or secondary outcomes are included and findings are summarized.
Table 1.
Published Clinical Studies on Anti-inflammatory Treatment Strategies for Depression.
| Study | Agent Studied and Site of Action | Total Number of Patients | Subjects | Depression Diagnosis | Comorbidities | Treatment | Outcomes |
|---|---|---|---|---|---|---|---|
| NSAID Monotherapy | |||||||
| Almeida et al. (2010) | Aspirin, COX-1 and COX-2 | 5556 | All males age 69–87. | GDS-15 | Smoking, cardiovascular disease, arthritis, cognitive impairment | Aspirin exposure in previous 5 years | Aspirin use not associated with lower odds of depression |
| Pasco et al. (2010) | Aspirin, COX-1 and COX-2 | 345 | All females above age 50. Twenty-two MDD and 323 controls | Structured Clinical Interview for DSM-IV-TR, research version, non-patient edition | Smoking, hyperlipidemia | Previous statin and aspirin exposure | Exposure to statins and aspirin associated with reduced MDD risk |
| Almeida et al. (2012) | Aspirin, COX-1 and COX-2 | 3687 | All males age 69–87 with increased homocysteine plasma levels | GDS-15 ≥ 7 | Smoking, others based on Charlson index | Aspirin exposure in previous 5 years | Aspirin use is associated with lower depression risk |
| Fields et al. (2012) | Celecoxib, COX-2; naproxen, COX-1 and COX-2 | 2312 | Age > 70. Depressive (449), controls (2079) | 30-item GDS score > 5 to | Family history of dementia | 12 months placebo (1,038) vs. celecoxib 400 mg (7 2 6) vs. naproxen 440 mg (7 1 9) daily | Celecoxib or naproxen did not improve depressive symptoms |
| Iyengar et al. (2013) | Celecoxib, COX-2; ibuprofen or naproxen, COX-1 and COX-2 | 1497 | Median age 61 in all treatment groups | PHQ-9 ≥ 10 | Active osteoarthritis | 6 weeks placebo (297) vs. ibuprofen 2400 mg or naproxen 1000 mg (593) vs. celecoxib200 mg (607) | All treatments superior to placebo |
| NSAID Augmentation Therapy | |||||||
| Muller et al. (2006) | Celecoxib, COX-2; reboxetine, noradrenaline reuptake | 40 | Mean age 44 in both treatment groups | HAMD 15–38 | None | 6 weeks reboxetine + placebo (20) vs. 400 mg (20) | Celecoxib group superior to reboxetine alone |
| Akhondzadeh et al. (2009) | Celecoxib, COX-2; fluoxetine, selective serotonin reuptake | 40 | Mean age 34 in both treatment groups | HAMD ≥ 18 | None | 6 weeks fluoxetine + placebo (20) vs. fluoxetine + celecoxib 400 mg (20) | Celecoxib group superior to fluoxetine alone |
| Abbasi et al. (2012) | Celecoxib, COX-2; sertraline, selective serotonin reuptake | 40 | Mean age of 35 in celecoxib group, 34 in placebo group | HAMD ≥ 18 | None | 6 weeks sertraline + placebo (20) vs. sertraline + celecoxib 400 mg (20) | Celecoxib group superior to sertraline alone. Baseline serum IL-6 predicted response |
| Majd et al. (2015) | Celecoxib, COX-2; fluoxetine, selective serotonin reuptake | 30 | Mean age 34 in celecoxib group, 36 in placebo group | HAMD ≥ 18 | None | 8 weeks fluoxetine + placebo (20) vs. fluoxetine + celecoxib 200 mg (20) | Celecoxib group superior to fluoxetine alone after 4 weeks but no difference after 8 weeks |
| Cytokine Inhibition | |||||||
| Tyring et al. (2006) | Etanercept, TNF-α | 618 | Mean age 45 in both treatment groups | HAMD, BDI | Psoriasis | 12 weeks placebo (309) vs. etanercept 50 mg (311) injections twice weekly | Etanercept superior to placebo for depression and fatigue |
| Menter et al. (2010) | Adalimumab, TNF-α | 96 | Mean age 45 in adalimumab group, 43 in placebo group | ZDS | Psoriasis | 12 weeks placebo (52) vs. adalimumab 40 mg (44) injectionsevery other week | Adalimumab superior in reducing depressive symptoms and improving QOL |
| Langley et al. (2010) | Ustekinumab, IL-12 and IL-23 | 1230 | Mean age 46 in ustekinumab group, 47 in placebo group | HADS-D | Psoriasis | 24 weeks placebo (410) vs. ustekinumab 45 mg (409) vs. ustekinumab 90 mg (411) | Both ustekinumab groups were superior to placebo in improving depressive symptoms and QOL |
| Raison et al. (2013) | Infliximab, TNF-α | 60 | Mean age 42 in infliximab group, 44 in placebo group | HAMD | None | 12 weeks three infusions placebo (30) vs. infliximab 5 mg/kg (30) | Infliximab superior if baseline CRP > 5mg/L |
| McIntyre et al. (2019) | Infliximab, TNF-α | 60 | Mean age 45 in infliximab group, 47 in placebo group | MADRS | History of childhood trauma (physical and/or sexual abuse) | 12 weeks three infusions placebo (31) vs. infliximab 5 mg/kg (29) | Infliximab superior only in pts with childhood trauma |
| Sun et al. (2017) | Sirukumab and siltuximab, IL-6 | 176 in sirukumab study, 65 in siltuximab study | Mean age 51 in sirukumab study, 44 in siltuximab study | PDMA criteria derived from SF-36 Health Survey | RA in sirukumab study, MCD in siltuximab study | Depressive symptoms assessed after 12 weeks of varying sirukumab doses and 15 weeks of siltuximab (11 mg/kg) intravenous infusion every 3 weeks | Siltuximab superior to placebo in improving depressive symptoms. Increased baseline IL-6R levels predicted sirukumab response |
| NMDA Receptor Antagonism | |||||||
| Yang et al. (2015) | Ketamine, NMDA receptor | 40 | 16 inpatient individuals, 24 healthy controls | HAMD and MADRS | None | Single intravenous infusion of ketamine 0.5 mg/kg over 40 min. | Baseline serum levels of IL-6 in the responder group were significantly higher than those in the nonresponder group. |
| Kiraly et al. (2017) | Ketamine, NMDA receptor | 59 | Mean age 45 in depressed group, 39 in control group | MADRS | Three patients had stable hypertension, two with hyperlipidemia, and one with mild type II diabetes. | Single intravenous infusion of ketamine 0.5 mg/Ag | Low pretreatment levels of fibroblast growth factor 2 were associated with ketamine treatment response. |
| Kynurenine Pathway Modulation | |||||||
| Miyaoka et al. (2012) | Minocycline, kynurenine pathway | 25 | Mean age 46.9 | HAMD | None | Non-placebo controlled, Open-label. 6 weeks SSRI + 150 mg minocycline | Minocycline add-on treatment showed significant and safe improvements in depressive symptoms |
| Dean et al. (2017) | Minocycline, kynurenine pathway | 71 | Mean age of 51 in minocycline group, 47.8 in placebo group | MADRS | Several, anxiety being the highest in both groups | 12 weeks of regular treatment + minocycline 200 mg/day or regular treatment + placebo | Minocycline add on treatment did not show significant improvements in depression scores |
| Husain et al. (2017) | Minocycline, kynurenine pathway | 41 | Mean age of 40 in minocycline group, 35 in placebo group | HAMD | None | 12 weeks of regular treatment + minocycline (100 mg/day, then 200 mg/day) or regular treatment + placebo | Minocycline add on treatment superior to placebo in improving depressive symptoms. |
| Emadi-Kouchak et al. (2016) | Minocycline, kynurenine pathway | 50 | Mean age of 34.7 in minocycline group, 36.4 in placebo group | HAMD | HIV (receiving HAART) | 6 weeks of minocycline 100 mg twice daily monotherapy or placebo | Minocycline monotherapy superior to placebo in decreasing depressive symptoms |
Abbreviations: NSAID, non-steroidal anti-inflammatory drug; COX, cyclooxygenase; GDS-15, 15-item geriatric depression scale; MDD, major depressive disorder; PHQ, patient health questionnaire-9; HAMD, hamilton depression scale; TNF-α, tumor necrosis factor-α; BDI, beck depression inventory; ZDS=zung self-rating depression scale; QOL, quality of life; HADS-D, hospital anxiety and depression scale for depression; CRP, c-reactive protein; IL-6, interleukin 6; PDMA, prevalent depressed mood and anhedonia; NMDA, N-methyl-d-aspartate; SF-36, short form survey; MCD, multicentric castleman’s disease; IL-6R, interleukin 6 receptor; NMDA, N-methyl-D-aspartate. GABAA, gamma-Aminobutyric acid-A.
