Abstract
Background:
Neutrophil-lymphocyte ratio (NLR) is one of the markers of systemic inflammation. Recent studies have associated NLR with diagnosis of preeclampsia (PE). However, due to small sample sizes and different research design, the diagnostic value of NLR in PE patients is not well understood. In this study, we evaluate the potential diagnostic value of NLR in PE.
Methods:
We searched PubMed, Embase, Cochrane Library, the Chinese National Knowledge Infrastructure (CNKI) databases, Wanfang data, VIP database and China Biomedical Literature Database systematically for relevant literatures up to May 20, 2018. All analyses were conducted using Meta-DiSc1.4 and Stata 12.0 software. Sensitivity, specificity and other measures of accuracy of NLR for the diagnosis of PE were pooled. Meta-regression was performed to identify the sources of heterogeneity.
Results:
This meta-analysis included a total of 7 studies. The pooled sensitivity and specificity were 0.74 (95% CI 0.71–0.76) and 0.64 (95%CI 0.61–0.68), positive likelihood ratio, 2.62 (95%CI1.79–3.84); negative likelihood ratio, 0.34 (95%CI 0.24–0.48); diagnostic odds ratio, 8.44 (95%CI 4–17.78), and area under the curve was 0.82. Meta regression showed that sample size was the main source of heterogeneity. Deeks funnel plot showed that there was no statistical significance for the evaluation of publication bias (P = .16).
Conclusion:
Current evidence suggests that the diagnostic accuracy of NLR has unsatisfactory specificity but acceptable sensitivity for diagnosis of PE. Further large-scale prospective studies are required to validate the potential applicability of using NLR alone or in combination other markers as PE diagnostic biomarker and explore potential factors that may influence the accuracy of NLR for PE diagnosis.
Keywords: biomarkers, meta-analysis, pre-eclampsia
1. Introduction
Preeclampsia (PE) is a unique vascular disease during pregnancy, with an incidence of 2% to 8%.[1] It was divided into 2 categories: mild PE and severe PE, causes adverse pregnancy outcomes between maternal and fetus, such as proteinuria, edema, multiple organ failure, fetal growth restriction, even intrauterine death. A World Health Organization survey on maternal mortality shows that the number of deaths due to pregnancy-induced hypertension worldwide is lower than 15%, it is still one of the top three causes of maternal mortality.[2]
There were a lot of researches on the pathogenesis of PE, one of the most recognized was: Inflammatory stimulation results in an abnormal immune response and induces vascular endothelial dysfunction those lead to hypertension.[3–6]
The term “low-grade inflammation” is used to define disease states that have no obvious clinical symptoms but have elevated immune cells and inflammatory factors.[7,8] Neutrophil-lymphocyte ratio (NLR) is one of the markers of systemic inflammation. It has become a research hotspot in recent years, participating in the development of various diseases and related to the prognosis of tumors.[9–14]
In recent years, some studies about the relationship between NLR and PE have been reported, but the conclusions were different due to the different research design, sample size and basic characteristics of the research subjects.[15–17] Meta-analysis is an effective analytical method to overcome the above shortcomings. In the present study, we conducted the meta-analysis using data from multiple studies to explore the diagnostic value of NLR for PE.
2. Materials and methods
2.1. Study search
The present study was conducted following the criteria of Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA).[18] We conducted a literature search using PubMed, The Cochrane Library, EMbase, Chinese National Knowledge Infrastructure (CNKI), Wanfang Medical Network, VIP (VIP), and China Biomedical Literature Database (CBM) without language limitation. The search time limitation was from the establishment of the database to May 31, 2018. The index words were as follows: “pre-eclampsia” “neutrophil-to-lymphocyte” “neutrophil-lymphocyte” “NLR” “Neutrophil/lymphocyte ratio”. All searches used a combination of subject words and free words. All search strategies were determined by multiple pre-searches, and the search formulas were adjusted according to the characteristics of each database.
Ethical approval was not necessary under the ethical committee of The People's Hospital of China Three Gorges University, since this study was a meta-analysis of previous literature works, which informed consents had been obtained by the previous clinical researchers.
2.2. Study selection
The included studies had to fulfill the following selection criteria:
-
(1)
evaluated diagnostic accuracy of NLR for PE;
-
(2)
Meet the following diagnostic criteria: the 8th edition of Obstetrics and Gynecology[19] or The American College of Obstetricians and Gynecologists (ACOG).[20] After reading the two diagnostic criterions carefully, we found both were the same standard. Such as: a systolic blood pressure of ≥140 mmHg or diastolic blood pressure ≥90 mmHg, measured twice in 4-hour intervals while resting, after the 20th gestational week, as well as 300 mg proteinuria detected in a 24-hour urine sample. Finally, ACOG criteria was chosen as standard for this study.
-
(3)
Clearly reporting sensitivity, specificity with 95% confidence interval or can calculate from 2 × 2 tables.
-
(4)
Duplicate documents of the same author or the same research institution, with closer publication.
-
(5)
the full-text article was available.
Studies with the following characteristics were excluded:
-
(1)
Unsuitable publication types: case reports, meetings, abstracts, reviews.
-
(2)
Animal experiments or non-clinical reports studies.
-
(3)
Incomplete data without original text.
-
(4)
Studies that overlapped the included studies (such as studies from the same study group, institution, and with the same results).
