Abstract
The incidence of breast cancer among Japanese women is substantially increasing. This study evaluated the effects of reproductive and lifestyle factors with respect to breast cancer overall and separately among pre- and postmenopausal women using data from the Three-Prefecture Cohort Study of Japan.
A total of 33,410 women aged 40 to 79 years completed a self-administered questionnaire, which included items about menstrual and reproductive history and other lifestyle factors. The follow-up period was from 1984 to 1992 in Miyagi and 1985 to 2000 in Aichi Prefectures. We used Cox proportional hazards regression models to estimate hazards ratios (HRs) and 95% confidence intervals (CIs) after adjusting for confounding factors.
After 9.8 mean years of follow-up, 287 cases of breast cancer were recorded. In the overall analysis, later menarche (≥16 years) and parity were significantly associated with a decreased risk of breast cancer, with HRs of 0.69 (95% CI 0.48–0.99) and 0.72 (95% CI 0.52–0.99), respectively. Further, there was a significant decline in the risk of breast cancer with increasing number of birth among parous women (P for trend = .010). On the contrary, a family history of breast cancer in the mother was significantly associated with an increased risk of breast cancer (HR 3.22, 95% CI 1.52–6.84). Analyses based on menopausal status at baseline indicated that height (≥160 cm) and weight (≥65 kg) were significantly associated with an increased risk of postmenopausal breast cancer, with HRs of 1.34 (95% CI 0.72–2.50) and 3.13 (95% CI 1.75–5.60), respectively. Risk associated with BMI significantly differs by menopausal status.
Our findings suggest the important role of reproductive factors in the development of breast cancer in Japanese women; however, body mass index (BMI) may have different effects on breast cancer in Japanese women compared with western women.
Keywords: body mass index, breast cancer, cohort, epidemiology, reproductive factors
1. Introduction
Breast cancer is the most common cancer among women worldwide, accounting for 25% of all cancer cases among women.[1] Although Japanese women have a relatively low risk of breast cancer compared with women in Western countries,[2] the incidence rate has been increasing rapidly,[3] and breast cancer is currently the leading cancer among Japanese women. The incidence rate of breast cancer among Japanese women increases with increasing age but decreases or flattens after 50 years of age, whereas it increases irrespective of age among women in Western countries.[4]
A number of epidemiological studies from Western countries with high incidence rates of breast cancer have been reported. Early age at menarche, null parity, later age at menopause, late age at first birth, less experience of breastfeeding, and family history of breast cancer were important risk factors for the development of breast cancer.[5–9] A meta-analysis of 8 case–control studies conducted from 1948 to 1993 in Japan revealed that reproductive factors for breast cancer risk were similar to those in Western countries.[10] However, different effects of body mass index (BMI) on breast cancer have been observed between women in Japan and Western countries.[11] For Japanese women, higher BMI was associated with an increased risk of breast cancer in both pre- and postmenopausal women.[12] In contrast, higher BMI in Western countries was associated with an increased risk of breast cancer in postmenopausal women and a decreased risk in premenopausal women.[13]
Lifestyle and reproductive patterns have changed among women in Japan. Although several Japanese cohort studies examined the association between known and suspected risk factors and breast cancer incidence,[14–16] a large-scale, population-based prospective survey (The Three-Prefecture Cohort Study)[17] enabled us to provide further evidence for the association between several risk factors and the risk of breast cancer among Japanese women. Using this dataset, we evaluated the effects of reproductive and lifestyle factors related to breast cancer to investigate differences in these associations between women in Japan and Western countries.
2. Methods
2.1. Study cohort
The present study was based on the Three-Prefecture Cohort Study, and its study design was previously reported in detail.[17] Briefly, a self-administered questionnaire regarding demographic factors and lifestyle characteristics was administered to participants living in Miyagi, Aichi, and Osaka Prefectures. This study did not include data from residents in Osaka Prefecture because information on reproductive factors was lacking. Of 35,136 women, 9 were excluded after the beginning date of follow-up was unified. In addition, 1411 elderly women aged ≥80 years and 306 women with cancer history were excluded. Finally, 33,410 women aged ≥40 years and without histories of cancer were eligible for our analyses (Fig. 1).
Figure 1.
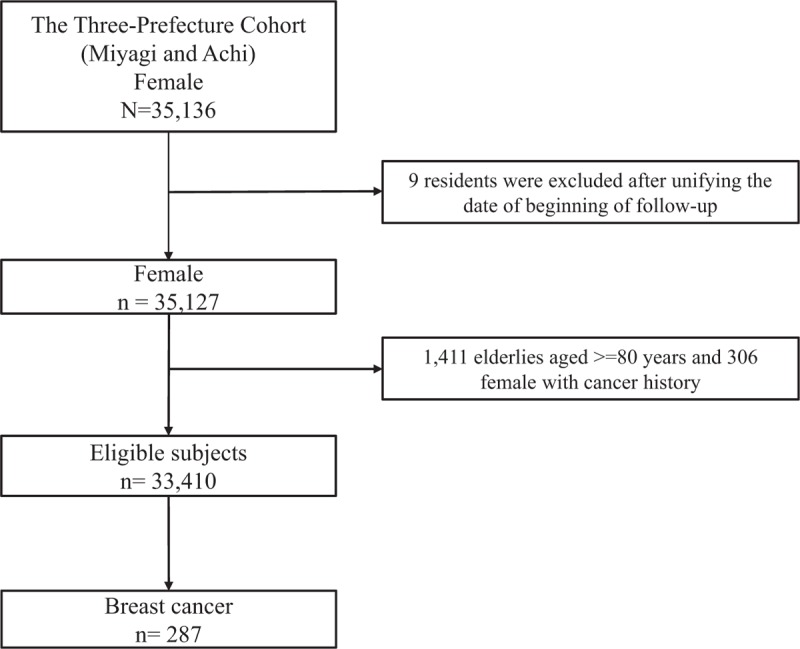
Participants from the Three-Prefecture Cohort study who were included in the current analysis.