5.1. Non-steroidal anti-inflammatory drug (NSAID) monotherapy
The concept of treating depression with NSAID has been extensively investigated in several clinical trials. NSAIDs exhibit their anti-inflammatory effects by inhibiting the enzymatic activity of cyclooxygenase (COX)-1 and −2. The studies, however, have shown mixed results with regards to the efficacy of these agents when used as a monotherapy.
A naturalistic observational retrospective study by Almeida et al. (2010) studied Australian men who had been exposed to aspirin 5 years prior to the study. Men who had used aspirin in the past 5 years but not at the time of assessment had greater odds of displaying clinically significant symptoms of depression than never users (odds ratio = 1.41, p = 0.047). The authors thus concluded that the use of aspirin is not associated with lower odds of depression (Almeida et al., 2010). One explanation for these results is that patients taking aspirin may have more chronic medical disease at baseline, thus putting them at higher risk of developing depression throughout their lifetime. Additionally, the discontinuation of aspirin in these patients could be indicative of worsening physical health, increased hospitalizations and subsequent depressive episodes. In another retrospective cohort study, estimated rates of depression were 1.7 per 1000 person-years for exposed subjects [statins (n = 94), aspirin (n = 104)] and 12.2 per 1000 person-years for the non-exposed subjects (Pasco et al., 2010). Although this study showed that exposure to statins and aspirin appears to be associated with a reduced risk of depression, it is important that to note that statin use is a significant confounder in terms of assessing aspirin’s true protective effect.
A subsequent Australian cross-sectional study also conducted by Almeida et al. assessed men with a history of depression and specifically looked at aspirin, B-vitamins and antidepressant medication use (SSRIs, SNRIs, MAO inhibitors) in this population. Study results showed that high total plasma homocysteine levels were associated with increased odds of depression (OR = 1.60). Other genetic epidemiological studies have shown that high total plasma homocysteine is associated with higher risk of cardiovascular events in general, and strokes in particular, because high total homocysteine promotes endothelial dysfunction and facilitates platelet activation. Additionally, there was a significant interaction between high homocysteine levels and use of aspirin (OR = 0.57), such that use of aspirin in the high homocysteine group was associated with lower risk of depression. The authors concluded that in a subset of geriatric depressed patients with elevated homocysteine levels, aspirin use may help prevent or lower depression risk (Almeida et al., 2012).
With regards to randomized placebo-controlled trials, Fields et al. performed a sub analysis on a depressed subset of the Alzheimer’s Disease Anti-inflammatory Prevention Trial. Individuals with normal cognition yet with a family history of Alzheimer-like dementia were randomly assigned to receive celecoxib 200 mg twice a day, naproxen sodium 220 mg twice a day, or placebo. Mean depressive symptoms scores in all three treatment groups remained similar over time (60 months). Furthermore, across all three treatment groups, there was no treatment effect on GDS scores in the significantly depressed patient subgroups (Fields et al., 2012). The authors concluded that neither celecoxib nor naproxen monotherapy was efficacious in treating depressive symptoms in this geriatric population.
Another recent analysis pooled data from 5 studies involving subjects with active osteoarthritis, and showed a detectable effect with a decrease PHQ-9 score in the ibuprofen or naproxen group (−0.31) and celecoxib group (−0.61) (P = 0.039). However, this study has poor external generalizability given that it only included a specific subset of depressed patients with osteoarthritis. Additionally, the study results could be confounded by the fact that NSAID usage likely improved these patients’ function status and this improvement could have subsequently led to higher quality of life and improvement in depressive symptoms (Iyengar et al., 2013).
Safety considerations of NSAID monotherapy also needs to be weighed, given that renal, gastric, cardiovascular side effects associated with the use of NSAIDs has been well documented in the literature. Nevertheless, clinical trials have shown that adjunctive antidepressant treatment with celecoxib can lead to rapid effects without gastric or cardiovascular adverse events (Muller et al., 2006; Akhondzadeh et al., 2009; Abbasi et al., 2012; Majd et al., 2015). Moreover, recent studies have identified that it is rofecoxib, but not celecoxib, treatment that increases cardiovascular adverse events as early as two months after treatment (Solomon et al., 2006).
5.2. NSAID augmentation therapy
Contemporary studies have also evaluated the efficacy of established NSAIDs as augmentation to mainstay antidepressant treatment. This section will focus on trials concerning celecoxib and its augmentation of SSRIs and SNRIs.
The oldest of these studies by Muller et al. (2006) was a prospective, double-blind, add-on trial of depressed individuals. Subjects were randomly assigned to either 4–10 mg reboxetine and 400 mg celecoxib group or to 4–10 mg reboxetine plus placebo group, and a statistically significant decrease in depressive symptoms was observed in both the reboxetine plus celecoxib and reboxetine only groups (F = 36 776, p < 0.0001). Importantly, the decrease in depressive symptom severity was more robust in the celecoxib add- on group (F = 3.220, p = 0.035). Thus, this study demonstrated that reboxetine plus celecoxib treatment was superior to reboxetine alone with regards to improving depressive symptoms (Muller et al., 2006).
Two more randomized, double blind, placebo-controlled studies demonstrated greater therapeutic efficacy of combination therapy with celecoxib and fluoxetine (SSRI) therapy as compared to fluoxetine alone in reducing symptoms in depressed individuals. (Akhondzadeh et al., 2009; Majd et al., 2015). In the Majd et al. study, however, the superior efficacy of SSRI and NSAID combination therapy dissipated after 8 weeks of treatment. One possible explanation for the lack of maintenance of treatment benefit in this study could be that celecoxib was dosed at only 200 mg daily compared to 400 mg used in other studies with positive outcomes lasting longer than 4 weeks of treatment (Majd et al., 2015).
