Abstract
Background
Healthcare workers (HCWs) have a high risk of tuberculosis (TB) infection. Since August 2017, Korea has mandated the testing of latent TB infection (LTBI) and recommended treatment from HCWs at medical institutions. However, the acceptance/completion rate and adverse events of LTBI treatment have not been analyzed.
Materials and Methods
From February to August 2017, we conducted a retrospective study at a referral university hospital in Korea, to screen the interferon-gamma release assay (IGRA) tests conducted for all HCWs for detecting and treating LTBI. HCWs diagnosed with LTBI were offered a 9-month isoniazid (9H), 3-month isoniazid/rifampin (3HR), or 4-month rifampin regimen. We investigated the acceptance/completion rate, adverse events, and causes of discontinuation or change in LTBI medication. A major adverse event was one wherein a patient had any adverse event ≥grade 3 causing LTBI treatment interruption.
Results
Of the 1,538 HCWs, 1,379 underwent IGRA testing for LTBI. Among them, 13.6% (187/1,379) tested positive and 73.3% (137/187) received treatment. The overall completion rate was 97.8% (134/137). HCWs were significantly more likely to complete first-line therapy with 3HR than with 9H (91.4% vs. 76.7%, P = 0.02). The most common major adverse event was hepatotoxicity (n = 7), followed by thrombocytopenia (n = 1) and anaphylactic shock (n = 1). Hepatotoxicity and hepatotoxicity (≥ grade 2) were more frequent in 9H than in 3HR (39.5% vs. 17.2%, P = 0.006 and 18.6% vs. 3.7%, P = 0.005, respectively). The median time to hepatotoxicity was 96 days (interquartile range, 20 – 103 days).
Conclusion
Completion of first-line therapy for LTBI is more likely with 3HR than with 9H. This might be related to the development of hepatotoxicity after around 3 months of treatment. Anaphylactic shock and platelet count should be carefully monitored in those receiving rifampin-containing regimens.
Keywords: Adverse drug reaction, Isoniazid, Rifampin, Latent tuberculosis infection
Introduction
Healthcare workers (HCWs) form a high-risk group having contact with patients with active pulmonary tuberculosis (TB) [1]. The annual conversion rate of latent TB infection (LTBI) among newly hired HCWs was at least 3% [2,3]. When in contact with patients with active TB, the 3-month conversion rate of LTBI was 9.2% [4]. Therefore, many national policies and guidelines recommend that HCWs should be included in LTBI screening programs [5,6]. The prevalence of LTBI based on analyses using the interferon-gamma release assay (IGRA) is 16–18% for existing HCWs in Korea [7,8]. Since 2017, in accordance with the Tuberculosis Prevention Act, the government of Korea has paid for LTBI screening and treatment for HCWs [9]. Accordingly, the screening and treatment of LTBI has become mandatory for HCWs in Korea.
Currently, the recommended treatment regimens for LTBI include 9 months of daily isoniazid (9H), 3 months of daily isoniazid plus rifampin (3HR), and 4 months of daily rifampin alone (4R). Since 2011, the Centers for Disease Control and Prevention has recommended the rifapentine plus isoniazid regimen for 12 weeks under directly observed therapy (DOT) for the general population [10]. The rifapentine plus isoniazid regimen has been a substitute to the LTBI treatment regimen with a higher completion rate in HCWs even when not utilizing DOT in the United States [11]. However, this regimen is not available in Korea.
Moreover, limited data were available on the acceptance/completion rate and adverse events associated with the available LTBI treatment regimens in Korea [12]. Therefore, the aim of this study was to investigate the acceptance rate, completion rate, characteristics of adverse events by regimen, and rate of discontinuation of LTBI treatment owing to adverse events among HCWs in Korea. In addition, we reviewed our institution's experience on the follow-up intervals for monitoring compliance and adverse events during LTBI treatment according to the different regimens.
Materials and Methods
1. Study population
This study was conducted at a 734-bed referral hospital located in Seoul, Korea. The study protocol was reviewed and approved by the Institutional Review Board of Soonchunhyang University, Seoul Hospital (approval No. 2018-08-008). Informed consent was waived by the board.
Since 2017, the government of Korea has paid for LTBI screening and treatment for HCWs [9]. From February to August 2017, we screened the IGRA tests conducted for all HCWs to detect and treat LTBI and excluded HCWs with a history of TB or LTBI treatment and a previous positive tuberculin skin test result.
