Abstract
Accumulating evidence suggests that the circadian rhythm plays a critical role in mood regulation, and circadian disturbances are often found in patients with major depressive disorder (MDD). The mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway is involved in mediating entrainment of the circadian system. Furthermore, the MAPK/ERK signaling pathway has been shown to be involved in the pathogenesis of MDD and the rapid onset of action of antidepressant therapies, both pharmaceutical and non-pharmaceutical. This review provides an overview of the involvement of the MAPK/ERK pathway in modulating the circadian system in the rapid action of antidepressant therapies. This pathway holds much promise for the development of novel, rapid-onset-of-action therapeutics for MDD.
Keywords: Major depressive disorder, MAPK pathway, Circadian system, Rapid antidepressant therapy
Introduction
Rapid-onset antidepressant therapies have been shown to be related to the circadian rhythm, but the specific molecular pathways have not been clarified [1–3]. The mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) signaling pathway is involved in major depressive disorder (MDD), but such an involvement has not been interpreted from the perspective of the circadian system [4]. The regulation of circadian genes by the MAPK/ERK pathway has been demonstrated elsewhere but not linked specifically to depression [5, 6]. In this review, we propose that rapid-onset pharmaceutical and non-pharmaceutical antidepressant therapies may modulate the circadian rhythm through the MAPK pathway.
Circadian Rhythm and Its Effect on Mood
The circadian rhythm in mammals is controlled by the suprachiasmatic nucleus (SCN), which is often referred to as the central circadian clock. It synchronizes the peripheral clock in various cells throughout the body. The molecular machinery of the circadian clock consists of interlocked molecular feedback loops [2, 7–9]. The core circadian loop consists of positive and negative branches. The positive branch consists of circadian locomotor output cycles kaput (CLOCK) and brain and muscle ARNT-like 1 (BMAL1) proteins, which heterodimerize and bind to the Enhancer Box of the period and cryptochrome genes to activate their transcription. The period (PER) and cryptochrome (CRY) proteins then heterodimerize in the cytoplasm and are phosphorylated by glycogen synthase kinase-3 and casein kinase Iε. Afterward, they shuttle back into the nucleus to inhibit the transcription of Clock and Bmal1 genes. Lastly, the PER and CRY proteins are degraded in the cytoplasm and release the inhibition of transcription. This process lasts about 24 h.
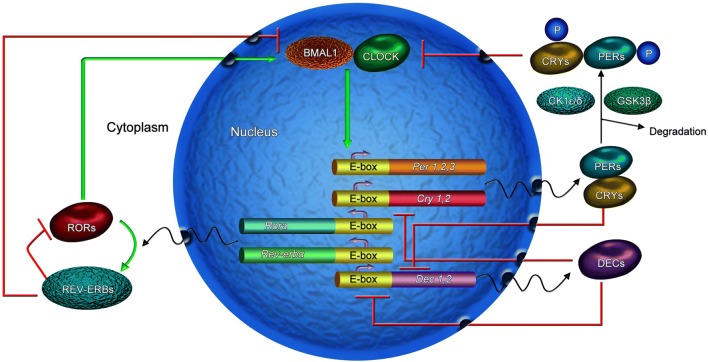
In addition to the core circadian feedback loop, interlocking feedback loops have also been explored with regard to their modulation of the circadian rhythm. The other feedback loop contains Rora and Rev-erbα, which regulate BMAL1 expression [10–13]. Rora activates the expression of BMAL1, and Rev-erbα inhibits it, which reinforces the oscillations. Another loop consists of Dec1 and Dec2, which negatively regulate circadian rhythms [14]. The CLOCK and BMAL1 complexes activate the transcription of the Dec1 and Dec2 genes, whereas the PER and CRY complexes inhibit them. In addition, differentiated embryo chondrocytes self-regulate to inhibit the transcription of their own genes. They also inhibit transcription of the Per and Cry genes [15]. These form multiple interlocked molecular feedback loops that provide stability and fine regulation of the circadian machinery (Fig. 1).
Fig. 1.
Circadian feedback loops in mammalian cells. The loops contain the classical core feedback loop (including BMAL1/CLOCK and PER/CRY proteins), an REV-ERB/ROR loop, and a Dec loop, which interlocks with the classical core loop. CLOCK and BMAL1 form heterodimers that activate the transcription of Cry and Per genes. PER dimerizes with CRY to inhibit CLOCK–BMAL1-mediated transcription. Rora activates the expression of BMAL1, and Rev-erbα inhibits it, forming a feedback loop. The CLOCK and BMAL1 complexes activate transcription of the Dec1 and Dec2 genes, whereas the PER and CRY complexes inhibit them. In addition, DECs self-regulate to inhibit the transcription of their own genes. This forms another loop. These loops are interlocked. CK1ε/δ, casein kinase 1ε/δ; CRY, cryptochrome; DEC, differentiated embryo chondrocyte; E-box, enhancer-box; PER, period; ROR, retinoic acid-related orphan receptor; P, phosphorylation.
Previous studies have shown that daily rhythms are prominent in every aspect of bodily function, such as sleep/wake periods, core body temperature, blood pressure, hormone secretion, cognition, and mood [16, 17]. Disturbances of the circadian rhythm may be related to mental disorders. For example, since the 1950s, circadian disturbances have been reported in patients with mood disorders [18]. Circadian clinical manifestations in MDD patients include social activity rhythm disorder, sleep/wake cycle disorder, blood pressure rhythm disorder, and hormone secretion rhythm disorder [19]. In the late 1980s, the Social Zeitgeber Theory of mood disorders proposed that stress leads to alterations of the circadian rhythms in susceptible individuals, resulting in depressive or manic episodes [20]. Research on patients with first-episode MDD has shown disturbances in circadian rhythms of the expression of PERIOD1, PERIOD2, CRY1, BMAL1, NPAS2, and GSK-3β, as well as abnormalities in the circadian rhythms of the secretion of melatonin, vasoactive intestinal peptide, cortisol, adrenocorticotropic hormone, insulin growth factor-1, and growth hormone [21]. And these abnormalities are correlated with the severity of depressive symptoms. Some studies have also reported that mood symptoms are alleviated along with the resumption of circadian rhythms with treatment [18, 19]. All therapeutic strategies that are employed for mood disorders alter or steady circadian rhythms [3]. Nevertheless, the mechanisms that underlie circadian rhythm disturbances that might induce mood alterations have not been clarified. Logan et al. found that chronic unpredictable mild stress (CUMS), a widely used animal model of depression, significantly reduces the rhythmic amplitude of activity and body temperature in mice [22]. These alterations of biological rhythms are directly correlated with depressive-like behaviors. Expression of the amplitude of the clock gene per2 rhythm is decreased in the SCN and increased in the nucleus accumbens (NAc) of CUMS mice. Molecular circadian changes in the SCN and NAc are directly correlated with mood-related behaviors [22]. CUMS also causes disturbances in the circadian rhythms of plasma corticosterone, melatonin, and vasoactive intestinal peptide in rats [23]. The functions of daily rhythms of the hypothalamus-pituitary-thyroid (HPT) axis are also reduced in rats exposed to CUMS [24]. In addition, CUMS induces alterations of the rPER2 rhythm in the rat SCN [25]. The hippocampal CLOCK protein has been shown to play an important part in the continuance of the depressive-like behaviors induced by CUMS [26].
