Abstract
Topical irritants such as capsaicin (CAP), peppermint oil (PO), and mustard oil (MO) are effective in relieving inflammatory muscle pain. We investigated the effects of topical irritants in a rat model of inflammatory muscle pain produced by injecting complete Freund’s adjuvant (CFA) into the tibialis anterior muscle. CFA-induced mechanical hypersensitivity and the spontaneous activity of muscular nociceptive afferents, and decreased weight-bearing of the hindlimb were relieved by topical application of CAP, PO, or MO on the skin overlying the inflamed muscle. The effects of topical irritants were abolished when applied to the skin on the ipsilateral plantar region or on the contralateral leg, or when the relevant cutaneous nerve or dorsal root was transected. Our results demonstrated that topical irritants may alleviate inflammatory muscle pain via activating cutaneous nociceptors and subsequently inhibiting the abnormal activity of muscular nociceptive neurons.
Keywords: Inflammatory muscle pain, Muscular nociceptor, Cutaneous nociceptor, Capsaicin
Introduction
Muscle pain is a common medical issue worldwide that can interfere with patients’ motor control [1, 2]. Besides genetic factors in diseases such as Duchenne’s syndrome and fibromyalgia [3–5], most muscle pain can be attributed to muscle strain, damage, and ischemia [6, 7]. For example, low back pain, which is a common complaint in patients with muscle strain or occupational muscle damage, can last for a long time and greatly impair the quality of life [8, 9].
However, most of our knowledge about pain is based on studies of cutaneous tissue and relatively less is known about muscle pain. In contrast to cutaneous pain, which is stabbing, tolerable, and well localized, muscle pain is more likely to be tearing, intolerable, and poorly localized and has a marked tendency of referred pain [1]. Previous studies have revealed that muscle nociceptors can respond to mechanical, thermal, and chemical stimuli, leading to muscle spasm and inflammatory muscle pain [10–13]. But almost all these results are based on muscular nerve filament recordings in rodent models, or the visual analogue pain scale in human subjects, so the electrophysiological characteristics of muscle nociceptors remain unclear.
In medical practice, topical application of irritant chemicals such as capsaicin (CAP) is effective in relieving many kinds of pain, including neuropathic pain, arthritis pain, neck pain, cluster headache, and post-mastectomy pain syndrome [14, 15]. Studies on transdermal absorption have revealed that the most concentrated topical drugs cannot penetrate the epidermis, suggesting an important role of cutaneous afferents in the analgesic effect of topical CAP [16]. Previously, we showed that topical analgesics might inhibit inflammatory muscle pain via the activation of cutaneous nociceptors in a rat model [17]. According to diffuse noxious inhibitory control theory [18], noxious stimulation from one location may be inhibited by another stimulus from a different location through convergent dorsal horn neurons. However, it is not clear whether the activity of muscle nociceptive neurons is inhibited by noxious stimuli to the skin, and whether this inhibition is associated with the activation of specific types of cutaneous nociceptors. In this study, we set out to answer the above questions with in vivo electrophysiological recordings from single muscle nociceptors in a rat model of inflammatory muscle pain.
Materials and Methods
Animals
Adult female Sprague-Dawley rats (specific pathogen free, 180 g–220 g, provided by the National Institutes for Food and Drug Control, Beijing, China) were randomly assigned to subgroups. All rats were housed at 23 °C ± 2 °C and a 12/12 h light/dark cycle-controlled room with free access to rodent chow and water. This study was approved by the Institutional Animal Care and Use Committees of the Chinese Academy of Medical Sciences and the Institute of Basic Medical Sciences (Approval Number: #211–2014).
Complete Freund’s Adjuvant (CFA) Injection
Under anesthesia (sodium pentobarbital, 50 mg/kg, i.p., Sigma Aldrich, St. Louis, MO, USA), the tibialis anterior muscle of the right (experimental) hindlimb was injected with 100 μL of CFA (#F5881, Sigma Aldrich) composed of inactivated and dried mycobacteria. The CFA was injected slowly into the muscle and no leakage was observed. The left (control) tibialis anterior muscle received 100 μL sterile saline. A total of 61 rats received CFA injections.
Surgery
Denervation of the Skin Over the Tibialis Anterior Muscle
Skin denervation surgery in 13 rats was conducted 3 days before injection of CFA. Under anesthesia (sodium pentobarbital, 50 mg/kg, i.p.), a longitudinal incision was made on the skin over the tibialis anterior. The lateral cutaneous branches of the superficial peroneal nerve were bluntly separated and transected. The day after surgery, failure to respond to a nociceptive pinch of the skin over tibialis anterior indicated successful denervation (see a previous report for detailed surgical procedures [17]). Transection of the cutaneous branches of the superficial peroneal nerve only causes loss of sensation in the corresponding receptive field but does not cause neuropathic pain, as previously reported in both rat models [19] and humans [20].
L4 Dorsal Rhizotomy
Three days before CFA injection, an L4 dorsal rhizotomy was performed in 2 rats. Under anesthesia (sodium pentobarbital, 50 mg/kg, i.p.), a skin incision was made in the midline L3–L5 region and muscles attached to the right L4 vertebra were removed. A hemilaminectomy was conducted with the dura matter and arachnoid membrane removed, then the L4 dorsal root was transected ~3 mm proximal to the L4 dorsal root ganglion (DRG). Damage to the L4 DRG and spinal cord was carefully avoided.
Assessment of CFA-Induced Inflammatory Muscle Pain
Hindlimb Perimeter Measurement
Rats (n = 5) were placed in the prone position. The two ends of the tibia were labeled on the skin, then the perimeter of limb was measured at the middle of the tibia.
