SUMMARY
The contribution of the different waves and sites of developmental hematopoiesis to fetal and adult blood production remains unclear. Here, we identify lymphatic vessel endothelial hyaluronan receptor-1 (LYVE1) as a marker of yolk sac (YS) endothelium and definitive hematopoietic stem and progenitor cells (HSPCs). Endothelium in mid-gestation YS and vitelline vessels, but not the dorsal aorta and placenta, were labeled by Lyve1-Cre. Most YSHSPCs and erythro-myeloid progenitors were Lyve1-Cre lineage traced, but primitive erythroid cells were not, suggesting that they represent distinct lineages. Fetal liver (FL) and adult HSPCs showed 35%–40% Lyve1-Cre marking. Analysis of circulation-deficient Ncx1−/− concepti identified the YS as a major source of Lyve1-Cre labeled HSPCs. FL proerythroblast marking was extensive at embryonic day (E) 11.5–13.5, but decreased to hematopoietic stem cell (HSC) levels by E16.5, suggesting that HSCs from multiple sources became responsible for erythropoiesis. Lyve1-Cre thus marks the divergence between YS primitive and definitive hematopoiesis and provides a tool for targeting YS definitive hematopoiesis and FL colonization.
Graphical Abstract

In Brief
Lee et al. identify LYVE1 as a marker that is highly enriched in yolk sac endothelium and definitive HSPCs during mid-gestation. Lyve1-Cre-labeled hematopoietic cells initiate fetal liver erythropoiesis and give rise to more than one-third of fetal and adult HSCs. The primitive erythroid lineage develops from LYVE1 negative precursors.
INTRODUCTION
Developmental hematopoiesis is composed of temporally orchestrated programs that generate both the differentiated blood cells to support embryonic growth and the undifferentiated hematopoietic stem cells (HSCs) to sustain post-natal hematopoiesis. This challenge is met by segregating hematopoiesis into multiple waves that occur in different anatomical locations. The earliest (“primitive”) hematopoietic wave starts in the extra-embryonic yolk sac (YS) at embryonic day (E) 7.5 and gives rise to erythroid cells that fulfill the immediate metabolic needs of the embryo (EM) (Moore and Metcalf, 1970). They are characterized by the expression of embryonic β globin (betaH1 in mice) (Haar and Ackerman, 1971). At E8.5–9.5, the YS also generates an intermediate (“transient-definitive”) wave of erythro-myeloid progenitors (EMPs) (McGrath et al., 2015; Mikkola and Orkin, 2006; Palis et al., 1999) that produce red cells expressing “definitive” adult type β globins (beta-major in mice) (McGrath et al., 2003). However, YS EMPs lack self-renewal ability and lymphoid potential, the hallmarks of HSCs. It has been proposed that EMPs initiate fetal liver (FL) definitive hematopoiesis and play roles in supporting embryonic survival (Chen et al., 2011), inflammatory signaling during HSC emergence (Es-pín-Palazón et al., 2014), and adult tissue-resident macrophage development (Gomez Perdiguero et al., 2015). However, the duration and significance of this intermediate hematopoietic wave are not fully understood.
The final (“definitive”) wave generates self-renewing, multipotent HSCs that sustain lifelong production of all blood cell types. Transplantable HSCs are first identified at E10.5–11.5 in the intra-embryonic para-aortic splanchnopleural/aorta-gonad-mesonephros region (pSP/AGM) and the vitelline and umbilical vessels that connect the pSP/AGM to extra-embryonic tissues (Medvinsky and Dzierzak, 1996; Müller et al., 1994). Several studies suggest that the YS and the placenta (PL) can also generate multipotent hematopoietic stem and progenitor cells (HSPCs) de novo (Inlay et al., 2014; Rhodes et al., 2008; Samokhvalov et al., 2007; Weissman et al., 1977, 1978; Zeigler et al., 2006). Observations from direct transplantation of pre-circulation YS into congenic embryos and inducible Runx1 lineage-tracing studies supported the hypothesis that YS contributes to adult hematopoiesis (Samokhvalov et al., 2007; Weissman et al., 1977, 1978). Furthermore, in vitro experiments with placental tissues isolated before or without the onset of blood circulation demonstrated multi-lineage hematopoietic potential (Rhodes et al., 2008; Zeigler et al., 2006). However, the degree to which each organ contributes to the post-natal HSC pool is unknown.
Lineage tracing and time-lapsed imaging of HSPC emergence in vitro and in vivo evidenced that definitive HSPCs arise from specialized endothelial cells, named hemogenic endothelium (Bertrand et al., 2010; Boisset et al., 2010; Chen et al., 2009; Eilken et al., 2009; Lancrin et al., 2009; Zovein et al., 2008). Hemogenic competence in the endothelium is established by the stem cell leukemia gene/T cell acute leukemia gene 1 (Scl/Tal1), which activates a broad transcriptional network of hematopoietic regulators and prevents misspecification of the endothelium to cardiac fate (Van Handel et al., 2012). SCL activates RUNX1 and other transcription factors that enable HSPC emergence from hemogenic endothelium (Org et al., 2015). SCL is also essential for the generation of primitive erythroid cells and controls the terminal maturation of erythroid cells at all stages of ontogeny (Mikkola et al., 2003a; Schlaeger et al., 2005; Shivdasani et al., 1995). The identity of the precursors for the primitive erythroid cells and the timing when they diverge from the definitive hematopoietic lineages are not known. The presumption that primitive and definitive hematopoietic waves represent distinct lineages that arise from their own precursors (“lineage switch” model) has been challenged by studies that show sequential expression of both the primitive and definitive globins (“maturational switch” model) during erythroid maturation (Kingsley et al., 2006; McGrath et al., 2011; Palis, 2008;Qiu et al., 2008).
Although the fundamentals of the developmental hematopoietic hierarchy are gradually being revealed, the contributions of each wave and hemogenic tissue to fetal and post-natal hematopoiesis remain poorly defined because of the lack of wave- and tissue-specific tools that would enable prospective isolation and tracking of the progeny of the temporally and spatially overlapping blood precursors. Most surface antigens expressed in the hemogenic endothelium or emerging HSPCs–e.g., Flk1, CD31, VE-Cadherin, Tie2, CD41, ckit, and Sca1–are developmentally regulated but not specific to any individual wave or anatomical site (McKinney-Freeman et al., 2009; Mikkola and Orkin, 2006). The use of inducible lineage-tracing studies using regulatory elements of hematopoietic transcription factors (e.g., Runx1) is complicated by the fact that the expression of such factors is not restricted to a particular hemogenic tissue (Samokhvalov et al., 2007). Thus, there are no robust methods to separate or genetically target in vivo individual hematopoietic waves or hemogenic tissues.
Here, we identify the lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1) as a marker that is differentially and highly expressed in YS endothelium during HSPC development. Lineage tracing by reporter gene expression and conditional deletion of the hematopoietic regulators Scl and Runx1 by Lyve1-Cre distinguished YS endothelium, EMPs, and definitive HSPCs from the primitive erythroid lineage. Our data suggest that Lyve1-Cre-labeled cells primarily originate from the YS and/or vitelline vessels and contribute to 35%–40% of fetal and adult HSC compartments. At E11.5, the FL pro-erythroblast pool is dominated by LYVE1-lineage cells, whereas by late gestation, the level of marking is similar to the HSC compartment, implying a temporal switch in the composition of precursors responsible for FL erythropoiesis. Lyve1-Cre thus provides a unique tool to investigate the contribution of YS hemogenic endothelium to fetal and adult hematopoiesis and the factors that regulate the fate of these cells.
RESULTS
LYVE1 Protein Is Expressed in YS Endothelium and Definitive Hematopoietic Cells
To define the precursors and respective contributions of the different waves to fetal and adult hematopoiesis, we sought for cell surface markers expressed in hemogenic endothelium that are tissue or wave specific. From a list of genes encoding surface proteins highly expressed in YS endothelium (Van Handel et al., 2012), Lyve1 emerged as a promising candidate. Initially reported as a marker for lymphatic vessels, LYVE1 expression was later documented also in mid-gestation YS vasculature, FL sinusoids, intersomitic vessels, and the cardinal vein, while being minimal in the dorsal aorta (DA) (Banerji et al., 1999; Gordon et al., 2008).
