Itch (also called pruritus) is an unpleasant somatosensation that evokes a desire or reflex to scratch in humans and other mammals [1]. Itch is now considered to be a unique sensory modality that is encoded by a labeled line that has genetically distinguishable neurons in the peripheral and central nervous systems [1]. Acute itch evokes scratching that may help to remove potentially harmful irritants from the skin, which is believed to be evolutionarily important for survival. Pathological chronic itch often occurs in patients with inflammatory skin diseases, systemic diseases, and neurological conditions. Importantly, chronic itch causes uncontrollable itch-scratch cycles that induce skin damage, affect sleep, and seriously reduce the quality of life [2]. At present, effective treatment for chronic itch is still lacking, possibly due to limited understanding of the mechanism of itch information-processing in the nervous system. Therefore, elucidation of the molecular, cellular, and circuitry mechanisms of itch will eventually help to develop new effective strategies for the management of chronic itch.
Itch sensation originates from the surface of the skin or mucosa. An itch stimulus is detected by the peripheral terminals of primary sensory neurons whose cell bodies are located in the dorsal root ganglia and trigeminal ganglia [3]. The central branches of primary sensory neurons, which contain glutamate and neuropeptides, project to the superficial dorsal horn of the spinal cord. In a simplified model, the central terminals of itch sensory neurons that contain the neuropeptide natriuretic polypeptide b (Nppb) make synaptic connections with gastrin-releasing peptide (GRP)-expressing neurons that express the Nppb receptor NPRA in lamina II of the spinal cord. GRP-expressing neurons release GRP to act on its receptor GRPR, which is expressed in a group of excitatory interneurons expressing vesicular glutamate transporter 2 (VGLUT2). GRPR-expressing neurons excite projection neurons in spinal cord lamina I, which send itch signals to the brain [3]. There are also several groups of itch inhibitory neurons, such as BHLHB5 (basic helix-loop-helix domain-containing protein, class b, 5)-expressing and neuropeptide Y-expressing interneurons, which act on GRPR-expressing neurons or their downstream neurons in the itch pathway to gate itch transmission in the dorsal horn [3]. Thus, there is a complicated gating mechanism for itch transmission in the spinal cord, which may involve distinct population of interneurons, including both excitatory and inhibitory interneurons [4, 5].
After processing in the spinal cord, axons of the itch projection neurons, possibly neurokinin 1 (NK1)-expressing neurons located in superficial lamina I, cross the midline to the contralateral side and join the spinothalamic tract projecting to the thalamus and/or the spinoparabrachial tract projecting to the parabrachial nucleus (PBN) [3], which in turn project into various brain areas. A subpopulation of PBN neurons, which require VGLUT2 for signal transmission, make disynaptic connections with GRPR+ neurons in the spinal cord. Positron emission tomography and functional magnetic resonance imaging have shown that primary and secondary somatosensory cortex are frequently activated in humans. In addition, other itch-activated areas include the cingulate and prefrontal cortex, amygdala, and limbic systems (possibly involved in emotional processing), as well as premotor, motor, and supplementary motor areas (possibly involved in scratching behavior) [3]. Although non-histaminergic itch induced by cowhage activates distinct brain areas, such as insular cortex, basal ganglia, claustrum, putamen, and thalamic nuclei, both histaminergic and non-histaminergic itch activate similar brain areas. Compared with pain, the precuneus and the posterior cingulate cortex are thought to be itch-specific, although it is still under debate whether there are itch-selective brain areas. Histamine-induced activation of some areas, such as the cingulate and prefrontal cortex, is more robust in chronic itch patients than in healthy individuals, indicating the central sensitization of itch under chronic itch conditions [3]. Nevertheless, our understanding of how itching and scratching are processed under physiological and pathophysiological conditions is still limited.
Like pain, itch-responsive neurons in the spinal cord are significantly affected by descending modulatory pathways from higher brain regions. Serotoninergic neurons in the nucleus raphe magnus send descending facilitatory projections to the spinal cord and directly stimulate GRPR+ neurons by acting on heterodimers of the 5-hydroxytryptamine (5-HT) 1A and GRP receptors [3]. Noradrenergic neurons in the locus coeruleus send descending inhibitory projections to the spinal cord, possibly via α-adrenergic receptors, to enhance the activity of inhibitory interneurons [3]. The periaqueductal gray (PAG), a major endogenous pain modulatory area and controller of morphine analgesia [6], is also activated during itch-induced scratching. Thus, activation of the PAG during scratching is traditionally considered to be correlated with descending itch suppression by opioids [3]. However, the precise neural circuitry of itch processing in the PAG remains unexplored.
