Abstract
To determine characteristics of diabetic macular edema patients with serous retinal detachment (SRD).
We classified naïve diabetic macular edema (DME) patients with or without SRD, and compared their baseline characteristics; intravitreal bevacizumab (IVB) responsiveness; aqueous concentrations of IL (interleukin)-1β, -2, -8, -10, -17, placental growth factor (PlGF), and vascular endothelial growth factor (VEGF). In addition, factors associated with the existence of SRD were identified.
Of the 64 DME patients, 14 had SRD. The average levels of aqueous VEGF and PlGF were significantly higher in the SRD group than in the control group (P = .022 and P = .041, respectively). The best-corrected visual acuity (BCVA) and central subfield thickness (CST) did not differ significantly between the 2 groups at baseline or after 3 consecutive monthly IVBs. In multivariate logistic regression analysis, the level of aqueous VEGF was the only factor associated with the existence of SRD (odds ratio: 1.03; P = .038).
Rather than aqueous inflammatory cytokines, levels of aqueous VEGFs were associated with the occurrence of SRD in DME patients. In terms of prognosis, the existence of SRD was not related with BCVA or CST changes.
Keywords: center-involving diabetic macular edema, diabetic macular edema, diabetic retinopatathy, intravitreal bevacizumab, serous retinal detachment, vascular endothelial growth factors
1. Introduction
Diabetic macular edema (DME) is one of the most significant causes of visual disturbance in patients with diabetic retinopathy (DR).[1] DME may be caused by damage to the blood–retina barrier induced by metabolic changes and inflammation.[1,2] DME is affected by not only cells in the retina but also the expressions of various molecules, including interleukins (ILs), vascular endothelial growth factors (VEGFs), tumor necrosis factor-α, and matrix metalloproteinase.[2–4]
Classification of DME into several types using various imaging tools, including fluorescein angiography, optical coherence tomography (OCT), and OCT angiography, has been investigated to identify causes, predict prognosis, and select appropriate treatments.[5–8] Of these techniques, OCT is a fast and noninvasive tool to quantify macular profiles. Recent studies using OCT have morphologically classified DME as cystoid macular edema (CME), diffuse retinal thickening (DRT), or serous retinal detachment (SRD).[9] Of these morphological classifications, the prognosis and mechanism of SRD is controversial. Some studies have reported that DME with SRD has a better responsiveness or better prognosis after anti-VEGF treatments, suggesting that there could be an association between VEGF and SRD.[10,11] However, there are other studies that reported contradicting results showing no association between inflammation and SRD.[12–14]
Therefore, in this study, we grouped naïve DME patients according to the presence of SRD, and compared their systemic and ocular factors including the levels of VEGFs and inflammatory cytokines in the aqueous humor. Then, we identified factors associated with SRD.
2. Methods
The study protocol adhered to the tenets of the Declaration of Helsinki. The protocol was approved by the institutional review/ethics board of the Catholic University of Korea. All participants gave written informed consent for the use of their clinical records.
We enrolled treatment-naïve center-involving DME (CIDME) eyes of central subfield thickness (CST) ≥300 μm from June, 2016 to November, 2016.[15] The criteria of exclusion included macular edema due to other causes including an epiretinal membrane or vitreo-macular traction. We also excluded eyes with histories of uveitis or intraocular surgery.
We measured glycated hemoglobin levels, and all patients took ophthalmic examinations, including measurements of the best corrected visual acuity (BCVA) and fundus examination. The CST was measured using OCT (Cirrus High-Definition OCT; Carl Zeiss Meditec, Dublin, CA) and axial length was measured using an IOL Master (Carl Zeiss Meditec). The hyperreflective foci (HF) were manually measured within 1500 μm, and ellipsoid zone (EZ) disruptions were manually measured within 1000 μm using a horizontal scan centered on the fovea.[6,16] EZ disruption was graded as 0 in case of intact, 1 in case of focal disruption ≤200 μm in length, and 2 in case of disruption >200 μm in length.
