Abstract
Previous researches have shown that anesthesia can affect the outcomes of many kinds of cancer after surgery. Here, we investigated the association between anesthesia and patient outcomes after elective open intrahepatic cholangiocarcinoma surgery.
This was a retrospective cohort study of patients who received elective open intrahepatic cholangiocarcinoma surgery between January 2005 and December 2014. Patients were grouped according to the anesthesia received, that is, propofol or desflurane anesthesia. Kaplan–Meier analysis was performed and survival curves were constructed from the date of surgery to death. After propensity matching, univariable and multivariable Cox regression models were used to compare hazard ratios for death. Subgroup analyses were performed for tumor node metastasis staging and postoperative metastasis and recurrence.
A total of 34 patients (21 deaths, 62.0%) with propofol anesthesia and 36 (31 deaths, 86.0%) with desflurane anesthesia were eligible for analysis. After propensity matching, 58 patients remained in each group. In the matched analysis, the propofol anesthesia had a better survival with hazard ratio of 0.51 (95% confidence interval, 0.28–0.94, P = .032) compared with desflurane anesthesia. In addition, subgroup analyses showed that patients under propofol anesthesia had less postoperative metastases (hazard ratio, 0.36; 95% confidence interval, 0.15–0.88; P = .025), but not fewer postoperative recurrence formation (hazard ratio, 1.17; 95% confidence interval 0.46–2.93; P = .746), than those under desflurane anesthesia in the matched groups.
In a limited sample size, propofol anesthesia was associated with better survival in open intrahepatic cholangiocarcinoma surgery. Prospective and large sample size researches are necessary to evaluate the effects of propofol anesthesia on the surgical outcomes of intrahepatic cholangiocarcinoma surgery.
Keywords: desflurane, intrahepatic cholangiocarcinoma surgery, propofol
1. Introduction
Intrahepatic cholangiocarcinoma (ICC) represents the second most common primary liver cancer and is increasing in incidence. Most patients are diagnosed at an advanced, nonsurgical stage and only about 1 in 5 cases are surgically resectable. Despite surgery, the recurrence rate is 60% to 70%, and the 5-year survival is only 30%.[1] Surgical resection is still the most effective treatment for patients with ICC for long-term survival.[2] Surgical procedure results in metabolic and neuroendocrine changes, which may lead to significant suppression of cell-mediated immunity and may eventually stimulate the implantation of circulating tumor cells.[3] This potential combination of tumor seeding and impaired immune responses enhances the susceptibility of patients undergoing cancer surgery to the development of metastasis and is associated with worse long-term outcomes. The potential role of anesthetic techniques and anesthetics in the process of cancer recurrence has attracted interest.[3]
Data from animal and human cancer cell line studies revealed that different anesthetics might influence the immune system in various ways.[4–9] Researches have shown that volatile anesthetics (VAs) are proinflammatory and may worse immune function, which may increase the incidence of cancer metastases.[8–12] In contrast, propofol seems to inhibit tumor growth and to reduce the risk of metastasis in mice and humans.[6,11–14]
Until now, no clinical study has compared the effects of the use of propofol- with desflurane-based anesthesia on patient outcomes after ICC surgery. We hypothesized that those patients receiving the desflurane anesthesia might have a subsequent worse outcome as our previous hepatocellular carcinoma (HCC) and colon cancer studies.[15,16] Therefore, we conducted a retrospective study to assess whether the type of anesthesia, desflurane anesthesia versus propofol anesthesia, is associated with long-term survival, postoperative recurrence, and metastasis after open ICC surgery.
2. Methods
2.1. Study design and setting
This retrospective cohort study was conducted at the Tri-Service General Hospital (TSGH), Taipei, Taiwan, Republic of China, a medical center.
2.2. Participants and data sources
The ethics committee of TSGH (Chairperson, Professor Mu-Hsien Yu) approved this retrospective study and waived the need for informed consent on May 18, 2017 (TSGHIRB No: 1-106-05-089). The relevant information was retrieved from the medical records and electronic database of TSGH. From January 2005 to December 2014, 70 patients with an American Society of Anesthesiologists (ASA) score of II to III who had undergone elective open ICC surgery for tumor node metastasis (TNM) stages I to IV ICC under propofol anesthesia (propofol group, n = 34) or desflurane anesthesia (desflurane group, n = 36) were included. The anesthetic technique was determined according to the anesthesiologist's preference. The exclusion criteria were combined propofol anesthesia with inhalation anesthesia or regional analgesia, incomplete data, and age <20 years (Fig. 1).
Figure 1.