Because LYVE1 expression has not been assessed in the context of developmental hematopoiesis, LYVE1 protein expression was surveyed in hemogenic organs by immunostaining and flow cytometry (FACS). Immunofluorescence (IF) at E10.5 demonstrated robust LYVE1 protein expression in YS and vitelline vasculature, whereas no signal was observed in the PL, umbilical vessels, and DA (Figure 1A). As reported previously, LYVE1 was also expressed in the cardinal vein at E10.5. At E9.5, LYVE1 protein expression in CD31+ endothelial cells was ubiquitous in the YS, but minimal in the EM and PL (Figures 1B and 1C). Scant LYVE1 protein was discernible in PL vasculature during HSPC emergence (E11.5) and expansion/maturation (E13.5); however, some LYVE1 protein could be detected at late gestation (E16.5) when the PL HSC pool has already diminished (Gekas et al., 2005) (Figure S1A). At E13.5, LYVE1 expression was also abundant in FL sinusoids and the azygous vein – tissues incapable of de novo HSPC generation–but not the aorta (Figure S1B).
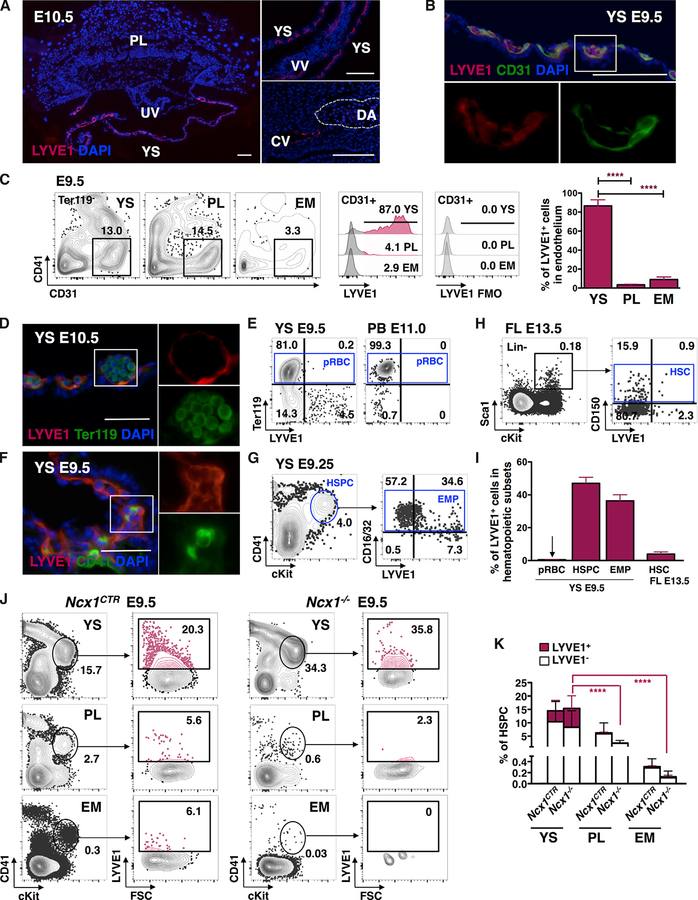
Figure 1. LYVE1 Protein Is Expressed in Yolk Sac Endothelium and Hematopoietic Stem and Progenitor Cells.
(A) LYVE1 protein is detected by IF in E10.5 YS, vitelline vessels (VVs), and the cardinal vein (CV), but is absent from the PL, umbilical vessels (UVs), and DA. Representative images from three independent experiments. Scale bar, 100 μm.
(B) IF staining shows co-localization of LYVE1 in E9.5 YS CD31+ endothelium. Representative image from three independent experiments. Scale bar, 100 μm.
(C) FACS analysis of CD31+CD41−Ter119− endothelium in YS, PL, and caudal half of the EM of one representative E9.5 conceptus. Corresponding histograms of LYVE1+ endothelial cells show robust LYVE1 expression in YS endothelium only. Data from n = 11 from three independent experiments are represented as mean ± SD; ***P ≤ 0.001.
(D) LYVE1 protein is not discernible by IF in Ter119+ primitive erythroid cells in E10.5 YS. Representative image from two independent experiments is shown. Scale bar, 50 μm.
(E) Representative FACS plots indicate that Ter119+ primitive erythroid cells (pRBCs) do not express LYVE1 in E9.5 YS or E11.0 peripheral blood (PB).
(F) IF staining of E9.5 YS shows co-expression of LYVE1 protein with HSPC marker CD41. Representative image from three independent experiments is shown. Scale bar, 50 μm.
(G) Representative FACS plots show LYVE1 expression in CD41midcKit+Ter119− HSPCs and its subset, CD16/32+ EMPs in E9.25 YS.
(H) Representative FACS plots of E13.5 FL document minimal LYVE1 expression in Lin−Sca1+cKit+ CD150+ HSC compartment.
(I) LYVE1 expression is prominent in mid-gestation YS HSPCs and EMPs. Data from n = 8, three independent experiments for YS pRBC; n = 28,10 independent experiments for YS HSPC; n = 8, three independent experiments for YS EMP; and n = 6, two independent experiments for FL HSC subset are shown as mean ± SD.
(J) Representative FACS plots of hemogenic tissues in Ncx1−/− E9.5 concepti demonstrate the enrichment of LYVE1-expressing CD41midcKit+Ter119− HSPCs in the YS.
(K) Significantly higher HSPC fraction in the YS than in the PL or EM expresses LYVE1 protein in Ncx1−/− concepti. Quantification of n = 23 for control and n = 11 for Ncx1−/− from five independent experiments is represented as mean ± SD. p values refer to the difference between LYVE1+ fractions; ****P ≤ 0.0001.
Analysis of the early concepti revealed LYVE1 expression in Tie2+CD31+ angioblasts already by E7.5 (Figure S1C). At E8.5, LYVE1 was present in YS Tie2+CD31+ angioblasts, but excluded from Ter119+ primitive erythroid cells (Figure S1C), the embryo, and allantois (data not shown). These data identify the YS and the vitelline vessels as the primary sites harboring LYVE1-expressing endothelium during HSPC emergence. Moreover, these data imply that LYVE1 protein is already expressed in the YS at the time when primitive hematopoiesis begins and the endothelium that gives rise to YS definitive lineages is specified.
We next assessed the expression of LYVE1 protein in YS hematopoietic cells. IF and FACS analyses showed that Ter119+ primitive erythroid cells were devoid of LYVE1 expression (Figures 1D, 1E, and 1I). In contrast, LYVE1 expression was observed in 47.0% ± 19.1% SD of E9.25–9.5 YS CD41midcKit+Ter119− HSPC population (Figures 1F, 1G, and 1I), which consists predominantly of EMPs (Ferkowicz et al., 2003; Mikkola et al., 2003b). Co-staining for the EMP marker CD16/32 (McGrath et al., 2015) suggested that most LYVE1+ HSPCs in E9.25 YS are EMPs (Figure 1G). In contrast to YS HSPCs and EMPs, the E13.5 FL LSK (Lin−Sca1+cKit+) CD150+ HSC subset showed minimal LYVE1 expression (Figures 1H and 1I).
To assess the origin of LYVE1+ HSPCs, LYVE1 expression was examined in concepti that were devoid of blood circulation due to a defective sodium calcium exchanger (Ncx1) required for cardiac activity (Koushik et al., 2001). FACS analysis of Ncx1−/− tissues showed that LYVE1 expression was robust in CD41midcKit+ HSPCs in the YS, but minimal in the PL or EM (Figures 1J and 1K). Comparable results were observed with CD16/32+CD41midcKit+ EMPs (Figure S1D). These data suggest that the YS is the primary source of LYVE1+-expressing HSPCs, including EMPs, in mid-gestation concepti.