2.2. Baseline questionnaire survey
The Three-Prefecture Cohort Study conducted baseline questionnaire surveys from February 1, 1983 to November 1, 1985.[17] A self-administered questionnaire in a sealed envelope was distributed by hand to participants. The questionnaire covered personal information as follows: area of residence, sex, height, weight, frequency of food intake, smoking, alcohol drinking status, family history of breast cancer in mother, age at menarche, menopausal status at baseline, age at menopause, parity history, parity number, and age at first birth. The agreement or permission of baseline survey for municipality residents was obtained from the municipal government with collaborators. The response to the questionnaire by participant was thought to be the agreement to the survey. The study was approved by the institutional review board of the National Cancer Center and the Ethics Committee of Osaka University School of Medicine.
2.3. Follow-up and identification of cancer cases
The study participants were followed from the start of the study, and the follow-up periods of cancer incidence were 9 years for Miyagi Prefecture (1984–1992) and 15 years for Aichi Prefecture (1985–2000). Vital status and dates of death and relocation were confirmed by the local government using residence certificates. During the study period, 7427 (22.2%) women moved out of the study area and 3201 (9.6%) died. Cancer incidence data were collected only for participants living in the study area. Cancer incidence and the date of diagnosis were obtained from local population-based cancer registries. The endpoint of this analysis was the incidence of breast cancer, defined as the International Classification of Disease 9th version (ICD9) codes 174 to 175.9 and 10th version (ICD10), codes C50 to C50.9. Up to the end of the cancer incidence follow-up period, 287 new breast cancer cases were identified in this population (Fig. 1).
2.4. Statistical analysis
The person-years of follow-up were calculated from the baseline survey for each participants until the date of diagnosis of breast cancer, or the date of emigration from the study area, or the date of death, or the end of follow-up (Miyagi, December 31, 1992; Aichi, December 31, 2000). The exposure variables analyzed in the present study were age at menarche, menopausal status, age at menopause, parity history, parity number, age at first birth, and family history of breast cancer in the mother. The crude incidence rate per 100,000 for breast cancer was calculated by dividing the number of breast cancer cases by the number of person-years. The Cox proportional hazards model was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of breast cancer incidence by the exposure variables using STATA version 13 MP (Stata Corp., College Station, TX).
The following variables were used for adjustment as potential confounders: age, area, region (urban/rural), BMI, alcohol consumption, smoking status, green-yellow vegetables consumption, other vegetables consumption, packed vegetables consumption, fruit consumption, miso soup consumption. Different sets of exposure variables were used for adjustment in respective estimated models (see footnotes in Tables 1–3). In our analyses, missing values in confounders were treated as an additional category and included in the models. Linear trends were assessed using the Cox proportional hazards models by treating each exposure category as a continuous variable. In the subgroup analyses, we excluded women without menopausal information at baseline. According to menopausal status (pre or post), we assessed breast cancer risk by reproductive factors and other variables, including height (<148, 148–151, 152–155, 156–159, or ≥160 cm), weight (<50, 50–54, 55–59, 60–64, or ≥65 kg), BMI (<18.5, 18.5–24.9, 25.0–26.9, or ≥27.0 kg/m2), smoking category (never, former, or current), and drinking category (never, former, sometimes, or almost daily). In addition, chi-square tests were used to determine differences the distributions of categorical variables of the baseline characteristics. All P values reported are 2-sided, and the significance level was set at P < .05.
Table 1.
Demographic and lifestyle characteristics of participants at baseline.