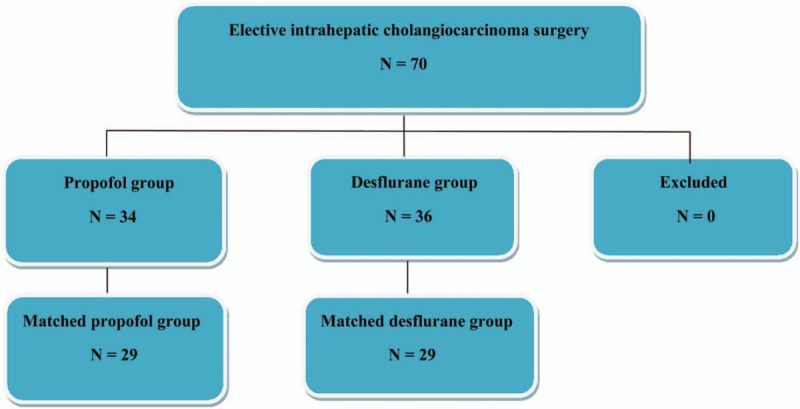
Flow diagram detailing the selection of patients included in the retrospective analysis. No patient was excluded due to combined propofol anesthesia with inhalation anesthesia or regional analgesia, incomplete data, and age <20 years.
No premedication was given before anesthesia induction. Standard monitoring, including noninvasive blood pressure, electrocardiography (lead II), pulse oximetry, end-tidal carbon dioxide, direct radial arterial blood pressure, and a central venous catheter were used for each patient. Anesthesia induction was with fentanyl, propofol, and cisatracurium or rocuronium for all cases.
In the propofol group, anesthesia was maintained with target-controlled infusion (TCI, Fresenius Orchestra Primea; Fresenius Kabi AG, Bad Homburg, Germany) using propofol at an effect site concentration (Ce) of 3 to 4 μg/mL in FiO2 of 100% oxygen at a flow rate of 0.3 L/min. In the desflurane group, the desflurane vaporizer was set between 4% and 10% in 100% oxygen at a flow of 300 mL/min in a closed breathing system. Repetitive bolus injections of cisatracurium and fentanyl were given as necessary throughout the operation.[15,16]
Maintenance of the Ce using TCI with propofol or desflurane was adjusted upward and downward by 0.2 to 0.5 μg/mL or 0.5% to 2%, respectively, when necessary according to the hemodynamics. The end-tidal carbon dioxide level was maintained at 35 to 45 mm Hg by adjusting the ventilation rate and maintained maximum airway pressure <30 cm H2O. After surgery patients were transferred to the postanesthesia care unit or intensive care unit for further care and were evaluated by the anesthesiologist in charge.[15,16]
2.3. Variables
We retrospectively collected the following patient data: anesthetic technique; time since the earliest included patient, which served as a surrogate of calendar year; sex; age at the time of surgery; serum hepatitis B surface antigen (HBsAg) positivity; serum hepatitis C virus (HCV) positivity; history of alcoholism; preoperative serum CA19-9 values; Child-Pugh score; and model for end-stage liver disease (MELD) score. For preoperative serum CA19-9 values, patients were grouped according to whether their CA19-9 levels were >37 or ≤37 U/mL, because a CA19-9 level >37 U/mL is associated with poor survival in ICC surgery.[17] The 10-year survival in patients with multiple comorbidities was predicted using the Charlson comorbidity index (CCI) of 0 (least comorbidity) to 37 (highest). Preoperative functional status was assessed in metabolic equivalents (METs), and patients were grouped according to whether their MET was ≥4 METs or <4 METs because the perioperative cardiac and long-term risks increase in patients with a capacity of <4 METs during most normal daily activities.[18] Other data included the ASA physical status score (from I indicating the lowest morbidity to V indicating the highest morbidity); TNM stage of the primary tumor; tumor size; tumor number; grade of surgical complications using the Clavien–Dindo classification [scaled from 0 (no) to V (most)]; intraoperative blood transfusion; postoperative chemotherapy; presence of postoperative recurrence; and presence of postoperative metastasis. Because these variables have been reported or posited to influence patient outcomes, they were chosen as potential confounders.
2.4. Statistical methods
The primary end-point was overall survival, which was compared between the groups receiving propofol or desflurane as the main anesthetic agent. Survival time was defined as the interval between the date of surgery and the date of death or August 31, 2017, for those who were censored. All data are presented as mean ± standard deviation (SD), number, or percentage.
Patient characteristics and death rates were compared between the groups treated with the different anesthetics using Student t test or the chi-square test. Survival according to the anesthetic technique was depicted visually in a Kaplan–Meier survival curve. The relationship between the anesthetic techniques (propofol or desflurane) and survival was analyzed using the Cox proportional-hazards model with and without adjustment for the abovementioned variables. Because significant interactions with the anesthetic techniques (propofol or desflurane) were found, we also conducted subgroup analyses for TNM stage and postoperative metastasis and recurrence.
