Abstract
Pancreatic cancer is one of the most malignant tumors worldwide. DNA replication plays a critical role in the occurrence and development of pancreatic cancer. TYMS encodes thymidylate synthase, which is important for DNA synthesis. The TYMS gene has been assessed in some tumors. However, the specific role of TYMS in pancreatic cancer has not been identified. This study was designed to clarify the diagnostic and prognostic significance of TYMS in pancreatic cancer.
The Cancer Genome Atlas (TCGA) database was used to compare TYMS expression in pancreatic cancer, and ROC curve analysis was used to investigate its diagnostic value. The correlation between clinical characteristics and TYMS expression was analyzed, and the prognostic value of TYMS expression in the patients with pancreatic cancer was assessed by Kaplan–Meier curves and Cox analysis.
TYMS was upregulated in pancreatic cancer and associated with poor overall survival (OS) and recurrence-free survival (RFS). Univariate and multivariate survival analysis demonstrated that TYMS is an independent risk factor for OS and RFS in patients with pancreatic cancer.
The upregulation of TYMS in pancreatic cancer leads to unfavorable OS and RFS in patients, and represents a diagnostic and prognostic biomarker for patients with pancreatic cancer.
Keywords: diagnosis, pancreatic cancer, prognosis, TYMS
1. Introduction
Pancreatic cancer is one of the most lethal malignant tumors worldwide, with an estimated five-year survival rate of only about 5%.[1] This means that early and accurate diagnosis of pancreatic cancer may help improve the survival of patients and is urgently required. To date, the diagnosis of pancreatic cancer relies on imaging methods, which are insufficient in some situations.[2] Therefore, identifying a diagnostic and prognostic biomarker has great clinical significance for patients with pancreatic cancer.
Cancer is regarded as a disease involving genome changes.[3] DNA replication, which affects genomic stability, plays an important role in the initiation and progression of tumors. TYMS, located on chromosome 18p, encodes thymidylate synthase (TS), an enzyme involved in the replication and repair of DNA.[4] TS plays an important role in DNA replication by catalyzing the methylation of dUMP to produce dTMP.[5] Some studies have described TYMS expression, suggesting that it plays a role in cancer.[4,6] However, the role of TYMS expression in the diagnostic and prognostic evaluation of patients with pancreatic cancer remains unclear.
In this study, we compared TYMS expression in patients with pancreatic cancer and healthy people, and evaluated the diagnostic value of TYMS in pancreatic cancer using ROC. We also investigated the correlation between clinical characteristics and TYMS expression, and discussed the prognostic role of TYMS in overall survival (OS) and recurrence-free survival (RFS) in patients. Finally, we explored whether TYMS could be a biomarker for the diagnostic and prognostic evaluation of patients with pancreatic cancer.
2. Methods
2.1. Data mining and collection
Data generated from patients with pancreatic cancer and RNA-seq results were downloaded using the RTCGAToolbox package in R (version 3.5.1) from The Cancer Genome Atlas (TCGA) database.[7,8] As a public database, ethics approval and patient consent were not applicable. The number of patients included in our analysis was 173, and the number of normal individuals was 4.
2.2. Statistical analysis
Box-plots were used to compare TYMS expression between different groups. ROC was applied to test the diagnostic value of TYMS, and patients were divided into 2 groups (high TYMS expression and low TYMS expression) based on thresholds set using pROC packages.[9] Chi-square and Fisher exact tests were used to examine the correlation between TYMS expression and clinical characteristics in patients. Kaplan–Meier curves were used to investigate OS and RFS, grouped by TYMS expression, using the Survival package in R.[10,11] Univariate Cox analysis was used to select the variables related to OS and RFS. Finally, Multivariate Cox analysis was used to check the effects of TYMS expression on the survival of patients, and other pathological features. P < .05 was considered statistically significant.
