Abstract
Background:
The correlation between single nucleotide polymorphism (SNP) rs12807809 in Neurogranin (NRGN) gene and Schizophrenia (SCZ) was investigated by several studies, whereas the results were conflicting. Thus, we performed the present meta-analysis to combine and analyze the available studies in order to provide a more accurate result on the association of rs12807809 polymorphism in NRGN gene and SCZ vulnerability.
Methods:
A comprehensive retrieval in PubMed, EMBASE, Web of Science, Cochrane Library and Wanfang was performed for relevant studies on the relationship of rs12807809 polymorphism and SCZ. Summary odds ratios (OR) with 95% confidence interval (95% CI) were calculated in allelic, homozygous, heterozygous, dominant and recessive model to appraise the association.
Results:
The meta-analysis included 8 studies containing 12552 SCZ cases and 34783 controls. The results showed a statistically significant correlation between SCZ and rs12807809 polymorphism in overall population in allelic model (OR = 1.10, 95%CI 1.04–1.17). However, subgroup analysis indicated the association only existed in Caucasians but not Asian.
Conclusion:
The results of present meta-analysis suggested significant association between SNP rs12807809 in NRGN gene and SCZ susceptibility in Caucasians but not Asians.
Keywords: meta-analysis, neurogranin, polymorphism, Rs12807809, schizophrenia
1. Introduction
SCZ is a destructive psychosis which onset time usually is in late adolescence or early adulthood, impacting around 0.5% to 1.2% of the population all over the world.[1] The main clinical manifestations are positive symptoms, especially hallucinations and delusions, and negative symptoms like decreased mood, speech and interest, and language and behavioral disorders.[2] These symptoms make the normal life of patients with SCZ difficult, resulting in inability to work, study and exercise their social responsibilities. Albeit pharmacological therapies are useful for SCZ, many patients have poor efficacy.[3] SCZ patients suffer a 10% to 15% suicide risk during their lifetime.[4] Therefore, there is a great need to find better treatments to reduce the effects of these symptoms in order to further meliorate prognosis and increase happiness index.
SCZ is a complicated psychosis involving many genetic factors as well as environmental factors.[5] Literature have reported that SCZ has a heritability about 80%, and first-degree relatives are 5 to 10 times more possible to have SCZ than the ordinary population.[6] In recent years, genome-wide association studies (GWASs) have authenticated many susceptible regions for SCZ.[7]
NRGN gene is located in chromosome 11q24.2.[8] NRGN encodes a post-synaptic protein kinase substrate that is exclusively expressed in human brain, binding to calmodulin (CaM) in the absence of calcium.[9] NRGN is a postsynaptic brain-specific protein that participates in signal transduction and regulates the interaction between calmodulin and calcium by binding to calmodulin.[10] NRGN has an important effect on Ca2+-CaM signaling pathway. NRGN oxidation induced by Ca2+ influx results in post-synaptic activation of CaM-dependent protein kinase II (CaMKII) in CaM, which is related to enhance N-methyl-D-aspartate (NMDA) receptor signaling. Thus, the change of NRGN activity may mediate the effects of NMDA dysfunction involved in the physiopathology of SCZ.[11,12]
In recent years, there have been several reports attempting to prove the association between rs12807809 polymorphism in RNGN gene and SCZ among different populations, but the results are controversial. For example, Sudesh et al reported that rs12807809 showed a significant association in South Indian population (cases = 1005 and controls = 1069).[13] However, the study by Li et al failed to observe an association between rs12807809 and SCZ in Chinese Han population (cases = 2496 and controls = 5184).[14] The reason for the conflicting results might be limited samples, insufficient statistical ability, ethnic specificity, participant heterogeneity. Thus, the meta-analysis aimed at synthetizing current studies on the association of rs12807809 polymorphism in RNGN gene and susceptibility to SCZ.