Weight-Bearing Measurement
Rats (n = 47) were positioned with the hindlimbs on force plates in an Incapacitance Tester (Institute of Biomedical Engineering, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China). When the animal was immobile and stable, the weight bearing capacity (g) of each hindlimb in 10 s was automatically averaged. The difference score (g) was defined as weight-bearing on the right (experimental) minus that on the left (control). The final difference score was determined as the average of 3 measurements.
Evan’s Blue Extravasation
A total of 4 rats were used in this experiment. Two days after CFA injection, 2 mL Evans blue (1%, i.v., Sigma Aldrich) was injected into the caudal vein. Transcardiac perfusion was conducted with 0.1 mol/L phosphate-buffered saline (PBS) 30 min later. After transcardial perfusion, the bilateral tibialis anterior muscles were harvested. Evan’s blue extravasation was measured as previously reported [21, 22].
Application of Topical Irritants
CAP, mustard oil (MO), and peppermint oil (PO) are known to activate cutaneous nociceptors [23–26]. Two days after CFA injection, hair was removed from the skin on the hindlimb and smeared with 0.5 mL 0.3% CAP, 50% MO, or 80% PO. Ethanol (ET) was used as vehicle control.
Immunofluorescent Staining
Six rats were used in this experiment. Under anesthesia (sodium pentobarbital, 50 mg/kg, i.p.), 100 μL of the fluorescent dye 1,1′-dioctadecyl-3,3,3′,3′-tetrame-thylindocarbocyanine perchlorate (DiI) (200 μg/mL, Sigma Aldrich) was slowly and carefully injected to the right tibialis anterior, and no obvious DiI leakage was observed. Immunofluorescent staining was conducted 8 days later. Briefly, isotonic saline was pumped into the left ventricle and flowed from the right atrial appendage to remove the blood, and then 4% paraformaldehyde was perfused to fix the tissue. The ipsilateral L2–L6 DRGs were collected and fixed in 4% paraformaldehyde overnight at 4 °C, followed by dehydration in 30% sucrose. The DRGs were then embedded and cut at 15 μm on a cryostat for immunofluorescent staining. After soaking in 0.2% Triton X-100 in PBS for 15 min and blocking in 10% normal donkey serum for 1 h at room temperature, sections of DRGs were incubated overnight at 4 °C in 10% normal donkey serum in PBS containing primary antibodies against PGP9.5, a ubiquitin hydrolase specifically expressed in neuronal tissue. The section was then incubated with secondary antibodies for 1 h. Finally, slides were washed in PBS and coverslipped with Vectashield mounting medium with DAPI. Images were captured using a confocal laser scanning microscope FV1000 (Olympus, Tokyo, Japan) and Olympus FluoView software. Positive neurons in each DRG were counted and then categorized into small (< 30 μm diameter), medium (30 μm–45 μm), and large (> 45 μm) neurons [27].
In Vivo Electrophysiological Recording
In vivo extracellular electrophysiological recordings from DRG neurons were performed in 67 DiI-labeled rats. Detailed information on the surgical exposure and extracellular recording from the L4 DRG was as in a previous report [28]. Briefly, under pentobarbital anesthesia (initial dose of 50 mg/kg i.p. followed by supplementary doses of 20 mg/kg i.p. whenever needed), the L5 transverse process was removed to expose the L4 DRG, and a laminectomy was made from L1 to L6. Oxygenated artificial cerebrospinal fluid at 35 °C was dripped onto the surface of the DRG during surgery and recording. Under a dissecting microscope, the perineurium and epineurium were carefully removed, the rat was transferred to the recording platform, and a pool was formed by attaching the skin to a metal ring. The receptive field of a DRG neuron was identified by exploring the tibialis anterior using a von Frey filament with the fixed tip diameter (1 mm) or a blunt probe. To ensure that the receptive field was in the muscle (not on the skin), the same mechanical stimuli were also applied to the skin. Von Frey filaments were applied to the tibialis anterior at different forces (40 mN, 80 mN, 100 mN, 200 mN, and 280 mN) and the mechanical threshold to evoke an action potential (AP) was repeatedly measured for 10 min. Spontaneous activity (SA) was defined as a continuous discharge lasting for 3 min without any external stimulus. Any electrical activity lasting < 3 s or induced by an external stimulus was excluded. Once SA was identified, the irritant was smeared on the skin and then SA was recorded continuously for at least 30 min.
Statistical Analysis
All data are presented as the mean and its standard error (mean ± SEM). Differences between two groups were analyzed using Student’s t-test. Differences among multiple groups were analyzed using one-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test. Two-way (time and group) ANOVA with Bonferroni the post hoc test was used to compare repeated measurements at different time points among groups. A statistically significant difference was defined as a two-sided P value < 0.05. IBM SPSS Statistics for Windows (version 21.0, Armonk, NY, USA) was used for statistical analysis.