3. Results
3.1. Patients’ characteristics
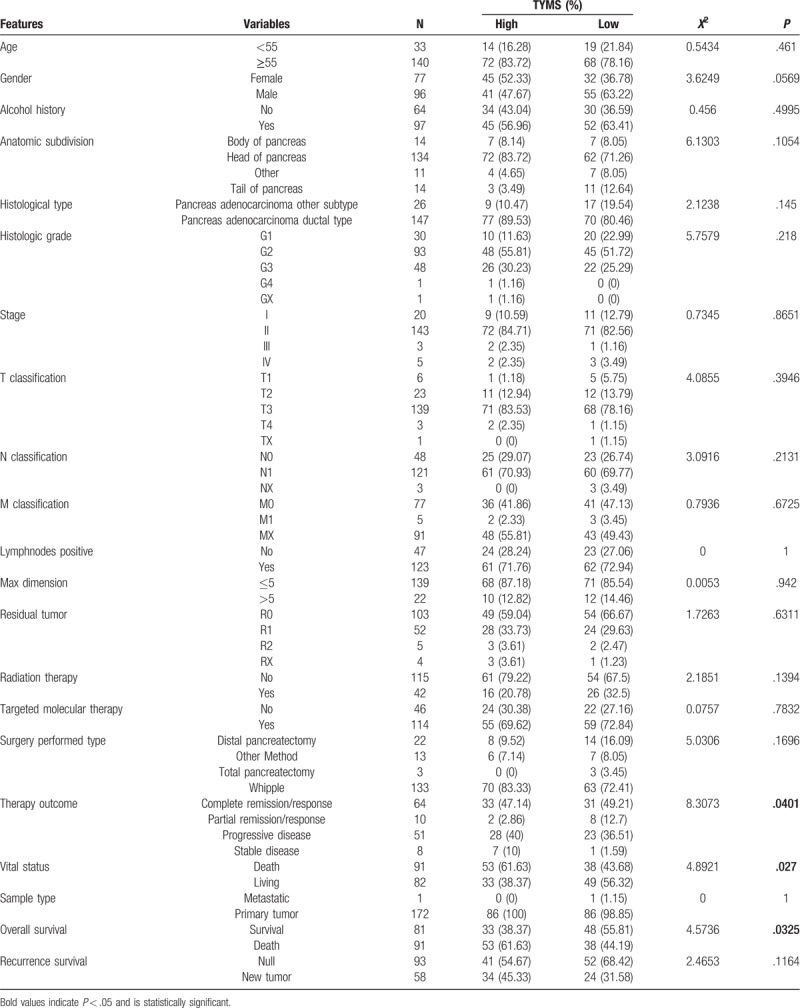
A total of 173 patients and 4 normal individuals were included in this analysis, and their clinical data were mined and downloaded from the TCGA database. Included in this study were 77 (44.51%) females and 96 (55.49%) males. There were 140 (80.92%) patients older than 55 years, and 33 (19.08%) patients younger than 55 years. Detailed patient characteristics are presented in Table 1 .
Table 1.
Clinical characteristics of the patients with pancreatic cancer.