Clock gene variants have often been associated with diurnal preference [19] and have been explored with regard to the mechanisms of mood disorders [27]. Preclinical and clinical reports suggest that mutations of both Clock [28, 29] and Per [30, 31] are associated with mood disorders. Previous research on circadian regulation has impressively exhibited its predictive value for the initiation of depression. Spulber et al. [32] studied the long-term behavioral changes induced by prenatal exposure to excessive glucocorticoids. They found that progressive changes in circadian entrainment precede depression. Circadian oscillations in clock gene mRNA expression are also diminished in skin fibroblasts before the initiation of depression. These results indicate that changes in the circadian entrainment of spontaneous activity and possibly clock gene expression in fibroblasts signal the development of depression. Other researchers have suggested that circadian disturbances might be the origin of mood disorders rather than their consequence [2].
Circadian rhythm disorders might be a part of the pathogenesis of mood disorders. However, detailed mechanisms, such as the cellular signaling pathways, have not been sufficiently clarified.
Regulation of the Circadian System by the MAPK Pathway
Light is known to be the strongest stimulus (zeitgeber) for entrainment of the circadian pacemaker [33, 34]. The retinohypothalamic tract (RHT) is located between the retina and the SCN in mammals. Even very dim light has been shown to entrain the circadian pacemaker [35–37]. However, non-photic stimuli have also been shown to exert weak but independent effects on the SCN. That is, other zeitgebers besides light can entrain the circadian pacemaker.
The MAPK signaling pathway may act as a critical common mediator of the circadian rhythm in the SCN, the periphery, and cultured cells, and circadian entrainment by photic and non-photic stimuli may be impacted by similar molecular mechanisms [38] (Fig. 2).
Fig. 2.
Photic- and non-photic-responsive MAPK signaling pathways in the brain. In photic circadian clock entrainment, the neurotransmitters glutamate and PACAP are released onto SCN neurons via the eye and RHT. The activation of NMDA and Pac1 receptors in turn results in the activation of Ras and heterotrimeric G proteins, which successively activate ERK and CREB. Non-photic stimulation by SD, ECT, or DBS leads to the activation of adenosine A1 receptors, which leads to the activation of ERK and CREB. Phosphorylated CREB is translocated to the nucleus and activates the transcription of immediate-early genes, including Per1. SD, sleep deprivation; ECT, electroconvulsive therapy; DBS, deep brain stimulation; P, phosphorylation; ER, endoplasmic reticulum; TSS, transcription start site.
MAPK Pathway Underlies Photic Entrainment of the Circadian System
The MAPK pathway [39] in mammals consists of ERK1/2, c-Jun N-terminal kinase (JNK), p38, and ERK5. The MAPK pathway has been suggested to be involved in entrainment of the circadian clock [38]. The potential involvement of Ras, part of the MAPK signaling pathway, in modulating the circadian rhythm has been proposed in several studies [40–48]. ERKs have also been shown to play a role in photic resetting of the clock in the rodent SCN [49, 50]. The role of the SCN clock as a master pacemaker is modulated by light via direct excitation from the eyes. Intrinsic photosensitive retinal ganglion cells detect light through melanopsin [51] and project directly to the SCN through the RHT. The terminals of the RHT release glutamate and pituitary adenylate cyclase-activating peptide (PACAP) in the SCN [52]. They are ligands for N-methyl-D-aspartate receptors (NMDARs) and Pac1 receptors, respectively, at postsynaptic neurons in the SCN. The activation of NMDARs is followed by an inflow of Ca2+ [53, 54], which stimulates Ca2+-calmodulin kinase II that successively activates cyclic adenosine monophosphate (cAMP) response element binding protein (CREB) [55, 56]. In parallel, the activation of NMDARs leads to the activation of Ras, with subsequent activation of the MAPK pathway. ERK1/2 phosphorylates p90 ribosomal S6 kinase, which successively phosphorylates CREB, which is indispensable for light-induced resetting of the circadian clock in the SCN [57, 58]. Pac1 receptors can be activated by both light and stress [59]. PACAP is a critical neurotransmitter that is released in stress transduction areas of the brain, including the paraventricular nucleus of the hypothalamus, the amygdala, extended amygdala nuclei, and the prefrontal cortex (PFC).
A previous study provided strong evidence that CREB plays a vital role as an endpoint of the MAPK pathway in the regulation of the mPer (mPer1 and mPer2) genes [58]. Additional studies suggested that the MAPK pathway mediates the photic input which is involved in immediate-early gene (e.g., c-fos and Per1) induction in the SCN [60–62]. Per1 is a clock gene in the negative feedback loop of the circadian system, the activation of which is thought to be an important event in modulating the clock [63]. The MAPK pathway has a series of downstream effector molecules that control the expression of related genes, including circadian genes [64] (Fig. 2). Moreover, ERK and MAPK interact with and phosphorylate CLOCK, BMAL1, CRY1, and CRY2 in the circadian system [6].
MAPK Pathway Underlies Non-photic Entrainment of the Circadian System
In addition to light, non-photic stimuli can also impact the circadian clock [65–67]. Numerous other stimuli have been shown to increase locomotor activity or arousal in animals, including injections of triazolam [68, 69] and morphine [70], confinement to a novel wheel [71, 72], social and sexual cues [73], and sleep deprivation [74]. Some research have also revealed that the circadian clock can be reset in humans by modulating mealtime, exercise, sleep-wake schedule, and social stimuli [75].
The intracellular biochemical cascades that underlie non-photic phase-shifts have gradually been identified. A study [76] showed that dark pulses decrease the levels of phosphorylated ERK1/2 but do not affect the levels of phosphorylated Ets-like gene 1. Another study revealed the involvement of the ERK/MAPK pathway in phase-shifts in response to 3 h of sleep deprivation initiated at midday in Syrian hamsters [67]. Cultured NIH-3T3 fibroblasts treated with tissue plasminogen activator exhibit circadian oscillations of gene expression that are restrained by a MEK inhibitor, and sustained activation of the MAPK pathway is sufficient to activate circadian gene expression [38]. These results demonstrate that the MAPK pathway acts as a critical mediator of signaling pathways that are involved in circadian entrainment by non-photic stimuli.
Research on the mechanisms that underlie physical therapy have revealed that sleep deprivation, electroconvulsive therapy (ECT), and deep brain stimulation (DBS) are associated with an increase in adenosinergic signaling [77–80]. Sleep deprivation increases the secretion of adenosine in the brain and upregulates adenosine A1 receptors in humans and rodents [81–83]. Two physical therapies for depression, ECT and DBS, increase the levels of adenosine and stimulate A1 receptors [77, 79, 84, 85]. Previous studies have shown that A1 receptors regulate phospholipase C [86–89] and the ERK pathway [90, 91] in cells where they are highly expressed (e.g., neurons and smooth muscle cells). The MAPK pathway is an important component of the circadian clock [6] and mediates the regulation of the circadian rhythm. Evidence suggests that A1 receptors mediate the circadian rhythm through the MAPK pathway and downstream CREB (Fig. 2).
To summarize, these investigations indicate that the MAPK pathway acts as a critical common mediator in the signaling pathways that regulate the circadian rhythm which is induced by both photic and non-photic stimuli. Regulation of the circadian system by the MAPK pathway may also play a critical role in depression.
