Abstract
To generate a nomogram to predict posthepatectomy liver failure (PHLF), we attempted to elucidate salient risk factors in patients with hepatocellular carcinoma (HCC).
We performed a retrospective review of 665 patients with HCC who received hepatectomy in 2 academic institutions in China. Independent risk factors for PHLF were identified from putative demographic, intrinsic, biochemical, surgery-related, and volumetric data. A predictive nomogram was formulated based on relevant risk factors, and we compared this with existing models.
We identified clinical signs of portal hypertension (P = .023), serum total bilirubin (P = .001), serum creatinine (P = .039), and intraoperative hemorrhage (P = .015) as being important risk factors in predicting PHLF. The nomogram had a C-index of 0.906 for the externally validated data. The nomogram displayed better predictive value than 2 of the other most cited models (C-indices of 0.641 and 0.616, respectively) in the current cohort. Additionally, we were able to patients into low- (<10%), intermediate- (10–30%), and high-risk (≥30%) groups based on the nomogram. This allows us to facilitate person-specific management.
Here, we constructed a simple nomogram for prediction of PHLF in patients with HCC weighted by independent risk factors. Further prospective studies are required to confirm the predictive ability of our nomogram.
Keywords: hepatocellular carcinoma, posthepatectomy liver failure, nomogram, prediction
1. Introduction
Recently, with advances in surgical technique and perioperative care, more patients are now able to receive hepatic reaction with curative intent.[1,2] Consequently, hepatobiliary surgeons are increasingly undertaking more extensive approaches, where extended liver resections are being offered postchemotherapy. Furthermore, major resections for patients with multiple comorbidities and those requiring vascular reconstruction are also being performed, with improved overall long-term survival the ultimate aim.[3] However, posthepatectomy liver failure (PHLF) is still a significant driver of morbidity and mortality.[4,5] Not only is it a leading cause of mortality, it is one of the primary drivers of life-threatening complications that are linked to operative treatment.[6–8]
There are numerous definitions of PHLF that have been used but it was only 2011 that a consensus was reached, after the International Study Group of Liver Surgery (ISGLS) defined PHLF as a “postoperatively acquired deterioration in the ability of the liver to maintain its synthetic, excretory and detoxifying functions, characterized by an increased international normalized ratio (INR) and concomitant hyperbilirubinemia on or after postoperative day 5.” This definition held true only in the absence of other causes for the relevant biochemical and clinical alterations such as biliary obstruction.[9] Although several articles relating to PHLF have been previously published,[3,10–13] an accurate and effective prediction system for PHLF is still lacking.[14]
Models incorporating End-Stage Liver Disease (MELD)[15] and albumin-bilirubin (ALBI) score[16] have been shown to have an increased incidence of PHLF. While a MELD score ≥10 appeared to be associated with an increased risk of PHLF, the MELD scoring system was not able to predict the severity of PHLF robustly.[17] Recently, Hu et al[13] reported that a newly designed nomogram was able to provide good preoperative prediction of PHLF in patients with resectable hepatocellular carcinoma (HCC). Nevertheless, a small sample size used to evaluate this nomogram and it was not externally validated. In addition, Dasari et al[3] also reported a PHLF risk score with high volume samples could stratify the PHLF risk in elective patients receiving hepatectomy. However, the surgical factors associated with PHLF were not included in this study and there was no external validation performed. Consequently, we conducted a retrospective study on patients with HCC who underwent “curative” liver resection with the aim of formulating a postoperative nomogram to accurately predict PHLF in these patients.
2. Materials and methods
2.1. Patients
We retrospectively collected the data from 665 patients with HCC who underwent attempted curative liver resection from 2 academic institutions in China. Patients in the Second Affiliated Hospital of Zhejiang University School of Medicine were selected as the training cohort (n = 325) while patients from the Eastern Hepatobiliary Surgery Hospital were chosen as the validation cohort (n = 340). These 2 institutions experience high volumes of patients requiring liver cancer surgery. Only patients receiving hepatectomy (R0 or R1 resection) and histopathologically confirmed as having HCC according to the European Association for the Study of the Liver (EASL) criteria[9] were included.
