Abstract
Background:
Cisplatin is often used for the treatment of oral cancer (OC). However, there are inconsistent results. Thus, this study plans to systematically assess the clinical efficacy and safety of cisplatin for adult patients with OC.
Methods:
We will search for PUBMED, EMBASE, Cochrane Library, AMED, Cumulative Index to Nursing and Allied Health Literature, Chinese Biomedical Literature Database, and China National Knowledge Infrastructure. All of them will be searched from the construction of each database up to the present with no restrictions of language and publication status. The data analysis will be conducted using RevMan 5.3 software to assess the efficacy and safety of cisplatin for adult patients with OC.
Results:
This study will summarize the most recent high-quality evidence and will provide helpful information about the efficacy and safety of cisplatin for adult patients with OC.
Conclusion:
The findings of this study will provide convinced evidence of cisplatin for adult patients with OC, and provide recommendations for clinical practice.
Systematic review registration:
PROSPERO CRD42019156558.
Keywords: cisplatin, efficacy, oral cancer, safety
1. Introduction
Oral cancer (OC) is one of the most common cancers worldwide, and has a 5-year survival rate of around 50%.[1–4] It has been reported about 354,864 new cases of OC were identified, and 177,384 people died from OC globally in 2018.[5] Although new patients have been diagnosed increasingly, most patients are still diagnosed at late and advanced stages at the initial diagnosis.[6–9] Due to its anatomic location, patients with such disorder often experience very poor quality of life, impairment of most vital functions, as well as their appearances.[10–12] Thus, it is very urgent to treat such condition timely and effectively.
Cisplatin has been reported to treat a variety of cancers effectively, especially for OC.[13–16] However, there is still no systematic evidence of cisplatin for the treatment of adult patients with OC.[17–21] Therefore, this study will assess the efficacy and safety of cisplatin for the treatment of adult patients with OC systematically.
2. Methods
2.1. Inclusion criteria
2.1.1. Types of studies
Any randomized controlled trials (RCTs) using cisplatin to treat adult patients with OC will be included, regardless of blinding, and allocation concealment.
2.1.2. Types of patients
This study will include adult patients (aged more than 18 years old) who were diagnosed as OC without limitations of gender, race, and duration of disease.
2.1.3. Types of interventions
The patients in the experimental group received cisplatin treatment, regardless its forms and dosage.
The patients in the control group received any routine treatments, such as radiation therapy, surgery, and any others, except cisplatin.
2.1.4. Type of outcome measurements
The primary outcomes are overall survival and pathological complete response. The secondary outcomes are recurrence-free survival; disease-free survival; quality of life, as measured by 36-Item Short Form Health Survey or any related tools; and toxicities.
2.2. Data sources and search strategy
2.2.1. Electronic searches
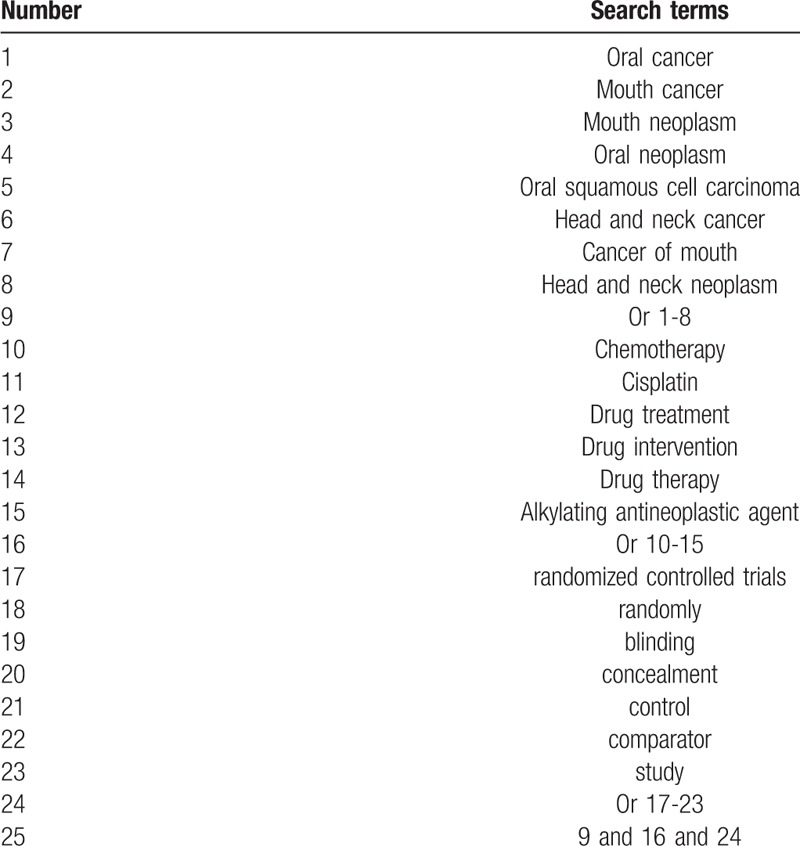
We will carry out comprehensive search from the construction of each database up to the present with no restrictions of language and publication status from following electronic databases: PUBMED, EMBASE, Cochrane Library, AMED, Cumulative Index to Nursing and Allied Health Literature, Chinese Biomedical Literature Database, and China National Knowledge Infrastructure. The search strategy for PUBMED is showed in Table 1. We also plan to build similar search strategies for other electronic databases.