Finally, a study by Abbasi et al. found that depressed individuals treated with celecoxib add-on group had significantly greater reduction in serum IL-6 concentrations (mean difference = 0.42 pg/ml, p = 0.001) as well as depressive symptoms scores (mean difference = 3.35, p = 0.005) compared to the placebo group. Moreover, the patients in the celecoxib add-on group had higher response (95%) and remission (35%) rates compared to the placebo group (50% and 5%, P = 0.003 and 0.04 respectively). Baseline IL-6 levels were significantly correlated with baseline depressive symptom scores (r = 0.378, P = 0.016). Importantly, reduction of depressive symptom scores were significantly correlated with reduction of serum IL-6 levels after 6 weeks of treatment (r = 0.673, P < 0.001). This study demonstrated that, compared to sertraline monotherapy, celecoxib and sertraline combination treatment was linked to greater reduction in IL-6 concentrations, superior reduction of depressive symptoms, and higher remission rates (Abbasi et al., 2012).
Although NSAID treatment appears to be a safe therapeutic option overall, one nationwide retrospective study from Korea with a cohort of over 4 million individuals investigated the risk of intracranial hemorrhage in patients being treated with antidepressants along with NSAIDs versus antidepressants alone. The time period examined was 30 days after initial antidepressant dose. The study found that there was increased intracranial hemorrhage risk with combination treatment and that this risk was higher in men compared to women (Shin et al., 2015). Important considerations for this study are the lack of information regarding whether or not age contributed to the observed increased hemorrhage risk and the uncertain external validity of these results given the absence of other studies confirming these findings in different ethnic groups.
5.3. Cytokine inhibition
In recent years, studies have focused on targeting cytokines for antidepressant therapy given the association of depression with inflammation. One such cytokine of interest has been TNF-α, which has been implicated in the neuroinflammatory pathophysiology of depression. In 2013, Raison et al. conducted a double-blind, placebo- controlled, clinical trial of medically stable depressed individuals with moderate treatment resistance and evaluated the antidepressant efficacy of infliximab, a TNF-α antagonist. Whereas there were no significant differences between infliximab- and placebo-treated patients on a measure of depressive symptom severity at 2 weeks, a post hoc analysis identified that infliximab showed an antidepressant effect among depressed who had high levels of inflammation at pretreatment (i.e., CRP > 5 mg/L). Depressed patients with a baseline serum levels of CRP > 5 mg/L had a 62% treatment response in the infliximab group compared to only 33% treatment response in the placebo group, although this difference was not significant (p = 0.19) due to the small sample size of these exploratory analysyes. Additionally, the generalizability of the effects of infliximab for treatment of depression may be limited; the majority of depressed patients seen in clinical practice would not actually show this level of increase in CRP (Raison et al., 2013). Importantly a recent study by McIntyre et al also failed to show that infliximab was efficacious in the treatment of depressive symptoms in a population of patients with bipolar I and II depression, and this result was found even though the sample was enriched a priori on the basis of inflammatory activation as indexed by at least clinical or phenotypic criteria (i.e., CRP of 5 mg/L or more; obesity and increased triglyceride levels, decreased high-density lipoprotein cholesterol level, or elevated blood pressure; type 1 or 2 diabetes; inflammatory bowel disorder; rheumatologic disorder; daily cigarette smoking; or migraine headaches) Whereas infliximab, as compared to placebo, did not significantly reduce depressive symptoms (relative risk, 1.09, p = 0.60), infliximab was superior to placebo in reducing depressive symptoms in patients with a history of childhood trauma (χ2 = 12.20; p = 0.02), although this exploratory result of a secondary outcome requires replication (McIntyre et al., 2019).
The antidepressant effects of adalimumab, another monoclonal anti-TNF α-antibody, were studied in the context of psoriasis patients. Menter et al. obtained data from patients with moderate to severe psoriasis. Patients in the original trial were randomized to the adalimumab or the placebo group. After 12 weeks of treatment, patients in the adalimumab group had an additional 6-point reduction in Zung Self-rating Depression Scale ZDS score compared to those in the placebo group (p < 0.001). Moreover, patients with 75% or greater reduction in baseline Psoriasis Area and Severity Index PASI score demonstrated greater ZDS score improvement compared to those with less than 75% reduction (10.6 and 1.4 respectively; p < 0.001) (Menter et al., 2010). Thus, based on the findings of this study, adalimumab treatment significantly reduced depressive symptoms and improved health-related quality of life compared to placebo. However, these results must be interpreted with caution because it is possible that adalimumab indirectly reduced depressive symptoms by improving quality of life and functionality in these patients.
Finally, another anti-TNF α medication etanercept (antibody fused with TNF α receptor) was similarly assessed for its antidepressant outcomes in psoriasis patients. In a study of patients with moderate to severe psoriasis, the authors demonstrated that etanercept treatment was superior to placebo in improving depressive symptoms (p = 0.0012, effect size = 0.25) and fatigue (p < 0.0001) (Tyring et al., 2006). However, these findings could be confounded by associated enhancements in fatigue symptoms and quality of life subsequently leading to a decrease in depressive symptoms.
A further study at Emory University (ClinicalTrials.gov Identifier: ) is also examining pre and post treatment changes in inflammatory cytokines (CRP, TNF-α, TNF-α receptors 1 and 2, IL-1, IL-1 A receptor agonist, IL-6, and IL-6 receptor) in 80 patients being treated with infliximab. This phase I clinical trial study, similar to the design of McIntyre et al. (McIntyre et al., 2019), will enrich the sample on the basis of inflammation, with selection of study participants with a baseline elevated peripheral levels of CRP (> 3 mg/L).
Other cytokines that have been the focus of immunologic antidepressant therapies include IL-12 and IL-23. A study of ustekinumab in patients with moderate to severe plaque psoriasis revealed that improvements in anxiety and depressive symptoms were greater in the ustekinumab treatment groups compared to placebo (p < 0.001) Examination of secondary endpoints in this study revealed that patients in both ustekinumab groups had greater improvement in quality of life and depressive symptom measurements compared to placebo (Langley et al., 2010). However, the above findings indicate that, although somewhat weak, improvements in psoriasis symptoms were occurring in parallel with depressive symptom response. Thus, it is difficult to assess if ustekinumab is directly improving depressive symptoms or if this finding is confounded by the aforementioned concomitant improvements in psoriasis symptoms.
IL-6 is a cytokine that has more recently gained attention for its potential role in depression pathophysiology and prediction of treatment response. A recent 2017 study by Sun et al. analyzed data from two trials: siltuximab (chimeric monoclonal antibody against IL-6) in the treatment of multicentric castleman’s disease (MCD) and sirukumab (human monoclonal antibody against IL-6) in the treatment of rheumatoid arthritis (RA). Overall, both sirukumab and siltuximab produced significantly greater improvements in prevalence of depressed mood and anhedonia group (PDMA) compared to placebo. The improvement in PDMA seen in the sirukumab study were positively correlated to baseline soluble IL-6 receptor levels. After the adjustment for covariates of disease severity, improvement in PDMA remained significant in the siltuximab but not the sirukumab study (Sun et al., 2017). The data from this study demonstrate the promising potential for IL-6 inhibition as an efficacious treatment strategy in patients with baseline inflammatory diathesis.
The safety consideration of cytokine antagonists are substantial with the chief safety concern of TNF-α inhibitor treatment being increased risk of opportunistic infections by tuberculosis, bacterial sepsis, and histoplasmosis. Additionally, lymphoma (including hepatosplenic T-cell lymphoma) and other malignancies, have been reported in children and adolescent patients, especially in those taking concomitant immunosuppressants (Toussi et al., 2013). On the contrary, recent systematic reviews and clinical trials have failed to identify any risk of infection among patients receiving cytokine inhibitor treatment (Kohler et al., 2016; Sun et al., 2017).