Propensity score (PS) matching with IBM SPSS Statistics 22.0 (IBM SPSS Inc., Chicago, IL) was used to select for the most similar PSs for preoperative variables (with calipers set at 0.2 SD of the logit of the PS) across each anesthesia: propofol or desflurane in a 1:1 ratio, to make sure the comparability between propofol and desflurane anesthesia before the surgery. Two-tailed P values <.05 were considered significant.
3. Results
The patient and treatment characteristics are shown in Table 1. The time since the earliest included patient, sex, age, HBsAg, HCV, Child-Pugh score, MELD score, preoperative CA19-9 level, CCI, preoperative functional status, ASA score, TNM stage of the primary tumor, tumor size, tumor number, grade of surgical complications, need for intraoperative blood transfusion, and the use of postoperative chemotherapy were insignificantly different between the 2 anesthetic techniques (Table 1).
Table 1.
Patients’ and treatment characteristics for overall group and matched group after propensity scoring.
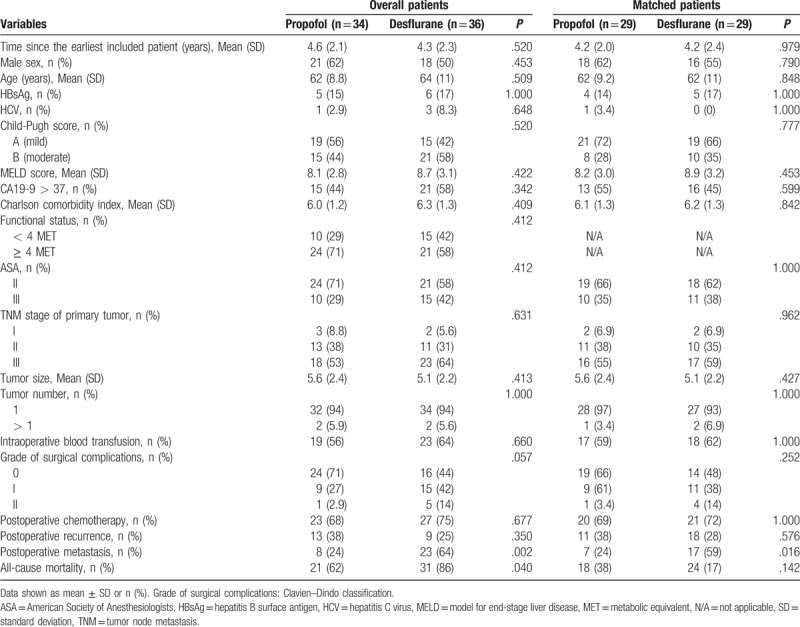
The overall mortality rate was significantly lower in the propofol anesthesia (62.0%) than in the desflurane anesthesia (86.0%) during follow-up (P = .04). In addition, in this study, the overall mortality equated to the cancer-specific mortality, because all postoperative deaths were resulting from cancer. Accordingly, the cancer-specific mortality rate was significantly lower in the propofol anesthesia (62.0%) than in the desflurane anesthesia (86.0%) during follow-up (P = .04). A lower percentage of patients in the propofol anesthesia (24.0%) exhibited postoperative metastasis compared with the desflurane anesthesia (64.0%; P = .002). The presence of postoperative recurrence did not differ between the 2 groups (Table 1). Kaplan–Meier survival curves for the 2 anesthetic techniques are shown in Figure 2A.
Figure 2.