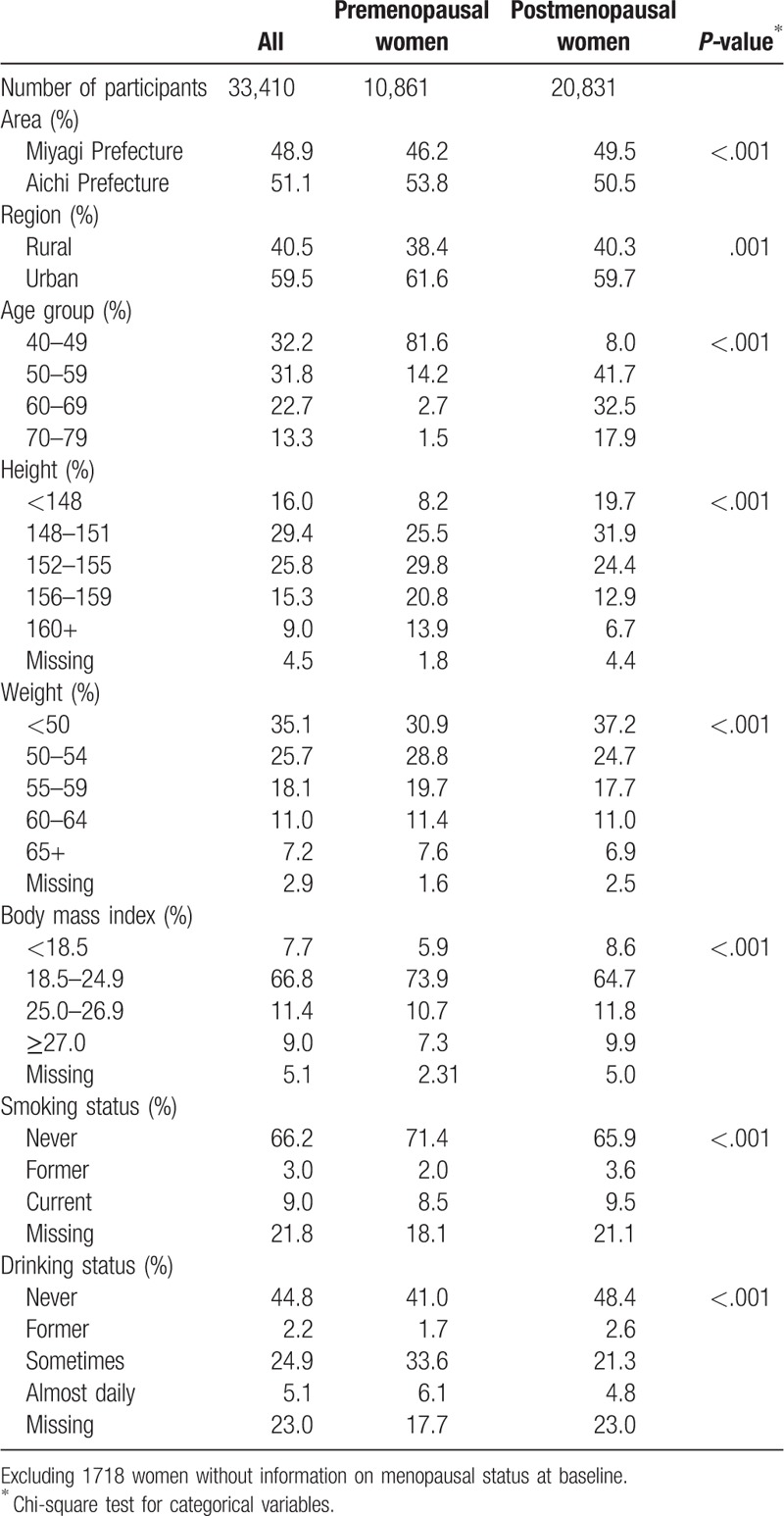
Table 3.
HR of breast cancer incidence according to reproductive factors.
3. Results
The demographic and lifestyle characteristics of the study participants at baseline with respect to menopausal status are presented in Table 1. Among the 33,410 participants, 10,861 (32.5%) were premenopausal and 20,831 (62.4%) were postmenopausal; the menopausal status was undefined in 1718 participants (5.1%). Compared with postmenopausal women, premenopausal women tended to be taller and thinner. Most of women were never smokers, and approximately half of women had never consumed alcohol.