Results
CFA-Induced Inflammatory Muscle Pain
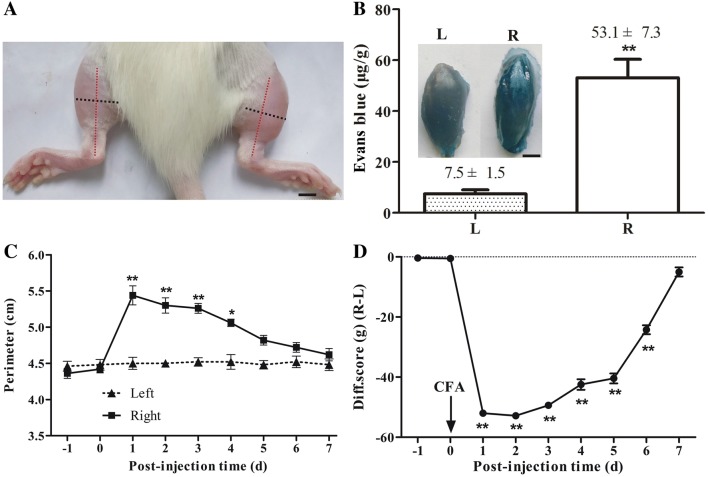
Two days after CFA injection into the right tibialis anterior muscle, the right hindlimb swelled (Fig. 1A) and Evans blue extravasation, an indicator of inflammation, was evident (n = 4, P < 0.01, Fig. 1B). The perimeter of the right hindlimb at the middle of the tibia peaked at 1 day after CFA injection (n = 5, P < 0.01) and then decreased gradually in the following days (Fig. 1C). The difference score for weight-bearing declined markedly with a minimum at 1 day after CFA injection (n = 6, P < 0.01), and recovered 7 days later (Fig. 1D).
Fig. 1.
Complete Freund’s adjuvant (CFA)-induced inflammatory muscle pain. A Representative photograph showing the swollen right hindlimb 2 days after CFA injection. Red and black dotted lines indicate the position of the tibia and the perimeter at the middle of the tibia, respectively. scale bar, 5 mm. B Evans blue extravasation from the ipsilateral (R) tibialis anterior 2 days after CFA injection; contralateral side (L) with injection of isotonic saline as control (n = 4). Inset, typical images of tibialis anterior muscles. Scale bar, 2 mm. C Perimeter of the ipsilateral hindlimb at the middle of the tibia peaked 1 day after CFA injection and then declined (n = 5). D Difference scores in weight-bearing (ipsilateral–contralateral) reached a minimum 1 day after CFA injection and was significantly reduced for 6 days (n = 6). *P < 0.05, **P < 0.01; ipsilateral vs contralateral in B and C, post-injection vs pre-injection −1 day in D.
Primary Sensory Neurons Innervating Tibialis Anterior Muscle
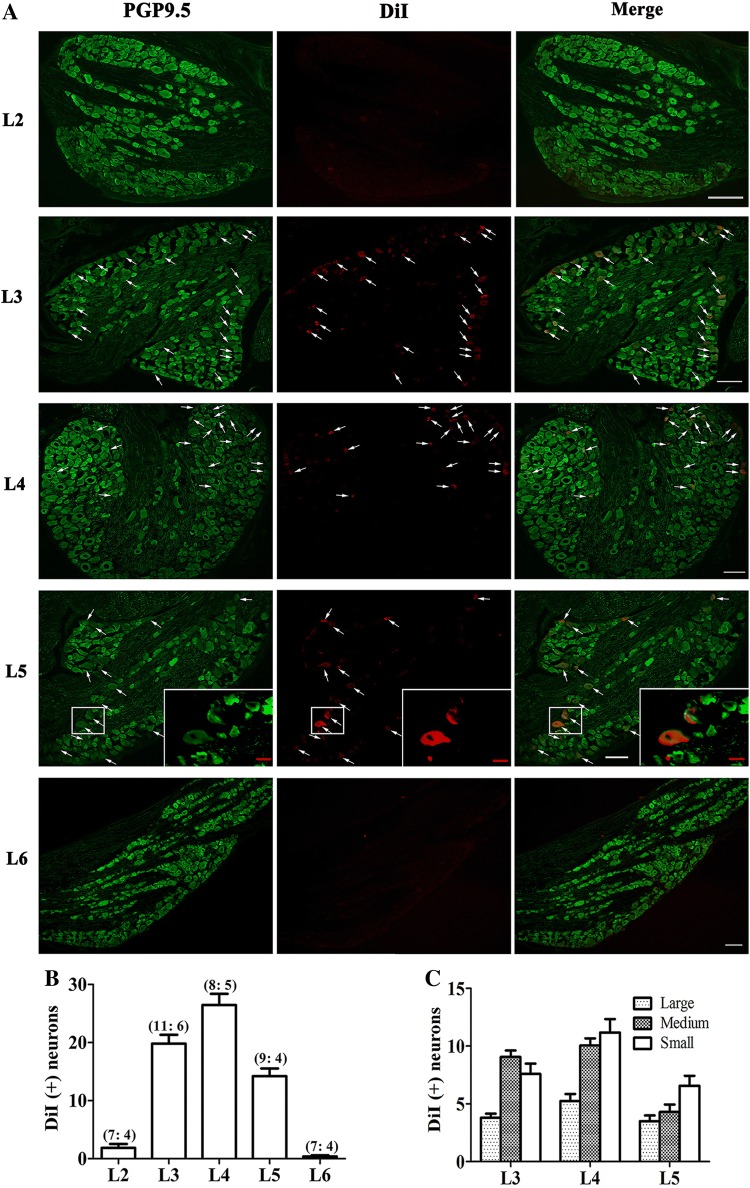
DiI-labeling and immunofluorescent staining with PGP9.5 in 6 rats demonstrated that the majority of primary sensory neurons specifically innervating tibialis anterior were in the L3, L4, and L5 DRGs (Fig. 2A, B). The majority of DiI-positive neurons were small- and medium-sized, and the L4 DRG contained the largest number of small DiI-positive neurons (Fig. 2C). Therefore the L4 DRG was chosen for recording the electrophysiological activity of muscular nociceptive C-neurons.
Fig. 2.