MAPK Pathway in Depression
The MAPK/ERK pathway has been shown to play a critical role in MDD [92, 93] and in the actions of antidepressants [4, 94, 95]. Elements of the MAPK/ERK signaling pathway, including MEK1, MEK2, and Rap1, are reduced in the frontal cortex in MDD patients [96]. The levels of CREB cAMP and Ca2+ signaling also reduced in MDD [96]. Evidence also suggests decreases in ERK1/2, ERK5, MEK1, and CREB in the hippocampus [97, 98] and Rap1 activity in the PFC and hippocampus [99] in depressed suicidal individuals. Postmortem investigations have reported that the mRNA and protein levels of ERK1/2 in prefrontal cortical areas and the hippocampus are significantly reduced in depressive suicide individuals [96, 99, 100]. MAPK-phosphatase 1 (MKP-1), a negative modulator of the MAPK pathway, is increased in the hippocampus of MDD patients [93]. The overexpression of MKP-1 is sufficient to induce depressive-like behavior in rodent models [93, 101]. The MAPK pathway has been suggested to play a vital role in the development of depression [95, 102].
Activity of the MAPK pathway is inhibited by chronic stress and restored by antidepressant treatment [103]. Inhibition of the MAPK pathway induces depressive- and anxiety-like behaviors [104]. Duman and colleagues demonstrated that inhibition of the MAPK pathway leads to depressive-like behaviors and blocks the behavioral actions of antidepressants on rodents [4]. Furthermore, depressive-like behavior is positively associated with a reduction of pERK in a rat model of depression [105]. Inhibition of the ERK pathway produces depressive-like behavior and blocks the antidepressant effects of ketamine [106]. Moreover, Pochwat et al. reported that ERK activation is crucial for both the short- and long-term antidepressant-like actions of NMDAR antagonists in the forced swim test in rats [107]. Recently, Labonté and colleagues reported that an increase in ERK signaling in glutamatergic pyramidal neurons in the ventromedial PFC (vmPFC) specifically increases the susceptibility of female rats to stress [108]. However, these results are in sharp contrast to those of previous studies in rodents [109–111] and postmortem human brains [96]. The study by Labonté and colleagues investigated the vmPFC, whereas most other previous studies focused on other cortical regions or the NAc. This may be the main reason for the discrepant findings. Further work is needed to illustrate why an increase in ERK signaling in the female vmPFC results in depressive-like behavior, while the same change in the male vmPFC has diverse outcomes.
In summary, abundant evidence indicates that the MAPK/ERK pathway is involved in the pathogenesis of depression and may be an attractive target for the development of new therapeutic strategies for MDD.
Regulation of the Circadian System by the MAPK Pathway: Involvement in Rapid Antidepressant Effects
Accumulating evidence suggests that MDD is a circadian-related disorder. Almost all patients with MDD suffer from alterations of diurnal rhythmicity (e.g., temperature, sleep, hormone secretion, and mood) during depressive episodes [3]. A review showed that both low-dose ketamine and sleep deprivation therapy (SDT) modulate the circadian rhythm in humans, animals, and neuronal cells [3]. The fact that both therapies impact sleep homeostasis and the circadian rhythm indicates that the circadian and sleep-wake systems and their interactions are related to rapid antidepressant effects.
Sleep Deprivation Therapy and Other Non-pharmaceutical Treatments
The advent SDT was a major revolution when considering its rapid remission of depressive manifestations [112]. SDT generally refers to keeping patients awake for ~36 h. The mechanism underlying the effects of SDT has been investigated for many years, but no mechanism has yet been demonstrated. Bunney et al. proposed that abnormal circadian clock genes which control rhythms are altered by a change of the sleep-wake cycle [7, 16]. SDT responders present distinct activation of circadian genes (Rora, Dec2, and Per1) after SDT, whereas non-responders present notable reductions in the expression of these genes afterwards [7]. A few studies of clock genes in mice showed that a subset of circadian clock genes (e.g., Per1 and Per2) appear to behave as immediate-early genes and are transcriptionally responsive within hours after sleep deprivation [113–116]. Depriving mice of sleep inhibits ~80% of rhythmic genes [114, 117].
The MAPK/ERK signaling pathway has been shown to be involved in the mechanism of action of SDT [118]. The diurnal activation of ERK in the dorsomedial SCN (i.e. the “shell”) is suppressed following sleep deprivation. A previous study indicated that adenosine A1 receptors are important for the antidepressant effects of sleep deprivation [119, 120]. Two other rapid-onset non-pharmaceutical therapies for depression, ECT and DBS, are also associated with an increase in the release of adenosine and the activation of A1 receptors [77, 79, 84]. Interestingly, A1 receptors regulate the ERK pathway [90, 91]. These studies suggest that the rapid-onset non-pharmaceutical treatments for depression exert their antidepressant effects by stimulating A1 receptor-ERK1/2 signaling (Fig. 2).
Besides, light therapy can also produce a rapid antidepressant effect. However, the parameters of light therapy varied among different clinical trials, so the results were different [121, 122]. Currently, there is no uniform procedure for light therapy. Above, we discussed the involvement of the MAPK/ERK pathway in both photic and non-photic entrainment of the circadian system. We propose that rapid-onset-of-action non-pharmaceutical treatments for depression exert their antidepressant effects by activating MAPK/ERK signaling, which mediates resetting of the circadian system, although no direct evidence is yet available to support our viewpoint.
Low-Dose Ketamine
Several studies indicate that the rapid antidepressant effects of low-dose ketamine are closely related to the regulation of circadian systems. Duncan et al. [123] found an association between the clinical antidepressant effects of ketamine and circadian timekeeping (i.e., amplitude and timing) using wrist-activity monitors in MDD patients. Bellet et al. [124] found that ketamine affects molecules associated with the central circadian clock. Specifically, ketamine decreases the amplitude of circadian transcription of the Bmal1, Per2, and Cry1 genes in a dose-dependent manner. Ma et al. [125] reported that ketamine accelerates the differentiation of double cortin-positive adult hippocampal neural progenitors into functionally mature neurons. This process requires activation of the tyrosine kinase receptor B (TrkB)-dependent ERK pathway. TrkB-dependent neuronal differentiation is related to the sustained antidepressant effects of ketamine. Moreover, another study reported that acute inhibition of the MAPK pathway produces depressive-like behavior and blocks the antidepressant effect of ketamine [106]. Recently, Yang et al. found that (R)-ketamine significantly attenuates the decrease in ERK phosphorylation and its upstream effector MAPK/ERK in the PFC and hippocampal dentate gyrus in susceptible mice following chronic social defeat stress [126]. Based on this evidence, we speculate that low-dose ketamine produces its rapid antidepressant effects through the MAPK pathway to regulate the circadian system.
Overall, these results suggest that both SDT and ketamine act on circadian genes through the ERK/MAPK pathway, which phosphorylates CREB to produce a rapid antidepressant response. Using comparative transcriptomics analyses, Orozco-Solis et al. found that both SDT and ketamine act on the circadian clock via the MAPK/ERK pathway in the anterior cingulate cortex [127]. These findings suggest that the mediation of entrainment of the circadian system by the MAPK/ERK pathway may be involved in neuropathological processes that are associated with depression and antidepressant therapy, although further studies are needed to test this possibility (Fig. 3).
Fig. 3.