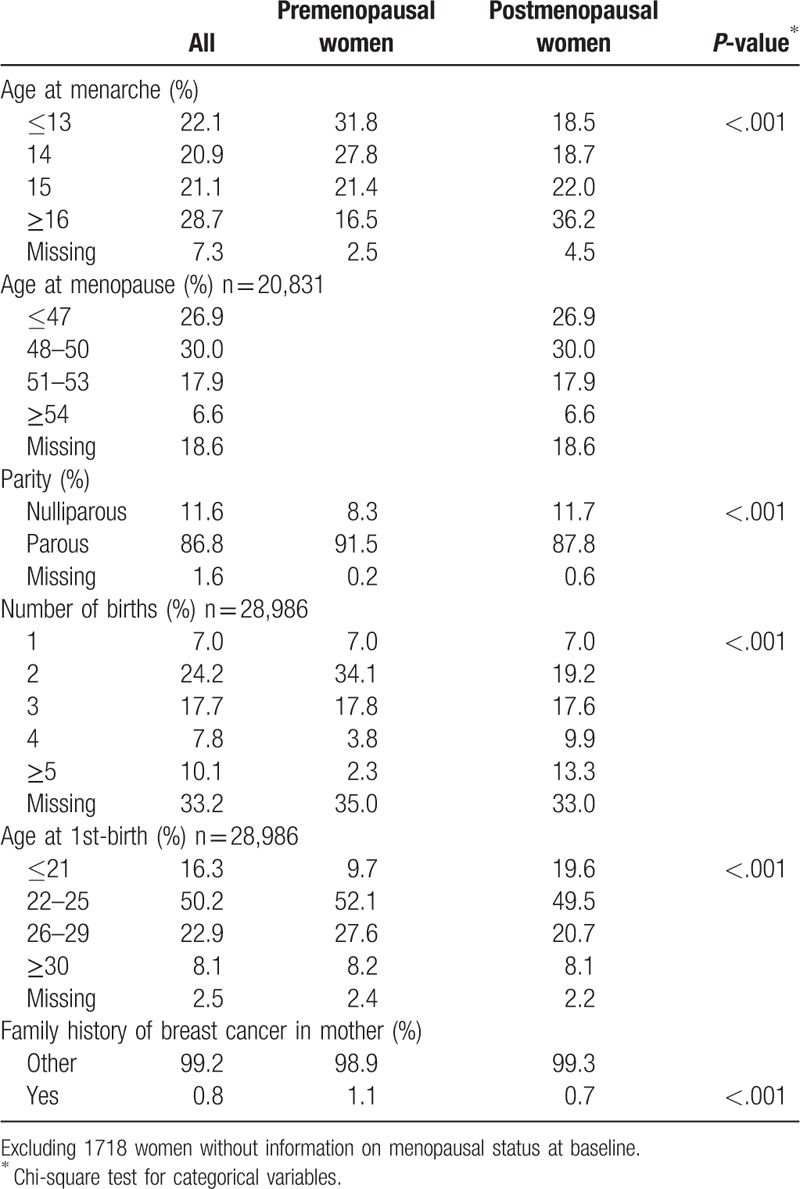
Table 2 shows the reproductive characteristics of participants at baseline by menopausal status. The proportions of those aged ≤13 years at menarche was 31.8% in premenopausal women and 18.5% in postmenopausal women. The proportion of postmenopausal women with no parous experience (11.7%) was higher than that among premenopausal women (8.3%). Among parous women, only 7.0% had 1 child no matter the menopause status. In addition, the proportion of women who had a first birth younger than 21 years was higher among postmenopausal women (19.6%) than that among premenopausal women (9.7%). Only 0.8% of women overall had a family history of breast cancer.
Table 2.
Reproductive characteristics of participants at baseline.
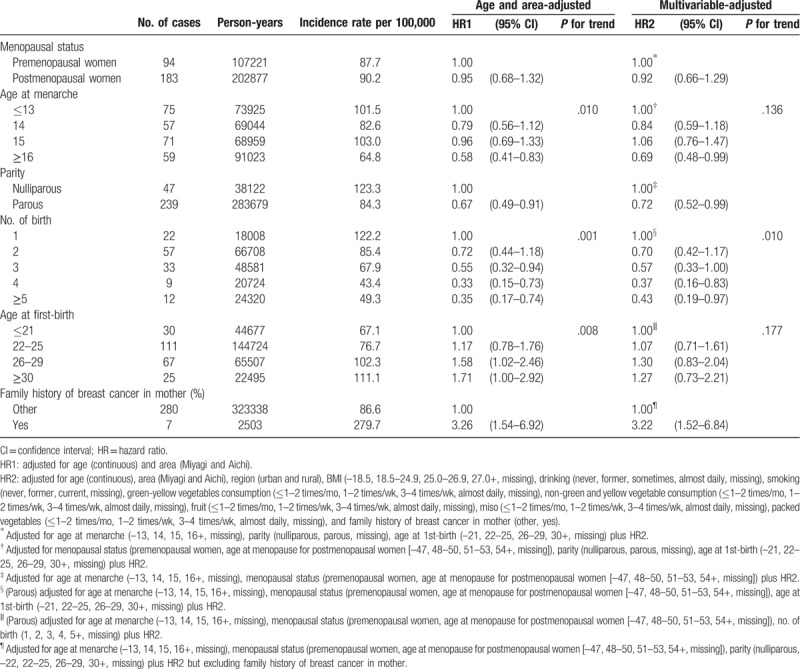
Of 325,840 person-years for 33,410 study participants (average follow-up period: 9.8 years), 94, 183, and 10 cases of breast cancer were recorded among premenopausal, postmenopausal, and undefined women at baseline, respectively. The HRs and 95% CIs of breast cancer according to menstrual and reproductive factors among the women overall are shown in Table 3. Compared with women with earlier menarche (≤13 years), women with later age at menarche (≥16 years) had a significant risk reduction of breast cancer incidence (HR 0.69; 95% CI 0.48–0.99). Relative to nulliparous women, the multi-adjusted HR for parous women was 0.72 (95% CI 0.52–0.99). Among parous women, the risk decreased significantly with an increasing number of births (P for trend = .010), even after adjusting for age at first birth. The risk of breast cancer incidence was significantly reduced for women with ≥5 births relative to those with 1 birth (HR 0.43; 95% CI 0.19–0.97). Women whose mother had a history of breast cancer had a significantly increased risk of breast cancer (HR 3.22; 95% CI 1.52–6.84).