2.3. Quality assessment and data extraction
Two reviewers (Wen-Fei Zheng, Jing-Qiong Zhan) independently screened the literature, extracted the data, including: author, year, country, ethnicity, sample size, diagnostic criteria, outcome indicators (sensitivity, specificity, true positive number (TP), false positive number (FP), false negative number (FN), true negative number (TN)). Missing data were supplemented by contacting authors as much as possible. Quality assessment included in the study was based on the QUADAS-2 scale.[21] Two reviewers independently evaluated the quality of the literature, in case of conflict, a third reviewer was consulted, and disagreement was settled through multilateral discussion.
2.4. Statistical analysis
Meta-disc 1.4 software (Cochrane Colloquium, Barcelona, Spain) and Stata 12.0 (Stata Corporation, College Station, USA) software were used for Statistical analysis. The sensitivity, specificity, diagnostic odds ratio (DOR), positive likelihood ratio (PLR), and negative likelihood ratio (NLR) were summarized. The area under the curve (AUC) was used for evaluating the overall accuracy. In addition, the threshold effect was assessed by the Spearman correlation coefficient (between the logic of sensitivity and logic of 1-specificity), a value of P less than .05 indicated significant threshold effect. The heterogeneities between studies were detected by chi-square and I2 test. P ≤ .05 or an I2 ≥ 50% indicated the existence of significant heterogeneity.[22] Meta-regression analyses and subgroup analysis were used to explore the potential sources of heterogeneity. Deeks’ funnel plot was performed to explore the possibility of publication bias, P < .01was considered representative of significant statistical publication bias.[23] All statistical tests were 2-sided, and P < .05 was considered statistically significant.
3. Results
3.1. Document screening process and results
A total of 130 related articles were obtained in the initial inspection. According to inclusion and exclusion criteria, 31 studies were excluded due to duplication, 56 studies were excluded as irrelevant study. After reading full-text articles, 11 studies were excluded for lacking necessary data. At last, 7 studies were determined to be eligible for meta-analysis (Fig. 1).
Figure 1.
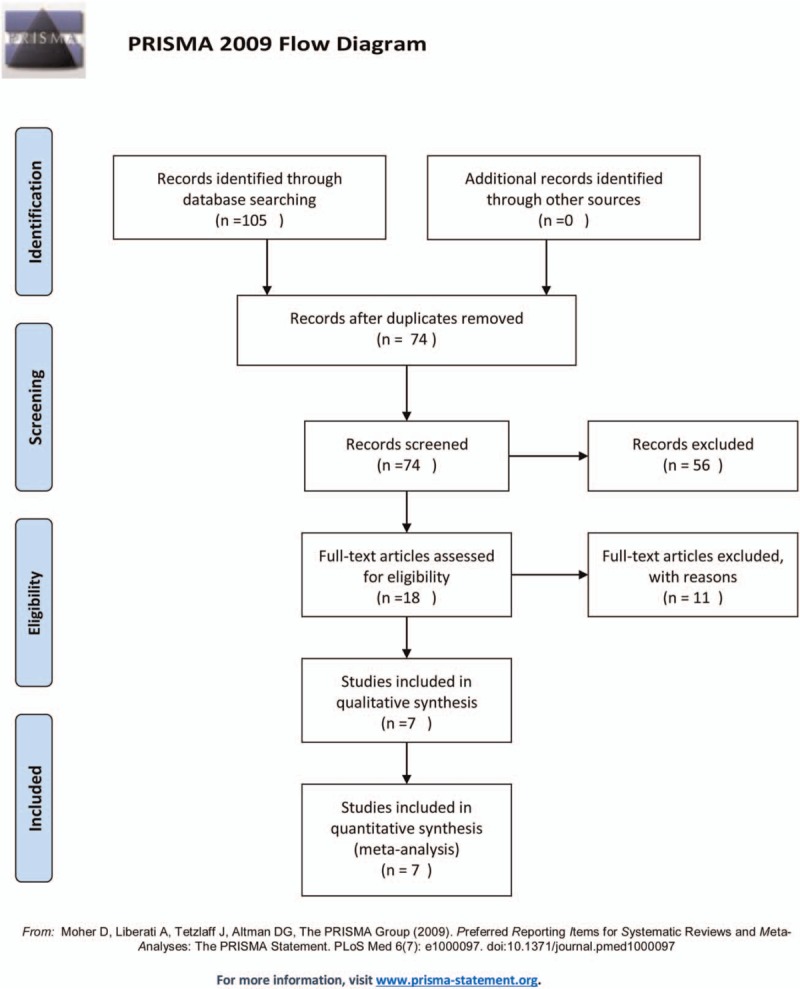
Flow chart showing the process for selecting eligible studies in the meta-analysis.
3.2. Inclusion of research basic characteristics and methodological quality evaluation
The basic characteristics of the study are shown in Table 1. In these 7 studies,[15,16,24–28] including 1298 cases of pre-eclampsia pregnant women (with or and) 894 cases of normal blood pressure pregnant women. All studies were published between 2014 and 2018. The methodological quality assessment is shown in Figure 2. The included studies basically met the criteria for quality assessment of diagnostic accuracy.
Table 1.
Basic characteristics of the included studies.
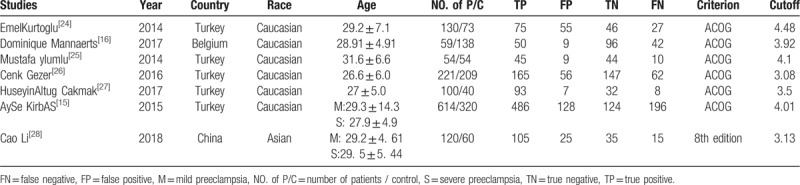
Figure 2.