2.1. Assay of cytokines and growth factors
We collected aqueous fluid specimens before first intravitreal bevacizumab (IVB) injection, and measured the concentrations of IL-1β, -2, -8, -10, -17, placental growth factor (PlGF), and VEGF in 75 μL aqueous humor. The antibodies were immobilized on beads, and samples with 75 μL Calibrator Diluent RD6–52 (R&D Systems, Minneapolis, MN) were added to the preparations. And the samples were incubated for 2 hours after adding beads, for 1 hour after adding detection antibodies, and for half-hour after adding the streptavidin-phycoerythrin reagent. Samples were read using the Luminex xMAP system (Luminex, Austin, TX).[17]
2.2. Statistical evaluation
Statistical analyses were performed using SPSS statistical software for Windows, version 20.0 (SPSS, Chicago, IL). The t test, Mann–Whitney U test, and chi-square test were used to compare the values or the ratios of the patient subgroups. Logistic regression was employed to identify factors associated with SRD occurrence.
3. Results
We enrolled 64 treatment-naïve CIDME eyes of 64 patients. The mean age was 55.85 ± 9.35 years, and there were 30 men and 34 women. In the DR staging, 47 patients had proliferative DR (73.44%) and 17 patients had non-proliferative DR (26.56%). All the patients who showed proliferative DR had received panretinal photocoagulation. The mean BCVA (LogMAR) was 0.45 ± 0.27, and the mean CST was 416.03 ± 81.14 μm at baseline. Based on DME morphology, 33 patients had CME and 31 had DRT. The systemic and ocular characteristics of the patients are summarized in Table 1.
Table 1.
Demographics and clinical characteristics of DME patients.
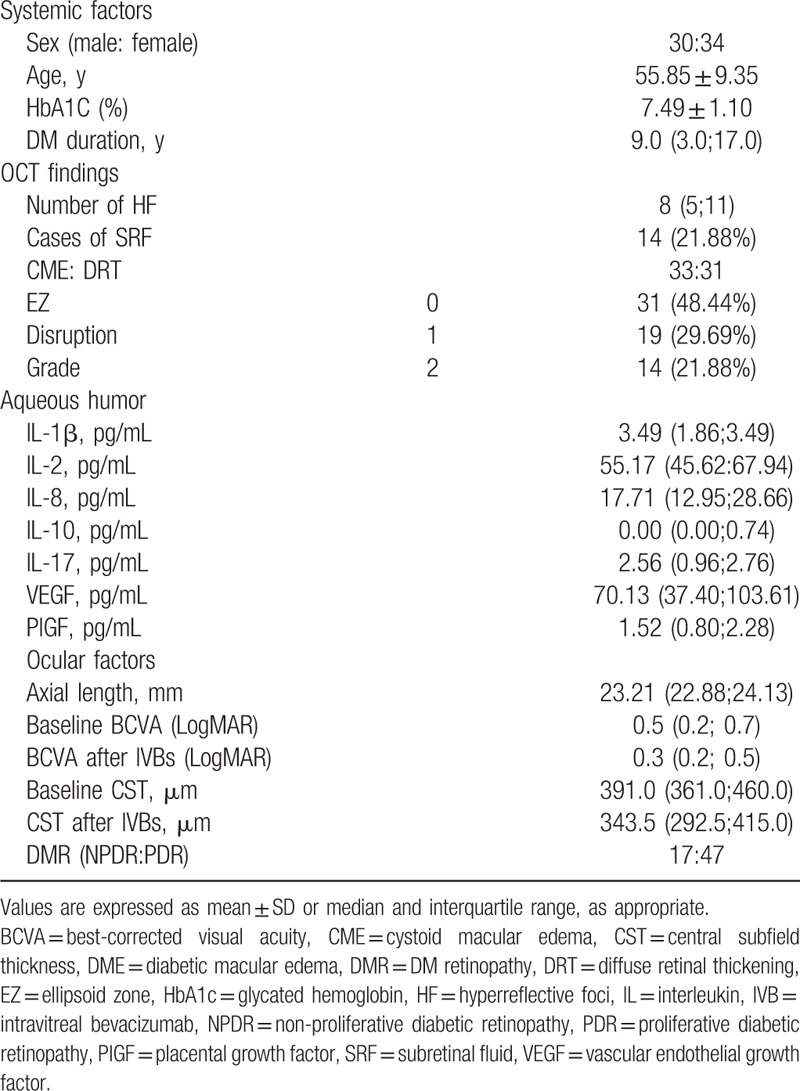
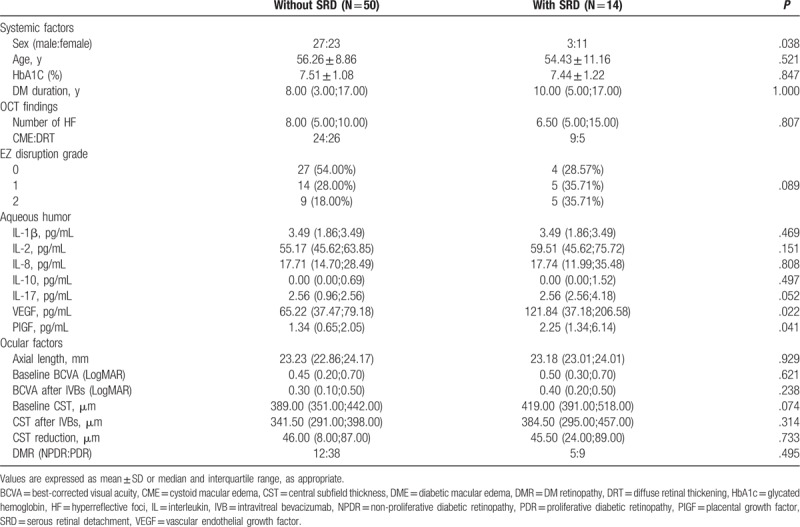