2. Methods
2.1. Literature search strategy
PubMed, EMBASE, Web of Science, Cochrane Library, and Wanfang were retrieved for relevant studies published before May 2019 by combining the Medical Subject Headings (MeSH) and free words: (Polymorphisms, Genetic OR Genetic Polymorphisms OR Genetic Polymorphism OR Polymorphism OR Polymorphisms OR variant OR variation OR mutant OR mutation OR “Polymorphism, Genetic”[Mesh]) AND (Schizophrenias OR Schizophrenic Disorders OR Disorder, Schizophrenic OR Disorders, Schizophrenic OR Schizophrenic Disorder OR Dementia Praecox OR “Schizophrenia”[Mesh]) AND (P17 Protein Kinase C Substrate OR RC3 Protein OR NRGN OR “Neurogranin”[Mesh] OR Neurogranin). Studies were regarded eligible if they examined the relation between rs12807809 polymorphism in RNGN gene and SCZ. Furthermore, we conducted a manual retrieval of the bibliography from related publications to obtain potential researches. The meta-analysis did not involve data related to patient personal information and therefore does not require ethical approval.
2.2. Inclusion and exclusion criteria
The criteria below need to be satisfied for inclusion studies:
-
(1)
published studies on the correlation of rs12807809 in the RNGN gene and SCZ;
-
(2)
case-control studies that included at least 50 cases and 50 controls;
-
(3)
healthy control subjects without any history of psychosis or family history of mental disorder;
-
(4)
studies with sufficient data to compute OR and 95% CI.
Exclusion criteria are as follows:
-
(1)
animal studies, expert opinions, reviews, and case studies;
-
(2)
pedigree studies;
-
(3)
studies without available allele and genotype frequencies.
2.3. Methodological quality assessment
Two reviewers (L Jin and Z An) utilized Newcastle-Ottawa scale (NOS) to evaluate the methodological quality of inclusion studies.[15] Guided by the Star system, an individual study was accessed from three broad perspectives: the selection of cases and controls, the comparability of cases and controls, and the determination of exposure or outcome of interest in case-control studies. Disagreements between reviewers were settled through discussion.
2.4. Data extraction
The data extraction of present study was independently performed by 2 reviewers. Information collected included first author, year of publication, country, ethnicity of study population, diagnostic criteria for SCZ, number of case and control subjects, allele and genotype frequencies of cases and controls. With regard to disagreements that emerged throughout the process, 2 investigators reviewed the literature and discussed with each other until consensus was reached.
2.5. Statistical analysis
The relationship rs12807809 polymorphism in RNGN and SCZ susceptibility was appraised by pooled ORs and 95% CIs in allelic comparison (T vs C), homozygous comparison (TT vs CC), heterozygous comparison (TC vs CC), dominant model (TT+TC vs CC), and recessive model (TT vs TC + CC). Z-test was employed to ascertain the summary OR results, and P < .05 was considered statistically significant. The data analysis was achieved using Review Manager 5.3 software (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
2.6. Heterogeneity and publication bias
Q-statistical test and I2 test are applied to appraise the heterogeneity across included studies, where test result was I2 ≥ 50% and P < .1 indicated the presence of heterogeneity.[16] When there was no significant heterogeneity (P > .1 or I2 < 50%) across studies, a fixed-effect model was selected. Conversely, the random-effect model was chosen. If there was heterogeneity, we would conduct a stratified analysis according to ethnicity to further determine the potential source of heterogeneity. Publication biases were examined by Funnel plot and asymmetry plots implied possible publication bias.[17] We conducted leave-one-out sensitivity analysis to appraise the stability of summary results.
3. Results
3.1. Literature search
Figure 1 displayed the literature identification and selection process. We identified 104 relevant literature after combining the search results. After removing the duplicate ones, 63 publications were retained. After scanning the titles and abstracts, a further 51 irrelevant studies were eliminated. Subsequently, the full-text review of the remaining 12 articles were performed to inspect their qualification according to the predetermined inclusion criteria, and 4 studies were excluded. Finally, 8 studies were considered to be qualified for the meta-analysis.
Figure 1.