In a new paper published in Neuron, Gao et al. have revealed that tachykinin 1 (Tac1)-expressing (Tac1+) neurons located in the PAG facilitate itch-scratch cycles via a descending rostral ventromedial medulla (RVM) pathway in mice [7]. First, they reported that neuronal activity in the lateral and ventrolateral PAG (l/vlPAG), reflected by an increased number of c-fos+ neurons and in extracellular electrophysiological recordings, significantly increases after intradermal injection of pruritogens (such as histamine or chloroquine). Pharmacogenetic inhibition of the l/vlPAG neurons significantly decreases the scratching behavior induced by pruritogens. Gao et al. further found that pharmacogenetic suppression of the RVM-projecting neurons, especially glutamatergic neurons, in the l/vlPAG significantly decreases itch behavior in mice. Thus, activation of glutamatergic PAG neurons promotes itch-scratch cycles via a descending RVM pathway in mice.
Next, Gao et al. found that ablation of a subpopulation of glutamatergic neurons in the l/vlPAG (Tac1+ neurons) significantly decreases the scratching behavior induced by acute and chronic itch in mice. In the mouse cheek model, ablation of Tac1+ neurons in the l/vlPAG significantly decreases itch-induced scratching, but not pain-induced wiping. Further, the activity of Tac1+ neurons in the l/vlPAG, reflected by fluorescent signal fluctuations, significantly increases during itch-induced scratching, but only slightly increases during pain-induced wiping. Thus, l/vlPAG Tac1+ neurons selectively respond to peripheral itch stimuli.
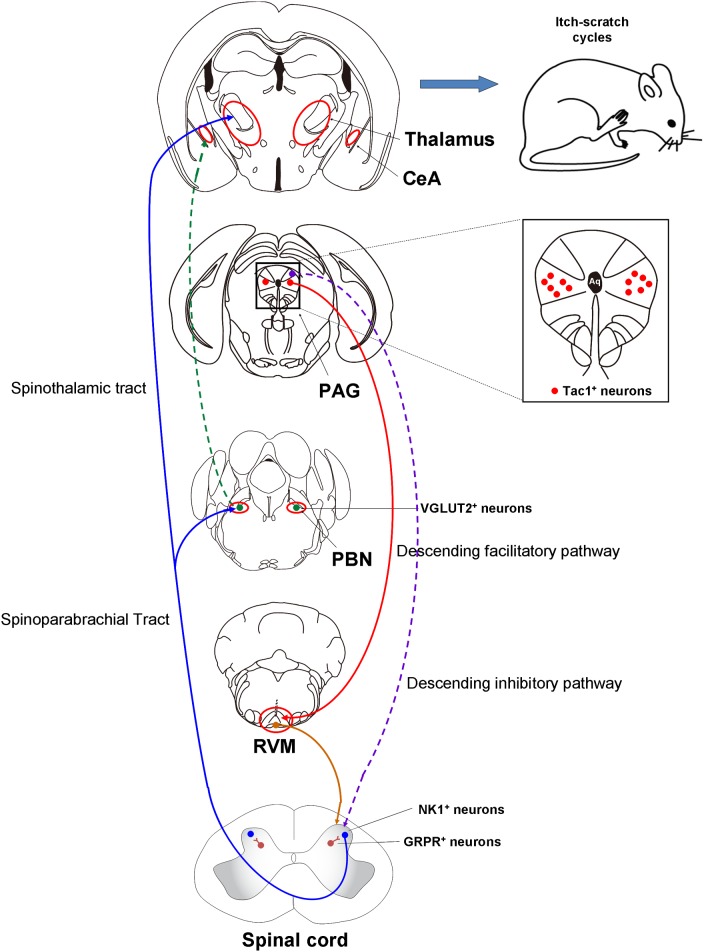
Further, Gao et al. showed that pharmacogenetic and optogenetic activation of Tac1+ neurons is sufficient to induce robust scratching behavior, which is inhibited by the ablation of spinal GRPR+ neurons by intrathecal injection of bombesin-saporin, but not GRPR itself, in mice. Finally, Gao et al. found that the scratching behavior induced by activation of l/vlPAG Tac1+ neurons is not affected by depletion of spinal 5-HT fibers or pharmacological blockade of spinal 5-HT1A receptors, suggesting that the descending 5-HT projection is not involved in the facilitatory role of l/vlPAG Tac1+ neurons in itch transmission. Interestingly, previous work has demonstrated that spinal GRPR+ neurons are not involved in chronic mechanical itch in mice [8]. Whether the l/vlPAG Tac1+ neurons are involved in mechanical itch processing warrants further investigation. Based on our understanding of the mechanisms underlying itch, we provide a simplified overview of the ascending and descending pathways for itch (Fig. 1).
Fig. 1.
Overview of the neural circuitry for itch-processing in the central nervous system in mice. CeA, central amygdala; GRPR, gastrin-releasing peptide receptor; NK1, neurokinin 1; PAG, periaqueductal gray; PBN, parabrachial nucleus; RVM, rostral ventromedial medulla; Tac1, tachykinin 1; VGLUT2, vesicular glutamate transporter 2.