Table 1.
Search strategy for PUBMED.
2.2.2. Other resources
We will also check dissertations, ongoing trials, and reference lists of identified studies to avoid losing any potential studies.
2.3. Data collection and analysis
2.3.1. Study selection
All identified papers will be checked through scanning their titles and abstracts by 2 independent authors. Then, we will exclude all irrelevant studies. The rest literatures will be read by full texts to further judge if they meet all inclusion criteria. Any excluded studies will be recorded with clear reasons. Any divergences between 2 authors will be settled down by a third author through discussion. The study selection will be presented in the flowchart.
2.3.2. Data collection process
Two authors will independently carry out data collection using pre-designed data extraction form. It consists of study characteristics (such as first author name, publication date, title, country, et al), patient characteristics (such as race, age, gender, inclusion criteria, et al), study methods (such as sample size calculation, methods of randomization, blind, et al), study treatments (such as intervention types, frequency, dosage, et al), all outcomes, safety, and other relevant information. When there is any missing or insufficient information, we will contact original authors by email to require them. Any divergences between 2 authors will be resolved by a third author via discussion.
2.4. Risk of bias assessment
Two authors will independently evaluate risk of bias of each included trial using Cochrane risk of bias tool according to the specific Guidelines of Cochrane Handbook for Systematic Reviews of Interventions. It has 7 detailed items, and each one is further assessed as “low risk of bias”, “unclear risk of bias”, and “high risk of bias”. Any disagreements will be solved by another author via discussion.
2.5. Assessment of heterogeneity
We will estimate heterogeneity among eligible studies using I2 statistic test. The value of I2 ≤ 50% refers low heterogeneity, while the value of I2 > 50% exerts significant heterogeneity.
2.6. Subgroup analysis
If there are sufficient studies for data pooling with substantial heterogeneity, we will perform subgroup analysis to explore reasons for such obvious heterogeneity according to the different risk of bias, treatments, comparators, and outcome measurements.
2.7. Sensitivity analysis
We will consider conducting sensitivity analysis to check stability of pooled results by eliminating low quality studies.
2.8. Reporting bias
If at least 10 included RCTs are entered in this study, we will perform funnel plots to check any potential reporting bias.[22]
2.9. Statistical analysis
RevMan 5.3 software will be used to carry out data analysis. For continuous data, mean difference or standardized mean difference and 95% confidence intervals (CIs) will be expressed. For dichotomous data, risk ratio and 95% CIs will be calculated. Heterogeneity across included trials will be identified using I2 statistic. I2 ≤ 50 indicates included studies have homogeneity, and a fixed-effects model will be selected. I2 > 50% means eligible studies have significant heterogeneity, and a random-effects model will be adopted. If there is homogeneity among more than 2 studies with similar treatments, controls, and outcomes, we will perform meta-analysis. If there is substantial heterogeneity, subgroup analysis will be operated. If there is still obvious heterogeneity after subgroup analysis, outcome data are not available for quantitative analysis, and we will report study results by qualitative description.
2.10. Ethics and dissemination
This study will not need research ethic, because we will use individual patient data. It is expected to be published on a peer-reviewed journal.
3. Discussion
OC is a devastating disease, which can result in highly morbidity and mortality in both males and females. Although promising advancements in its management approaches available, the overall efficacy is still not satisfied. Fortunately, previous studies have reported that cisplatin can benefit adult patients with OC. However, there is still not systematic review to address it. Therefore, this study will assess the efficacy and safety of cisplatin for the treatment of adult patients with OC. The results of this study will provide helpful evidence for OC treatment, and helpful recommendation for clinical practice.
Author contributions
Conceptualization: Yao Feng, Dian-song Yang, Hai-bo Tang, Yuan-sheng Ding, Xiao-guang Li.
Data curation: Dian-song Yang, Hai-bo Tang, Yuan-sheng Ding, Xiao-guang Li.
Formal analysis: Yao Feng, Hai-bo Tang, Yuan-sheng Ding.
Funding acquisition: Yao Feng.
Investigation: Yao Feng, Dian-song Yang, Xiao-guang Li.
Methodology: Dian-song Yang, Hai-bo Tang, Yuan-sheng Ding, Xiao-guang Li.
Project administration: Yao Feng, Dian-song Yang.
Resources: Hai-bo Tang, Yuan-sheng Ding, Xiao-guang Li.
Software: Dian-song Yang, Hai-bo Tang, Yuan-sheng Ding, Xiao-guang Li.
Supervision: Yao Feng.
Validation: Yao Feng, Dian-song Yang, Hai-bo Tang, Yuan-sheng Ding, Xiao-guang Li.
Visualization: Yao Feng, Hai-bo Tang, Yuan-sheng Ding, Xiao-guang Li.
Writing – original draft: Yao Feng, Yuan-sheng Ding, Xiao-guang Li.