After excluding 1718 women whose information of menopausal status were missing at baseline, the HRs of breast cancer by menopausal status were showed in Table 4. Compared with an age of 13 at menarche, the risk of breast cancer incidence decreased marginally for those ≥16 years in postmenopausal women (HR 0.66; 95% CI 0.43–1.02). Women with a later age at menopause (≥54 years) had a higher risk of breast cancer (HR 1.72; 95% CI 0.98–3.02) compared with those with age at menopause ≤47 years. Regardless of the menopausal status, the risk of breast cancer incidence was significantly reduced for parous women (HR 0.54; 95% CI 0.30–0.99 among premenopausal women and HR 0.64; 95% CI 0.43–0.94 among postmenopausal women). Although the risk of breast cancer incidence among parous women decreased with increasing number of births in both pre- and postmenopausal women, only the risk for premenopausal women was statistically significant (P for trend = .010). Women whose mother had breast cancer had increased risks for the disease, although a significant association was observed only among postmenopausal women (HR: 4.49; 95% CI 1.83–11.00).
Table 4.
HR of breast cancer incidence according to reproductive factors by menopausal status.
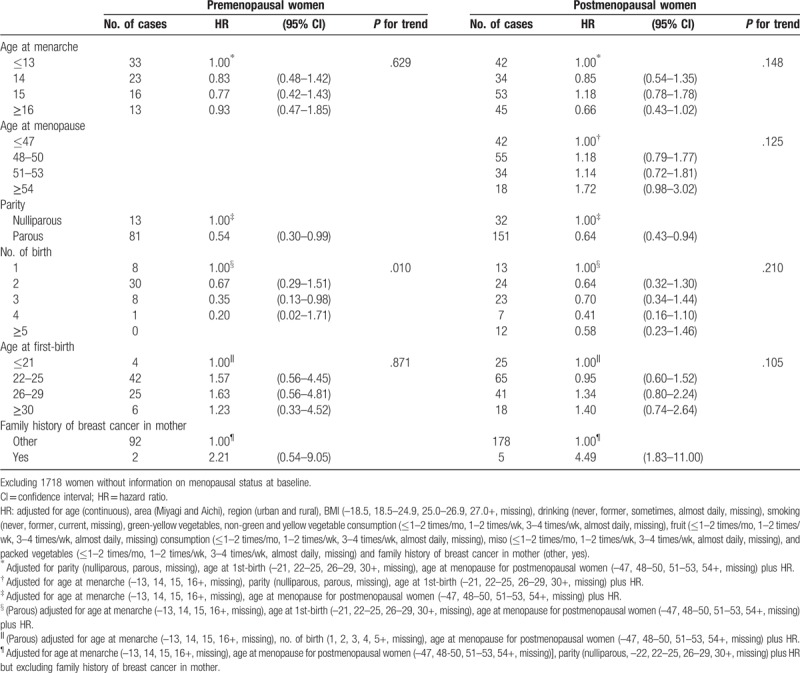
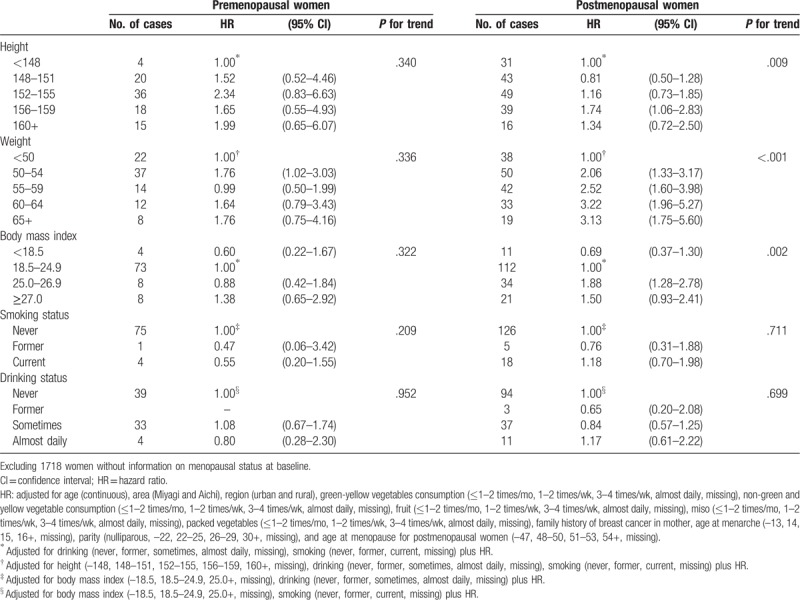
Table 5 shows the HRs of breast cancer incidence by height, weight, BMI, smoking status, and drinking category according to menopausal status. Overall, a positive association between height and breast cancer risk was observed for both pre- and postmenopausal women (P for trend = .340 and .009, respectively). Weight was also associated with an increased risk of breast cancer in both pre- and postmenopausal women, but the association was more evident in postmenopausal women (P for trend < .001). Among postmenopausal women, a high BMI was associated with high HRs of breast cancer incidence compared with that in women with a low BMI (P for trend = .002), while it was not associated in premenopausal women with breast cancer. In contrast, smoking and drinking category were not associated with the risk of breast cancer incidence irrespective of the menopausal status.