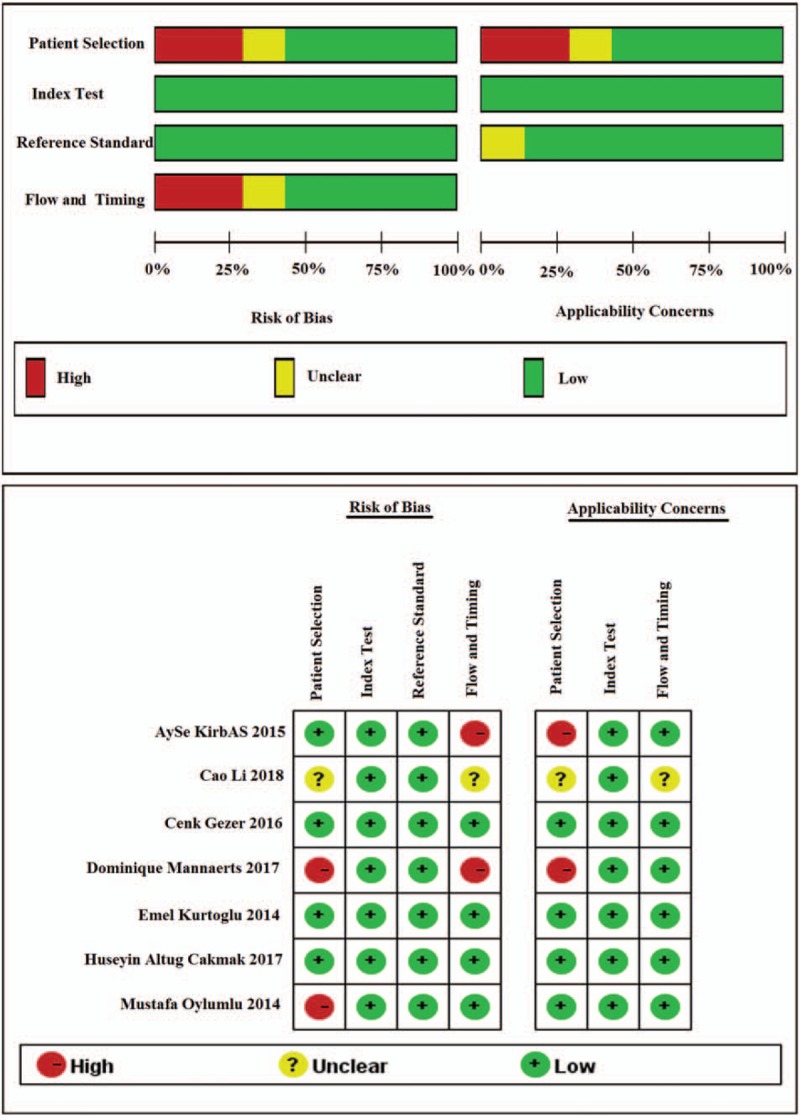
Quality as assessment of the studies selected for the meta-analysis (QUADAS-2).
3.3. Diagnostic accuracy
Seven studies were included and the random effects model was used to evaluate the overall effect of NLR in the diagnosis of PE Figure (Fig. 3 ). The combined sensitivity and specificity were 0.74 (95% CI 0.71–0.76) (Fig. 3 A) and 0.64 (95% CI 0.61–0.68) (Fig. 3 B). In addition, the PLR was 2.62 (95% CI 1.79–3.84) (Fig. 3 C), the negative likelihood ratio was 0.34 (95% CI 0.24–0.48) (Fig. 3 D), the DOR was 8.44 (95% CI 4–17.78) (Fig. 3 E). The SROC curve for the studies was shown in Figure 4, AUC was 0.82. The above results indicated that NLR had a moderate diagnostic value for the diagnosis of PE. Through the test of post-test probability calculation, it was further shown that NLR could improve the diagnostic value of pre-eclampsia, as shown in Figure 5.
Figure 3.