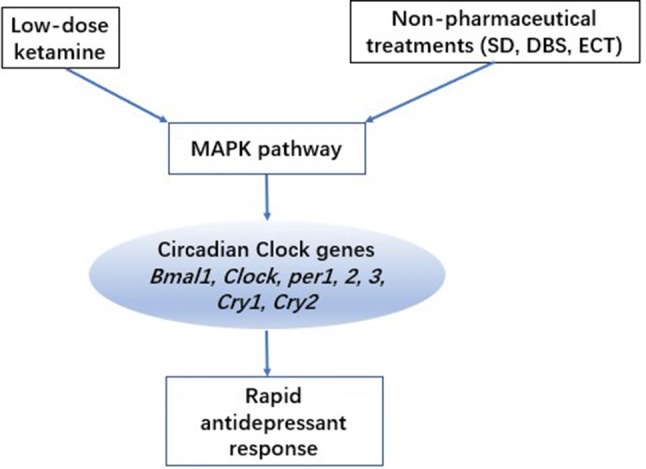
Low-dose ketamine and non-pharmaceutical treatments, including SD, DBS, and ECT, regulate the circadian system through the MAPK pathway, which phosphorylates CREB to produce a rapid antidepressant response. Phosphorylated CREB directly binds to the CRE sequence of the per1 and per2 genes and regulates their transcription. ERK/MAPK interacts with and phosphorylates CLOCK, BMAL1, CRY1, and CRY2 in the circadian system, leading to a rapid antidepressant effect. SD, sleep deprivation; ECT, electroconvulsive therapy; DBS, deep brain stimulation.
At present, most of the commonly-used first-line antidepressants target monoamine neurotransmitters, such as the selective serotonin reuptake inhibitors. Although the MAPK signaling pathway is involved in almost all of their mechanisms, they do not directly target this signaling pathway, so the onset of their action takes a relatively long time. Sleep deprivation can have a rapid antidepressant action but this only lasts for a short period and is easy to reverse. Depressive symptoms can recur as sleep recovers. However, the other rapid antidepressant methods, such as DBS, modified ECT, and low-dose of ketamine, whose mechanisms of action are still in the exploratory stage, are not well established. So we cannot provide the long-term outcomes of the currently available interventions targeting the MAPK pathway for antidepressant effects.
Conclusions and Future Directions
To date, accumulating evidence has indicated that the MAPK/ERK pathway is involved in the pathogenesis of depression and the mechanism of action of both pharmaceutical and non-pharmaceutical antidepressant therapies. Increasing evidence also demonstrates the mediation of entrainment of the circadian system by the MAPK/ERK pathway. However, little evidence links these systems to depression. To our knowledge, only one study has directly demonstrated such a mechanism in depression [127] using comparative transcriptomics analyses. Future studies are needed to test this hypothesis using various models, including behavioral tests, western blot, virus microinjection into specific brain areas, and conditioned intervention genes in animals.
Some basic questions still need to be answered. First, does the circadian system play an important or indispensable role in the pathogenesis of depression? Second, do other pathways mediate entrainment of the circadian system? Third, how do these pathways interact with and affect the circadian system? These are critical questions that need to be explored. The MAPK pathway is a promising target for novel therapeutics with a rapid onset of action for the long-term treatment of severe, refractory MDD.
Based on previous studies, we propose that the regulation of circadian genes by the MAPK pathway is involved in the mechanism of depression. Nonetheless, direct evidence is needed to demonstrate that the rapid antidepressant effects of non-pharmaceutical and pharmaceutical therapies occur through entrainment of the circadian system mediated by the MAPK/ERK pathway. In fact, not all MDD patients respond to one kind of rapid antidepressant therapy, such as sleep deprivation, DBS or low-dose ketamine. This indicates that the mechanism underlying depression differs in different individuals. Nevertheless, the circadian rhythm involvement in depression and antidepressant therapy may also be complicated. Lazzerini Ospri et al. argued that circadian rhythms and mood could have synergistic effects but not be causally linked [128]. They proposed that mood could be affected by a comparison of the incidental properties of the output of circadian oscillators. Nevertheless, direct evidence or detailed mechanisms have not been provided. Above all, we need to explore the mechanism underlying this phenomenon. The MAPK pathway is important for mood regulation and clock entrainment, but the existing evidence cannot explain this complicated phenomenon.
In conclusion, most researchers in this field support the idea that circadian rhythm and mood are closely related, and some believe that there may be a causal link between them. However, so far there is no direct evidence of such a link. In this review, we propose that rapid-onset antidepressant therapies, both pharmaceutical and non-pharmaceutical, may regulate the circadian rhythm through the MAPK pathway. As far as we know, this is the first review to link the MAPK pathway, the circadian system, and antidepressant action, and only one report supports this link. Hence, in the future, we need use various animal models, specific gene interventions, virus microinjection into specific brain areas, or conditioned intervention genes in animals to test this hypothesis. And this may provide strong support for the Social Zeitgeber Theory of mood disorder.
Acknowledgements
This review was supported by the National Basic Research Development Program of China (2015CB856400 and 2015CB553503), the National Natural Science Foundation of China (81521063), and the Natural Science Foundation of Beijing Municipality, China (7162101).
Contributor Information
Su-Xia Li, Email: li313@bjmu.edu.cn.
George Fu Gao, Email: gaof@im.ac.cn.
Lin Lu, Email: linlu@bjmu.edu.cn.