Anterograde labelling of primary sensory neurons innervating tibialis anterior. A Immunofluorescent staining of L2–L6 dorsal root ganglia (DRGs) with the neuronal marker PGP9.5 showing primary sensory neurons in the L3–L5 DRGs innervating tibialis anterior anterogradely labeled with DiI (arrows). Inset, enlarged image of labeled neurons in L5 DRG. Scale bars, 100 μm (white); 20 μm (red). B Numbers of neurons labeled by DiI in L2–L6 DRGs (number of DRGs and rats in each group listed in brackets). C Numbers of large-(> 40 μm), medium (30 μm–40 μm), and small (< 30 μm) neurons labeled in L3–L5 DRGs. DiI, 1,1′-dioctadecyl-3,3,3′,3′-tetrame-thylindocarbocyanine perchlorate.
CFA-Induced Mechanical Hypersensitivity in Muscular Nociceptive Neurons and Inhibitory Effects of Topical Irritants
A total of 216 DiI-labeled L4 DRG neurons were recorded from 61 CFA-injected and 6 control (naïve) rats. Among the 185 neurons recorded from the CFA-injected rats, 60 were C-neurons and 2 were A -neurons (all small-diameter) responding to localized mechanical stimuli to tibialis anterior (Fig. 3A–D); 102 were muscle spindles (69 large, 21 medium, and 11 small neurons); and the receptive fields of the remaining 17 (7 large, 2 medium, and 8 small neurons) could not be found. Among the 31 neurons recorded from naïve rats, 7 were C-type muscular nociceptive neurons (all small-diameter), 24 were muscle spindles (10 large, 4 medium, and 10 small neurons) and the receptive fields of the remaining 4 (2 large, 1 medium, and 1 small neurons) could not be found.
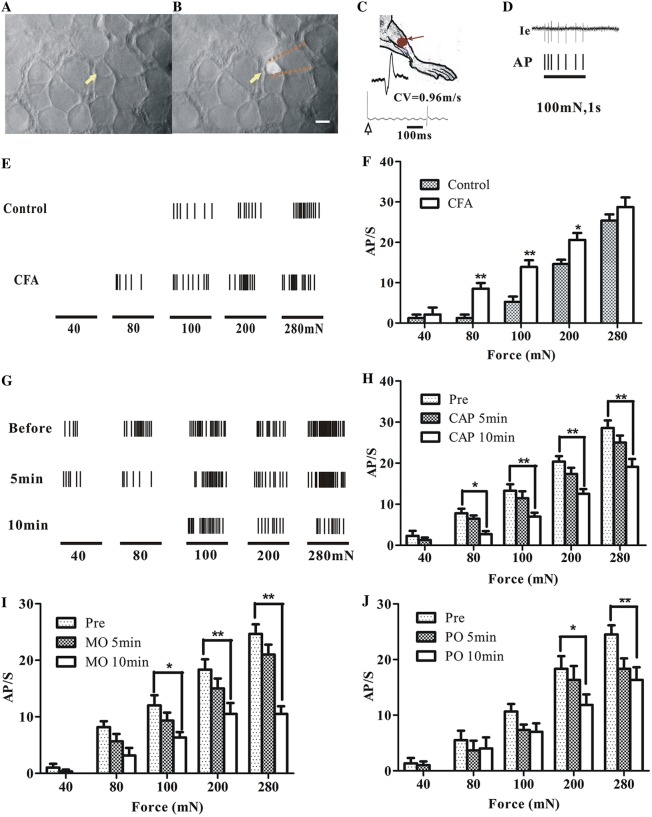
Fig. 3.
Mechanical thresholds of muscular C-nociceptive neurons in rat dorsal root ganglion (DRG). A Bright-field image of the surface of an L4 DRG; arrow indicates a small neuron. B Fluorescent image of the same cell body recorded by an extracellular glass micropipette (dotted lines). Scale bar, 20 μm. C Cartoon of measurement of conduction velocity of the sciatic nerve. Receptive field in tibialis anterior labeled by a red spot. D Single-neuron extracellular recording of a typical response of a fluorescence-labeled small neuron to 100 mN mechanical stimulation. Action potentials (APs) in the original recording trace (Ie) are presented as corresponding ticks below. E Responses of muscle nociceptive C-neurons to von Frey filaments of several forces (40 mN, 80 mN, 100 mN, 200 mN, and 280 mN) in CFA-injected and control rats. F Action potential discharge rates (AP/s) of muscular nociceptive C-neurons evoked by mechanical stimuli in complete Freund’s adjuvant (CFA)-injected (n = 11) and control rats (n = 6). G Typical recordings of the responses of a muscular C-nociceptive neuron from a CFA-injected rat to different forces applied to the receptive field in tibialis anterior before and after topical capsaicin (CAP) application. H–J Summary of responses (AP/s) of muscular C-nociceptive neurons to different forces before and after topical application of CAP (n = 11 in H), mustard oil (MO, n = 6 in I), and peppermint oil (PO, n = 6 in J) on the skin over tibialis anterior in CFA-injected rats. *P < 0.05, **P < 0.01; CFA vs control (F), post- vs pre-application (H–J).
Muscular nociceptive neurons in CFA-injected rats showed enhanced responses to mechanical stimuli (Fig. 3E, F). Compared with the naïve rats (n = 6), the action potential discharge rates evoked by mechanical stimuli in CFA-injected rats (n = 11) increased significantly (80 mN, 7.82 ± 2.0 vs 1.25 ± 1.53, P < 0.01; 100 mN, 13.27 ± 2.16 vs 5.25 ± 1.92, P < 0.01; 200 mN, 20.36 ± 1.18 vs 14.62 ± 1.73, P < 0.05), indicating mechanical hypersensitivity in CFA-inflamed muscle.