2. Study protocol and data collection
We retrospectively investigated age, sex, professions, comorbidity, TB or LTBI treatment history, and TB risk classification of the HCWs. TB risk classification was as follows: the first group was of HCWs working in the department that examine, diagnose, and treat patients with respiratory TB (e.g., pulmonary/infection clinic, respiratory/TB ward, emergency department, medical intensive care unit, bronchoscopy suite, and TB-related laboratory); the second group was of HCWs who served immunocompromised patients (e.g., in an organ transplantation unit or newborn nursery); the third group was of HCWs in regular contact with patients with a low chance of contact with patients with respiratory TB; and the fourth group was of HCWs who do not have contact with patients. All HCWs who were IGRA positive were interviewed by one pulmonologist. An IGRA, the QuantiFERON-TB Gold in-tube test, was performed according to the manufacturer's instructions. A positive result was defined as an interferon concentration ≥0.35 IU/mL and ≥25% of the negative control [13]. Plain chest radiography or computed tomography (CT) was performed to exclude active pulmonary TB. If necessary, sputum acid-fast bacilli staining and culture were performed at least twice to exclude active pulmonary TB.
Three treatment options of LTBI are available in Korea: isoniazid (5 mg/kg/day; maximum 300 mg/day) mono-treatment for 9 months (9H), isoniazid/rifampin combination regimen for 3 months (3HR), and rifampin mono-treatment for 4 months (4R). Rifapentine is not yet available in Korea. The HCWs' medical history and drug interactions were investigated, and they were allowed to select their preferred treatment regimen. Basal complete blood count and liver function test were performed before LTBI treatment. The first follow-up blood tests were performed within approximately 2 – 3 weeks after starting medication; treatment compliance and adverse events were also investigated at this time point. If compliance was good and adverse events were absent, the follow-up interval was changed to 2 or 3 months.
All adverse events were graded using the National Cancer Institute's Common Terminology Criteria for Adverse Effects (CTCAE) version 5.0 [14]. The definition of hepatotoxicity was based on increased alanine aminotransferase (ALT) levels, and the severity of hepatotoxicity was classified according to the CTCAE [13,14]. During treatment, if HCWs had an ALT level five times the upper limit of normal or an ALT level three times the upper limit of normal together with nausea, vomiting, abdominal pain, jaundice, or unexplained fatigue, after excluding other liver problems, their LTBI treatment was interrupted [15]. A major adverse event was defined as one wherein a patient had any adverse event ≥grade 3, which caused LTBI treatment interruption.
3. Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows/Macintosh, Version 21.0 (IBM Corp., Armonk, NY, USA). Categorical variables were compared using the chi-square or Fisher's exact test, as appropriate. Continuous variables were compared using the Mann-Whitney U-test. Variables with P value ≤ 0.05 were considered significant.