There were 50 eyes with DME without SRD and 14 eyes with SRD. There was a significant difference in sex distribution, but no significant differences were found in age, glycated hemoglobin level, duration of diabetes, DR stage, BCVA, or CST between the 2 groups. The OCT findings such as number of HF and EZ grade also showed no significant differences. The BCVA and CST after 3 consecutive monthly IVB injections did not significantly differ (P = .238 and P = .314, respectively). In the comparison of cytokine levels in the aqueous humor, VEGF and PlGF levels were significantly higher in the SRD group (P = .022 and P = .041, respectively) (Table 2).
Table 2.
Demographics and clinical characteristics of DME patients depending on the existence of SRD.
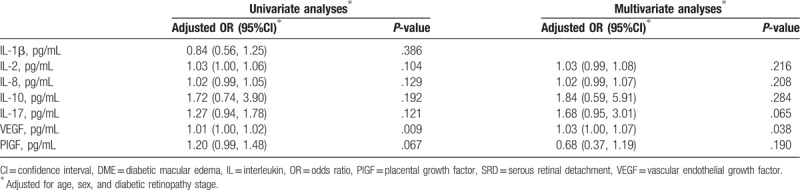
The factors identified as associated with SRD are summarized in Table 3. In univariate and multivariate logistic analyses, VEGF level was the only factor associated with the existence of SRD (odds ratio [OR]: 1.01, P = .009 and OR: 1.03, P = .038, respectively).
Table 3.
Results of logistic regression of the effects of SRD in DME patients.
4. Discussion
Many studies have reported that DME is mediated by inflammation and VEGFs, and their actions are closely interconnected.[2,18] DME treatment by suppressing VEGF levels or the controlling inflammation has been spotlighted. Studies using imaging tools or biomarkers to find early and effective treatments among the various options have been reported.[16,19] This study also focused on finding more effective treatment regimen among various treatment options by using an imaging tool and biomarker; we are the first study to find that SRD in DME is associated with VEGF levels.