We used the following inclusion criteria: aged 18 to 85 years; patients with resectable HCC eligible for hepatectomy; no prior treatment; and Child–Pugh A or B liver function. We excluded patients with a history of any other malignant tumor or recurrent tumors before hepatectomy. Both institutional ethics committees of Second Affiliated Hospital of Zhejiang University School of Medicine and institutional ethics committees of Eastern Hepatobiliary Surgery Hospital approved this study. We obtained written informed consent from patients for use of their clinical data in this research study.
2.2. Data collection
The data for patients undergoing hepatectomy included sex, age, body weight, body mass index (BMI), activity of daily living (ADL) scores, history of etiology, comorbidity, hepatitis, cirrhosis, preoperative laboratory test, clinical signs of portal hypertension (CSPH), Child–Pugh classification, indocyanine green retention rate (ICGR15) test, radiological data (including computed tomography [CT] or magnetic resonance imaging [MRI]-based volumetric factors) and surgical information. Patients who underwent cholecystectomy along with hepatectomy were enrolled in the study.
The CSPH was defined as “a hepatic venous pressure gradient (HVPG) ≥10 mm Hg, with the presence of porto-systemic collaterals on imaging or patients with gastroesophageal varices on endoscopy.”[18] We defined resection of 4 or more liver segments as major hepatectomy and defined resection of 3 or less liver segments as minor hepatectomy.[19] As no consensus has been reached for determining standardized liver volume (SLV),[14] we used total functional liver volume (TFLV). Total liver volume (TLV) incorporated total tumor volume to replace SLV, and the following formula was generated to calculate future liver remnant (FLR) ratio: FLR ratio = (FLR/TFLV) × 100% (Fig. 1). Spleen volume (Sp) may also be a critical factor in posthepatectomy outcomes. Indeed Truant et al reported that increased Sp/FLR (Fig. 2) appeared to correlate with PHLF.[20]
Figure 1.
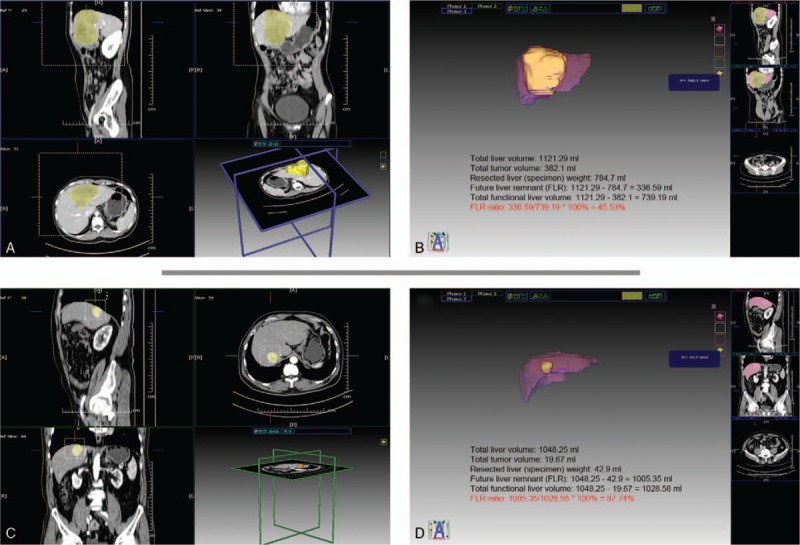
(A,B) Liver volume obtained from a 58-year-old woman with posthepatectomy liver failure and future liver remnant (FLR) ratio of 45.53%. (C,D) Liver volume obtained from a 66-year-old man with normal posthepatectomy liver function and an FLR ratio of 97.74%.
Figure 2.