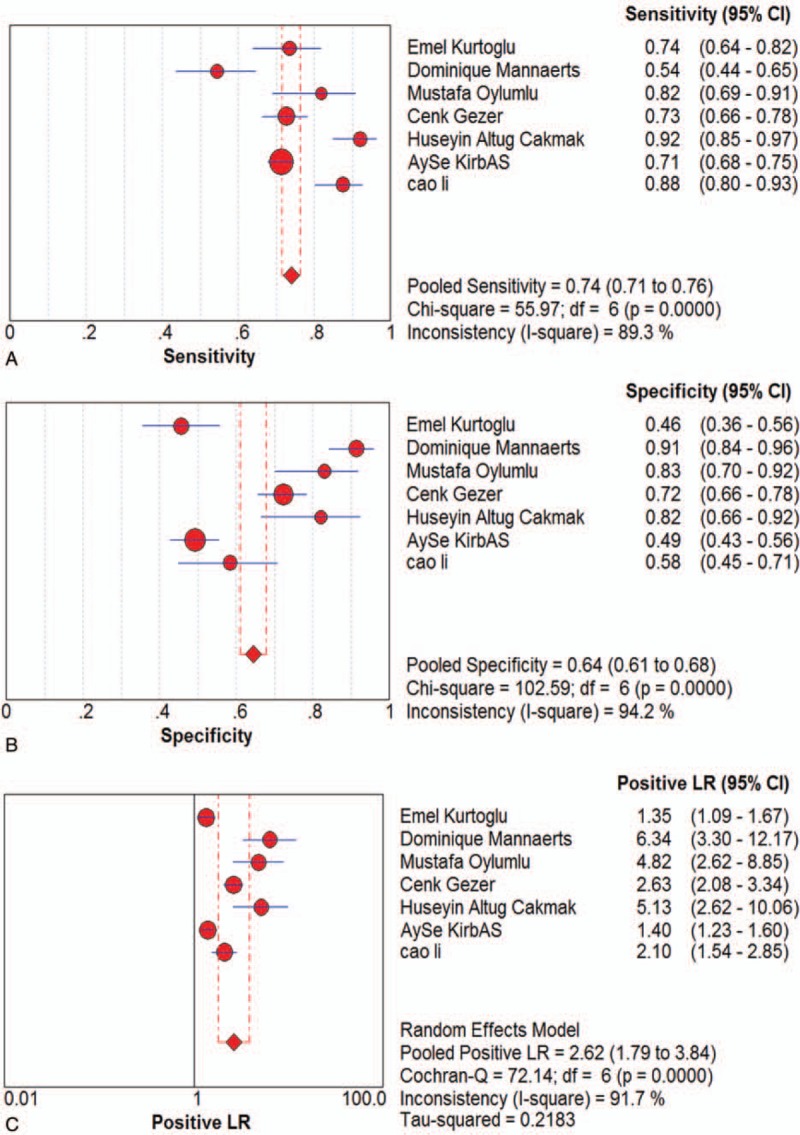
The indicator of effect. Forest plot of estimated sensitivity (A) specificity (B) positive likelihood ratio (C) negative likelihood ratio (D) diagnostic odds ratio (E) for quantitative analysis of Neutrophil-Lymphocyte Ratio in the diagnosis of preeclampsia.
Figure 3 (Continued).
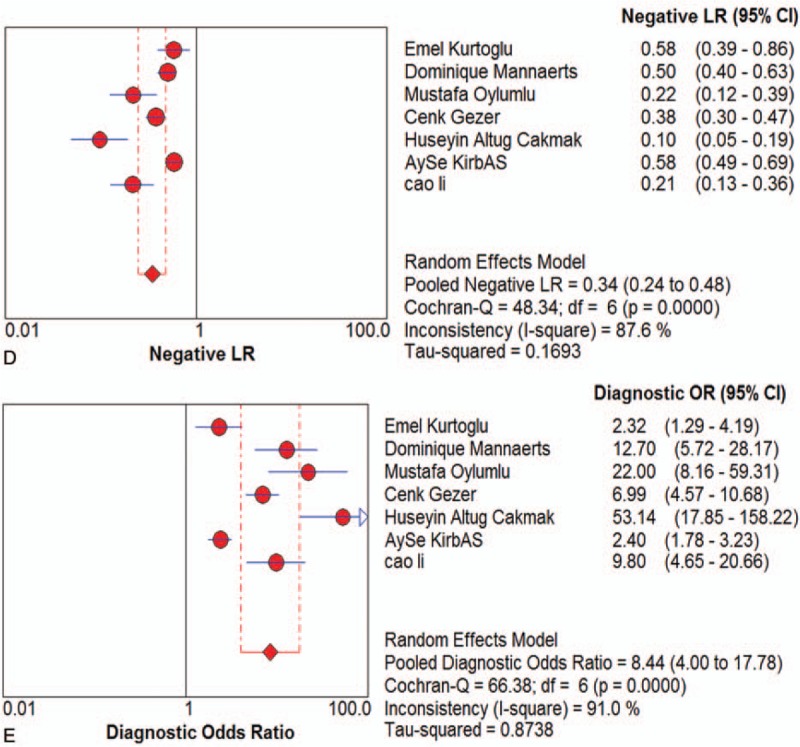
The indicator of effect. Forest plot of estimated sensitivity (A) specificity (B) positive likelihood ratio (C) negative likelihood ratio (D) diagnostic odds ratio (E) for quantitative analysis of Neutrophil-Lymphocyte Ratio in the diagnosis of preeclampsia.
Figure 4.
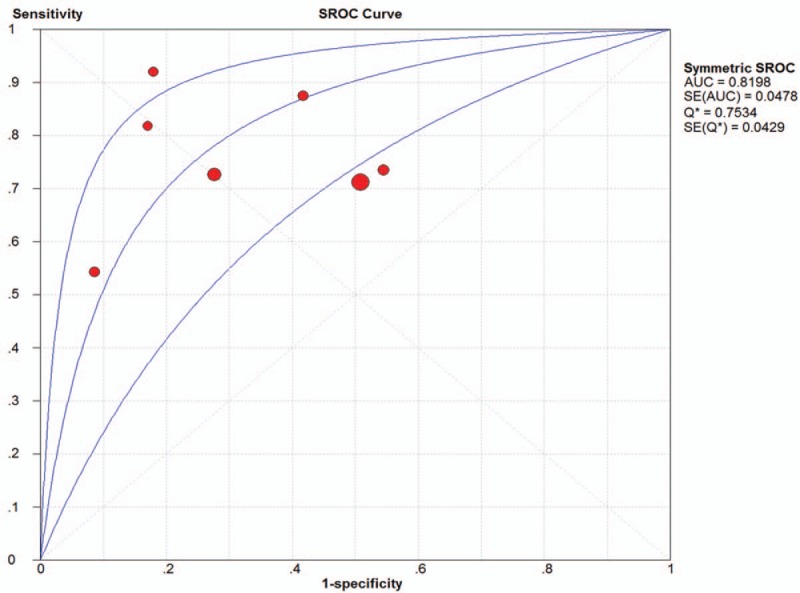
The SROC curve for quantitative analysis of Neutrophil-Lymphocyte Ratio in the diagnosis of preeclampsia.