Several treatment options for DME are now available. Photocoagulation of the leaking point with a focal laser is used to treat non-CIDME patients.[20] Removal of traction, clearing the inflammatory cytokines and growth factors, and increasing the oxygen levels of the vitreous and retina via vitrectomy have also been used to treat refractory DME.[8,21–23] However, the main treatment of DME is intravitreal injection of anti-VEGF agents or steroids.[24–26] Many studies have shown that visual disturbances are associated with the degree of macular thickness, and long-lasting and chronic DME can compromise visual functions.[27–29] Thus, early and optimal treatments are required to recover and acquire a normal macular contour. If proper treatments are delayed, permanent visual disturbance can occur.[29,30] Thus, it is necessary to identify more relevant mechanisms in DME subtypes, so that customized treatments can be selected. Although SRD is one of the most common manifestations in DME, the mechanism of action is still unclear. We found SRD group had significantly higher aqueous levels of VEGF and PlGF (P = .022 and P = .041, respectively). Additionally, in the logistic analyses, the occurrence of SRD was significantly associated with VEGF levels (OR: 1.03; P = .038). The PlGF, a member of the VEGF family, is induced by ischemic reinal condition, and has a key role in angiogenesis and vasculogenesis in retina.[31] Based on these results, SRD seems to be more associated with VEGFs than with inflammation.
Although vitreous samples adequately reflect retinal status,[32] obtaining vitreous samples is invasive and difficult because few patients are treated with vitrectomy due to DME. In addition, posterior vitreous detachment or blood contamination could compromise data quality of vitreous samples. The aqueous humor, which can easily be obtained during intravitreal injection, can also reflect retinal status. The levels of many cytokines are increased under conditions such as retinal hypoxia or inflammation,[33,34] and most studies that have used aqueous humor samples have shown that the concentrations of various molecules from DME patients differ from those of controls.[17,35] However, few studies have investigated the associations between responsiveness of treatment and the levels of these biomarkers.[19] We suggest that aqueous humor samples can be used to identify the major mechanisms of DME. Our results could be useful in selecting appropriate treatments or predicting the prognoses of patients.
There is a consensus that the subretinal fluid in DME is resolved with anti-VEGF treatment,[10] but the prognosis regarding BCVA and CST is controversial. Some studies have reported that SRD patients showed better responsiveness or prognoses in CST reduction or BCVA improvement with anti-VEGF treatments,[10,36,37] but others have reported no better responsiveness with the same treatments.[11,14] The mechanisms of SRD are also controversial. In contrast to our results, some studies have reported that there is an association between SRD and inflammation[12,38] and that steroid implants can result in a positive response.[39,40] On the other hand, other studies have shown that there is an association between systemic status or glycemic control and SRD.[41,42] Although there was no remaining SRD after 3 consecutive IVBs in all patients in the SRD group (Fig. 1), the degree of CST reduction in these patients did not differ from that of the DME patients without SRD in our study, and we did not detect a difference in glycated hemoglobin levels between the 2 groups. As the mechanism of DME is complicated and there is a lack of studies about the responsiveness of DME, more investigations and studies are needed to clarify the origin and responsiveness of SRD in DME.