5.4. Ketamine
While it is known that ketamine is a non-competitive NMDA receptor antagonist, its exact antidepressant mechanism of action is complex and not completely understood. As discussed above, ketamine has been shown to decrease IDO and the kynurenine/tryptophan ratio within the CNS (Wang et al., 2015). Ketamine also plays a role in regulation of synaptogenesis, via potentiation of brain-derived neurotrophic factor (BDNF). Additionally, ketamine modulates α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors and stimulates the mammalian target of rapamycin (mTOR), which all play key roles in neuronal plasticity (Zunszain et al., 2013).
With regards to specific effects on inflammatory mediators, murine models of depression have shown that ketamine administration lowered levels of IL-6, TNF-α, and IL-1β (Wang et al., 2015). In addition to its effects on pro-inflammatory cytokines, ketamine has been shown to suppress levels of NF-κB, a molecule whose translocation is thought to play a role in the pathogenesis of depression (Tan et al., 2015).
Ketamine’s robust and rapidly acting antidepressant effects have been well documented in the literature over the past decade or so (Zarate et al., 2006; Murrough et al., 2013; Lapidus et al., 2014). More specifically, ketamine has shown promising results with respect to improving depressive symptoms in treatment resistant depressed patients (Zarate et al., 2006). Furthermore, emerging evidence suggests that patients with treatment resistant depression might have elevated levels of inflammatory cytokines, and that such abnormalities might predict treatment outcome in response to ketamine. Kiraly et al. demonstrated that levels of IL-6 were elevated in treatment resistant depressed patients, and found that low pretreatment levels of fibroblast growth factor 2, but not inflammatory cytokines were associated with ketamine treatment response (Kiraly et al., 2017).
A current study in Mexico (ClinicalTrials.gov Identifier: ) is evaluating the use of intravenous low-dose ketamine in the treatment of treatment-resistant depression, as well as the changes in glutamate neurotransmission and inflammatory serum markers. Specifically, this trial is examining baseline and post treatment levels of IL-1 and TNF-α, thus potentially providing additional beneficial screening tools and predictive biomarkers in this patient population.
5.5. Minocycline
As discussed earlier, both the kynurenine pathway and the interplay of its components with cytokines and CNS neurons are implicated in the inflammatory pathophysiology of depression. More specifically, it is believed that this pathway influences mood disorders by dictating the balance between neuroprotection and neurotoxicity in the brain. It is hypothesized that minocycline exerts its antidepressant effects by inhibiting neurotoxic factors (pro-inflammatory cytokines, reactive oxygen species) released by microglia and activating neuroprotective agents (anti-inflammatory cytokines, antioxidants, neurotrophic factors) released by astrocytes (Soczynska et al., 2012).
A number of recent studies have evaluated minocycline’s efficacy in the treatment of depression via its anti-inflammatory effects. There is only one study to date that has evaluated minocycline as monotherapy in treatment of depressed patients with comorbid HIV on highly active antiretroviral therapy (HAART) (Emadi-Kouchak et al., 2016). Given the direct effects of the HIV virus on the cortical and subcortical areas of the brain, which cause an neuro-inflammatory state, neuronal death and damage, as well as peripheral immune system alterations, the authors of this study hypothesized that administration of minocycline could possibly reduce depressive symptoms in this subset of patients through its anti-inflammatory, anti-oxidative, and neuroprotective effects on the CNS. Results demonstrated significant effect for time by treatment interaction on the HDRS score [F(2, 88) = 7.50, P = 0.001]. Moreover, there was greater improvement in depressive symptoms scores in the minocycline group compared with the placebo group (p = 0.001) (Emadi-Kouchak et al., 2016). Thus, it can be concluded that minocycline monotherapy was safe and produced clinically significant and superior improvements in depressive symptoms compared to placebo.
An open label, non-placebo trial by Miyaoka et al. evaluated minocycline as an adjunctive treatment in patients diagnosed with major depression with psychotic features. The study demonstrated that the addition of minocycline to existing antidepressant treatment (fulvoxamine, paroxetine, and sertraline) resulted in stastically significant reductions in both depressive and psychotic symptoms (Miyaoka et al., 2012). Thus, this study showed that minocycline add-on treatment, for patients with unipolar psychotic depression being treated with SSRIs, resulted in a safe and significant improvement in depressive symptoms.
More recently, two trials have also evaluated minocycline as add on therapy for treatment of depressed patients who did not have co morbid psychosis. The first, by Dean et al. compared minocycline add on therapy to treatment as usual with placebo and demonstrated that minocycline adjunctive therapy did not produce significant improvements in depressive symptoms but that this therapy could indirectly induce a reduction in depressive symptoms through its positive impact on quality of life and social/occupational functioning (Dean et al., 2017). The second trial by Husain et al. also compared minocycline add on to treatment as usual, mood stabilizers (with the exception of valproic acid) and antipsychotics to placebo but differed in that patients in this study had previously failed to respond to at least two antidepressants. After 12 weeks of treatment, the minocycline add-on group had greater reductions in depressive symptoms compared to the placebo group (effect size = 1.21, p < 0.001) (Husain et al., 2017).
Aside from initial differences in dosing, one possible explanation for the different minocycline adjunctive treatment outcomes observed in the two aforementioned studies could be that treatment as usual was unknown in the Dean et al. study and varied in the Husain et al. study. Another explanation could be that the treatment resistant patients in the Husain et al. study have different baseline inflammatory profiles compared to the Dean et al. patients thus leading to more treatment responsiveness in the Husain et al. group.
5.6. Other novel immunotherapies
An ongoing study by Janssen Research & Development, LLC (ClinicalTrials.gov Identifier: ) is investigating the safety and tolerability of a novel agent, JNJ 54175446, in participants with depression. JNJ 54175446 is a purinergic P2X7 receptor antagonist, thus modulating extracellular mechanisms of inflammation in the CNS, PNS, microglia, and macrophages. This phase I study is composed of three treatment arms: JNJ 54175446, JNJ 54175446 + placebo, and placebo. Additionally, one of the secondary outcomes of this study is the effect of JNJ-54175446 versus placebo on total sleep deprivation-induced changes in IL-1β and cortisol levels.
As discussed above, depressed individuals tend to have increased metabolism of tryptophan to kynurenine with resultant production of neurotoxic quinolinic acid. Recent murine studies have shown that kynurenine must first be taken up into CNS cells via large neutral amino acid transporters (LAT). A recent animal study demonstrated that leucine, among other amino acids, significantly decreased production of downstream kynurenine metabolites via inhibition of kynurenine uptake in CNS astrocytes (Sekine et al., 2015). Building upon this novel idea of utilizing amino acids as antidepressant agents, a current study at the University of Texas Southwestern Medical Center (ClinicalTrials.gov Identifier: ) is assessing the antidepressant effect of L-leucine (large, neutral, essential amino acid). Aside from measuring pre and post treatment changes in depression symptom severity, this pilot phase II clinical trial will explore the influence of increased inflammation on treatment response via post-hoc sub analysis.
6. Future directions
Together these data point to several key areas for further investigation, which are listed below:
6.1. Utility of inflammation in diagnosing depression and guiding treatment decision-making
Clinical translational research is needed to further evaluate the link between inflammatory biomarkers and the diagnosis and detection of individuals who possess treatment refractory major depressive disorder. To date, research has been limited to clinical samples being evaluated in controlled trial or psychiatric settings. Additionally, it is not known whether these findings generalize to depressed patients seen in the community or in primary care settings, and to what extent broad efforts that characterize inflammation might inform antidepressant treatment algorithms to optimize response and remission of depression.