Results
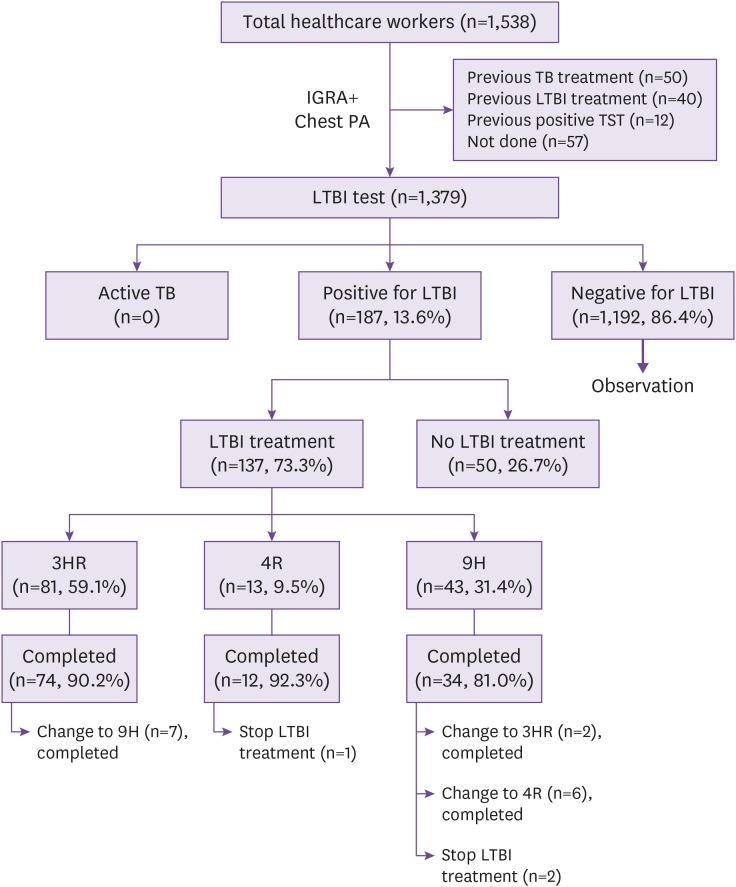
The total number of HCWs was 1,538 in August 2017. HCWs with a previous history of TB treatment (n = 50), LTBI treatment (n = 40), or positive tuberculin skin test result (n = 12) and those not tested using the IGRA (n = 57) were excluded. Detailed characteristics of the HCWs included are shown in Supplementary Table 1. Finally, 1,379 HCWs were tested for LTBI using the IGRA test; among them, 187 (13.6%) tested positive for IGRA. Their median age was 46 years (interquartile range [IQR], 39 – 52 years), and 64 (34.2%) were men (Table 1). IGRA positivity increased significantly with increasing age (P <0.001) (Supplementary Table 2). Additional examinations for excluding active pulmonary TB were performed in 9 and 3 HCWs using chest CT and sputum acid-fast bacilli stain/culture, respectively. None of the HCWs had active TB. The overall acceptance rate of LTBI treatment was 73.3% (137/187) (3HR: n = 81 [59.1%]; 9H: n = 43 [31.4%]; and 4R: n = 13 [9.5%]) (Table 1 and Fig. 1). The acceptance rate was significantly higher among nurses than among doctors (85.7% vs. 55.9%, P < 0.001) or other HCWs (85.7% vs. 66.7%, P = 0.002).
Table 1. Baseline characteristics of healthcare workers positive for interferon-gamma release assay, with acceptance and completion rates of latent tuberculosis infection.
| Variable | Total (N = 187) | Acceptance rate | Completion rate | |
|---|---|---|---|---|
| Gender | ||||
| Male | 64 (32.2) | 41 (64.1) | 35 (85.4) | |
| Female | 123 (65.8) | 96 (78.0) | 84 (87.5) | |
| Age, median (IQR) | 46 (39–52) | NA | NA | |
| Age group | ||||
| 20–29 | 12 (6.4) | 9 (75.0) | 8 (88.9) | |
| 30–39 | 36 (19.3) | 22 (61.1) | 21 (95.5) | |
| 40–49 | 70 (37.4) | 58 (82.9) | 50 (86.2) | |
| 50–59 | 55 (29.4) | 37 (67.3) | 30 (81.1) | |
| 60–69 | 13 (7.0) | 10 (76.9) | 9 (90.0) | |
| 70–79 | 5 (0.3) | 1 (100.0) | 1 (100.0) | |
| Job classification | ||||
| Nurse | 34 (18.2) | 78 (85.7) | 18 (94.7) | |
| Doctors | 91 (48.7) | 19 (55.9) | 68 (87.2) | |
| Others | 62 (33.2) | 40 (64.5) | 33 (82.5) | |
| Risk classification | ||||
| First | 32 (17.1) | 10 (31.3) | 9 (90.0) | |
| Second | 18 (9.6) | 16 (88.9) | 15 (93.8) | |
| Third | 112 (59.9) | 88 (78.6) | 77 (87.5) | |
| Fourth | 25 (13.4) | 23 (92.0) | 18 (78.3) | |
| Acceptance of LTBI treatment | 137 (73.3) | |||
| LTBI treatment regimen | ||||
| 9H | 43 (23.0) | NA | 33 (76.7) | |
| 3HR | 81 (43.3) | NA | 74 (91.4) | |
| 4R | 13 (7.0) | NA | 12 (92.3) | |
Data are presented as number of healthcare workers (%), unless otherwise specified.
IQR, interquartile range; NA, not available; LTBI, latent tuberculosis infection; 9H, 9-month isoniazid regimen; 3HR, 3-month isoniazid/rifampin regimen; 4R, 4-month rifampin regimen.
Figure 1. Study flow diagram.
IGRA, interferon-gamma release assay; PA, posteroanterior view; TB, tuberculosis; TST, tuberculin skin test; LTBI, latent tuberculosis infection; 3HR, 3-month isoniazid/rifampin regimen; 4R, 4-month rifampin regimen; 9H, 9-month isoniazid regimen.