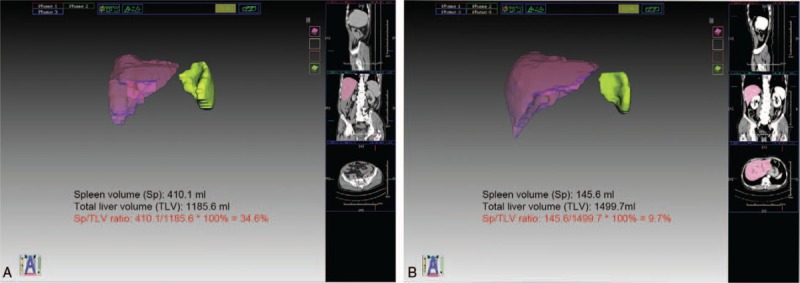
(A) Spleen volume obtained from a 44-year-old man with clinical signs of portal hypertension (CSPH) and spleen volume (Sp)/total liver volume (TLV) ratio of 34.6%. (B) Sp obtained from a 65-year-old man with non-CSPH and Sp/TLV ratio of 9.7%.
2.3. Statistical analysis
All statistical analyses were performed using version 7.00 (GraphPad Software, San Diego, CA) and SPSS, version 22.0 for Windows (IBM Corp, Armonk, NY). Categorical variables were evaluated using either Fisher exact test or Chi-squared test. Continuous variables were compared using the Mann–Whitney U test (for variables that were not normally distributed) or the Student t test. All variables were incorporated into a univariate analysis and only those variables showing statistical significance (P < .05) were included in a multivariate logistic regression model. The corresponding area under the curve (AUC) and receiver operating characteristic (ROC) curves and were used to assess how well the test data affected performance of the predictive model.
The nomogram was formulated based on the results of multivariate logistic regression analysis, using the rms package of R, version 3.1.1 (http://www.r-project.org/). In constructing the model, each of the independent factors was assigned points proportionate to the value of the regression coefficient. Calculating the concordance index (C-index) was used to measure the predictive performance of the nomogram.
3. Results
3.1. Patient characteristics
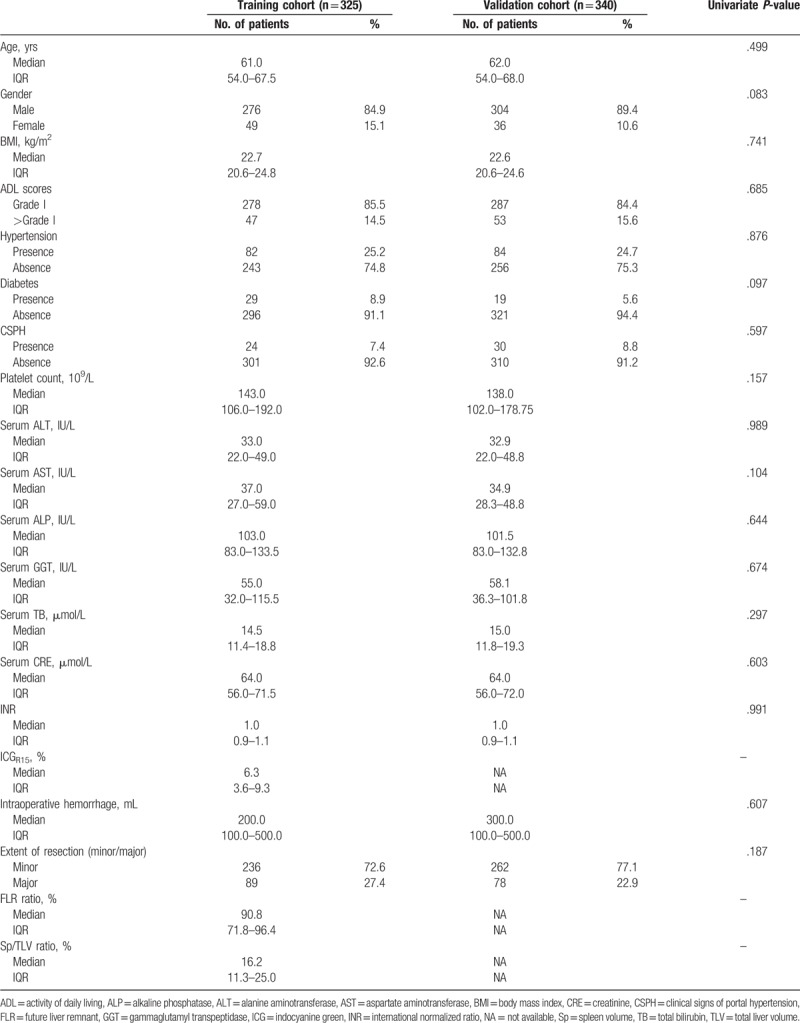
The final cohort of patients was 665 (580 males and 85 females) with a mean age of 61 years. Patients from our center were chosen as the training cohort (n = 325) while patients from the remaining center were selected as the externally validated cohort (n = 340). The ratio of the patients in these 2 groups was approximately 1 to 1. Reassuringly, the baseline patient characteristics did not differ significantly between the 2 groups (Table 1). The PHLF percentage was 8.3% (n = 27) in the training cohort and 7.1% (n = 24) in the externally validated cohort.