6.2. Value of assessment of inflammation in evaluating disease burden and relapse risk
Although CNS levels of inflammatory cytokines may be difficult to measure, several studies to date have demonstrated the relative ease with which peripheral serum cytokines can be measured and profiled in individuals receiving antidepressant treatment. However, naturalistic, observational studies are needed to determine whether assessment of inflammatory biomarkers can be utilized for monitoring the accumulated burden of depressive disorders, and/or informing the identification of those at greatest risk for relapse. Nevertheless, as noted above, Setiawan et al. cross-sectionally showed that microglial activation as indexed by TSPO VT, was associated with depression disease burden (Setiawan et al., 2018), which suggests that monitoring of inflammatory dynamics, such as TSPO, might aid in the management of depression, a life-long, chronic medical condition comparable to diabetes or hypertension.
6.3. New directions in targeting inflammatory mechanisms in the treatment of depression
Although the clinical studies mentioned in this review have identified certain serum inflammatory markers that predict treatment response, these results are mixed with regards to whether elevated circulating levels predict better or worse treatment response. This observation is particularly evident with regards to studies evaluating IL-6 levels. Thus, it is possible that there are other factors influencing the production of this cytokine that could in turn offer more reliable as sociations with treatment response. One such factor is gp130, but additional research is needed to understand whether increasing gp130 inhibition of IL-6 trans-signaling might serve as a more specific target to block the pro-inflammatory effects of IL-6, as compared to IL-6 antibodies, while preserving beneficial homeostatic function of IL-6.
Other novel research approaches that could potentially aid in further understanding the inflammatory mechanisms underlying the treatment of depression include focusing on cytokines with anti-inflammatory properties, such as IL-10. Further research on the efficacy of IL-10 agonists is needed to determine whether such agents might have given protective effects. Moreover, future studies are needed to assess efficacy and safety profiles of IL-10 agonists.
Acknowledgements
The authors acknowledge research support provided by the National Institutes of Health, USA (R01AG051944, R01AG056424-01, R01AG026364, R01CA160245, R01AG057750 and R01CA207130).
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2019.09.016.
References
- Abbasi SH, Hosseini F, Modabbernia A, Ashrafi M, Akhondzadeh S, 2012. Effect of celecoxib add-on treatment on symptoms and serum il-6 concentrations in patients with major depressive disorder: Randomized double-blind placebo-controlled study. J. Affect. Disord 141, 308–314. 10.1016/j.jad.2012.03.033. [DOI] [PubMed] [Google Scholar]
- Aguzzi A, Barres BA, Bennett ML, 2013. Microglia: scapegoat, saboteur, or something else? Science 339, 156–161. 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhondzadeh S, Jafari S, Raisi F, Nasehi AA, Ghoreishi A, Salehi B, Mohebbi-Rasa S, Raznahan M, Kamalipour A, 2009. Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depress Anxiety 26, 607–611. 10.1002/da.20589. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, 2005. Depression in the elderly. Lancet 365, 1961–1970. 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Alfonso H, Jamrozik K, Hankey GJ, Flicker L, 2010. Aspirin use, depression, and cognitive impairment in later life: the health in men study. J. Am. Geriatr. Soc 58, 990–992. 10.1111/j.1532-5415.2010.02827.x. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Flicker L, Yeap BB, Alfonso H, McCaul K, Hankey GJ, 2012. Aspirin decreases the risk of depression in older men with high plasma homocysteine. Transl. Psychiatry 2, e151 10.1038/tp.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews G, Issakidis C, Sanderson K, Corry J, Lapsley H, 2004. Utilising survey data to inform public policy: comparison of the cost-effectiveness of treatment of ten mental disorders. Br. J. Psychiatry 184, 526–533. [DOI] [PubMed] [Google Scholar]
- Anthony DC, Couch Y, Losey P, Evans MC, 2012. The systemic response to brain injury and disease. Brain Behav. Immun 26, 534–540. 10.1016/j.bbi.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Banks WA, 2015. The blood-brain barrier in neuroimmunology: tales of separation and assimilation. Brain Behav. Immun 44, 1–8. 10.1016/j.bbi.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baune BT, Dannlowski U, Domschke K, Janssen DG, Jordan MA, Ohrmann P, Bauer J, Biros E, Arolt V, Kugel H, Baxter AG, Suslow T, 2010. The interleukin 1 beta (il1b) gene is associated with failure to achieve remission and impaired emotion processing in major depression. Biol. Psychiatry 67, 543–549. 10.1016/j.biopsych.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Ben Achour S, Pascual O, 2010. Glia: the many ways to modulate synaptic plasticity. Neurochem. Int 57, 440–445. 10.1016/j.neuint.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Benros ME, Waltoft BL, Nordentoft M, Ostergaard SD, Eaton WW, Krogh J, Mortensen PB, 2013. Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry 70, 812–820. 10.1001/jamapsychiatry.2013.1111. [DOI] [PubMed] [Google Scholar]
- Bentivoglio M, Mariotti R, Bertini G, 2011. Neuroinflammation and brain infections: historical context and current perspectives. Brain Res. Rev 66, 152–173. 10.1016/j.brainresrev.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Drevets WC, 2016. Role of neuro-immunological factors in the pathophysiology of mood disorders: implications for novel therapeutics for treatment resistant depression. Curr. Top. Behav. Neurosci 31, 339–356. 10.1007/7854_2016_43. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, Miller AH, 2012. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch. Gen. Psychiatry 69, 1044–1053. 10.1001/archgenpsychiatry.2011.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho LA, Torre JP, Papadopoulos AS, Poon L, Juruena MF, Markopoulou K, Cleare AJ, Pariante CM, 2013. Lack of clinical therapeutic benefit of antidepressants is associated overall activation of the inflammatory system. J. Affect. Disord 148, 136–140. 10.1016/j.jad.2012.10.036. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Cavanagh J, de Boer P, Mondelli V, Jones DNC, Drevets WC, Cowen PJ, Harrison NA, Pointon L, Pariante CM, Bullmore ET, 2019. Treatment-resistant depression and peripheral c-reactive protein. Br. J. Psychiatry 214, 11–19. 10.1192/bjp.2018.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm D, Sanderson K, Ayuso-Mateos JL, Saxena S, 2004. Reducing the global burden of depression: population-level analysis of intervention cost-effectiveness in 14 world regions. Br. J. Psychiatry 184, 393–403. [DOI] [PubMed] [Google Scholar]
- Chung WS, Barres BA, 2012. The role of glial cells in synapse elimination. Curr. Opin. Neurobiol 22, 438–445. 10.1016/j.conb.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl J, Ormstad H, Aass HC, Malt UF, Bendz LT, Sandvik L, Brundin L, Andreassen OA, 2014. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology 45, 77–86. 10.1016/j.psyneuen.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Dantzer R, 2012. Depression and inflammation: an intricate relationship. Biol. Psychiatry 71, 4–5. 10.1016/j.biopsych.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Lawson MA, Kelley KW, 2011. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology 36, 426–436. 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean OM, Kanchanatawan B, Ashton M, Mohebbi M, Ng CH, Maes M, Berk L, Sughondhabirom A, Tangwongchai S, Singh AB, McKenzie H, Smith DJ, Malhi GS, Dowling N, Berk M, 2017. Adjunctive minocycline treatment for major depressive disorder: a proof of concept trial. Aust. N. Z. J. Psychiatry 51, 829–840. 10.1177/0004867417709357. [DOI] [PubMed] [Google Scholar]