Lyve1-Cre Labels YS Endothelium and Vitelline Vessels
To define the contribution of LYVE1+ candidate hemogenic endothelial cells to fetal and adult hematopoiesis, lineage tracing was performed by breeding Lyve1-eGFP-hCre knockin mice (Pham et al., 2010) (referred as “Lyve1-Cre” hereafter) with reporter mouse lines. As originally reported, endogenous eGFP signal from the Lyve1-Cre line was weak and did not interfere with the signal from the reporter GFP. We first used RosamT/mG fluorescent reporter mice (Figure 2A), in which all cells of the conceptus initially express tdTomato. Upon activation of Cre re-combinase, the floxed membrane-targeted tdTomato cassette is excised, allowing the expression of the downstream membrane-targeted EGFP in Cre-expressing cells and their progeny (Muzumdar et al., 2007). Maternal blood cells in the PL could be separated from the cells of the conceptus because they were devoid of any fluorescence.
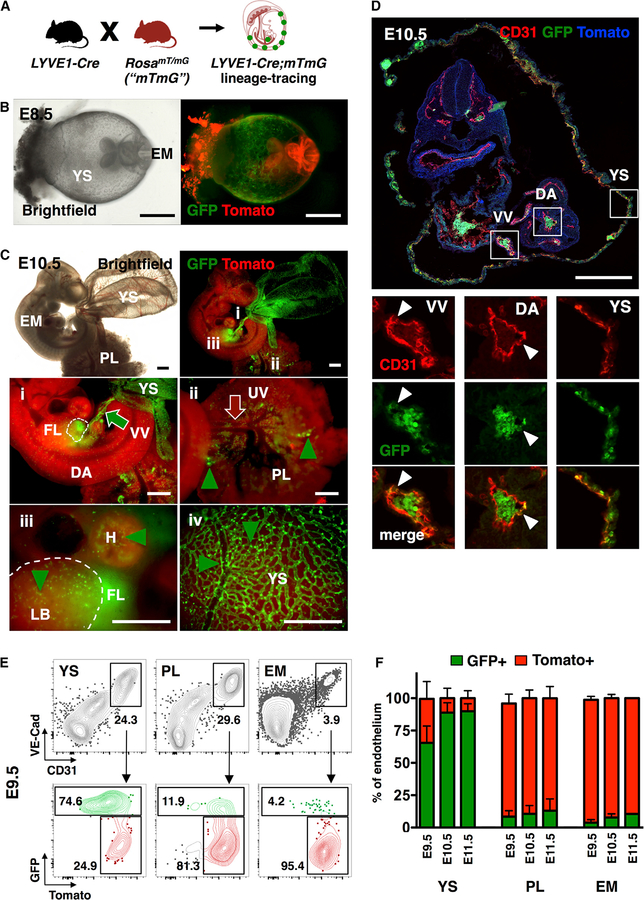
Figure 2. Lyve1-Cre Labels Yolk Sac Endothelium and Vitelline Vessels.
(A) Schematic of mouse model for tracing Lyve1-Cre lineage cells using the RosamT/mG reporter line.
(B) Brightfield and whole mount fluorescence microscopy images of Lyve1-Cre;mTmG E8.5 concepti show the replacement of Tomato (red) by GFP (green) expression in the YS. Representative image from n = 8 concepti from two independent experiments. Scale bar, 500 μm.
(C) Brightfield and whole mount fluorescence microscopy images of YS, EM, and PL at E10.5. (i) shows GFP expression in the fetal liver (FL; dashed outline) and the vitelline vessels (VV; green arrow). (ii) shows intact Tomato activity in PL and umbilical vessels (UV; red arrow) and in the dorsal aorta (DA). GFP+ hematopoietic cells (green arrowheads) are found in PL vasculature (ii), heart (H) and limb bud (LB; dashed outline) (iii), and YS (iv). GFP expression is prominent throughout YS vasculature (iv). Representative image of n = 18 embryos from three independent experiments. Scale bar, 500 μm.
(D) At E10.5, GFP labeling is widespread in YS and VV CD31+ endothelium, but minimal in the DA. Representative image from two independent experiments. Scale bar, 250 μm.
(E) Representative FACS plots of GFP labeling of VE-Cad+CD31+ endothelial cells in E9.5 YS, PL, and caudal half of the EM.
(F) Bar graphs of endothelial cells show robust Lyve1-Cre lineage tracing in YS at E9.5–E11.5. EM refers to the caudal half of the embryo at E9.5 and the aorta-gonad-mesonephros region at E10.5 and E11.5. Data are from pools of embryos from three (E9.5), seven (E10.5), and three (E11.5) independent experiments and are represented as mean ± SD.
See also Table S1.
Analysis of Lyve1-Cre;RosamT/mG concepti by fluorescence microscopy demonstrated Lyve1-Cre labeling already in E8.5 YS (Figure 2B). By E10.5, marking was apparent in YS and vitelline vessels as well as the FL (Figures 2Ci, 2Ciii, and 2Civ), but absent from umbilical and placental large vessels (Figure 2Cii). In contrast to the robust labeling of CD31+ YS and vitelline vessels, the dorsal aorta showed minimal Lyve1-Cre labeling (Figures 2C and 2D).
FACS analysis of hemogenic tissues from Lyve1-Cre; RosamT/mG concepti at E9.5–E11.5 further documented that VE-Cad+CD31+ endothelial cells were GFP labeled abundantly in the YS but minimally in the PL and EM (the latter includes the caudal half at E9.5 and the aorta-gonad-mesonephros region at later ages) (Figures 2E and 2F). These data raised the hypothesis that Lyve1-Cre labels YS hemogenic endothelium.
Lyve1-Cre Lineage Traces YS Definitive HSPCs but Bypasses the Primitive Erythroid Lineage
We next interrogated the contribution of LYVE1 lineage cells to the different hematopoietic compartments. Lyve1-Cre was crossed with a R26-stop-YFP strain, where deletion of the transcriptional stop preceding the YFP sequence in the Rosa26 locus leads to marking of all progenies with YFP (Figure 3A) (Friedrich and Soriano, 1991).
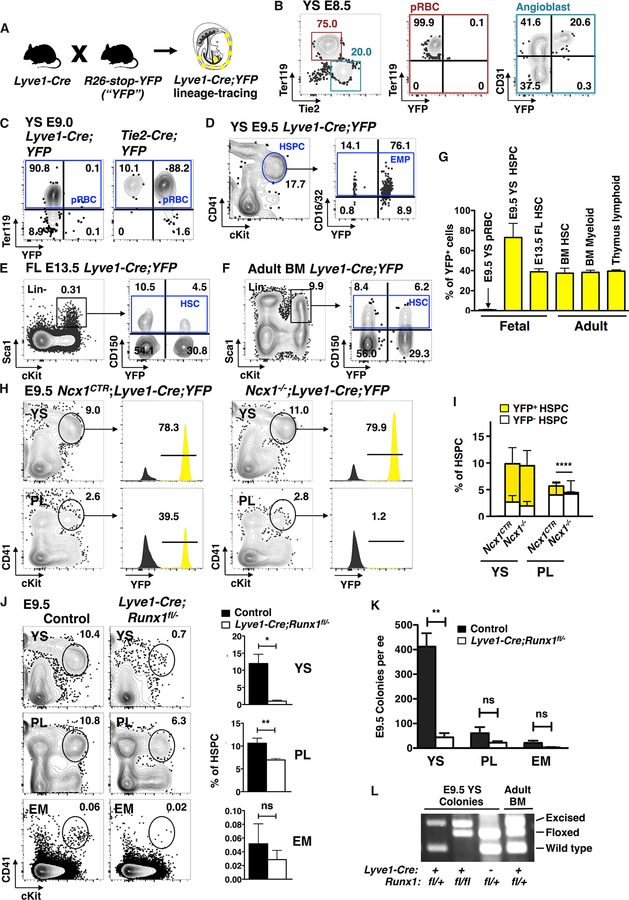
Figure 3. Lyve1-Cre Targets Yolk-Sac-Definitive Hematopoiesis.
(A) Schematic of mouse model for tracing Lyve1-Cre-marked cells using the R26-stop-YFP reporter.
(B) Representative FACS plots of E8.5 conceptus show Lyve1-Cre;YFP labeling in Tie2+CD31+ angioblasts, but not in Ter119+ primitive erythroid cells (pRBCs).
(C) Representative FACS plots of E9.0 YS demonstrate labeling of Ter119+ pRBC by Tie2-Cre, but not by Lyve1-Cre.