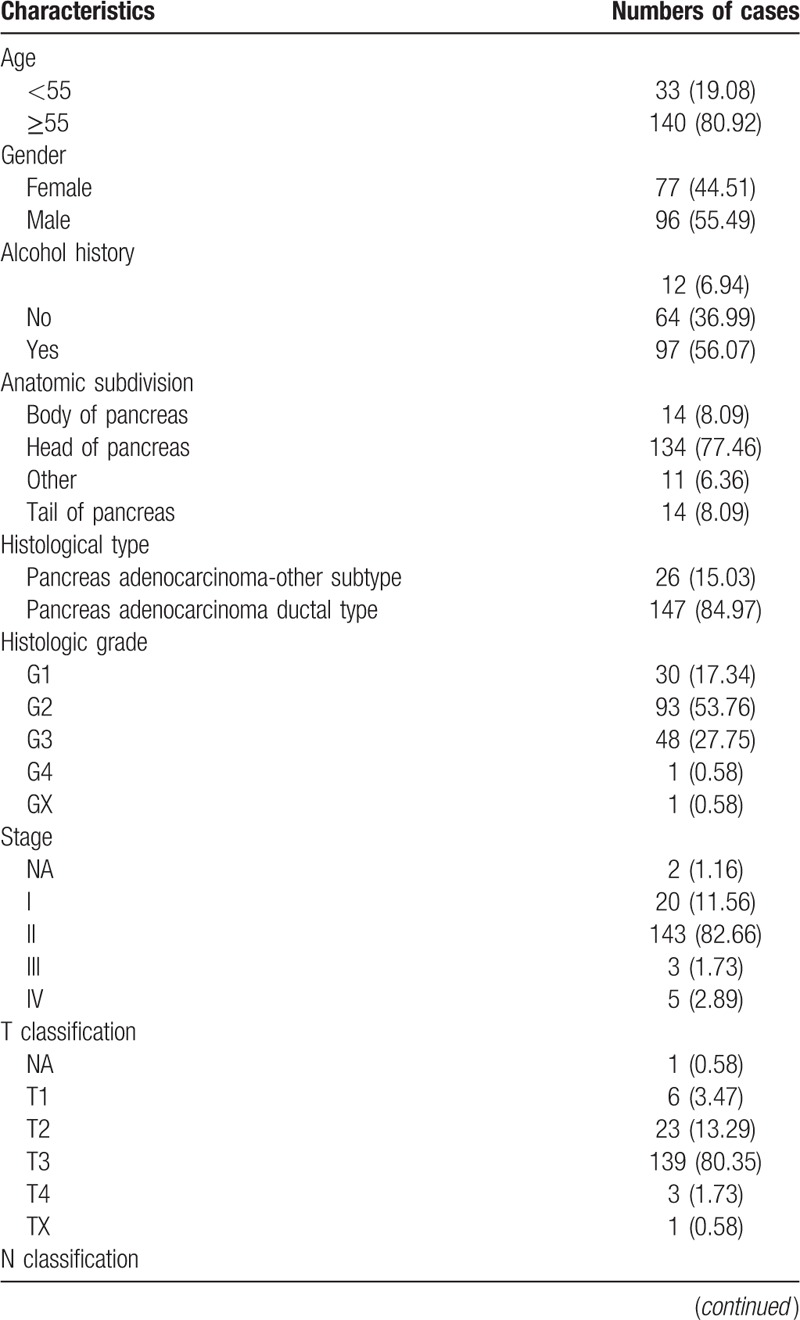
3.2. TYMS is upregulated in pancreatic cancer
TYMS expression was compared between pancreatic cancer samples and normal tissues (Fig. 1). Our results show that TYMS is highly expressed in pancreatic cancer. Moreover, TYMS expression significantly differed in vital status (P = .0038) subgroups.
Figure 1.
TYMS was upregulated in pancreatic cancer. Expression of TYMS in normal tissues and pancreatic cancer samples with different stages, T classification, N classification, M classification, histologic grade, residual tumor, and vital status.
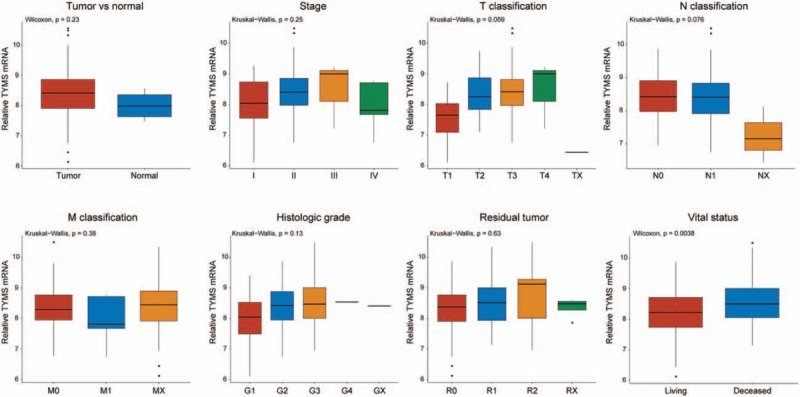
3.3. Diagnostic value of TYMS
We performed ROC analysis of data from patients and healthy people to measure the diagnostic value of TYMS. The AUC was 0.675, representing a moderate diagnostic value for pancreatic cancer (Fig. 2). Subgroup analysis further demonstrated the diagnostic value of PES1 expression in distinct stage, with AUC of 0.562 for stage I, 0.691 for stage II, 0.667 for stage III and 0.450 for stage IV.