References
- 1.Albrecht U. Molecular mechanisms in mood regulation involving the circadian clock. Frontiers In Neurology 2017,8. [DOI] [PMC free article] [PubMed]
- 2.McClung CA. How might circadian rhythms control mood? Let me count the ways. Biol Psychiatry. 2013;74:242–249. doi: 10.1016/j.biopsych.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunney BG, Li JZ, Walsh DM, Stein R, Vawter MP, Cartagena P, et al. Circadian dysregulation of clock genes: clues to rapid treatments in major depressive disorder. Mol Psychiatry. 2015;20:48–55. doi: 10.1038/mp.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 5.Serchov T, Heumann R. Ras activity tunes the period and modulates the entrainment of the suprachiasmatic clock. Frontiers In Neurology 2017,8. [DOI] [PMC free article] [PubMed]
- 6.Goldsmith CS, Bell-Pedersen D. Diverse roles for MAPK signaling in circadian clocks. Adv Genet. 2013;84:1–39. doi: 10.1016/B978-0-12-407703-4.00001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunney BG, Bunney WE. Mechanisms of rapid antidepressant effects of sleep deprivation therapy: clock genes and circadian rhythms. Biol Psychiatry. 2013;73:1164–1171. doi: 10.1016/j.biopsych.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang EE, Kay SA. Clocks not winding down: unravelling circadian networks. Nat Rev Mol Cell Biol. 2010;11:764–776. doi: 10.1038/nrm2995. [DOI] [PubMed] [Google Scholar]
- 10.Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, et al. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 11.Honma S, Ikeda M, Abe H, Tanahashi Y, Namihira M, Honma K, et al. Circadian oscillation of BMAL1, a partner of a mammalian clock gene Clock, in rat suprachiasmatic nucleus. Biochem Biophys Res Commun. 1998;250:83–87. doi: 10.1006/bbrc.1998.9275. [DOI] [PubMed] [Google Scholar]
- 12.Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, et al. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43:527–537. doi: 10.1016/j.neuron.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 14.Honma S, Kawamoto T, Takagi Y, Fujimoto K, Sato F, Noshiro M, et al. Dec1 and Dec2 are regulators of the mammalian molecular clock. Nature. 2002;419:841–844. doi: 10.1038/nature01123. [DOI] [PubMed] [Google Scholar]
- 15.Ueda HR, Hayashi S, Chen W, Sano M, Machida M, Shigeyoshi Y, et al. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat Genet. 2005;37:187–192. doi: 10.1038/ng1504. [DOI] [PubMed] [Google Scholar]
- 16.Bunney WE, Bunney BG. Molecular clock genes in man and lower animals: possible implications for circadian abnormalities in depression. Neuropsychopharmacology. 2000;22:335–345. doi: 10.1016/S0893-133X(99)00145-1. [DOI] [PubMed] [Google Scholar]
- 17.Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- 18.Wirz-Justice A. Biological rhythm disturbances in mood disorders. Int Clin Psychopharmacol. 2006;21(Suppl 1):S11–15. doi: 10.1097/01.yic.0000195660.37267.cf. [DOI] [PubMed] [Google Scholar]
- 19.McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007;114:222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehlers Cindy L. Social Zeitgebers and Biological Rhythms. Archives of General Psychiatry. 1988;45(10):948. doi: 10.1001/archpsyc.1988.01800340076012. [DOI] [PubMed] [Google Scholar]
- 21.Li SX, Liu LJ, Xu LZ, Gao L, Wang XF, Zhang JT, et al. Diurnal alterations in circadian genes and peptides in major depressive disorder before and after escitalopram treatment. Psychoneuroendocrinology. 2013;38:2789–2799. doi: 10.1016/j.psyneuen.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Logan RW, Edgar N, Gillman AG, Hoffman D, Zhu XY, McClung CA. Chronic stress induces brain region-specific alterations of molecular rhythms that correlate with depression-like behavior in mice. Biol Psychiatry. 2015;78:249–258. doi: 10.1016/j.biopsych.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y, Liu LJ, Wang C, Li SX. Swimming exercise may not alleviate the depressive-like behaviors and circadian alterations of neuroendocrine induced by chronic unpredictable mild stress in rats. Neurol, Pychiatry Brain Res. 2012;18:202–207. [Google Scholar]
- 24.Guo TY, Liu LJ, Xu LZ, Zhang JC, Li SX, Chen C, et al. Alterations of the daily rhythms of HPT axis induced by chronic unpredicted mild stress in rats. Endocrine. 2015;48:637–643. doi: 10.1007/s12020-014-0314-y. [DOI] [PubMed] [Google Scholar]
- 25.Jiang WG, Li SX, Zhou SJ, Sun Y, Shi J, Lu L. Chronic unpredictable stress induces a reversible change of PER2 rhythm in the suprachiasmatic nucleus. Brain Res. 2011;1399:25–32. doi: 10.1016/j.brainres.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Jiang WG, Li SX, Liu JF, Sun Y, Zhou SJ, Zhu WL, et al. Hippocampal CLOCK protein participates in the persistence of depressive-like behavior induced by chronic unpredictable stress. Psychopharmacology (Berl) 2013;227:79–92. doi: 10.1007/s00213-012-2941-4. [DOI] [PubMed] [Google Scholar]
- 27.Partonen T. Clock gene variants in mood and anxiety disorders. J Neural Transm (Vienna) 2012;119:1133–1145. doi: 10.1007/s00702-012-0810-2. [DOI] [PubMed] [Google Scholar]
- 28.McClung CA. Circadian rhythms and mood regulation: insights from pre-clinical models. Eur Neuropsychopharmacol. 2011;21(Suppl 4):S683–693. doi: 10.1016/j.euroneuro.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hampp G, Albrecht U. The circadian clock and mood-related behavior. Commun Integr Biol. 2008;1:1–3. doi: 10.4161/cib.1.1.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, et al. Regulation of monoamine oxidase A by circadian-clock components implies clock influence on mood. Curr Biol. 2008;18:678–683. doi: 10.1016/j.cub.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Spulber S, Conti M, DuPont C, Raciti M, Bose R, Onishchenko N, et al. Alterations in circadian entrainment precede the onset of depression-like behavior that does not respond to fluoxetine. Transl Psychiatry. 2015;5:e603. doi: 10.1038/tp.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright KP, Jr, Czeisler CA. Absence of circadian phase resetting in response to bright light behind the knees. Science. 2002;297:571. doi: 10.1126/science.1071697. [DOI] [PubMed] [Google Scholar]
- 34.Honma S. The mammalian circadian system: a hierarchical multi-oscillator structure for generating circadian rhythm. J Physiol Sci. 2018;68:207–219. doi: 10.1007/s12576-018-0597-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeitzer JM, Kronauer RE, Czeisler CA. Photopic transduction implicated in human circadian entrainment. Neurosci Lett. 1997;232:135–138. doi: 10.1016/s0304-3940(97)00599-5. [DOI] [PubMed] [Google Scholar]
- 36.Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526(Pt 3):695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duffy JF, Wright KP., Jr Entrainment of the human circadian system by light. J Biol Rhythms. 2005;20:326–338. doi: 10.1177/0748730405277983. [DOI] [PubMed] [Google Scholar]
- 38.Akashi M, Nishida E. Involvement of the MAP kinase cascade in resetting of the mammalian circadian clock. Genes & Development. 2000;14:645–649. [PMC free article] [PubMed] [Google Scholar]