Table 1.
Patient baseline characteristics by cohort.
3.2. Factors associated with PHLF
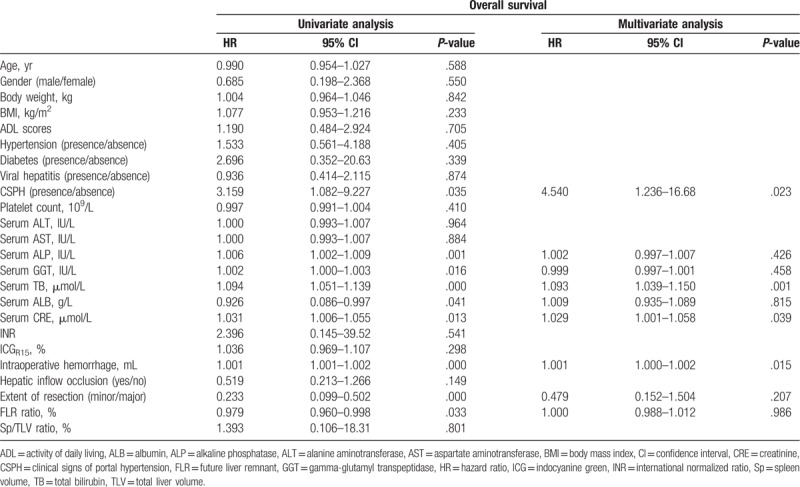
In the training cohort, univariate analysis showed that CSPH, serum alkaline phosphatase (ALP), serum gamma-glutamyl transpeptidase (GGT), serum total bilirubin (TB), serum albumin (ALB), serum creatinine (CRE), intraoperative hemorrhage, and extent of resection and FLR ratio were all significantly associated with PHLF (Table 2). However, the multivariate analysis revealed that only CSPH (P = .023), serum TB (P = .001), serum CRE (P = .039), and intraoperative hemorrhage (P = .015) were independently associated with PHLF (Table 2).
Table 2.
Univariate and multivariate analyses of factors associated with posthepatectomy liver failure.
3.3. Construction of the predictive nomogram
We constructed the nomogram to predict the probability of PHLF by using the 4 aforementioned independent risk factors (Fig. 3). The points assigned to each factor were weighted by their hazard ratios (HRs). The total score was used to calculate the probability of PHLF. For example, a patient with HCC who underwent hepatectomy had 55 μmol/L preoperative TB (84 points), 60 μmol/L preoperative CRE (20 points), 1000 mL intraoperative hemorrhage (6 points), and CSPH (23 points). Thus, the total score for this patient was 133, indicating a >90% probability of developing PHLF.
Figure 3.
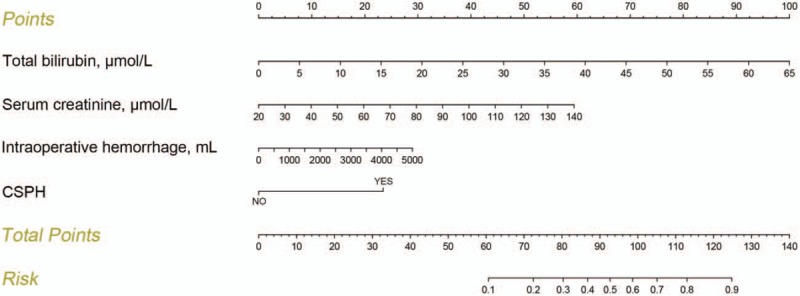
Predictive nomogram for assessing probability of posthepatectomy liver failure in patients with hepatocellular carcinoma.