After topical irritants (CAP, n = 11; MO, n = 6; PO, n = 6) were smeared on the skin over the inflamed muscle in CFA-injected rats, the responses of muscular nociceptive neurons to mechanical stimuli started to decrease in 5 min–10 min (Fig. 3G). When the same series of mechanical stimuli were given, the average discharge rate for all 3 irritants dropped significantly. The most dramatic change occurred after the application of CAP, suggesting a strong analgesic effect (Fig. 3H–J).
CFA-Induced Spontaneous Activity (SA) in Muscular Nociceptive Neurons and Inhibitory Effects of Topical Irritants
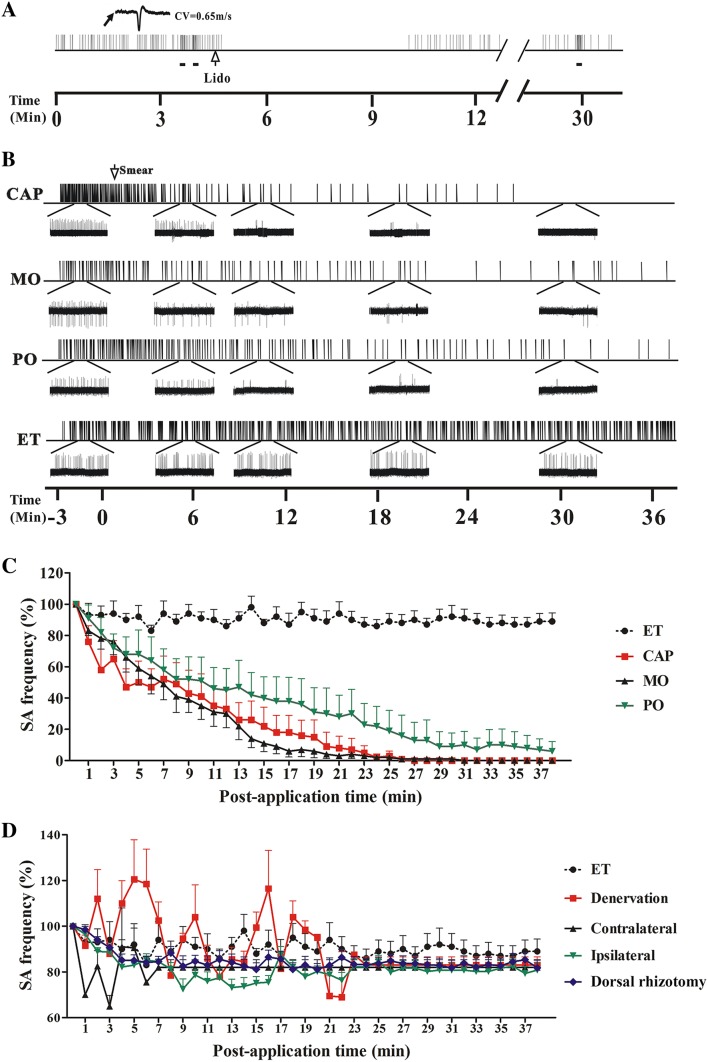
Out of the 62 muscular nociceptive DRG neurons recorded from the CFA-injected rats, SA was recorded from 30 (all were C-type and small-diameter). In contrast, none of the 7 muscular nociceptive neurons from the naïve rats showed SA. The average discharge rate of these muscular nociceptive neurons was 0.75 Hz (0.33 to 1.7 Hz). The SA was inhibited by intramuscular injection of 10 μL lidocaine (0.02 g/mL) (Fig. 4A).
Fig. 4.
Spontaneous activity (SA) of muscular nociceptive C-neurons recorded in L4 dorsal root ganglion (DRG) neurons after applying irritants in rats injected with complete Freund’s adjuvant (CFA). A Representative recording of SA in a muscular C-nociceptive neuron (conduction velocity [CV] = 0.65 m/s) 2 days after CFA injection. The SA was blocked within 1 min after intramuscular injection of lidocaine (Lido, 10 μL, blank arrow) into the receptive field, and then recovered ~6 min later. Horizontal bars below recording indicate mechanical stimuli (100 mN) to the receptive field. B Examples of SA in muscular nociceptive C-neurons that was gradually inhibited by capsaicin (CAP), mustard oil (MO), and peppermint oil (PO) on the skin overlying the inflamed muscle in CFA-injected rats. Ethanol (ET) was used as control. Insets below, expanded traces at different time points. C Statistics of SA frequency after applying CAP (n = 10), MO (n = 6), PO (n = 6), and ET (n = 8) on the skin overlying the inflamed muscle in CFA-injected rats. The average SA frequency within 3 min before irritant application was defined as 100%. CAP, MO and PO vs ET; CAP group: P < 0.05 at 5 min post-application and later; MO and PO groups: P < 0.05 at 12 min post-application and later. D Statistics of SA frequency after applying CAP to the skin overlying the inflamed muscle after cutaneous denervation (n = 6) or L4 dorsal rhizotomy (n = 2); the skin overlying the contralateral tibialis anterior (Contralateral) (n = 5); and the ipsilateral plantar skin (Ipsilateral) (n = 6) in CFA-injected rats. Denervation, L4 dorsal rhizotomy, Contralateral and Ipsilateral vs ET (n = 8); P > 0.05 at all tested time points.
After topical application of the irritants CAP (n = 10), MO (n = 6), or PO (n = 6) to the skin over the inflamed muscle in CFA-injected rats, the discharge rate of SA declined significantly within a few minutes in all groups (Fig. 4B). No significant change occurred in the ET group (vehicle control) (n = 8) (P > 0.05). The application of CAP induced a stronger early response while both MO and CAP completely blocked SA within 30 min (Fig. 4B, C).