Table 5.
HR of breast cancer incidence by height, weight, body mass index, smoking, and drinking category according to the menopausal status.
4. Discussion
This population-based prospective cohort study from 3 prefectures in Japan demonstrated that several menstrual and reproductive factors; family history of breast cancer in the mother; height; weight; and high BMI were associated with an increased risk of breast cancer.
Estrogen plays an important role in the development of breast cancer.[18] Women who start menstruating early in life have an increased risk of developing breast cancer.[19] Early menarche was associated with the early onset and increased frequency of ovulatory circles. Women with an early age of menarche are earlier exposed to increased ovarian hormone levels and have higher estrogen levels for several years longer. Several years of exposure to high-level estrogen stimulus increased the risk of breast cancer.[20,21] In other words, an older age of menarche is associated with a lower risk of breast cancer. For every year delay in menarche, the risk decreases by around 5%.[22] In the present study, a risk decrement was observed among women with later age at menarche (women aged ≥16 years, HR, 0.69; 95% CI 0.48–0.99) and the risk decreased nearly 6% for each 1-year delay in menarche (data not shown).
Pregnancy promotes the differentiation of mammary gland epithelium, and the differentiated cells are not susceptible to undergo neoplastic transformation. Therefore, when the early first full pregnancy occurs early, the early differentiation of mammary gland cells are induced.[23] In addition, the first full pregnancy changes the long-term hormonal levels including decreased prolactin, higher sex hormone-binding globulin, and lower estrogen, which may be associated with the decreased of breast cancer.[16] Compared with nulliparous women, women who have had at least one full-term pregnancy have an approximately 25% reduction in breast cancer risk.[6,7] In addition, a younger age at first birth was associated with greater protection against breast cancer.[22,24] A previous study showed that women with first delivery after 35 years of age had a risk about 40% higher than that in those with a first birth before 20 years.[7] In addition, women with multi-parity had a lower risk of breast cancer than that in women with low parity. The risk of breast cancer significantly declined with increasing number of birth even after controlling for the influence of various risk factors related to breast cancer. Our result was similar to one from other previous studies[5–10,14,16] and would reinforce the importance of the association between high parity and the decreased risk of breast cancer. In addition, this phenomenon would be partially explained by the long-lasting protective effect which has been enhanced by every new full-term pregnancy after the first. The diminution in breast cancer risk with increasing parity may be related to changes in plasma prolactin levels, and high parity was associated with low prolactin concentration both in pre- and postmenopausal women.[22] Our results were similar to those of other studies in which parous women had a 28% reduction in breast cancer risk relative to nulliparous women. Women with >5 children had exceedingly lower risks than those of women with only 1 child. Furthermore, women with a later age at first birth tended to have a higher risk of breast cancer, although the results were not statistically significant.
The results of this study underscored that women with a family history of breast cancer were at an increased risk of the disease, which could be explained partially by shared genes. Mutations in BRCA1, BRCA2, P53, PTEN, and ATM are associated with breast cancer risk, especially those in BRCA1 and BRCA2.[22]BRCA2 plays a more important role than BRCA1 in Japanese familial breast cancers.[25,26] The pooled estimate of relative risk (RR) of women with a single affected first-degree relative (mother, sister, or daughter) was 2.1 (95% CI: 2.0–2.2).[27] In the present study, the HR of women with a family history of breast cancer in mother was about 3 times, which was similar to that in previous study in Japan (HR: 2.79, 95% CI 1.59–4.87).[15]
Obesity increased the risk of breast cancer in postmenopausal women.[22,27] After menopause, instead of ovarian, aromatization of adrenal androgens to estrogens in adipose tissue become the main estrogen source.[28,29] As BMI increases, estradiol increases and sex hormone-binding globulin concentration deceases in postmenopausal women. Therefore, obese postmenopausal women have higher levels of bioavailable estrogens, resulting in increased risks of breast cancer.[30] A meta-analysis showed that a 5 kg/m2 increase in BMI was positively associated with postmenopausal breast cancer (RR: 1.12, 95% CI 1.08–1.16).[13] In this study, HR per 5 kg/m2 increased of BMI: 1.07 (95% CI: 1.03–1.10) in postmenopausal women (data not shown in Table).
Circulating insulin-like growth factor (IGF)-1 concentration was also positively related to the risk of premenopausal breast cancer. IGF-1 concentration is low among women with low BMI and increases with increasing BMI but decreases again with obesity.[31] In addition, obese premenopausal women tend to have an increased frequency of anovulatory menstrual cycles and lower estrogen level.[30] These might be associated with an inverse association between increased BMI and premenopausal breast cancer. A significant inverse association was observed among women with BMI ≥31 kg/m2.[11] However, a previous study suggested an inverse association between increased BMI and premenopausal breast cancer in North American, European, and Australian but a positive association in Asian women.[13] Compared with Western women, Japanese women have a low prevalence of obesity (BMI ≥30 kg/m2), at ∼3%,[32] compared with ∼33% in the United States,[33] and ∼19% in the European Union.[34] This may be one reason for the lack of inverse association in Japan.