(D) Representative FACS plots of E9.5 YS demonstrate labeling of YS CD41midcKit+Ter119− HSPCs and CD16/32+ EMPs by Lyve1-Cre.
(E and F) Representative FACS plots display similar YFP marking in the Lin−Sca1+cKit+CD150+ HSC of E13.5 FL (E) and 8-month-old adult BM (F).
(G) Quantification shows a contrast of YFP marking between pRBCs (n = 4, two independent experiments) and YS HSPCs (n = 15, 7 independent experiments). Lyve1-Cre marking in E13.5 FL Lin−Sca1+cKit+CD150+ HSCs (n = 15, 4 independent experiments) is similar to 8-month-old adult BM HSCs, BM Mac1+Gr1+ myeloid cells (n = 8, two independent experiments), and thymus CD4+CD8+ T lymphocytes (n = 7, two experiments). No comparison of marking between populations yielded statistical difference. Data are represented as mean ± SD.
(H) Representative FACS plots of E9.5 Lyve1-Cre;YFP Ncx1−/− concepti show preservation of YFP+ HSPCs in the YS but near depletion in the PL when blood circulation is absent.
(I) Quantification of YFP+ fraction in HSPCs of E9.5 Ncx1−/− concepti with impaired circulation. Data from n = 16 for Ncx1CTR and n = 5 for Ncx1−/− from three independent experiments are represented as mean ± SD; **p value of the difference in YFP+ fractions at ≤ 0.01.
(J) Representative FACS plots and quantification of HSPCs in hemogenic organs of E9.5 LYve1-Cre;Runx1fl/− tissues show depletion of HSPCs from the YS upon conditional deletion of Runx1 in LYVE1 lineage cells. The loss in HSPCs is partial in the PL and insignificant in the EM. Data from n = 4 for mutants and n = 9 for controls from three independent experiments are represented as mean ± SD. p values are shown as ns if > 0.05; *P ≤ 0.05; **P ≤ 0.01.
(K) Quantification of myelo-erythroid colonies per embryo equivalent (ee) from each organ. Data from n = 4 for mutants and n = 3 for controls from two independent experiments are represented as mean ± SD.
(L) Genotyping of Runx1 alleles in representative colonies.
FACS analysis of E8.5 Lyve1-Cre;YFP YS showed labeling in one-third of angioblasts, whereas none of the Ter119+ primitive erythroid cells were marked (Figure 3B). Likewise, no labeling was observed in Ter119+ cells in E9.0 Lyve1-Cre;YFP YS, although 90% of erythroid cells were labeled when the YFP reporter strain was crossed with Tie2/Tek-Cre mouse, in which Cre is activated in hemato-vascular precursors after gastrulation (Figure 3C) (Kisanuki et al., 2001). 100% labeling was observed with the germ-cell-specific Vasa-Cre mouse, in which Cre is active during fertilization, enabling Cre recombination in all cells of the conceptus (data not shown) (Gallardo et al., 2007). Hence, using two different Cre lines, we verified that the absence of YFP signal in Lyve1-Cre;YFP E9.0 primitive erythroid cells was not explained by silencing of the Rosa26 locus. Later in development, however, Lyve1-Cre tracing of primitive erythroid cells could not be tracked because reporter expression from the Rosa26 locus was silenced as the primitive erythroid cells matured, irrespective of the Cre line used (data not shown).
Lack of Lyve1-Cre labeling in primitive erythroid cells was confirmed by analyzing E9.5 Lyve1-Cre;RosamT/mG concepti. Although many CD43+ hematopoietic cells in YS had switched from tdTomato to GFP expression, CD71+Ter119+ primitive erythroid cells retained tdTomato expression (Figure S2A), documenting absent Lyve1-Cre marking, despite intact activity of the Rosa26 locus. These data confirm that the primitive erythroid lineage does not originate from LYVE1-expressing hemogenic endothelium.
In contrast to the unlabeled primitive erythroid cells, 69.7% ± 14.8% SD of CD41midcKit+ HSPCs in E9.5 Lyve1-Cre;YFP YS was marked by YFP (Figures 3D and 3G). These data imply that Lyve1-Cre marks the stage when the precursors generating YS definitive hemogenic endothelium and HSPCs have separated from the primitive erythroid lineage.
Lyve1-Cre Labels a Subset of Fetal and Adult HSCs
To investigate whether Lyve1-Cre marking is restricted to EMPs or includes HSCs, we assessed the contribution of lineage-traced cells to fetal and adult HSCs. At E13.5, when the FL HSC compartment has become established and is rapidly expanding, 38.9% ± 11.6% SD of the LSK CD150+ HSCs were YFP marked (Figures 3E and 3G). In the adult, comparable fractions of YFP labeling were found among LSK CD150+ HSCs (37.4% ± 11.1% SD) and Mac1+Gr1+ myeloid cells (38.2% ± 6.0% SD) in the bone marrow (BM) and CD4+CD8+ T lymphocytes (39.4% ± 3.5% SD) in the thymus (Figures 3F and 3G). The uniform degree of post-natal Lyve1-Cre marking in all major lineages is in stark contrast to the absent or low expression of LYVE1 protein in HSCs or in cells committed to the different hematopoietic lineages (Figure S2B). These data establish that more than one-third of long-term repopulating HSCs derive from the Lyve1-Cre lineage, and that they were labeled during embryonic development.
YS Is the Primary Source of Lyve1-Cre Lineage-Derived HSPCs at Mid-gestation
Although the YS was the only hemogenic organ that showed extensive Lyve1-Cre labeling of endothelium during HSPC emergence (Figure 2F), the PL (Figures 3H and 3I) and EM (data not shown) also harbored CD41midcKit+ HSPCs with reporter expression. To examine how circulating cells contribute to the HSPC census in each hemogenic organ, YFP fluorescence was examined in blood cells of heartbeat-deficient Ncx1−/− concepti. Although the YS maintained the richly labeled HSPC pool (79% ± 2.8% SD), the absence of circulation in Ncx1−/− mutants depleted YFP+ labeled HSPCs from the PL (Figures 3H and 3I). The CD16/32+ EMP subset in Ncx1−/− mutants also showed a similar redistribution of YFP+ fractions (Figure S2C). These data suggested that the YS is the main source of Lyve1-Cre-line-age-traced HSPCs and EMPs at mid-gestation. Due to the severely defective generation of HSPCs in the Ncx1−/− mutant embryos, labeling of HSPCs generated in this organ could not be reliably assessed.
To target the hematopoietic cells derived from the LYVE1 lineage, Lyve1-Cre was used to delete the Runt-related transcription factor 1 (Runx1) gene, which is required for the generation of EMPs and HSCs. Comparison of Lyve1-Cre;Runx1fl/− concepti to control tissues at E9.5 showed near depletion of CD41midckit+ HSPCs in the conditional knockout YS. Both the PL and EM showed a partial reduction in their HSPC populations, likely reflecting the lack of migration of Lyve1-Cre-depleted HSPCs from the YS. The HSPC populations observed in the PL and EM likely represent the HSPCs generated in situ, which were unaffected by Lyve1-Cre mediated Runx1 deletion (Figure 3J). A parallel defect in myelo-erythroid colony formation was evident in Lyve1-Cre;Runx1fl/− YS (Figure 3K). As expected from the known function of Runx1 in hemogenic endothelium (Chen et al., 2009), genotyping of YS colonies confirmed that only rare cells that had escaped Lyve1-Cre-mediated Runx1 deletion formed colonies (Figure 3L). These genetic labeling and gene ablation data verified that YS HSPCs are efficiently targeted by Lyve1-Cre, whereas the PL and EM harbor native HSPCs that do not originate from the LYVE1 lineage.