Figure 2.
TYMS represented a moderate diagnostic value. The ROC of normal tissues and all pancreatic cancer samples, and subgroup analysis for stage I, II, III, and IV pancreatic cancer.
3.4. Correlation between clinical characteristics and TYMS expression in pancreatic cancer
We divided patients into TYMS high and low expression groups and examined the correlation between clinical characteristics and TYMS expression in patients with pancreatic cancer (Table 2). The correlation between TYMS expression and clinical characteristics, including therapy outcome (P = .0401), vital status (P = .027), and OS (P = .0325) was significant.
Table 1 (Continued).
Clinical characteristics of the patients with pancreatic cancer.
3.5. High TYMS expression is associated with poor OS in patients with pancreatic cancer
Kaplan–Meier curves of OS showed that higher TYMS expression was associated with worse OS (P = .014) (Fig. 3). Subgroup analysis further indicated that high TYMS expression significantly affected the OS of patients in histologic grades G1/G2 (P = .025) and clinical stages I/II (P = .023). Univariate analysis revealed that histological type (P = .001), T classification (P = .009), N classification (P = .002), residual tumor (P = .018), and TYMS expression (P = .014) were associated with poor OS. Multivariate analysis including age, gender, histological type, stage, T/N/M classification, residual tumor, and TYMS expression parameters further showed that TYMS is an independent risk factor for OS in patients with pancreatic cancer (HR = 1.62, 95% CI: 1.05–2.48, P = .028; Table 3).
Figure 3.
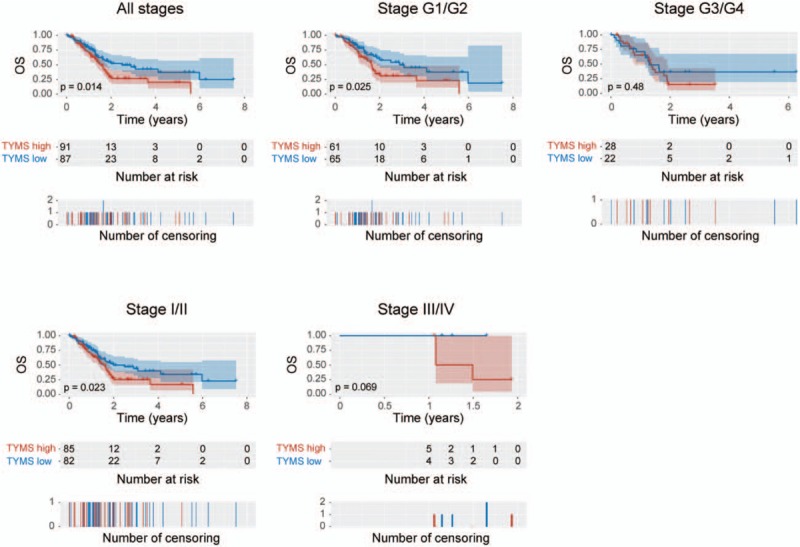
Kaplan–Meier curves of overall survival in patients with pancreatic cancer: in all tumors; histologic grades G1/G2 and G3/G4; and clinical stages I/II and III/IV.
Table 2.
Association between the clinical features and TYMS expression in patients with pancreatic cancer.