Among the HCWs who agreed to receive LTBI treatment, the overall completion rate was 97.8% (134/137). The completion rate of first-line therapy was 86.9% (119/137). In total, 15 HCWs required a change in their LTBI regimen before completing the treatment. HCWs were significantly more likely to complete first-line therapy with 3HR than with 9H (91.4% vs. 76.7%, P = 0.02). Adverse events and treatment outcomes according to the LTBI treatment regimens are shown in Table 2 and 3.
Table 2. Adverse events according to the latent tuberculosis infection treatment regimens.
| Variable | 9H (n = 43) | 3HR (n = 81) | 4R (n = 13) | P valuea | ||
|---|---|---|---|---|---|---|
| Adverse events, n (%) | 25 (58.1) | 37 (45.7) | 3 (23.1) | 0.26 | ||
| Number of adverse events, n (%) | ||||||
| 0 | 18 (41.9) | 43 (53.1) | 11 (84.6) | 0.26 | ||
| 1 | 17 (39.5) | 33 (40.7) | 2 (15.4) | 1.00 | ||
| 2 | 7 (16.3) | 3 (3.7) | 0 | 0.32 | ||
| 3 | 1 (2.3) | 1 (1.2) | 0 | 1.00 | ||
| Grade of adverse eventsb, n (%) | ||||||
| 1 | 14 (32.6) | 29 (35.8) | 1 (7.7) | 0.70 | ||
| 2 | 10 (23.3) | 7 (8.6) | 0 | 0.03 | ||
| 3 | 2 (4.7) | 1 (1.2) | 1 (7.7) | 0.28 | ||
| 4 | 0 | 1 (1.2) | 0 | 1.00 | ||
| Hepatotoxicity, n (%) | 17 (39.5) | 14 (17.3) | 0 | 0.009 | ||
| Hepatotoxicity (≥grade 2) | 8 (18.6) | 3 (3.7) | 0 | 0.02 | ||
| Time to hepatotoxicity, median (IQR) | 102 (96–105) | 20 (15–89) | NA | <0.001 | ||
| Major adverse eventsc, n (%) | 7 (16.3) | 1 (1.2) | 1 (7.7) | 0.02 | ||
| Hepatotoxicity | ||||||
| ALT elevation more than 3 times the ULN in the presence of hepatitis symptoms | 4 (9.3) | 0 | 0 | 0.12 | ||
| ALT elevation more than 5 times the ULN in the absence of symptoms | 3 (7.0) | 0 | 0 | 0.05 | ||
| Anaphylaxis, n (%) | 0 | 0 | 1 (7.7) | NA | ||
| Thrombocytopenia, n (%) | 0 | 1 (1.2)d | 0 | 1.00 | ||
Data are presented as number of healthcare workers (%), unless otherwise specified.
aP value for comparison between the 9-month isoniazid and 3-month isoniazid/rifampin.
bGrade 1, Mild: asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated. Grade 2, Moderate: minimal, local, or noninvasive intervention indicated; limiting age-appropriate instrumental activities of daily living (ADL). Grade 3, Severe or medically significant but not immediately life-threatening: hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care ADL. Grade 4, Life-threatening consequences: urgent intervention indicated. Grade 5, Death related to adverse events.
cMajor adverse event was defined as one wherein a patient had any adverse event ≥ grade 3, which caused an interruption in latent tuberculosis infection treatment.
dAt day 85 of 3HR treatment, a decreased platelet count below 5,000 mm3 was identified.
9H, 9-month isoniazid; 3HR, 3-month isoniazid/rifampin; 4R, 4-month rifampin; IQR, interquartile range; NA, not applicable; ALT, alanine aminotransferase; ULN, upper limit of normal.
Table 3. Treatment outcomes according to the latent tuberculosis infection treatment regimens.
| Variable | 9H (n = 43) | 3HR (n = 81) | 4R (n = 13) | P valuea | |
|---|---|---|---|---|---|
| Total number of visits, median (IQR) | 6 (5–7) | 3 (3–4) | 4 (4–4) | <0.001 | |
| Number of visits in patients without adverse events | 5 (5–5) | 3 (3–3) | 4 (4–4) | <0.001 | |
| Number of visits in patients with adverse events | 7 (6–9) | 4 (3–5) | 4 (4–NA) | <0.001 | |
| Time to treatment interruption, median (IQR) | 103 (25–143) | 23 (6–93) | 31 (NA) | 0.16 | |
| Treatment outcome, n (%) | |||||
| Complete without a change in the 1st regimen | 34 (81.0) | 74 (90.2) | 12 (92.3) | 0.03 | |
| Complete with a change in the 1st regimen | 8 (18.6) | 7 (8.6) | 0 | 0.15 | |
| Incomplete | 2 (4.7) | 0 | 1 (7.7) | 0.12 | |
Data are presented as number of healthcare workers (%), unless otherwise specified.
aP value for comparison between the 9-month isoniazid and 3-month isoniazid/rifampin.