Lyve1-Cre Lineage Tracing Identifies a Temporal Switch in the Precursors Responsible for FL Erythropoiesis
To investigate the contribution of Lyve1-Cre lineage cells to FL hematopoiesis, we assessed the fraction of lineage-traced cells in different hematopoietic populations from mid to late gestation. Lyve1-Cre marking in the FL Lin−cKit+CD34+Sca1+ HSC compartment peaked at E11.5 (61.3% ± 17.8% SD), when the FL seeding by HSCs begins, and leveled to 36.4% ± 11.7% SD at E13.5 and 41.4% ± 10.4% SD at E16.5 (Figures 4A and 4D). Of note, CD150, the Slam family marker associated with long-term reconstituting HSCs in mice (Kiel et al., 2005) was not used in E11.5 FL because it is not reliably expressed in HSCs during early FL colonization (McKinney-Freeman et al., 2009). However, incorporating CD150 expression to further characterize HSCs at E13.5 did not change their fraction of Lyve1-Cre marking (data not shown). These data suggest that LYVE1-lineage cells are major contributors to initially colonize the FL HSC compartment, whereas by E13.5, non-lineage-traced HSCs co-seed the FL. The uniform degree of HSC marking from E13.5 to adult implies that the respective contributions of Lyve1-Cre labeled and non-labeled HSCs to the HSC pool remains stable after the FL HSC compartment has been established by E13.5.
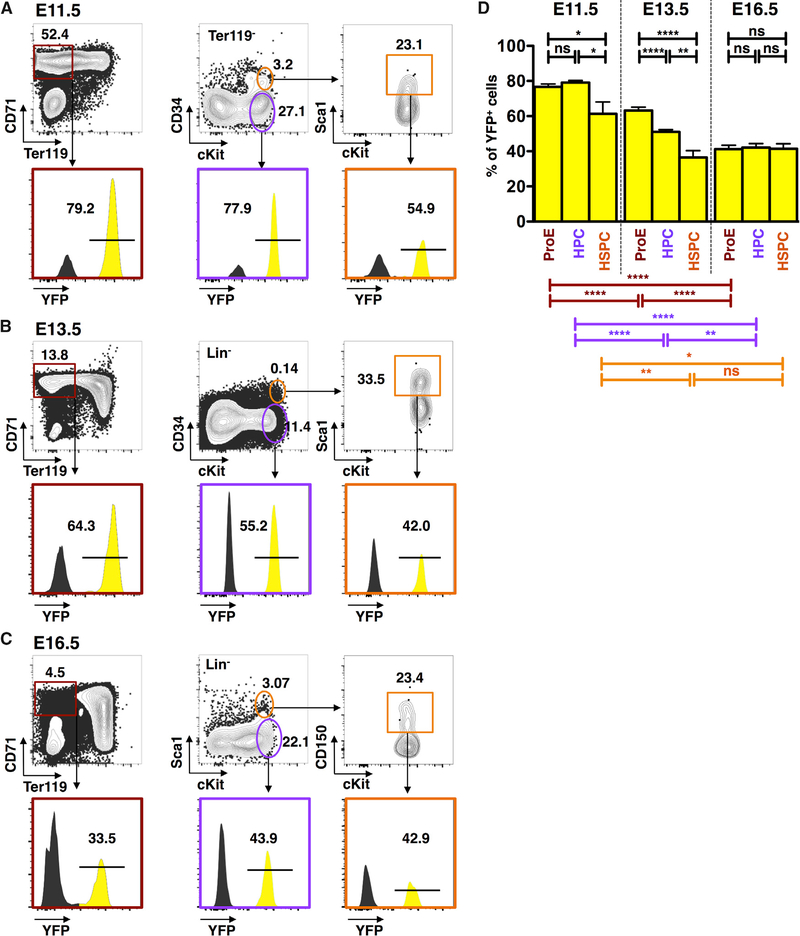
Figure 4. Progenitors of Lyve1-Cre Lineage Initiate Fetal Liver Hematopoiesis.
(A) Representative FACS plots of Lyve1-Cre marking in hematopoietic subpopulations in one representative E11.5 FL: Ter119− CD71+ proerythroblasts (ProEs), Ter119−cKit+ HPCs, and Ter119−CD34+cKit+Sca1+ HSPCs.
(B) Representative FACS plots of FL subsets at E13.5.
(C) Representative FACS plots of FL subsets at E16.5.
(D) Comparative analysis of E11.5, E13.5, and E16.5 FL illustrates a shift in hematopoietic populations derived from the Lyve1-Cre lineage. Data from n = 9, three independent experiments for E11.5, n = 18, three independent experiments for E13.5, and n = 13, two independent experiments for E16.5, are represented as mean ± SD. p values are shown as ns if > 0.05; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001.
See also Table S1.
In addition to serving as a site for HSC expansion, the FL supports definitive erythropoiesis through most of the fetal life. Whereas the primitive erythroid cells differentiate in the YS and undergo maturation in circulation, the progenitors for definitive erythroid cells must transit through the FL to differentiate and enucleate (McGrath et al., 2011). It has been proposed that the precursors that first colonize the FL and initiate definitive erythropoiesis derive from the YS, but their origin and the duration of their contribution have not been experimentally proven.
FACS analysis of Lyve1-Cre;YFP FL at E11.5 revealed that it contained CD71+Ter119− pro-erythroblasts (ProE) and ckit+ CD34− hematopoietic progenitor cells (HPCs) with extensive YFP marking (76.7% ± 4.7% SD and 79.3% ± 3.0% SD, respectively) (Figures 4A and 4D), which was comparable to the level measured in E9.5 YS CD41midcKit+ HSPCs (Figures 3D and 3G). This suggests that the YS supplies the precursors that first colonize the FL to initiate definitive erythropoiesis. By E13.5, 63.2% ± 8.7% SD of the ProE and 51.0% ± 5.5% SD of FL HPCs derived from the LYVE1 lineage (Figures 4B and 4D). These compartments displayed significantly higher marking than the Lin−Sca1+cKit+CD34+ HSC subset (36.4% ± 11.7% SD), implicating that the progenitors responsible for FL-definitive erythropoiesis derive from a different source than most HSCs at this stage. By E16.5, the ProE- and HSC-enriched populations exhibited similar degrees of Lyve1-Cre marking (41.2% ± 8.1% SD and 41.4% ± 10.3% SD, respectively), suggesting that by late gestation, HSCs become responsible for FL erythropoiesis (Figures 4C and 4D).
Lyve1-Cre-Mediated Deletion of Scl/Tal1 Targets Early FL Definitive Erythropoiesis but Spares the Primitive Erythroid Lineage
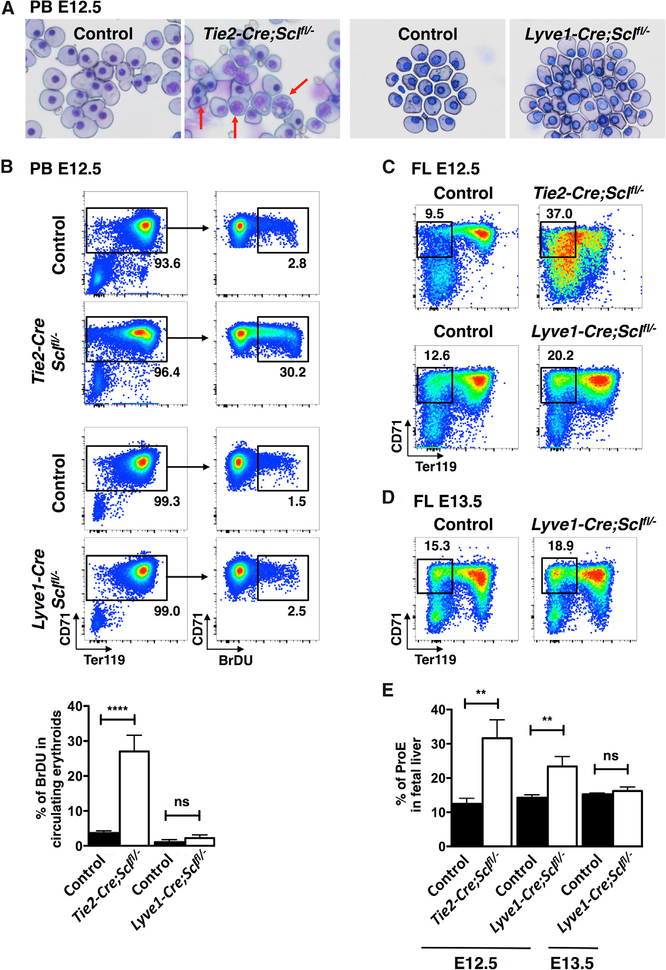
To confirm that the Lyve1-Cre strain can be used to manipulate early definitive erythropoiesis without affecting primitive erythropoiesis, we created a Lyve1-Cre-mediated conditional knockout of the transcription factor SCL. Although SCL is essential for the specification of hemogenic endothelium (Porcher et al., 1996; Van Handel et al., 2012), it is dispensable for HSC maintenance (Mikkola et al., 2003a) because of redundancy with LYL1 (Souroullas et al., 2009). Because SCL is required for the terminal differentiation of all erythroid cells, inactivation of Scl by Tie2-Cre results in defects in both primitive and definitive erythropoiesis (Schlaeger et al., 2005). As previously reported, May-Grunwald Giemsa stain of E12.5 peripheral blood from Tie2-Cre;Sclfl/− mutants displayed abnormal primitive erythroid cells that were mitotic, binucleated, or had a larger nucleus-to-cytoplasm ratio than control littermates, implying defective maturation (Figure 5A). Furthermore, 2-hr 5-Bromo-2’-deoxyuridine (BrDU) pulse analysis showed a high fraction of mutant erythroid cells undergoing DNA replication (Figure 5B). In contrast, when Scl was excised in Lyve1-Cre-expressing cells, the primitive erythroid cells did not show abnormal morphology or proliferation, indicating that they had been spared from Scl inactivation (Figures 5A and 5B).