3.4. Validation of the predictive accuracy for PHLF using the nomogram
The calibration curve showed a good agreement between the likelihood of PHLF using our nomogram and the actual observed disease in both cohorts (Fig. 4A, B). In addition, The C-index of the nomogram for prediction of PHLF was 0.818 (95% confidence interval [CI]: 0.735–0.901) for the training cohort and 0.906 (95% CI: 0.833–0.979) for the validation cohort (Fig. 5A, B).
Figure 4.
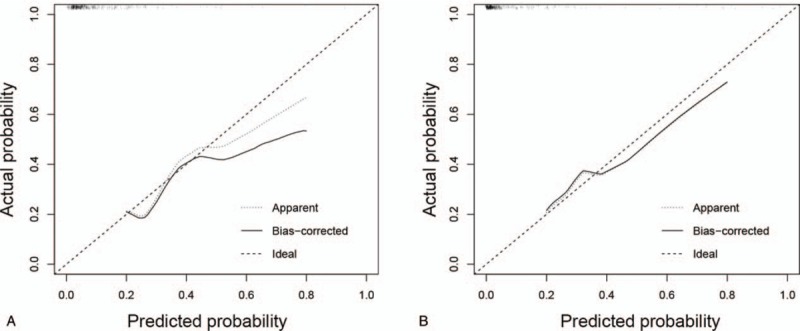
(A) Calibration curves for predicted probability of posthepatectomy liver failure (PHLF) in training cohort (using the nomogram values). (B) Calibration curves for predicted probability of PHLF in external validation cohort (using the nomogram values).
Figure 5.
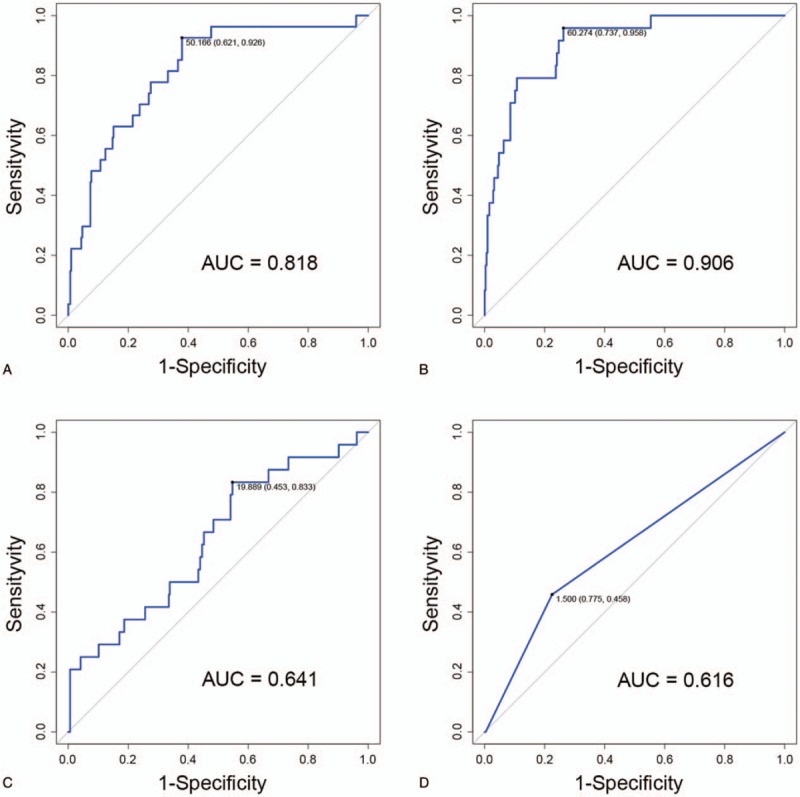
(A) Receiver-operating characteristics curve for posthepatectomy liver failure (PHLF) in training cohort (using the nomogram values). The C-index is 0.818. (B) Receiver-operating characteristics curve for PHLF in external validation cohort (using the nomogram values). The C-index is 0.906. (C) Receiver-operating characteristics curve for PHLF in external validation cohort (using the albumin-bilirubin values). The C-index is 0.641. (D) Receiver-operating characteristics curve for PHLF in external validation cohort (using the Models incorporating End-Stage Liver Disease values). The C-index is 0.616. AUC = area under the curve.