9H, 9-month isoniazid; 3HR, 3-month isoniazid/rifampin; 4R, 4-month rifampin; IQR, interquartile range; NA, not applicable.
Adverse events according to treatment regimens are shown in Supplementary Table 3. In total, 31 (22.6%) HCWs had hepatotoxicity as an adverse event; among these, 11 (8 HCWs in 9H and 3 in 3HR) had ≥ grade 2 hepatotoxicity. No difference was observed in hepatotoxicity incidence according to the age groups (P = 0.77) (Supplementary Table 4). Hepatotoxicity and hepatotoxicity (≥ grade 2) were more frequent in 9H than in 3HR (39.5% vs. 17.2%, P = 0.006 and 18.6% vs. 3.7%, P = 0.005, respectively). None of the HCWs on 4R showed hepatotoxicity. The median time to confirmed hepatotoxicity was 96 days (IQR, 20–103 days). Approximately one-third (32.3%, 7/31) of hepatotoxicity cases was documented within 1 month. Hepatotoxicity ≥grade 2 cases were documented at a median of 97 days (IQR, 21-105 days). Hepatotoxicity ≥ grade 2 were documented within 1 month in 3 of 11 HCWs (13, 20, and 21 days). After 132 days of LTBI treatment, one HCW had grade 2 hepatotoxicity in 9H regimen.
Major adverse events occurred in nine HCWs (seven receiving 9H, one receiving 3HR, and one receiving 4R). The most common major adverse event was hepatotoxicity (n = 7), followed by thrombocytopenia (n = 1) and anaphylaxis (n = 1) (Table 2). A detailed profile of adverse events that caused a change in or discontinuation of TB medication is shown in Table 4.
Table 4. Detailed profile of adverse events causing a change or discontinuation of latent tuberculosis infection medication.
| Regimen | Treatment outcome | Adverse event | |
|---|---|---|---|
| 9H | |||
| 1 | Change to 4R, then complete | LFT elevation (ALT, grade 3; AST/ALT, 84/212) without symptoms | |
| 2 | Change to 4R, then complete | LFT elevation (ALT, grade 2; AST/ALT, 105/126) with symptoms | |
| 3 | Stop, reluctance to other regimens | LFT elevation (ALT, grade 2; AST/ALT, 129/135) Recurrence of sudden sensorineural hearing loss | |
| 4 | Change to 3HR, then complete | GI trouble including nausea; fatigue, grade 1 | |
| 5 | Change to 4R, without completion | Headache, grade 2 (9H); eye disorder, grade 1 (4R) | |
| 6 | Change to 4R, then complete | LFT elevation (ALT, grade 3; AST/ALT, 338/670) with symptoms | |
| 7 | Change to 3HR | Hyperuricemia grade 1 | |
| 8 | Change to 4R, then complete | LFT elevation (ALT, grade 1; AST/ALT, 100/106) with symptoms | |
| 9 | Change to 4R, then complete | LFT elevation (ALT, grade 2; AST/ALT, 102/140) with symptoms | |
| 10 | Change to 4R, then complete | LFT elevation (ALT, grade 3; AST/ALT, 105/678) without symptoms | |
| 3HR | |||
| 1 | Change to 9H, then complete | GI trouble including nausea, grade 2 | |
| 2 | Change to 9H, then complete | GI trouble including gastroparesis, grade 2 | |
| 3 | Change to 9H, then complete | Platelet count decrease, grade 4; purpura, grade 1 | |
| 4 | Change to 9H, then complete | GI trouble including vomiting, grade 1; rash, grade 1 | |
| 5 | Change to 9H, then complete | Dyspnea, grade 2; headache, grade 2; myalgia, grade 2 | |
| 6 | Change to 9H, then complete | GI trouble including nausea, grade 2 | |
| 7 | Change to 9H, then complete | Unwanted urine color change | |
| 4R | |||
| 1 | Stop, reluctance to other regimens | Anaphylactic shock, grade 3 | |
9H, 9-month isoniazid; 4R, 4-month rifampin; LFT, liver function test; ALT, alanine aminotransferase; AST, aspartate transaminase; 3HR, 3-month isoniazid/rifampin; GI, gastrointestinal.