3.6. High TYMS expression is associated with poor RFS in patients with pancreatic cancer
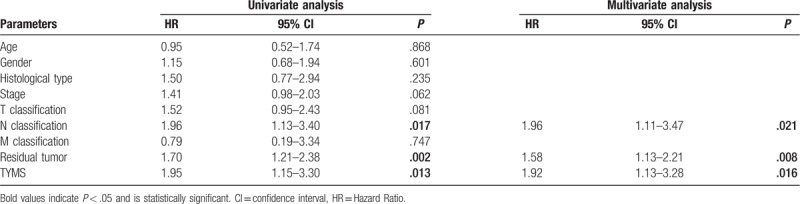
We generated Kaplan–Meier curves of RFS (Fig. 4). Upregulation of TYMS was associated with poor RFS in patients with pancreatic cancer (P = .021). Subgroup analysis revealed that TYMS upregulation was associated with poor RFS in clinical stage I/II (P = .0038). Univariate analysis revealed that N classification (P = .017), residue tumor (P = .002), and TYMS expression (P = .013) were associated with poor RFS (Table 4). Multivariate analysis further confirmed TYMS as an independent risk factor for RFS in patients with pancreatic cancer (HR = 1.92, 95% CI: 1.13–3.28, P = .016).
Figure 4.
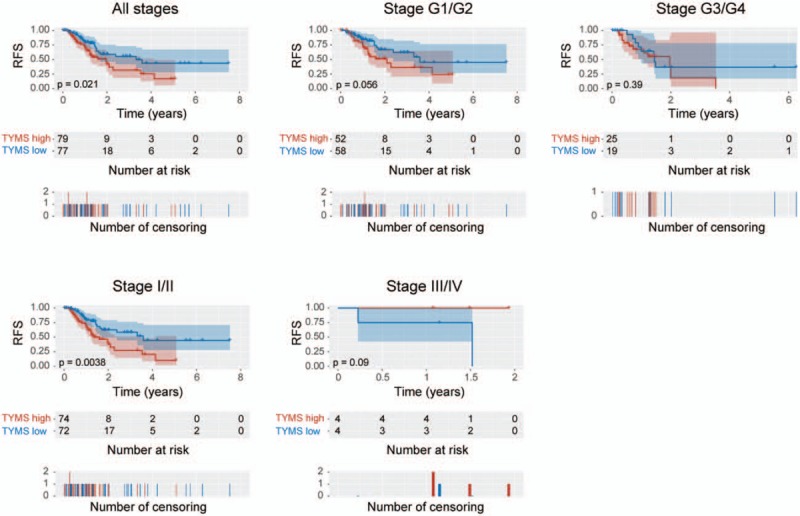
Kaplan–Meier curves of recurrence-free survival in patients with pancreatic cancer: in all tumors; histologic grades G1/G2 and G3/G4; and clinical stages I/II and III/IV.
Table 3.
Univariate and multivariate analysis of overall survival in patients with pancreatic cancer.
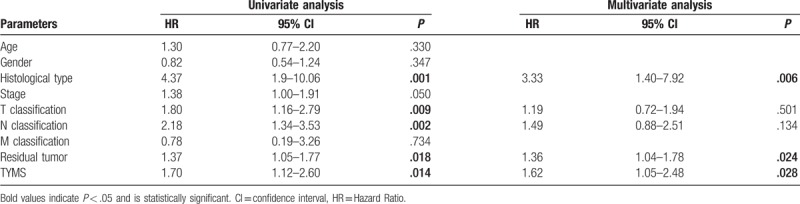
Table 4.
Univariate and multivariate analysis of recurrence-free survival in patients with pancreatic cancer.
4. Discussion
Our team has been working toward developing novel biomarkers for distinct tumors.[12,13] In this study, we report that TYMS is upregulated in pancreatic cancer and that elevated TYMS expression is associated with therapy outcome, vital status, and OS of patients. Kaplan–Meier curves of OS and RFS also showed that high TYMS expression is correlated with poor survival in patients. Moreover, univariate and multivariate analyses suggest that TYMS expression plays a prognostic role in patients with pancreatic cancer. Our results indicate that TYMS is insufficient for use as a diagnostic biomarker, but the prognostic capacity of TYMS is sufficient.