3.4. Subgroup analysis and meta-regression analysis
I2 test showed obvious heterogeneity among the 7 studies (sensitivity: I2 = 89.3%, specificity: I2 = 94.2%, DOR: I2 = 91%, P < .01). The threshold effect was the primary cause of heterogeneity. The spearman correlation coefficient was 0.179 (P = .702), indicating that there was no threshold effect and the heterogeneity caused by other reasons. To further explore the source of heterogeneity, subgroup analysis was performed based on ethnicity, sample size (total number of samples >200 and total sample size <200), cutoff values (>4 and <4). In the racial subgroup, there was only 1 study on Asian populations, we found that studies on Asian populations group had a better overall accuracy as compared with that of those on non-Asian populations, with sensitivity of 0.88 vs 0.65, specificity of 0.58 vs 0.57, PLR of 2.1 vs 1.36, NLR of 0.38 vs 0.30, RR of 9.8 vs 1.96, only NLR was poor, 0.21 vs 0.71, respectively. Furthermore, the larger sample size group had a better specificity, PLR, negative likelihood ratio, risk ratio and AUC than smaller. In addition, we also found that the cutoff value of NLR (>4 or <4) cannot indicate the accuracy well, the group of NLR>4 only had a bit better sensitivity, PLR and risk ratio, but a poor of specificity, negative likelihood ratio and AUC.
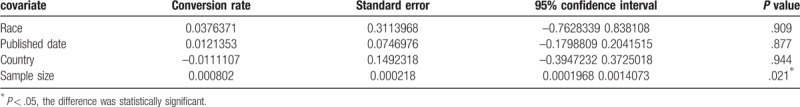
To explore probable sources of the heterogeneity, we utilized a meta-regression analysis to assess covariates used in the seven studies. We found sample size maybe a source of heterogeneity, race, country and published date of articles did not influence on heterogeneity. The pooled data about Subgroup analysis and meta-regression analysis are shown in Tables 2 and 3, respectively.
Table 2.
Subgroup analysis results.
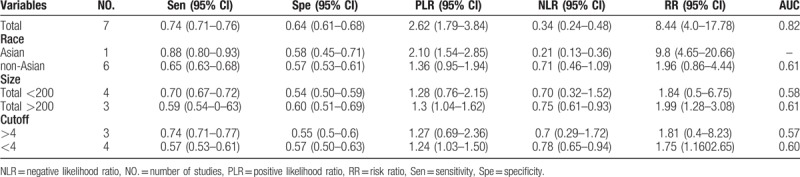
Table 3.
Meta-regression analysis results.
3.5. Publishing bias
The publication bias was visually displayed by using Deek's funnel plots. P value was .16, which indicated there was no publication bias (Fig. 6)
Figure 5.
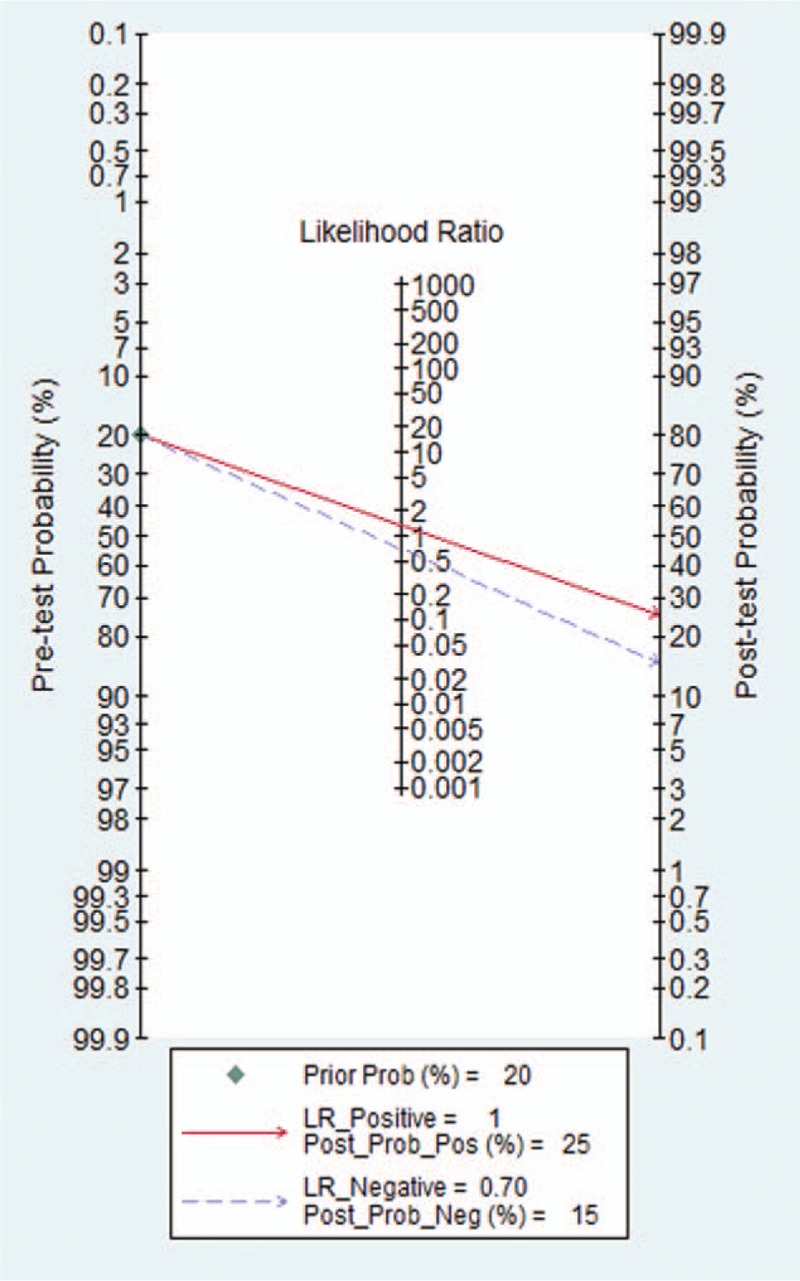
The Fagan for assessment of Neutrophil-Lymphocyte Ratio diagnostic probability.