To compare the MELD[15] and ALBI[16] score, the data from the externally validated cohort were used to assess the performance of the 2 scoring systems. The C-indices were 0.616 (95% CI: 0.512–0.720) for ALBI (Fig. 5C) and 0.641 (95% CI: 0.522–0.760) for MELD (Fig. 5D), respectively. Therefore, the criteria used to generate a C-index of 0.906 were considered to be the best among all the aforementioned criteria.
3.5. Risk groups
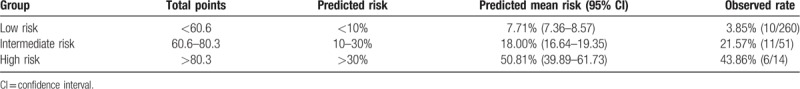
Based on the risk estimated by the nomogram, we believe that patients could be separated into 3 risk groups (Table 3): a low-risk group (total points <60.6 and predicted PHLF rate <10%), with a predicted mean risk of venous invasion of 7.71% (95% CI: 7.36–8.57); an intermediate-risk group (total points 60.6–80.3 and predicted PHLF rate 10–30%), with a predicted mean risk of PHLF of 18% (95% CI: 16.64–19.35); and a high-risk group (total points >80.3 with a predicted PHLF percentage exceeding 30%), and a predicted mean risk of 50.81% PHLF (95% CI: 39.89–61.73). The observed PHLF percentages were essentially equal to the predicted mean risks.
Table 3.
Risk groups based on the predicted nomogram.
4. Discussion
The prediction of PHLF is still evolving mainly due to its multifactorial causative factors.[3] Even so, many risk factors[10–12] have been identified and several prediction models[3,13] have been developed for PHLF. However, their clinical utility has some limitations.[14] We performed this study to identify the risk factors of PHLF in patients with resectable HCC to construct a nomogram for postsurgical prediction of PHLF.
A number of biologic markers appear to be associated with PHLF.[14] However, none of these markers individually have been shown to provide an adequate assessment of liver function.[10] TB was recognized as one of the most important factors of liver function and is a component of the ISGLS definition.[9] Preoperative TB levels of ≥20.3 mmol/L have been reported to be a strongly independent risk factor for PHLF.[21] Dasari et al[3] reported that patients with raised preoperative CRE, indicative of impaired renal function, exhibited a higher incidence of PHLF when analysis was multivariate. This may be due to the fact that elevated preoperative CRE is an indicator of poor general health and may suggest other coexisting medical disorders. In our study, preoperative TB and CRE were also found to be important indicators of PHLF and included in the nomogram. In addition, intraoperative hemorrhage was the other independent risk factor of PHLF in our study (P = .015).[22]
The CSPH is associated with an augmented likelihood of developing varices,[23] overt clinical decompensation (ascites, variceal hemorrhage, and encephalopathy),[24] postoperative decompensation,[25] and HCC. The American Association for the Study of Liver Diseases (AASLD) guidelines and the EASL guidelines consider elevated portal hypertension to be a contraindication for resection due to the higher relative risk of liver decompensation after surgery.[26] A n increasing number of studies have confirmed that increased portal venous pressure is associated with a higher risk of liver function decompensation after surgery, at least in the short term.[13] In our study, CSPH was demonstrated to be an important independent risk factor of PHLF. The definition of “the presence of porto-systemic collaterals on imaging or patients with gastroesophageal varices on endoscopy” was used to assess the presence of CSPH. Given that HVPG is an invasive technique, it is not used widely throughout the world.