High TYMS expression has been reported in breast cancer,[14] non-small cell lung cancers,[15] and prostate cancer.[6,16] However, TYMS expression levels in pancreatic cancer were unknown. Our results demonstrate that TYMS is upregulated in pancreatic cancer. This result is consistent with those previously reported in other tumors. Moreover, different TYMS expression levels were found in distinct histologic grades and vital status, suggesting that TYMS plays an important role in the progress and prognosis of pancreatic cancer. However, because the AUC of ROC was relatively low, the diagnostic value of TYMS was moderate.
TYMS, encodes an enzyme that participates in the synthesis of DNA and its role in the proliferation of tumor cells has been reported. Patients with advanced clinical stage prostate cancer more commonly express TYMS than do those with less advanced disease.[16] In colorectal cancer, knockdown of TYMS inhibits the proliferation of tumor cells and promotes apoptosis.[17] Our results, that patients with poorer vital status or OS had higher TYMS expression levels, are consistent with these observations.
TYMS expression is associated with decreased survival in lung carcinoma,[15,18] metastatic colorectal cancer,[19] and hepatocellular carcinoma.[20] Our results, consistent with those reported in other cancers, show that high TYMS expression correlates with poorer OS in patients with pancreatic cancer. We also found that high TYMS expression affects the OS of patients in histologic grades G1/G2 and clinical stages I/II, but not in histologic grades G3/G4 and clinical stages III/IV. These data suggest that TYMS has different roles in patients with distinct stages.
Surgery is the only way to cure pancreatic cancer.[21] However, tumor recurrence adversely impacts the effectiveness of surgery, which complicates treatment choice. Here, we also show that high TYMS expression leads to poorer RFS in patients with pancreatic cancer, indicating that TYMS expression levels can be used to inform treatment choice. Moreover, we found high TYMS expression significantly affected the RFS of patients in clinical stages I/II, rather than in clinical stages III/IV, indicating a powerful prognostic capacity during an early stage. However, the prognostic role of TYMS in patients was limited in histologic grades G3/G4. Exploring more potential grade G1/G2 biomarkers in patients with pancreatic cancer in the near future is important. Limitations of our study included the small sample size in some comparison groups, and that tissue collection was very difficult in some cases given that operations were not suitable for patients with late stage pancreatic cancer. In addition, some types of pancreatic cancer were rarely observed.
Our results show that TYMS plays a significant role in the diagnosis and prognosis of patients with pancreatic cancer. These results contribute to the collective understanding of the role of TYMS in the diagnosis and prognosis of patients with cancer. The application of TYMS in prognosis is limited in histologic grades G3/G4, and a novel biomarker in histologic grades G1/G2 of patients with pancreatic cancer is urgently required in the future.
In conclusion, we show that, in patients with pancreatic cancer, higher TYMS expression is associated with advanced clinical stages and undesirable prognosis, and that TYMS could be used as a biomarker for diagnostic and prognostic evaluation in patients with pancreatic cancer.
Acknowledgments
We thank Rebecca Porter, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author contributions
Conceptualization: Bin Liu.
Data curation: Yan Jiao.
Formal analysis: Bai Ji.
Funding acquisition: Bin Liu.
Investigation: Yanqing Li.
Methodology: Yan Jiao.
Resources: Bai Ji.
Software: Yanqing Li.
Supervision: Baoxing Jia.
Writing – original draft: Zhuo Fu.
Writing – review & editing: Baoxing Jia.
Bin Liu orcid: 0000-0002-0648-0573.
Footnotes
Abbreviations: OS = overall survival, RFS = recurrence-free survival, TCGA = The Cancer Genome Atlas.
How to cite this article: Fu Z, Jiao Y, Li Y, Ji B, Jia B, Liu B. TYMS presents a novel biomarker for diagnosis and prognosis in patients with pancreatic cancer. Medicine. 2019;98:51(e18487).
This study was supported by the Project of Talent Development Funds of Jilin Province (grant number: 802160100428). The funding sources had no involvement in design, analysis or submission of this article.
The authors have no conflicts of interests to disclose.