Figure 6.
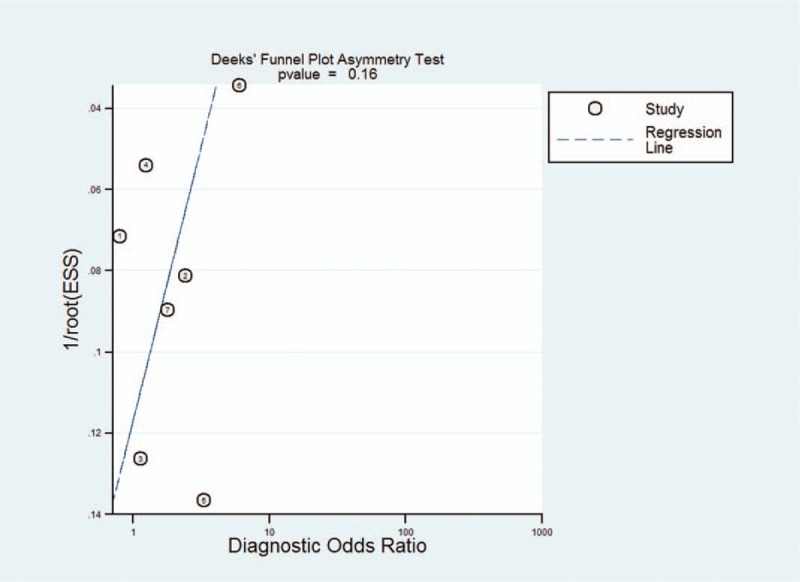
The Deeks’ funnel plot for the assessment of potential publication bias of the included studies.
4. Discussion
The present systematic review and meta-analysis of 7 studies including 1298 cases of pre-eclampsia pregnant women, and or with 894 cases of normal blood pressure pregnant women investigated the potential of NLR for identification of PE. In recent years, NLR has attracted more attention as a marker of systemic inflammatory response. A previous study confirmed[29,30] that NLR had a certain value for the prediction and prognosis in some cancers and cardiovascular diseases. There are so many methods to predict the diagnose of PE, including traditional prediction, biophysical prediction and serum marker prediction.[31–34] There are abundant studies about serum markers, such as Liu's study[35] about the relationship between body mass index (BMI), hematocrit (HCT), platelets (PLT), miR-210 and PE in early pregnancy show the AUCs were 0.69, 0.67, 0.73, and 0.75, respectively. The AUC of the above indicators in the second trimester were 0.71, 0.81, 0.65, and 0.81, respectively. Jin et al[36] used a combined detection of homocysteine + triglyceride + lipoprotein B + age + BMI (Hcy + TG + Apo-B + folic acid + age + BMI) to predict the PE, and the AUC was 0.77. Gan et al[34] showed that PE can be predicted by miR-210 and miR-155, AUC was 0.75 and 0.703, respectively. All of these studies have shown that some serologic markers are valuable in predicting PE, but none of them are acknowledged and reliable.
The present study showed that NLR had a good diagnostic value for the diagnosis of PE, the AUC is 0.82. Unfortunately, less research has been done to compare NLR and other conventional markers, so we could not clarify whether the individual or combined NLR can improve the diagnostic accuracy of PE. The pooled sensitivity and specificity of NLR were 0.74 (95% CI 0.71–0.76) and 0.64 (95% CI 0.61–0.68), respectively, revealing the quantitative analysis of NLR has an acceptable sensitive but a poor specificity for diagnosis of PE. The likelihood ratio is an independent indicator to assess the authenticity, which can simultaneously reflect sensitivity and specificity. When the PLR is >10 or the negative likelihood ratio is <0.1, the probability of diagnosing or excluding a certain disease is significantly increased,[37] Likelihood ratio is more clinically significant than SROC curve and DOR value. In our study, the PLR and the negative likelihood ratio were 2.62 (95% CI 1.79–3.84) and 0.34 (95% CI 0.24–0.48), respectively. The results showed that the diagnostic accuracy of NLR in PE patients was nearly 3 times of the healthy patients, but with 34% error rate. These results indicated that the likelihood ratio obtained in Meta-analysis may be poor stability and accuracy. Moreover, when the odds ratio is equal to 1, indicate a test cannot distinguish between patients with a disease or not. The DOR value of our study is 8.44 (95% CI 4–17.78), indicated that NLR had a certain accuracy in the diagnosis of patients with PE. According to Fagan's analysis, we knew that the post-test probability of PLR can be increased by 25% when the pre-test probability was 20%, the negative likelihood ratio was reduced to 15%. The above results showed that there was a good diagnostic value of NLR for PE.