In contrast, no significant change was found in the SA frequency of muscular nociceptive neurons after topical application of CAP to the ipsilateral plantar skin (n = 6), or the skin over the contralateral tibialis anterior (n = 5), or when the skin was denervated (n = 6) or after an ipsilateral L4 dorsal rhizotomy (n = 2) (P > 0.05) (Fig. 4D).
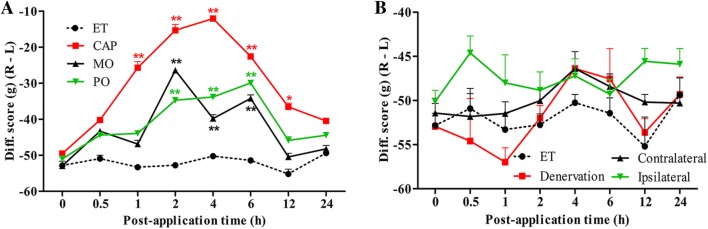
Inhibitory Effect of Irritants on Unbalanced Weight-Bearing
Two days after CFA injection, the difference score (ipsilateral–contralateral) for weight-bearing decreased from 0 to about −50 g. Following the application of irritants, the weight-bearing was monitored periodically for up to 24 h. In the CAP group (n = 4), the difference score started to recover as early as 1 h after application and lasted for ~12 h, with a peak at ~4 h (Fig. 5A). The changes in weight-bearing were less in the MO (n = 4) and PO (n = 4) groups and lasted for only ~6 h (Fig. 5A). No significant change was found in the ET group (n = 4, P > 0.05).
Fig. 5.
Weight-bearing after application of topical irritants in CFA-injected rats. A Difference scores for weight-bearing (ipsilateral–contralateral) increased after the application of capsaicin (CAP) (n = 4), mustard oil (MO) (n = 4), peppermint oil (PO) (n = 4), and ethanol (ET) (n = 4) to the skin over the ipsilateral tibialis anterior. B Difference score for weight-bearing remained unchanged following the application of CAP to the denervated skin over the ipsilateral tibialis anterior (Denervation) (n = 7), skin over the contralateral tibialis anterior (Contralateral) (n = 6), and the ipsilateral plantar skin (Ipsilateral) (n = 6). ET on the skin overlying the inflamed muscle was taken as control in both A and B. *P < 0.05, **P < 0.01; CAP, MO and PO vs ET (A); Denervation, Contralateral and Ipsilateral vs ET (B).
Similarly, there was no significant change in the weight-bearing scores of CFA-injected rats after application of CAP to the ipsilateral plantar skin (n = 6), or the skin over the contralateral tibialis anterior (n = 6), or when the skin was denervated (n = 7, P > 0.05) (Fig. 5B).
Discussion
In clinical practice, muscle pain is a frequent complaint in patients with muscle strain, muscle damage, or ischemia. Topical analgesics such as capsaicin cream are effective for the relief of inflammatory muscle pain, possibly through the activation of cutaneous nociceptors, but without a clear understanding of the mechanism [17]. In this study, inflammatory muscle pain was successfully induced by CFA, as indicated by intolerable weight-bearing, massive Evans blue extravasation, mechanical hypersensitivity and SA of muscular nociceptive neurons. Nociceptive irritants applied to the skin over the inflamed muscle, but not to remote skin, were able to relieve muscle inflammation. No analgesic effect was found either after transection of the L4 dorsal root or denervation of the skin over the inflamed muscle. These findings provided strong evidence that the activation of cutaneous nociceptors by topical irritants inhibit the mechanical hypersensitivity and abnormal spontaneous activity of muscular nociceptive neurons, and thus alleviate inflammatory muscle pain.
Distinct from cutaneous pain, muscle pain is more tearing and diffuse, and often accompanied by an extended area of referred pain [29]. Compared with cutaneous sensory neurons, muscular sensory neurons have different actions on pain signal conduction and central sensitization [30], and vary in their response to peripheral nerve injury [31]. All these differences indicate the need to explore the characteristics of muscular nociceptive neurons. The electrophysiological characteristics of cutaneous nociceptive neurons have been reported in many studies, but the characteristics of muscular nociceptive neurons are barely understood because of the complexity and difficulty of in vivo single-neuron electrophysiological recording. In this study, the fluorescent dye DiI was used to track primary sensory neurons, allowing us to record from nociceptive neurons specifically innervating the inflamed muscle. To our knowledge, this study is the first to successfully record the electrophysiological properties of single muscular nociceptive neurons in the rat model, and this might help to better understand the mechanisms underlying muscle pain.
SA in primary nociceptive neurons is considered to be an indicator of spontaneous pain and an important factor in neuropathic pain [32]. In this study, robust SA was recorded from the nociceptive neurons innervating the inflamed muscle, and was blocked by the application of irritants to the skin over the inflamed muscle. The CFA-induced mechanical hypersensitivity of the muscular nociceptive neurons was also alleviated by these topical irritants. These effects could not be induced by mechanical stimuli or innocuous stimuli such as light touch or wiping with a Q-tip (data not shown). In traditional Chinese medicine, noxious stimuli that may irritate cutaneous nociceptors such as scraping, cupping, and certain plasters were widely used to relieve muscle pain. In modern medicine, the burning sensation of CAP and other chemical irritants is also used to treat various pains, including neuropathic pain and joint pain [14, 15].