- 39.Plotnikov A, Zehorai E, Procaccia S, Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta. 2011;1813:1619–1633. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 40.Serchov T, Jilg A, Wolf CT, Radtke I, Stehle JH, Heumann R. Ras activity oscillates in the mouse suprachiasmatic nucleus and modulates circadian clock dynamics. Mol Neurobiol. 2016;53:1843–1855. doi: 10.1007/s12035-015-9135-0. [DOI] [PubMed] [Google Scholar]
- 41.Butcher GQ, Lee B, Obrietan K. Temporal regulation of light-induced extracellular signal-regulated kinase activation in the suprachiasmatic nucleus. J Neurophysiol. 2003;90:3854–3863. doi: 10.1152/jn.00524.2003. [DOI] [PubMed] [Google Scholar]
- 42.Cheng P, He Q, He Q, Wang L, Liu Y. Regulation of the Neurospora circadian clock by an RNA helicase. Genes Dev. 2005;19:234–241. doi: 10.1101/gad.1266805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eckel-Mahan KL, Phan T, Han S, Wang H, Chan GC, Scheiner ZS, et al. Circadian oscillation of hippocampal MAPK activity and cAMP: implications for memory persistence. Nat Neurosci. 2008;11:1074–1082. doi: 10.1038/nn.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayashi Y, Sanada K, Fukada Y. Circadian and photic regulation of MAP kinase by Ras- and protein phosphatase-dependent pathways in the chick pineal gland. FEBS Lett. 2001;491:71–75. doi: 10.1016/s0014-5793(01)02153-6. [DOI] [PubMed] [Google Scholar]
- 45.Relogio A, Thomas P, Medina-Perez P, Reischl S, Bervoets S, Gloc E, et al. Ras-mediated deregulation of the circadian clock in cancer. PLoS Genet. 2014;10:e1004338. doi: 10.1371/journal.pgen.1004338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serchov T, Heumann R. Constitutive activation of ras in neurons: implications for the regulation of the mammalian circadian clock. Chronobiol Int. 2006;23:191–200. doi: 10.1080/07420520500521970. [DOI] [PubMed] [Google Scholar]
- 47.Tsuchiya Y, Minami I, Kadotani H, Todo T, Nishida E. Circadian clock-controlled diurnal oscillation of Ras/ERK signaling in mouse liver. Proc Jpn Acad Ser B Phys Biol Sci. 2013;89:59–65. doi: 10.2183/pjab.89.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weber F, Hung HC, Maurer C, Kay SA. Second messenger and Ras/MAPK signalling pathways regulate CLOCK/CYCLE-dependent transcription. J Neurochem. 2006;98:248–257. doi: 10.1111/j.1471-4159.2006.03865.x. [DOI] [PubMed] [Google Scholar]
- 49.Butcher GQ, Doner J, Dziema H, Collamore M, Burgoon PW, Obrietan K. The p42/44 mitogen-activated protein kinase pathway couples photic input to circadian clock entrainment. J Biol Chem. 2002;277:29519–29525. doi: 10.1074/jbc.M203301200. [DOI] [PubMed] [Google Scholar]
- 50.Coogan AN, Piggins HD. MAP kinases in the mammalian circadian system–key regulators of clock function. J Neurochem. 2004;90:769–775. doi: 10.1111/j.1471-4159.2004.02554.x. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011;34:572–580. doi: 10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 53.Tischkau SA, Gallman EA, Buchanan GF, Gillette MU. Differential cAMP gating of glutamatergic signaling regulates long-term state changes in the suprachiasmatic circadian clock. J Neurosci. 2000;20:7830–7837. doi: 10.1523/JNEUROSCI.20-20-07830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding JM, Chen D, Weber ET, Faiman LE, Rea MA, Gillette MU. Resetting the biological clock - mediation of nocturnal circadian shifts by glutamate and No. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- 55.Farnsworth CL, Freshney NW, Rosen LB, Ghosh A, Greenberg ME, Feig LA. Calcium Activation Of Ras Mediated by Neuronal Exchange Factor Ras-Grf. Nature. 1995;376:524–527. doi: 10.1038/376524a0. [DOI] [PubMed] [Google Scholar]
- 56.Wang JQ, Tang QS, Parelkar NK, Liu ZG, Samdani S, Choe ES, et al. Glutamate signaling to Ras-MAPK in striatal neurons - Mechanisms for inducible gene expression and plasticity. Mol Neurobiol. 2004;29:1–14. doi: 10.1385/MN:29:1:01. [DOI] [PubMed] [Google Scholar]
- 57.Butcher GQ, Lee BY, Hsieh F, Obrietan K. Light- and clock-dependent regulation of ribosomal S6 kinase activity in the suprachiasmatic nucleus. Eur J Neurosci. 2004;19:907–915. doi: 10.1111/j.0953-816x.2004.03155.x. [DOI] [PubMed] [Google Scholar]
- 58.Travnickova-Bendova Z, Cermakian N, Reppert SM, Sassone-Corsi P. Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. Proc Natl Acad Sci U S A. 2002;99:7728–7733. doi: 10.1073/pnas.102075599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eiden LE, Emery AC, Zhang LM, Smith CB. PACAP signaling in stress: insights from the chromaffin cell. Pflugers Arch. 2018;470:79–88. doi: 10.1007/s00424-017-2062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dziema H, Oatis B, Butcher GQ, Yates R, Hoyt KR, Obrietan K. The ERK/MAP kinase pathway couples light to immediate-early gene expression in the suprachiasmatic nucleus. Eur J Neurosci. 2003;17:1617–1627. doi: 10.1046/j.1460-9568.2003.02592.x. [DOI] [PubMed] [Google Scholar]
- 61.Kornhauser JM, Nelson DE, Mayo KE, Takahashi JS. Photic and circadian regulation of c-fos gene expression in the hamster suprachiasmatic nucleus. Neuron. 1990;5:127–134. doi: 10.1016/0896-6273(90)90303-w. [DOI] [PubMed] [Google Scholar]
- 62.Abe H, Rusak B. Physiological mechanisms regulating photic induction of Fos-like protein in hamster suprachiasmatic nucleus. Neurosci Biobehav Rev. 1994;18:531–536. doi: 10.1016/0149-7634(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 63.Tischkau SA, Mitchell JW, Tyan SH, Buchanan GF, Gillette MU. Ca2+/cAMP response element-binding protein (CREB)-dependent activation of Per1 is required for light-induced signaling in the suprachiasmatic nucleus circadian clock. J Biol Chem. 2003;278:718–723. doi: 10.1074/jbc.M209241200. [DOI] [PubMed] [Google Scholar]
- 64.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mrosovsky N. A non-photic gateway to the circadian clock of hamsters. Ciba Found Symp 1995,183:154–167; discussion 167–174. [DOI] [PubMed]
- 66.Mrosovsky N. Locomotor activity and non-photic influences on circadian clocks. Biol Rev Camb Philos Soc. 1996;71:343–372. doi: 10.1111/j.1469-185x.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 67.Antle MC, Tse F, Koke SJ, Sterniczuk R, Hagel K. Non-photic phase shifting of the circadian clock: role of the extracellular signal-responsive kinases I/II/mitogen-activated protein kinase pathway. Eur J Neurosci. 2008;28:2511–2518. doi: 10.1111/j.1460-9568.2008.06533.x. [DOI] [PubMed] [Google Scholar]
- 68.Van Reeth O, Turek FW. Stimulated activity mediates phase shifts in the hamster circadian clock induced by dark pulses or benzodiazepines. Nature. 1989;339:49–51. doi: 10.1038/339049a0. [DOI] [PubMed] [Google Scholar]
- 69.Mrosovsky N, Salmon PA. Triazolam and phase-shifting acceleration re-evaluated. Chronobiol Int. 1990;7:35–41. doi: 10.3109/07420529009056952. [DOI] [PubMed] [Google Scholar]
- 70.Marchant EG, Mistlberger RE. Morphine phase-shifts circadian rhythms in mice: role of behavioural activation. Neuroreport. 1995;7:209–212. [PubMed] [Google Scholar]
- 71.Reebs SG, Mrosovsky N. Effects of induced wheel running on the circadian activity rhythms of Syrian hamsters: entrainment and phase response curve. J Biol Rhythms. 1989;4:39–48. doi: 10.1177/074873048900400103. [DOI] [PubMed] [Google Scholar]
- 72.Wickland CR, Turek FW. Phase-shifting effects of acute increases in activity on circadian locomotor rhythms in hamsters. Am J Physiol. 1991;261:R1109–1117. doi: 10.1152/ajpregu.1991.261.5.R1109. [DOI] [PubMed] [Google Scholar]