Figure 5. Lyve1-Cre Targets Early Fetal Liver Definitive Hematopoiesis.
(A) May-Grunwald Giemsa stain of E12.5 peripheral blood (PB) shows primitive erythroid defects in Tie2-Cre;Sclfl/− blood cells (arrows), but not in Lyve1-Cre;Sclfl/− blood. Representative images from three independent experiments are shown.
(B) Representative FACS plots of BrDU staining of E12.5 PB illustrate excessive proliferative activity in Tie2-Cre;Sclfl/− primitive erythroid cells, but not in Lyve1-Cre;Sclfl/− cells. Bar graphs from n = 22 controls and n = 4 knockouts from three independent Tie2-Cre;Sclfl/− experiments and n = 6 controls and n = 3 knockouts from two independent Lyve1-Cre;Sclfl/− experiments are shown as mean ± SD.
(C) E12.5 FLs from both Tie2-Cre;Sclfl/− (upper panel) and Lyve1-Cre;Sclfl/− (lower panel) embryos exhibit accumulation of proerythroblasts (ProEs).
(D) At E13.5, the defect in ProE fraction in Lyve1-Cre;Sclfl/− FL has corrected.
(E) Quantification of ProE fractions in E12.5 and E13.5 FL. Data from n = 2 (E12.5 Tie2-Cre;Sclfl/−) and n = 6 (control), n = 3 (E12.5 Lyve1-Cre;Sclfl/−) and n = 7 (control), and n = 4 (E13.5 Lyve1-Cre; Sclfl/−) and n = 14 (control) from two independent experiments for each genotype and age are shown as mean ± SD.
See also Table S1.
Both Tie2-Cre;Sclfl/− and Lyve1-Cre;Sclfl/− E12.5 fetal livers showed an increase in the CD71+Ter119− ProE fraction (Figures 5C and 5E), consistent with delayed maturation reported in Scl-deficient definitive erythroid cells (Mikkola et al., 2003a; Schlaeger et al., 2005). This defect was ameliorated by E13.5 (Figures 5D and 5E), likely due to replenishment of the FL ProE pool by a new source of unaffected precursors. These data verify that Lyve1-Cre deletes Scl specifically from the transient-definitive, but not primitive erythroid cells, enabling studies that address the impact of the different erythroid waves on embryonic development.
DISCUSSION
The challenge of tracking the origin and contribution of the different waves of developmental hematopoiesis in vivo has limited the understanding of their unique roles in the embryo and adult. We report that LYVE1 expression and lineage tracing can be used to specifically examine the definitive hematopoiesis originating from the YS and/or vitelline vessels without targeting the primitive erythroid lineage or the majority of the HSC compartment generated elsewhere in the conceptus.
LYVE1 protein expression and Lyve1-Cre fate mapping distinguished endothelial cells from the YS and vitelline vessels from those from other hemogenic organs at the developmental window when HSPCs emerge. Although LYVE1+ EMPs and HSPCs could be detected in all hemogenic organs in concepti with normal circulation, analysis of heartbeat-deficient Ncx1−/− concepti implied that the YS is the main source of LYVE1+ HSPC and EMPs. These data propose that some, if not all, HSCs of Lyve1-Cre lineage, which constitute more than one-third of the fetal and the adult HSC pools, originate from the YS and/or vitelline vessels. However, because LYVE1 protein expression appears in PL vasculature at late gestation and a low level of Lyve1-Cre marking is noted in PL and EM endothelium at mid-gestation, our data do not exclude the possibility that other hemogenic organs may also contribute to the LYVE1-lineage-traced HSC pool. The Ncx1−/− model cannot be used to assess adult-repo-pulating HSC activity of Lyve1-Cre-marked cells because these embryos die by E10.0–before transplantable HSCs can be har-vested–and because heartbeat is critical for proper HSC development (North et al., 2009). Hence, the question of whether all or most of the 35%−40% Lyve1-Cre-marked fetal and adult HSPCs arise from the YS and/or vitelline vessels requires further studies using different experimental systems. Nevertheless, because the YS generates predominantly Lyve1-Cre-marked HSPCs, while the PL and the EM produce mostly unmarked ones, our data propose that HSCs from multiple anatomical sites contribute to adult hematopoiesis. How these different lineages of HSCs contribute to the various hematopoietic subsets in the adult can now be approached experimentally in vivo using the Lyve1-Cre model. This is important because HSCs are heterogeneous and can show cell intrinsic biases to different hematopoietic lineages in an age-dependent manner (Beerman et al., 2010; Benz et al., 2012; Pang et al., 2011; Sieburg et al., 2006; Yamamoto et al., 2013).
Our data document that the LYVE1+ endothelium in the YS does not give rise to primitive erythroid cells. The absence of LYVE1 protein expression and marking in the primitive erythroid cell contrasts with the abundant protein expression and marking in YS definitive HSPC. These data imply that these two hematopoietic lineages have separated from a common mesodermal precursor before Lyve1-Cre becomes active. The YS thus gives rise to at least two lineages of erythroid cells that originate from distinct precursors. The Lyve1-Cre lineage trace may thus provide clarity to studies that challenged the “lineage switch model” by reporting that the types of globins expressed in primitive or definitive erythroid lineages are not mutually exclusive because the cells undergo “maturational globin switch” during erythroid differentiation (Kingsley et al., 2006; McGrath et al., 2011; Palis, 2008; Qiu et al., 2008).
LYVE1 expression and lineage tracing offer a powerful tool to separate primitive erythroid lineage from early fetal definitive erythropoiesis. The defects in both primitive and definitive erythroid cell differentiation caused by the conditional deletion of Scl by Tie2-Cre (Schlaeger et al., 2005) were uncoupled using Lyve1-Cre, which disrupted only the early FL definitive erythropoiesis. Selective inactivation of key hematopoietic regulators in Lyve1-Cre progenies makes it possible to study the requirement of such factors in early FL erythropoiesis without the confounding effects of early embryonic lethality, as in Scl null embryos (Porcher et al., 1996; Shivdasani et al., 1995), or primitive erythroid defects, as in Tie2-Cre;Sclfl/− concepti.
The Lyve1-Cre model also provides in vivo evidence that the earliest progenitors seeding and initiating FL erythropoiesis are products of a distinct lineage that likely emerges from the YS. However, the deletion of Scl in LYVE1-lineage cells causes only a temporary effect on FL erythropoiesis, possibly because progenitors from other sources that were spared from Lyve1-Cre deletion also converge to the FL and replenish the initially attenuated erythroid compartment. Future studies will inform whether the erythroid progenitors or HSCs belonging to the LYVE1 lineage exhibit different properties than those arising from non-LYVE1-lineage precursors.