It is worth noting that there were discrepancies in our behavioral and electrophysiological findings. For example, although the inhibitory effect of MO in electrophysiology studies was stronger than that after CAP, the analgesia induced by MO was less than that induced by CAP in behavioral tests. This discrepancy might be due to the difference in the durations of the behavioral and electrophysiological recordings. In the behavioral experiments, we found that the strongest analgesic effect occurred a few hours after applying the irritants. In the electrophysiological recordings, we were only able to record the discharges of primary neurons for as long as 40 min after the skin was smeared with irritant. Although the recordings were conducted with great care, the activity of DRG neurons tends to attenuate after prolonged recording. This technical limitation might be the reason for the discrepancies between the behavioral and electrophysiological results, and will be further explored in future studies.
CAP, MO, and PO evoke pain sensation in the skin by activating different subtypes of nociceptive neuronal ending through different receptors (TRPV1, TRPM8, and TRPA1, respectively) [33]. We believe that the inhibitory effect on the evoked and spontaneous activity of muscular DRG neurons by these topical irritants may be attributed to the activation of small-diameter nociceptive cutaneous afferents, regardless of the subtypes of these neurons. So far, the mechanism of this inhibitory effect of cutaneous nociceptors on muscular nociceptors has not been fully clarified. Using behavioral and in vivo electrophysiological methods, we first confirmed a previous finding that application of the analgesic CAP to the skin over the inflamed muscle alleviates inflammatory muscle pain [17]. Furthermore, the analgesic effects disappeared when the sensory input was blocked by dorsal rhizotomy or skin denervation, or when the irritants were applied to remote skin. These results suggest that activated cutaneous nociceptive afferents inhibit muscular nociceptive neurons through an inhibitory interaction in the same spinal segment. This potential mechanism might be different from the theory of diffuse noxious inhibitory control [18]. We speculate that activated cutaneous nociceptive afferents inhibit the dorsal root reflex [34] of adjacent nociceptive fibers innervating the inflamed muscle, thus reducing the antidromic discharge of muscular C-fibers and the release of inflammatory mediators in the peripheral nerve terminals. The hypothesis involving spinal mechanisms needs to be further investigated in future experiments.
We studied the electrophysiological characteristics of muscular nociceptive neurons in a rat model of inflammatory muscle pain. The topical irritants CAP, MO, and PO, applied to the skin over the inflamed muscle inhibited muscular nociceptive neurons and alleviated muscle pain via the activation of cutaneous nociceptors. Our findings suggest novel therapeutic strategies for the treatment of inflammatory muscle pain.
Acknowledgements
We thank Bo Yuan and Tao Wang from the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, Beijing, China, for technical assistance. This work was supported by the National Natural Science Foundation of China (81771205 and 91632113), the Natural Science Foundation and Major Basic Research Program of Shanghai Municipality, China (16JC1420500 and 16JC1420502), the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2017-I2M-3-008), and the National Natural Science Foundation for Young Scientists of China (81600956).
Conflict of interest
All authors claim that there are no conflicts of interest.
Footnotes
Yehong Fang and Jie Zhu have contributed equally to the work and share first authorship.
References
- 1.Harden RN. Muscle pain syndromes. Am J Phys Med Rehabil. 2007;86:S47–S58. doi: 10.1097/PHM.0b013e31802ba648. [DOI] [PubMed] [Google Scholar]
- 2.Staud R. Future perspectives: pathogenesis of chronic muscle pain. Best Pract Res Clin Rheumatol. 2007;21:581–596. doi: 10.1016/j.berh.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Sumpton JE, Moulin DE. Fibromyalgia. Handb Clin Neurol. 2014;119:513–527. doi: 10.1016/B978-0-7020-4086-3.00033-3. [DOI] [PubMed] [Google Scholar]
- 4.Borg-Stein J, Iaccarino MA. Myofascial pain syndrome treatments. Phys Med Rehabil Clin N Am. 2014;25:357–374. doi: 10.1016/j.pmr.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Segal LS, Odgers R, Carpentieri D, Shrader MW. Back pain in Duchenne muscular dystrophy: steroids are not always the culprit. J Pediatr Orthop B. 2016;25:81–85. doi: 10.1097/BPB.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 6.Mense S. Muscle pain: mechanisms and clinical significance. Dtsch Arztebl Int. 2008;105:214–219. doi: 10.3238/artzebl.2008.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graven-Nielsen T, Arendt-Nielsen L. Induction and assessment of muscle pain, referred pain, and muscular hyperalgesia. Curr Pain Headache Rep. 2003;7:443–451. doi: 10.1007/s11916-003-0060-y. [DOI] [PubMed] [Google Scholar]
- 8.Salzberg L. The physiology of low back pain. Prim Care. 2012;39:487–498. doi: 10.1016/j.pop.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Golob AL, Wipf JE. Low back pain. Med Clin North Am. 2014;98:405–428. doi: 10.1016/j.mcna.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Ambalavanar R, Moritani M, Haines A, Hilton T, Dessem D. Chemical phenotypes of muscle and cutaneous afferent neurons in the rat trigeminal ganglion. J Comp Neurol. 2003;460:167–179. doi: 10.1002/cne.10655. [DOI] [PubMed] [Google Scholar]