Discussion
In the present study, the overall acceptance rate was 73.3% and completion rate was 97.8%, among HCWs who accepted LTBI treatment. The completion rate with the first-line therapy regimen was significantly higher with 3HR than with 9H. Approximately half of the HCWs (47.4%, 65/137) experienced adverse events, and major adverse events occurred in 6.6% (9/137). Hepatotoxicity was documented in 22.6% (31/137), and seven HCWs had to change or discontinue their LTBI treatment; all of these seven HCWs were in the 9H group. Treatment interruption occurred at a median of 92 days after LTBI treatment initiation (108 days in 9H and 16 days in 3HR). The major reason for treatment interruption differed according to the LTBI regimen: hepatotoxicity in 9H and gastrointestinal abnormality in 3HR.
Notably, the completion rate was very high irrespective of the LTBI treatment regimen. Only three HCWs discontinued LTBI treatment; among them, one discontinued treatment because of non-compliance. Limited comparable data were available regarding the acceptance or completion rate of LTBI treatment among HCWs in Korea [12]. According to previous studies, the acceptance rate of LTBI treatment ranged between 39% and 84%, with the completion rate ranging between 69% and 91% [16,17,18]. At the study institution, one pulmonologist performed all LTBI diagnosis and treatment. Moreover, an outpatient clinic nurse left important text messages or placed phone calls during regular visits in the absence of DOT. This result was consistent with that of a previous randomized control study comparing self-administrated versus directly observed once-weekly isoniazid and isoniazid/rifapentine treatment for LTBI. Self-administrated treatment with/without a reminder was not inferior to DOT [18].
There is a paucity of data on the reasonable frequency of visits to monitor adverse events [19]. The American Thoracic Society recommended baseline and follow-up visits for patients with a possible liver disorder, symptom or sign of hepatotoxicity, or age >36 years [15]. In a previous study, among 3,377 patients receiving LTBI treatment with isoniazid, the number of hepatotoxic events after 1, 3, and 6 months was 2.75, 7.20, and 4.20 per 1,000 patients, respectively [20]. In the present study, hepatotoxicity was documented at a median of 96 days. Approximately one-third (32.3%) of hepatotoxicity cases was documented within 1 month. Hepatotoxicity (≥ grade 2) cases were documented within 1 month in 3 out of 11 HCWs (13, 20, and 21 days). These findings suggest that follow-up after treatment initiation is necessary at least within a range of 1 – 3 months. However, we could not confirm the usefulness of the 2-month follow-up test because we did not perform this test.
Among HCWs receiving rifampin-containing LTBI treatment regimens, three receiving 3HR had hepatotoxicity (≥ grade 2). However, we did not discontinue or change the first regimen because of the short treatment duration. If a hepatotoxic event occurred in the late phase of treatment, a physician can decide to continue LTBI treatment. Among HCWs receiving 3HR, four of seven discontinued or changed the regimen because of gastrointestinal discomfort, and the remaining three discontinued because of decreased platelet count, dyspnea, and urine color change. Therefore, HCWs receiving 3HR require careful monitoring. In our study, the median time to a change in medication was 23 days. In a previous study on patients receiving LTBI treatment with anti-tumor necrosis factor, the mean time from treatment initiation to regimen change was 117 days in the 9H group, 39.1 days in the 3HR group, and 30.1 days in the 4R group [21]. Therefore, a 1-month follow-up is necessary after initiating treatment with a rifampin-containing regimen.
We could not evaluate the effects of the isoniazid plus rifapentine regimen because this regimen has not yet been introduced in Korea. There is still concern regarding rifampin-containing regimens because of life-threatening events such as anaphylactic shock. In this study, an anaphylactic shock event occurred in the 4R group. In a previous study, no difference was observed in the proportion of subjects with grade 3 or 4 adverse events or in the risk of death between 3 months of starting the rifapentine plus isoniazid regimen and 9 months of starting the isoniazid regimen [16]. In a systematic review and meta-analysis, the risk of adverse events, discontinuation of treatment because of adverse events, and death was similar for the isoniazid plus rifapentine regimen and other LTBI treatment regimens [22].