- Dinan TG, 2009. Inflammatory markers in depression. Curr. Opin. Psychiatry 22, 32–36 10.1097/YCO.0b013e328315a56100001504-200901000-00007. [DOI] [PubMed] [Google Scholar]
- D’Mello C, Le T, Swain MG, 2009. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J. Neurosci 29, 2089–2102. 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL, 2010. A meta-analysis of cytokines in major depression. Biol. Psychiatry 67, 446–457. 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Duseja R, Heir R, Lewitus GM, Altimimi HF, Stellwagen D, 2015. Astrocytic tnfalpha regulates the behavioral response to antidepressants. Brain Behav. Immun 44, 187–194. 10.1016/j.bbi.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Eggermont AM, Suciu S, Santinami M, Testori A, Kruit WH, Marsden J, Punt CJ, Sales F, Gore M, Mackie R, Kusic Z, Dummer R, Hauschild A, Musat E, Spatz A, Keilholz U, 2008. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage iii melanoma: final results of eortc 18991, a randomised phase iii trial. Lancet 372, 117–126. 10.1016/s0140-6736(08), 61033-8. [DOI] [PubMed] [Google Scholar]
- Eller T, Vasar V, Shlik J, Maron E, 2008. Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 32, 445–450 S0278-5846(07)00344-2 [pii] 10.1016/j.pnpbp.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Emadi-Kouchak H, Mohammadinejad P, Asadollahi-Amin A, Rasoulinejad M, Zeinoddini A, Yalda A, Akhondzadeh S, 2016. Therapeutic effects of minocycline on mild-to-moderate depression in hiv patients: a double-blind, placebo-controlled, randomized trial. Int. Clin. Psychopharmacol 31, 20–26. 10.1097/yic.0000000000000098. [DOI] [PubMed] [Google Scholar]
- Enache D, Pariante CM, Mondelli V, 2019. Markers of central inflammation in major depressive disorder: a systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav. Immun 10.1016/j.bbi.2019.06.015. [DOI] [PubMed] [Google Scholar]
- Fava M, 2003. Diagnosis and definition of treatment-resistant depression. Biol. Psychiatry 53, 649–659. [DOI] [PubMed] [Google Scholar]
- Felger JC, Haroon E, Patel TA, Goldsmith DR, Wommack EC, Woolwine BJ, Le N-A, Feinberg R, Tansey MG, Miller AH, 2018. What does plasma crp tell us about peripheral and central inflammation in depression? Mol. Psychiatry, 10.1038/s41380-018-0096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields C, Drye L, Vaidya V, Lyketsos C, 2012. Celecoxib or naproxen treatment does not benefit depressive symptoms in persons age 70 and older: findings from a randomized controlled trial. Am. J. Geriatr. Psychiatry 20, 505–513. 10.1097/JGP.0b013e318227f4da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebe A, Horn M, Schmidt F, Janssen G, Schmid-Wendtner MH, Volkenandt M, Hauschild A, Goldsmith CH, Schaefer M, 2010. Dose-dependent development of depressive symptoms during adjuvant interferon-1 alpha} treatment of patients with malignant melanoma. Psychosomatics 51, 466–473. 10.1176/appi.psy.51.6.466. [DOI] [PubMed] [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M, 2015. Cumulative meta-analysis of interleukins 6 and lbeta, tumour necrosis factor alpha and c-reactive protein in patients with major depressive disorder. Brain Behav. Immun 49, 206–215. 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, Bloch M, 2011. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology 36, 2452–2459. 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Fleischer CC, Felger JC, Chen X, Woolwine BJ, Patel T, Hu XP, Miller AH, 2016. Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol. Psychiatry 10.1038/mp.2015.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Daguanno AW, Woolwine BJ, Goldsmith DR, Baer WM, Wommack EC, Felger JC, Miller AH, 2018. Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive dis order. Psychoneuroendocrinology 95, 43–49. 10.1016/j.psyneuen.2018.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner FL, Ransohoff RM, Becher B, 2015. Immune attack: the role of inflammation in alzheimer disease. Nat. Rev. Neurosci 16, 358–372. 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Rebusi N, Heshmati M, Aleyasin H, Warren BL, Lebonte B, Horn S, Lapidus KA, Stelzhammer V, Wong EH, Bahn S, Krishnan V, Bolanos-Guzman CA, Murrough JW, Merad M, Russo SJ, 2014. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci. U.S.A Ill, 16136–16141. 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz MA, Wertz J, Zhu D, Cattaneo A, Musaelyan K, Nikkheslat N, Thuret S, Pariante CM, Zunszain PA, 2014. Antidepressant compounds can be both pro- and anti-inflammatory in human hippocampal cells. Int. J. Neuropsychopharmacol 18 10.1093/ijnp/pyu076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain MI, Chaudhry IB, Husain N, Khoso AB, Rahman RR, Hamirani MM, Hodsoll J, Qurashi I, Deakin JF, Young AH, 2017. Minocycline as an adjunct for treatment-resistant depressive symptoms: a pilot randomised placebo-controlled trial. J. Psychopharmacol 31, 1166–1175. 10.1177/0269881117724352. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Miller AH, 2007. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav. Immun 21, 374–383. [DOI] [PubMed] [Google Scholar]
- Iyengar RL, Gandhi S, Aneja A, Thorpe K, Razzouk L, Greenberg J, Mosovich S, Farkouh ME, 2013. Nsaids are associated with lower depression scores in patients with osteoarthritis. 1017.e1011–1018. Am. J. Med 126 10.1016/jamjmed.2013.02.037. [DOI] [PubMed] [Google Scholar]
- Jacob L, Rockel T, Kostev K, 2017. Depression risk in patients with rheumatoid arthritis in the United Kingdom. Rheumatol. Therapy 4, 195–200. 10.1007/S40744-017-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen DG, Caniato RN, Verster JC, Baune BT, 2010. A psychoneuroimmunological review on cytokines involved in antidepressant treatment response. Hum. Psychopharmacol 25, 201–215. 10.1002/hup.1103. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Jiang JX, Zhang GX, 2014. Macrophages: a double-edged sword in experimental autoimmune encephalomyelitis. Immunol. Lett 160, 17–22. 10.1016/j.imlet.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Horn SR, Van Dam NT, Costi S, Schwartz J, Kim-Schulze S, Patel M, Hodes GE, Russo SJ, Merad M, Iosifescu DV, Charney DS, Murrough JW, 2017. Altered peripheral immune profiles in treatment-resistant depression: response to ketamine and prediction of treatment outcome. Transl. Psychiatry 7, e1065 10.1038/tp.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler O, Benros M, Krogh J, 2015. Anti-inflammatory intervention in depression—reply. JAMA Psychiatry 72, 512–513. 10.1001/jamapsychiatry.2014.3186. [DOI] [PubMed] [Google Scholar]
- Kohler O, Krogh J, Mors O, Benros ME, 2016. Inflammation in depression and the potential for anti-inflammatory treatment. Curr. Neuropharmacol 14, 732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopschina Feltes P, Doorduin J, Klein HC, Juarez-Orozco LE, Dierckx RA, Moriguchi-Jeckel CM, de Vries EF, 2017. Anti-inflammatory treatment for major depressive disorder: implications for patients with an elevated immune profile and non-responders to standard antidepressant therapy. J. Psychopharmacol 31, 1149–1165. 10.1177/0269881117711708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korczak DJ, Pereira S, Koulajian K, Matejcek A, Giacca A, 2011. Type 1 diabetes mellitus and major depressive disorder: evidence for a biological link. Diabetologia 54, 2483–2493. 10.1007/s00125-011-2240-3. [DOI] [PubMed] [Google Scholar]