- 11.Hoheisel U, Reinohl J, Unger T, Mense S. Acidic pH and capsaicin activate mechanosensitive group IV muscle receptors in the rat. Pain. 2004;110:149–157. doi: 10.1016/j.pain.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 12.Hoheisel U, Unger T, Mense S. Excitatory and modulatory effects of inflammatory cytokines and neurotrophins on mechanosensitive group IV muscle afferents in the rat. Pain. 2005;114:168–176. doi: 10.1016/j.pain.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Kirillova I, Rausch VH, Tode J, Baron R, Janig W. Mechano- and thermosensitivity of injured muscle afferents. J Neurophysiol. 2011;105:2058–2073. doi: 10.1152/jn.00938.2010. [DOI] [PubMed] [Google Scholar]
- 14.Hautkappe M, Roizen MF, Toledano A, Roth S, Jeffries JA, Ostermeier AM. Review of the effectiveness of capsaicin for painful cutaneous disorders and neural dysfunction. Clin J Pain. 1998;14:97–106. doi: 10.1097/00002508-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Sawynok J. Topical analgesics in neuropathic pain. Curr Pharm Des. 2005;11:2995–3004. doi: 10.2174/1381612054865019. [DOI] [PubMed] [Google Scholar]
- 16.Cal K. Skin disposition of menthol after its application in the presence of drug substances. Biopharm Drug Dispos. 2008;29:449–454. doi: 10.1002/bdd.631. [DOI] [PubMed] [Google Scholar]
- 17.Duan WR, Lu J, Xie YK. Mechanisms of topical analgesics in relieving pain in an animal model of muscular inflammation. Pain Med. 2013;14:1381–1387. doi: 10.1111/pme.12199. [DOI] [PubMed] [Google Scholar]
- 18.Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). II. Lack of effect on non-convergent neurones, supraspinal involvement and theoretical implications. Pain. 1979;6:305–327. doi: 10.1016/0304-3959(79)90050-2. [DOI] [PubMed] [Google Scholar]
- 19.Zhou LJ, Ren WJ, Zhong Y, Yang T, Wei XH, Xin WJ, et al. Limited BDNF contributes to the failure of injury to skin afferents to produce a neuropathic pain condition. Pain. 2010;148:148–157. doi: 10.1016/j.pain.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 20.Ijpma FF, Nicolai JP, Meek MF. Sural nerve donor-site morbidity: thirty-four years of follow-up. Ann Plast Surg. 2006;57:391–395. doi: 10.1097/01.sap.0000221963.66229.b6. [DOI] [PubMed] [Google Scholar]
- 21.Cooksey CJ. Quirks of dye nomenclature 1 Evans blue. Biotech Histochem. 2014;89:111–113. doi: 10.3109/10520295.2013.822560. [DOI] [PubMed] [Google Scholar]
- 22.Donelan J, Boucher W, Papadopoulou N, Lytinas M, Papaliodis D, Dobner P, et al. Corticotropin-releasing hormone induces skin vascular permeability through a neurotensin-dependent process. Proc Natl Acad Sci U S A. 2006;103:7759–7764. doi: 10.1073/pnas.0602210103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue H, Asaka T, Nagata N, Koshihara Y. Mechanism of mustard oil-induced skin inflammation in mice. Eur J Pharmacol. 1997;333:231–240. doi: 10.1016/S0014-2999(97)01040-6. [DOI] [PubMed] [Google Scholar]
- 24.Banvolgyi A, Pozsgai G, Brain SD, Helyes ZS, Szolcsanyi J, Ghosh M, et al. Mustard oil induces a transient receptor potential vanilloid 1 receptor-independent neurogenic inflammation and a non-neurogenic cellular inflammatory component in mice. Neuroscience. 2004;125:449–459. doi: 10.1016/j.neuroscience.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Elsaie LT, El Mohsen AM, Ibrahim IM, Mohey-Eddin MH, Elsaie ML. Effectiveness of topical peppermint oil on symptomatic treatment of chronic pruritus. Clin Cosmet Investig Dermatol. 2016;9:333–338. doi: 10.2147/CCID.S116995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Argoff CE. Topical analgesics in the management of acute and chronic pain. Mayo Clin Proc. 2013;88:195–205. doi: 10.1016/j.mayocp.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Tandrup T. Unbiased estimates of number and size of rat dorsal root ganglion cells in studies of structure and cell survival. J Neurocytol. 2004;33:173–192. doi: 10.1023/B:NEUR.0000030693.91881.53. [DOI] [PubMed] [Google Scholar]
- 28.Ma C, LaMotte RH. Multiple sites for generation of ectopic spontaneous activity in neurons of the chronically compressed dorsal root ganglion. J Neurosci. 2007;27:14059–14068. doi: 10.1523/JNEUROSCI.3699-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arendt-Nielsen L, Graven-Nielsen T. Muscle pain: sensory implications and interaction with motor control. Clin J Pain. 2008;24:291–298. doi: 10.1097/AJP.0b013e31815b608f. [DOI] [PubMed] [Google Scholar]
- 30.Andersen OK, Graven-Nielsen T, Matre D, Arendt-Nielsen L, Schomburg ED. Interaction between cutaneous and muscle afferent activity in polysynaptic reflex pathways: a human experimental study. Pain. 2000;84:29–36. doi: 10.1016/S0304-3959(99)00174-8. [DOI] [PubMed] [Google Scholar]
- 31.Hu P, McLachlan EM. Selective reactions of cutaneous and muscle afferent neurons to peripheral nerve transection in rats. J Neurosci. 2003;23:10559–10567. doi: 10.1523/JNEUROSCI.23-33-10559.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci. 2006;26:1281–1292. doi: 10.1523/JNEUROSCI.3388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore C, Gupta R, Jordt SE, Chen Y, Liedtke WB. Regulation of pain and itch by TRP channels. Neurosci Bull. 2018;34:120–142. doi: 10.1007/s12264-017-0200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin Q, Wu J, Willis WD. Dorsal root reflexes and cutaneous neurogenic inflammation after intradermal injection of capsaicin in rats. J Neurophysiol. 1999;82:2602–2611. doi: 10.1152/jn.1999.82.5.2602. [DOI] [PubMed] [Google Scholar]