This study has several strengths. Ours is the first study to provide a whole picture of LTBI screening and treatment results, including acceptance, completion rate, and complications in a referral hospital after the initiation of national act for screening of LTBI in HCWs in Korea. In the study institution, all HCWs positive for LTBI were treated and monitored by one pulmonologist. Therefore, we could minimize the difference in judgement by observers. In addition, we showed a very high completion rate with 97.8%, which is a very high compared with previous studies [11,16,17,18,23]. The present study included information on monitoring intervals for hepatotoxicity and provided insights on the difference in completion rates between 9H and 3HR.
This study also has some limitations. Firstly, because this study was conducted at a single teaching hospital, we cannot generalize the results to other institutions. Second, we could not evaluate the effectiveness of LTBI treatment.
Screening and self-administered treatment of LTBI among HCWs in Korea were well maintained, with a very high overall completion rate. Regular monitoring via liver function tests is necessary between 1 and 3 months after LTBI treatment initiation when using 9H, and careful monitoring of treatment-related symptoms or signs is necessary when using rifampin-containing regimens. Compared to the isoniazid-containing regimens, the rifampin-containing regimens have an acceptable completion rate, but adverse events such as anaphylactic shock and decreased platelet count should be carefully monitored.
Footnotes
Conflict of Interest: No conflicts of interest.
- Conceptualization: PSY, KYK.
- Data curation: PSY, LE, LEJ, KYK.
- Formal analysis: PSY, KYK.
- Investigation: PSY, KYK.
- Methodology: PSY, KTH, KYK.
- Supervision: LE, LEJ, KTH, KYK.
- Writing - original draft: PSY.
- Writing - review & editing: LE, LEJ, KTH, KYK.
SUPPLEMENTARY MATERIALS
Baseline characteristics of all healthcare workers in the study institution
Interferon-gamma release assay positivity according to age group
Adverse events according to the latent tuberculosis infection treatment regimens
Hepatotoxicity according to age group
References
- 1.Lee JE, Kim YK, Kim TH, Kim KH, Lee EJ, Uh ST, Choi TY. What strategy can be applied to the patients with culture positive tuberculosis to reduce treatment delay in a private tertiary healthcare center? Infect Chemother. 2011;43:42–47. [Google Scholar]
- 2.Park HY, Jeon K, Suh GY, Kwon OJ, Chung DR, Yoonchang SW, Kang ES, Koh WJ. Interferon-γ release assay for tuberculosis screening of healthcare workers at a Korean tertiary hospital. Scand J Infect Dis. 2010;42:943–945. doi: 10.3109/00365548.2010.524658. [DOI] [PubMed] [Google Scholar]
- 3.Lee K, Han MK, Choi HR, Choi CM, Oh YM, Lee SD, Kim WS, Kim DS, Woo JH, Shim TS. Annual incidence of latent tuberculosis infection among newly employed nurses at a tertiary care university hospital. Infect Control Hosp Epidemiol. 2009;30:1218–1222. doi: 10.1086/648082. [DOI] [PubMed] [Google Scholar]
- 4.Park SY, Lee EJ, Kim YK, Lee SY, Kim GE, Jeong YS, Kim JH, Kim TH. Aggressive contact investigation of in-hospital exposure to active pulmonary tuberculosis. J Korean Med Sci. 2019;34:e58. doi: 10.3346/jkms.2019.34.e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen PA, Lambert LA, Iademarco MF, Ridzon R, CDC Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1–141. [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence (NICE) Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. [Accessed 27 June 2019]. Available at: http://www.nice.org.uk/guidance/cg117/chapter/1-Guideline.