LYVE1 is expressed abundantly in lymphatic vessels and lymph node sinus endothelial cells, but its expression has also been described in other cell types, such as liver blood sinusoids and tissue macrophages (Mouta Carreira et al., 2001; Pham et al., 2010; Schledzewski et al., 2006). In the YS, LYVE1 expression is not related to lymphatic identity because the YS does not contain lymphatic vasculature (Banerji et al., 1999; Gordon et al., 2008). It is unknown how the YS- and vitelline-vessel-restricted expression pattern of LYVE1 among hemogenic vessels is established, and whether this is intrinsically regulated or if it involves signals from the microenvironment. It will be interesting to determine whether LYVE1 expression is also enriched in YS and vitelline vessels and HSPCs during human development, and how the expression pattern is preserved during ESC differentiation.
The functional significance of LYVE1 expression in YS endothelial or hematopoietic cells is unknown. Although it was suggested to serve in cell adhesion/transmigration and as a scavenger for hyaluronan turnover, mouse knockout studies in either 129/C57BL/6 or C57BL/6 background were unable to demonstrate a critical function for LYVE1 in these processes or in embryonic development in general (Gale et al., 2007). The Cre cassette was inserted into the 3′UTR of the endogenous Lyve1 locus, avoiding disruption in the LYVE1 protein. Hence, our model cannot be used to answer whether loss of LYVE1 causes subtle effects in hematopoiesis that would be observed upon more detailed examination or backcrossing to other genetic backgrounds. However, because of the hematopoietic wave- and tissue-restricted expression of LYVE1, the Lyve1-Cre model can be exploited to uncover new functions for the different blood progenitor lineages and HSC sources during developmental and adult hematopoiesis.
EXPERIMENTAL PROCEDURES
Mouse Models
Lyve1-eGFP-hCre knockin mice (C57BL/6) (Pham et al., 2010) were crossed with two reporter lines: (1) R26-YFP carrying a floxed transcriptional stop upstream of the YFP in Rosa26 locus (Srinivas et al., 2001), and (2) double-fluorescent reporter (RosamT/mG), where cells express Tomato constitutively but switch to GFP upon excision (Muzumdar et al., 2007). Whereas YFP or GFP fluorescence rendered from crossing with the reporter lines could clearly highlight the Cre-excised cells, eGFP from Lyve1-eGFP-hCre alone could not be discerned by fluorescence microscopy, as previously reported (Pham et al., 2010). FACS analysis confirmed the low eGFP signal from Lyve1-eGFP-hCre, which did not overlap with the intense YFP or GFP signal. Hence, for simplicity, the Lyve1-eGFP-hCre is referred as Lyve1-Cre. To label the entire hemato-vascular compartment, Tie-2/Tek-Cre mice (Kisanuki et al., 2001) were bred with the R26-YFP line. To label all cells of the conceptus, the germ-cell-specific Vasa-Cre was crossed with the R26-YFP line. Lyve1-Cre;YFP mice were further crossed with Ncx1+/− mice (Koushik et al., 2001) to generate Ncx1−/− mutants that underwent Lyve1-Cre labeling and lacked circulation. Although some contaminating embryonic red blood cells (RBCs) could occasionally be found in the embryo proper of Ncx1−/− concepti, only embryos with less than 5% RBC contamination were used in the analysis. Lyve1-Cre was also bred with Runx1fl/− (Growney et al., 2005; Wang et al., 1996) and Sclfl/− (Mikkola et al., 2003a; Shivdasani et al., 1995). Embryos were not separated based on sex, and both female and male embryos were studied. Mice were maintained according to protocols of Animal Research Committee at the University of California, Los Angeles, and the University of California, Irvine, who approved these studies.
See the Supplemental Experimental Procedures for preparation of tissue sections, immunostaining, flow cytometry, clonogenic progenitor assay, cytospin, cell proliferation assay, and statistical analysis.
Supplementary Material
Highlights.
LYVE1 is expressed in yolk sac endothelium and hematopoietic stem and progenitor cells
Lyve1-Cre marks yolk sac definitive hematopoiesis but bypasses primitive erythropoiesis
LYVE1 lineage cells initiate fetal liver definitive erythropoiesis
A subset of fetal and adult hematopoietic stem cells are derived from the LYVE1 lineage
ACKNOWLEDGMENTS
We thank Drs. Calvanese, Montecino-Rodriguez, and Dorshkind for discussions. We thank the UCLA Translational Pathology Core Laboratory for preparing the paraffin tissue sections, and Vanessa Scarfone and the UCI Sue and Bill Gross Stem Cell Research Center FACS core for flow cytometry. We thank Drs. Speck and Gilliland for providing the Runx1fl/fl and Runx1 knockout mice and Dr. Orkin for providing the Sclfl/fl and Scl knockout mice.
This work was supported by NIH R01 HL097766, NIH R01 DK100959, and Leukemia Lymphoma Society Scholar Award (1269-12) to H.K.A.M, NIH R56 HL133656 to M.A.I, TG2-01169 CIRM Type I Training Grant, the American Association of Obstetricians and Gynecologists Foundation Scholarship, and the Specialty Training and Advanced Research Training Fellowship at UCLA to L.K.L, and by the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA and Jonsson Cancer Center Foundation.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, two figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2016.10.080.
REFERENCES
- Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, and Jackson DG (1999). LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 144, 789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerman I, Bhattacharya D, Zandi S, Sigvardsson M, Weissman IL, Bryder D, and Rossi DJ (2010). Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl. Acad. Sci. USA 107, 5465–5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz C, Copley MR, Kent DG, Wohrer S, Cortes A, Aghaeepour N, Ma E, Mader H, Rowe K, Day C, et al. (2012). Hematopoietic stem cell subtypes expand differentially during development and display distinct lymphopoietic programs. Cell Stem Cell 10, 273–283. [DOI] [PubMed] [Google Scholar]
- Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, and Traver D (2010). Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464, 108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, and Robin C (2010). In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464, 116–120. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, and Speck NA (2009). Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 457, 887–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Li Y, De Obaldia ME, Yang Q, Yzaguirre AD, Yamada-Ina-gawa T, Vink CS, Bhandoola A, Dzierzak E, and Speck NA (2011). Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell 9, 541–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilken HM, Nishikawa S, and Schroeder T (2009). Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature 457, 896–900. [DOI] [PubMed] [Google Scholar]
- Espín-Palazcón R, Stachura DL, Campbell CA, García-Moreno D, Del Cid N, Kim AD, Candel S, Meseguer J, Mulero V, and Traver D (2014). Proinflammatory signaling regulates hematopoietic stem cell emergence. Cell 159, 1070–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferkowicz MJ, Starr M, Xie X, Li W, Johnson SA, Shelley WC, Morrison PR, and Yoder MC (2003). CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development 130,4393–4403. [DOI] [PubMed] [Google Scholar]
- Friedrich G, and Soriano P (1991). Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 5, 1513–1523. [DOI] [PubMed] [Google Scholar]
- Gale NW, Prevo R, Espinosa J, Ferguson DJ, Dominguez MG, Yanco-poulos GD, Thurston G, and Jackson DG (2007). Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol. Cell. Biol 27, 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo T, Shirley L, John GB, and Castrillon DH (2007). Generation of a germ cell-specific mouse transgenic Cre line, Vasa-Cre. Genesis 45, 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekas C, Dieterlen-Lièvre F, Orkin SH, and Mikkola HK (2005). The placenta is a niche for hematopoietic stem cells. Dev. Cell 8, 365–375. [DOI] [PubMed] [Google Scholar]
- Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, and Rodewald HR (2015). Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518, 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EJ, Gale NW, and Harvey NL (2008). Expression of the hyaluronan receptor LYVE-1 is not restricted to the lymphatic vasculature; LYVE-1 is also expressed on embryonic blood vessels. Dev. Dyn 237, 1901–1909. [DOI] [PubMed] [Google Scholar]
- Growney JD, Shigematsu H, Li Z, Lee BH, Adelsperger J, Rowan R, Curley DP, Kutok JL, Akashi K, Williams IR, et al. (2005). Loss of Runx1 perturbs adult hematopoiesis and is associated with a myeloproliferative phenotype. Blood 106, 494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haar JL, and Ackerman GA (1971). A phase and electron microscopic study of vasculogenesis and erythropoiesis in the yolk sac of the mouse. Anat. Rec 170, 199–223. [DOI] [PubMed] [Google Scholar]