Figure 1.
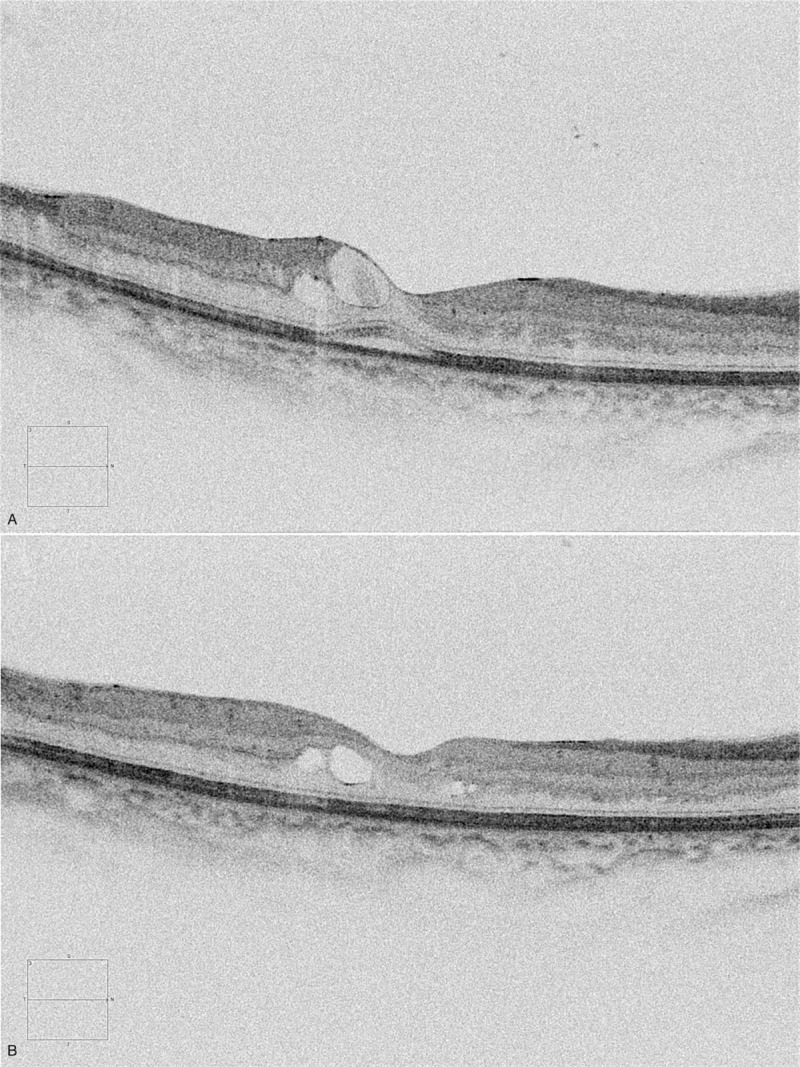
A representative patient who had diabetic macular edema (DME) with a serous retinal detachment (SRD) pattern. (A) The baseline spectral domain-optical coherence tomography image shows center-involving DME with an SRD pattern. (B) After 3 consecutive intravitreal bevacizumab injections, the SRD pattern disappeared and the DME decreased.
Our study had some limitations. First, our sample size was relatively small and the follow-up duration was short. The aqueous levels showed no significant difference in SRD group may be attributable to the small number of control patients. We also judge that there is significantly higher ratio of women in SRD group by chance due to small sample size. Long-term changes in CST and BCVA must be evaluated with the treatments. In addition, although we made an effort to statistically factor in the systemic and ocular status of patients, other factors we did not consider might have affected our results. Second, the relationships between cytokine levels and other imaging techniques like fluorescein angiography or OCT angiography should be studied in terms of DME pathogenesis.[8]
In summary, the occurrence of SRD was associated with VEGF levels. Additional studies with more patients are required to confirm our results and to elucidate the pathogenesis of DME, which may provide the basis for novel therapeutic approaches.
Acknowledgments
The authors acknowledge financial support from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2017R1C1B5017408). This research was also supported by a Grant of Translational R&D Project through the Institute for Bio-Medical Convergence, Incheon St. Mary's Hospital, The Catholic University of Korea.
Author contributions
Conceptualization: Jin-woo Kwon.
Data curation: Jin-woo Kwon.
Formal analysis: Jin-woo Kwon.
Funding acquisition: Hyung Bin Hwang.
Investigation: Hyung Bin Hwang.
Supervision: Hyung Bin Hwang, Donghyun Jee.
Validation: Jin-woo Kwon.
Visualization: Jin-woo Kwon.
Writing – original draft: Jin-woo Kwon.
Jin-woo Kwon orcid: 0000-0003-2093-4284.
Footnotes
Abbreviations: BCVA = best corrected visual acuity, CIDME = center-involving diabetic macular edema, CME = cystoid macular edema, CST = central subfield thickness, DME = diabetic macular edema, DR = diabetic retinopathy, DRT = diffuse retinal thickening, EZ = ellipsoid zone, HF = hyperreflective foci, IL = interleukins, OCT = optical coherence tomography, PlGF = placental growth factor, SRD = serous retinal detachment, VEGF = vascular endothelial growth factors.
How to cite this article: Hwang HB, Jee D, Kwon Jw. Characteristics of diabetic macular edema patients with serous retinal detachment. Medicine. 2019;98:51(e18333).
No author has a financial and proprietary interest in any material or method mentioned.
The authors have no conflicts of interest to disclose.