- 7.Jo KW, Hong Y, Park JS, Bae IG, Eom JS, Lee SR, Cho OH, Choo EJ, Heo JY, Woo JH, Shim TS. Prevalence of latent tuberculosis infection among health care workers in South Korea: a multicenter study. Tuberc Respir Dis (Seoul) 2013;75:18–24. doi: 10.4046/trd.2013.75.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeon JH, Seong H, Hur H, Park Y, Kim YA, Park YS, Han CH, Lee SM, Seo JH, Kang JG. Prevalence and risk factors of latent tuberculosis among Korean healthcare workers using whole-blood interferon-γ release assay. Sci Rep. 2018;8:10113. doi: 10.1038/s41598-018-28430-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho KS. Tuberculosis control in the Republic of Korea. Epidemiol Health. 2018;40:e2018036. doi: 10.4178/epih.e2018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMRW Morb Mortal Wkly Rep. 2011;60:1650–1653. [PubMed] [Google Scholar]
- 11.Arquello Perez E, Seo SK, Schneider WJ, Eisenstein C, Brown AE. Management of latent tuberculosis infection among healthcare workers: 10-year experience at a single center. Clin Infect Dis. 2017;65:2105–2111. doi: 10.1093/cid/cix725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H, Koo GW, Min JH, Park TS, Park DW, Moon JY, Kim SH, Kim TH, Yoon HJ, Sohn JW. Factors associated with non-initiation of latent tuberculosis treatment among healthcare workers with a positive interferon-gamma releasing assay. Sci Rep. 2019;9:61. doi: 10.1038/s41598-018-37319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khanna P, Nikolayevskyy V, Warburton F, Dobson E, Drobniewski F. Rate of latent tuberculosis infection detected by occupational health screening of nurses new to a London teaching hospital. Infect Control Hosp Epidemiol. 2009;30:581–584. doi: 10.1086/597546. [DOI] [PubMed] [Google Scholar]
- 14.NCI Common Terminology Criteria for Adverse Events. (CTCAE) CTCAE files. [Accessed 27 June 2019]. Available at: http://evs.nci.nih.gov/ftp1/CTCAE/About.html.
- 15.Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, Peloquin CA, Gordin FM, Nunes D, Strader DB, Bernardo J, Venkataramanan R, Sterling TR ATS (American Thoracic Society) Hepatotoxicity of Antituberculosis Therapy Subcommittee. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174:935–952. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 16.Schein YL, Madebo T, Andersen HE, Arnesen TM, Dyrhol-Riise AM, Tveiten H, White RA, Winje BA. Treatment completion for latent tuberculosis infection in Norway: a prospective cohort study. BMC Infect Dis. 2018;18:587. doi: 10.1186/s12879-018-3468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, Hackman J, Hamilton CD, Menzies D, Kerrigan A, Weis SE, Weiner M, Wing D, Conde MB, Bozeman L, Horsburgh CR, Jr, Chaisson RE TB Trials Consortium PREVENT TB Study Team. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155–2166. doi: 10.1056/NEJMoa1104875. [DOI] [PubMed] [Google Scholar]
- 18.Belknap R, Holland D, Fenq PJ, Milet JP, Caylà JA, Martinson NA, Wright A, Chen MP, Moro RN, Scott NA, Arevalo B, Miró JM, Villarino ME, Weiner M, Borisov AS TB Trials Consortium iAdhere Study Team. Self-administered versus directly observed once-weekly isoniazid and rifapentine treatment of latent tuberculosis infection: a randomized trial. Ann Intern Med. 2017;167:689–697. doi: 10.7326/M17-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Getahun H, Matteelli A, Chaisson RE, Raviglione M. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015;372:2127–2135. doi: 10.1056/NEJMra1405427. [DOI] [PubMed] [Google Scholar]
- 20.Fountain FF, Tolley E, Chrisman CR, Self TH. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: a 7-year evaluation from a public health tuberculosis clinic. Chest. 2005;128:116–123. doi: 10.1378/chest.128.1.116. [DOI] [PubMed] [Google Scholar]
- 21.Park SJ, Jo KW, Yoo B, Lee CK, Kim YG, Yang SK, Byeon JS, Kim KJ, Ye BD, Park SH, Shim TS. Comparison of LTBI treatment regimens for patients receiving anti-tumor necrosis factor therapy. Int J Tuberc Lung Dis. 2015;19:342–348. doi: 10.5588/ijtld.14.0554. [DOI] [PubMed] [Google Scholar]
- 22.Njie GJ, Morris SB, Woodruff RY, Moro RN, Vernon AA, Borisov AS. Isoniazid-rifapentine for latent tuberculosis infection: a systematic review and meta-analysis. Am J Prev Med. 2018;55:244–252. doi: 10.1016/j.amepre.2018.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menzies D, Adjobimey M, Ruslami R, Trajman A, Sow O, Kim H, Obeng Baah J, Marks GB, Long R, Hoeppner V, Elwood K, Al-Jahdali H, Gninafon M, Apriani L, Koesoemadinata RC, Kritski A, Rolla V, Bah B, Camara A, Boakye I, Cook VJ, Goldberg H, Valiquette C, Hornby K, Dion MJ, Li PZ, Hill PC, Schwartzman K, Benedetti A. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med. 2018;379:440–453. doi: 10.1056/NEJMoa1714283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics of all healthcare workers in the study institution
Interferon-gamma release assay positivity according to age group
Adverse events according to the latent tuberculosis infection treatment regimens
Hepatotoxicity according to age group