This work provides important insight into the neural circuitry underlying a descending modulatory pathway of itch sensation in mice. However, it also raises a series of interesting questions. First, under acute itch conditions, scratching is very effective for relieving itch. In contrast, under chronic itch conditions, an itch-scratch-itch cycle occurs [2], suggesting that chronic itch is no longer inhibited by scratching; rather, scratching may promote itch. Do Tac1+ neurons in the PAG play a distinct role in acute and chronic itch? Second, given that acute itch can be inhibited by pain or scratching [1], can pain stimuli or scratching itself affect the activity of Tac1+ neurons in the PAG? Do Tac1+ neurons in the PAG play any role in the inhibition of itch induced by painful stimulation? Third, opioids such as morphine can induce or exacerbate itch sensation in humans and animal models. Given that the PAG is a major area for the action of morphine (such as in analgesia), does morphine have any effect on the activity of Tac1+ neurons in the PAG to modulate itch processing? Interestingly, one study has shown that local injection of bombesin, a GRPR ligand, into the PAG reduces scratching behavior followed by immobility in an animal model [9]. Fourth, what is the role of GRP-GRPR signaling in the PAG and its relationship with Tac1+ neurons in itch processing? Finally, if the descending 5-HT projection is not involved in the facilitatory role of PAG Tac1+ neurons in itch processing, what transmitter is involved in facilitating the modulation of itch?
Interestingly, grooming is an innate behavior with an evolutionarily-conserved pattern and is frequently used as a measure of repetitive behavior in rodent models of autism spectrum disorder and obsessive compulsive disorder [10]. Gao et al. found that activation of PAG Tac1+ neurons also evokes robust grooming behavior, and scratching and grooming behaviors are likely mediated by different subsets of these neurons. To further identify the roles of these subpopulations of PAG Tac1+ neurons based on their molecular identity or connectivity in distinct functions (such as grooming and scratching) is extremely urgent.
In summary, Gao et al. clearly demonstrated that Tac1+ neurons in the PAG facilitate the itch-scratching cycle via a descending RVM pathway. We also speculate that dysfunction of the descending modulatory system of itch may play a critical role in the development of chronic itch. This has important clinical implications for the discovery of descending neural circuitry to drive the itch-scratching cycle, given that the cycle is a common phenomenon under chronic itch conditions. Thus, this newly identified top-down pathway may provide potential therapeutic targets in the central nervous system for the management of chronic itch
Acknowledgements
TL was supported by grants from the National Natural Science Foundation of China (81870874, 31371179, and 81300968) and the Natural Science Foundation of Jiangsu Province, China (BK20170004 and 2015-JY-029). XL was supported by the Postgraduate Research and Practice Innovation Program of Jiangsu Province, China (KYCX17_2034).
Contributor Information
Xiu-Hua Miao, Email: xiuhua_miao@126.com.
Tong Liu, Email: liutong80@suda.edu.cn.
References
- 1.LaMotte RH, Dong X, Ringkamp M. Sensory neurons and circuits mediating itch. Nat. Rev. Neurosci. 2014;15:19–31. doi: 10.1038/nrn3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N. Engl. J. Med. 2013;368:1625–1634. doi: 10.1056/NEJMcp1208814. [DOI] [PubMed] [Google Scholar]
- 3.Dong X, Dong X. Peripheral and Central Mechanisms of Itch. Neuron. 2018;98:482–494. doi: 10.1016/j.neuron.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji RR. Recent progress in understanding the mechanisms of pain and itch: the second special issue. Neurosci Bull. 2018;34:1–3. doi: 10.1007/s12264-018-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miao X, Huang Y, Liu TT, Guo R, Wang B, Wang XL, et al. TNF-alpha/TNFR1 signaling is required for the full expression of acute and chronic itch in mice via peripheral and central mechanisms. Neurosci Bull. 2018;34:42–53. doi: 10.1007/s12264-017-0124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 7.Gao ZR, Chen WZ, Liu MZ, Chen XJ, Wan L, Zhang XY, et al. Tac1-expressing neurons in the periaqueductal gray facilitate the itch-scratching cycle via descending regulation. Neuron. 2019;101(45–59):e49. doi: 10.1016/j.neuron.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Bourane S, Duan B, Koch SC, Dalet A, Britz O, Garcia-Campmany L, et al. Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science. 2015;350:550–554. doi: 10.1126/science.aac8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spruijt BM, Cools AR, Gispen WH. The periaqueductal gray: a prerequisite for ACTH-induced excessive grooming. Behav Brain Res. 1986;20:19–25. doi: 10.1016/0166-4328(86)90097-5. [DOI] [PubMed] [Google Scholar]
- 10.Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci. 2016;17:45–59. doi: 10.1038/nrn.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]