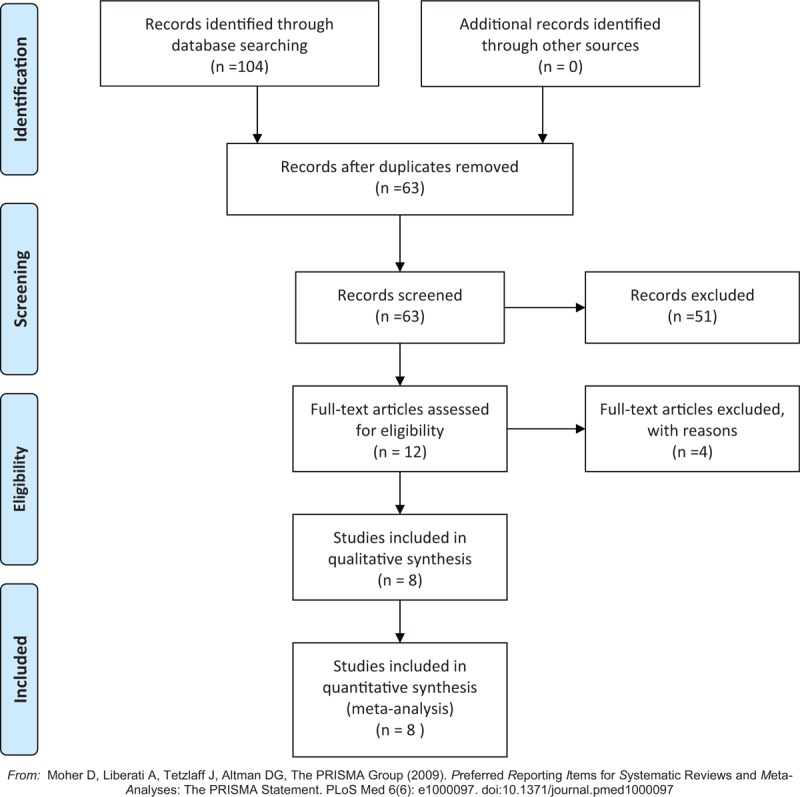
Flow diagram of literature retrieval and selection.
3.2. Study characteristics
Eight case-control studies with a total of 47,335 subjects (12,552 SCZ cases and 34,783 healthy controls) were included in this study.[9,13,18–23] The sample sizes varied from 158 to 12,080. Table 1 generalized the main characteristics and allele and genotype frequencies of inclusion studies. Two studies were conducted among Caucasian population, and the others were across Asian population. The study by Zhang et al focused on the association between SNP rs12807809 and resting-state hippocampal functional connectivity in SCZ.[18] The study by Shen et al carried out a correlation and functional study between 7 common SNPs in NRGN gene and SCZ.[19] Wen et al's study investigated a multi-disease association between 3 SNPs in NRGN and Chinese patients with SCZ, depression and bipolar disorder.[20] All inclusion studies accorded with HWE. NOS scores of inclusion studies were greater than 6, which indicated they had a good methodological quality (Table 2).
Table 1.
Main characteristics of included studies and genotype frequencies of cases and controls.

Table 2.
Quality assessment of included studies according to the Newcastle-Ottawa Scale.

3.3. Meta-analysis and subgroup-analysis
The summary data under allelic model (T vs C, OR = 1.10, 95%CI: 1.04–1.17, P = .0005; Fig. 2) indicated a significant association between rs12807809 and SCZ in the overall population. However, none significant correlation was observed in homozygous, heterozygous, dominant model or recessive models (Table 3). We performed subgroup analysis by ethnicity, the result revealed a statistically significant association between SNP rs12807809 and SCZ risk in Caucasian population (T vs C, OR = 1.14, 95%CI, 1.08–1.20, P < .00001; Fig. 3), but not in Asian population. Because of insufficient data, we did not analyze the association between rs12807809 and SCZ in Caucasians under homozygous, heterozygous, dominant, and recessive models. We analyzed the association between rs12807809 variant and risk of SCZ in the Asian population, but no significant association was observed.
Figure 2.