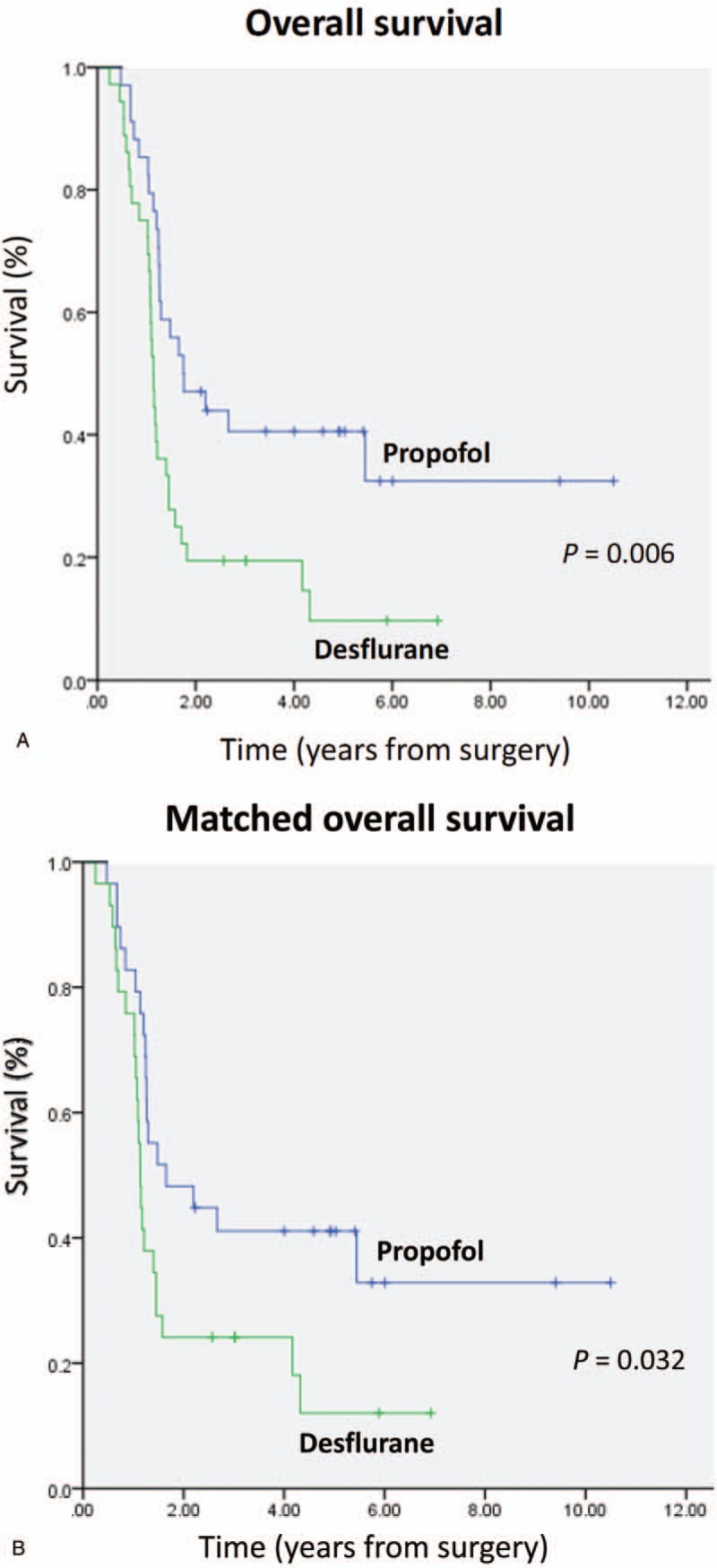
A, Overall survival curves from the date of surgery by anesthesia type. B, Overall survival curves from the date of surgery by anesthesia type after propensity score matching.
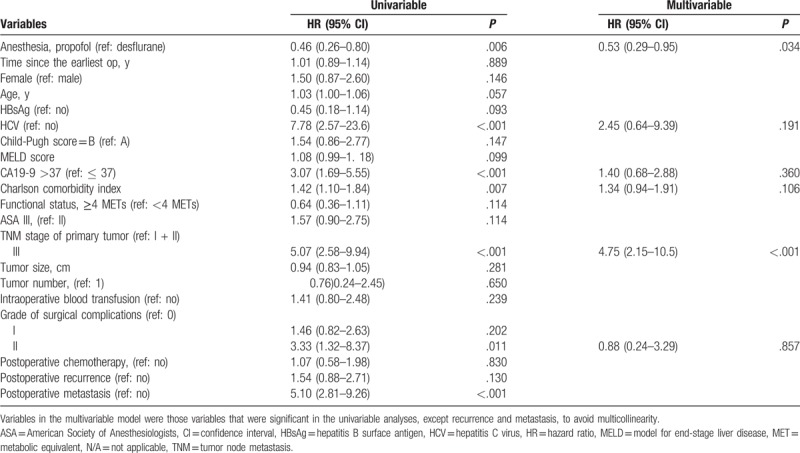
The overall mortality risk associated with the use of propofol and desflurane anesthesia during ICC surgery is shown in Table 2. Overall survival from the date of surgery grouped according to the anesthetic technique and other variables was compared separately in a univariable Cox model and subsequently in a multivariable Cox regression model. Patients who received propofol anesthesia exhibited better overall survival than those who received desflurane anesthesia [overall survival 38.2% vs 13.9%, respectively; the crude hazard ratio (HR) was 0.46; 95% confidence interval (CI), 0.26–0.80; P = .006]. Variables in the multivariable model were those variables that were significant in the univariable analysis, except recurrence and metastasis, to avoid multicollinearity, and the finding did not change (HR, 0.53; 95% CI, 0.29–0.95; P = .034). Other variable that significantly increased the risk of death after the multivariable analysis was higher TNM stage (Table 2).