Heterogeneity is an important part of the meta-analysis. The I2 test in our study showed a high degree of heterogeneity among the seven studies included. The threshold effect is a main source of heterogeneity, the spearman correlation coefficient of this study was 0.179. (P = .702), indicating that heterogeneity was caused by not a threshold effect but other reasons. To explore the potential sources of heterogeneity, we conducted a meta-regression of the country, publication time, sample size, and ethnicity. The results showed that sample size was the heterogeneity source of the study, the test of P value was .021. In addition, publication bias is not significant, indicating that our meta-analysis results were reliable.
There are still some limitations in our study:
-
(1)
NLR is a new indicator of systemic inflammation, and the numbers of document included were limited in this meta-analysis. Some defects exist in robustness.
-
(2)
Five of the 7 studies were from Turkey and one from China, involved English and Chinese, so selective bias for specific populations or languages may exist.
-
(3)
Some data (such as TP, FP, TN, FN) were obtained not directly from the original articles but indirectly calculated by AUC, which may affect the accuracy of the diagnosis.
-
(4)
The sample size included affected the results in a way.
-
(5)
The time of test NLR is different, some in first trimester, some are not. Time span is different, it is difficult to do subgroup analysis, which may be a factor of heterogeneity.
5. Conclusion
In summary, NLR has an acceptable sensitivity as a diagnostic marker for PE, but not better specificity. Prospective studies with larger sample will be needed in the future to verify the potential applicability of NLR alone or combination with other markers for PE.
Acknowledgments
Thanks all authors and patients of the previous research for providing data for our article.
Author contributions
Conceptualization: Wenfei Zheng
Data curation: Jingqiong Zhan, Huigai Ma
Formal analysis: Wenfei Zheng, Aihua Chen
Methodology: Huaijie Yang
Writing – original draft: Wenfei Zheng,
Writing – review & editing: Rashmisha Maharjan
Conceptualization: Wenfei Zheng.
Data curation: Jingqiong Zhan, Huigai Ma.
Formal analysis: Wenfei Zheng, Aihua Chen.
Methodology: Huaijie Yang.
Writing – original draft: Wenfei Zheng.
Writing – review & editing: Rashmisha Maharjan.
Footnotes
Abbreviations: AUC = the area under the curve, BMI = Body Mass Index, CBM = China Biomedical Literature Database, CI = confidence interval, CNKI = Chinese National Knowledge Infrastructure, DOR = diagnostic odds ratio, Fig = Figure, FN = false negative number, FP = false positive number, HCT = hematocrit, NLR = negative likelihood ratio/ Neutrophil-lymphocyte ratio, PE = preeclampsia, PLR = positive likelihood ratio, PLT = platelets, TN = true negative number, TP = true positive number.
How to cite this article: Zheng WF, Zhan J, Chen A, Ma H, Yang H, Maharjan R. Diagnostic value of neutrophil-lymphocyte ratio in preeclampsia: A PRISMA-compliant systematic review and meta-analysis. Medicine. 2019;98:51(e18496).
This study was financially supported by the National Natural Science Foundation of China (No. 81701484) and medical and health research projects of Yichang (A19–301–28).
The authors have no conflicts of interests to disclose.
References
- [1].Wong T, Groen H, Faas M, et al. Clinical risk factors for gestational hypertensive disorders in pregnant women at high risk for developing preeclampsia. Pregnancy Hypertens 2013;3:248–53. [DOI] [PubMed] [Google Scholar]
- [2].Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, et al. Global, regional, and national levels and causes of maternal mortality during 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet (London, England) 2014;384:980–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rovere-Querini P, Castiglioni MT, Sabbadini MG, et al. Signals of cell death and tissue turnover during physiological pregnancy, pre-eclampsia, and autoimmunity. Autoimmunity 2007;40:290–4. [DOI] [PubMed] [Google Scholar]
- [4].Messerli M, May K, Hansson SR, et al. Feto-maternal interactions in pregnancies: Placental microparticles activate peripheral blood monocytes. Placenta 2010;31:106–12. [DOI] [PubMed] [Google Scholar]
- [5].Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 2011;123:2856–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Laresgoiti-Servitje E, Gomez-Lopez N, Olson DM. An immunological insight into the origins of pre-eclampsia. Hum Reprod Update 2010;16:510–24. [DOI] [PubMed] [Google Scholar]
- [7].Fowler A, Agha R. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography--the growing versatility of NLR. Atherosclerosis 2013;228:44–5. [DOI] [PubMed] [Google Scholar]
- [8].Guthrie G, Charles K, Roxburgh C, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218–30. [DOI] [PubMed] [Google Scholar]