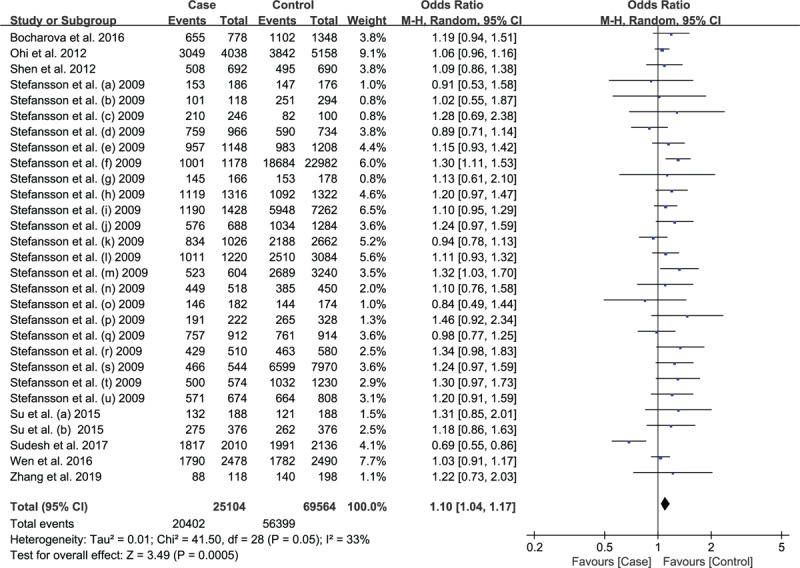
Calculated OR and 95% CI for the association rs12807809 polymorphism and Schizophrenia risk in the allelic model for overall populations. Forest plot of association between rs12807809 and risk of Schizophrenia for allelic model (T vs C).
Table 3.
The pooled ORs and 95% CIs for the association between rs12807809 polymorphism in NRGN gene and Schizophrenia susceptibility.

Figure 3.
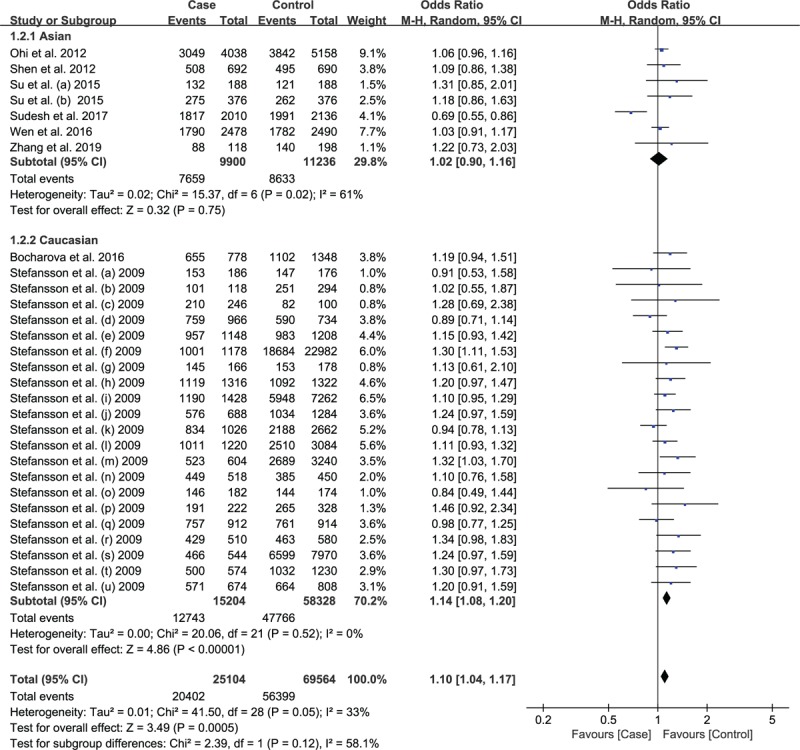
Forest plot of rs12807809 polymorphism and risk of Schizophrenia using a random-effect model: subgroup-analysis by ethnicity.
3.4. Sensitivity analysis
We removed each study in this study to judge the effect of individual dataset on the summary ORs. When the study by Sudesh et al was removed, the heterogeneity changed from light heterogeneity (P = .05; I2 = 33%) to non-heterogeneity (P = .61, I2 = 0%) while the pooled OR value did not change much (from OR = 1.10 95%CI: 1.05–1.14 to OR = 1.11 95%CI: 1.07–1.16, Fig. 4) under allelic model. But for recessive model, the overall effect reversed without no heterogeneity (Fig. 5). After the deletion of study by Sudesh et al, the pooled ORs did not change significantly for homozygous, heterozygous and dominant model. After removing this article, the effects on the results of the 5 models are different, so this article is retained. After careful reinvestigation, it was found that there was pedigree study in the case group, of which 25.2% had consanguinity and 32.4% had SCZ family history, which may result in high heterogeneity.