Table 2.
Cox proportional-hazards regression for mortality: univariable and multivariable models for overall patients.
Because PS matching is considered the best method to minimize confounding in observational studies.[19] We used the PS from logistic regression to adjust the baseline characteristics and the choice of therapy between the 2 groups. After matching, 29 pairs were formed (Table 1). Patient characteristics and prognostic factors of ICC were insignificantly different between the matched groups. Kaplan–Meier survival curves for the 2 anesthetic techniques are shown in Figure 2B.
3.1. Subgroup analyses for presence of postoperative metastasis, postoperative recurrence, TNM stage, and disease progression
In the nonstratified analysis, patients with propofol anesthesia showed better survival than those with desflurane; the crude HR was 0.46 (95% CI, 0.26–0.80; P = .006), and the PS-matched HR was 0.51 (95% CI, 0.28–0.94; P = .032) (Table 3; Fig. 3).
Table 3.
Subgroup analyses for presence of postoperative recurrence, postoperative metastasis, TNM stage, and disease progression.
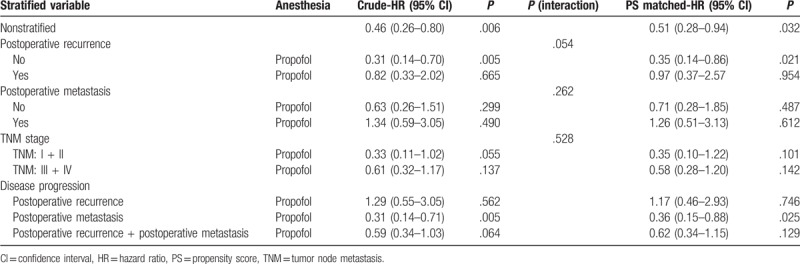
Figure 3.
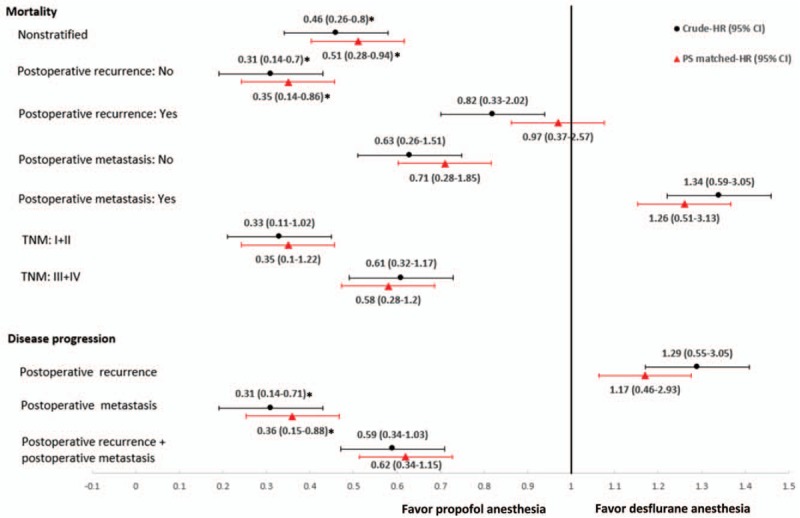
Subgroup analyses for presence of postoperative recurrence, postoperative metastasis, TNM stage, and disease progression. Data were presented as hazard ratio (HR) with 95% confidence interval (CI). ∗P < .05 compared with desflurane anesthesia. PS = propensity score, TNM = tumor node metastasis.
There was marginally significant interaction between the anesthetic techniques and postoperative recurrence formation (P = .054). However, there was insignificant interaction between the anesthetic techniques and postoperative metastasis (P = .262) or TNM stage (P = .528) (Table 3).
Patients without postoperative recurrence who received propofol had better survival than those who received desflurane. For patients with no postoperative recurrence, the crude HR was 0.31 (95% CI, 0.14–0.70; P = .005), and the PS-matched HR was 0.35 (95% CI, 0.14–0.86; P = .021). However, the mortality rate was no significant difference in patients with postoperative recurrence between 2 techniques as the crude HR was 0.82 (95% CI, 0.33–2.02; P = .665), and the PS-matched HR was 0.97 (95% CI, 0.37–2.57; P = .954) (Table 3; Fig. 3).