The mechanisms underlying the association between height and breast cancer risk are not completely understood. Height is primarily determined by genetic factors. However, early energy and nutrition restriction, resulting in short height, may inhibit cell proliferation and early events in tumorigenesis.[11] Women in this study born between 1905 and 1945 might have been experienced nutritional inadequacy in childhood and adolescence during World War II, resulting in sufficient variation in energy intake, which has been proposed as an explanation for the positive association between height and breast cancer risk.[35] Pooled analysis of Western countries[11] showed a significant positive association between height and the risk of postmenopausal breast cancer, while the association was not significant for premenopausal women. One study from Japan also indicated that the positive association was more evident in postmenopausal women.[35]
This study had several limitations. First, the follow-up period of the cohort study was 1984 to 1992 in Miyagi Prefecture and 1985 to 2000 in Achi Prefecture, and the reproductive and life style patterns of this study might differ from the present situation in 2019. Further, we did not have information on menopausal status after the start of follow-up. In addition, the information on BMI and other confounders were also based on the baseline questionnaire; thus, we were unable to consider changes over time of those confounders. Second, this study obtained information about family history of breast cancer, however, since our questionnaire could not differentiate between “no,” “unknown,” or “missing” responses, we treated these as 1 item. Therefore, this study likely underestimated the effect of a family history of breast cancer. Third, reproductive information such as breastfeeding, and the information on exogenous female hormone use were lacking. In addition, we could not evaluate the association of menstrual and reproductive factors with hormone receptor-defined breast cancer.[17]
In conclusion, in this large-scale prospective cohort study among Japanese women, we showed that multi-parity was associated with a decreased risk of breast cancer. Early age at menarche (≤13 years) and family history of breast cancer in the mother were related to an increased risk of breast cancer. Height, weight, and BMI ≥25.0 kg/m2 were also associated with an increased risk of breast cancer in postmenopausal women.
Acknowledgments
The authors thank the staff within each study area for their time and efforts in the collection and processing of data. We also express our gratitude to all study participants. This study was supported by a Grant-in-Aid for Scientific Research (25460752) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author contributions
Data curation: Rong Liu.
Formal analysis: Rong Liu.
Methodology: Rong Liu, Tetsuhisa Kitamura, Tomotaka Sobue, Junya Sado.
Project administration: Yuri Kitamura, Tomotaka Sobue, Junya Sado, Yumi Sugawara, Keitaro Matsuo, Tomio Nakayama, Ichiro Tsuji, Hidemi Ito, Takaichiro Suzuki, Kota Katanoda, Suketami Tominaga.
Software: Rong Liu, Junya Sado.
Supervision: Yuri Kitamura, Tetsuhisa Kitamura, Tomotaka Sobue, Yumi Sugawara, Keitaro Matsuo, Tomio Nakayama, Ichiro Tsuji, Hidemi Ito, Takaichiro Suzuki, Kota Katanoda, Suketami Tominaga.
Writing – original draft: Rong Liu.
Writing – review & editing: Rong Liu, Yuri Kitamura, Tetsuhisa Kitamura, Tomotaka Sobue, Junya Sado.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, HR = hazard ratio, ICD = International Classification of Disease, IGF-1 = insulin-like growth factor, RR = relative risk.
How to cite this article: Liu R, Kitamura Y, Kitamura T, Sobue T, Sado J, Sugawara Y, Matsuo K, Nakayama T, Tsuji I, Ito H, Suzuki T, Katanoda K, Tominaga S. Reproductive and lifestyle factors related to breast cancer among Japanese women: An observational cohort study. Medicine. 2019;98:51(e18315).
Source of Funding: None.
The authors have no conflicts of interest to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Parkin DM, Muir CS. Cancer Incidence in Five Continents. Comparability and quality of data. IARC Sci Publ 1992;45–173. [PubMed] [Google Scholar]
- [3].Katanoda K, Hori M, Matsuda T, et al. An updated report on the trends in cancer incidence and mortality in Japan, 1958-2013. Jpn J Clin Oncol 2015;45:390–401. [DOI] [PubMed] [Google Scholar]
- [4].Matsuno RK, Anderson WF, Yamamoto S, et al. Early- and late-onset breast cancer types among women in the United States and Japan. Cancer Epidemiol Biomarkers Prev 2007;16:1437–42. [DOI] [PubMed] [Google Scholar]
- [5].Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev 1993;15:36–47. [DOI] [PubMed] [Google Scholar]
- [6].Layde PM, Webster LA, Baughman AL, et al. The independent associations of parity, age at first full term pregnancy, and duration of breastfeeding with the risk of breast cancer. Cancer and Steroid Hormone Study Group. J Clin Epidemiol 1989;42:963–73. [DOI] [PubMed] [Google Scholar]