Development of PHLF is strongly associated with the volume and function of the remaining healthy liver.[14] However, there is no consensus on how much remaining liver is considered to be “enough” due to the diverse characteristics of such affected patients. Volumetric factors including FLR ratio, Sp/TLV ratio, and the extent of resection and functional factors such as ICGR15 were included in our study. Unfortunately, none of these factors were associated with the presence of PHLF in multivariable analysis. However, this is explicable since every patient received a rigorous preoperative examination. Patients with less of their remaining functional liver volume or poor liver function would not receive surgery. Therefore, the lack of volumetric factors did not affect the application of our nomogram.
The performance of our nomogram in predicting PHLF could be considered to be acceptable given that the C-index was 0.818 and 0.906 in both cohorts. The calibration curves were also suggestive of a relatively accurate nomogram. When compared with other criteria (ALBI, AUC = 0.616; MELD, AUC = 0.641), our nomogram showed superior accuracy. By using the nomogram, we were able to stratify patients with HCC into 3 distinct risk groups. By doing so, specific management strategies can be established according to the specific risk categories. We suggest early use of hepatic protectant, close observation and intensive care should be planned after surgery especially in the intermediate- and high-risk groups.
This study has some limitations. First, although CT or MRI-based volumetric factors were considered in our study, parameters of ultrasonic elastography imaging were not included. Second, although we had 665 patients in this study sample, the sample size is still considered to be quite small. Larger cohorts across multiple sites are needed to further evaluate the clinical utility of our nomogram in the future.
5. Conclusion
We present a novel prediction nomogram of PHLF based on 4 essential independent prognostic risk factors. This proposed nomogram showed acceptable performance, with a C-index of 0.906 that represents robust external validation. The nomogram may be a convenient tool for facilitating decisions regarding posthepatectomy treatment approaches.
Author contributions
Conceptualization: Xueli Bai, Tingbo Liang.
Data curation: Tianyu Tang, Weiyun Yao, Cheng-Xiang Guo, Wei Song.
Software: Tianyu Tang.
Validation: Yi Zong.
Writing – original draft: Yinan Shen.
Footnotes
Abbreviations: AUC = area under the curve, C-index, concordance index, HCC = hepatocellular carcinoma, PHLF = posthepatectomy liver failure, ROC = receiver operating characteristic curves, SLV = standardized liver volume, TFLV = total functional liver volume.
How to cite this article: Shen YN, Tang TY, Yao WY, Guo CX, YZ, Song W, Liang TB, Bai XL. A nomogram for prediction of posthepatectomy liver failure in patients with hepatocellular carcinoma: a retrospective study. Medicine. 2019;98:51(e18490).
YNS, TYT, and WYY contributed equally to the work.
This study is supported by National Program on Key Basic Research Project (973 Program) (grant no: 2014CB542101), The National Natural Science Foundation of China (grant no: 81472212), The Key Program of Medical Scientific Research Foundation of Zhejiang Province (grant no: WKJ-ZJ-1410), The Key Program of Administration of Traditional Chinese Medicine of Zhejiang Province (grant no: 2014ZZ007), Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents, National nonprofit industry research sub project (grant no: 201502014), Zhejiang Provincial Key Innovation Team of Pancreatic Cancer Diagnosis & Treatment (grant no: 2013TD06), and China Scholarship Council (grant no: 201706320169).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
The authors have no conflicts of interest to disclose.