Patients with propofol anesthesia had less postoperative metastasis than those with desflurane; the crude HR was 0.31 (95% CI, 0.14–0.71; P = .005), and the PS-matched HR was 0.36 (95% CI, 0.15–0.88; P = .025). However, with regard to postoperative recurrence, propofol anesthesia was similar to desflurane anesthesia, the crude HR was 1.29 (95% CI, 0.55–3.05; P = .562) and the PS-matched HR was 1.17 (95% CI, 0.46–2.93; P = .746) (Table 3; Fig. 3).
In summary, patients with ICC with propofol anesthesia had lower all-cause mortality, lower cancer-specific mortality, and fewer postoperative metastasis. In addition, propofol anesthesia seemed to reduce recurrence-free mortality compared with desflurane anesthesia.
4. Discussion
The major finding in the present study is that propofol anesthesia in open ICC surgery improves survival and reduces risk of postoperative metastasis compared with desflurane anesthesia. These findings are consistent with those of previous researches of propofol anesthesia that demonstrated better survival after surgery for gastrointestinal cancers, such as esophageal, gastric, HCC, or colon cancer compared with VAs.[15,16,20,21] In addition, we found that a higher TNM stage was associated with poor survival after ICC surgery, as has also been observed previously.[22]
Surgical resection is the only potentially curative treatment for ICC and is associated with 5-year overall survival rates between 15% and 40%.[1] However, up to two-thirds of the patients with ICC have postoperative cancer recurrence, most commonly in the remnant liver.[1] Other common sites of recurrence include the peritoneum and abdominal lymph nodes.[1] Prognostic factors associated with cancer recurrence are vascular invasion, multiple tumors, and lymph node metastases.[1] The likelihood of tumor metastasis depends on the balance between the metastatic potential of the tumor and the antimetastatic host defenses, of which cell-mediated immunity, and natural killer cell function in particular, is a critical component.[23] Data from studies of human cancer cell lines and animal showed that various anesthetics or anesthetic techniques might affect the immune system in various ways[4–9] and might therefore influence the cancer patient's survival or risk of recurrence or metastasis.[6,8–11]
In this study, we found a 50% lower death rate with propofol anesthesia than with desflurane anesthesia in patients after ICC surgery. We also found that propofol anesthesia was related to a lower incidence of postoperative recurrence and metastasis compared with desflurane anesthesia in HCC or colon cancer surgery.[15,16] By contrast, recent retrospective studies reported insignificant differences in overall survival between the use propofol and VAs in breast cancer and HCC surgery.[11,24–26] So far, there are very few researches of the effects of the anesthetic techniques in ICC surgery; further investigations are needed to illuminate the effects of the anesthetic techniques on ICC recurrence and metastasis.
To our best knowledge, very few researches of human ICC cell lines reported the influences of propofol on cancer cell growth and survival. Recently, using human cholangiocarcinoma cell lines, Zhang et al[27] reported that propofol inhibited the proliferation, migration, and invasion of cholangiocarcinoma cells, and induced apoptosis and cell cycle arrest. The mechanism may be associated with the Wnt/β-catenin signaling pathway. This finding suggested that propofol may be an effective drug for treating ICC, although further clinical studies are warranted.
Upregulation of hypoxia-inducible factor (HIF) was associated with a poor prognosis in colorectal cancer study.[28] In addition, HIF-1α overexpression correlated significantly with higher stage, and tended to correlate with tumor diameter (>4 cm), vessels infiltration, and intrahepatic metastasis in ICC surgery.[29] HIF-1α overexpression was also identified as an independent prognostic factor for both overall and disease-free survival in ICC.[29] Propofol inhibited tumor aggressiveness by suppressing HIF-1α expression in non–small cell lung cancer cells.[30] By contrast, isoflurane enhanced renal cancer cell growth and malignant potential via upregulating HIF-1α levels in vitro.[31] Sevoflurane also promoted the expansion of human glioma stem cells through activation of HIF-1α in vitro.[32] Taken together, these limited reports suggest that isoflurane or sevoflurane may promote cancer cell growth or invasion, whereas propofol has a beneficial effect by suppressing cancer cell proliferation, migration, or invasion.[27] However, to our knowledge, the mechanism by which desflurane anesthesia influences the recurrence or metastasis of ICC remains unknown.
There were some limitations in this study. First, it was a retrospective study and the 70 patients were not randomly allocated. However, we used all available patients from January 2005 to December 2014 from our medical center, and patient characteristics were similar between 2 anesthetic techniques. Moreover, we still conducted PS matching to minimize confounding in this observational study,[19] and further prospective multicenter study is warranted. Second, different VAs may have different effects on ICC. We analyzed only desflurane because it is the most frequently used VA in our hospital. Finally, in our hospital, we do not routinely use nonsteroidal anti-inflammatory drugs during ICC surgery because of the risk of life-threatening complications such as peptic ulceration.[33] However, nonsteroidal anti-inflammatory drugs may be linked to better survival in ICC populations,[34] although further investigation is necessary.