- [7].Ewertz M, Duffy SW, Adami HO, et al. Age at first birth, parity and risk of breast cancer: a meta-analysis of 8 studies from the Nordic countries. Int J Cancer 1990;46:597–603. [DOI] [PubMed] [Google Scholar]
- [8].Albrektsen G, Heuch I, Tretli S, et al. Breast cancer incidence before age 55 in relation to parity and age at first and last births: a prospective study of one million Norwegian women. Epidemiology 1994;5:604–11. [DOI] [PubMed] [Google Scholar]
- [9].Clavel-Chapelon F. Differential effects of reproductive factors on the risk of pre- and postmenopausal breast cancer. Results from a large cohort of French women. Br J Cancer 2002;86:723–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nagata C, Hu YH, Shimizu H. Effects of menstrual and reproductive factors on the risk of breast cancer: meta-analysis of the case-control studies in Japan. Jpn J Cancer Res 1995;86:910–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol 2000;152:514–27. [DOI] [PubMed] [Google Scholar]
- [12].Wada K, Nagata C, Tamakoshi A, et al. Body mass index and breast cancer risk in Japan: a pooled analysis of eight population-based cohort studies. Ann Oncol 2014;25:519–24. [DOI] [PubMed] [Google Scholar]
- [13].Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–78. [DOI] [PubMed] [Google Scholar]
- [14].Iwasaki M, Otani T, Inoue M, et al. Role and impact of menstrual and reproductive factors on breast cancer risk in Japan. Eur J Cancer Prev 2007;16:116–23. [DOI] [PubMed] [Google Scholar]
- [15].Kawai M, Minami Y, Kuriyama S, et al. Reproductive factors, exogenous female hormone use and breast cancer risk in Japanese: the Miyagi Cohort Study. Cancer Causes Control 2010;21:135–45. [DOI] [PubMed] [Google Scholar]
- [16].Tamakoshi K, Yatsuya H, Wakai K, et al. Impact of menstrual and reproductive factors on breast cancer risk in Japan: results of the JACC study. Cancer Sci 2005;96:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sado J, Kitamura T, Kitamura Y, et al. Rationale, design, and profile of the three-prefecture Cohort in Japan: a 15-year follow-up. J Epidemiol 2017;27:193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med 2001;344:276–85. [DOI] [PubMed] [Google Scholar]
- [19].McPherson K, Steel CM, Dixon JM. ABC of breast diseases. Breast cancer-epidemiology, risk factors, and genetics. BMJ 2000;321:624–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Apter D, Vihko R. Early menarche, a risk factor for breast cancer, indicates early onset of ovulatory cycles. J Clin Endocrinol Metab 1983;57:82–6. [DOI] [PubMed] [Google Scholar]
- [21].Vihko R, Apter D. Endocrine characteristics of adolescent menstrual cycles: impact of early menarche. J Steroid Biochem 1984;20:231–6. [DOI] [PubMed] [Google Scholar]
- [22].Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol 2001;2:133–40. [DOI] [PubMed] [Google Scholar]
- [23].Russo J, Moral R, Balogh GA, et al. The protective role of pregnancy in breast cancer. Breast Cancer Res 2005;7:131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lee SH, Akuete K, Fulton J, et al. An increased risk of breast cancer after delayed first parity. Am J Surg 2003;186:409–12. [DOI] [PubMed] [Google Scholar]
- [25].Fukutomi T, Ushijima T, Inoue R, et al. BRCA1 and BRCA2 germline mutations in Japanese with hereditary breast cancer families. Breast Cancer 1997;4:256–8. [DOI] [PubMed] [Google Scholar]
- [26].Inoue R, Ushijima T, Fukutomi T, et al. BRCA2 germline mutations in Japanese breast cancer families. Int J Cancer 1997;74:199–204. [DOI] [PubMed] [Google Scholar]
- [27].Pharoah PD, Day NE, Duffy S, et al. Family history and the risk of breast cancer: a systematic review and meta-analysis. Int J Cancer 1997;71:800–9. [DOI] [PubMed] [Google Scholar]
- [28].Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol 2002;3:565–74. [DOI] [PubMed] [Google Scholar]
- [29].Bernstein L, Ross RK. Endogenous hormones and breast cancer risk. Epidemiol Rev 1993;15:48–65. [DOI] [PubMed] [Google Scholar]
- [30].Potischman N, Swanson CA, Siiteri P, et al. Reversal of relation between body mass and endogenous estrogen concentrations with menopausal status. J Natl Cancer Inst 1996;88:756–8. [DOI] [PubMed] [Google Scholar]
- [31].Renehan AG, Zwahlen M, Minder C, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet 2004;363:1346–53. [DOI] [PubMed] [Google Scholar]
- [32].Yoshiike N, Seino F, Tajima S, et al. Twenty-year changes in the prevalence of overweight in Japanese adults: the National Nutrition Survey 1976-95. Obes Rev 2002;3:183–90. [DOI] [PubMed] [Google Scholar]
- [33].Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999-2000. JAMA 2002;288:1723–7. [DOI] [PubMed] [Google Scholar]
- [34].Bergstrom A, Pisani P, Tenet V, et al. Overweight as an avoidable cause of cancer in Europe. Int J Cancer 2001;91:421–30. [DOI] [PubMed] [Google Scholar]
- [35].Iwasaki M, Otani T, Inoue M, et al. Body size and risk for breast cancer in relation to estrogen and progesterone receptor status in Japan. Ann Epidemiol 2007;17:304–12. [DOI] [PubMed] [Google Scholar]