Figure 4.
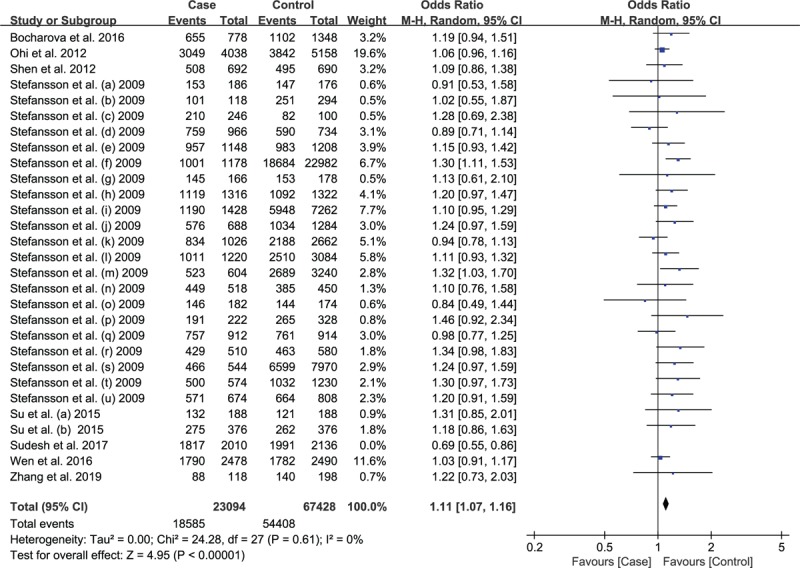
Association between rs12807809 polymorphism and risk of Schizophrenia under allelic model with Sudesh et al's study excluded.
Figure 5.
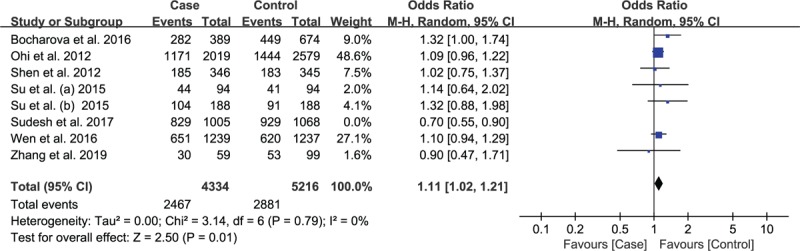
Association between rs12807809 polymorphism and risk of Schizophrenia under recessive model with Sudesh et al's study excluded.
3.5. Publication bias
The publication bias of the inclusion studies was assessed by Funnel plot. There was no obvious asymmetry according to shape of the funnel plots (Fig. 6).
Figure 6.
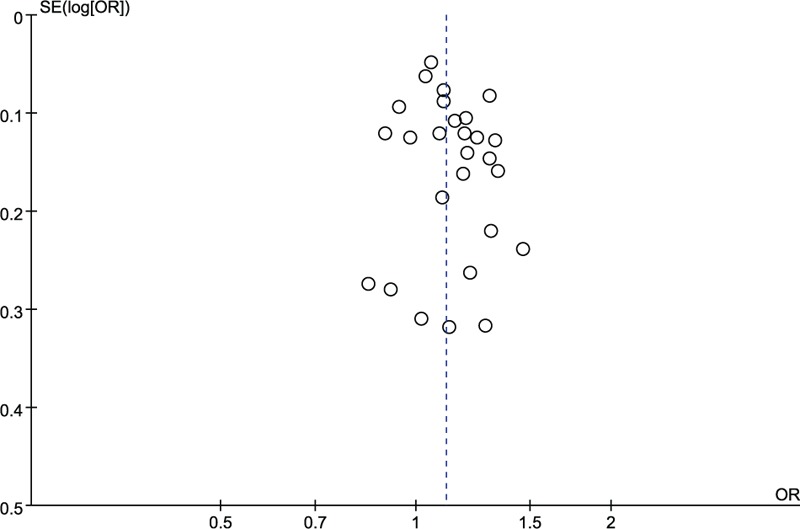
Funnel plot of rs12807809 polymorphism and risk of Schizophrenia.