In conclusion, during open ICC surgery, propofol anesthesia improved survival compared with desflurane anesthesia. Patients under desflurane anesthesia had more postoperative metastasis, but not postoperative recurrence formation.
Acknowledgments
The authors thank the Cancer Registry Group of Tri-Service General Hospital for the clinical data support.
Author contributions
Conceptualization: Hou-Chuan Lai.
Data curation: Meei-Shyuan Lee, Kuen-Tze Lin, Shun-Ming Chan, Jen-Yin Chen, Yao-Tsung Lin.
Formal analysis: Meei-Shyuan Lee, Kuen-Tze Lin, Shun-Ming Chan, Jen-Yin Chen, Yao-Tsung Lin.
Investigation: Hou-Chuan Lai, Zhi-Fu Wu.
Methodology: Meei-Shyuan Lee, Zhi-Fu Wu.
Supervision: Zhi-Fu Wu.
Writing – original draft: Hou-Chuan Lai.
Writing – review and editing: Hou-Chuan Lai, Zhi-Fu Wu.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, CCI = Charlson comorbidity index, Ce = effect site concentration, CI = confidence interval, HBsAg = hepatitis B surface antigen, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, HIF = hypoxia-inducible factor, HR = hazard ratio, ICC = intrahepatic cholangiocarcinoma, MELD = model for end-stage liver disease, MET = metabolic equivalent, PS = propensity score, SD = standard deviation, TCI = target-controlled infusion, TNM = tumor node metastasis, TSGH = Tri-Service General Hospital, VA = volatile anesthetic.
How to cite this article: Lai HC, Lee MS, Lin KT, Chan SM, Chen JY, Lin YT, Wu ZF. Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in intrahepatic cholangiocarcinoma surgery. Medicine. 2019;98:51(e18472).
The authors have no funding and conflicts of interest to disclose.
IRB: The Ethics Committee of the Tri-Service General Hospital (Taipei, Taiwan, Republic of China; TSGHIRB No: 1-106-05-089) approved the study Institution: Institutional Review Board of Tri-Service General Hospital, National Defense Medical Center. No 325, Section 2, Chenggong Road, Neihu 114, Taipei, Taiwan, Republic of China. Phone: 886-2-87923311 Ext. 10552,17672,17673. Email: tsghirb@ndmctsgh.edu.tw
References
- [1].Chun YS, Javle M. Systemic and adjuvant therapies for intrahepatic cholangiocarcinoma. Cancer Control 2017;24:1073274817729241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lendoire JC, Gil L, Imventarza O. Intrahepatic cholangiocarcinoma surgery: the impact of lymphadenectomy. Chin Clin Oncol 2018;7:53. [DOI] [PubMed] [Google Scholar]
- [3].Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology 2016;124:69–79. [DOI] [PubMed] [Google Scholar]
- [4].Inada T, Kubo K, Kambara T, et al. Propofol inhibits cyclo-oxygenase activity in human monocytic THP-1 cells. Can J Anesth 2009;56:222–9. [DOI] [PubMed] [Google Scholar]
- [5].Inada T, Yamanouchi Y, Jomura S, et al. Effect of propofol and isoflurane anaesthesia on the immune response to surgery. Anaesthesia 2004;59:954–9. [DOI] [PubMed] [Google Scholar]
- [6].Kushida A, Inada T, Shingu K. Enhancement of antitumor immunity after propofol treatment in mice. Immunopharmacol Immunotoxicol 2007;29:477–86. [DOI] [PubMed] [Google Scholar]
- [7].Loop T, Dovi-Akue D, Frick M, et al. Volatile anesthetics induce caspasedependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology 2005;102:1147–57. [DOI] [PubMed] [Google Scholar]
- [8].Melamed R, Bar-Yosef S, Shakhar G, et al. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg 2003;97:1331–9. [DOI] [PubMed] [Google Scholar]
- [9].Shapiro J, Jersky J, Katzav S, et al. Anesthetic drugs accelerate the progression of postoperative metastases of mouse tumors. J Clin Invest 1981;68:678–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Moudgil GC, Singal DP. Halothane and isoflurane enhance melanoma tumour metastasis in mice. Can J Anaesth 1997;44:90–4. [DOI] [PubMed] [Google Scholar]
- [11].Lee JH, Kang SH, Kim Y, et al. Effects of propofol-based total intravenous anesthesia on recurrence and overall survival in patients after modified radical mastectomy: a retrospective study. Korean J Anesthesiol 2016;69:126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Enlund M, Berglund A, Andreasson K, et al. The choice of anaesthetic--sevoflurane or propofol--and outcome from cancer surgery: a retrospective analysis. Ups J Med Sci 2014;119:251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gilliland HE, Armstrong MA, Carabine U, et al. The choice of anesthetic maintenance technique influences the antiinflammatory cytokine response to abdominal surgery. Anesth Analg 1997;85:1394–8. [DOI] [PubMed] [Google Scholar]