- 73.Mrosovsky N. Phase response curves for social entrainment. J Comp Physiol A. 1988;162:35–46. doi: 10.1007/BF01342701. [DOI] [PubMed] [Google Scholar]
- 74.Antle MC, Mistlberger RE. Circadian clock resetting by sleep deprivation without exercise in the Syrian hamster. J Neurosci. 2000;20:9326–9332. doi: 10.1523/JNEUROSCI.20-24-09326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mistlberger RE, Skene DJ. Nonphotic entrainment in humans? J Biol Rhythms. 2005;20:339–352. doi: 10.1177/0748730405277982. [DOI] [PubMed] [Google Scholar]
- 76.Coogan AN, Piggins HD. Dark pulse suppression of P-ERK and c-Fos in the hamster suprachiasmatic nuclei. Eur J Neurosci. 2005;22:158–168. doi: 10.1111/j.1460-9568.2005.04193.x. [DOI] [PubMed] [Google Scholar]
- 77.van Calker D, Biber K. The role of glial adenosine receptors in neural resilience and the neurobiology of mood disorders. Neurochem Res. 2005;30:1205–1217. doi: 10.1007/s11064-005-8792-1. [DOI] [PubMed] [Google Scholar]
- 78.Burnstock G, Krugel U, Abbracchio MP, Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol. 2011;95:229–274. doi: 10.1016/j.pneurobio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 79.Sadek AR, Knight GE, Burnstock G. Electroconvulsive therapy: a novel hypothesis for the involvement of purinergic signalling. Purinergic Signal. 2011;7:447–452. doi: 10.1007/s11302-011-9242-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Serchov T, Heumann R, van Calker D, Biber K. Signaling pathways regulating Homer1a expression: implications for antidepressant therapy. Biol Chem. 2016;397:207–214. doi: 10.1515/hsz-2015-0267. [DOI] [PubMed] [Google Scholar]
- 81.Basheer R, Bauer A, Elmenhorst D, Ramesh V, McCarley RW. Sleep deprivation upregulates A1 adenosine receptors in the rat basal forebrain. Neuroreport. 2007;18:1895–1899. doi: 10.1097/WNR.0b013e3282f262f6. [DOI] [PubMed] [Google Scholar]
- 82.Elmenhorst D, Basheer R, McCarley RW, Bauer A. Sleep deprivation increases A(1) adenosine receptor density in the rat brain. Brain Res. 2009;1258:53–58. doi: 10.1016/j.brainres.2008.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elmenhorst D, Meyer PT, Winz OH, Matusch A, Ermert J, Coenen HH, et al. Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study. J Neurosci. 2007;27:2410–2415. doi: 10.1523/JNEUROSCI.5066-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bekar L, Libionka W, Tian GF, Xu Q, Torres A, Wang X, et al. Adenosine is crucial for deep brain stimulation-mediated attenuation of tremor. Nat Med. 2008;14:75–80. doi: 10.1038/nm1693. [DOI] [PubMed] [Google Scholar]
- 85.Hamani C, Diwan M, Macedo CE, Brandao ML, Shumake J, Gonzalez-Lima F, et al. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol Psychiatry. 2010;67:117–124. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 86.Biber K, Klotz KN, Berger M, Gebicke-Harter PJ, van Calker D. Adenosine A1 receptor-mediated activation of phospholipase C in cultured astrocytes depends on the level of receptor expression. J Neurosci. 1997;17:4956–4964. doi: 10.1523/JNEUROSCI.17-13-04956.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rogel A, Bromberg Y, Sperling O, Zoref-Shani E. The neuroprotective adenosine-activated signal transduction pathway involves activation of phospholipase C. Nucleosides Nucleotides Nucleic Acids. 2006;25:1283–1286. doi: 10.1080/15257770600890939. [DOI] [PubMed] [Google Scholar]
- 88.Fenton RA, Shea LG, Doddi C, Dobson JG., Jr Myocardial adenosine A(1)-receptor-mediated adenoprotection involves phospholipase C, PKC-epsilon, and p38 MAPK, but not HSP27. Am J Physiol Heart Circ Physiol. 2010;298:H1671–1678. doi: 10.1152/ajpheart.01028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Robin E, Sabourin J, Benoit R, Pedretti S, Raddatz E. Adenosine A1 receptor activation is arrhythmogenic in the developing heart through NADPH oxidase/ERK- and PLC/PKC-dependent mechanisms. J Mol Cell Cardiol. 2011;51:945–954. doi: 10.1016/j.yjmcc.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 90.Migita H, Kominami K, Higashida M, Maruyama R, Tuchida N, McDonald F, et al. Activation of adenosine A1 receptor-induced neural stem cell proliferation via MEK/ERK and Akt signaling pathways. J Neurosci Res. 2008;86:2820–2828. doi: 10.1002/jnr.21742. [DOI] [PubMed] [Google Scholar]
- 91.Kunduri SS, Mustafa SJ, Ponnoth DS, Dick GM, Nayeem MA. Adenosine A1 receptors link to smooth muscle contraction via CYP4a, protein kinase C-alpha, and ERK1/2. J Cardiovasc Pharmacol. 2013;62:78–83. doi: 10.1097/FJC.0b013e3182919591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Qi H, Mailliet F, Spedding M, Rocher C, Zhang X, Delagrange P, et al. Antidepressants reverse the attenuation of the neurotrophic MEK/MAPK cascade in frontal cortex by elevated platform stress; reversal of effects on LTP is associated with GluA1 phosphorylation. Neuropharmacology. 2009;56:37–46. doi: 10.1016/j.neuropharm.2008.06.068. [DOI] [PubMed] [Google Scholar]
- 93.Duric V, Banasr M, Licznerski P, Schmidt HD, Stockmeier CA, Simen AA, et al. A negative regulator of MAP kinase causes depressive behavior. Nat Med. 2010;16:1328–1332. doi: 10.1038/nm.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang L, Xu T, Wang S, Yu L, Liu D, Zhan R, et al. Curcumin produces antidepressant effects via activating MAPK/ERK-dependent brain-derived neurotrophic factor expression in the amygdala of mice. Behav Brain Res. 2012;235:67–72. doi: 10.1016/j.bbr.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 95.Di Benedetto B, Radecke J, Schmidt MV, Rupprecht R. Acute antidepressant treatment differently modulates ERK/MAPK activation in neurons and astrocytes of the adult mouse prefrontal cortex. Neuroscience. 2013;232:161–168. doi: 10.1016/j.neuroscience.2012.11.061. [DOI] [PubMed] [Google Scholar]
- 96.Yuan P, Zhou R, Wang Y, Li X, Li J, Chen G, et al. Altered levels of extracellular signal-regulated kinase signaling proteins in postmortem frontal cortex of individuals with mood disorders and schizophrenia. J Affect Disord. 2010;124:164–169. doi: 10.1016/j.jad.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dwivedi Y, Rizavi HS, Teppen T, Sasaki N, Chen H, Zhang H, et al. Aberrant extracellular signal-regulated kinase (ERK) 5 signaling in hippocampus of suicide subjects. Neuropsychopharmacology. 2007;32:2338–2350. doi: 10.1038/sj.npp.1301372. [DOI] [PubMed] [Google Scholar]
- 98.Dwivedi Y, Rizavi HS, Zhang H, Mondal AC, Roberts RC, Conley RR, et al. Neurotrophin receptor activation and expression in human postmortem brain: effect of suicide. Biol Psychiatry. 2009;65:319–328. doi: 10.1016/j.biopsych.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dwivedi Y, Rizavi HS, Conley RR, Pandey GN. ERK MAP kinase signaling in post-mortem brain of suicide subjects: differential regulation of upstream Raf kinases Raf-1 and B-Raf. Mol Psychiatry. 2006;11:86–98. doi: 10.1038/sj.mp.4001744. [DOI] [PubMed] [Google Scholar]