4. Discussion
With the development of society, mental health has received increasing attention. Mental health is as important as physical health. SCZ is a chronic and recurrent mental illness that causes high morbidity and mortality and a huge emotional and financial burden on society.[24] Albeit the concrete pathogenesis of SCZ is still unclear, genetic risk factors play an essential role in SCZ. Identifying polymorphisms in potentially pathogenic genes enable us to forecast disease and take preventions. GWAS have identified many variants that are related to SCZ, where NRGN gene is one of the most commonly investigated candidate genes.
NRGN is largely expressed in brain regions that are crucial for cognitive functions, particularly in CA1 pyramidal neurons in the hippocampus.[20] Furthermore, NRGN gene knockout mice shows neurological and behavioral deficits related to learning and memory.[25] Broadbent et al found NRGN immunostaining decreased in areas 9 and 32 of prefrontal cortex involved in advanced cognitive function and working memory in postmortem SCZ brains.[26] These evidences indicate NRGN gene may play an important part in learning and memory. NRGN gene has been proved to play a crucial role in synaptic signaling, plasticity, neurodevelopment, learning, and memory.[27]
Besides, there have been several studies investigated the effects of SNP rs12807809. An fMRI study carried out in 94 healthy subjects reported TT homozygous carriers of rs12807809 showed higher activation than C-carriers in the anterior cingulate cortex during episodic memory encoding.[28] Pohlack’ study found the hippocampal activity of homozygous T-allele carriers was impaired during the acquisition of situational fear.[29] Similarly, a recent study has shown that NRGN rs12807809 TT genotype was associated with abnormal hippocampal functional connection at rest in SCZ.[18] Taken together, these evidences suggest that rs12807809 is related to the function of hippocampus and cingulate gyrus. The effect of rs12807809 on the hippocampus function is likely to be one of the reasons for the change of cognitive function of SCZ.[30] However, other studies showed discordant results. Donohoe et al failed to detect significant difference between TT homozygotes and C-carriers on neuropsychological performance.[31] Therefore, more studies on rs12807809 are extremely necessary in order to fully explore the potential of rs12807809 TT genotype as a SCZ biomarker, which will contribute to the clinical diagnosis of the disease and take preventions.
A series of studies have been conducted to detect the relationship between rs12807809 and SCZ vulnerability, whereas some studies indicated that rs12807809 was related to SCZ risk, others did not observe an association. A meta-analysis of these studies could provide a more convincing result on the association between rs12807809 and SCZ vulnerability. The present study involved 12552 patients and 34783 controls, and the findings suggested that rs12807809 was significantly correlated with SCZ vulnerability in allelic model. However, in the subgroup analysis of ethnicity stratification, a significant association of rs12807809 with SCZ susceptibility was observed only in the Caucasian population, but not in the Asian population.
It should be noted that Wen et al performed a meta-analysis indicating there was no significant association of rs12807809 with SCZ across Han Chinese population, which is similar with our result of Asian in subgroup analysis.[20] But our study has several methodological strengths. First of all, we attempted to carry out a more comprehensive literature retrieval strategy, and literature search and screening obtained 8 studies containing 12,552 patients and 34,783 healthy controls across Caucasian and Asian, while Wen et al only investigated 4 studies with 4269 patients and 6962 controls from Han Chinese population. We further conducted an ethnicity subgroup analysis to investigate whether there was a race-specific effect in this association. In addition, for each study included, we utilized NOS to conduct a quality assessment, which enabled us to determine the potential bias risk. Besides, our study assessed the HWE of healthy controls of each included study, which also conduced to the creditability of the results. Finally, we executed a sensitivity analysis to confirm the stability and reliability of the results. Therefore, these advantages strongly promote us to draw more accurate and credible conclusions.