References
- [1].Thompson HH, Tompkins RK, Longmire WP. Major hepatic resection. A 25-year experience. Ann Surg 1983;197:375–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fan ST, Lai EC, Lo CM, et al. Hospital mortality of major hepatectomy for hepatocellular carcinoma associated with cirrhosis. Arch Surg 1995;130:198–203. [DOI] [PubMed] [Google Scholar]
- [3].Dasari BVM, Hodson J, Roberts KJ, et al. Developing and validating a pre-operative risk score to predict post-hepatectomy liver failure. HPB (Oxford) 2019;21:539–46. [DOI] [PubMed] [Google Scholar]
- [4].Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg 2007;204:854–62. [DOI] [PubMed] [Google Scholar]
- [5].Farges O, Malassagne B, Flejou JF, et al. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg 1999;229:210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schindl MJ, Millar AM, Redhead DN, et al. The adaptive response of the reticuloendothelial system to major liver resection in humans. Ann Surg 2006;243:507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Breitenstein S, DeOliveira ML, Raptis DA, et al. Novel and simple preoperative score predicting complications after liver resection in noncirrhotic patients. Ann Surg 2010;252:726–34. [DOI] [PubMed] [Google Scholar]
- [8].Paugam-Burtz C, Janny S, Delefosse D, et al. Prospective validation of the “fifty-fifty” criteria as an early and accurate predictor of death after liver resection in intensive care unit patients. Ann Surg 2009;249:124–8. [DOI] [PubMed] [Google Scholar]
- [9].Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713–24. [DOI] [PubMed] [Google Scholar]
- [10].Lafaro K, Buettner S, Maqsood H, et al. Defining post hepatectomy liver insufficiency: where do we stand. J Gastrointest Surg 2015;19:2079–92. [DOI] [PubMed] [Google Scholar]
- [11].van den Broek MA, Olde DSW, Dejong CH, et al. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int 2008;28:767–80. [DOI] [PubMed] [Google Scholar]
- [12].Khan AS, Garcia-Aroz S, Ansari MA, et al. Assessment and optimization of liver volume before major hepatic resection: current guidelines and a narrative review. Int J Surg 2018;52:74–81. [DOI] [PubMed] [Google Scholar]
- [13].Hu H, Han H, Han XK, et al. Nomogram for individualised prediction of liver failure risk after hepatectomy in patients with resectable hepatocellular carcinoma: the evidence from ultrasound data. Eur Radiol 2018;28:877–85. [DOI] [PubMed] [Google Scholar]
- [14].Shen YN, Zheng ML, Guo CX, et al. The role of imaging in prediction of post-hepatectomy liver failure. Clin Imaging 2018;52:137–45. [DOI] [PubMed] [Google Scholar]
- [15].Delis SG, Bakoyiannis A, Dervenis C, et al. Perioperative risk assessment for hepatocellular carcinoma by using the MELD score. J Gastrointest Surg 2009;13:2268–75. [DOI] [PubMed] [Google Scholar]
- [16].Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 2015;33:550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Andreatos N, Amini N, Gani F, et al. Albumin-bilirubin score: predicting short-term outcomes including bile leak and post-hepatectomy liver failure following hepatic resection. J Gastrointest Surg 2017;21:238–48. [DOI] [PubMed] [Google Scholar]
- [18].Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017;65:310–35. [DOI] [PubMed] [Google Scholar]
- [19].Reddy SK, Barbas AS, Turley RS, et al. A standard definition of major hepatectomy: resection of four or more liver segments. HPB (Oxford) 2011;13:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Truant S, Oberlin O, Sergent G, et al. Remnant liver volume to body weight ratio > or = 0.5%: a new cut-off to estimate postoperative risks after extended resection in noncirrhotic liver. J Am Coll Surg 2007;204:22–33. [DOI] [PubMed] [Google Scholar]
- [21].Shen Y, Shi G, Huang C, et al. Prediction of post-operative liver dysfunction by serum markers of liver fibrosis in hepatocellular carcinoma. PLoS One 2015;10:e0140932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].de Franchis R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63:743–52. [DOI] [PubMed] [Google Scholar]
- [23].Groszmann RJ, Garcia-Tsao G, Bosch J, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med 2005;353:2254–61. [DOI] [PubMed] [Google Scholar]
- [24].Ripoll C, Groszmann R, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 2007;133:481–8. [DOI] [PubMed] [Google Scholar]
- [25].Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 1996;111:1018–22. [DOI] [PubMed] [Google Scholar]
- [26].Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42:1208–36. [DOI] [PubMed] [Google Scholar]