- 100.Dwivedi Y, Rizavi HS, Roberts RC, Conley RC, Tamminga CA, Pandey GN. Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects. J Neurochem. 2001;77:916–928. doi: 10.1046/j.1471-4159.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- 101.Barthas F, Humo M, Gilsbach R, Waltisperger E, Karatas M, Leman S, et al. Cingulate Overexpression of Mitogen-Activated Protein Kinase Phosphatase-1 as a Key Factor for Depression. Biol Psychiatry. 2017;82:370–379. doi: 10.1016/j.biopsych.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 102.Marsden WN. Synaptic plasticity in depression: Molecular, cellular and functional correlates. Progress In Neuro-Psychopharmacology & Biological Psychiatry. 2013;43:168–184. doi: 10.1016/j.pnpbp.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 103.First M, Gil-Ad I, Taler M, Tarasenko I, Novak N, Weizman A. The effects of fluoxetine treatment in a chronic mild stress rat model on depression-related behavior, brain neurotrophins and ERK expression. J Mol Neurosci. 2011;45:246–255. doi: 10.1007/s12031-011-9515-5. [DOI] [PubMed] [Google Scholar]
- 104.Qi X, Lin W, Wang D, Pan Y, Wang W, Sun M. A role for the extracellular signal-regulated kinase signal pathway in depressive-like behavior. Behav Brain Res. 2009;199:203–209. doi: 10.1016/j.bbr.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 105.Qi XL, Lin WJ, Li JF, Pan YQ, Wang WW. The depressive-like behaviors are correlated with decreased phosphorylation of mitogen-activated protein kinases in rat brain following chronic forced swim stress. Behavioural Brain Research. 2006;175:233–240. doi: 10.1016/j.bbr.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 106.Reus GZ, Vieira FG, Abelaira HM, Michels M, Tomaz DB, dos Santos MA, et al. MAPK signaling correlates with the antidepressant effects of ketamine. J Psychiatr Res. 2014;55:15–21. doi: 10.1016/j.jpsychires.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 107.Pochwat B, Rafalo-Ulinska A, Domin H, Misztak P, Nowak G, Szewczyk B. Involvement of extracellular signal-regulated kinase (ERK) in the short and long-lasting antidepressant-like activity of NMDA receptor antagonists (zinc and Ro 25-6981) in the forced swim test in rats. Neuropharmacology. 2017;125:333–342. doi: 10.1016/j.neuropharm.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 108.Labonte B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, et al. Sex-specific transcriptional signatures in human depression. Nat Med. 2017;23:1102–1111. doi: 10.1038/nm.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Quan MN, Zhang N, Wang YY, Zhang T, Yang Z. Possible antidepressant effects and mechanisms of memantine in behaviors and synaptic plasticity of a depression rat model. Neuroscience. 2011;182:88–97. doi: 10.1016/j.neuroscience.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 110.Covington HE, 3rd, Vialou V, Nestler EJ. From synapse to nucleus: novel targets for treating depression. Neuropharmacology. 2010;58:683–693. doi: 10.1016/j.neuropharm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mailliet F, Qi H, Rocher C, Spedding M, Svenningsson P, Jay TM. Protection of stress-induced impairment of hippocampal/prefrontal LTP through blockade of glucocorticoid receptors: implication of MEK signaling. Exp Neurol. 2008;211:593–596. doi: 10.1016/j.expneurol.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 112.Pflug B, Tolle R. Disturbance of the 24-hour rhythm in endogenous depression and the treatment of endogenous depression by sleep deprivation. Int Pharmacopsychiatry. 1971;6:187–196. doi: 10.1159/000468269. [DOI] [PubMed] [Google Scholar]
- 113.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 114.Thompson CL, Wisor JP, Lee CK, Pathak SD, Gerashchenko D, Smith KA, et al. Molecular and anatomical signatures of sleep deprivation in the mouse brain. Front Neurosci. 2010;4:165. doi: 10.3389/fnins.2010.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wisor JP, Pasumarthi RK, Gerashchenko D, Thompson CL, Pathak S, Sancar A, et al. Sleep deprivation effects on circadian clock gene expression in the cerebral cortex parallel electroencephalographic differences among mouse strains (vol 28, pg 7193, 2008) J Neurosci. 2008;28:7929–7929. doi: 10.1523/JNEUROSCI.1150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wisorl JP, O’Hara BF, Terao A, Selby CP, Kilduff TS, Sancar A, et al. A role for cryptochromes in sleep regulation. Bmc Neuroscience 2002,3. [DOI] [PMC free article] [PubMed]
- 117.Maret S, Dorsaz S, Gurcel L, Pradervand S, Petit B, Pfister C, et al. Homer1a is a core brain molecular correlate of sleep loss. Proc Natl Acad Sci U S A. 2007;104:20090–20095. doi: 10.1073/pnas.0710131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zagaar M, Dao A, Levine A, Alhaider I, Alkadhi K. Regular exercise prevents sleep deprivation associated impairment of long-term memory and synaptic plasticity in the CA1 area of the hippocampus. Sleep. 2013;36:751–761. doi: 10.5665/sleep.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Serchov T, Clement HW, Schwarz MK, Iasevoli F, Tosh DK, Idzko M, et al. Increased Signaling via Adenosine A1 Receptors, Sleep Deprivation, Imipramine, and Ketamine Inhibit Depressive-like Behavior via Induction of Homer1a. Neuron. 2015;87:549–562. doi: 10.1016/j.neuron.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hines DJ, Schmitt LI, Hines RM, Moss SJ, Haydon PG. Antidepressant effects of sleep deprivation require astrocyte-dependent adenosine mediated signaling. Transl Psychiatry. 2013;3:e212. doi: 10.1038/tp.2012.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014;15:443–454. doi: 10.1038/nrn3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li X, Li X. The antidepressant effect of light therapy from retinal projections. Neurosci Bull. 2018;34:359–368. doi: 10.1007/s12264-018-0210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Duncan WC, Jr., Slonena E, Hejazi NS, Brutsche N, Yu KC, Park L, et al. Motor-activity markers of circadian timekeeping are related to ketamine’s rapid antidepressant properties. Biol Psychiatry 2017. [DOI] [PMC free article] [PubMed]
- 124.Bellet MM, Vawter MP, Bunney BG, Bunney WE, Sassone-Corsi P. Ketamine influences CLOCK:BMAL1 function leading to altered circadian gene expression. PLoS One. 2011;6:e23982. doi: 10.1371/journal.pone.0023982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ma Z, Zang T, Birnbaum SG, Wang Z, Johnson JE, Zhang CL, et al. TrkB dependent adult hippocampal progenitor differentiation mediates sustained ketamine antidepressant response. Nat Commun. 2017;8:1668. doi: 10.1038/s41467-017-01709-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang C, Ren Q, Qu YG, Zhang JC, Ma M, Dong C, et al. Mechanistic target of rapamycin-independent antidepressant effects of (R)-Ketamine in a social defeat stress model. Biol Psychiatry. 2018;83:18–28. doi: 10.1016/j.biopsych.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 127.Orozco-Solis R, Montellier E, Aguilar-Arnal L, Sato S, Vawter MP, Bunney BG, et al. A circadian genomic signature common to ketamine and sleep deprivation in the anterior cingulate cortex. Biol Psychiatry. 2017;82:351–360. doi: 10.1016/j.biopsych.2017.02.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lazzerini Ospri L, Prusky G, Hattar S. Mood, the circadian system, and melanopsin retinal ganglion cells. Annu Rev Neurosci. 2017;40:539–556. doi: 10.1146/annurev-neuro-072116-031324. [DOI] [PMC free article] [PubMed] [Google Scholar]