Writing – review & editing: Yao Feng, Dian-song Yang, Hai-bo Tang, Yuan-sheng Ding, Xiao-guang Li.
Footnotes
Abbreviations: CIs = confidence intervals, OC = oral cancer, RCTs = randomized controlled trials.
How to cite this article: Feng Y, Yang Ds, Tang Hb, Ding Ys, Li Xg. Efficacy and safety of cisplatin for the management of adult patients with oral cancer: a protocol for systematic review. Medicine. 2019;98:51(e18210).
YF and D-sY contributed equally to this study.
This study was supported by Heilongjiang Provincial Health and Family Planning Commission Research Project (2017-417). The funding institute was not allowed to involve any sections of this study.
The authors have no conflicts of interests to disclose.
References
- [1].Madera Anaya MV, Franco JV, Merchán-Galvis ÁM, et al. Quality assessment of clinical practice guidelines on treatments for oral cancer. Cancer Treat Rev 2018;65:47–53. [DOI] [PubMed] [Google Scholar]
- [2].Gupta N, Gupta R, Acharya AK, et al. Changing trends in oral cancer - a global scenario. Nepal J Epidemiol 2016;6:613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rhodus NL. Oral cancer update: 2017. Northwest Dent 2017;96:21–3. [PubMed] [Google Scholar]
- [4].Calixto G, Bernegossi J, Fonseca-Santos B, et al. Nanotechnology-based drug delivery systems for treatment of oral cancer: a review. Int J Nanomedicine 2014;9:3719–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [6].Kao SY, Mao L, Jian XC, et al. Expert consensus on the detection and screening of oral cancer and precancer. Chin J Dent Res 2015;18:79–83. [PubMed] [Google Scholar]
- [7].Mehrotra R, Gupta DK. Exciting new advances in oral cancer diagnosis: avenues to early detection. Head Neck Oncol 2011;3:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Güneri P, Epstein JB. Late stage diagnosis of oral cancer: components and possible solutions. Oral Oncol 2014;50:1131–6. [DOI] [PubMed] [Google Scholar]
- [9].Chen XJ, Zhang XQ, Liu Q, et al. Nanotechnology: a promising method for oral cancer detection and diagnosis. J Nanobiotechnol 2018;16:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rivera C. Essentials of oral cancer. Int J Clin Exp Pathol 2015;8:11884–94. [PMC free article] [PubMed] [Google Scholar]
- [11].Prince VM, Papagerakis S, Prince ME. Oral cancer and cancer stem cells: relevance to oral cancer risk factors, premalignant lesions, and treatment. Curr Oral Heal Rep 2016;3:65–73. [Google Scholar]
- [12].Patton DW, Ali A, Davies R, et al. Oral rehabilitation and quality of life following the treatment of oral cancer. Dent Update 1994;21:231–4. [PubMed] [Google Scholar]
- [13].Sato K, Hayashi Y, Watanabe K, et al. Concurrent chemoradiotherapy with intravenous cisplatin and docetaxel for advanced oral cancer. Nagoya J Med Sci 2019;81:407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chiang TE, Ho CL, Lin CS, et al. Complete remission in very advanced oral cancer by docetaxel, cisplatin, 5-fluorouracil based induction chemotherapy followed by concurrent chemoradiation. J Dent Sci 2018;13:82–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen CF, Lu CC, Chiang JH, et al. Synergistic inhibitory effects of cetuximab and curcumin on human cisplatin-resistant oral cancer CAR cells through intrinsic apoptotic process. Oncol Lett 2018;16:6323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tang X, Hu YJ, Ju WT, et al. Elevated growth differentiating factor 15 expression predicts long-term benefit of docetaxel, cisplatin and 5-fluorouracil induction chemotherapy in patients with oral cancer. Oncol Lett 2018;15:8118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rohde S, Kovács AF, Turowski B, et al. Intra-arterial high-dose chemotherapy with cisplatin as part of a palliative treatment concept in oral cancer. AJNR Am J Neuroradiol 2005;26:1804–9. [PMC free article] [PubMed] [Google Scholar]
- [18].Kovács AF. Chemoembolization using cisplatin crystals as neoadjuvant treatment of oral cancer. Cancer Biother Radiopharm 2005;20:267–79. [DOI] [PubMed] [Google Scholar]
- [19].Tegeder I, Bräutigam L, Seegel M, et al. Cisplatin tumor concentrations after intra-arterial cisplatin infusion or embolization in patients with oral cancer. Clin Pharmacol Ther 2003;73:417–26. [DOI] [PubMed] [Google Scholar]
- [20].Andreadis C, Vahtsevanos K, Sidiras T, et al. 5-Fluorouracil and cisplatin in the treatment of advanced oral cancer. Oral Oncol 2003;39:380–5. [DOI] [PubMed] [Google Scholar]
- [21].Wada T, Harada M, Morita N, et al. Chemotherapy administered using two-route infusion of cisplatin and sodium thiosulfate and intravenous infusion of vinblastine and peplomycin in patients with oral cancer. Clin Ther 1995;17:280–9. [DOI] [PubMed] [Google Scholar]
- [22].Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882–93. [DOI] [PubMed] [Google Scholar]