- [9].Ethier JL, Desautels D, Templeton A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res 2017;19:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wei B, Yao M, Xing C, et al. The neutrophil lymphocyte ratio is associated with breast cancer prognosis: an updated systematic review and meta-analysis. OncoTargets Ther 2016;9:5567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu JF, Ba L, Lv H, et al. Association between neutrophil-to-lymphocyte ratio and differentiated thyroid cancer: a meta-analysis. Sci Rep 2016;6:38551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kanatsios S, Melanoma Project M, Li Wai Suen CSN, et al. Neutrophil to lymphocyte ratio is an independent predictor of outcome for patients undergoing definitive resection for stage iv melanoma. J Surg Oncol 2018;118:915–21. [DOI] [PubMed] [Google Scholar]
- [13].Chan JY, Zhang Z, Chew W, et al. Biological significance and prognostic relevance of peripheral blood neutrophil-to-lymphocyte ratio in soft tissue sarcoma. Sci Rep 2018;8:11959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Thio Q, Goudriaan WA, Janssen SJ, et al. Prognostic role of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with bone metastases. Br J Cancer 2018;119:737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kirbas A, Ersoy AO, Daglar K, et al. Prediction of preeclampsia by first trimester combined test and simple complete blood count parameters. J Clin Diagn Res 2015;9:Qc20–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mannaerts D, Heyvaert S. Are neutrophil/lymphocyte ratio (nlr), platelet/lymphocyte ratio (plr), and/or mean platelet volume (mpv) clinically useful as predictive parameters for preeclampsia? J Matern Fetal Neonatal Med 2019;32:1412–9. [DOI] [PubMed] [Google Scholar]
- [17].Yucel B, Ustun B. Neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, mean platelet volume, red cell distribution width and plateletcrit in preeclampsia. Pregnancy hypertens 2017;7:29–32. [DOI] [PubMed] [Google Scholar]
- [18].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. Ann Intern Med 2009;151:264–9. w264. [DOI] [PubMed] [Google Scholar]
- [19].Xie Xing GW. Obstetrics and Gynaecology. 2013;Beijing, China: People's Medical Publishing House, 66-67. [Google Scholar]
- [20].Tamás P, Hantosi E, Farkas B, et al. Preliminary study of the effects of furosemide on blood pressure during late-onset pre-eclampsia in patients with high cardiac output. Int J Gynaecol Obstet 2017;136:87–90. [DOI] [PubMed] [Google Scholar]
- [21].Whiting P, Rutjes A, Westwood M, et al. Quadas-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- [22].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882–93. [DOI] [PubMed] [Google Scholar]
- [24].Kurtoglu E, Kokcu A, Celik H, et al. May ratio of neutrophil to lymphocyte be useful in predicting the risk of developing preeclampsia? A pilot study. J Matern Fetal Neonatal Med 2015;28:97–9. [DOI] [PubMed] [Google Scholar]
- [25].Oylumlu M, Ozler A, Yildiz A, et al. New inflammatory markers in pre-eclampsia: Echocardiographic epicardial fat thickness and neutrophil to lymphocyte ratio. Clin Exp Hypertens 2014;36:503–7. [DOI] [PubMed] [Google Scholar]
- [26].Gezer C, Ekin A, Ertas IE, et al. High first-trimester neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios are indicators for early diagnosis of preeclampsia. Ginekol Pol 2016;87:431–5. [DOI] [PubMed] [Google Scholar]
- [27].Cakmak HA, Dincgez Cakmak B, Abide Yayla C, et al. Assessment of relationships between novel inflammatory markers and presence and severity of preeclampsia: Epicardial fat thickness, pentraxin-3, and neutrophil-to-lymphocyte ratio. Hypertens Pregnancy 2017;36:233–9. [DOI] [PubMed] [Google Scholar]
- [28].Cao Li LH. Correlation between neutrophil - lymphocyte ratio and preeclampsia. Chin J Matern Child Health 2018;33:3. [Google Scholar]
- [29].Kaya H, Ertas F, Soydinc MS. Association between neutrophil to lymphocyte ratio and severity of coronary artery disease. Clin Appl Thromb Hemost 2014;20:50–4. [DOI] [PubMed] [Google Scholar]
- [30].Unal D, Eroglu C, Kurtul N, et al. Are neutrophil/lymphocyte and platelet/lymphocyte rates in patients with non-small cell lung cancer associated with treatment response and prognosis? Asian Pac J Cancer Prev 2013;14:5237–42. [DOI] [PubMed] [Google Scholar]
- [31].Pillay P, Moodley K, Moodley J, et al. Placenta-derived exosomes: potential biomarkers of preeclampsia. Int J Nanomedicine 2017;12:8009–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Erez O, Romero R, Maymon E, et al. The prediction of late-onset preeclampsia: results from a longitudinal proteomics study 2017;12:e0181468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fung MF, Reid A, Faught W, et al. Prospective longitudinal study of ultrasound screening for endometrial abnormalities in women with breast cancer receiving tamoxifen. Gynecol Oncol 2003;91:154–9. [DOI] [PubMed] [Google Scholar]
- [34].Gan L, Liu Z, Wei M, et al. Mir-210 and mir-155 as potential diagnostic markers for pre-eclampsia pregnancies. Medicine 2017;96:e7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu Liyi LM, Li Wei, Han Luhao, et al. The predictive value of serum mir-210 in severe preeclampsia. Chin J Eugen Genet 2018;26:50–4. [Google Scholar]
- [36].Jin Y, Feng S, Yuna G, et al. The predictive value of homocysteine and blood lipid levels in early pregnancy for severe preeclampsia. Int J Gynecol Obstet 2018;45: 51-54+84. [Google Scholar]
- [37].Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ (Clinical research ed) 2004;329:168–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