References
- [1].Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology 2015;122:1375–94. [DOI] [PubMed] [Google Scholar]
- [2].Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res 2011;30:343–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Aveleira CA, Lin CM, Abcouwer SF, et al. TNF-alpha signals through PKCzeta/NF-kappaB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes 2010;59:2872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kwon JW, Choi JA, Jee D. Matrix Metalloproteinase-1 and matrix Metalloproteinase-9 in the aqueous humor of diabetic macular edema patients. PLoS One 2016;11:e0159720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yeung L, Lima VC, Garcia P, et al. Correlation between spectral domain optical coherence tomography findings and fluorescein angiography patterns in diabetic macular edema. Ophthalmology 2009;116:1158–67. [DOI] [PubMed] [Google Scholar]
- [6].Maheshwary AS, Oster SF, Yuson RM, et al. The association between percent disruption of the photoreceptor inner segment-outer segment junction and visual acuity in diabetic macular edema. Am J Ophthalmol 2010;150:63.e1–7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kozak I, El-Emam SY, Cheng L, et al. Fluorescein angiography versus optical coherence tomography-guided planning for macular laser photocoagulation in diabetic macular edema. Retina 2014;34:1600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee J, Moon BG, Cho AR, et al. Optical coherence tomography angiography of DME and its association with anti-VEGF treatment response. Ophthalmology 2016;123:2368–75. [DOI] [PubMed] [Google Scholar]
- [9].Kim BY, Smith SD, Kaiser PK. Optical coherence tomographic patterns of diabetic macular edema. Am J Ophthalmol 2006;142:405–12. [DOI] [PubMed] [Google Scholar]
- [10].Sophie R, Lu N, Campochiaro PA. Predictors of functional and anatomic outcomes in patients with diabetic macular edema treated with ranibizumab. Ophthalmology 2015;122:1395–401. [DOI] [PubMed] [Google Scholar]
- [11].Shimura M, Yasuda K, Yasuda M, et al. Visual outcome after intravitreal bevacizumab depends on the optical coherence tomographic patterns of patients with diffuse diabetic macular edema. Retina 2013;33:740–7. [DOI] [PubMed] [Google Scholar]
- [12].Sonoda S, Sakamoto T, Yamashita T, et al. Retinal morphologic changes and concentrations of cytokines in eyes with diabetic macular edema. Retina 2014;34:741–8. [DOI] [PubMed] [Google Scholar]
- [13].Kim M, Kim Y, Lee SJ. Comparison of aqueous concentrations of angiogenic and in fl ammatory cytokines based on optical coherence tomography patterns of diabetic macular edema. Indian J Ophthalmol 2015;63:312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kaya M, Karahan E, Ozturk T, et al. Effectiveness of intravitreal ranibizumab for diabetic macular edema with serous retinal detachment. Korean J Ophthalmol 2018;32:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sadda SR, Tan O, Walsh AC, et al. Automated detection of clinically significant macular edema by grid scanning optical coherence tomography. Ophthalmology 2006;113:1187.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kang JW, Chung H, Chan Kim H. Correlation of optical coherence tomographic hyperreflective foci with visual outcomes in different patterns of diabetic macular edema. Retina 2016;36:1630–9. [DOI] [PubMed] [Google Scholar]
- [17].Jonas JB, Jonas RA, Neumaier M, et al. Cytokine concentration in aqueous humor of eyes with diabetic macular edema. Retina 2012;32:2150–7. [DOI] [PubMed] [Google Scholar]
- [18].Wang J, Xu X, Elliott MH, et al. Muller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes 2010;59:2297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kwon JW, Jee D. Aqueous humor cytokine levels in patients with diabetic macular edema refractory to anti-VEGF treatment. PLoS One 2018;13:e0203408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Scott IU, Edwards AR, Beck RW, et al. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology 2007;114:1860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Haller JA, Qin H, Apte RS, et al. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology 2010;117:1087.e3–93.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Uji A, Murakami T, Suzuma K, et al. Influence of vitrectomy surgery on the integrity of outer retinal layers in diabetic macular edema. Retina 2018;38:163–72. [DOI] [PubMed] [Google Scholar]