- Krogh J, Benros ME, Jorgensen MB, Vesterager L, Elfving B, Nordentoft M, 2014. The association between depressive symptoms, cognitive function, and inflammation in major depression. Brain Behav. Immun 35, 70–76. 10.1016/j.bbi.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Kruse JL, Congdon E, Olmstead R, Njau S, Breen EC, Narr KL, Espinoza R, Irwin MR, 2018. Inflammation and improvement of depression following electroconvulsive therapy in treatment-resistant depression. J. Clin. Psychiatry 79 10.4088/JCP.17m11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley RG, Feldman SR, Han C, Schenkel B, Szapary P, Hsu MC, Ortonne JP, Gordon KB, Kimball AB, 2010. Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: results from a randomized, double-blind, placebo-controlled phase iii trial. J. Am. Acad. Dermatol 63, 457–465. 10.1016/j.jaad.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H, 2000. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology 22, 370–379 S0893-133X(99)00134-7 [pii] 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Lapidus KA, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, Feder A, Iosifescu DV, Charney DS, Murrough JW, 2014. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol. Psychiatry 76, 970–976. 10.1016/j.biopsych.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard B, Maes M, 2012. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav. Rev 36, 764–785. 10.1016/j.neubiorev.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ho RC, Mak A, 2012. Interleukin (il)-6, tumour necrosis factor alpha (tnfalpha) and soluble interleukin-2 receptors (sil-2r) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J. Affect. Disord 139, 230–239. 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Liu RP, Zou M, Wang JY, Zhu JJ, Lai JM, Zhou LL, Chen SF, Zhang X, Zhu JH, 2014. Paroxetine ameliorates lipopolysaccharide-induced microglia activation via differential regulation of mapk signaling. J Neuroinflammation 11, 47 10.1186/1742-2094-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, 2015. Inflammatory cytokine-associated depression. Brain Res. 1617, 113–125. 10.1016/j.brainres.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, 2011. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog. Neuro-Psychopharm. Biol. Psychiatry 35, 664–675. 10.1016/j.pnpbp.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Yirmiya R, 2012. Targeting il-1 in depression. Expert Opin. Ther. Targets 16, 1097–1112. 10.1517/14728222.2012.718331. [DOI] [PubMed] [Google Scholar]
- Majd M, Hashemian F, Hosseini SM, Vahdat Shariatpanahi M, Sharifi A, 2015. A randomized, double-blind, placebo-controlled trial of celecoxib augmentation of sertraline in treatment of drug-naive depressed women: a pilot study. Iranian J. Pharm. Res.: IJPR 14, 891–899. [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Subramaniapillai M, Lee Y, Pan Z, Carmona NE, Shekotikhina M, Rosenblat JD, Brietzke E, Soczynska JK, Cosgrove VE, Miller S, Fischer EG, Kramer NE, Dunlap K, Suppes T, Mansur RB, 2019. Efficacy of adjunctive infliximab vs placebo in the treatment of adults with bipolar i/ii depression: a randomized clinical trial. JAMA Psychiatry, 10.1001/jamapsychiatry.2019.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, 2008. Origin and physiological roles of inflammation. Nature 454, 428–435. 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Menter A, Augustin M, Signorovitch J, Yu AP, Wu EQ, Gupta SR, Bao Y, Mulani P, 2010. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J. Am. Acad. Dermatol 62, 812–818. 10.1016/j.jaad.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL, 2009. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 65, 732–741 S0006-3223(08)01532-1 [pii] 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL, 2016. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol 16, 22–34. 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaoka T, Wake R, Furuya M, Liaury K, Ieda M, Kawakami K, Tsuchie K, Taki M, Ishihara K, Araki T, Horiguchi J, 2012. Minocycline as adjunctive therapy for patients with unipolar psychotic depression: an open-label study. ProgNeuro-Psychopharm. Biol. Psychiatry 37, 222–226. 10.1016/j-pnpbp.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, Spellmann I, Hetzel G, Maino K, Kleindienst N, Moller HJ, Arolt V, Riedel M, 2006. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: Results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol. Psychiatry 11, 680–684 4001805 [pii] 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ, 2007. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol. Psychiatry 12, 988–1000. 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- Munzer A, Sack U, Mergl R, Schonherr J, Petersein C, Bartsch S, Kirkby KC, Bauer K, Himmerich H, 2013. Impact of antidepressants on cytokine production of depressed patients in vitro. Toxins 5, 2227–2240. 10.3390/toxins5112227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, A1 Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ, 2013. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am. J. Psychiatry 170, 1134–1142. 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JC, Papakostas GI, 2009. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am. J. Psychiatry 166, 980–991. 10.1176/appi.ajp.2009.09030312. [DOI] [PubMed] [Google Scholar]
- O’Brien SM, Scully P, Fitzgerald P, Scott LV, Dinan TG, 2007. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J. Psychiatr. Res 41, 326–331. 10.1016/j.jpsychires.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Obuchowicz E, Bielecka AM, Paul-Samojedny M, Pudelko A, Kowalski J, 2014. Imipramine and fluoxetine inhibit lps-induced activation and affect morphology of microglial cells in the rat glial culture. Pharmacol. Rep.: PR 66, 34–43. 10.1016/j.pharep.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Pariante CM, 2016. Neuroscience, mental health and the immune system: overcoming the brain-mind-body trichotomy. Epidemiol. Psychiatric Sci 25, 101–105. 10.1017/s204579601500089x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasco JA, Jacka FN, Williams LJ, Henry MJ, Nicholson GC, Kotowicz MA, Berk M, 2010. Clinical implications of the cytokine hypothesis of depression: the association between use of statins and aspirin and the risk of major depression. Psychother. Psychosom 79, 323–325. 10.1159/000319530. [DOI] [PubMed] [Google Scholar]
- Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC, 2015. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin. Ther 37, 984–995. 10.1016/j.clinthera.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH, 2013. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 70, 31–41. 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Gorman JM, Dieterich DT, 2005. Interferon-induced depression and cognitive impairment in hepatitis c virus patients: a 72 week prospective study. Aids 19 (Suppl 3), S174–S178. [DOI] [PubMed] [Google Scholar]
- Roose SP, Schatzberg AF, 2005. The efficacy of antidepressants in the treatment of late-life depression. J. Clin. Psychopharmacol 25, S1–S7. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M, 2006. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a star*d report. Am. J. Psychiatry 163, 1905–1917. 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Schaefer M, 2014. Antidepressant pretreatment for the prevention of interferon alfa-associated depression: a systematic review and meta-analysis. Psychosomatics 55, 221–234. 10.1016/j.psym.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Sekine A, Okamoto M, Kanatani Y, Sano M, Shibata K, Fukuwatari T, 2015. Amino acids inhibit kynurenic acid formation via suppression of kynurenine uptake or kynurenic acid synthesis in rat brain in vitro. SpringerPlus 4 10.1186/S40064-015-0826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E, Attwells S, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Xu C, Sharma S, Kish S, Houle S, Meyer JH, 2018. Association of translocator protein total distribution volume with duration of untreated major depressive disorder: a cross-sectional study. Lancet Psychiatry 5, 339–347. 10.1016/S2215-0366(18), 30048-8. [DOI] [PubMed] [Google Scholar]