- [14].Mammoto T, Mukai M, Mammoto A, et al. Intravenous anesthetic, propofol inhibits invasion of cancer cells. Cancer Lett 2002;184:165–70. [DOI] [PubMed] [Google Scholar]
- [15].Lai HC, Lee MS, Lin C, et al. Propofol-based total intravenous anaesthesia is associated with better survival than desflurane anaesthesia in hepatectomy for hepatocellular carcinoma. Br J Anaesth 2019;123:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wu ZF, Lee MS, Wong CS, et al. Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in colon cancer surgery. Anesthesiology 2018;129:932–41. [DOI] [PubMed] [Google Scholar]
- [17].Shen WF, Zhong W, Xu F, et al. Clinicopathological and prognostic analysis of 429 patients with intrahepatic cholangiocarcinoma. World J Gastroenterol 2009;15:5976–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Eagle KA, Berger PB, Calkins H, et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J Am Coll Cardiol 2002;39:542–53. [DOI] [PubMed] [Google Scholar]
- [19].Gaudino M, Di Franco A, Rahouma M, et al. Unmeasured confounders in observational studies comparing bilateral versus single internal thoracic artery for coronary artery bypass grafting: a meta-analysis. J Am Heart Assoc 2018;7:e008010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jun IJ, Jo JY, Kim JI, et al. Impact of anesthetic agents on overall and recurrence-free survival in patients undergoing esophageal cancer surgery: a retrospective observational study. Sci Rep 2017;7:14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sessler DI, Riedel B. Anesthesia and cancer recurrence: context for divergent study outcomes. Anesthesiology 2019;130:3–5. [DOI] [PubMed] [Google Scholar]
- [22].Choi SB, Kim KS, Choi JY, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol 2009;16:3048–56. [DOI] [PubMed] [Google Scholar]
- [23].Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth 2010;105:106–15. [DOI] [PubMed] [Google Scholar]
- [24].Yoo S, Lee HB, Han W, et al. Total intravenous anesthesia versus inhalation anesthesia for breast cancer surgery: a retrospective cohort study. Anesthesiology 2019;130:31–40. [DOI] [PubMed] [Google Scholar]
- [25].Yan T, Zhao JJ, Bi XY, et al. Effect of different anesthetic techniques on the prognosis of hepatocellular carcinoma after hepatectomy. Zhonghua Yi Xue Za Zhi 2017;97:1719–23. [DOI] [PubMed] [Google Scholar]
- [26].Huang YH, Lee MS, Lou YS, et al. Propofol-based total intravenous anesthesia did not improve survival compared to desflurane anesthesia in breast cancer surgery. PLoS One 2019;14:e0224728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang Z, Zang M, Wang S, et al. Effects of propofol on human cholangiocarcinoma and the associated mechanisms. Exp Ther Med 2019;17:472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Baba Y, Nosho K, Shima K, et al. HIF1A overexpression is associated with poor prognosis in a cohort of 731 colorectal cancers. Am J Pathol 2010;176:2292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Morine Y, Shimada M, Utsunomiya T, et al. Hypoxia inducible factor expression in intrahepatic cholangiocarcinoma. Hepatogastroenterology 2011;58:1439–44. [DOI] [PubMed] [Google Scholar]
- [30].Yang N, Liang Y, Yang P, et al. Propofol suppresses LPS-induced nuclear accumulation of HIF-1α and tumor aggressiveness in non-small cell lung cancer. Oncol Rep 2017;37:2611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Benzonana LL, Perry NJ, Watts HR, et al. Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology 2013;119:593–605. [DOI] [PubMed] [Google Scholar]
- [32].Shi QY, Zhang SJ, Liu L, et al. Sevoflurane promotes the expansion of glioma stem cells through activation of hypoxia-inducible factors in vitro. Br J Anaesth 2015;114:825–30. [DOI] [PubMed] [Google Scholar]
- [33].Armstrong CP, Blower AL. Non-steroidal anti-inflammatory drugs and life threatening complications of peptic ulceration. Gut 1987;28:527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yeh CN, Chiang KC, Juang HH, et al. Reappraisal of the therapeutic role of celecoxib in cholangiocarcinoma. PLoS One 2013;8:e69928. [DOI] [PMC free article] [PubMed] [Google Scholar]