References
- [1].Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016;22:9694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhang L, Sanagapalli S, Stoita A. Challenges in diagnosis of pancreatic cancer. World J Gastroenterol 2018;24:2047–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hanahan D, Weinberg RA. The hallmark of cancer. Cell 2000;100:57–71. [DOI] [PubMed] [Google Scholar]
- [4].Gallegos-Arreola MP, Zuniga-Gonzalez GM, Sanchez-Lopez JY, et al. TYMS 2R3R polymorphism and DPYD [IVS]14+1G>A gene mutation in Mexican colorectal cancer patients. Acta Biochim Pol 2018;65:227–34. [DOI] [PubMed] [Google Scholar]
- [5].Carreras CW, Santi DV. The catalytic mechanism and structure of thymidylate synthase. Annu Rev Biochem 1995;64:721–62. [DOI] [PubMed] [Google Scholar]
- [6].Burdelski C, Strauss C, Tsourlakis MC, et al. Overexpression of thymidylate synthase (TYMS) is associated with aggressive tumor features and early PSA recurrence in prostate cancer. Oncotarget 2015;6:8377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Team RDC. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Computing 2012;1:12–21. [Google Scholar]
- [8].Samur MK. RTCGAToolbox: a new tool for exporting TCGA firehose data. Plos One 2014;9:e106397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. Bmc Bioinformatics 2011;12:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Therneau TM, April A Package for Survival Analysis in S. 2015. [Google Scholar]
- [11].Therneau TM, Grambsch PM. Modeling survival data: extending the cox model. Technometrics 2000;97:353–4. [Google Scholar]
- [12].Jiao Y, Fu Z, Li Y, et al. High EIF2B5 mRNA expression and its prognostic significance in liver cancer: a study based on the TCGA and GEO database. Cancer Manag Res 2018;10:6003–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jiao Y, Fu Z, Li Y, et al. Aberrant FAM64A mRNA expression is an independent predictor of poor survival in pancreatic cancer. PLoS One 2019;14:e0211291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gupta P, Suman S, Mishra M, et al. Autoantibodies against TYMS and PDLIM1 proteins detected as circulatory signatures in Indian breast cancer patients. Proteomics Clin Appl 2016;10:564–73. [DOI] [PubMed] [Google Scholar]
- [15].Sun S, Shi W, Wu Z, et al. Prognostic significance of the mRNA expression of ERCC1, RRM1, TUBB3 and TYMS genes in patients with non-small cell lung cancer. Exp Ther Med 2015;10:937–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Russo GI, Bier S, Hennenlotter J, et al. Expression of tumour progression-associated genes in circulating tumour cells of patients at different stages of prostate cancer. BJU Int 2018;122:152–9. [DOI] [PubMed] [Google Scholar]
- [17].Xu W, Jiang H, Zhang F, et al. MicroRNA-330 inhibited cell proliferation and enhanced chemosensitivity to 5-fluorouracil in colorectal cancer by directly targeting thymidylate synthase. Oncol Lett 2017;13:3387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jiang H, Wang H, Wang S, et al. Expression of ERCC1, TYMS, RRM1, TUBB3, non-muscle myosin II, myoglobin and MyoD1 in lung adenocarcinoma pleural effusions predicts survival in patients receiving platinum-based chemotherapy. Mol Med Rep 2015;11:3523–32. [DOI] [PubMed] [Google Scholar]
- [19].Abdallah EA, Fanelli MF, Buim ME, et al. Thymidylate synthase expression in circulating tumor cells: a new tool to predict 5-fluorouracil resistance in metastatic colorectal cancer patients. Int J Cancer 2015;137:1397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yeh HW, Lee SS, Chang CY, et al. Pyrimidine metabolic rate limiting enzymes in poorly-differentiated hepatocellular carcinoma are signature genes of cancer stemness and associated with poor prognosis. Oncotarget 2017;8:77734–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kamisawa T, Wood L, Itoi T, et al. Pancreatic cancer. Lancet 2016;388:73–85. [DOI] [PubMed] [Google Scholar]