Similar to other studies, there were several limitations in this study. First of all, we only searched the literature issued in English and Chinese. Thus, potentially relevant papers published in other languages may not be identified, which might introduce potential selection bias. Secondly, a stratified analysis by ethnicities could not be done under homozygous, heterozygous, dominant and recessive model because insufficient frequencies data. Last, this meta-analysis exclusively concentrated on the association between rs12807809 and SCZ risk without considering gene-gene or gene-environment interactions. Hence, in order to comprehensively illustrate the pathogenesis of SCZ, it is extremely necessary to study the combined interaction of these related genes.
5. Conclusion
Rs12807809 polymorphism in NRGN gene is significantly associated with SCZ susceptibility in Caucasians but not Asians.
Author contributions
Conceptualization: Yongyong Shi.
Data curation: Songnian Fu.
Funding acquisition: QiZhong Yi.
Investigation: Lu Jin.
Methodology: Lu Jin, Zhiguo An.
Project administration: Bin Xu.
Resources: Hongxing Hu.
Software: Lu Jin, Xiao Luo.
Supervision: QiZhong Yi.
Validation: Lu Jin.
Visualization: Lu Jin, Daibin Mu.
Writing – original draft: Lu Jin.
Writing – review & editing: Lu Jin, QiZhong Yi.
Footnotes
Abbreviations: CI = confidence interval, HWE = Hardy-Weinberg Equilibrium, NOS = Newcastle-Ottawa Scale, NRGN = Neurogranin, OR = odds ratio, SCZ = schizophrenia, SNP = single nucleotide polymorphism.
How to cite this article: Jin L, An Z, Xu B, Mu D, Fu S, Hu H, Shi Y, Luo X, Yi Q. The association between rs12807809 polymorphism in neurogranin gene and risk of schizophrenia: A meta-analysis. Medicine. 2019;98:51(e18518).
The present study was supported by National Natural Science Foundation of China (Nos. 81660233 and 81360209).
The authors report no conflicts of interest.
References
- [1].Rethelyi JM, Benkovits J, Bitter I. Genes and environments in schizophrenia: The different pieces of a manifold puzzle. Neurosci Biobehav Rev 2013;37:2424–37. [DOI] [PubMed] [Google Scholar]
- [2].Gejman PV, Sanders AR, Kendler KS. Genetics of schizophrenia: new findings and challenges. Annu Rev Genomics Hum Genet 2011;12:121–44. [DOI] [PubMed] [Google Scholar]
- [3].Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014;511:421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhou Y, Dong F, Lanz TA, et al. Interactome analysis reveals ZNF804A, a schizophrenia risk gene, as a novel component of protein translational machinery critical for embryonic neurodevelopment. Mol Psychiatry 2018;23:952–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Girard SL, Dion PA, Rouleau GA. Schizophrenia genetics: putting all the pieces together. Curr Neurol Neurosci Rep 2012;12:261–6. [DOI] [PubMed] [Google Scholar]
- [6].Nieratschker V, Nothen MM, Rietschel M. New genetic findings in schizophrenia: is there still room for the dopamine hypothesis of schizophrenia? Front Behav Neurosci 2010;4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jiang H, Qiao F, Li Z, et al. Evaluating the association between CACNA1C rs1006737 and schizophrenia risk: a meta-analysis. Asia Pac Psychiatry 2015;7:260–7. [DOI] [PubMed] [Google Scholar]
- [8].Smith RL, Knight D, Williams H, et al. Analysis of neurogranin (NRGN) in schizophrenia. Am J Med Genet B Neuropsychiatr Genet 2011;156b:532–5. [DOI] [PubMed] [Google Scholar]
- [9].Stefansson H, Ophoff RA, Steinberg S, et al. Common variants conferring risk of schizophrenia. Nature 2009;460:744–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].John JP, Thirunavukkarasu P, Halahalli HN, et al. A systematic review of the effect of genes mediating neurodevelopment and neurotransmission on brain morphology: Focus on schizophrenia 2015;21:1–26. [Google Scholar]
- [11].Ohi K, Hashimoto R, Yasuda Y, et al. Influence of the NRGN gene on intellectual ability in schizophrenia. J Hum Genet 2013;58:700–5. [DOI] [PubMed] [Google Scholar]