- Shin J-Y, Park M-J, Lee SH, Choi S-H, Kim M-H, Choi N-K, Lee J, Park B-J, 2015. Risk of intracranial haemorrhage in antidepressant users with concurrent use of non-steroidal anti-inflammatory drugs: nationwide propensity score matched study. BMJ: British Med. J 351 10.1136/bmj.h3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR, 2014. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol. Bull 140, 774–815. 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soczynska JK, Mansur RB, Brietzke E, Swardfager W, Kennedy SH, Woldeyohannes HO, Powell AM, Manierka MS, McIntyre RS, 2012. Novel therapeutic targets in depression: minocycline as a candidate treatment. Behav. Brain Res 235, 302–317. 10.1016/j.bbr.2012.07.026. [DOI] [PubMed] [Google Scholar]
- Sokol CL, Luster AD, 2015. The chemokine system in innate immunity. Cold Spring Harbor Perspect. Biol 7 10.1101/cshperspect.a016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon DH, Avorn J, Sturmer T, Glynn RJ, Mogun H, Schneeweiss S, 2006. Cardiovascular outcomes in new users of coxibs and nonsteroidal antiinflammatory drugs: high-risk subgroups and time course of risk. Arthritis Rheum. 54, 1378–1389. 10.1002/art.21887. [DOI] [PubMed] [Google Scholar]
- Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, Sarnyai Z, Mawrin C, Brisch R, Bielau H, Meyer zu Schwabedissen L, Bogerts B, Myint AM, 2011. Severe depression is associated with increased microglial quinolinic acid in sub-regions of the anterior cingulate gyrus: evidence for an immune-modulated gluta-matergic neurotransmission? J. Neuroinflammation 8, 94 10.1186/1742-2094-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ, 2015. Inflammation and clinical response to treatment in depression: a meta-analysis. Eur. Neuropsychopharmacol 25, 1532–1543. 10.1016/j.euroneuro.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang D, Salvadore G, Hsu B, Curran M, Casper C, Vermeulen J, Kent JM, Singh J, Drevets WC, Wittenberg GM, Chen G, 2017. The effects of interleukin-6 neutralizing antibodies on symptoms of depressed mood and anhedonia in patients with rheumatoid arthritis and multicentric castleman’s disease. Brain Behav. Immun 66, 156–164. 10.1016/j.bbi.2017.06.014. [DOI] [PubMed] [Google Scholar]
- Tan Y, Wang Q, She Y, Bi X, Zhao B, 2015. Ketamine reduces lps-induced hmgb1 via activation of the nrf2/ho-1 pathway and nf-kappab suppression. J. Trauma Acute Care Surg 78, 784–792. 10.1097/ta.0000000000000588. [DOI] [PubMed] [Google Scholar]
- Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N, 2014. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav. Immun 42, 50–59. 10.1016/j.bbi.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Toussi SS, Pan N, Walters HM, Walsh TJ, 2013. Infections in children and adolescents with juvenile idiopathic arthritis and inflammatory bowel disease treated with tumor necrosis factor-alpha inhibitors: systematic review of the literature. Clin. Infect. Dis 57, 1318–1330. 10.1093/cid/cit489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Celia D, Krishnan R, 2006. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase iii trial. Lancet 367, 29–35. [DOI] [PubMed] [Google Scholar]
- Uher R, Tansey KE, Dew T, Maier W, Mors O, Hauser J, Dernovsek MZ, Henigsberg N, Souery D, Farmer A, McGuffin P, 2014. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am. J. Psychiatry 171, 1278–1286. 10.1176/appi.ajp.2014.14010094. [DOI] [PubMed] [Google Scholar]
- Valkanova V, Ebmeier KP, Allan CL, 2013. Crp, il-6 and depression: a systematic review and meta-analysis of longitudinal studies. J. Affect. Disord 150, 736–744. 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Vismari L, Alves GJ, Muscara MN, Palermo-Neto J, 2012. A possible role to nitric oxide in the anti-inflammatory effects of amitriptyline. Immunopharmacol. Immunotoxicol 34, 578–585. 10.3109/08923973.2011.638305. [DOI] [PubMed] [Google Scholar]
- Vitkovic L, Bockaert J, Jacque C, 2000. “Inflammatory” cytokines: neuromodulators in normal brain? J. Neurochem 74, 457–471. [DOI] [PubMed] [Google Scholar]
- Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, Kelley KW, Dantzer R, 2013. Nmda receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in c57bl/6j mice. Neuropsychopharm 38, 1609–1616. 10.1038/npp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Yu H-Y, Shen X-F, Gao Z-Q, Yang C, Yang J-J, Zhang G-F, 2015. The rapid antidepressant effect of ketamine in rats is associated with down-regulation of pro-inflammatory cytokines in the hippocampus. Upsala J. Med. Sci 120, 241–248. 10.3109/03009734.2015.1060281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T, 2013. Global burden of disease attributable to mental and substance use disorders: findings from the global burden of disease study 2010. Lancet 382, 1575–1586. 10.1016/s0140-6736(13), 61611-6. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Franklin T, Iwata M, Duman RS, 2016. Integrating neuroimmune systems in the neurobiology of depression. Nat. Rev. Neurosci 17, 497–511. 10.1038/nrn.2016.69. [DOI] [PubMed] [Google Scholar]
- Yang JJ, Wang N, Yang C, Shi JY, Yu HY, Hashimoto K, 2015. Serum interleukin-6 is a predictive biomarker for ketamine’s antidepressant effect in treatment-resistant patients with major depression. Biol. Psychiatry 77, el9–e20. 10.1016/j.biopsych.2014.06.021. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Rimmerman N, Reshef R, 2015. Depression as a microglial disease. Trends Neurosci. 38, 637–658. 10.1016/j.tins.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Yohn SE, Errante EE, Rosenbloom-Snow A, Somerville M, Rowland M, Tokarski K, Zafar N, Correa M, Salamone JD, 2016. Blockade of uptake for dopamine, but not norepinephrine or 5-ht, increases selection of high effort instrumental activity: implications for treatment of effort-re la ted motivational symptoms in psycho pathology. Neuropharm 109, 270–280. 10.1016/j.neuropharm.2016.06.018. [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Hori H, Ikenouchi-Sugita A, Umene-Nakano W, Ueda N, Nakamura J, 2009. Higher plasma interleukin-6 (il-6) level is associated with ssri- or snri-refractory depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 33, 722–726. 10.1016/j.pnpbp.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK, 2006. A randomized trial of an n-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63, 856–864. 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D, Gross CT, 2014. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci 17, 400–406. 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- Zoga M, Oulis P, Chatzipanagiotou S, Masdrakis VG, Pliatsika P, Boufidou F, Foteli S, Soldatos CR, Nikolaou C, Papageorgiou C, 2014. Indoleamine 2,3-dioxygenase and immune changes under antidepressive treatment in major depression in females. Vivo 28, 633–638. [PubMed] [Google Scholar]
- Zunszain PA, Horowitz MA, Cattaneo A, Lupi MM, Pariante CM, 2013. Ketamine: synaptogenesis, immunomodulation and glycogen synthase kinase-3 as underlying mechanisms of its antidepressant properties. Mol. Psychiatry 18, 1236–1241. 10.1038/mp.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]