- [23].Jackson TL, Nicod E, Angelis A, et al. Pars plana vitrectomy for diabetic macular edema: a systematic review, meta-analysis, and synthesis of safety literature. Retina 2017;37:886–95. [DOI] [PubMed] [Google Scholar]
- [24].Boyer DS, Yoon YH, Belfort R, Jr, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 2014;121:1904–14. [DOI] [PubMed] [Google Scholar]
- [25].Gillies MC, Lim LL, Campain A, et al. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology 2014;121:2473–81. [DOI] [PubMed] [Google Scholar]
- [26].Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 2015;372:1193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Browning DJ, Glassman AR, Aiello LP, et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology 2007;114:525–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chakravarthy U, Yang Y, Lotery A, et al. Clinical evidence of the multifactorial nature of diabetic macular edema. Retina 2018;38:343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bressler SB, Ayala AR, Bressler NM, et al. Persistent macular thickening after ranibizumab treatment for diabetic macular edema with vision impairment. JAMA Ophthalmol 2016;134:278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Boyer DS, Nguyen QD, Brown DM, et al. Outcomes with As-needed ranibizumab after initial monthly therapy: long-term outcomes of the phase III RIDE and RISE trials. Ophthalmology 2015;122:2504.e1–13.e1. [DOI] [PubMed] [Google Scholar]
- [31].Kovacs K, Marra KV, Yu G, et al. Angiogenic and inflammatory vitreous biomarkers associated with increasing levels of retinal ischemia. Invest Ophthalmol Vis Sci 2015;56:6523–30. [DOI] [PubMed] [Google Scholar]
- [32].Iglicki M, Lavaque A, Ozimek M, et al. Biomarkers and predictors for functional and anatomic outcomes for small gauge pars plana vitrectomy and peeling of the internal limiting membrane in naive diabetic macular edema: The VITAL Study. PLoS One 2018;13:e0200365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jung SH, Kim KA, Sohn SW, et al. Association of aqueous humor cytokines with the development of retinal ischemia and recurrent macular edema in retinal vein occlusion. Invest Ophthalmol Vis Sci 2014;55:2290–6. [DOI] [PubMed] [Google Scholar]
- [34].Feng J, Zhao T, Zhang Y, et al. Differences in aqueous concentrations of cytokines in macular edema secondary to branch and central retinal vein occlusion. PLoS One 2013;8:e68149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dong N, Xu B, Chu L, et al. Study of 27 aqueous humor cytokines in type 2 diabetic patients with or without macular edema. PLoS One 2015;10:e0125329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gerendas BS, Prager S, Deak G, et al. Predictive imaging biomarkers relevant for functional and anatomical outcomes during ranibizumab therapy of diabetic macular oedema. Br J Ophthalmol 2018;102:195–203. [DOI] [PubMed] [Google Scholar]
- [37].Fickweiler W, Schauwvlieghe AME, Schlingemann RO, et al. Predictive value of optical coherence tomographic features in the bevacizumab and ranibizumab in patients with diabetic macular edema (BRDME) study. Retina 2018;38:812–9. [DOI] [PubMed] [Google Scholar]
- [38].Yenihayat F, Ozkan B, Kasap M, et al. Vitreous IL-8 and VEGF levels in diabetic macular edema with or without subretinal fluid. Int Ophthalmol 2019;39:821–8. [DOI] [PubMed] [Google Scholar]
- [39].Lee H, Kang KE, Chung H, et al. Prognostic factors for functional and anatomic outcomes in patients with diabetic macular edema treated with dexamethasone implant. Korean J Ophthalmol 2018;32:116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zur D, Iglicki M, Busch C, et al. OCT biomarkers as functional outcome predictors in diabetic macular edema treated with dexamethasone implant. Ophthalmology 2018;125:267–75. [DOI] [PubMed] [Google Scholar]
- [41].Tsai MJ, Hsieh YT, Shen EP, et al. Systemic associations with residual subretinal fluid after ranibizumab in diabetic macular edema. J Ophthalmol 2017;2017:4834201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Turgut B, Gul FC, Ilhan N, et al. Comparison of serum glycosylated hemoglobin levels in patients with diabetic cystoid macular edema with and without serous macular detachment. Indian J Ophthalmol 2010;58:381–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