- [12].Ohi K, Hashimoto R, Yasuda Y, et al. Impact of the genome wide supported NRGN gene on anterior cingulate morphology in schizophrenia. PloS One 2012;7:e29780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sudesh R, Priyadarshini T, Preeti R, et al. Minor allele C of rs12807809 polymorphism in NRGN contributes to the severity of psychosis in patients with Schizophrenia in South Indian population. Neurosci Lett 2017;649:107–11. [DOI] [PubMed] [Google Scholar]
- [14].Li T, Li Z, Chen P, et al. Common variants in major histocompatibility complex region and TCF4 gene are significantly associated with schizophrenia in Han Chinese. Biol Psychiatry 2010;68:671–3. [DOI] [PubMed] [Google Scholar]
- [15].Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in metaanalyses. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed July 1, 2019. [Google Scholar]
- [16].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ 2007;176:1091–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang Y, Gong X, Yin Z, et al. Association between NRGN gene polymorphism and resting-state hippocampal functional connectivity in schizophrenia. BMC Psychiatry 2019;19:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shen YC, Tsai HM, Cheng MC, et al. Genetic and functional analysis of the gene encoding neurogranin in schizophrenia. Schizophr Res 2012;137:7–13. [DOI] [PubMed] [Google Scholar]
- [20].Wen Z, Chen J, Khan RA, et al. Polymorphisms in NRGN are associated with schizophrenia, major depressive disorder and bipolar disorder in the Han Chinese population. J Affect Disord 2016;194:180–7. [DOI] [PubMed] [Google Scholar]
- [21].Bocharova AV, Stepanov VA, Marusin AV, et al. Association study of genetic markers of schizophrenia and its cognitive endophenotypes. Genetika 2017;53:100–8. [PubMed] [Google Scholar]
- [22].Ohi K, Hashimoto R, Yasuda Y, et al. Functional genetic variation at the NRGN gene and schizophrenia: evidence from a gene-based case-control study and gene expression analysis. Am J Med Genet B Neuropsychiatr Genet 2012;159b:405–13. [DOI] [PubMed] [Google Scholar]
- [23].Su L, Long J, Pan R, et al. Influence of NRGN rs12807809 polymorphism on symptom severity in individuals with schizophrenia in the Han population but not the Zhuang population of south China. Acta Neuropsychiatr 2015;27:221–7. [DOI] [PubMed] [Google Scholar]
- [24].Gurung R, Prata DP. What is the impact of genome-wide supported risk variants for schizophrenia and bipolar disorder on brain structure and function? A systematic review. Psychol Med 2015;45:2461–80. [DOI] [PubMed] [Google Scholar]
- [25].Walton E, Geisler D, Hass J, et al. The impact of genome-wide supported schizophrenia risk variants in the neurogranin gene on brain structure and function. PloS One 2013;8:e76815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Broadbelt K, Ramprasaud A, Jones LB. Evidence of altered neurogranin immunoreactivity in areas 9 and 32 of schizophrenic prefrontal cortex. Schizophr Res 2006;87:6–14. [DOI] [PubMed] [Google Scholar]
- [27].Thong JY, Qiu A, Sum MY, et al. Effects of the neurogranin variant rs12807809 on thalamocortical morphology in schizophrenia. PloS One 2013;8:e85603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Krug A, Krach S, Jansen A, et al. The effect of neurogranin on neural correlates of episodic memory encoding and retrieval. Schizophr Bull 2013;39:141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pohlack ST, Nees F, Ruttorf M, et al. Risk variant for schizophrenia in the neurogranin gene impacts on hippocampus activation during contextual fear conditioning. Mol Psychiatry 2011;16:1072–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rose EJ, Morris DW, Fahey C, et al. The effect of the neurogranin schizophrenia risk variant rs12807809 on brain structure and function. Twin Res Hum Genet 2012;15:296–303. [DOI] [PubMed] [Google Scholar]
- [31].Donohoe G, Walters J, Morris DW, et al. A neuropsychological investigation of the genome wide associated schizophrenia risk variant NRGN rs12807809. Schizophr Res 2011;125:304–6. [DOI] [PubMed] [Google Scholar]