- Inlay MA, Serwold T, Mosley A, Fathman JW, Dimov IK, Seita J, and Weissman IL (2014). Identification of multipotent progenitors that emerge prior to hematopoietic stem cells in embryonic development. Stem Cell Reports 2, 457–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, and Morrison SJ (2005). SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121. [DOI] [PubMed] [Google Scholar]
- Kingsley PD, Malik J, Emerson RL, Bushnell TP, McGrath KE, Bloedorn LA, Bulger M, and Palis J (2006). “Maturational” globin switching in primary primitive erythroid cells. Blood 107, 1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, and Yanagisawa M (2001). Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev. Biol 230, 230–242. [DOI] [PubMed] [Google Scholar]
- Koushik SV, Wang J, Rogers R, Moskophidis D, Lambert NA, Creazzo TL, and Conway SJ (2001). Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. FASEB J. 15, 1209–1211. [DOI] [PubMed] [Google Scholar]
- Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, and Lacaud G (2009). The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature 457, 892–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath KE, Koniski AD, Malik J, and Palis J (2003). Circulation is established in a stepwise pattern in the mammalian embryo. Blood 101, 1669–1676. [DOI] [PubMed] [Google Scholar]
- McGrath KE, Frame JM, Fromm GJ, Koniski AD, Kingsley PD, Little J, Bulger M, and Palis J (2011). A transient definitive erythroid lineage with unique regulation of the β-globin locus in the mammalian embryo. Blood 117, 4600–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath KE, Frame JM, Fegan KH, Bowen JR, Conway SJ, Catherman SC, Kingsley PD, Koniski AD, and Palis J (2015). Distinct sources of hematopoietic progenitors emerge before HSCs and provide functional blood cells in the mammalian embryo. Cell Rep. 11, 1892–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney-Freeman SL, Naveiras O, Yates F, Loewer S, Philitas M,Curran M, Park PJ, and Daley GQ (2009). Surface antigen phenotypes of hematopoietic stem cells from embryos and murine embryonic stem cells. Blood 114, 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvinsky A, and Dzierzak E (1996). Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 86, 897–906. [DOI] [PubMed] [Google Scholar]
- Mikkola HK, and Orkin SH (2006). The journey of developing hematopoietic stem cells. Development 133, 3733–3744. [DOI] [PubMed] [Google Scholar]
- Mikkola HK, Klintman J, Yang H, Hock H, Schlaeger TM, Fujiwara Y, and Orkin SH (2003a). Haematopoietic stem cells retain long-term repopulating activity and multipotency in the absence of stem-cell leukaemia SCL tal-1 gene. Nature 421, 547–551. [DOI] [PubMed] [Google Scholar]
- Mikkola HKA, Fujiwara Y, Schlaeger TM, Traver D, and Orkin SH (2003b). Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood 101, 508–516. [DOI] [PubMed] [Google Scholar]
- Moore MA, and Metcalf D (1970). Ontogeny ofthe haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br. J. Haematol 18, 279–296. [DOI] [PubMed] [Google Scholar]
- Mouta Carreira C, Nasser SM, di Tomaso E, Padera TP, Boucher Y, Tomarev SI, and Jain RK (2001). LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res. 61, 8079–8084. [PubMed] [Google Scholar]
- Müller AM, Medvinsky A, Strouboulis J, Grosveld F, and Dzierzak E (1994). Development of hematopoietic stem cell activity in the mouse embryo. Immunity 1, 291–301. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, and Luo L (2007). A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605. [DOI] [PubMed] [Google Scholar]
- North TE, Goessling W, Peeters M, Li P, Ceol C, Lord AM, Weber GJ, Harris J, Cutting CC, Huang P, et al. (2009). Hematopoietic stem cell development is dependent on blood flow. Cell 137, 736–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Org T, Duan D, Ferrari R, Montel-Hagen A, Van Handel B, Kerényi MA, Sasidharan R, Rubbi L, Fujiwara Y, Pellegrini M, et al. (2015). Scl binds to primed enhancers in mesoderm to regulate hematopoietic and cardiac fate divergence. EMBO J. 34, 759–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palis J, Robertson S, Kennedy M, Wall C, and Keller G (1999). Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 126, 5073–5084. [DOI] [PubMed] [Google Scholar]
- Palis J (2008). Ontogeny of erythropoiesis. Curr. Opin. Hematol 15, 155–161. [DOI] [PubMed] [Google Scholar]
- Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, Schrier SL, and Weissman IL (2011). Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl. Acad. Sci. USA 108, 20012–20017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham THM, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R, Coughlin SR, McDonald DM, Schwab SR, and Cyster JG (2010). Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med 207, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcher C, Swat W, Rockwell K, Fujiwara Y, Alt FW, and Orkin SH (1996). The T cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. Cell 86, 47–57. [DOI] [PubMed] [Google Scholar]
- Qiu C, Olivier EN, Velho M, and Bouhassira EE (2008). Globin switches in yolk sac-like primitive and fetal-like definitive red blood cells produced from human embryonic stem cells. Blood 111, 2400–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, Conway S, Orkin SH, Yoder MC, and Mikkola HK (2008). The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell 2, 252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samokhvalov IM, Samokhvalova NI, and Nishikawa S (2007). Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature 446, 1056–1061. [DOI] [PubMed] [Google Scholar]
- Schlaeger TM, Mikkola HK, Gekas C, Helgadottir HB, and Orkin SH (2005). Tie2Cre-mediated gene ablation defines the stem-cell leukemia gene (SCL/tal1)-dependent window during hematopoietic stem-cell development. Blood 105, 3871–3874. [DOI] [PubMed] [Google Scholar]
- Schledzewski K, Falkowski M, Moldenhauer G, Metharom P, Kzhysh-kowska J, Ganss R, Demory A, Falkowska-Hansen B, Kurzen H, Ugurel S, et al. (2006). Lymphatic endothelium-specific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80+, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: implications for the assessment of lymphangiogenesis. J. Pathol 209, 67–77. [DOI] [PubMed] [Google Scholar]
- Shivdasani RA, Mayer EL, and Orkin SH (1995). Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature 373, 432–434. [DOI] [PubMed] [Google Scholar]
- Sieburg HB, Cho RH, Dykstra B, Uchida N, Eaves CJ, and Muller-Sieburg CE (2006). The hematopoietic stem compartment consists of a limited number of discrete stem cell subsets. Blood 107, 2311–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souroullas GP, Salmon JM, Sablitzky F, Curtis DJ, and Goodell MA (2009). Adult hematopoietic stem and progenitor cells require either Lyl1 or Scl for survival. Cell Stem Cell 4, 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, and Costantini F (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol 1, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Handel B, Montel-Hagen A, Sasidharan R, Nakano H, Ferrari R, Boogerd CJ, Schredelseker J, Wang Y, Hunter S, Org T, et al. (2012). Scl represses cardiomyogenesis in prospective hemogenic endothelium and endocardium. Cell 150, 590–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Stacy T, Binder M, Marin-Padilla M, Sharpe AH, and Speck NA (1996). Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc. Natl. Acad. Sci. USA 93, 3444–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman IL, Baird S, Gardner RL, Papaioannou VE, and Raschke W (1977). Normal and neoplastic maturation of T-lineage lymphocytes. Cold Spring Harb. Symp. Quant. Biol 41, 9–21. [DOI] [PubMed] [Google Scholar]
- Weissman I, Papaioannou V, and Gardner R (1978). Fetal hematopoietic origins of the adult hematolymphoid system In Differentiation of Normal and Neoplastic Hematopoietic Cells, Clarkson B, Mark P, and Till J, eds. (Cold Spring Harbor Laboratory Press; ), pp. 33–47. [Google Scholar]
- Yamamoto R, Morita Y, Ooehara J, Hamanaka S, Onodera M, Rudolph KL, Ema H, and Nakauchi H (2013). Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell 154, 1112–1126. [DOI] [PubMed] [Google Scholar]
- Zeigler BM, Sugiyama D, Chen M, Guo Y, Downs KM, and Speck NA (2006). The allantois and chorion, when isolated before circulation or chorioallantoic fusion, have hematopoietic potential. Development 133, 4183–4192. [DOI] [PubMed] [Google Scholar]
- Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, et al. (2008). Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell 3, 625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.